Abstract
Purpose
Most candidate gene studies on the neurobiology of voluntary exercise behavior have focused on the dopaminergic signaling pathway and its role in the mesolimbic reward system. We hypothesized that dopaminergic candidate genes may influence exercise behavior through additional effects on executive functioning and that these effects are only detected when the types of exercise activity are taken into account.
Methods
Data on voluntary exercise behavior and at least one SNP/VNTR were available for 12,929 participants of the Netherlands Twin Registry. Exercise activity was classified as externally paced if a high level of executive function skill was required. The total volume of voluntary exercise (minutes per week) as well as the volume specifically spent on externally paced activities were tested for association with nine functional dopaminergic polymorphisms (DRD1: rs265981, DRD2/ANKK1: rs1800497, DRD3: rs6280, DRD4: VNTR 48bp, DRD5: VNTR 130–166bp, DBH: rs2519152, DAT1: VNTR 40bp, COMT: rs4680, MAOA: VNTR 30bp), a polygenic score (PGS) based on nine alleles leading to lower dopamine responsiveness, and a PGS based on three alleles associated with both higher reward sensitivity and better executive functioning (DRD2/ANKK1: ‘G’ allele, COMT: Met allele, DAT1: 440bp allele).
Results
No association with total exercise volume or externally paced exercise volume was found for individual alleles or the nine-allele polygenic score. The volume of externally paced exercise behavior was significantly associated with the reward and executive function congruent PGS. This association was driven by the DAT1 440bp and COMT Met allele which acted as increaser alleles for externally paced exercise behavior.
Conclusion
Taking into account the types of exercise activity may increase the success of identifying genetic variants and unraveling the neurobiology of voluntary exercise behavior.
Keywords: candidate gene, exercise behavior, reward sensitivity, executive functioning
Introduction
Despite the substantial heritability of exercise behavior (1,2), genetic association studies have not yet been successful in uncovering the causative variants for the initiation and maintenance of voluntary exercise behavior (3,4). Most candidate gene studies on the neurobiology of voluntary exercise behavior to date have focused on the dopaminergic signaling pathway (as reviewed in 5). Three of these studies found a significant association between genetic variants in the dopaminergic system and exercise behavior in humans. The first study found that women reporting European ancestry (N=256) who are homozygous for the DRD2/ANKK1 ‘A’ allele (which is associated with decreased levels of DRD2 receptors) had 25–38% lower past year general physical activity levels compared to carriers of the ‘C’ allele (6). The second study found no significant differences in past year physical activity levels or exercise habit among 648 Japanese men and women with respect to the DRD2/ANKK1 gene. However, they did find a significant association between the DRD2/ANKK1 genotype and exercise habits in the period from childhood to adolescence, in which homozygotes of the ‘A’ allele were again less likely to be exercisers (7). The third study, in 54 month old children (N=651), found that individual carriers of the 3.5–4 repeat MAO-A VNTR (high activity version) showed lower overall parent-reported activity levels than the carriers of the 3 repeat MAO-A VNTR( low activity version) (8).
After these promising first findings, subsequent larger studies have failed to find an association with these and other genes involved in the dopaminergic system and either daily physical activity or the more narrow trait of voluntary exercise behavior. A study by Jozkow et al. (2012) in 900 Polish men investigating the relationship between physical activity and the DRD2 C313T and the DRD4 48-bp VNTR polymorphisms did not find an association (9). A study by Huppertz et al. (2014) investigated whether functional single nucleotide polymorphisms (SNPs) or variable number of tandem repeats (VNTRs) in several dopaminergic genes, including DRD2/ANKK1, involved in the mesolimbic reward system could explain the heritability of voluntary exercise behavior. Despite their large number of participants (N=8768) they did not find an association between any of the dopaminergic SNPs or VNTRs and exercise behavior in leisure time.
A shared limitation of the studies on this topic so far is that they lumped together all regular moderate to vigorous exercise activities into a single measure. Landers & Esch (2015) have stressed that taking into account the nature of the set of skills required in exercise activity is of major importance when investigating the neurobiology of individual differences in exercise behavior, most notably for the engagement in sports (10). They hypothesize that the required level of externally versus internally paced skills is of key importance in the endorsement of sports and exercise as a regular leisure time activity. As defined by Galligan (2000), each exercise/sports activity is located somewhere on the external-internal paced continuum. Internally paced or self-paced activities are exercise/sports activities in which the performer controls the rate at which the activity is executed (11). Such activities usually rely on closed skills including for example a javelin throw or the discus. In externally paced activities, the environment (which may include opponents or natural elements) controls the rate of performing the activity. The performer must pay attention to external events in order to control his/her rate of movement. These activities require reactive skills, and are usually open skills (i.e. in ball games the performer must time his actions with the actions of other players and the ball) (11,12). As is clear from these definitions, externally paced exercise activities rely much more on executive functions (e.g. task switching, inhibition, and planning) than more internally paced activities, like jogging.
Based on the simple principle that people are more motivated to repeat a behavior that they are good at, a tight fit of one’s skills to the exercise activities chosen can be expected to increase the chance of long-term adherence to those exercise activities. Because executive functioning is critical to performance in externally paced sports, ones executive function abilities might drive the motivation to voluntarily engage in such exercise behaviors. Various twin studies have shown substantial heritability of performance in executive function tasks (13,14) and, intriguingly, genetic variation in dopaminergic signaling has been widely regarded as a major contributor to this heritability (13,15). In fact, the same genetic polymorphisms that have been investigated in the context of reward processing in the striatal brain regions have been hypothesized to have an effect on executive functioning in prefrontal brain regions. Table 1 summarizes the reported association of functional genetic polymorphisms in the dopamine system with reward sensitivity and executive functioning. More detail on the variants of these nine polymorphisms and their hypothesized neurobiological effects is provided in the appendix (see Appendix, Supplemental Digital Content 1, Functional variants in dopaminergic genes and their association with reward sensitivity and executive functioning).
Table 1.
The effect of various functional genetic polymorphism of the dopamine system and their reported effect on dopamine levels, reward sensitivity and executive functioning.
| Polymorphism | Alleles | Variant | Effect on dopamine | Effect on reward sensitivity | Effect on executive functioning | Reference in Supp. material |
|---|---|---|---|---|---|---|
| DRD1 (SNP rs265981) | A G | A | Lower dopamine responsiveness | ↑ | ↓ | (1–6) |
| DRD2/ANKK1 (SNP rs1800497) | A G | A | Higher dopamine responsiveness | ↓ | ↓ | (11–20) |
| DRD3 (SNP rs6280) | T C | C (Gly) | Lower dopamine responsiveness | ↑ | ? | (22–27) |
| DBH (SNP rs2519152) | T C | T | Higher dopamine levels | ? | ↓ | (28–34) |
| COMT (SNP rs4680) | A G | A (Met) | Higher dopamine levels | ↑ | ↑ | (35–39) |
| DAT1 (VNTR 40bp) | 440 480 | 440 | Higher dopamine levels | ↑ | ↑ | (39–43) |
| DRD4 (VNTR 48bp) | 7r vs. all other | 7 repeat | Higher dopamine responsiveness | ↑ | ↓ | (39,45–54) |
| DRD5 (VNTR 130–166bp) | 148bp vs. all other | 148bp | Lower dopamine responsiveness | ↑ | ↓ | (30,56–60) |
| MAOA (VNTR 30bp) | < 3.5 vs. >=3.5 | >=3.5 | Lower dopamine levels | ↑ | ↓ | (61–63) |
For detailed information and references see supplementary material
Table 1 suggests that most of these genetic polymorphisms have a dual effect on reward sensitivity and executive function. These pleiotropic genetic effects are sometimes aligned such that the same allelic variant is associated with both higher reward sensitivity and better executive functioning (DRD2/ANKK1 (rs1800497) ‘T’ allele, COMT (rs4680) ‘A’ allele, DAT1 (VNTR 40bp) 440bp repeat) whereas for other genes the same allele has opposing or unclear effects on both traits (DRD1 (rs265981) ‘G’ allele), DRD3 (rs6280) ‘C’ allele, DBH (rs2519152) ‘T’ allele, DRD4 (VNTR 48bp) 7 repeat, DRD5 (VNTR 130–166bp) 148bp repeat, MAOA (VNTR 30bp) >=3.5 repeats). We currently do not know whether the putative effects of these dopaminergic variants on exercise behavior depend more on reward sensitivity or on executive function. We hypothesize that the latter effect cannot be ignored. If dopaminergic effects on executive function are present, the genetic association with voluntary exercise behavior as a whole, which is a mixture of internally and externally paced exercise activities, could be diluted by opposite effects of the same allele on externally versus internally paced exercise activities.
In the current study we test whether the genetic polymorphisms in dopaminergic pathways listed in Table 1 are associated more strongly with the total weekly minutes of voluntary externally paced exercise behavior than with the total weekly minutes of voluntary exercise behavior as a whole. We further expect this to be most prominent in those alleles associated with both higher reward sensitivity and better executive functioning (DRD2/ANKK1 (rs1800497) ‘T’ allele, COMT (rs4680) ‘A’ allele, DAT1 (VNTR 40bp) 440bp repeat).
Materials and Methods
Participants
The participants of this study were drawn from the larger cohort of twins and their family members that agreed to participate in the study on individual differences and behavior by the Netherlands Twin Registry (NTR). The Medical Research Ethics Committee of the VU University Medical Centre approved the protocol for data collection. All participants of 18 years and older signed written informed consent. For participants under the age of 18 the primary caregiver gave written informed consent. Characteristics and recruitment of participants are described elsewhere (16,17). Only individuals with a Dutch/Western European background for whom both genotyping data and at least one measure of leisure time exercise behavior through self-report was available were eligible for inclusion. Because the heritability of exercise behavior is highest during adolescence (70–80% vs. 20% in children and 50–60% in adulthood (18)) the exercise data drawn from several longitudinal questionnaires were optimized to find genetic associations. The optimization consisted of choosing the data from the questionnaire in which the participants’ age was closest to the age of 18 years. The final sample consisted of 12,929 individuals (4393 families, including 1671 MZ twin pairs), 39.8% males, with an age range of 12 – 90 years (M=32.45, SD=15.95) in which 59.6% were adults (age range=20–90) and 40.4% were adolescents (age range=12–19).
Phenotyping
The phenotype of interest for this study was regular voluntary exercise behavior. Data on exercise behavior were collected by self-report questionnaires in which participants were asked to indicate (1) which exercises activities they participated in regularly (maximum number of five activities), (2) how many times per week they participated in the respective activity on average and (3) how many minutes per instance they participated in the respective activity on average. Previous studies (19) have shown that the test-retest reliability of this questionnaire is high (>0.82) and that its outcome is associated with that of other instruments measuring regular moderate to vigorous physical activity (20). Based on the questionnaire, a variable coding for regular exercise (“Do you regularly participate in sports/exercise activities - Yes/No”) was created, in which regular voluntary exercisers were given a value of 1 (Yes) and non-exercisers were given a value of 0 (No). We were not interested in activities that are (1) irregularly engaged in (such as ski holidays, swimming on holidays), (2) non-leisure time activities (e.g., cycling or walking as a form of transportation), (3) are related to gardening or house-cleaning, and (4) (for younger participants) compulsory physical education classes. These activities were therefore excluded. Participants that are only engaged in the excluded exercise activities are thus classified as non-exercisers and given a value of 0.
For each exercise activity reported, it was determined whether this activity was internally paced or externally paced. Based on the external-internal paced continuum of Galligan (2000) (11) we defined four levels of internally vs. externally paced exercise to which an activity was assigned: (1) highly externally paced (pace is influenced by both teammates and opponents; e.g. soccer, basketball), (2) intermediate externally paced (pace is influenced by opponents or teammates only; e.g. martial arts, tennis), (3) low externally paced (pace is influenced by dynamic external elements like wind/water/music/synchronic team movements; e.g. sailing, street dance) and (4) internally paced (pace is mostly or entirely self-directed; e.g. running, yoga). In this study, we focus on the end of the continuum, namely: highly externally paced exercise. Based on the above classification, participants were given a value of 1 (Yes) if they engaged in highly externally paced exercise activities (22.2%) and a value of 0 (No) if they engaged in intermediate (10.1%) or low (8.3%) externally paced activities only or if they did not engage in externally paced exercise (59.4%).
For each indicated exercise activity the total number of minutes per week participated in the respective activity was calculated by multiplying the number of times per week with the number of minutes per time for that activity. The total number of minutes per week engaged in all exercise behaviors was calculated by summing over all eligible activities an individual was engaged in. The number of minutes per week spent on externally paced activities was calculated by summing over the relevant activities the participant had reported. Due to the high degree of skewedness for all the exercise activity variables we decided to log10 transform them for further analysis.
Genotyping and imputation
The SNP genotyping was done on several platforms, including sequencing for the Netherlands reference genome project GONL (21). Platform priority was set as follows, GONL sequence (N = 368) > Illumnia Omni 1M (N=257) > Illumina Human Beadchip 660 (N=1439) > Affymetrix 6.0 (N=8940) > Affymetrix-Perlegen (N=1142). If a sample was done multiple times, the sample with the highest number of genotyped quality controlled SNPs was selected. Samples were removed if they failed to fulfill the quality control (QC) criteria as described previously in (22).
The genotype data of all platforms, except the GONL sequence individuals, were then merged into a single dataset. Subsequently, the missing SNP genotypes between each platform were cross-platform imputed using the GONL reference dataset (23). A second round of QC was applied to the cross-platform imputed data (21). After this step the 386 GONL samples from the NTR were re-added to the data for the SNPs that survived the QC. Ethnic outliers were detected (N=666) using ten principal components (PCs), which were calculated for each individual in the data using the approach as described in (24). Subsequently, 20 PCs were computed within the Dutch sample to capture possible population clustering within The Netherlands (24). Finally, a second round of imputations was done with the 1000G Phase 3 all ancestries reference panel using the Michigan Imputation Server.
In addition to these SNPs, Variable Number Tandem Repeats (VNTRs), namely the classic DAT1, DRD4, DRD5 and MAOA polymorphisms were available for 3363–3680 participants. The genotyping was done with PCR assays, and details about the laboratory procedures are described in Beijsterveldt et al (2013). Participants with mendelian errors were removed from the analyses. VNTRs were tested for HWE p > .001 and MAF > .01. None of the VNTRs failed the thresholds for HWE and MAF. The HWE and MAF for the SNPs and VNTRs in the final dataset are shown in Table 2.
Table 2.
Number of individuals with complete genotype and phenotype data (N), their mean age (SD), % male, allele/VNTR coding, minor allele frequency (MAF) and the P value of the test for Hardy-Weinberg Equilibrium (HWE).
| Gene | N | Age | Male | Minor allele | 0 | 1 | 2 | MAF | HWE |
|---|---|---|---|---|---|---|---|---|---|
| μ (SD) | % | Allele/repeat | Allele/repeat | Allele/repeat | |||||
| DRD1 (rs265981) | 10244 | 34.46 (16.00) | 38.8 | A | GG | AG | AA | .36 | .05 |
| DRD2/ANKK1 (rs1800497) | 10247 | 32.37 (15.98) | 39.5 | A | GG | AG | AA | .19 | .23 |
| DRD3 (rs6280) | 10247 | 32.46 (16.00) | 38.8 | C | TT | TC | CC | .31 | .36 |
| DBH (rs2519152) | 10245 | 32.46 (16.00) | 38.8 | C | CC | TC | TT | .47 | .43 |
| COMT (rs4680) | 10246 | 32.46 (16.00) | 38.8 | A | GG | AG | AA | .45 | .68 |
| DAT1 (VNTR 40bp) | 3586 | 27.78 (14.87) | 43.1 | 440bp | Two 480bp | 440bp – 480bp | Two 440 bp | .25 | .37 |
| DRD4 (VNTR 48bp) | 3363 | 28.61 (15.08) | 43.7 | 7r | No 7r | One 7r | Two 7r | .18 | .54 |
| DRD5 (VNTR 130–166bp) | 3680 | 28.60 (14.97) | 42.2 | 148bp | No 148bp | One 148bp | Two 148bp | .49 | .43 |
| MAOA (VNTR 30bp) | |||||||||
| - Males | 1564 | 29.38 (15.59) | - | >=3.5r | One <3.5r | - | One >=3.5r | .34 | n.a. |
| - Females | 2052 | 28.28 (14.55) | - | >=3.5r | Two <3.5r | <3.5r – >=3.5r | Two >=3.5r | .37 | .86 |
Genotype coding and polygenic scores
The SNPs were coded based on the presence of one of the two alleles in the genotype ranging from 0 to 2. The VNTRs were coded based on the presence or absence of a specific number of repeats ranging from 0 to 2. An exception was the X-linked MAOA VNTR where males received a code of 2 if the specific repeat was present on their single X-chromosome. Coding of the SNPs and VNTRs is based on concordance with previous research findings on the polymorphism with regard to reward sensitivity, executive functioning and exercise behavior (Table 1). An overview of the exact coding of the alleles/repeats per polymorphism can be found in Table 2.
In addition, two polygenic scores were calculated. The first polygenic score (‘low DA response PGS’) was calculated by summing the number of alleles associated with lower dopamine responsiveness (DRD1 (rs265981) ‘A’ allele, DRD2/ANKK1 (rs1800497) ‘G’ allele, DRD3 (rs628) ‘C’ allele, DRD4 (VNTR 48bp) no 7 repeat, DRD5 (VNTR 130–166bp) 148bp) or higher dopamine levels (DBH (rs2519152) ‘T’ allele, COMT (rs4690) ‘A’ allele, DAT1 (VNTR 40bp) 440bp, MAOA (VNTR 30bp) <3.5 repeats) for each participant, referred to as the number of increaser alleles. To calculate the second polygenic score (‘executive and reward congruency PGS’) we summed the number of alleles associated with both higher reward sensitivity and better executive functioning (DRD2/ANKK1 (rs1800497) ‘G’ allele, COMT (rs4680) ‘A’ allele, DAT1 (VNTR 40bp) 440bp repeat) for each participant, referred to as the number of increaser alleles.
Statistical analysis
To map the differences in exercise behavior (defined as: % regular exercisers, % regular externally paced exercisers, number of minutes spent on exercise as a whole and number of minutes spent on externally paced exercise) with regard to sex and age (binned in three age groups: 18– <25 years, 25– <55 years and 55 years and older) a chi-square and an ANOVA analysis were performed respectively.
General linear model analyses were performed in SPSS for windows (version 23.0, SPSS Inc.) to investigate the association between the genetic polymorphisms and polygenic scores versus the total weekly minutes of voluntary exercise behavior as a whole and/or the total weekly minutes of voluntary externally paced exercise behavior. In each linear model analysis, the genetic polymorphisms and polygenic scores were entered as independent variables separately. Non-exercisers (N=4926) are retained in these analyses and given a weekly volume of zero. As a sensitivity analysis, we repeated the analyses in exercisers only.
In the genetic association analyses the following variables were included as covariates: sex (0 = male, 1 = female), age, age squared, sex × age interaction and the first 20 genetic principal components (PCs). Family was included as a random factor to account for clustering due to relatedness. Preliminary analyses showed that correction for the Batch effect of genotyping SNPs was not necessary. For the analyses including VNTRs the following variables were included as covariates: sex, age, age squared, sex × age interaction and batch effect for study origin. Family was included as a random factor to account for clustering due to relatedness. For a subset of the participants in the VNTR analyses, the first 20 genetic PCs were available due to concurrent availability of genome-wide SNP data (~70%). For this subset, all analyses were repeated including the before mentioned covariates with addition of the 20 PCs.
We corrected for multiple testing by dividing the p-value by the number of polymorphisms and two polygenic scores (0.05/11), resulting in a significance threshold of p = 0.005.
Results
Table 3 depicts the differences between sex and age groups with regard to voluntary exercise behavior. Males spent more minutes per week engaged in exercise behavior than females, both in exercise as a whole and externally paced activities. Both males and females show a significant decrease in the time spent on exercise behavior with increasing age, and this decrease is much more pronounced when looking at externally paced exercise only.
Table 3.
Sex and age differences in voluntary exercise behavior.
| All participants | |||||
|---|---|---|---|---|---|
|
| |||||
| N | % exercisers | % exercisers engaged in externally paced activities | # weekly minutes exercise μ (SD) | # weekly minutes externally paced exercise μ (SD) | |
| All | 12929 | 61.9 | 22.2 | 132.44 (172.39) | 45.01 (100.35) |
| Males | 5144 | 62.0 | 30.8 | 152.67 (194.06) | 64.10 (116.59) |
| Females | 7786 | 61.9 | 16.5* | 119.07 (154.99)* | 32.39 (85.71)* |
|
| |||||
| 18–25 years | 6143 | 72.4 | 35.2 | 173.52 (182.14) | 78.55 (126.43) |
| Males | 2490 | 75.1 | 46.9 | 207.40 (201.25) | 109.40 (141.41) |
| Females | 3653 | 70.6 | 27.2 | 150.43 (163.93) | 57.53 (110.29) |
| 25–55 years | 5650 | 54.2 | 11.5 | 97.77 (148.07) | 16.28 (55.40) |
| Males | 2118 | 51.8 | 17.8 | 102.78 (160.94) | 24.79 (66.69) |
| Females | 3532 | 55.7 | 7.7 | 93.17 (139.69) | 11.19 (46.63) |
| 55+ years | 1136 | 43.4# | 4.9# | 87.69 (179.93)# | 6.48 (35.39)# |
| Males | 536 | 41.0 | 7.5 | 95.52 (209.88) | 9.02 (36.45) |
| Females | 600 | 45.5 | 2.7 | 80.70 (147.97) | 4.22 (34.30) |
|
| |||||
| Exercisers only | |||||
|
| |||||
| All | 8007 | - | 35.8 | 213.85 (174.86) | 72.68 (119.38) |
| Males | 3187 | - | 49.7 | 246.42 (194.13) | 103.47 (113.67) |
| Females | 4820 | - | 26.6* | 192.32 (157.21)* | 52.32 (104.03)* |
|
| |||||
| 18–25 years | 4449 | - | 48.6 | 239.59 (173.13) | 108.46 (137.21) |
| Males | 1870 | - | 62.5 | 276.17 (186.91) | 145.68 (146.09) |
| Females | 2579 | - | 38.5 | 213.06 (157.21) | 81.48 (123.61) |
| 25–55 years | 3065 | - | 21.2 | 178.39 (160.81) | 30.02 (72.43) |
| Males | 1097 | - | 34.3 | 198.44 (176.15) | 57.86 (86.52) |
| Females | 1968 | - | 13.9 | 167.22 (150.47) | 20.08 (61.04) |
| 55+ years | 493 | - | 11.4# | 202.07 (226.99)# | 14.94 (52.57)# |
| Males | 220 | - | 18.2 | 232.73 (274.83) | 21.98 (54.40) |
| Females | 273 | - | 5.9 | 177.36 (176.11) | 9.27 (50.43) |
Chi-square analysis showed that males and females differed significantly, p < .001
Post- hoc Bonferroni ANOVA analyses showed that all age groups differed significantly, p < .001
The genetic association analyses for each of the nine genes separately showed no evidence for an association of the dopaminergic alleles with either exercise as a whole or externally paced exercise (Table 4). The low DA response PGS also did not yield a significant association with either exercise behavior as a whole or externally paced exercise behavior.
Table 4.
The effect of genotype on the total weekly number of minutes spend on voluntary exercise behavior.
| # minutes exercise | # minutes externally paced exercise | # minutes externally paced exercise in exercisers only | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Gene (allele)* | N | B | SE | P | N | B | SE | P | N | B | SE | P |
| DRD1 (A)* | 10244 | −0.011 | 0.0171 | .51 | 10244 | −0.008 | 0.0147 | .59 | 6362 | −0.010 | 0.0211 | .64 |
| DRD2/ANKK1 (A) | 10247 | 0.005 | 0.0211 | .82 | 10247 | −0.002 | 0.0179 | .90 | 6364 | −0.006 | 0.0255 | .81 |
| DRD3 (C)* | 10247 | −0.020 | 0.0177 | .26 | 10247 | −0.020 | 0.0155 | .20 | 6364 | −0.025 | 0.0219 | .25 |
| DBH (T)* | 10245 | 0.014 | 0.0168 | .39 | 10245 | −0.004 | 0.0144 | .77 | 6362 | −0.013 | 0.0206 | .52 |
| COMT (A) | 10246 | −0.012 | 0.0171 | .47 | 10246 | 0.024 | 0.0145 | .093 | 6363 | 0.041 | 0.0208 | .050 |
|
| ||||||||||||
| DAT1 (440bp) | 3586 | 0.053 | 0.0316 | .095 | 3586 | 0.085 | 0.0309 | .006 | 2300 | 0.104 | 0.0403 | .010 |
| DAT1 (440bp)* | 2511 | 0.034 | 0.0388 | .37 | 2511 | 0.093 | 0.0393 | .018 | 1683 | 0.122 | 0.0479 | .011 |
| DRD4 (7r) | 3363 | −0.022 | 0.0388 | .58 | 3363 | 0.061 | 0.0357 | .086 | 2118 | 0.099 | 0.0495 | .045 |
| DRD4 (7r)* | 2512 | −0.017 | 0.0442 | .71 | 2512 | 0.058 | 0.0439 | .19 | 1683 | 0.103 | 0.0578 | .075 |
| DRD5 (148bp) | 3680 | 0.002 | 0.0282 | .95 | 3680 | 0.006 | 0.0259 | .82 | 2364 | −0.003 | 0.0352 | .93 |
| DRD5(148bp)* | 2568 | −0.011 | 0.0342 | .74 | 2568 | 0.015 | 0.0326 | .65 | 1723 | 0.021 | 0.0423 | .62 |
| MAOA (>=3.5r) | 3616 | −0.007 | 0.0244 | .77 | 3616 | −0.011 | 0.0234 | .64 | 2327 | −0.021 | 0.0313 | .50 |
| MAOA (>=3.5r)* | 2523 | 0.004 | 0.0290 | .90 | 2523 | 0.010 | 0.0287 | .71 | 1699 | 0.010 | 0.0366 | .78 |
|
| ||||||||||||
| low DA response PGS | 2512 | 0.009 | 0.0117 | .45 | 2512 | 0.019 | 0.0113 | .09 | 1685 | 0.024 | 0.0145 | .10 |
| low DA response PGS* | 2403 | 0.009 | 0.0119 | .47 | 2403 | 0.019 | 0.0115 | .09 | 1614 | 0.021 | 0.0148 | .15 |
| ‘executive and reward congruency PGS | 3586 | 0.005 | 0.0172 | .78 | 3586 | 0.047 | 0.0162 | .003 | 2300 | 0.071 | 0.0220 | .001 |
| executive and reward congruency PGS’* | 2511 | −0.008 | 0.0208 | .71 | 2511 | 0.058 | 0.0208 | .005 | 1683 | 0.084 | 0.0267 | .002 |
SNPs: model corrected for sex, age, age squared, age × sex interaction and 20 principal component on Dutch ancestry; VNTRs & PGSs: model corrected for sex, age, age squared, age × sex interaction and batch effect.
Indicates model including VNTRs additionally corrected for the 20 principal component on Dutch ancestry (which were only available for a subset of the participants, hence the smaller sample sizes).
Significant effects (p < .005) are depicted in bold.
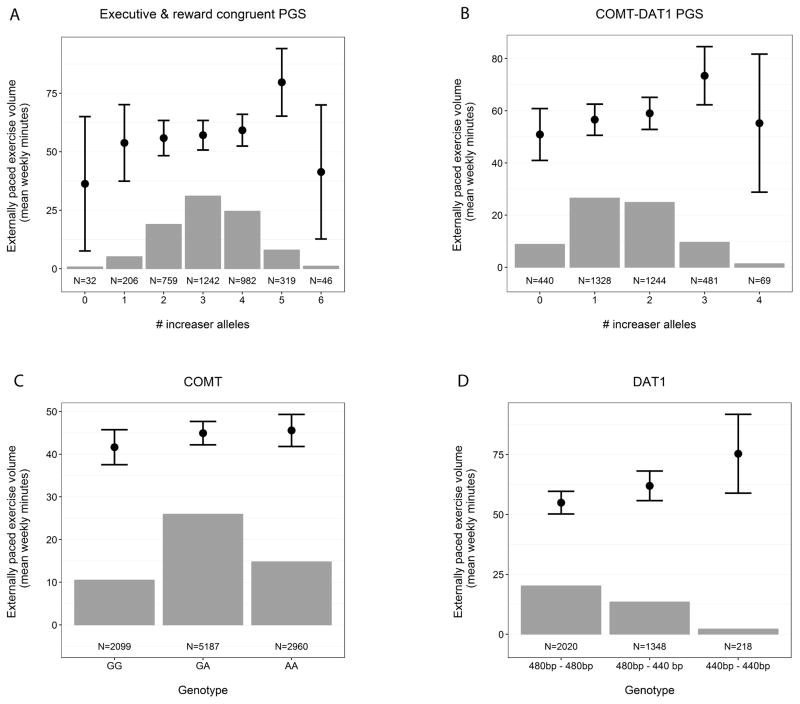
In contrast, the executive and reward congruency PGS did show a significant association with the number of minutes per week spent on externally paced exercise behavior (p=0.003; Table 4, Figure 1a). The association of this PGS remains significant and consistent in direction after correcting for the genetic PCs and when computed in exercisers only (p=0.005; Table 4). However, the observed association appears to be entirely attributable to the DAT1 and COMT genes with no additional contribution of DRD2/ANKK1. When testing this assumption, a PGS based on DAT1 and COMT indeed showed an even stronger effect (total population: N = 3562, B = 0.057, SE = 0.0195, p = .003; exercisers only: N = 2287, B = 0.089, SE = 0.0263, p = .001, Figure 1b). Independently, an increase in the number of DAT1 and COMT alleles that are positively associated with reward and executive functioning (440bp allele and MET allele, respectively) is associated with an increase in minutes spent on externally paced exercise (Figure 1c, d). However, both executive and reward congruency PGS measures are indicative of an inverted U-shape, in which having both the minimal and the maximal number of increaser alleles is non beneficial.
Figure 1. The association between genotype and externally paced exercise volume.
(A & B) Dots with error bars show a positive relationship between the number of executive and reward congruent PGS (composed of DRD2/ANKK1, COMT & DAT1) and COMT-DAT1 PGS increaser alleles, respectively, and externally paced exercise volume. The solid bars depict the number of participants for each PGS allele count. (C & D) Dots with error bars show a positive relationship between the COMT A and DAT1 440bp alleles and externally paced exercise volume. The solid bars depict the number of participants for each genotype. Error bars depict 95% CI.
Discussion
In this paper we aimed to expand the body of research on causative biological factors for the initiation and maintenance of voluntary exercise behavior. We focused on genetic variants with functional effects on dopaminergic signalling with reported downstream effects on executive functioning and reward sensitivity. We gained new insight by explicitly taking into account the types of voluntary exercise activity participants were regularly engaged in.
In a large dataset (N=12,929), we classified all self-reported voluntary exercise activities as either highly externally paced or otherwise based on the external-internal paced continuum of Galligan et al. (2000). This continuum takes into account the nature of the set of skills required in exercise activities, specifically executive function skills. We hypothesized that the effects of executive skills on the motivation to engage in exercise cannot be ignored if the exercise activities depend on these skills. Genetic association with nine functional dopaminergic allelic variants and two polygenic scores based on these variants yielded only a single result that survived multiple testing and correction for possible stratification. The polygenic score of increaser alleles in DRD2/ANKK1, COMT and DAT1 for executive function and reward sensitivity showed a consistent association with a higher volume of externally paced exercise activities. The effect appeared to be driven entirely by the COMT Met and DAT1 440bp alleles, with the DRD2/ANKK1 ‘A’ allele having no additional contribution.
The COMT and DAT1 alleles, here associated with higher weekly externally paced exercise volume have been previously linked to higher dopamine levels, better executive functioning and higher reward sensitivity. The COMT gene encodes the dopamine degrading catechol-O-methyltransferase enzyme and is highly expressed in the prefrontal cortex (PFC) and to a lesser extent in the striatum (25). The Met variant degrades dopamine less efficiently compared to the COMT-Val enzyme, resulting in higher dopamine levels (26). Homozygosity of the COMT-Met allele has been associated with both better performance on neuropsychological measures of executive function (Trail Making Test (TMT, Part B) or the Delis-Kaplan Executive Function System Trail Making subtest (DKEFS, Trial 4)) (27), better performance on the Frontal Assessment Battery (FAB) (28) and relatively increased reward learning (29) and reward responsiveness (30).
The DAT1 gene encodes the dopamine active transporter which clears dopamine from the synapse by depositing it back into the cell. It is thought to be particularly important as a regulator of phasic dopamine release in subcortical regions where DAT1 is most abundant (31,32). The 440bp allele is less transcriptionally active compared to the 480bp allele (25) resulting in lower dopamine reuptake and thus higher dopamine levels in the synapse. The less transcriptionally active 440bp allele is associated with better performance on a continuous performance task in children with ADHD (15), a less steep decline in performance over time on the Psychomotor Vigilance Test in healthy adults (33) and higher reward anticipation and responsiveness (30).
Across the largest part of the range of combined genotypes, the COMT Met and DAT1 440bp synaptic dopamine level increaser alleles had an additive effect on externally paced exercise behavior (figure 1B). However, the additivity of COMT and DAT1 seems to break down in the combination of the two most extreme homozygotes of COMT Met/Met and DAT1 440bp/440bp. Double homozygotes spent much less than the expected time on externally paced exercise. Caution is in order in interpreting this finding. There were relatively few (N=69) participants in this genotype group and the within-group individual differences were large. Our finding nonetheless resonates with similar previous findings. A study by Yacubian et al. (2007) found an inverted U-shape typed interaction effect of COMT and DAT1 haplotypes on striatal reward sensitivity during a reward sensitive guessing task. Specifically, the combination of the COMT Met allele with the DAT1 480bp allele and the COMT Val allele with the DAT1 440bp allele showed blunted responses.
A comparable interaction between the COMT Met and DAT1 440bp allele has been observed with regard to executive functioning. During a verbal fluency task, the COMT Met allele homozygotes who also carried the DAT1 440bp allele showed more activation in the left parietal cortex compared to COMT Met allele homozygotes with 2 copies of the DAT1 480bp allele (34). Similar interactions have been found in the hippocampus, and tentatively in the prefrontal cortex during two memory tasks (35).
The deviant pattern in the double COMT/DAT1 homozygotes has been explained by an interaction between tonic and phasic striatal DA levels (36). When tonic DA levels are higher than average due to the COMT Met enzyme, phasic DA release is likely to be more strongly inhibited through stimulation of presynaptic auto receptors. However, reduced synaptic DA clearance due to the DAT1 440bp variant might augment phasic DA levels leading to an optimal balance between phasic and tonic striatal DA levels resulting in optimal dopaminergic signaling.
The association of exercise behavior with the COMT and DAT1 alleles was limited to externally paced exercise activities. No association of the increaser alleles in COMT and DAT1 for executive function and reward sensitivity was seen with the total weekly minutes of voluntary exercise behavior as a whole, which includes the large group of exercisers (42.5%) engaged in internally paced activities (like jogging, swimming and bicycle racing). In fact, none of the nine polymorphisms, nor a polygenic score based on their increaser allele effects on dopamine availability, were associated with total volume of exercise behavior. This is consistent with the prior two largest studies on the association between dopaminergic variants and moderate to vigorous physical activity (9) or voluntary exercise behavior (5).
That the association with dopaminergic candidates can be restricted to a particular type of exercise activity immediately suggests that the age and sex composition of a sample may influence the association. In our data, we observed that both younger participants and males were the most likely to be engaged in a form of externally paced exercise behavior. Younger participants and males also spent more minutes per week on externally paced exercise behavior when compared to older participants and females respectively. These observations confirm previous suggestions from twin studies that the genetic contribution to the total volume of exercise behavior changes over time (37) and that the influence of genetic variants may be different at different ages. Many genes are differentially expressed across the lifespan, for instance through epigenetic modifications (38,39). It is clearly advisable to take the type of exercise behaviors into account in genetic association studies on exercise behavior, but also sex and age effects on the preferred activities. This advice should not be restricted to candidate genes in the dopaminergic system.
A question that remains unsolved is whether the association of the two dopaminergic genes with externally paced exercise behavior is more strongly mediated by the effects of the COMT and DAT1 variants on executive function skills rather than reward sensitivity, or that both pathways are of relevance. In this article we suggest that executive skills are of influence on the motivation to engage in exercise activities that depend on these skills, based on the simple principle that people are more motivated to repeat a behavior that they are good at. However, dopaminergic effects on reward sensitivity could also directly act to influence either the acute affective response to exercise, or the ‘feeling good’ often reported shortly after exercise has terminated. Large individual differences in the acute affective response to exercise as well as feeling good after exercising have been reported, which are partly heritable (40). The COMT and DAT1 variants could well be part of that heritability, because engagement in exercise behavior leads to the production of dopamine (41). Genetic variation in the mesolimbic reward system could amplify the feelings of reward more in exercisers than others, increasing the motivation to repeat this behavior (42).
The above reasoning contrasts with recent insights from contemporary exercise psychology. Evidence from a systematic review favored the acute response to exercise as the stronger determinant of future exercise behavior than the affective state after exercise (43). The majority of people, including regular exercisers, report feeling ‘bad’ rather than ‘good’ during exercise when compared to their positive affect at rest (44). An aversion to exercise, in the absence of immediate utility, would make evolutionary sense as it leads to energy conservation (45). If exercising is not rewarding at all, it would seem doubtful that genetic effects on reward sensitivity play a major role. However, we cannot exclude the possibility that more positive affect is awarded during externally paced exercise activities than during internally paced activities. Externally paced activities differ in more aspects from internally paced activities then just their dependency on good executive function. They are far more often present in competitive team sports than in solitary and/or non-competitive exercise. Rewarding effects of the social context and feelings of mastery (e.g. winning a game) may therefore be more abundant in externally paced exercise. To untangle the relative importance of genetic effects on executive function versus reward sensitivity for the engagement in different types of exercise behavior, more in-depth phenotyping of exercise activities may be necessary, i.e. establishing whether they are performed in a competitive or recreational setting and in which social context.
The strengths of the current study include the large sample size, carefully chosen functional genetic polymorphisms influencing the dopamine circuit, executive functioning and reward sensitivity and well-defined classification of exercise behavior on the external-internal paced continuum. Furthermore, we corrected for ancestry and repeated the VNTR analyses in a subset of the participants for whom the first 20 genetic PCs were available. Lastly, we performed a sensitivity analysis in exercisers to determine whether the observed effects were not due to exercise status (Yes/No). A limitation of this study is that it was built on a theoretical framework deriving largely from candidate gene studies, not just with regard to exercise behavior but also with regard to reward sensitivity and executive function. Large scale meta-analytic consortia using a hypothesis-free genome wide association study (GWAS) approach have only rarely confirmed findings of hypothesis-driven candidate gene studies which have been notably difficult to replicate (46,47). The value of theoretical frameworks building on candidate gene findings can therefore be contested.
We present the first large-scale study that investigated the effect of genetic polymorphisms in the dopaminergic signaling pathway with time spent on voluntary externally paced exercise behavior. We conclude that genetic variation in dopaminergic signaling involved in both executive functioning and reward sensitivity may influence the preference for this type of exercise behavior. More generally, we conclude that taking into account the type of exercise activities, rather than total volumes expressed (e.g. weekly amount of time exercising), can increase the success of genetic association studies aiming to unravel the neurobiology of voluntary exercise behavior.
Supplementary Material
Appendix: Functional variants in dopaminergic genes and their association with reward sensitivity and executive functioning.
Acknowledgments
We thank the members of the twin families registered with The Netherlands Twin Register for their continued support of scientific research.
Funding for the NTR was obtained from the Netherlands Organization for Scientific Research (NWO) and The Netherlands Organisation for Health Research and Development (ZonMW) grants 904-61-090, 985-10-002, 912-10-020, 904-61-193,480-04-004, 463-06-001, 451-04-034, 400-05-717, Addiction-31160008, Middelgroot-911-09-032, Spinozapremie 56-464-14192, Biobanking and Biomolecular Resources Research Infrastructure (BBMRI–NL, 184.021.007, NWO-Groot 480-15-001/674) ; VU Institute for Health and Care Research (EMGO+ ); the European Community’s Seventh Framework Program (FP7/2007-2013), ENGAGE (HEALTH-F4-2007-201413); the European Research Council (ERC Advanced, 230374, ERC Starting grant 284167), Rutgers University Cell and DNA Repository (NIMH U24 MH068457-06), the Avera Institute, Sioux Falls, South Dakota (USA) and the National Institutes of Health (NIH, R01D0042157-01A, MH081802; R01 DK092127-04, Grand Opportunity grants 1RC2 MH089951 and 1RC2 MH089995).
Footnotes
The authors declare no conflict of interest.
The results of the present study do not constitute endorsement by American College of Sports Medicine and are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
References
- 1.Stubbe JH, Boomsma DI, De Geus EJC. Sports Participation during Adolescence: A Shift from Environmental to Genetic Factors. Med Sci Sport Exerc. 2005;37(4):563–70. doi: 10.1249/01.mss.0000158181.75442.8b. [DOI] [PubMed] [Google Scholar]
- 2.Stubbe JH, de Geus EJC. Handbook of Behavior Genetics. New York, NY: Springer New York; 2009. Genetics of Exercise Behavior; pp. 343–358. [Google Scholar]
- 3.Knab AM, Bowen RS, Hamilton AT, Gulledge AA, Lightfoot JT. Altered dopaminergic profiles: Implications for the regulation of voluntary physical activity. Behav Brain Res. 2009;204(1):147–152. doi: 10.1016/j.bbr.2009.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rankinen T, Roth SM, Bray MS, et al. Advances in exercise, fitness, and performance genomics. Med Sci Sport Exerc. 2010;42(5):835–846. doi: 10.1249/MSS.0b013e3181d86cec. [DOI] [PubMed] [Google Scholar]
- 5.Huppertz C, Bartels M, Groen-Blokhuis MM, et al. The dopaminergic reward system and leisure time exercise behavior: a candidate allele study. Biomed Res Int. 2014;2014:591717. doi: 10.1155/2014/591717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simonen RL, Rankinen T, Pérusse L, et al. A dopamine D2 receptor gene polymorphism and physical activity in two family studies. Physiol Behav. 2003;78(4):751–757. doi: 10.1016/s0031-9384(03)00084-2. [DOI] [PubMed] [Google Scholar]
- 7.Murakami H, Fuku N, Kawakami R, et al. DRD2/ANKK1 gene polymorphism rs1800497 is associated with exercise habit in the period from childhood to adolescence in Japanese. J Phys Fit Sport Med. 2017;6(2):95–102. [Google Scholar]
- 8.Good DJ, Li M, Deater-Deckard K. A Genetic Basis for Motivated Exercise. Exerc Sport Sci Rev. 2015;43(4):231–237. doi: 10.1249/JES.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 9.Jozkow P, Slowinska-Lisowska M, Laczmanski L, Medras M. DRD2 C313T and DRD4 48-bp VNTR polymorphisms and physical activity of healthy men in Lower Silesia, Poland (HALS study) Ann Hum Biol. 2013;40(2):186–190. doi: 10.3109/03014460.2012.748829. [DOI] [PubMed] [Google Scholar]
- 10.Landers JG, Esch T. Sport physiology, dopamine and nitric oxide – Some speculations and hypothesis generation. Med Hypotheses. 2015;85(6):905–909. doi: 10.1016/j.mehy.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Galligan F. Advanced PE for Edexcel. Heinemann Educational. 2000:490. [Google Scholar]
- 12.Vestberg T, Gustafson R, Maurex L, Ingvar M, Petrovic P. Executive Functions Predict the Success of Top-Soccer Players. In: García AV, editor. PLoS One. 4. Vol. 7. 2012. p. e34731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan J, Wu Y, Fossella JA, Posner MI. Assessing the heritability of attentional networks. BMC Neurosci. 2001;2(1):14. doi: 10.1186/1471-2202-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. J Exp Psychol Gen. 2008;137(2):201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barnes JJM, Dean AJ, Nandam LS, O’Connell RG, Bellgrove MA. The Molecular Genetics of Executive Function: Role of Monoamine System Genes. Biol Psychiatry. 2011;69(12):127–143. doi: 10.1016/j.biopsych.2010.12.040. [DOI] [PubMed] [Google Scholar]
- 16.Van Beijsterveldt CEM, Groen-Blokhuis M, Hottenga JJ, et al. The Young Netherlands Twin Register (YNTR): Longitudinal Twin and Family Studies in Over 70,000 Children. Twin Res Hum Genet. 2013;16(1):252–267. doi: 10.1017/thg.2012.118. [DOI] [PubMed] [Google Scholar]
- 17.Willemsen G, Vink JM, Abdellaoui A, et al. The Adult Netherlands Twin Register: Twenty-Five Years of Survey and Biological Data Collection. Twin Res Hum Genet. 2013;16(1):271–281. doi: 10.1017/thg.2012.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Geus EJC, Bartels M, Kaprio J, Lightfoot JT, Thomis M. Genetics of Regular Exercise and Sedentary Behaviors. Twin Res Hum Genet. 2014;17(4):262–271. doi: 10.1017/thg.2014.42. [DOI] [PubMed] [Google Scholar]
- 19.Stubbe JH, de Moor MHM, Boomsma DI, de Geus EJC. The association between exercise participation and well-being: A co-twin study. Prev Med (Baltim) 2007;44(2):148–152. doi: 10.1016/j.ypmed.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 20.de Moor M, De Geus E. Lifestyle Medicine. 2. CRC Press; 2013. Genetic Influences on Regular Exercise Behavior; pp. 1367–1378. [Google Scholar]
- 21.Franciolo LC, Menelaou A, Pulit SL, et al. Whole-genome sequence variation, population structure and demographic history of the Dutch population. Nat Genet. 2014;46(8):818–825. doi: 10.1038/ng.3021. [DOI] [PubMed] [Google Scholar]
- 22.Mbarek H, Milaneschi Y, Hottenga J-J, et al. Genome-Wide Significance for PCLO as a Gene for Major Depressive Disorder. Twin Res Hum Genet. 2017;20(4):267–270. doi: 10.1017/thg.2017.30. [DOI] [PubMed] [Google Scholar]
- 23.Fedko IO, Hottenga J-J, Medina-Gomez C, et al. Estimation of Genetic Relationships Between Individuals Across Cohorts and Platforms: Application to Childhood Height. Behav Genet. 2015;45(5):514–528. doi: 10.1007/s10519-015-9725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdellaoui A, Hottenga J-J, de Knijff P, et al. Population structure, migration, and diversifying selection in the Netherlands. Eur J Hum Genet. 2013;21(11):1277–1285. doi: 10.1038/ejhg.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yacubian J, Sommer T, Schroeder K, et al. Gene– gene interaction associated with neural reward sensitivity. Proc Natl Acad Sci. 2007;104(19):8125–8130. doi: 10.1073/pnas.0702029104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer-Lindenberg A, Kohn P, Kolachana B. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nature. 2005;8(5):594–596. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- 27.Wishart H, Roth R, Saykin A. COMT Val158Met genotype and individual differences in executive function in healthy adults. J Int Neuropsych Soc. 2011;17(1):174–180. doi: 10.1017/S1355617710001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitaki S, Isomura M, Maniwa K. Impact of five SNPs in dopamine-related genes on executive function. Acta Neurol (Napoli) 2013;127(1):70–76. doi: 10.1111/j.1600-0404.2012.01673.x. [DOI] [PubMed] [Google Scholar]
- 29.Corral-Frías NS, Pizzagalli DA, Carré JM, et al. COMT Val 158 Met genotype is associated with reward learning: a replication study and meta-analysis. Genes, Brain Behav. 2016;15(5):503–513. doi: 10.1111/gbb.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dreher J-C, Kohn P, Kolachana B, Weinberger DR, Berman KF. Variation in dopamine genes influences responsivity of the human reward system. Proc Natl Acad Sci U S A. 2009;106(2):617–622. doi: 10.1073/pnas.0805517106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sesack S, Carr D. Selective prefrontal cortex inputs to dopamine cells: implications for schizophrenia. Physiol Behav. 2002;77(4):513–517. doi: 10.1016/s0031-9384(02)00931-9. [DOI] [PubMed] [Google Scholar]
- 32.Lewis D, Melchitzky D, Sesack S. Dopamine transporter immunoreactivity in monkey cerebral cortex: regional, laminar, and ultrastructural localization. J Comp Neurol. 2001;432(1):199–236. doi: 10.1002/cne.1092. [DOI] [PubMed] [Google Scholar]
- 33.Lim J, Ebstein R, Tse C, Monakhov M, Lai PS. Dopaminergic polymorphisms associated with time-on-task declines and fatigue in the Psychomotor Vigilance Test. PLoS One. 2012;7(3):e33767. doi: 10.1371/journal.pone.0033767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prata DP, Mechelli A, Fu CHY, et al. Epistasis between the DAT 3′ UTR VNTR and the COMT Val158Met SNP on cortical function in healthy subjects and patients with schizophrenia. Proc Natl Acad Sci. 2009;106(32):13600–13605. doi: 10.1073/pnas.0903007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertolino A, Di Giorgio A, Blasi G, et al. Epistasis between Dopamine Regulating Genes Identifies a Nonlinear Response of the Human Hippocampus During Memory Tasks. Biol Psychiatry. 2008;64(3):226–234. doi: 10.1016/j.biopsych.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Bilder RM, Volavka J, Lachman HM, Grace AA. The Catechol-O-Methyltransferase Polymorphism: Relations to the Tonic–Phasic Dopamine Hypothesis and Neuropsychiatric Phenotypes. Neuropsychopharmacology. 2004;29(11):1943. doi: 10.1038/sj.npp.1300542. [DOI] [PubMed] [Google Scholar]
- 37.Huppertz C, Bartels M, de Zeeuw EL, et al. Individual Differences in Exercise Behavior: Stability and Change in Genetic and Environmental Determinants From Age 7 to 18. Behav Genet. 2016;46(5):665–679. doi: 10.1007/s10519-016-9799-x. [DOI] [PubMed] [Google Scholar]
- 38.Van Dongen J, Nivard MG, Willemsen G, et al. Genetic and environmental influences interact with age and sex in shaping the human methylome. Nat Commun. 2016:7. doi: 10.1038/ncomms11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slieker RC, Van Iterson M, Luijk R, et al. Age-related accrual of methylomic variability is linked to fundamental ageing mechanisms. Genome Biol. 2016;17(1):191. doi: 10.1186/s13059-016-1053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schutte NM, Nederend I, Hudziak JJ, Bartels M, De Geus EJC. Heritability of the affective response to exercise and its correlation to exercise behavior. Psychol Sport Exerc. 2017;31:139–148. doi: 10.1016/j.psychsport.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meeusen R, De Meirleir K. Exercise and Brain Neurotransmission. Sport Med. 1995;20(3):160–188. doi: 10.2165/00007256-199520030-00004. [DOI] [PubMed] [Google Scholar]
- 42.Greenwood BN, Foley TE, Le TV, et al. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav Brain Res. 2011;217(2):354–362. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rhodes RE, Kates A. Can the Affective Response to Exercise Predict Future Motives and Physical Activity Behavior? A Systematic Review of Published Evidence. Ann Behav Med. 2015;49(5):715–731. doi: 10.1007/s12160-015-9704-5. [DOI] [PubMed] [Google Scholar]
- 44.Ekkekakis P, Parfitt G, Petruzzello SJ. The Pleasure and Displeasure People Feel When they Exercise at Different Intensities. Sport Med. 2011;41(8):641–671. doi: 10.2165/11590680-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 45.Lee HH, Emerson JA, Williams DM. The Exercise-Affect-Adherence Pathway: An Evolutionary Perspective. Front Psychol. 2016;7:1285. doi: 10.3389/fpsyg.2016.01285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ioannidis JPA. Non-replication and inconsistency in the genome-wide association setting. Hum Hered. 2007;64(4):203–213. doi: 10.1159/000103512. [DOI] [PubMed] [Google Scholar]
- 47.Sullivan PF. Spurious Genetic Associations. Biol Psychiatry. 2007;61(10):1121–1126. doi: 10.1016/j.biopsych.2006.11.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix: Functional variants in dopaminergic genes and their association with reward sensitivity and executive functioning.