Abstract
We recently found that thymidine phosphorylase (TYMP), also known as platelet-derived endothelial cell growth factor, plays an important role in platelet activation in vitro and thrombosis in vivo by participating in multiple signaling pathways. Platelets are a major source of TYMP. Since platelet-mediated clot formation is a key event in several fatal diseases, such as myocardial infarction, stroke and pulmonary embolism, understanding TYMP in depth may lead to uncovering novel mechanisms in the development of cardiovascular diseases. Targeting TYMP may become a novel therapeutic for cardiovascular disorders. In this review paper, we summarize the discovery of TYMP and the potential molecular mechanisms of TYMP involved in the development of various diseases, especially cardiovascular diseases. We also offer insights regarding future studies exploring the role of TYMP in the development of cardiovascular disease as well as in therapy.
Keywords: thymidine phosphorylase, platelets, thrombosis, cancer
1. TYMP discovery
As early as 1930, it was found that a deoxyribose was released from thymidine by the enzymatic action of a nucleotidase prepared from animal tissues [1]. About two decades later, Manson and Lampen discovered that thymidine was cleaved by a phosphorolytic process to release a sugar phosphate ester [2]. Friedkin and Roberts purified and characterized the enzyme from animal tissues in 1954 [3], and named this thymidine catalytic enzyme thymidine phosphorylase (TYMP). In the early literature, thymidine phosphorylase was abbreviated as TP. To distinguish it from the thromboxane receptor, also abbreviated as TP, we use “TYMP” in most of our recent publications. Human TYMP was first isolated from the amniochorion [4]. In 1987 and 1992, two Japanese groups reported two “new” human proteins, namely, platelet-derived endothelial cell growth factor (PD-ECGF) [5] and gliostatin [6]. Sequence analysis found that these two “new” proteins were identical to human TYMP [7–9], and from then on, TYMP was also called PD-ECGF or gliostatin, and characterized as having a strong angiogenic effect and in inhibiting glial cell growth. Of note, TYMP/PD-ECGF/gliostatin protein is not identical to platelet-derived growth factor (PDGF) or epithelial cell growth factor (ECGF).
TYMP is known now to express by various cells [10]. TYMP is also highly expressed in solid tumors, such as in breast and colorectal cancers; its biological effects in cancer are mainly recognized as being strongly pro-angiogenic [7] and anti-apoptotic [11], and these will be discussed in Section 3. There is no evidence that a TYMP gene mutation leads to the development of tumor cells. However, loss-of-function mutations of the TYMP gene cause mitochondrial neurogastrointestinal encephalopathy (MNGIE), a rare autosomal recessive human disease [12]. Somjen et al., as well as our group, have demonstrated that TYMP inhibits vascular smooth muscle cell (VSMC) DNA synthesis and thus inhibits VSMC proliferation [13–15]. TYMP is mainly found inside the cell due to its lack of an amino-terminal hydrophobic leader sequence, required for cell secretion [16]. The following discussion thus mainly describes its intracellular functions. Although several studies implied that TYMP affected focal adhesion kinase (FAK) and integrin activity [17], which is related to its enzymatic activity, studies have not revealed whether TYMP itself participates in signaling transduction. In this regard, our recent studies are the first to show that TYMP acts as a signaling molecule by directly binding to Src family kinase (SFK) through its N-terminus residue, and also plays an important role in platelet activation and thrombosis [18]. Figure 1 outlines a time course of the discovery of TYMP, with accumulating data indicating that TYMP may be associated with the development of various diseases, especially cardiovascular disorders, leading to the conclusion that targeting TYMP may yield novel therapeutics for these diseases. In this review, we will summarize the cognition and function of TYMP and its relationship to disease, and provide the principal basis for targeting TYMP as a possible therapeutic strategy to counter the development of cardiovascular diseases.
Figure 1.
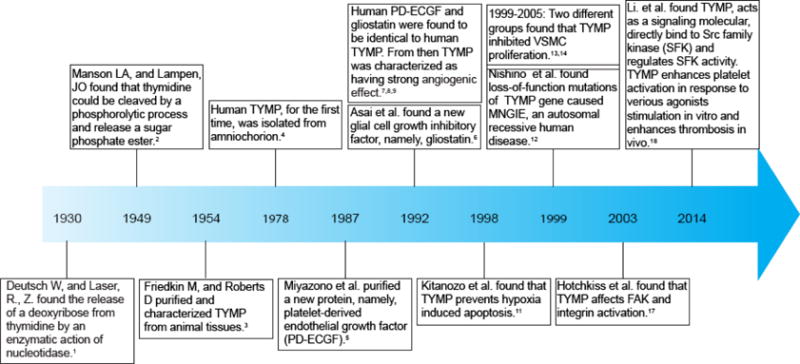
History of TYMP development and timeline of the discovery of functions.
2. TYMP structure
The human gene encoding TYMP is located on chromosome 22q13.32-qter [12], which contains 10 exons spanning more than 4.3 kb [19]. TYMP mRNA has a length of 1.8 kb and codes for a 482-residue protein with a molecular weight of ~50 kDa [20]. The first TYMP structure to be described with a thymine in its active site was from Escherichia coli, as outlined by Walter and his coworkers [21]. Norman et al. described the first structure of human TYMP in a binding mode in the presence of 5-chloro-6-(1-(2-iminopyrrolidinyl)methyl) uracil (TPI), a specific and potent TYMP inhibitor (Figure 2) [22]. Additional structural studies were also conducted on human TYMP in the presence of thymidine/thymine [23], two major substrates of TYMP, and KIN59 [24], a non-competitive TYMP inhibitor. The binding of substrates or inhibitors to TYMP induces a closed conformational change, which is essential for a TYMP-mediated phosphorolytic reaction to occur. The inhibitory role of KIN59 in binding TYMP, without affecting the binding of substrates to the enzyme, strongly suggests that a conformational change in TYMP influences its function.
Figure 2. A crystal structure of human thymidine phosphorylase (TYMP).
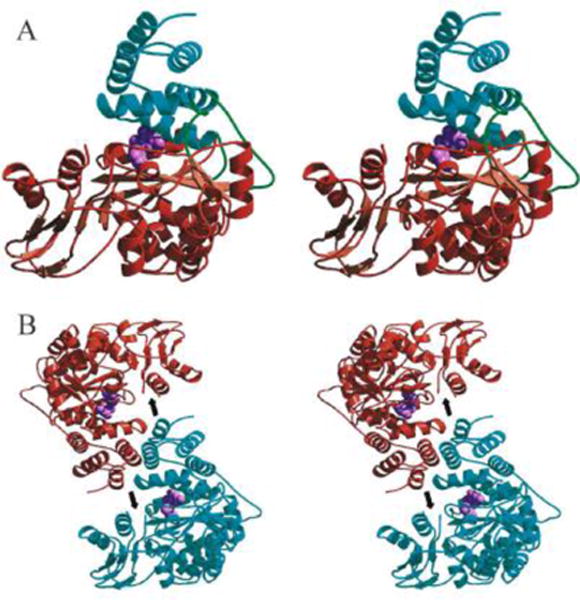
A. Stereo view of the human TYMP monomer showing the main-chain trace of the α domain (cyan), hinge regions (green), and the mixed α/β domain (brown). The TYMP inhibitor, 5-chloro-6-(1-(2-iminopyrrolidinyl)methyl) uracil (TPI; light purple) is shown as a cpk model in the active site situated between the α and α/β domains. B. Stereo view of the human TP dimer generated by applying two-fold crystallographic symmetry to the monomer. Human TYMP monomers are colored cyan and brown, and TPI (light purple) is shown as a cpk model. The position of the proteolytically cleaved loop is indicated by an arrow. (Adopted from Norman et al. Structure 2004, 12:75–85, Figure 3, with permission from Elsevier).
Both bacterial and mammal TYMPs are made up of homodimers. Each TYMP monomer consists of a small α-helical domain (α-domain), and a large mixed α-helical and β-sheet domain (α/β domain) separated by a large cleft containing the substrate binding site [22–24]. Without substrate binding, TYMP is usually in an open conformation; after binding by substrates, the two domains move toward each other to generate a closed conformation [22–24]. Krenitsky and colleagues have conducted kinetic studies using both E. coli and rabbit TYMPs and found that TYMP follows a sequential mechanism whereby phosphate is the first substrate to bind to the enzyme, with 2DDRP the last product to be released from TYMP [25]. Site-directed mutation analysis of human TYMP has revealed that Lys115, His116, Leu148, Tyr199, Arg202, Ile214 and Ser217 are essential for enzyme activity [26, 27]. Bronckaers et al. also demonstrated Asp203 plays an important role in allowing the loop stabilization that is required for the catalytic efficiency of this enzyme [24]. All of these key sites are conserved among different organisms (Figure 3A). An ability to inhibit such binding sites is particularly significant not only for the regulation of the enzyme’s activity [24, 26, 27], but also likely affects the signaling function of TYMP [15, 18]. Therefore, these key positions may be important targeting sites for drug development.
Figure 3. Analysis of TYMP protein sequences.
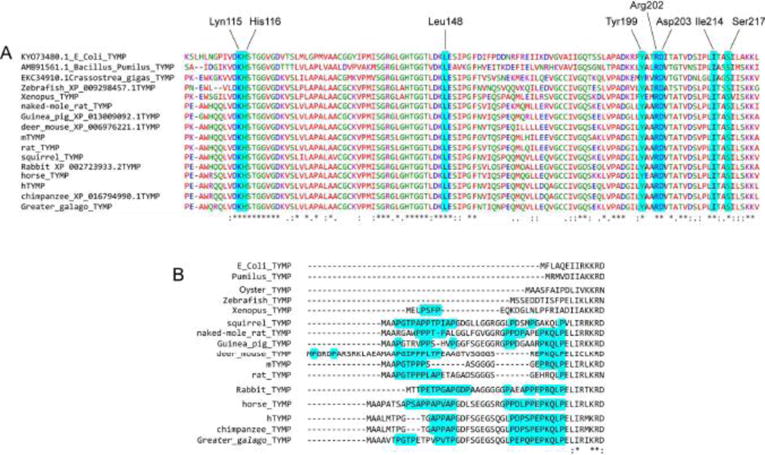
A. The key amino acid residues, in which a mutation diminishes TYMP activity, are preserved in all species. B. Alignment of the N-terminus of TYMP proteins revealed that a proline-rich N-terminus is only present in mammals.
By analyzing sequences of TYMP proteins from different organisms, we found that mammalian TYMP contains a proline-rich N-terminus (Figure 3B) [18] that is lacking in bacteria, fish and frogs as well as invertebrate animals. Hemostasis is not applicable to bacteria, while frogs and fish have nucleated thrombocytes in lieu of platelets, whether clot formation is similar to that observed in mammals is unclear. Thus, the evolution of a well-conserved proline-rich N-terminus in mammals may be indicative of its necessity for hemostasis. Our recent study is the first to demonstrate that TYMP functions as a signaling molecule and plays an essential role in regulating platelet activation and clot formation in response to vascular injury [18]. This effect occurs through the binding of TYMP to several members of the Src family of protein tyrosine kinases (SFK), possibly via the proline-rich domain in the TYMP N-terminus and the SH3 domain in SFK. Unfortunately, all structural studies of human TYMP, to date, show up to 34 missing amino acid N-terminal residues [23, 26]. Whether and how this N-terminal peptide can affect TYMP function requires further study.
In summary, in addition to playing an important role in cancer through enhancing angiogenesis in the cancer microenvironment, increasing evidence suggests that TYMP is essential for platelet activation and thrombosis that may lead to fatal cardiovascular diseases, such as myocardial infarction and stroke. Therefore, understanding the structure of TYMP and mechanisms of substrate binding to TYMP may be essential to support a rationale for new drug investigations into cardiovascular diseases.
3. TYMP functions
3.1) TYMP acts as a pyrimidine nucleoside phosphorylase
TYMP belongs to the pyrimidine nucleoside phosphorylase (PyNP) family, and its primary function is to drive the salvage pathway of pyrimidine nucleosides [24, 28]. In the presence of inorganic phosphate, TYMP reverse catalyzes thymidine to thymine and 2-D-deoxyribose-1-phosphate (2DDRP); the latter is further degraded to 2-D-deoxyribose (2DDR; Figure 4, left) [22]. TYMP also has deoxyribosyl transferase activity that involves the transfer of a deoxyribosyl moiety from a pyrimidine nucleoside to another pyrimidine base (Figure 4, right), resulting in the formation of a new pyrimidine nucleoside [29]. This reaction is most likely via an SN2-like transition state involving a nucleobase, 2′-deoxyribose and phosphate [26, 30]. Therefore, functional TYMP acts as a homodimer, plays a key role in pyrimidine nucleoside metabolism, and ensures a sufficient pyrimidine nucleotide pool for DNA repair and replication.
Figure 4.
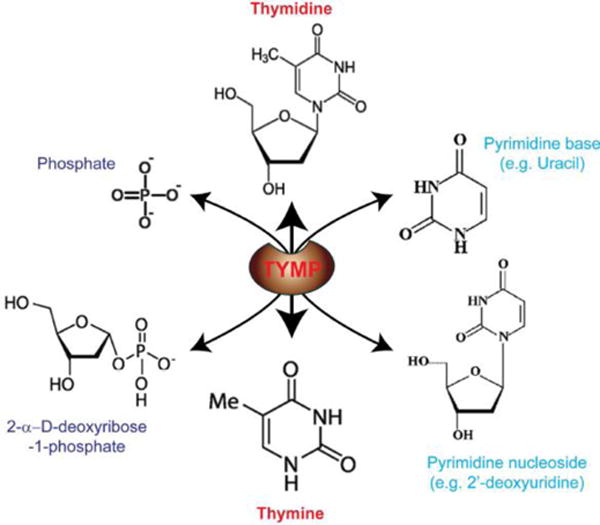
Catalytic functions of TYMP.
TYMP also recognizes several pyrimidines or pyrimidine nucleoside analogs endowed with antiviral and antitumoral activity. These include 5-(E)-(2-bromovinyl)-2′-deoxyuridine (BVDU), 5-trifluorothymidine (TFT) and 5-fluorouracil (5FU), as well as 5-fluoro-5′-deoxyuridine (5′DFUR), an intermediate metabolite of capecitabine [28, 31–35]. The rationale for the design of capecitabine is to take the advantage of increased levels of TYMP found in several tumors in contrast to normal tissues, potentially allowing for selective tumor toxicity [36] since TYMP acts in the last step to catalyze capecitabine to 5FU, an antineoplastic agent.
3.2) TYMP has a strong pro-angiogenic effect
In 1987, an “endothelial cell growth factor” was purified from human platelets and named PD-ECGF. Based on a radiolabeled-thymidine incorporation assay, PD-ECGF had a strong mitogenic effect on endothelial cells [5]. Five years later, human PD-ECGF was found to have the same protein sequence as human TYMP [9], meaning they were the same protein. Since then, as a pro-angiogenic factor expressed at high levels in several solid cancers as mentioned above, TYMP has undergone both in vitro and in vivo investigations, especially with regard to tumor-associated angiogenesis [37–42]. By the direct injection of a plasmid vector encoding human TYMP cDNA into an ischemic canine myocardium or rabbit hindlimb, we demonstrated that TYMP promotes angiogenesis in cardiac and skeletal muscles [43–45]. Angiogenesis is the formation of new blood vessels from a preexisting vasculature [46], which is essential for tumor growth and cancer progression [47]. How TYMP enhances angiogenesis is still not fully understood, and is hitherto considered in the following main mechanisms:
Firstly, TYMP enhances endothelial cell migration through its metabolite 2DDR [48]. Therefore, the angiogenic function of TYMP relates to its enzymatic activity and it follows that blocking the enzymatic function of TYMP abolishes its angiogenic effect [49]. FAK is a non-receptor protein tyrosine kinase required for the formation of focal adhesions. FAK acts as a molecular bridge between intracellular and extracellular spaces that integrates a variety of environmental stimuli and mediates a two-way crosstalk between the extracellular matrix and cytoskeleton [50]. Both TYMP and 2DDR stimulate the phosphorylation of FAK at tyrosines 397 and 925 to induce the formation of focal adhesions; they therefore play an important role in endothelial cell migration and angiogenesis [51]. This effect relates to the activation of the integrins α5β1 and αVβ3 rather than a change in their expression on the cell surface or in their total cellular levels [51]. We also found that TYMP deficiency did not affect the expression of β1 and β3 on the platelet surface [18], in line with the findings of others. By adding 2DDR to cultured cells, one recent study concluded that 2DDR bound to α5β1 and αVβ3 and induced phosphatidyl inositol 3-kinase (PI3K)/AKT activation [52] based on an antibody-blocking assay as published previously by Hotchkiss et al. [51]; however, this finding was not supported by convincing results from binding assays. Indeed, using [3H]-2DDR, Brown and Bicknell revealed a lack of any specific binding of 2DDR to endothelial cells, which express both integrins α5β1 and αVβ3, indicating that the effects of 2DDR are not receptor-mediated [53]. Therefore, how TYMP induces integrin activation is remains unclear. Our recent study demonstrated that platelet TYMP, acting as a signaling molecule, can directly bind to SH3 domain-containing proteins, including Lyn, Fyn and Yes, and thus plays an important role in intracellular signal transduction upon platelet activation [18]. FAK also has a SH3-binding motif (PXXP); therefore, TYMP may fine-tune FAK function by regulating SH3 domain–containing proteins in a coordinated manner. It is possible that the binding of substrate to TYMP causes a conformational change that affects its binding to other signaling molecules. We hypothesize that TYMP will function similarly in endothelial cells and activate integrins α5β1 and αVβ3 via “inside-out”, but not “outside-in” signaling. This theory is under further investigation.
Secondly, in addition to activating integrin proteins, 2DDR also activates p70/s6k, an important downstream kinase of the mechanistic target of rapamycin (mTOR), which also regulates cell proliferation and angiogenesis [54]. 2DDR-mediated angiogenesis is inhibited by rapamycin blocking p70/s6k kinase activation. An association between rapamycin and TYMP is also shown by evidence of a protective role for thymidine in rapamycin-induced cytotoxicity [55]. The TYMP inhibitor, TPI, inhibits the protective effect of thymidine in rapamycin-induced cytotoxicity, suggesting this protective effect depends on TYMP activity. TYMP may also mediate FAK signaling, since downstream of FAK, PI3K/AKT is necessary for activating mTOR signaling. In our recent study, we found that a TYMP deficiency or the inhibition of TYMP activity dramatically attenuated AKT activation in response to the activation of glycoprotein VI (GPVI) signaling [18], further suggesting a link between TYMP and AKT. Clarification of this pathway may provide novel insights into TYMP-mediated angiogenesis.
Thirdly, TYMP may enhance angiogenesis by directly stimulating the expression of pro-angiogenic factors, including vascular endothelial growth factor (VEGF), matrix metalloproteinase (MMP)-2, urokinase plasminogen activator (uPA), tumor necrosis factor alpha (TNFα) and interleukin (IL)-8, among others. [42, 48, 56, 57]. In general, the expression and function of angiogenesis-related proteins are tightly regulated at different levels. For example, MMP-2 is regulated at three levels: gene expression, pro-enzyme activation, and enzyme inactivation by related inhibitory proteins [46, 57]. Tabata and colleagues have found that the angiogenic effect of TYMP is mediated by up-regulating the expression of IL-8 and NFκB [42]. TYMP, possibly through increasing reactive oxygen species (ROS) generation, promotes the transcription of IL-8 and other NFκB target genes, including IL-6 and fibronectin-1 [42]. TYMP has been found in nuclei, especially in actively proliferating cells such as endothelial cells or macrophages, suggesting that TYMP may directly participate in regulating the transcriptional expression of genes.
Whether and how TYMP regulates gene expression is unknown. DNA methylation plays an important role in the programming of gene expression; in mammals, about 40% of genes contain dinucleotide cytosine-guanine (CpG) islands, the targets of DNA methylation in their promoters [58]. Liu et al. demonstrated that through increasing expression of DNA methyltransferase 3A (DNMT3A), TYMP up-regulates methylation of several genes related to bone formation [52]. This effect is also mediated by TYMP-induced PI3K/AKT activation [52], as also reported in many other studies [18]. We previously also showed a positive correlation between TYMP expression and increased expression and activities of MMP-2/9 and uPA in a canine model of transmyocardial laser revascularization [46, 57]. Interestingly, the treatment of invasive breast cancer cells, MCF-7 and ZR-75-1, with the methyltransferase inhibitor 5′-aza-2′-deoxycytidine (5-AzaCdR) dramatically enhanced the invasiveness of these cells by inducing the expression of uPA and MMP-2 [59]. Therefore, uncovering the role of TYMP in DNA methylation and its relationship to the expression of pro-angiogenic factors in endothelial and vascular smooth muscle cells may help us understand how TYMP enhances angiogenesis.
Lastly, TYMP may promote angiogenesis by enhancing the survival of endothelial progenitor cells (EPCs). Preclinical and clinical studies have suggested that EPCs contribute to the reparative processes of the vascular endothelium, including participating in angiogenesis [60, 61]. Using proteomics analysis, Pula and colleagues demonstrated that EPCs secrete TYMP [62]. Inhibition of TYMP with an inhibitor diminished EPC colony formation and increased cell apoptosis; such inhibitory effects were reversed by 2DDR, suggesting that TYMP has a paracrine effect on EPCs [62].
Taken together, the role of TYMP on angiogenesis is still unclear and additional studies are necessary. In particular, focusing on the signaling pathways mediated by TYMP in endothelial cells may clarify its angiogenic effect and lead to the development of additional pro- or anti-angiogenic therapeutics.
3.3) TYMP has an anti-apoptotic effect
In 1998, Kitanozo et al. found that expression of TYMP in colon and esophageal tumors was a prognostic factor independent of microvessel density [11]. This finding led them to speculate that TYMP may affect tumor progression independently of its angiogenic effect. The same group found that overexpression of TYMP in epidermoid carcinoma KB cells prevented hypoxia-induced apoptosis [11] in the first paper to show evidence that TYMP inhibits apoptosis. Since then, several studies from different institutes found reverse correlations of apoptosis and the expression of TYMP in different cancers, including colorectal carcinomas [63], esophageal carcinomas [64, 65], epithelial ovarian cancer [66], and gastric carcinomas [67]. We presented direct evidence that the injection of a mammalian expression plasmid vector encoding human TYMP into canine ischemic heart significantly inhibited apoptosis [45, 68]. The potential mechanisms that mediate the inhibitory role of TYMP on apoptosis correlated to the suppression of p38 mitogen-activated protein kinase (MAPK) [69], the inhibition of Fas-induced caspase-8 activation [70], a decrease in expression of Bax and the attenuation of caspase-3 activation [45, 68], as well as an increase in PI3K/AKT activity [71]. However, how TYMP affects the activity of these molecules is unknown. Mori and colleagues showed that inhibition of TYMP by TPI did not affect Fas-induced apoptosis [72], indicating that the role of TYMP in Fas-induced apoptotic signal transduction was independent of its enzymatic activity. Ikeda et al. also demonstrated that both wild type and activity-null mutant TYMP overexpression inhibited cisplatin-induced apoptosis in human leukaemia Jurkat cells [73], further suggesting that TYMP has an unknown biologic role that is independent of its enzymatic activity. Based on our recent finding that TYMP acts as a signaling molecule [18], we believe that the anti-apoptotic effect of TYMP is mediated by this protein but not its enzymatic substrates. Additional studies focusing on TYMP signal transduction under apoptotic conditions are necessary to resolve this issue.
3.4) TYMP as a novel pro-thrombotic protein
Platelets are the major source of TYMP [5], with each human platelet containing from 5,400 to 11,600 copies of TYMP [74, 75]. This is more than several major platelet surface receptors, such as GPVI, or signaling molecules such as Lyn and Fyn. However, whether and how TYMP affected platelet function under either physiological or pathophysiological conditions was unknown prior to our recent publication [18]. TYMP is highly expressed in several chronic diseases including atherosclerosis, several solid cancers, and type II diabetes as well as in viral infections as will be discussed in the following section. Increased expression of TYMP in human hepatocellular carcinoma correlated with a high incidence of portal vein tumor thrombosis [76]. The perfusion of erythrocyte-encapsulated TYMP into mice also increased the incidence of thrombosis in the lung [77]. These findings suggested that TYMP may play a role in platelet activation and increase the risk of thrombosis. Such a hypothesis was clearly confirmed in our recent study [18], which combined studies using an in vivo FeCl3-induced mouse carotid artery thrombosis model [78, 79] and in vitro studies using both human and mouse platelets. We found that: (1) TYMP deficiency in mice significantly prolongs the time to occlusive thrombosis formation in response to FeCl3-induced carotid artery injury; (2) TYMP deficiency significantly attenuates platelet adhesion to collagen and reduces platelet agonist-induced platelet aggregation and activation; (3) TYMP forms a complex with Lyn, Fyn and Yes in human platelets and modulates the activities of Lyn and downstream signaling molecules of Lyn, such as PECAM1 and AKT; (4) Lyn haploinsufficiency diminishes the phenotype found in Tymp+/− mice; and (5) Pharmacologic inhibition of TYMP activity reduces agonist-induced human and murine platelet aggregation in vitro and prolongs murine thrombosis times in vivo without affecting hemostasis. Although high TYMP expression in gastric cancer positively correlates with thrombocytosis [80], TYMP deficiency does not affect platelet counts [18]. Interestingly, aspirin, an anti-platelet drug, inhibits TYMP production in a human monocyte cell line, THP1 [81], suggesting that the long-term use of aspirin may also inhibit TYMP production in platelets and thus result in an anti-thrombotic effect. This study indicates that targeting TYMP may lead to novel anti-platelet and anti-thrombotic therapies.
In our studies, substrates of TYMP, including thymidine, 2DDR and 2DDRP, did not affect ADP- and collagen-induced platelet activation [18]. Using mass spectrometry, Vara and colleagues demonstrated that 2DDRP is present in platelets at concentrations of 80–400 μM; the addition of 2DDRP to human platelets dramatically increased low-dose thrombin-induced platelet aggregation [82]. Such interesting data support our studies and the suggestion that TYMP metabolites secreted from platelets potentiate the aggregative response to low-dose agonist stimulation, thus enhancing thrombosis in vivo. Whether this effect is mediated by a 2DDRP-induced TYMP conformational change is unknown. Additional studies are necessary to identify the detailed mechanism that mediates a TYMP-associated prothrombotic effect, including the effect of TYMP on GPVI and GPCR signaling pathways.
3.5) TYMP inhibits DNA synthesis and proliferation of vascular smooth muscle cells
Immunohistochemical staining was used to detect TYMP in VSMC as early as 1995 [83]. Somjen et al. were the first to report that treatment of human umbilical arterial smooth muscle cells in culture with TYMP/PD-ECGF protein significantly inhibited cellular DNA synthesis [13]. We further demonstrated that the TYMP-mediated inhibitory effect on VSMC proliferation was mediated by the induction of p27KIP1 and heme oxygenase-1 [14, 84]. Additionally, our recent study revealed that the Src family kinase, Lyn, played an important role in mediating the inhibitory effect of TYMP on VSMC [15]. We found that TYMP induced the activation of a signal transducer and activator of transcription 3 (STAT3) in a Lyn-dependent manner. This subsequently upregulated the expression of unphosphorylated STAT3, which also plays a critical role in inhibiting VSMC proliferation [15]. Interestingly, Kirchmer et al. recently demonstrated that STAT-3 promoted VSMC contractile gene expression, with high levels of STAT-3 driving VSMC into a more mature phenotype [85], which supports our findings. Our recent study further demonstrated that TYMP overexpression increased ATP or extracellular calcium overload that induced intracellular calcium sparking as well as enhanced VSMC contraction (unpublished data, not shown), suggesting that TYMP may drive VSMC into a more mature contractile phenotype, and thus may regulate vessel wall tension and blood pressure. Taken together, our knowledge regarding TYMP in VSMC is limited. Elucidating the mechanistic role of TYMP on VSMC biology may provide novel insights into understanding the development of atherosclerotic diseases and hypertension.
3.6) TYMP inhibits glial cell growth
As mentioned previously, TYMP is also known as gliostatin, which was originally purified from extracts of neurofibroma [6]. TYMP shows a strong inhibitory effect on all glial cells at nanomolar concentrations [8]. Glial cells are non-neuronal cells that maintain homeostasis, form myelin, and provide support and protection for neurons in the central and peripheral nervous systems [86]. Interestingly, the expression of TYMP increases in neurons after ischemic injury [87], suggesting TYMP may directly act on neurons to protect these from ischemic injury. However, the detailed mechanisms of action of TYMP on both neurons and glial cells are not well studied. A recent investigation has also led to the suggestion that TYMP, together with VEGFA, promotes the breakdown of the blood–brain barrier (BBB) [88], highlighting the urgency of investigating how TYMP affects the neurological system. Clarification of the role of TYMP within the nervous system may lead to the development of novel therapeutics for ischemic stroke and other brain traumas.
4. The role of TYMP in the development of chronic diseases
4.1) TYMP and cancer development
Several excellent reviews have summarized the role of TYMP in cancer [89–91]. TYMP is highly expressed by several solid cancers, including head–neck [92, 93], breast [94, 95], lung [96, 97], oral squamous carcinoma [98], esophageal [99], gastric [100–102], colorectal [103, 104], bladder [40, 105], prostate [106, 107], ovarian [108, 109], and cervical [110, 111] cancers. However, some cancers, such as the oral squamous carcinoma cell lines HSC3, SasH1 and SasL1 as well as SCC9, do not express TYMP (Figure 5). The reason for this is unknown, but strongly suggests that TYMP is not essential for the development of cancers. Comparing a normal urine proteome, Kiprijanovska and colleagues revealed that 11 proteins are distinctively expressed in prostate cancer [107]. Of these, TYMP was one of three proteins that was associated with cellular growth and proliferation [107]. Since evidence does not exist for a TYMP gene mutation in the development of tumor cells, these facts suggest that TYMP may be important for tumor growth by enhancing angiogenesis and inhibiting apoptosis, but is not characteristic of tumor development.
Figure 5. TYMP protein expression in different cancer cell lines.
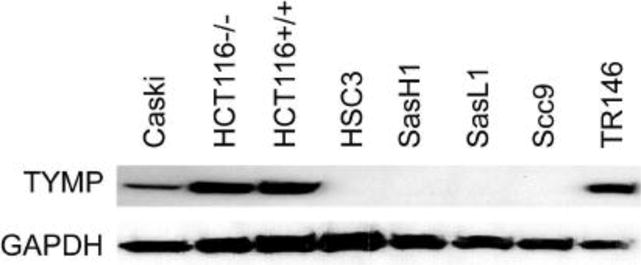
Cancer cells in culture were grown to 80% confluence. Cells were lysed and protein samples electrophoresed and western blotted using anti-TYMP antibody (sc-56584, Santa Cruz, Dallas, TX, USA). GAPDH was used as a loading control. The experiment was repeated with a similar result.
In the cancer microenvironment, both tumor cells and cells in the surrounding matrix, including fibroblasts, macrophages and lymphocytes, express TYMP. The high expression of TYMP in infiltrating, non-malignant cells suggests this may be influenced by local inflammation and stress conditions such as hypoxia in the cancer environment, rather than genetic changes that may function as paracrine stimuli for cancer growth. Many studies have indicated that high levels of TYMP in cancer are also associated with poor patient outcomes.
In addition to a pro-angiogenic effect as discussed above in section 3.2), TYMP may also enhance epithelial to mesenchymal transition (EMT) or endothelial–mesenchymal transition (EndMT), and thus may increase tumor invasiveness and angiogenesis by upregulating the expression of genes associated with these events. We have found that TYMP expression is associated with the up-regulated expression of MMP1, 2 and 9, as well as uPA, in a canine model of transmyocardial laser revascularization [46, 57]. A recent study has demonstrated that TYMP drives the opening of the BBB and increases BBB permeability by downregulating claudin 5 and occludin expression [88]. The decreased expression of claudin and occludin is necessary for the dissociation of tight junctions during EMT [112]. Overexpressed TYMP also significantly up-regulates Rho-associated coiled–coil domain kinase (ROCK1) expression and dramatically enhances cell motility [113]. This evidence suggests that TYMP may play an important role in EMT, and consequently may contribute to cancer invasion into tissues.
Targeting TYMP may be considered a potential immunotherapy for cancer. Guillem and colleagues recently provided the first epidemiological evidence of the TYMP gene SNP (rs112723255) effectively acting as a minor histocompatibility antigen [114]. This finding is in agreement with a previous study and suggests that TYMP is a potential target for cancer immunotherapy [115]. This may be due to that peptide-associated epitope, which can be influenced by polymorphisms [115], is involved in an antigenic recognition. Patients carrying the T allele of TYMP, rs11479, are likely to show a high level of TYMP expression in tumor tissue [116], suggesting this may be a specific target for TYMP targeted antitumor immunotherapy.
4.2) TYMP may have dual roles in atherosclerosis
The formation of atherosclerotic plaque is characterized by pathologic intimal hyperplasia and lipid accumulation in the vessel wall; macrophages play a crucial role in the progression of this process [117]. TYMP has been found in the atherosclerotic plaque [118, 119] and is considered to induce plaque angiogenesis that contributes to plaque growth and rupture. Macrophages contain a large amount of pro-angiogenic factors. Therefore, plaque angiogenesis may just be a physiological response to the pro-angiogenic factors released by these cells, rather than playing an atherogenic role [120]. Indeed, as mentioned above, we and other groups have found that TYMP inhibits VSMC proliferation [13–15, 121]. The adventitial delivery of the human TYMP gene significantly decreased neointimal hyperplasia in both balloon-injured rat carotid arteries and rabbit venous grafts that were implanted into the carotid artery [14, 121]. Both TYMP gene overexpression and the addition of exogenous TYMP inhibited VSMC proliferation [15], suggesting that high TYMP expression in the atherosclerotic plaque may actually attenuate the response of the VSMC vessel wall to pathophysiological stimulation. However, how plaque TYMP affects patient outcomes is still unclear since further human studies were not conducted after these early reports [118, 119].
IL-1β, a key driver of inflammatory lesion pathogenesis and reactive astrogliosis, exacerbates atherosclerosis development. Alexander and colleagues have reported that IL-1 has a protective effect on late atherosclerosis development through an IL-1β–dependent decrease of SMC proliferation [122]. IL-1 is known to upregulate TYMP expression through the NFκB pathway. Therefore, their finding may be due to IL-1β–induced TYMP expression as increased TYMP will contribute to switching the SMC phenotype from a proliferative to a mature, contractile one [15]. Inhibition of IL-1 may reduce TYMP expression and thus attenuate the inhibitory effect of TYMP on VSMC. TYMP has been examined in the plaque; however, its detailed distribution and origin is unclear.
In addition to TNFα and IL-1 mentioned above, other inflammatory cytokines, including IL-6, IL-8, IL-17, interferon- γ (IFN- γ), and granulocyte-colony stimulating factor (rhG-CSF) among others, also induce TYMP expression [123–125]. Exposure of tumor cells to stress factors, such as hypoxia, hypoglycemia, chemotherapy and radiotherapy, also stimulate the expression of TYMP [126–130]. These findings suggest TYMP may be a mediator in the response to inflammatory and stress stimulation. In addition to its function in hemostasis and thrombosis, platelets are essential for inflammation. Activated platelets release their components, which comprise a multitude of inflammatory and vasoactive substances, and attract atherogenic leukocytes from the circulation [131]. Therefore, platelet-associated inflammation has been recognized as a culprit that enhances atherosclerosis [132]. From this viewpoint, TYMP-enhanced platelet activation may thus promote the development of atherosclerosis.
Overall, the role of TYMP in atherosclerosis has been poorly studied. Elucidating its contribution to inflammation, as well as its integrated role in the functions of platelets, endothelial cells, VSMC and macrophages, will provide novel insights in understanding the vascular microenvironment. This may lead to the development of strategies to enhance healthy vessel remodeling, and thus contribute to the development of novel therapeutics for atherosclerotic diseases.
4.3) The TYMP gene may be responsible for mitochondrial neurogastrointestinal encephalomyopathy
MNGIE is an extremely rare inherited autosomal recessive disease showing multiple deletions in mitochondrial DNA; it is the only disease that is reportedly related to a loss of function mutation of the TYMP gene [12, 133]. For more detailed information, please find a recent update by Dr. Hirano [134]. A diagnosis of MNGIE relies on the following criteria: (1) biallelic pathogenic variants in TYMP; (2) significantly reduced TYMP activity; and (3) dramatically increased plasma concentrations of thymidine and deoxyuridine [134]. Hitherto, allogeneic hematopoietic stem cell transplantation is the only therapy that has been demonstrated to achieve long-term clinical efficacy [135]. However, high morbidity and mortality rates are associated with this procedure and this thus necessitates further research to find safer alternatives. TYMP protein is highly expressed in the liver. Therefore, orthotopic liver transplantation has been suggested to be a therapeutic alternative for patients with MNGIE [136]; however, the long-term outcome of this potential strategy remains to be investigated. By using a liver-targeted AAV vector, Torres-Torronteras and colleagues demonstrated that liver-directed TYMP expression is feasible as a potentially safe genetic therapeutic agent for MNGIE [137]. However, hitherto, such gene therapy has not been undertaken in humans.
Of note, some studies have suggested that a functional mutation of the TYMP gene is not necessary to induce MNGIE. A study by Kumagai and colleagues indicated that a mutation of the TYMP gene was not the primary cause of MNGIE because TYMP mutations were also present in several unrelated healthy individuals [138]. Furthermore, in patients who were diagnosed as having 22q13.3 deletion syndrome, a TYMP deletion was found in some cases [139, 140]; however, such patients did not show symptoms similar to those diagnosed with MNGIE. Such data suggest that the development of MNGIE may be more complex than the presence of a single TYMP gene mutation.
5. Regulation of TYMP expression
5.1) Regulation of TYMP expression at the transcriptional level
Regulatory mechanisms of TYMP expression have not been well studied, either in hematopoietic cells or in cancers. By analyzing the promoter region of TYMP for up to −3,000 bp using ConSite, a Web-based tool for finding cis-regulatory elements in genomic sequences [141, 142], we found two n-myc and six Snail binding sites using a 100% cutoff transcription factor (TF) score (relative matrix score thresholds [default 80%]). Max, USF, ARNT, SAP-1, E74A, COUP-TF and SOX17 are also potential TFs if the TF cutoff score is lowered to 95%. Such TFs participate in the regulation of angiogenesis, cellular survival, apoptosis, adhesion and migration as well as EMT, processes in which TYMP also plays a role. However, the circumstances under which such TFs regulate TYMP expression have not been elucidated. A transcriptional activator (Gli3) regulates the expression of several proangiogenic factors, including TYMP, both in vitro and in vivo [143]. A minimal GLI-consensus sequence 5′-GGGTGGTC-3, a Gli3 binding site, is present in the TYMP promoter region (not shown). SP1, a zinc finger TF that binds to GC-rich motifs of many promoters, mediates TNFα-induced TYMP gene expression in rheumatoid fibroblast-like synoviocytes [144] and in human colon carcinoma cells [145]. Two SP1 binding sites were found using ConSite when the TF cutoff score was lowered to 90%. These data suggest that in spite of a lack of direct evidence, the TFs identified above by ConSite with high TF cutoff scores may actually regulate TYMP expression. Clarifying how such TFs regulate TYMP expression may lead to the development of novel therapeutic strategies to treat cancer and other diseases in which high TYMP expression has been shown to contribute to the development of disease.
Multiple lines of evidence from the published literature also suggest that the expression of TYMP may be regulated by female sex hormones. In normal endometrium, TYMP expression has a cyclical pattern and mirrors changing endometrial patterns of the menstrual cycle [146, 147], suggesting that estrogen or other ovarian hormones may regulate TYMP expression. Activated estrogen receptors (ER) interact with the transcription factor, SP-1 [148]. As mentioned above, SP-1 is known to upregulate TYMP expression [149]. Tamoxifen, which acts as both an ER agonist and antagonist in a cell-dependent manner, upregulates [150] or downregulates [151] TYMP expression. Several studies have also indicated that progestogens upregulate TYMP expression [152–154]. However, the function of female sex hormone-mediated TYMP expression is unknown. It may play a role in promoting neovascularization after regression of microvessels in the endometrium. It may also mediate contraceptive pill-associated thrombophilia, since TYMP plays an important role in thrombosis [18].
5.2) Regulation of TYMP expression at the epigenetic level
The expression of TYMP may also be epigenetically regulated. Histone deacetylases (HDACs), by removing the acetyl groups, increase the positive charge of histone tails to encourage high-affinity binding between histones and the DNA backbone. The increased histone/DNA binding prevents transcription, and thus controls cellular functions including cell proliferation, survival and differentiation. Terranova–Barberio and colleagues found that HDAC inhibitors (HDACi) up-regulated TYMP expression at both the transcriptional and protein levels in breast cancer cells, but not in the non-tumorigenic breast cell line, MCF-10A, in a dose- and time-dependent manner [155]. By knocking down HDACs using siRNA or isoform-specific HDACi, they concluded that HDAC3 was the main isoform involved in the modulation of TYMP by inhibition [155]. Another mechanism of epigenetic regulation of TYMP is mediated by DNA methylation, in which a methyl group is added to the TYMP promoter segment at CpG sites by DNA methyltransferase [156]. By studying cancer cell lines with different TYMP expression levels, Guarcello et al. found TYMP expression was down-regulated in cancer cells by a mechanism that involved methylation of the TYMP promoter [156]. Increased methylation of the TYMP promoter was also found in mesothelioma compared to normal pericardium [157], and was responsible for the lack of any effect of capecitabine treatment in this disease, since TYMP is required by capecitabine to convert it to an active form.
5.3) Regulation of TYMP expression at the post-translational level
A paucity of information exists regarding how TYMP function is affected by post-translational modification. TYMP is not glycosylated [20]. Human TYMP has a consensus sequence (ser/Thr-X-Lys/Arg) for phosphorylation by protein kinase C (PKC) [158]. However, a PKC inducer does not induce TYMP phosphorylation in vivo [159]. Based on results analyzed by online Ubiquitination Prediction software (CKSAAP_UbSite Prediction and UbPred), human TYMP does not have any potential ubiquitination sites (data not shown). Heat shock protein (HSP) 90, as a chaperone protein, can bind to TYMP and prevent the ubiquitin-26S proteasome pathway mediating TYMP degradation [160]. However, inhibition of HSP90 using 17-allylamino-17-demethoxygeldanamycin (17-AAG) resulted in the decreased expression of TYMP not only at the protein level, but also at the mRNA level, suggesting a lack of post-translational regulation.
6. TYMP is a potential drug target
6.1) TYMP as a target of anti-cancer drugs
Nucleoside analogs have been used as antiviral and anticancer agents. However, the efficiency of some of these drugs can be diminished by TYMP-mediated degradation. Inhibition of TYMP activity thus may increase the therapeutic efficiency of these drugs. In addition, as mentioned above, TYMP has strong pro-angiogenic and anti-apoptotic effects, and is highly expressed in several cancers or cancer matrix cells. Therefore, TYMP has become a target of anti-cancer drugs since inhibition of TYMP can achieve synergic anti-cancer therapeutic activities through: 1) increasing the efficiency of anticancer drugs; 2) inhibiting angiogenesis; and 3) enhancing apoptosis. Several excellent review papers have summarized recent approaches in the search for novel TYMP inhibitors and outlined the potential therapeutic applications of such inhibitors [89, 161]. Among these compounds, only TPI, the most potent TYMP inhibitor, [162] has been approved by the US FDA for clinical use. TPI is a component of an oral anti-cancer drug, TAS-102 (brand name LONSURF® [trifluridine and tipiracil hydrochloride]), that is associated with a significant improvement in the overall survival of patients with refractory colorectal cancer [104]. Other inhibitors, such as KIN59, which also has a strong inhibitory effect on angiogenesis without interacting with the substrate-binding sites of TYMP [163], also look promising. However, additional studies are needed to clarify their therapeutic potential.
6.2) TYMP as a target of anti-thrombotic drugs
As mentioned above, our recent study demonstrated that targeting TYMP dramatically inhibits thrombosis without affecting hemostasis [18]. This provides a promising new direction for developing a novel anti-platelet and anti-thrombotic drug by targeting TYMP for various diseases associated with a high risk of thrombosis. Venous and arterial thromboembolism events (VTEs and ATEs) are a major cause of death in patients with cancer, accounting for one fifth of all VTEs occurring in the community [164]. Several hypotheses have been tested to identify the causal relationship between active cancer and the development of VTE, with the coagulation system most likely activated during disease [164]. Platelets are thought to contribute to the hypercoagulable state in patients with cancer [165]. In addition to their central role in hemostasis and thrombosis, platelets also contribute to the development of a variety of diseases, including malignancies. Therefore, targeting platelets may achieve: 1) the prevention of VTEs or ATEs, and 2) negatively influence the development of cancer, thus significantly improving the quality of life of patients with cancer. Taken together, targeting TYMP may be a novel anti-thrombotic therapy, especially for cancer-associated thrombophilia.
6.3) Safety of TYMP inhibitors
Information regarding the potential side effects of TYMP inhibition is lacking. We found that the inhibitory effect of KIN59 on collagen-induced platelet aggregation was reversible, with the ability of platelets to respond to collagen returning to normal approximately 30 minutes after KIN59 treatment [18]. This is in line with a previous finding by Liekens et al. [166] on the reversible inhibition of TYMP by KIN59. Signs of toxicity or inflammation around the site of drug exposure were not found in mice treated with KIN59 [166]. However, whether TPI also reversibly inhibited TYMP function has not been reported. TPI partially suppressed the growth of TYMP-expressing tumors but did not significantly suppress tumor angiogenesis, also suggesting a lack of toxicity in vivo by TPI [167]. Human uridine phosphorylase 2 shares substrates, including thymidine, with TYMP [168, 169], and may thus compensate for TYMP inhibition. Our recent study also demonstrated that TPI inhibits platelet activation in vitro, and thrombosis in vivo without disturbing hemostasis (unpublished data, not shown). Taken together, the above suggest that targeting TYMP may be a safe, effective therapy for inhibiting thrombosis without any obvious side effects. However, long-term rigorous studies are necessary to confirm this conclusion.
7. Conclusion
In the present review, we aimed to summarize the main molecular mechanisms of TYMP as they relate to various diseases. We focused on reported roles and undertook predictive analysis. Based on this body of knowledge on TYMP, we suggest targeting TYMP as a potential new therapy for preventing various diseases, especially those with a high risk of thrombosis.
Acknowledgments
This paper has been edited by native English-speaking experts from BioMed Proofreading® LLC.
The preparation of this review was not supported by any external funding. Dr. Li is salaried in part by NIH grants: NIH R01HL129179 (PI: Anirban Sen Gupta) and R01HL130090-01A1 (PI: Thomas M McIntyre).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no potential conflicts of interest to disclose.
References
- 1.Deutsch W, Laser RZ. Physiol Chem. 1930;186 [Google Scholar]
- 2.Manson LA, adn Lampen JO. Federation Proc. 1949;8:224. [Google Scholar]
- 3.Friedkin M, Roberts D. The enzymatic synthesis of nucleosides. I. Thymidine phosphorylase in mammalian tissue. The Journal of biological chemistry. 1954;207:245–56. [PubMed] [Google Scholar]
- 4.Kubilus J, Lee LD, Baden HP. Purification of thymidine phosphorylase from human amniochorion. Biochimica et Biophysica Acta (BBA) - Enzymology. 1978;527:221–8. doi: 10.1016/0005-2744(78)90271-1. [DOI] [PubMed] [Google Scholar]
- 5.Miyazono K, Okabe T, Urabe A, Takaku F, Heldin CH. Purification and properties of an endothelial cell growth factor from human platelets. The Journal of biological chemistry. 1987;262:4098–103. [PubMed] [Google Scholar]
- 6.Asai K, Hirano T, Kaneko S, Moriyama A, Nakanishi K, Isobe I, et al. A novel glial growth inhibitory factor, gliostatin, derived from neurofibroma. J Neurochem. 1992;59:307–17. doi: 10.1111/j.1471-4159.1992.tb08905.x. [DOI] [PubMed] [Google Scholar]
- 7.Furukawa T, Yoshimura A, Sumizawa T, Haraguchi M, Akiyama S, Fukui K, et al. Angiogenic factor. Nature. 1992;356:668. doi: 10.1038/356668a0. [DOI] [PubMed] [Google Scholar]
- 8.Asai K, Nakanishi K, Isobe I, Eksioglu YZ, Hirano A, Hama K, et al. Neurotrophic action of gliostatin on cortical neurons. Identity of gliostatin and platelet-derived endothelial cell growth factor. J Biol Chem. 1992;267:20311–6. [PubMed] [Google Scholar]
- 9.Usuki K, Saras J, Waltenberger J, Miyazono K, Pierce G, Thomason A, et al. Platelet-derived endothelial cell growth factor has thymidine phosphorylase activity. Biochem Biophys Res Commun. 1992;184:1311–6. doi: 10.1016/s0006-291x(05)80025-7. [DOI] [PubMed] [Google Scholar]
- 10.Fox SB, Moghaddam A, Westwood M, Turley H, Bicknell R, Gatter KC, et al. Platelet-derived endothelial cell growth factor/thymidine phosphorylase expression in normal tissues: an immunohistochemical study. J Pathol. 1995;176:183–90. doi: 10.1002/path.1711760212. [DOI] [PubMed] [Google Scholar]
- 11.Kitazono M, Takebayashi Y, Ishitsuka K, Takao S, Tani A, Furukawa T, et al. Prevention of hypoxia-induced apoptosis by the angiogenic factor thymidine phosphorylase. Biochem Biophys Res Commun. 1998;253:797–803. doi: 10.1006/bbrc.1998.9852. [DOI] [PubMed] [Google Scholar]
- 12.Nishino I, Spinazzola A, Hirano M. Thymidine phosphorylase gene mutations in MNGIE, a human mitochondrial disorder. Science. 1999;283:689–92. doi: 10.1126/science.283.5402.689. [DOI] [PubMed] [Google Scholar]
- 13.Somjen D, Jaffe A, Knoll E, Kohen F, Amir-Zaltsman Y, Stern N. Platelet-derived endothelial cell growth factor inhibits DNA synthesis in vascular smooth muscle cells. American journal of hypertension. 1999;12:882–9. doi: 10.1016/s0895-7061(99)00063-1. [DOI] [PubMed] [Google Scholar]
- 14.Li W, Tanaka K, Morioka K, Uesaka T, Yamada N, Takamori A, et al. Thymidine phosphorylase gene transfer inhibits vascular smooth muscle cell proliferation by upregulating heme oxygenase-1 and p27KIP1. Arterioscler Thromb Vasc Biol. 2005;25:1370–5. doi: 10.1161/01.ATV.0000168914.85107.64. [DOI] [PubMed] [Google Scholar]
- 15.Yue H, Tanaka K, Furukawa T, Karnik SS, Li W. Thymidine phosphorylase inhibits vascular smooth muscle cell proliferation via upregulation of STAT3. Biochim Biophys Acta. 2012;1823:1316–23. doi: 10.1016/j.bbamcr.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desgranges C, Razaka G, Rabaud M, Bricaud H. Catabolism of thymidine in human blood platelets: purification and properties of thymidine phosphorylase. Biochimica et biophysica acta. 1981;654:211–8. doi: 10.1016/0005-2787(81)90174-x. [DOI] [PubMed] [Google Scholar]
- 17.Hotchkiss KA, Ashton AW, Schwartz EL. Thymidine phosphorylase and 2-deoxyribose stimulate human endothelial cell migration by specific activation of the integrins alpha 5 beta 1 and alpha V beta 3. The Journal of biological chemistry. 2003;278:19272–9. doi: 10.1074/jbc.M212670200. [DOI] [PubMed] [Google Scholar]
- 18.Li W, Gigante A, Perez-Perez MJ, Yue H, Hirano M, McIntyre TM, et al. Thymidine phosphorylase participates in platelet signaling and promotes thrombosis. Circ Res. 2014;115:997–1006. doi: 10.1161/CIRCRESAHA.115.304591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stenman G, Sahlin P, Dumanski JP, Hagiwara K, Ishikawa F, Miyazono K, et al. Regional localization of the human platelet-derived endothelial cell growth factor (ECGF1) gene to chromosome 22q13. Cytogenet Cell Genet. 1992;59:22–3. doi: 10.1159/000133191. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa F, Miyazono K, Hellman U, Drexler H, Wernstedt C, Hagiwara K, et al. Identification of angiogenic activity and the cloning and expression of platelet-derived endothelial cell growth factor. Nature. 1989;338:557–62. doi: 10.1038/338557a0. [DOI] [PubMed] [Google Scholar]
- 21.Walter MR, Cook WJ, Cole LB, Short SA, Koszalka GW, Krenitsky TA, et al. Three-dimensional structure of thymidine phosphorylase from Escherichia coli at 2.8 A resolution. J Biol Chem. 1990;265:14016–22. doi: 10.2210/pdb1tpt/pdb. [DOI] [PubMed] [Google Scholar]
- 22.Norman RA, Barry ST, Bate M, Breed J, Colls JG, Ernill RJ, et al. Crystal structure of human thymidine phosphorylase in complex with a small molecule inhibitor. Structure. 2004;12:75–84. doi: 10.1016/j.str.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 23.El Omari K, Bronckaers A, Liekens S, Perez-Perez MJ, Balzarini J, Stammers DK. Structural basis for non-competitive product inhibition in human thymidine phosphorylase: implications for drug design. Biochem J. 2006;399:199–204. doi: 10.1042/BJ20060513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bronckaers A, Aguado L, Negri A, Camarasa MJ, Balzarini J, Perez-Perez MJ, et al. Identification of aspartic acid-203 in human thymidine phosphorylase as an important residue for both catalysis and non-competitive inhibition by the small molecule “crystallization chaperone” 5′-O-tritylinosine (KIN59) Biochemical pharmacology. 2009;78:231–40. doi: 10.1016/j.bcp.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Krenitsky TA. Pentosyl transfer mechanisms of the mammalian nucleoside phosphorylases. J Biol Chem. 1968;243:2871–5. [PubMed] [Google Scholar]
- 26.Mitsiki E, Papageorgiou AC, Iyer S, Thiyagarajan N, Prior SH, Sleep D, et al. Structures of native human thymidine phosphorylase and in complex with 5-iodouracil. Biochem Biophys Res Commun. 2009;386:666–70. doi: 10.1016/j.bbrc.2009.06.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyadera K, Sumizawa T, Haraguchi M, Yoshida H, Konstanty W, Yamada Y, et al. Role of thymidine phosphorylase activity in the angiogenic effect of platelet derived endothelial cell growth factor/thymidine phosphorylase. Cancer research. 1995;55:1687–90. [PubMed] [Google Scholar]
- 28.Liekens S, Bronckaers A, Perez-Perez MJ, Balzarini J. Targeting platelet-derived endothelial cell growth factor/thymidine phosphorylase for cancer therapy. Biochem Pharmacol. 2007;74:1555–67. doi: 10.1016/j.bcp.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz M. Thymidine phosphorylase from Escherichia coli. Properties and kinetics. European journal of biochemistry / FEBS. 1971;21:191–8. doi: 10.1111/j.1432-1033.1971.tb01455.x. [DOI] [PubMed] [Google Scholar]
- 30.Pugmire MJ, Cook WJ, Jasanoff A, Walter MR, Ealick SE. Structural and theoretical studies suggest domain movement produces an active conformation of thymidine phosphorylase. Journal of molecular biology. 1998;281:285–99. doi: 10.1006/jmbi.1998.1941. [DOI] [PubMed] [Google Scholar]
- 31.Nakayama C, Wataya Y, Meyer RB, Jr, Santi DV, Saneyoshi M, Ueda T. Thymidine phosphorylase. Substrate specificity for 5-substituted 2′-deoxyuridines. Journal of medicinal chemistry. 1980;23:962–4. doi: 10.1021/jm00182a029. [DOI] [PubMed] [Google Scholar]
- 32.Desgranges C, Razaka G, Rabaud M, Bricaud H, Balzarini J, De Clercq E. Phosphorolysis of (E)-5-(2-bromovinyl)-2′-deoxyuridine (BVDU) and other 5-substituted-2′-deoxyuridines by purified human thymidine phosphorylase and intact blood platelets. Biochem Pharmacol. 1983;32:3583–90. doi: 10.1016/0006-2952(83)90307-6. [DOI] [PubMed] [Google Scholar]
- 33.de Bruin M, van Capel T, Smid K, van der Born K, Fukushima M, Hoekman K, et al. Role of platelet derived endothelial cell growth factor/thymidine phosphorylase in fluoropyrimidine sensitivity and potential role of deoxyribose-1-phosphate. Nucleosides Nucleotides Nucleic Acids. 2004;23:1485–90. doi: 10.1081/NCN-200027702. [DOI] [PubMed] [Google Scholar]
- 34.Bronckaers A, Balzarini J, Liekens S. The cytostatic activity of pyrimidine nucleosides is strongly modulated by Mycoplasma hyorhinis infection: Implications for cancer therapy. Biochem Pharmacol. 2008;76:188–97. doi: 10.1016/j.bcp.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz EL, Baptiste N, Wadler S, Makower D. Thymidine phosphorylase mediates the sensitivity of human colon carcinoma cells to 5-fluorouracil. J Biol Chem. 1995;270:19073–7. doi: 10.1074/jbc.270.32.19073. [DOI] [PubMed] [Google Scholar]
- 36.Di Gennaro E, Piro G, Chianese MI, Franco R, Di Cintio A, Moccia T, et al. Vorinostat synergises with capecitabine through upregulation of thymidine phosphorylase. Br J Cancer. 2010;103:1680–91. doi: 10.1038/sj.bjc.6605969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hata K, Fujiwaki R, Nakayama K, Maede Y, Fukumoto M, Miyazaki K. Expression of thymidine phosphorylase and vascular endothelial growth factor in epithelial ovarian cancer: correlation with angiogenesis and progression of the tumor. Anticancer Res. 2000;20:3941–9. [PubMed] [Google Scholar]
- 38.Goto T, Shinmura K, Yokomizo K, Sakuraba K, Kitamura Y, Shirahata A, et al. Expression levels of thymidylate synthase, dihydropyrimidine dehydrogenase, and thymidine phosphorylase in patients with colorectal cancer. Anticancer Res. 2012;32:1757–62. [PubMed] [Google Scholar]
- 39.Giatromanolaki A, Koukourakis MI, Kakolyris S, Kaklamanis L, Barbatis K, O’Byrne KJ, et al. Focal expression of thymidine phosphorylase associates with CD31 positive lymphocytic aggregation and local neo-angiogenesis in non-small cell lung cancer. Anticancer Res. 1998;18:71–6. [PubMed] [Google Scholar]
- 40.O’Brien TS, Fox SB, Dickinson AJ, Turley H, Westwood M, Moghaddam A, et al. Expression of the angiogenic factor thymidine phosphorylase/platelet-derived endothelial cell growth factor in primary bladder cancers. Cancer Res. 1996;56:4799–804. [PubMed] [Google Scholar]
- 41.Leek RD, Talks KL, Pezzella F, Turley H, Campo L, Brown NS, et al. Relation of hypoxia-inducible factor-2 alpha (HIF-2 alpha) expression in tumor-infiltrative macrophages to tumor angiogenesis and the oxidative thymidine phosphorylase pathway in Human breast cancer. Cancer Res. 2002;62:1326–9. [PubMed] [Google Scholar]
- 42.Tabata S, Ikeda R, Yamamoto M, Shimaoka S, Mukaida N, Takeda Y, et al. Thymidine phosphorylase activates NFkappaB and stimulates the expression of angiogenic and metastatic factors in human cancer cells. Oncotarget. 2014;5:10473–85. doi: 10.18632/oncotarget.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li PG, Xu JW, Ikeda K, Kobayakawa A, Kayano Y, Mitani T, et al. Caffeic acid inhibits vascular smooth muscle cell proliferation induced by angiotensin II in stroke-prone spontaneously hypertensive rats. Hypertens Res. 2005;28:369–77. doi: 10.1291/hypres.28.369. [DOI] [PubMed] [Google Scholar]
- 44.Yamada N, Li W, Ihaya A, Kimura T, Morioka K, Uesaka T, et al. Platelet-derived endothelial cell growth factor gene therapy for limb ischemia. J Vasc Surg. 2006;44:1322–8. doi: 10.1016/j.jvs.2006.07.051. [DOI] [PubMed] [Google Scholar]
- 45.Li W, Tanaka K, Morioka K, Takamori A, Handa M, Yamada N, et al. Long-term effect of gene therapy for chronic ischemic myocardium using platelet-derived endothelial cell growth factor in dogs. J Gene Med. 2008;10:412–20. doi: 10.1002/jgm.1156. [DOI] [PubMed] [Google Scholar]
- 46.Li W, Chiba Y, Kimura T, Morioka K, Uesaka T, Ihaya A, et al. Transmyocardial laser revascularization induced angiogenesis correlated with the expression of matrix metalloproteinases and platelet-derived endothelial cell growth factor. Eur J Cardiothorac Surg. 2001;19:156–63. doi: 10.1016/s1010-7940(00)00649-7. [DOI] [PubMed] [Google Scholar]
- 47.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nature medicine. 2011;17:1359–70. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 48.Bijnsdorp IV, Capriotti F, Kruyt FA, Losekoot N, Fukushima M, Griffioen AW, et al. Thymidine phosphorylase in cancer cells stimulates human endothelial cell migration and invasion by the secretion of angiogenic factors. Br J Cancer. 2011;104:1185–92. doi: 10.1038/bjc.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hotchkiss KA, Ashton AW, Klein RS, Lenzi ML, Zhu GH, Schwartz EL. Mechanisms by which tumor cells and monocytes expressing the angiogenic factor thymidine phosphorylase mediate human endothelial cell migration. Cancer Res. 2003;63:527–33. [PubMed] [Google Scholar]
- 50.Romer LH, Birukov KG, Garcia JG. Focal adhesions: paradigm for a signaling nexus. Circ Res. 2006;98:606–16. doi: 10.1161/01.RES.0000207408.31270.db. [DOI] [PubMed] [Google Scholar]
- 51.Hotchkiss KA, Ashton AW, Schwartz EL. Thymidine phosphorylase and 2-deoxyribose stimulate human endothelial cell migration by specific activation of the integrins alpha 5 beta 1 and alpha V beta 3. The Journal of biological chemistry. 2003;278:19272–9. doi: 10.1074/jbc.M212670200. [DOI] [PubMed] [Google Scholar]
- 52.Liu H, Liu Z, Du J, He J, Lin P, Amini B, et al. Thymidine phosphorylase exerts complex effects on bone resorption and formation in myeloma. Sci Transl Med. 2016;8:353ra113. doi: 10.1126/scitranslmed.aad8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brown NS, Bicknell R. Thymidine phosphorylase, 2-deoxy-D-ribose and angiogenesis. Biochem J. 1998;334(Pt 1):1–8. doi: 10.1042/bj3340001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bijnsdorp IV, Peters GJ. Deoxyribose protects against rapamycin-induced cytotoxicity in colorectal cancer cells in vitro. Nucleosides Nucleotides Nucleic Acids. 2011;30:1197–202. doi: 10.1080/15257770.2011.602657. [DOI] [PubMed] [Google Scholar]
- 55.Peters GJ, Bijnsdorp IV, Fukushima M. Thymidine phoshorylase as a target for antiangiogenesis treatment. Nucleic acids symposium series. 2008:629. doi: 10.1093/nass/nrn318. [DOI] [PubMed] [Google Scholar]
- 56.Tabata S, Ikeda R, Yamamoto M, Furukawa T, Kuramoto T, Takeda Y, et al. Thymidine phosphorylase enhances reactive oxygen species generation and interleukin-8 expression in human cancer cells. Oncol Rep. 2012;28:895–902. doi: 10.3892/or.2012.1887. [DOI] [PubMed] [Google Scholar]
- 57.Li W, Tanaka K, Chiba Y, Kimura T, Morioka K, Uesaka T, et al. Role of MMPs and plasminogen activators in angiogenesis after transmyocardial laser revascularization in dogs. Am J Physiol Heart Circ Physiol. 2003;284:H23–30. doi: 10.1152/ajpheart.00240.2002. [DOI] [PubMed] [Google Scholar]
- 58.Fatemi M, Pao MM, Jeong S, Gal-Yam EN, Egger G, Weisenberger DJ, et al. Footprinting of mammalian promoters: use of a CpG DNA methyltransferase revealing nucleosome positions at a single molecule level. Nucleic Acids Res. 2005;33:e176. doi: 10.1093/nar/gni180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chik F, Szyf M. Effects of specific DNMT gene depletion on cancer cell transformation and breast cancer cell invasion; toward selective DNMT inhibitors. Carcinogenesis. 2011;32:224–32. doi: 10.1093/carcin/bgq221. [DOI] [PubMed] [Google Scholar]
- 60.Jujo K, Ii M, Losordo DW. Endothelial progenitor cells in neovascularization of infarcted myocardium. J Mol Cell Cardiol. 2008;45:530–44. doi: 10.1016/j.yjmcc.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jimenez-Quevedo P, Gonzalez-Ferrer JJ, Sabate M, Garcia-Moll X, Delgado-Bolton R, Llorente L, et al. Selected CD133(+) progenitor cells to promote angiogenesis in patients with refractory angina: final results of the PROGENITOR randomized trial. Circ Res. 2014;115:950–60. doi: 10.1161/CIRCRESAHA.115.303463. [DOI] [PubMed] [Google Scholar]
- 62.Pula G, Mayr U, Evans C, Prokopi M, Vara DS, Yin X, et al. Proteomics identifies thymidine phosphorylase as a key regulator of the angiogenic potential of colony-forming units and endothelial progenitor cell cultures. Circulation research. 2009;104:32–40. doi: 10.1161/CIRCRESAHA.108.182261. [DOI] [PubMed] [Google Scholar]
- 63.Matsuura T, Kuratate I, Teramachi K, Osaki M, Fukuda Y, Ito H. Thymidine phosphorylase expression is associated with both increase of intratumoral microvessels and decrease of apoptosis in human colorectal carcinomas. Cancer Res. 1999;59:5037–40. [PubMed] [Google Scholar]
- 64.Okamoto E, Osaki M, Kase S, Adachi H, Kaibara N, Ito H. Thymidine phosphorylase expression causes both the increase of intratumoral microvessels and decrease of apoptosis in human esophageal carcinomas. Pathology international. 2001;51:158–64. doi: 10.1046/j.1440-1827.2001.01184.x. [DOI] [PubMed] [Google Scholar]
- 65.Ikeguchi M, Sakatani T, Ueta T, Fukuda K, Yamaguchi K, Tsujitani S, et al. The expression of thymidine phosphorylase suppresses spontaneous apoptosis of cancer cells in esophageal squamous cell carcinoma. Pathobiology : journal of immunopathology, molecular and cellular biology. 2001;69:36–43. doi: 10.1159/000048756. [DOI] [PubMed] [Google Scholar]
- 66.Hata K, Fujiwaki R, Maede Y, Nakayama K, Fukumoto M, Miyazaki K. Expression of thymidine phosphorylase in epithelial ovarian cancer: correlation with angiogenesis, apoptosis, and ultrasound-derived peak systolic velocity. Gynecologic oncology. 2000;77:26–34. doi: 10.1006/gyno.1999.5651. [DOI] [PubMed] [Google Scholar]
- 67.Osaki M, Sakatani T, Okamoto E, Goto E, Adachi H, Ito H. Thymidine phosphorylase expression results in a decrease in apoptosis and increase in intratumoral microvessel density in human gastric carcinomas. Virchows Archiv : an international journal of pathology. 2000;437:31–6. doi: 10.1007/s004280000205. [DOI] [PubMed] [Google Scholar]
- 68.Li W, Tanaka K, Ihaya A, Fujibayashi Y, Takamatsu S, Morioka K, et al. Gene therapy for chronic myocardial ischemia using platelet-derived endothelial cell growth factor in dogs. Am J Physiol Heart Circ Physiol. 2005;288:H408–15. doi: 10.1152/ajpheart.00176.2004. [DOI] [PubMed] [Google Scholar]
- 69.Ikeda R, Che XF, Ushiyama M, Yamaguchi T, Okumura H, Nakajima Y, et al. 2-Deoxy-D-ribose inhibits hypoxia-induced apoptosis by suppressing the phosphorylation of p38 MAPK. Biochem Biophys Res Commun. 2006;342:280–5. doi: 10.1016/j.bbrc.2006.01.142. [DOI] [PubMed] [Google Scholar]
- 70.Mori S, Takao S, Ikeda R, Noma H, Mataki Y, Wang X, et al. Role of thymidine phosphorylase in Fas-induced apoptosis. Human cell. 2001;14:323–30. [PubMed] [Google Scholar]
- 71.Jeung H-C, Che X-F, Haraguchi M, Zhao H-Y, Furukawa T, Gotanda T, et al. Protection against DNA damage-induced apoptosis by the angiogenic factor thymidine phosphorylase. FEBS Lett. 2006;580:1294–302. doi: 10.1016/j.febslet.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 72.Mori S, Takao S, Ikeda R, Noma H, Mataki Y, Wang X, et al. Thymidine phosphorylase suppresses Fas-induced apoptotic signal transduction independent of its enzymatic activity. Biochem Biophys Res Commun. 2002;295:300–5. doi: 10.1016/s0006-291x(02)00662-9. [DOI] [PubMed] [Google Scholar]
- 73.Ikeda R, Furukawa T, Mitsuo R, Noguchi T, Kitazono M, Okumura H, et al. Thymidine phosphorylase inhibits apoptosis induced by cisplatin. Biochem Biophys Res Commun. 2003;301:358–63. doi: 10.1016/s0006-291x(02)03034-6. [DOI] [PubMed] [Google Scholar]
- 74.Burkhart JM, Vaudel M, Gambaryan S, Radau S, Walter U, Martens L, et al. The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood. 2012;120:e73–82. doi: 10.1182/blood-2012-04-416594. [DOI] [PubMed] [Google Scholar]
- 75.Wijten P, van Holten T, Woo LL, Bleijerveld OB, Roest M, Heck AJ, et al. High precision platelet releasate definition by quantitative reversed protein profiling–brief report. Arterioscler Thromb Vasc Biol. 2013;33:1635–8. doi: 10.1161/ATVBAHA.113.301147. [DOI] [PubMed] [Google Scholar]
- 76.Guo L, Kuroda N, Toi M, Miyazaki E, Hayashi Y, Enzan H, et al. Increased expression of platelet-derived endothelial cell growth factor in human hepatocellular carcinomas correlated with high Edmondson grades and portal vein tumor thrombosis. Oncol Rep. 2001;8:871–6. doi: 10.3892/or.8.4.871. [DOI] [PubMed] [Google Scholar]
- 77.Levene M, Coleman DG, Kilpatrick HC, Fairbanks LD, Gangadharan B, Gasson C, et al. Preclinical toxicity evaluation of erythrocyte-encapsulated thymidine phosphorylase in BALB/c mice and Beagle dogs: an enzyme replacement therapy for mitochondrial neurogastrointestinal encephalomyopathy. Toxicol Sci. 2012 doi: 10.1093/toxsci/kfs278. [DOI] [PubMed] [Google Scholar]
- 78.Li W, McIntyre TM, Silverstein RL. Ferric chloride-induced murine carotid arterial injury: A model of redox pathology. Redox Biol. 2013;1:50–5. doi: 10.1016/j.redox.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li W, Nieman M, Sen Gupta A. Ferric Chloride-induced Murine Thrombosis Models. Journal of visualized experiments : JoVE. 2016 doi: 10.3791/54479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang L, Huang X, Chen Y, Jin X, Li Q, Yi TN. Prognostic value of TP/PD-ECGF and thrombocytosis in gastric carcinoma. Eur J Surg Oncol. 2012;38:568–73. doi: 10.1016/j.ejso.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 81.Zhu GH, Schwartz EL. Expression of the angiogenic factor thymidine phosphorylase in THP-1 monocytes: induction by autocrine tumor necrosis factor-alpha and inhibition by aspirin. Molecular pharmacology. 2003;64:1251–8. doi: 10.1124/mol.64.5.1251. [DOI] [PubMed] [Google Scholar]
- 82.Vara DS, Campanella M, Canobbio I, Dunn WB, Pizzorno G, Hirano M, et al. Autocrine amplification of integrin alphaIIbbeta3 activation and platelet adhesive responses by deoxyribose-1-phosphate. Thromb Haemost. 2013;109:1108–19. doi: 10.1160/TH12-10-0751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fox SB, Moghaddam A, Westwood M, Turley H, Bicknell R, Gatter KC, et al. Platelet-derived endothelial cell growth factor/thymidine phosphorylase expression in normal tissues: an immunohistochemical study. The Journal of pathology. 1995;176:183–90. doi: 10.1002/path.1711760212. [DOI] [PubMed] [Google Scholar]
- 84.Handa M, Li W, Morioka K, Takamori A, Yamada N, Ihaya A. Adventitial delivery of platelet-derived endothelial cell growth factor gene prevented intimal hyperplasia of vein graft. J Vasc Surg. 2008;48:1566–74. doi: 10.1016/j.jvs.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 85.Kirchmer MN, Franco A, Albasanz-Puig A, Murray J, Yagi M, Gao L, et al. Modulation of vascular smooth muscle cell phenotype by STAT-1 and STAT-3. Atherosclerosis. 2014;234:169–75. doi: 10.1016/j.atherosclerosis.2014.02.029. [DOI] [PubMed] [Google Scholar]
- 86.Jessen KR, Mirsky R. Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature. 1980;286:736–7. doi: 10.1038/286736a0. [DOI] [PubMed] [Google Scholar]
- 87.Hayashi T, Wang XQ, Zhang HZ, Deguchi K, Nagotani S, Sehara Y, et al. Induction of platelet derived-endothelial cell growth factor in the brain after ischemia. Neurol Res. 2007;29:463–8. doi: 10.1179/016164107X164139. [DOI] [PubMed] [Google Scholar]
- 88.Chapouly C, Tadesse Argaw A, Horng S, Castro K, Zhang J, Asp L, et al. Astrocytic TYMP and VEGFA drive blood-brain barrier opening in inflammatory central nervous system lesions. Brain. 2015;138:1548–67. doi: 10.1093/brain/awv077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bronckaers A, Gago F, Balzarini J, Liekens S. The dual role of thymidine phosphorylase in cancer development and chemotherapy. Medicinal research reviews. 2009;29:903–53. doi: 10.1002/med.20159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bijnsdorp IV, de Bruin M, Laan AC, Fukushima M, Peters GJ. The role of platelet-derived endothelial cell growth factor/thymidine phosphorylase in tumor behavior. Nucleosides Nucleotides Nucleic Acids. 2008;27:681–91. doi: 10.1080/15257770802143988. [DOI] [PubMed] [Google Scholar]
- 91.Elamin YY, Rafee S, Osman N, KJ OB, Gately K. Thymidine Phosphorylase in Cancer; Enemy or Friend? Cancer Microenviron. 2016;9:33–43. doi: 10.1007/s12307-015-0173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Giatromanolaki A, Fountzilas G, Koukourakis MI, Arapandoni P, Theologi V, Kakolyris S, et al. Neo-angiogenesis in locally advanced squamous cell head and neck cancer correlates with thymidine phosphorylase expression and p53 nuclear oncoprotein accumulation. Clinical & experimental metastasis. 1998;16:665–72. doi: 10.1023/a:1006554512338. [DOI] [PubMed] [Google Scholar]
- 93.Saito K, Khan K, Yu SZ, Ronson S, Rhee J, Li G, et al. The predictive and therapeutic value of thymidine phosphorylase and dihydropyrimidine dehydrogenase in capecitabine (Xeloda)-based chemotherapy for head and neck cancer. The Laryngoscope. 2009;119:82–8. doi: 10.1002/lary.20003. [DOI] [PubMed] [Google Scholar]
- 94.Ruckhaberle E, Karn T, Engels K, Turley H, Hanker L, Muller V, et al. Prognostic impact of thymidine phosphorylase expression in breast cancer–comparison of microarray and immunohistochemical data. European journal of cancer. 2010;46:549–57. doi: 10.1016/j.ejca.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 95.Fox SB, Westwood M, Moghaddam A, Comley M, Turley H, Whitehouse RM, et al. The angiogenic factor platelet-derived endothelial cell growth factor/thymidine phosphorylase is up-regulated in breast cancer epithelium and endothelium. Br J Cancer. 1996;73:275–80. doi: 10.1038/bjc.1996.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Giatromanolaki A, Koukourakis MI, Comley M, Kaklamanis L, Turley H, O’Byrne K, et al. Platelet-derived endothelial cell growth factor (thymidine phosphorylase) expression in lung cancer. J Pathol. 1997;181:196–9. doi: 10.1002/(SICI)1096-9896(199702)181:2<196::AID-PATH763>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 97.Ko JC, Chiu HC, Syu JJ, Jian YJ, Chen CY, Jian YT, et al. Tamoxifen enhances erlotinib-induced cytotoxicity through down-regulating AKT-mediated thymidine phosphorylase expression in human non-small-cell lung cancer cells. Biochem Pharmacol. 2014;88:119–27. doi: 10.1016/j.bcp.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 98.Ranieri G, Labriola A, Achille G, Florio G, Zito AF, Grammatica L, et al. Microvessel density, mast cell density and thymidine phosphorylase expression in oral squamous carcinoma. International journal of oncology. 2002;21:1317–23. [PubMed] [Google Scholar]
- 99.Lee S, Park YH, Kim KH, Cho EY, Ahn YC, Kim K, et al. Thymidine synthase, thymidine phosphorylase, and excision repair cross-complementation group 1 expression as predictive markers of capecitabine plus cisplatin chemotherapy as first-line treatment for patients with advanced oesophageal squamous cell carcinoma. Br J Cancer. 2010;103:845–51. doi: 10.1038/sj.bjc.6605831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang L, Liu S, Lei Y, Wang K, Xu M, Chen Y, et al. Systemic immune-inflammation index, thymidine phosphorylase and survival of localized gastric cancer patients after curative resection. Oncotarget. 2016 doi: 10.18632/oncotarget.9923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang X, Zheng Z, Shin YK, Kim KY, Rha SY, Noh SH, et al. Angiogenic factor thymidine phosphorylase associates with angiogenesis and lymphangiogenesis in the intestinal-type gastric cancer. Pathology. 2014;46:316–24. doi: 10.1097/PAT.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 102.Giatromanolaki A, Koukourakis MI, Stathopoulos GP, Kapsoritakis A, Paspatis G, Kakolyris S, et al. Angiogenic interactions of vascular endothelial growth factor, of thymidine phosphorylase, and of p53 protein expression in locally advanced gastric cancer. Oncology research. 2000;12:33–41. doi: 10.3727/000000001108747426. [DOI] [PubMed] [Google Scholar]
- 103.Iwagaki H, Hizuta A, Mori K, Yoshino T, Kawahara K, Tanaka N, et al. Endogenous gamma-interferon activates thymidine phosphorylase in colorectal cancer tissue. Research communications in molecular pathology and pharmacology. 1995;87:345–52. [PubMed] [Google Scholar]
- 104.Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909–19. doi: 10.1056/NEJMoa1414325. [DOI] [PubMed] [Google Scholar]
- 105.Aoki S, Yamada Y, Nakamura K, Taki T, Tobiume M, Honda N. Thymidine phosphorylase expression as a prognostic marker for predicting recurrence in primary superficial bladder cancer. Oncol Rep. 2006;16:279–84. [PubMed] [Google Scholar]
- 106.Kikuno N, Moriyama-Gonda N, Yoshino T, Yoneda T, Urakami S, Terashima M, et al. Blockade of paclitaxel-induced thymidine phosphorylase expression can accelerate apoptosis in human prostate cancer cells. Cancer Res. 2004;64:7526–32. doi: 10.1158/0008-5472.CAN-04-0996. [DOI] [PubMed] [Google Scholar]
- 107.Kiprijanovska S, Stavridis S, Stankov O, Komina S, Petrusevska G, Polenakovic M, et al. Mapping and Identification of the Urine Proteome of Prostate Cancer Patients by 2D PAGE/MS. Int J Proteomics. 2014;2014:594761. doi: 10.1155/2014/594761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stevenson DP, Collins WP, Farzaneh F, Hata K, Miyazaki K. Thymidine phosphorylase activity and prodrug effects in a three-dimensional model of angiogenesis: implications for the treatment of ovarian cancer. Am J Pathol. 1998;153:1573–8. doi: 10.1016/S0002-9440(10)65745-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tamura R, Yokoyama Y, Yoshida H, Imaizumi T, Mizunuma H. 4-Methylumbelliferone inhibits ovarian cancer growth by suppressing thymidine phosphorylase expression. J Ovarian Res. 2014;7:94. doi: 10.1186/s13048-014-0094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fujiwaki R, Hata K, Iida K, Maede Y, Koike M, Miyazaki K. Thymidine phosphorylase expression in progression of cervical cancer: correlation with microvessel count, proliferating cell nuclear antigen, and apoptosis. J Clin Pathol. 1999;52:598–603. doi: 10.1136/jcp.52.8.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sato S, Yajima A, Sasaki H, Mizutani K, Honjo H, Yamamoto K, et al. Prognostic value of thymidine phosphorylase immunostaining in patients with uterine cervical cancer treated concurrently with doxifluridine, radiotherapy and immunotherapy. Oncol Rep. 2001;8:239–44. doi: 10.3892/or.8.2.239. [DOI] [PubMed] [Google Scholar]
- 112.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yoshinaga K, Inoue H, Tanaka F, Mimori K, Utsunomiya T, Mori M. Platelet-derived endothelial cell growth factor mediates Rho-associated coiled-coil domain kinase messenger RNA expression and promotes cell motility. Ann Surg Oncol. 2003;10:582–7. doi: 10.1245/aso.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 114.Guillem V, Hernandez-Boluda JC, Gallardo D, Buno I, Bosch A, Martinez-Laperche C, et al. A polymorphism in the TYMP gene is associated with the outcome of HLA-identical sibling allogeneic stem cell transplantation. Am J Hematol. 2013;88:883–9. doi: 10.1002/ajh.23523. [DOI] [PubMed] [Google Scholar]
- 115.Slager EH, Honders MW, van der Meijden ED, van Luxemburg-Heijs SA, Kloosterboer FM, Kester MG, et al. Identification of the angiogenic endothelial-cell growth factor-1/thymidine phosphorylase as a potential target for immunotherapy of cancer. Blood. 2006;107:4954–60. doi: 10.1182/blood-2005-09-3883. [DOI] [PubMed] [Google Scholar]
- 116.Huang L, Chen F, Chen Y, Yang X, Xu S, Ge S, et al. Thymidine phosphorylase gene variant, platelet counts and survival in gastrointestinal cancer patients treated by fluoropyrimidines. Sci Rep. 2014;4:5697. doi: 10.1038/srep05697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Otsuka F, Yasuda S, Noguchi T, Ishibashi-Ueda H. Pathology of coronary atherosclerosis and thrombosis. Cardiovasc Diagn Ther. 2016;6:396–408. doi: 10.21037/cdt.2016.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ignatescu MC, Gharehbaghi-Schnell E, Hassan A, Rezaie-Majd S, Korschineck I, Schleef RR, et al. Expression of the angiogenic protein, platelet-derived endothelial cell growth factor, in coronary atherosclerotic plaques: In vivo correlation of lesional microvessel density and constrictive vascular remodeling. Arteriosclerosis, thrombosis, and vascular biology. 1999;19:2340–7. doi: 10.1161/01.atv.19.10.2340. [DOI] [PubMed] [Google Scholar]
- 119.Boyle JJ, Wilson B, Bicknell R, Harrower S, Weissberg PL, Fan TP. Expression of angiogenic factor thymidine phosphorylase and angiogenesis in human atherosclerosis. J Pathol. 2000;192:234–42. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH699>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 120.Sluimer JC, Daemen MJ. Novel concepts in atherogenesis: angiogenesis and hypoxia in atherosclerosis. J Pathol. 2009;218:7–29. doi: 10.1002/path.2518. [DOI] [PubMed] [Google Scholar]
- 121.Handa M, Li W, Morioka K, Takamori A, Yamada N, Ihaya A. Adventitial delivery of platelet-derived endothelial cell growth factor gene prevented intimal hyperplasia of vein graft. J Vasc Surg. 2008;48:1566–74. doi: 10.1016/j.jvs.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 122.Alexander MR, Moehle CW, Johnson JL, Yang Z, Lee JK, Jackson CL, et al. Genetic inactivation of IL-1 signaling enhances atherosclerotic plaque instability and reduces outward vessel remodeling in advanced atherosclerosis in mice. J Clin Invest. 2012;122:70–9. doi: 10.1172/JCI43713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Waguri Y, Otsuka T, Sugimura I, Matsui N, Asai K, Moriyama A, et al. Gliostatin/platelet-derived endothelial cell growth factor as a clinical marker of rheumatoid arthritis and its regulation in fibroblast-like synoviocytes. Br J Rheumatol. 1997;36:315–21. doi: 10.1093/rheumatology/36.3.315. [DOI] [PubMed] [Google Scholar]
- 124.Toyoda Y, Tabata S, Kishi J, Kuramoto T, Mitsuhashi A, Saijo A, et al. Thymidine phosphorylase regulates the expression of CXCL10 in rheumatoid arthritis fibroblast-like synoviocytes. Arthritis Rheumatol. 2014;66:560–8. doi: 10.1002/art.38263. [DOI] [PubMed] [Google Scholar]
- 125.Rahmati M, Petitbarat M, Dubanchet S, Bensussan A, Chaouat G, Ledee N. Granulocyte-Colony Stimulating Factor related pathways tested on an endometrial ex-vivo model. PLoS One. 2014;9:e102286. doi: 10.1371/journal.pone.0102286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Eda H, Fujimoto K, Watanabe S, Ura M, Hino A, Tanaka Y, et al. Cytokines induce thymidine phosphorylase expression in tumor cells and make them more susceptible to 5′-deoxy-5-fluorouridine. Cancer chemotherapy and pharmacology. 1993;32:333–8. doi: 10.1007/BF00735915. [DOI] [PubMed] [Google Scholar]
- 127.Griffiths L, Dachs GU, Bicknell R, Harris AL, Stratford IJ. The influence of oxygen tension and pH on the expression of platelet-derived endothelial cell growth factor/thymidine phosphorylase in human breast tumor cells grown in vitro and in vivo. Cancer Res. 1997;57:570–2. [PubMed] [Google Scholar]
- 128.Sawada N, Ishikawa T, Fukase Y, Nishida M, Yoshikubo T, Ishitsuka H. Induction of thymidine phosphorylase activity and enhancement of capecitabine efficacy by taxol/taxotere in human cancer xenografts. Clin Cancer Res. 1998;4:1013–9. [PubMed] [Google Scholar]
- 129.Endo M, Shinbori N, Fukase Y, Sawada N, Ishikawa T, Ishitsuka H, et al. Induction of thymidine phosphorylase expression and enhancement of efficacy of capecitabine or 5′-deoxy-5-fluorouridine by cyclophosphamide in mammary tumor models. Int J Cancer. 1999;83:127–34. doi: 10.1002/(sici)1097-0215(19990924)83:1<127::aid-ijc22>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 130.Sawada N, Ishikawa T, Sekiguchi F, Tanaka Y, Ishitsuka H. X-ray irradiation induces thymidine phosphorylase and enhances the efficacy of capecitabine (Xeloda) in human cancer xenografts. Clin Cancer Res. 1999;5:2948–53. [PubMed] [Google Scholar]
- 131.Coppinger JA, Maguire PB. Insights into the platelet releasate. Curr Pharm Des. 2007;13:2640–6. doi: 10.2174/138161207781662885. [DOI] [PubMed] [Google Scholar]
- 132.Lievens D, von Hundelshausen P. Platelets in atherosclerosis. Thromb Haemost. 2011;106:827–38. doi: 10.1160/TH11-08-0592. [DOI] [PubMed] [Google Scholar]
- 133.Hirano M. Mitochondrial Neurogastrointestinal Encephalopathy Disease. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, et al., editors. GeneReviews(R) Seattle (WA): 1993. [Google Scholar]
- 134.Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews® [Internet] Seattle (WA): University of Washington; Seattle: 2016. M. H. Mitochondrial Neurogastrointestinal Encephalopathy Disease; pp. 1993–2016. 2005 Apr 22 [Updated 2016 Jan14] [Google Scholar]
- 135.Halter JP, Michael W, Schupbach M, Mandel H, Casali C, Orchard K, et al. Allogeneic haematopoietic stem cell transplantation for mitochondrial neurogastrointestinal encephalomyopathy. Brain. 2015;138:2847–58. doi: 10.1093/brain/awv226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Boschetti E, D’Alessandro R, Bianco F, Carelli V, Cenacchi G, Pinna AD, et al. Liver as a source for thymidine phosphorylase replacement in mitochondrial neurogastrointestinal encephalomyopathy. PLoS One. 2014;9:e96692. doi: 10.1371/journal.pone.0096692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Torres-Torronteras J, Viscomi C, Cabrera-Perez R, Camara Y, Di Meo I, Barquinero J, et al. Gene therapy using a liver-targeted AAV vector restores nucleoside and nucleotide homeostasis in a murine model of MNGIE. Mol Ther. 2014;22:901–7. doi: 10.1038/mt.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kumagai Y, Sugiura Y, Sugeno H, Takebayashi Y, Takenoshita S, Yamamoto T. Thymidine phosphorylase gene mutation is not a primary cause of mitochondrial neurogastrointestinal encephalomyopathy (MNGIE) Internal medicine. 2006;45:443–6. doi: 10.2169/internalmedicine.45.1371. [DOI] [PubMed] [Google Scholar]
- 139.Figura MG, Coppola A, Bottitta M, Calabrese G, Grillo L, Luciano D, et al. Seizures and EEG pattern in the 22q13.3 deletion syndrome: clinical report of six Italian cases. Seizure. 2014;23:774–9. doi: 10.1016/j.seizure.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 140.Bartsch O. Encyclopedia of molecular mechanisms of disease. 2009:504–6. [Google Scholar]
- 141.Sandelin A, Wasserman WW, Lenhard B. ConSite: web-based prediction of regulatory elements using cross-species comparison. Nucleic Acids Res. 2004;32:W249–52. doi: 10.1093/nar/gkh372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yao B, Wang Q, Liu CF, Bhattaram P, Li W, Mead TJ, et al. The SOX9 upstream region prone to chromosomal aberrations causing campomelic dysplasia contains multiple cartilage enhancers. Nucleic Acids Res. 2015;43:5394–408. doi: 10.1093/nar/gkv426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Renault MA, Vandierdonck S, Chapouly C, Yu Y, Qin G, Metras A, et al. Gli3 regulation of myogenesis is necessary for ischemia-induced angiogenesis. Circ Res. 2013;113:1148–58. doi: 10.1161/CIRCRESAHA.113.301546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ikuta K, Waguri-Nagaya Y, Kikuchi K, Yamagami T, Nozaki M, Aoyama M, et al. The Sp1 transcription factor is essential for the expression of gliostatin/thymidine phosphorylase in rheumatoid fibroblast-like synoviocytes. Arthritis research & therapy. 2012;14:R87. doi: 10.1186/ar3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhu GH, Lenzi M, Schwartz EL. The Sp1 transcription factor contributes to the tumor necrosis factor-induced expression of the angiogenic factor thymidine phosphorylase in human colon carcinoma cells. Oncogene. 2002;21:8477–85. doi: 10.1038/sj.onc.1206030. [DOI] [PubMed] [Google Scholar]
- 146.Sivridis E, Giatromanolaki A, Koukourakis M, Bicknell R, Harris A, Gatter K. Thymidine phosphorylase expression in normal and hyperplastic endometrium. J Clin Pathol. 2000;53:704–8. doi: 10.1136/jcp.53.9.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fujimoto J, Ichigo S, Sakaguchi H, Hirose R, Tamaya T. Expression of platelet-derived endothelial cell growth factor and its mRNA in uterine endometrium during the menstrual cycle. Mol Hum Reprod. 1998;4:509–13. doi: 10.1093/molehr/4.5.509. [DOI] [PubMed] [Google Scholar]
- 148.Kushner PJ, Agard D, Feng WJ, Lopez G, Schiau A, Uht R, et al. Oestrogen receptor function at classical and alternative response elements. Novartis Found Symp. 2000;230:20–6. doi: 10.1002/0470870818.ch3. discussion 7-40. [DOI] [PubMed] [Google Scholar]
- 149.Usuki K, Gonez LJ, Wernstedt C, Moren A, Miyazono K, Claesson-Welsh L, et al. Structural properties of 3.0 kb and 3.2 kb transcripts encoding platelet-derived endothelial cell growth factor/thymidine phosphorylase in A431 cells. Biochim Biophys Acta. 1994;1222:411–4. doi: 10.1016/0167-4889(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 150.Kataoka M, Yamaguchi Y, Moriya Y, Sawada N, Yasuno H, Kondoh K, et al. Antitumor activity of chemoendocrine therapy in premenopausal and postmenopausal models with human breast cancer xenografts. Oncol Rep. 2012;27:303–10. doi: 10.3892/or.2011.1541. [DOI] [PubMed] [Google Scholar]
- 151.Ko J-C, Chiu H-C, Syu J-J, Jian Y-J, Chen C-Y, Jian Y-T, et al. Tamoxifen enhances erlotinib-induced cytotoxicity through down-regulating AKT-mediated thymidine phosphorylase expression in human non-small-cell lung cancer cells. Biochemical Pharmacology. 2014;88:119–27. doi: 10.1016/j.bcp.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 152.Aoki I, Fujimoto J, Tamaya T. Effects of various steroids on platelet-derived endothelial cell growth factor (PD-ECGF) and its mRNA expression in uterine endometrial cancer cells. The Journal of Steroid Biochemistry and Molecular Biology. 2003;84:217–22. doi: 10.1016/s0960-0760(03)00031-1. [DOI] [PubMed] [Google Scholar]
- 153.Osuga Y, Toyoshima H, Mitsuhashi N, Taketani Y. The presence of platelet-derived endothelial cell growth factor in human endometrium and its characteristic expression during the menstrual cycle and early gestational period. Hum Reprod. 1995;10:989–93. doi: 10.1093/oxfordjournals.humrep.a136083. [DOI] [PubMed] [Google Scholar]
- 154.Zhang L, Mackenzie IZ, Rees MC, Bicknell R. Regulation of the expression of the angiogenic enzyme platelet-derived endothelial cell growth factor/thymidine phosphorylase in endometrial isolates by ovarian steroids and cytokines. Endocrinology. 1997;138:4921–30. doi: 10.1210/endo.138.11.5517. [DOI] [PubMed] [Google Scholar]
- 155.Terranova-Barberio M, Roca MS, Zotti AI, Leone A, Bruzzese F, Vitagliano C, et al. Valproic acid potentiates the anticancer activity of capecitabine in vitro and in vivo in breast cancer models via induction of thymidine phosphorylase expression. Oncotarget. 2015 doi: 10.18632/oncotarget.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Guarcello V, Blanquicett C, Naguib FNM, el Kouni MH. Suppression of thymidine phosphorylase expression by promoter methylation in human cancer cells lacking enzyme activit. Cancer chemotherapy and pharmacology. 2008;62:85–96. doi: 10.1007/s00280-007-0578-5. [DOI] [PubMed] [Google Scholar]
- 157.Kosuri KV, Wu X, Wang L, Villalona-Calero MA, Otterson GA. An epigenetic mechanism for capecitabine resistance in mesothelioma. Biochem Biophys Res Commun. 2010;391:1465–70. doi: 10.1016/j.bbrc.2009.12.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Woodgett JR, Gould KL, Hunter T. Substrate specificity of protein kinase C. Use of synthetic peptides corresponding to physiological sites as probes for substrate recognition requirements. European journal of biochemistry / FEBS. 1986;161:177–84. doi: 10.1111/j.1432-1033.1986.tb10139.x. [DOI] [PubMed] [Google Scholar]
- 159.Usuki K, Miyazono K, Heldin CH. Covalent linkage between nucleotides and platelet-derived endothelial cell growth factor. J Biol Chem. 1991;266:20525–31. [PubMed] [Google Scholar]
- 160.Weng SH, Tseng SC, Huang YC, Chen HJ, Lin YW. Inhibition of thymidine phosphorylase expression by using an HSP90 inhibitor potentiates the cytotoxic effect of cisplatin in non-small-cell lung cancer cells. Biochemical pharmacology. 2012;84:126–36. doi: 10.1016/j.bcp.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 161.Perez-Perez MJ, Priego EM, Hernandez AI, Camarasa MJ, Balzarini J, Liekens S. Thymidine phosphorylase inhibitors: recent developments and potential therapeutic applications. Mini reviews in medicinal chemistry. 2005;5:1113–23. doi: 10.2174/138955705774933301. [DOI] [PubMed] [Google Scholar]
- 162.Fukushima M, Suzuki N, Emura T, Yano S, Kazuno H, Tada Y, et al. Structure and activity of specific inhibitors of thymidine phosphorylase to potentiate the function of antitumor 2′-deoxyribonucleosides. Biochem Pharmacol. 2000;59:1227–36. doi: 10.1016/s0006-2952(00)00253-7. [DOI] [PubMed] [Google Scholar]
- 163.Liekens S, Hernandez AI, Ribatti D, De Clercq E, Camarasa MJ, Perez-Perez MJ, et al. The nucleoside derivative 5′-O-trityl-inosine (KIN59) suppresses thymidine phosphorylase-triggered angiogenesis via a noncompetitive mechanism of action. The Journal of biological chemistry. 2004;279:29598–605. doi: 10.1074/jbc.M402602200. [DOI] [PubMed] [Google Scholar]
- 164.Heit JA. Cancer and venous thromboembolism: scope of the problem. Cancer Control. 2005;12(Suppl 1):5–10. doi: 10.1177/1073274805012003S02. [DOI] [PubMed] [Google Scholar]
- 165.Mezouar S, Frere C, Darbousset R, Mege D, Crescence L, Dignat-George F, et al. Role of platelets in cancer and cancer-associated thrombosis: Experimental and clinical evidences. Thromb Res. 2016;139:65–76. doi: 10.1016/j.thromres.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 166.Liekens S, Hernandez AI, Ribatti D, De Clercq E, Camarasa MJ, Perez-Perez MJ, et al. The nucleoside derivative 5′-O-trityl-inosine (KIN59) suppresses thymidine phosphorylase-triggered angiogenesis via a noncompetitive mechanism of action. The Journal of biological chemistry. 2004;279:29598–605. doi: 10.1074/jbc.M402602200. [DOI] [PubMed] [Google Scholar]
- 167.Matsushita S, Nitanda T, Furukawa T, Sumizawa T, Tani A, Nishimoto K, et al. The effect of a thymidine phosphorylase inhibitor on angiogenesis and apoptosis in tumors. Cancer Res. 1999;59:1911–6. [PubMed] [Google Scholar]
- 168.Johansson M. Identification of a novel human uridine phosphorylase. Biochem Biophys Res Commun. 2003;307:41–6. doi: 10.1016/s0006-291x(03)01062-3. [DOI] [PubMed] [Google Scholar]
- 169.Roosild TP, Castronovo S, Villoso A, Ziemba A, Pizzorno G. A novel structural mechanism for redox regulation of uridine phosphorylase 2 activity. J Struct Biol. 2011;176:229–37. doi: 10.1016/j.jsb.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


