Abstract
Research into the biology of extracellular vesicles (EVs), including exosomes and microvesicles, has expanded significantly with advances in EV isolation techniques, a better understanding of the surface markers that characterize exosomes and microvesicles, and greater information derived from –omics approaches on the proteins, lipids, mRNAs, and microRNAs (miRNAs) transported by EVs. We have recently discovered a role for exosome-derived miRNAs in age-related bone loss and osteoarthritis, two conditions that impose a significant public health burden on the aging global population. Previous work has also revealed multiple roles for EVs and their miRNAs in muscle regeneration and congenital myopathies. Thus, EVs appear to be involved in a number of degenerative conditions that impact the musculoskeletal system, indicating that the musculoskeletal system is an excellent model for investigating the role of EVs in tissue maintenance and repair. This review highlights the role of EVs in bone, skeletal muscle, and joint health, including both normal tissue metabolism as well as tissue injury repair and regeneration. A consistent theme that emerges from study of musculoskeletal EVs is that various miRNAs appear to mediate a number of key pathological processes. These findings point to a potential therapeutic opportunity to target EV-derived miRNAs as a strategy for improving musculoskeletal function.
Keywords: exosomes, muscular dystrophy, osteoporosis, osteoarthritis, microRNA
Introduction
Research on extracellular vesicles (EVs), including exosomes and microvesicles, has expanded significantly in recent years. This expanded knowledge is due in part to advances in EV isolation techniques, a better understanding of the surface markers that characterize exosomes and microvesicles, and greater information derived from –omics approaches on the proteins, lipids, mRNAs, and microRNAs (miRNAs) transported by EVs [1–4]. Exosomes and microvesicles differ in size, with exosomes being smaller (40–150 nm) in diameter and microvesicles being larger (>150 nm). These EVs also differ in their biogenesis, where exosomes develop by inward budding of the plasma membrane, formation of a multivesicular body (MVB), and then MVB fusion with the plasma membrane to release the exosome particles. In contrast, microvesicles form by direct outward budding of the plasma membrane. EVs can be isolated by sequential ultracentrifugation, precipitation using polyethylene glycol-based reagents, or size exclusion chromatography and are now characterized by well-established markers (http://exocarta.org/exosome_markers). Together, this work has established EV-mediated transport of signaling factors as a novel mode of intercellular communication and epigenetic regulation [5]. EVs are thought to contribute to a number of important physiological functions such as immune responses, tissue repair, and neural communication via their role(s) in intercellular transport [6]. In addition, EVs appear to play key roles in cancer progression and metastasis [7]. Because EVs have been shown to play important roles in both normal and abnormal physiology, they are also likely to be involved in the normal maintenance and degeneration of musculoskeletal tissues. Indeed, we have recently highlighted a role for exosome-derived miRNAs in age-related bone loss and osteoarthritis [8, 9], two conditions that impose a significant public health burden on the aging global population. This review highlights the role of EVs in bone, skeletal muscle, and joint health, including normal tissue metabolism as well as tissue injury repair and regeneration.
Extracellular vesicles in muscle cell differentiation, muscle regeneration, and congenital myopathies
Skeletal muscle comprises approximately 30–40% of body weight. Myofibers are prone to injury because they are regularly exposed to high contractile forces that can damage the sarcolemma, especially during eccentric muscle contraction [10]. The response of muscle fibers to injury and mechanical stress ranges from plasma membrane repair to a regenerative process involving the recruitment of satellite cells. The congenital absence of dystrophin elevates the incidence of muscle injury, ultimately leading to long-term skeletal muscle dysfunction. Muscle regeneration requires the coordinate, sequential expression of various factors including secreted proteins, inflammatory cytokines, miRNAs, and membrane lipids [10–12]. EVs are actively secreted by differentiating myoblasts throughout this process [13], these EVs can enhance muscle regeneration, and circulating muscle-derived EVs may serve as biomarkers of disease progression in congenital myopathies.
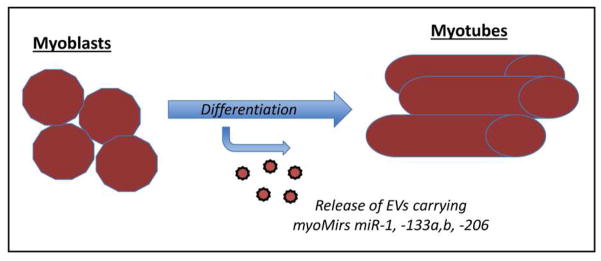
Evidence from several in vitro studies reveals that the secretion of EVs increases during muscle differentiation (Fig. 1). This has been demonstrated using mouse C2C12 cells [14] as well as primary human myoblasts [15]. EVs derived from myoblasts contain growth factors that act as potential regulators of development, function, and repair such as basic fibroblast growth factor (bFGF), insulin-like growth factor-1 (IGF-1), transforming growth factor-beta1 (TGF-B1), and vascular endothelial growth factor (VEGF), among others (VEGF R3, PDGF) [16–18]. Importantly, these paracrine growth factors play a significant role in muscle satellite cell chemotaxis and lineage commitment. Additional studies have sought to define the biological activity of EVs derived from differentiating myoblasts and fully differentiated myotubes. Fluorescent labeling approaches have been employed to detect EVs in the cytoplasm of treated myoblasts [19, 20]. Thus, muscle cells not only secrete EVs but muscle cells also readily endocytose these vesicles. In vitro studies also provide insights into the potential functions of these EVs. Myotube-derived EVs can promote the differentiation of myoblasts by altering expression of cyclin-D1 and myogenin [19, 21]. It is important to note here that while EVs are secreted by both myoblasts and myotubes (Fig. 1), telocytes may also represent another source of EVs in muscle. These stromal cells have projections called telopods that extend into skeletal muscle interstitium [22]. Importantly, these projects are found to be associated in situ with exosomes and other shed vesicles, suggesting a potential role for telocyte-derived EVs in cellular signaling during muscle regeneration [22].
Figure 1.
Muscle regeneration requires the commitment of satellite cells to the myoblast lineage, and their further maturation into myotubes. Specific muscle-derived microRNAs termed myoMirs, including miR-1, 133a,b, and -206 show increased expression during myoblast differentiation. These microRNAs are also secreted in EVs during myogenic differentiation, presumably favoring the differentiation and maturation of neighboring myoblasts [24].
The in vitro studies referenced above suggest that muscle cells secrete EVs, which may in turn be endocytosed by neighboring myoblasts, impacting their potential for proliferation and differentiation. These observations point to a role for muscle-derived EVs in mediating muscle regeneration. This potential role for EVs is supported by work indicating that treatment of wounded muscle with muscle-derived EVs reduces fibrosis and increases the number of regenerating myofibers [15]. A set of muscle-specific miRNAs termed myoMirs, which includes miR-1, -133a,b, and -206, have been identified in skeletal muscle [11, 19, 23–25]. Not surprisingly, EVs released from skeletal muscle are enriched in these miRNAs, and circulating muscle-derived EVs transport these miRNAs [26]. Moreover, muscle injury and calcium influx stimulate EV release [24], further implicating EVs as signaling molecules involved in communication during injury and repair. MiR-206 is induced by MyoD, and miR-206 can in turn promote myoblast differentiation through the downregulation of Twist-1 [27]. EVs released following muscle injury may therefore promote repair in neighboring fibers by transporting myoMirs such as miR-206 (Table 1) (Fig. 1).
Table 1.
Summary of cells secreting extracellular vesicles in various musculoskeletal tissues, their miRNA cargo, and proposed role in disease development and progression.
| Tissue/Cell Source | miRNA cargo | Target cell | Function in Disease | Reference |
|---|---|---|---|---|
| Myoblasts & myotubes | mir-1, 133a, b, -206 | Neighboring muscle cells | Promote tissue regeneration in Duchenne Muscular Dystrophy and myotonic dystrophy | [27, 28, 30] |
| Endothelial cells | mir-31 | Bone marrow mesenchymal stem cells | Suppress osteogenic differentiation in osteoporosis | [38] |
| Bone marrow stem cells | mir-183 | Bone marrow mesenchymal stem cells, osteoclasts | Induce stem cell senescence and promote bone resorption in osteoporosis | [8] |
| Osteoclasts | mir-214 | Osteoblasts | Suppress bone resorption in osteoporosis | [37] |
| Dendritic cells | mir-146a, -155 | Dendritic cells | Modulate inflammatory gene expression in rheumatoid arthritis | [42] |
| Fibroblast-like synoviocytes | miR-26a | Articular chondrocytes | Modulation of NF-κB signaling in osteoarthritis | [9, 62, 63] |
Serum levels of miR-1, -133a, and -206 are increased in patients with Duchenne muscular dystrophy as well as in dystrophin-deficient mdx mice (Table 1) [28]. These miRNas are in some cases associated with the CD63 antigen, which is located on the surface on EVs, consistent with an exosomal origin for these circulating miRNAs [24]. Exposure of myoblasts to EVs from serum of mdx mice, or overexpression of myoMirs themselves, promotes cell survival and reduces cell death [23]. EVs may also play a role in myotonic dystrophy (DM1), the most common form adult-onset muscular dystrophy (Table 1) [30]. In the case of DM1 the myoMirs miR-1, -133a,b, and miR-206 are all elevated in DM1 patients that had progressive disease; however, these same markers are not elevated in those who did not demonstrate disease progression [30]. It is important to note here that these miRNAs were not directly isolated from exosomes, and so they could be circulating bound to other proteins or lipids. Nevertheless, these studies and those from healthy human subjects [26] suggest that monitoring EV-derived miRNAs may serve as one approach for tracking disease progression or non-invasively assessing muscle injury following exercise.
Extracellular vesicles in bone metabolism and age-related bone loss
Bone is a tissue that is constantly undergoing modeling and remodeling resulting from the coordinated activity of osteoblasts, which synthesize bone, and osteoclasts, which break down or resorb bone. Osteoblasts and osteoclasts communicate with other cells, as well as with each other, through multiple mechanisms including hormones, growth factors, mRNA, and miRNA. In addition, the stem cell populations within bone actively secrete EVs, which are involved in normal bone homeostasis, repair, and disease pathology [31, 32]. We review below evidence from a number of studies showing that EVs can directly impact osteoclasts, osteoblasts, and bone stromal (stem) progenitor cells (BMSCs) to regulate bone formation and resorption.
Osteoblast-derived EVs contain bone regulatory proteins that can stimulate osteoclast formation, representing a mechanism for intercellular communication in bone tissue [33]. Specifically, EVs secreted by osteoblasts contain receptor activator of nuclear factor kappa beta ligand (RANKL), and parathyroid hormone (PTH) was found to promote RANKL release. EVs carrying RANKL then fuse to osteoclasts to deliver RANKL [33] (Fig 2). RANKL belongs to the tumor necrosis factor (TNF) family and is a cytokine that plays a role in bone metabolism as it regulates the development, maintenance, and activation of osteoclasts [34]. EVs of older patients can also inhibit osteogenesis when compared to EVs isolated from younger individuals, and the inhibitory response is inversely correlated with levels of Galectin-3 [35]. Galectin-3 is a lectin that enhances osteoblastogenesis by protecting β-Catenin from degradation [35]. Galectin-3 is reduced in EVs of the elderly, and in vitro assays demonstrate that overexpression of Galectin-3 results in enhanced osteogenic differentiation capacity of BMSCs.
Figure 2.
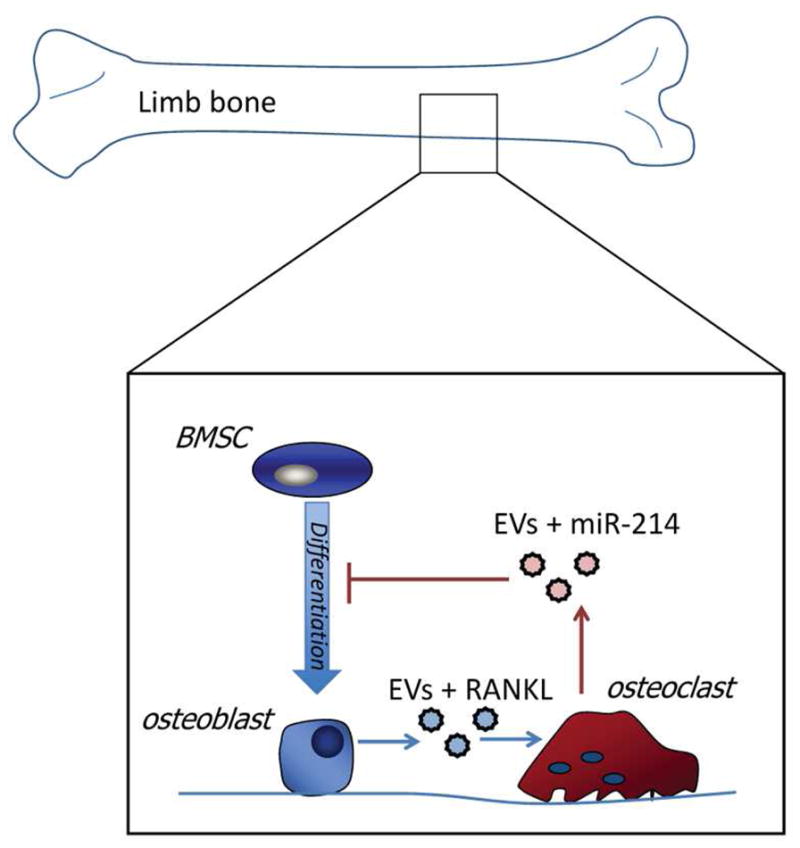
Bone mass is maintained by the coordinated activity of bone-forming cells (osteoblasts) and bone-resorbing cells (osteoclasts). Bone-forming osteoblasts secrete EVs carrying RANKL, which stimulates bone resorption by osteoclasts. Osteoclasts can in turn suppress osteogenic differentiation of bone marrow stromal cells (BMSCs) by secreting EVs carrying miR-214. EVs carrying miR-183 increase in bone marrow with aging, contributing to BMSC senescence and the activity of osteoclasts [8].
EVs derived from mineralizing osteoblasts have also been shown to contain miRNAs such as miR-1192, -680, and -302a, and these EVs can be taken up by bone marrow stromal cells (BMSCs) to stimulate osteogenic differentiation [36]. The mechanism(s) underlying the potential of osteoblast-derived EVs to stimulate the osteogenic differentiation of BMSCs appear to involve the capacity of EV-derived miRNAs to activate the Wnt/β-catenin pathway [36]. The canonical Wnt pathway is a key regulator of BMSC differentiation, and Wnt pathway activation will inhibit the expression of adipogenic factors such as PPARγ and CCAAT/enhancer binding protein α and stimulate the expression of osteogenic factors Runx2, Dlx5 and Osterix. EVs not only appear to play a role in stimulating bone formation, but EVs secreted by osteoclasts may also suppress osteoblast activity through the action of miRNAs. Specifically, elderly women with fractures and ovariectomized mice with bone loss are both found to have elevated levels of EVs carrying miR-214-3p (Table 1) [37]. Osteoclasts were observed to be the primary source of EV-derived miR-214-3p, which is transferred to osteoblasts in vitro [37] (Fig. 2). This suggests that osteoclasts communicate with osteoblasts via both EVs and miRNAs, and that inhibition of miR-214-3p could possibly attenuate bone loss with aging by enhancing bone formation.
Additional miRNAs carried via exosomes may also contribute to osteoporosis and age-related bone loss. The miRNA miR-31 is abundant within EVs secreted by endothelial cells in elderly and osteoporotic patients [38]. When miR-31 is taken up by BMSCs, it inhibits osteogenic differentiation (Table 1). We recently isolated EVs from the bone marrow interstitial fluid of aged and young mice to identify miRNAs that might be altered in aging bone and therefore contribute to bone loss [8]. Aged mice were shown to have elevated levels of miRNA-183, a member of the functional miRNA cluster including miR-182 and miR-96. Our in vitro experiments showed that miR-183 could be increased in exosomes from MSCs following exposure to oxidative stress, and that miR-183 could induce senescence in BMSCs. These findings implicating miR-183 in bone loss and stem cell senescence are consistent with previous studies showing that miR-183 can induce senescence in other cell types (Table 1) [10], and that miR-183 can induce osteoclastogenesis [40]. Moreover miR-182, which shares many gene targets with miR-183, is known to suppress bone formation [41]. Together these findings point to an emerging role for extracellular vesicles in mediating bone loss through their effects on BMSCs, osteoclasts, and osteoblasts (Fig. 2).
Extracellular vesicles in degenerative joint diseases: rheumatoid arthritis and osteoarthritis
Musculoskeletal tissues provide a broad range of cell types in which EVs appear to be involved in cell-cell communication, and limb joints provide further evidence that EVs are active within the tissue microenvironment. The articular ends of limb bones are covered with hyaline cartilage, consisting of chondrocytes surrounded by extracellular matrix (ECM) rich in collagen and proteoglycans. The cartilage is bathed in synovial fluid, which is secreted by fibroblast-like synoviocytes (FLS) surrounding the joint. The chronic inflammation of the joints that accompanies rheumatoid arthritis RA), and the mechanical degradation of articular cartilage that accompanies osteoarthritis (OA), each appear to involve changes in the EVs circulating within the joint space.
In the case of RA, EVs can present antigens to activate the innate and adaptive immune system, leading to inflammation within the joint, and the EVs also transport inflammatory cytokines such as TNF-α and IL-1 [42]. These findings are also consistent with other studies showing that EVs produced by synovial fibroblasts from RA patients contain a membrane bound form of TNF-α, whereas EVs from patient samples with OA do not carry this form of TNF-α [43]. EVs carry catabolic proteases such as MMPs and ADAMTS-5 which can cause breakdown of the ECM [42]. For example, EVs from RA synovial fibroblasts can cleave aggrecan, and this process is inhibited by TIMP-3, meaning that the activity could be due to ADAMTS or another metalloproteinase [44]. Another example of a catabolic protein carried by EVs is Aminopeptidase N/CD13. This metalloproteinase is found in EVs and can be increased in RA synovium and synovial fluid via transport by EVs [45]. Inhibition of CD13 results in decreased growth and migration of fibroblast like synoviocytes, meaning that CD13 may have a role in the hyperplasia that creates pathology in RA [45].
EVs in muscle and bone are highly enriched in miRNAs, and not surprisingly miRNAs are also abundant in EVs from the limb joints. In the case of RA, miR-155 and miR 146a are known to be involved in disease development [42]. Both of these miRNAs are stimulated by TNF-α and indirectly affect the inflammatory response, with miR-155 increasing inflammation and miR-146a decreasing inflammation. Both of these miRNAs are up-regulated in patients with RA compared to normal controls, and when miR-155 is inhibited in vivo TNF-α production is decreased [42, 46, 47]. Elevated miR-155 is expression is also associated with increased production of cytokines leading to an inflammatory state [46]. Mir-146a may have the ability to modulate the immune and inflammatory response in patients with RA. For example, miR-146a overexpression suppresses IL-6 and IL-8 and down regulates IL-1β and proteins associated with TNF receptors [48–50]. Mir-155 and miR-146a are also found in EVs released by dendritic cells, and when these EVs are taken up by recipient dendritic cells miR-155 promotes inflammatory gene expression whereas miR-146a reduces inflammatory gene expression (Table 1) [42,51].
The pathogenesis of OA is complicated, environmental and genetic factors interacting in a manner that ultimately leads to articular cartilage damage and degeneration. ECM maintenance is very important for joint health and there is a balance between synthesis and degradation of ECM; when this balance is altered, the patient is at risk for the pathology seen in OA [42]. The two main important cells related to these processes are chondrocytes, which help synthesize the extracellular matrix of articular cartilage and fibroblast-like synoviocytes (FLS), which secrete synovial fluid. EVs are involved in communication between these different cell types. For example, EVs isolated from chondrocytes treated with IL-1β can directly increase MMP-13 production in FLS cells [42]. MMPs are known to breakdown ECM, leading to progression of OA. EVs isolated from synovial fluid of inflamed joints stimulates the release of inflammatory cytokines, MMPs, and chemokines by macrophages, showing that they are biologically active and play a role in joint disease progression [54]. Similarly, EVs from T cells and monocytes induce MMP-1, MMP-3, and MMP-9 production in synovial fibroblasts [55–57]. Another study found that articular cartilage from knee and hip joints of OA patients contain numerous EVs, and that these EVs drive cartilage mineralization that in turn increases mechanical stress on articular cartilage [55, 56, 58]. These negative effects of EVs could be potential targets for inhibition in order to delay progression of OA.
miRNAs carried by EVs are also implicated in the development of OA. When FLS cells are treated with Il-1β, EVs are secreted that show elevated levels of miR-500B, miR-4454, miR-720, miR-199b, and miR-3154 [59]. Recently, EVs from synovial fluid of patient with OA were compared to those without OA [42]. The cargo of the OA EVs showed a 2.5-fold increase in miR-200c, which is known to be up regulated with oxidative stress and indirectly represses ZEB1, which has a role in maintaining articular cartilage [42]. Further, there are gender specific miRNAs in the patients with OA, which we have shown by profiling miRNA from both male and female OA synovial fluid samples [9]. In this study, EVs from synovial fluid from healthy men and women, as well as men and women with OA were characterized. Not only was there differential expression of several miRNAs in the OA group, it was also found that the female OA group had a greater number of miRNAs that were differentially regulated as compared to males. Certain of these miRNAs, such as miR-26a which was downregulated in female OA patients, are also estrogen responsive (Fig. 3). Estrogen is known to increase expression of miR-26a, which targets the toll-like receptor TLR3, whereas estrogen inhibitors suppress miR-26a in FLS cells (Table 1) [9]. The incidence of OA is higher in females than in males, and the data from EV-derived miRNAs in OA patients may provide some molecular mechanisms to explain this disparity.
Figure 3.
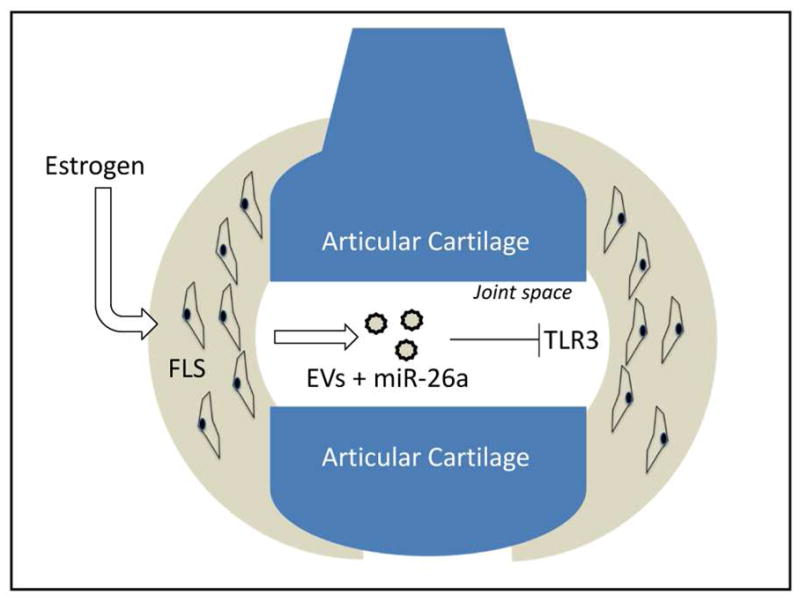
MicroRNAs detected in extracellular vesicles in synovial fluid of patients with osteoarthritis (OA) differ between men and women, and are secreted by fibroblast-like synoviocytes (FLS). In particular, women with OA show a marked downregulation of miR-26a, which is known to target toll-like receptors such as TLR3 in articular chondrocytes. Estrogen is known to stimulate miR-26a production, whereas estrogen inhibitors will suppress miR-23a expression [9].
Discussion & Conclusions
Exosomes and microvesicles have received considerable attention for their roles in cancer progression and metastasis, their potential role as biomarkers, and their therapeutic application as a stem cell product useful for enhancing tissue repair. Less attention has been paid to the role(s) of EVs in normal tissue physiology, or how EVs may be involved in age-related degeneration of various tissue types. Emerging evidence on the function of EVs in musculoskeletal diseases suggest that the musculoskeletal system is an excellent model for investigating the role of EVs in tissue maintenance and repair (Table 1). For example, a number of studies now show that EVs are actively released during the processes of muscle development and differentiation, and that these EVs have significant biological activity mediated in part by their cargo of miRNAs. In addition, the cycle of muscle damage and regeneration observed in myopathies such as Duchennne muscular dystrophy and myotonic dystrophy suggest that monitoring EV-derived miRNAs may serve as one approach for tracking disease progression or even non-invasively assessing muscle injury following exercise.
A consistent theme that emerges from study of musculoskeletal EVs is that various miRNAs appear to mediate a number of key pathological processes. These findings point to a potential therapeutic opportunity to target EV-derived miRNAs as a strategy for improving musculoskeletal function. In the case of age-related bone loss and osteoporosis, miRNAs such as miR-183 may stimulate bone resorption and induce bone marrow stem cell senescence, whereas others such as miR-214 can inhibit the osteogenic differentiation of bone marrow stromal cells. Delivering antagonists to these miRNAs, or targeting the factors upstream of these miRNAs that trigger their expression (e.g., reactive oxygen species), may represent new approaches for slowing the loss of bone mass with age. Likewise, the cartilage degeneration that is involved in osteoarthritis is characterized by marked changes in the expression of EV-derived miRNAs, and these miRNAs differ markedly between men and women. This important finding underscores the fact that there may be no “on size fits all” approach for OA prevention, and that personalized medicine strategies that incorporate specific aspects of EV biology and signaling may be effective.
We have defined specific miRNAs and proteins carried by EVs in muscle, bone, and limb joints, but it is important to realize that these tissues do not function in isolation from one another. That is, EVs and their cargo secreted during muscle regeneration may directly impact bone, and vice versa. This point is underscored by recent evidence that the myokine myostatin can mediate EV cargo in osteocytes, directly linking muscle-derived factors with bone-derived EVs and their miRNAs regulating bone formation [60]. Moreover, many of the myoMirs released from skeletal muscle with muscle injury and regeneration circulate throughout the body, and are likely to impact a number of different organs and tissues. This is further supported by recent experiments demonstrating that EVs produced peripherally by hematopoietic cells are endocytosed by neurons in the brain [61]. Future research is likely to provide new insights into mechanisms of crosstalk among organ systems in both normal and pathological conditions that are regulated by extracellular vesicles and their cargo. We expect that musculoskeletal tissues are likely to be involved in this network of inter-organ communication.
Acknowledgments
Funding for this research was provided in part by the National Institute on Aging (P01 AG036675) and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR070029).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Helwa I, et al. A Comparative Study of Serum Exosome Isolation Using Differential Ultracentrifugation and Three Commercial Reagents. PLoS One. 2017;12:1–22. doi: 10.1371/journal.pone.0170628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lobb RJ, et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4:27031. doi: 10.3402/jev.v4.27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rider MA, Hurwitz SN, Meckes DG., Jr ExtraPEG: A Polyethylene Glycol-Based Method for Enrichment of Extracellular Vesicles. SciRep. 2016;6:23978. doi: 10.1038/srep23978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haraszti RA, et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J Extracell Vesicles. 2016;5:32570. doi: 10.3402/jev.v5.32570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collino F, et al. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One. 2010;5(7):e11803. doi: 10.1371/journal.pone.0011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yáñez-Mó M, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoshino A, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis C, et al. MicroRNA-183-5p increases with age in bone-derived extracellular vesicles, suppresses bone marrow stromal (stem) cell proliferation, and induces stem cell senescence. Tissue Eng Part A. 2017 doi: 10.1089/ten.tea.2016.0525. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kolhe R, et al. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci Rep. 2017;7(1):20–29. doi: 10.1038/s41598-017-01905-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demonbreun AR, McNally EM. Muscle cell communication in development and repair. Curr Opin Pharmacol. 2017;34:7–14. doi: 10.1016/j.coph.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, Wang B. Extracellular vesicle microRNAs mediate skeletal muscle myogenesis and disease. Biomed Rep. 2016;5(3):296–300. doi: 10.3892/br.2016.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tidball JG. Inflammatory processes in muscle injury and repair. Am J Phys Regul Integr Comp Physiol. 2005;288(2):345–353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- 13.Le Bihan MC, et al. In-depth analysis of the secretome identifies three major independent secretory pathways in differentiating human myoblasts. J Proteomics. 2012;77:344–356. doi: 10.1016/j.jprot.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Romancino DP, et al. Identification and characterization of the nano-sized vesicles released by muscle cells. FEBS Letters. 2017;587(9):1379–1384. doi: 10.1016/j.febslet.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi JS, et al. Exosomes from differentiating human skeletal muscle cells trigger myogenesis of stem cells and provide biochemical cues for skeletal muscle regeneration. J Control Release. 2016;222:107–115. doi: 10.1016/j.jconrel.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Do MKQ, et al. Time-coordinated prevalence of extracellular HGF, FGF2 and TGF-β3 in crush-injured skeletal muscle. Animal Science Journal. 2017;83(10):712–717. doi: 10.1111/j.1740-0929.2012.01057.x. [DOI] [PubMed] [Google Scholar]
- 17.Yao-Hua Song JLS, Delafontaine Patrice, Godard Michael P. The therapeutic potential of IGF-I in skeletal muscle repair. Trends Endocrinol Metab. 2013;24(6):310–319. doi: 10.1016/j.tem.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoier B, et al. Subcellular localization and mechanism of secretion of vascular endothelial growth factor in human skeletal muscle. Faseb j. 2013;27(9):3496–3504. doi: 10.1096/fj.12-224618. [DOI] [PubMed] [Google Scholar]
- 19.Braun T, Gautel M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nature Reviews Molecular Cell Biology. 2011;12(6):349–361. doi: 10.1038/nrm3118. [DOI] [PubMed] [Google Scholar]
- 20.Quattrocelli M, Sampaolesi M. The mesmiRizing complexity of microRNAs for striated muscle tissue engineering. Adv Drug Deliv Rev. 2015;88:37–52. doi: 10.1016/j.addr.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Forterre A, et al. Proteomic analysis of C2C12 myoblast and myotube exosome-like vesicles: a new paradigm for myoblast-myotube cross talk? PLoS One. 2014;9(1):e84153. doi: 10.1371/journal.pone.0084153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Popescu LM, et al. Identification of telocytes in skeletal muscle interstitium: implication for muscle regeneration. J Cell Mol Med. 2011;15(6):1379–1392. doi: 10.1111/j.1582-4934.2011.01330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aoi W. Frontier impact of microRNAs in skeletal muscle research: a future perspective. Front Physiol. 2014;5:495. doi: 10.3389/fphys.2014.00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuzaka Y, et al. Characterization and functional analysis of extracellular vesicles and muscle-abundant miRNAs (miR-1, miR-133a, and miR-206) in C2C12 myocytes and mdx mice. PLoS One. 2016;11(12):e0167811. doi: 10.1371/journal.pone.0167811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forterre A, et al. Myotube-derived exosomal miRNAs downregulate Sirtuin1 in myoblasts during muscle cell differentiation. Cell Cycle. 2014;13(1):78–89. doi: 10.4161/cc.26808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guescini M, et al. Muscle Releases Alpha-Sarcoglycan Positive Extracellular Vesicles Carrying miRNAs in the Bloodstream. PLoS One. 2015;10(5):e0125094. doi: 10.1371/journal.pone.0125094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koutalianos D, et al. MyoD transcription factor induces myogenesis by inhibiting Twist-1 through miR-206. J Cell Sci. 2015;128(19):3631–3645. doi: 10.1242/jcs.172288. [DOI] [PubMed] [Google Scholar]
- 28.Aoi W, Sakuma K. Does regulation of skeletal muscle function involve circulating microRNAs? Front Physiol. 2014;5:39. doi: 10.3389/fphys.2014.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koutsoulidou A, et al. Elevated Muscle-Specific miRNAs in Serum of Myotonic Dystrophy Patients Relate to Muscle Disease Progress. PLoS One. 2015;10(4):e0125341. doi: 10.1371/journal.pone.0125341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roccaro AM, et al. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013;123(4):542–555. doi: 10.1172/JCI66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanghavi PYP, Upadhyay, Hamrick MW, Tang Y, Dawn B. Exosomes for bone disease. Mesenchymal Stem Cell Derived Exosomes: The Potential for Translational Nanomedicine. 2015;72:207–221. [Google Scholar]
- 33.Deng L, et al. Osteoblast-derived microvesicles: A novel mechanism for communication between osteoblasts and osteoclasts. Bone. 2015;79:37–42. doi: 10.1016/j.bone.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 34.Kohli SS, Kohli VS. Role of RANKL–RANK/osteoprotegerin molecular complex in bone remodeling and its immunopathologic implications, in Indian. J Endocrinol Metab. 2011;32:175–181. doi: 10.4103/2230-8210.83401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weilner S, et al. Vesicular Galectin-3 levels decrease with donor age and contribute to the reduced osteo-inductive potential of human plasma derived extracellular vesicles. Aging. 2016;8(1):16–33. doi: 10.18632/aging.100865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui Y, et al. Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression. FEBS Letters. 2016;590(1):185–92. doi: 10.1002/1873-3468.12024. [DOI] [PubMed] [Google Scholar]
- 37.Li D, et al. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat Commun. 2016;7:872. doi: 10.1038/ncomms10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weilner S, et al. Secreted microvesicular miR-31 inhibits osteogenic differentiation of mesenchymal stem cells. Aging Cell. 2016;15(4):744–754. doi: 10.1111/acel.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ke K, et al. MicroRNA-183 increases osteoclastogenesis by repressing heme oxygenase-1. Bone. 2015;81:237–246. doi: 10.1016/j.bone.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 41.Kim KM, et al. miR-182 is a negative regulator of osteoblast proliferation, differentiation, and skeletogenesis through targeting FoxO1. J Bone Miner Res. 2012;27(8):1669–1679. doi: 10.1002/jbmr.1604. [DOI] [PubMed] [Google Scholar]
- 42.Withrow J, et al. Extracellular vesicles in the pathogenesis of rheumatoid arthritis and osteoarthritis. Arthritis Res Ther. 2016;18(1):286. doi: 10.1186/s13075-016-1178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang HG, et al. A membrane form of TNF-alpha presented by exosomes delays T cell activation-induced cell death. J Immunol. 2006;176(12):7385–7393. doi: 10.4049/jimmunol.176.12.7385. [DOI] [PubMed] [Google Scholar]
- 44.Lo Cicero A, et al. Microvesicles shed by oligodendroglioma cells and rheumatoid synovial fibroblasts contain aggrecanase activity. Matrix Biol. 2012;31(4):229–233. doi: 10.1016/j.matbio.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morgan RL, et al. Localization, Shedding, Regulation and Function of Aminopeptidase N/CD13 on Fibroblast like Synoviocytes. PLoS One. 2016;11(9):e0162008. doi: 10.1371/journal.pone.0162008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stanczyk J, et al. Altered expression of MicroRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum. 2008;58(4):1001–1009. doi: 10.1002/art.23386. [DOI] [PubMed] [Google Scholar]
- 47.Kurowska-Stolarska M, et al. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc Natl Acad Sci. 2011;108(27):1193–1198. doi: 10.1073/pnas.1019536108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakasa T, et al. Expression of microRNA-146 in rheumatoid arthritis synovial tissue. Arthritis Rheum. 2008;58(5):1284–1292. doi: 10.1002/art.23429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taganov KD, et al. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci. 2006;103(33):12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhaumik D, et al. MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging. 2009;1(4):402–411. doi: 10.18632/aging.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alexander M, et al. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat Commun. 2015;6:7321. doi: 10.1038/ncomms8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gyorgy B, et al. Improved flow cytometric assessment reveals distinct microvesicle (cell-derived microparticle) signatures in joint diseases. PLoS One. 2012;7(11):e49726. doi: 10.1371/journal.pone.0049726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skriner K, et al. Association of citrullinated proteins with synovial exosomes. Arthritis Rheum. 2006;54(12):3809–3814. doi: 10.1002/art.22276. [DOI] [PubMed] [Google Scholar]
- 54.Domenis R, et al. Characterization of the Proinflammatory Profile of Synovial Fluid-Derived Exosomes of Patients with Osteoarthritis. Mediators Inflamm. 2017;20(17):48–87. doi: 10.1155/2017/4814987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anderson HC, Mulhall D, Garimella R. Role of extracellular membrane vesicles in the pathogenesis of various diseases, including cancer, renal diseases, atherosclerosis, and arthritis. Lab Invest. 2010;90(11):1549–1557. doi: 10.1038/labinvest.2010.152. [DOI] [PubMed] [Google Scholar]
- 56.Blom AB, et al. Crucial role of macrophages in matrix metalloproteinase-mediated cartilage destruction during experimental osteoarthritis: involvement of matrix metalloproteinase 3. Arthritis Rheum. 2007;56(1):147–157. doi: 10.1002/art.22337. [DOI] [PubMed] [Google Scholar]
- 57.Distler JH, et al. The induction of matrix metalloproteinase and cytokine expression in synovial fibroblasts stimulated with immune cell microparticles. Proc Natl Acad Sci. 2005;102(8):2892–2897. doi: 10.1073/pnas.0409781102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ali SY, Griffiths S. Formation of calcium phosphate crystals in normal and osteoarthritic cartilage. Ann Rheum Dis. 1983;42(1):45–48. doi: 10.1136/ard.42.suppl_1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kato T, et al. Exosomes from IL-1beta stimulated synovial fibroblasts induce osteoarthritic changes in articular chondrocytes. Arthritis Res Ther. 2014;16(4):163. doi: 10.1186/ar4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qin Y, et al. Myostatin inhibits osteoblastic differentiation by suppressing osteocyte-derived exosomal microRNA-218: A novel mechanism in muscle-bone communication. J Biol Chem. 2017;292(26):11021–11033. doi: 10.1074/jbc.M116.770941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ridder K, et al. Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol. 2014;12(6):e1001874. doi: 10.1371/journal.pbio.1001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xie Q, Wei M, Kang X, Liu D, Quan Y, Pan X, Liu X, Liao D, Liu J, Zhang B. Reciprocal inhibition between miR-26a and NF-κB regulates obesity-related chronic inflammation in chondrocytes. Biosci Rep. 2015;35(3):e00204. doi: 10.1042/BSR20150071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yin X, Wang JQ, Yan SY. Reduced miR-26a and miR-26b expression contributes to the pathogenesis of osteoarthritis via the promotion of p65 translocation. Mol Med Rep. 2017;15(2):551–558. doi: 10.3892/mmr.2016.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]