Abstract
Our previous studies suggest that prenatal arsenic exposure (50 ppb) modifies epigenetic control of the programming of the glucocorticoid receptor (GR) signaling system in the developing mouse brain. These deficits may lead to long-lasting consequences, including deficits in learning and memory, increased depressive-like behaviors, and an altered set-point of GR feedback throughout life. To understand the arsenic-induced changes within the GR system, we assessed the impact of in utero arsenic exposure on the levels of the GR and growth arrest-specific-5 (Gas5), a noncoding RNA, across a key gestational period for GR programming (gestational days, GD 14–18) in mice. Gas5 contains a glucocorticoid response element (GRE)-like sequence that binds the GR, thereby decreasing GR-GRE-dependent gene transcription and potentially altering GR programming. Prenatal arsenic exposure resulted in sex-dependent and age-dependent shifts in the levels of GR and Gas5 expression in fetal telencephalon. Nuclear GR levels were reduced in males, but unchanged in females, at all gestational time points tested. Total cellular Gas5 levels were lower in arsenic-exposed males with no changes seen in arsenic-exposed females at GD16 and 18. An increase in total cellular Gas-5 along with increased nuclear levels in GD14 arsenic-exposed females, suggests a differential regulation of cellular compartmentalization of Gas5. RIP assays revealed reduced Gas5 associated with the GR on GD14 in the nuclear fraction prepared from arsenic-exposed males and females. This decrease in levels of GR-Gas5 binding continued only in the females at GD18. Thus, nuclear GR signaling potential is decreased in prenatal arsenic-exposed males, while it is increased or maintained at levels approaching normal in prenatal arsenic-exposed females. These findings suggest that females, but not males, exposed to arsenic are able to regulate the levels of nuclear free GR by altering Gas5 levels, thereby keeping GR nuclear signaling closer to control (unexposed) levels.
Keywords: Development, brain, arsenic, lncRNA, Gas5, glucocorticoid, sex differences
Introduction
Arsenic readily crosses the placenta during development [1,34,86] and all forms of arsenic, including inorganic and methylated arsenicals, accumulate in the brain [74]. Several epidemiological studies have demonstrated that arsenic exposure is associated with neurological and cognitive deficits [12,22,27,37,64,70,71,95]. Many of the cognitive deficits seen following arsenic exposure may be mediated by the glucocorticoid receptor (GR) and hypothalamic pituitary adrenal (HPA) signaling system [44,50,82,94]. In our previous work, we observed significant cognitive deficits, which corresponded with alterations in GR signaling, in mice that were exposed to arsenic throughout gestation and during the early postnatal period [2,9,26,52,53,81,83]. We have shown that exposure to 50 ppb arsenic, a concentration that is moderately higher than the EPA recommended limit of 10ppb, affects glucocorticoid signaling including reduced expression of GR in both the embryonic and adult mouse brain [2,9,26], suggesting that arsenic disrupts the fetal programming of the GR system. Aberrant GR programming during development has lasting effects into adulthood, including increased susceptibility to psychiatric disorders [50,61,98]. Most of our published studies using this model of prenatal arsenic exposure have used males. However, we recently reported that sex-specific effects within the glucocorticoid signaling system in fetal placenta and brain [9]. In an effort to identify mechanisms through which arsenic exerts these actions, we conducted a series of studies assessing the effects of arsenic on regulators of GR signaling in the embryonic brain, focusing on noncoding RNAs.
Noncoding RNAs are functional RNA molecules that control gene expression and are not thought to be translated into proteins; however, recent studies suggest that some lncRNAs may be translated into small polypeptides [16,51]. These molecules regulate gene expression at both the transcriptional and post-transcriptional level [13]. Examples of noncoding RNAs include microRNAs, which act as RNA silencers by base-pairing with a complementary sequence on mRNA, small nucleolar RNAs (snoRNAs), which guide chemical modifications of other RNAs, and long noncoding RNAs (lncRNAs), which are distinguished from other noncoding RNAs by having a transcript length greater than 200 nucleotides [33]. While more than one-fifth of the genome has been determined to consist of these new classes of RNA molecules, the physiologic roles of most of them are still unknown [42]. The abundance of these noncoding RNA molecules, combined with data suggesting that certain types of noncoding RNAs are highly conserved throughout species, suggests that they may play important roles as epigenetic gene regulators [41].
Growth arrest-specific-5 (Gas5) is a lnc RNA which has been implicated in a variety of cellular processes including cell cycle control and growth arrest [48,57,75] and apoptosis [39,57,58,66]. Gas5 expression has been shown to arrest cells in the G1-S phase of growth, suggesting that it may act as a cell cycle regulator [30]. Prolonged growth arrest in cells with high levels of Gas5 has been shown to cause a decrease in the transcription of the inhibitor of apoptosis 2 (IAP2) gene, thereby sensitizing cells to apoptosis [45]. It is important to note that the IAP2 gene contains a glucocorticoid response element (GRE) site within its promoter and its expression is induced by dexamethasone [91]. Gas5 reduces GR-dependent transcription by forming a double helical, hairpin structure which mimics a GRE site, and which competes with GREs for binding to the DNA binding domain of the GR [39,45,80]. Gas5 also is capable of binding to and blocking the transcriptional activity of the mineralocorticoid, progesterone and androgen, but not estrogen, receptors [39,45]. In addition to binding steroid hormone receptors, Gas5 is a multiple snoRNA host gene that encodes 9 (10 in humans) C/D box snoRNAs within 11 introns [76]. Recently, He et al. [35] reported that, in humans, five of these snoRNAs contain sequences that appear to be PIWI-interacting RNAs (piRNAs). Thus, there are several likely sources of transcriptional and translational regulation contained within the Gas5 gene.
Gas5 expression is detected as early as the 8-cell stage of mouse development [24] and continues throughout embryogenesis and fetal development with relatively high expression in the developing brain [15]. Fetal mouse Gas5 expression peaks at gestational day (GD) 16 [15]. In the adult mouse, Gas5 expression is high in brain and low in liver and spleen [15]. Morellini and colleagues [55] have shown that Gas5 is expressed in the hippocampus at higher levels than found throughout the rest of the brain [55].
The role of Gas5 as a regulator of GR-associated transcription suggests that it may play a significant role in the programming of the HPA axis during development and throughout life. Programming of the HPA axis is believed to be most important during the second trimester (~GD10-19 in the mouse) of fetal development [38,69]. Gas5 expression has been shown to be increased during periods of psychogenic stress, suggesting that Gas5 is also a likely regulator of HPA axis programming postnatally [55].
The role of lncRNAs in prenatal arsenic-mediated changes in GR programming has not been explored. The goal of the present studies was to assess the impact of prenatal arsenic exposure during fetal brain development on both Gas5 and GR levels to determine if changes in the lncRNA may be responsible for altered GR programming following in utero exposure to arsenic.
Materials and Methods
Prenatal Arsenic Exposure Model
All procedures involving animals, including the prenatal arsenic exposure model, were approved by the Institutional Animal Care and Use Committee at the University of New Mexico and followed the National Institutes of Health Guide for the care and use of laboratory animals (8th edition, 2011). Male and female C57BL/6J mice were obtained from Jackson Laboratory and were raised in a reverse light/dark cycle with lights on at 20:00 and off at 08:00 daily. Female animals were exposed to arsenic in their drinking water, as previously described [53,85]. Prior to mating, female mice were acclimated to arsenic exposure by drinking 50 parts-per-billion (ppb) arsenic (sodium arsenate, Sigma Aldrich) water, prepared using standard tap water. Control mice were provided with tap water, which contained approximately 5 ppb arsenic. Mating occurred over a single 8 hour period during the dark cycle. After mating, females were maintained on arsenic water throughout pregnancy. Dams were decapitated, fetuses were removed and the telencephalons were dissected, snap frozen in liquid nitrogen and stored at −80° C at GD14, 16 or 18. Mice were euthanized between 0800–1000hr.
Sex Determination qPCR Analysis
Fetal sex was determined by measuring genomic DNA (gDNA) expression of the Sry gene. Sry gene primers were purchased from Invitrogen (cat: A156612) with the following sequences: forward: GCTGGGATGCAGGTGGAAAA; reverse: CCCTCCGATGAGGCTGATATT (PrimerBank ID: 6766761a1). qPCR was carried out in duplicate using Roche FastStart Universal SYBR Green Master (ROX) (cat: 04913850001, Roche Diagnostics) following the manufacturer’s suggested protocol. Sex was determined by comparative analysis of Ct values [84].
Tissue Extraction for Protein Quantification
Fetal telencephalons were homogenized according to previously established protocols [7]. Individual telencephalons were homogenized in a Kontes RNase-Free Pellet Pestle Grinder (cat: KT749520-0090, VWR) in 100 μL ice cold homogenization buffer (HB) (20 mM Tris-HCl, pH 7.4, 1 mM EDTA, 320 mM sucrose, 1:1000 protease inhibitor cocktail (Sigma P8340), 200 μM sodium orthovanadate). Samples were centrifuged at 1,000 ×gmax for 6 minutes, the supernatant was removed, the pellet was resuspended in 75 μL of HB and spun again at 1,000 ×gmax for 10 minutes. The supernatant was removed, combined with the first supernatant and frozen at −80° C; the preparation was designated the post-nuclear lysate (PNL) and contains cytosolic and membrane proteins. The pellet was resuspended in extraction buffer (EB) (HB containing 75 mM NaCl, 75 mM KCl and 1% v/v Triton X-100). Samples were sonicated (2× 15 sec) in ice then kept in ice for 20 minutes. Samples were centrifuged at 15,000 ×gmax for 15 min and the supernatant was collected as the soluble nuclear (nuc) fraction, and frozen at −80° C. Protein concentration was determined using the Qubit® Protein Assay Kit following the manual method (cat: Q33212, ThermoFisher Scientific) on a Qubit® 2.0 Fluorometer.
Evaluation of Protein Expression
Immunoblotting was carried out following established protocols [2,84]. Antibody concentrations and loaded total proteins amounts (15 μg) of male and female PNL and nuc fractions were optimized to fit the linear range of signal detection. Protein expression was evaluated in GD14, GD16 and GD18, male and female, PNL and nuc fractions (n ≥ 5). Transfer was done onto Immobilon-FL Transfer Membrane (cat: IPFL07810, Millipore) using a Mini Blot Module (cat: B1000, Life Technologies). Blots were incubated in in LI-COR Blocking Buffer (cat: 927-40000, LI-COR) overnight at +4° C. Membranes were then incubated with anti-GR antibody (cat: sc-1004, Santa Cruz Biotechnology) diluted 1:1,000 in PBS-T (10x PBS (cat: 70011-044, Life Technologies) diluted in deionized water, 1% v/v Tween-20) for 3 hours at room temperature. An immunofluorescent secondary antibody was applied (IRDye 680 Donkey anti-Rabbit IgG (cat: 926-68073) diluted 1:10,000 in PBS-T) for 45 minutes at room temperature without light exposure. Imaging of the immunoblots was conducted using a two-channel infrared detection Odyssey Infrared Imaging System (cat: LIC-8201-00, LI-COR) and quantified using Image Studio version 5.0 (LI-COR Biosciences). Protein detected by immunofluorescence was corrected to the total protein as quantified by Coomassie staining [65].
Nucleic Acid Purification
gDNA and RNA were extracted from 20 mg frozen telencephalon using the DNeasy Blood and Tissue Mini Kit (cat: 69504, Qiagen) and RNeasy Plus Mini Kit (cat: 74134, Qiagen), respectively, according to the manual methods. The gDNA and RNA were stored at −20° C and −80° C, respectively. Integrity was tested using a NanoDrop 1000 Spectrophotometer (Thermo Scientific) followed by quantification using the Qubit dsDNA HS Assay Kit (cat: Q32851, Thermo Scientific) or using the Qubit RNA BR Assay Kit (cat: Q10211, Thermo Scientific) on a Qubit® 2.0 Fluorometer.
Quantitative PCR assessment of microRNA expression
Ten ng of total RNA was reverse transcribed with the TaqMan® MicroRNA Reverse Transcription Kit (cat: 4366596; Life Technologies) using primer sets for TaqMan® MicroRNA Assay (cat: 4427975; Life Technologies) for miR-21 (mmu-mir21 Accession MI0000569) according to the manufacturer’s instructions. To produce cDNA suitable for the endogenous control, snoRNA202 (Snord68, cat:4427975; ThermoFisher) RNA was reverse transcribed with the TaqMan® MicroRNA Reverse Transcription Kit and Random Hexamers (50 μM, cat: N8080127; Life Technologies). Previous work identified this snoRNA as a stable endogenous control for assessments of microRNA expression in our prenatal arsenic exposure model [9,84].
Gas5 RNA Assessment
Reverse transcription was carried out on 1 μg of RNA using the Roche Transcriptor First Strand cDNA Synthesis Kit (cat: 04897030001, Roche Diagnostics) according to the associated manual method. cDNA was further quantified using NanoDrop and stored at −20°C. Quantitative analysis of Gas5 lncRNA expression was carried out using qPCR. qPCR primers for Gas5 lncRNA were developed using the NCBI Primer-Blast software: forward: GGAAGCTGGATAACAGAGCGA; reverse: GGTATTCCTTGTAATGGGACCAC. Hprt was used as an endogenous control using the following primer set, forward: TGACACTGGCAAAACAATGCA; reverse: GGTCCTTTTCACCAGCAAGCT. Primer specificity was determined using qPCR dissociation curves and agarose gel electrophoresis and efficiency was established to be between 90% and 110%. Samples were run in triplicate on a LightCycler 96 instrument (Roche Diagnostics) using Roche FastStart Universal SYBR Green Master (Rox) (cat: 04913850001, Roche Diagnostics) under standard conditions. Template and reverse transcription negative controls were confirmed negative. To assess the levels of Gas5 in the nuclear fraction, the RNA extracted from the input fraction of the RIP was converted to cDNA and Gas5 levels were quantified by qPCR. Analysis of fold expression was carried out using the Comparative CT method.
RNA Binding Protein Co-Immunoprecipitation (RIP)
The level of nuclear Gas5 bound with GR was measured by immunoprecipitation of the GR and assessment of associated Gas5 by qPCR. Using the Magna Nuclear RIP (Cross-linked) Nuclear RNA-Binding Protein Immunoprecipitation Kit (Millipore, #17-10520), frozen whole telecephalon was homogenized in ice-cold Nuclease Free PBS (kit) with Protease Inhibitor Cocktail (kit) and RNase Inhibitor (kit). Half of this cellular homogenate was treated with formaldehyde to cross-link associated molecules. The cytoplasmic membrane was lysed and the nuclei were isolated by centrifugation at 1,000 ×gmax for 5 minutes at +4*C. Following probe sonication of the nuclear lysate, 10uL of each nuclear lysate was removed and labeled “Input” and 50uL of each nuclear lysate was used for the immunoprecipitation reaction and labeled “IP.” Using anti-GR antibody (M-20; Santa Cruz, sc-1004x), GR and associated molecules were isolated using magnetic beads (kit). Following elution and cross-link reversal, the RNA was purified with Trizol (Thermo 10296028) and chloroform. Pellet Paint (Millipore, 69049-3), a precipitate enhancer, was used to aid the RNA purification. Following qPCR, the data were analyzed as % input, following Thermo Fisher’s online guide for ChIP data analysis.
Statistical Analysis
Protein and RNA expression data were analyzed using SPSS (v.24, IBM) and GraphPad Prism 6 software (GraphPad Software, Inc). Two-way or one-way ANOVAs, followed with post hoc comparison using bonferroni corrected Student’s t-test was done with significance determined by a p value of <.05. Data are presented as mean ± SEM normalized to control. A power calculation (G*power v3.1.9.2) for a two way ANOVA revealed an expected 80% power to detect an effect of .65 with 5 per group and for a one-way ANOVA with n= 5 per group the effect size detected is .68. The number of samples per group ranged from 5–9.
Results
Developmental expression of Gas5: influence of sex and arsenic exposure
We assessed the developmental changes in Gas5 levels in both controls and prenatal arsenic exposed fetal brains at GD14-18, a period critical to glucocorticoid programming [38,69,77]. To make the comparison across the developmental time points, the data in Figure 1 are corrected to the control expression levels at GD14 within each sex. There is a common pattern of Gas5 expression in the male and female control brain with a large peak at GD16 and precipitous decline by GD18 (Figure 1 column). One-way ANOVA found a significant effect of age on Gas5 levels [F(2,20)= 58.44, p=.0001] for males and females [(F(2,18)=33.806, p=.0001]. Compared to E14, there was a significant increase in Gas5 levels at E16 [(t(12)=4.73, p=.0001] and a significant decrease at E18 (t(12)= 2.564, p= .025] for females. Similarly for the males, levels of Gas5 at E16 were greater then E14 [t(12)=3.089, p=.009 ] and there was a significant decrease at E18 [t(14)=6.643, p=.0001] relative to E14 levels.
Figure 1.
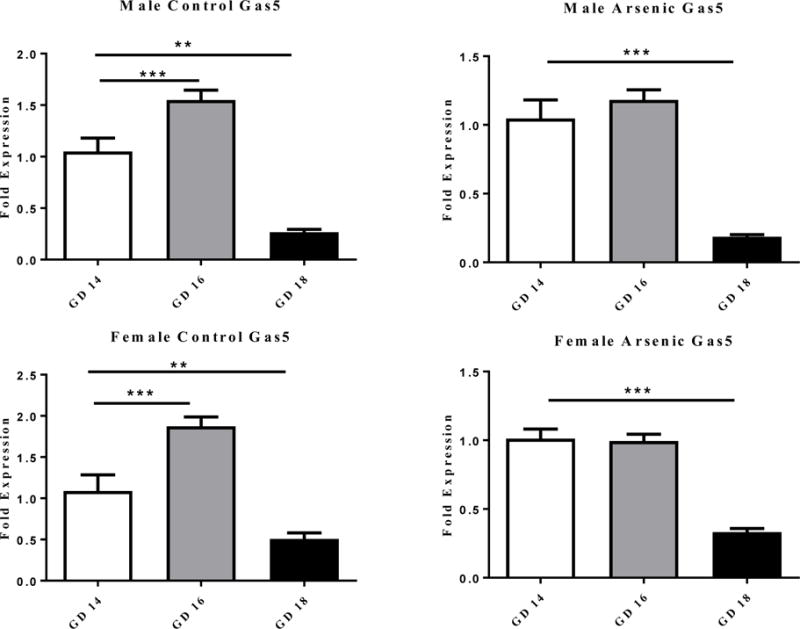
The effect on the level of Gas5 RNA expression in male (top row of figures) and female (bottom row of figures) brain at GD 14, 16 and18. The developmental pattern of Gas5 expression is shown for the control (right column) and the prenatal arsenic exposed (left column) fetal brains. Fold expression of Gas5 was acquired by the ΔΔCt (Comparative CT) method. Arsenic exposed are presented in the filled columns and controls presented in the unfilled columns. Data presented are mean ±SEM n = 6–9, **p<.003, ***p< .0001.
In general, prenatal arsenic exposure blunted the developmental-dependent increase in Gas5 at GD16 (Figure 1 right column) in both the male and female brains; however, the reduction at GD 18 was still present. The age analysis revealed a significant developmental effect of age on Gas5 RNA in the arsenic females [F(1,17)= 58.998, p=.0001] and arsenic males [F(1,19)=43.315, p=.0001]. Unlike the control condition, there was no significant increase in Gas5 levels at E16 but there was a significant decrease from GD14 to GD18 [t(13)=6.805, p=.0001] for the males and for the females [t(11)= 6.435, p=.0001].
Developmental expression of GR: influence of sex and arsenic exposure
Gas5 binds to the DNA binding domain of the GR via two GRE-like sequences in Gas5, thereby suppressing the transcriptional activity of the GR [39,45]. Because of this, Gas5 has often been referred to as a ‘GR decoy’ or a riborepressor for GR [45]. It has been suggested that high levels of Gas5 can alter glucocorticoid responding, potentially reducing GR ability to autoregulate [49]. We assessed the levels of the GR in both the nuclear and PNL fractions in arsenic-exposed and control male and female fetal brains at GD14-18 using immunoblotting techniques (Figure 2). Two-way ANOVAs were conducted on the separate fractions and developmental time-points. For the GD14 nuclear fraction (Figure 2A), there was no significant treatment effect [F(1,16)= 3.924, p=.065] or sex effect [F(1,16)= 3.318, p= .087], but there was a significant treatment by sex interaction [F(1,16)= 7.793, p=.012]. There was a decrease in the levels of nuclear GR in the males exposed to arsenic [t(8)=3.966, p=.004] with no decrease present in the arsenic-exposed females. In the GD14 PNL fraction (Figure 2B) there was a significant effect of arsenic treatment [F(1,18)= 7.202, p=.015] and no significant sex effect [F(1,18)= 0.145, p= .707], but there was a significant treatment by sex interaction [F(1,18)= 4.478, p=.05]. Post hoc analysis showed a significant decrease in the levels of GR in the PNL fraction in arsenic males [t(10)= 2.876, p=.0165] but not in females. In the nuclear fraction at GD16 (Figure 2C), two-way ANOVA revealed that there was no significant treatment effect [F(1,36)= 3.0173, p=.091] but there was a significant sex effect [F(1,36)= 91.05, p= .0001], as well as a significant treatment by sex interaction [F(1,36)= 6.361, p=.016]. Again, we found that there was a decrease in GR levels in the arsenic-exposed male nuclear fraction [t(12)=2.7, p=.0137] with no effect on female GD16 nuclear levels. In the PNL fraction at GD16 (figure 2D), there was no significant treatment effect [F(1,21)= 2.071, p=.165] but there was a significant sex effect [F(1,21)= 27.39, p= .0001], and no significant treatment by sex interaction [F(1,21)= 1.498, p=.235]. In the nuclear fraction from GD18 brain (Figure 2E), two-way ANOVA revealed that there was no significant treatment effect [F(1,21)= 0.096, p=.760] or sex effect [F(1,21)= 1.253, p= .276], but there was a significant treatment by sex interaction [F(1,21)= 8.30, p=.009]. Post hoc testing showed a significant decrease in nuclear GR levels in male arsenic-exposed [t(12)= 2.283, p= .0415] with no reduction in female arsenic-exposed. In the PNL fraction at GD18 (Figure 2F) there were no changes noted due to sex or arsenic treatment. Two-way ANOVA revealed that there was no significant treatment effect [F(1,21)= .0096, p=.760] and no significant sex effect [F(1,21)= 1.253, p= .276], but there was a significant treatment by sex interaction [F(1,21)= 8.3098, p=.009]. We note that the effects of prenatal arsenic exposure on nuclear and PNL GR levels in GD14 and GD18 males observed in the present studies replicate our previous findings [9].
Figure 2.
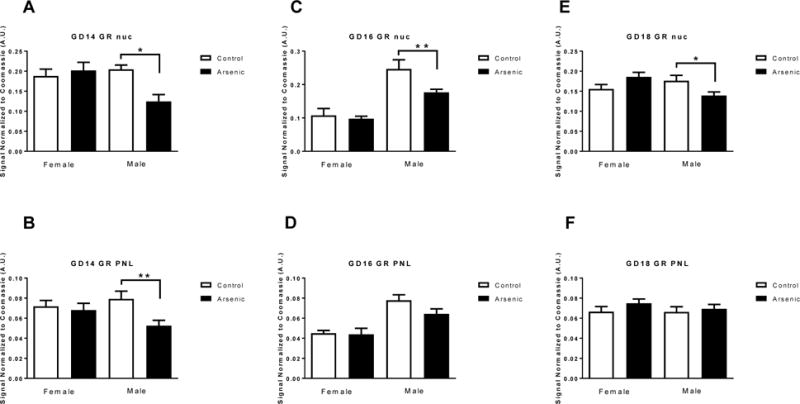
The effect of prenatal arsenic on glucocorticoid receptor (GR) protein expression assessed by immunoblot in nuclear (Nuc, Figs. A, C, E) and post nuclear (PNL, Figs. B, D, F) fractions from GD14 (Figs.A and B), GD16 (Figs. C and D) and GD18 (Figs. E and F) in arsenic (filled bars) and control (open bars) male and female brains. Data are mean ± SEM, n=7–9, * p<.05 and ** p<.004. Antibody intensity was corrected to within blot Coomassie stain.
Sex dependent changes in Gas5 in response to arsenic exposure
We measured the impact of prenatal arsenic exposure on total Gas5 in male and female brain at each of the developmental time-points (Figure 3). The fold expression data in Figure 3 are expressed relative to female controls within gestational day. At GD14 (Figure 3 left panel), a two-way ANOVA revealed a significant effect of treatment [F(1,24)= 7.857, p=.010], no effect of sex [F(1,24)= .107, p=.746] and a treatment by sex interaction [F(1,24)=9.772, p=.005]. Post hoc analysis revealed a significant increase in Gas5 levels in arsenic-exposed females [t(12)=4.602, p=.001], but not males. This sex difference was reversed by GD16 (Figure 3 center panel); there was a significant effect of treatment [F(1,24)= 12.803, p=.002], no effect of sex [F(1,24)= 1.230, p=.278] and no treatment by sex interaction [F(1,24)=2.145, p=.156]. By GD16, arsenic-exposed males displayed a significant decrease in Gas5 [t(12)= 3.744, p=.003], while females showed no effect of arsenic on Gas5 levels. The reduction in Gas5 levels in male arsenic exposed brains continued into GD18 (Figure 3 right panel). We found there was no significant overall effect of treatment [F(1,28)= 1.548, p=.224], a significant effect of sex [F(1,28)= 28.73, p=.0001] and no treatment by sex interaction [F(1,28)=2.162, p=.153]. The overall sex difference in Gas5 levels at GD18 was due to a lower level of Gas5 in the male brain overall. In addition, there was a significant Gas5 decrease in male arsenic-exposed [t(15)=3.347, p=.004], similar to that seen at GD16.
Figure 3.
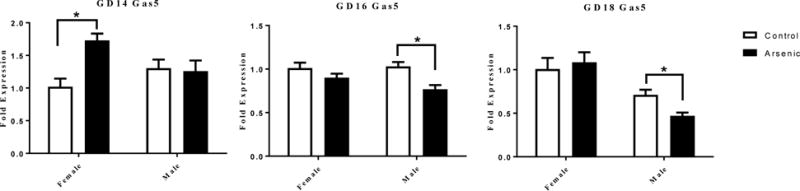
The effect of prenatal arsenic on Gas5 RNA expression assessed by qPCR in whole homogenate from GD14 (left panel), GD16 (center panel) and GD18 (right panel) in arsenic (filled bars) and control (open bars) male and female brains. Fold expression of Gas5 was acquired by the ΔΔCt (Comparative CT) method. Data are mean ± SEM, n=7–9, * p<.05.
Effect of prenatal arsenic on levels of miR-21
Sodium arsenite has been shown to increase microRNA-21 (miR-21) expression in cultured cells [28,47,68]. Gas5 and miR-21 are known to negatively regulate each other’s levels [97]. mir-21 is expressed prior to GD12 in the mouse embryo [56], with expression in the GD18 mouse brain being enriched in the hippocampal formation and cortex [67]. To determine if the arsenic-associated changes in Gas5 levels were due to reciprocal changes in the levels of miR-21, we measured levels of miR-21 in brain samples taken from littermates analyzed for Gas5 levels (Figure 4). The fold expression data presented are expressed relative to female control levels of miR-21. At GD14 (Figure 4 left panel) a two way ANOVA revealed no significant effect of treatment [F(1,24)= 2.084, p=.162], no effect of sex [F(1,24)=0.048, p=.829] and no treatment by sex interaction [F(1,24)=0.007, p=.936]. At GD16 (figure 4 center panel) there was an overall effect of treatment [F(1,24)= 4.693, p=.04], no effect of sex [F(1,24)=0.001, p=.984] and no treatment by sex interaction [F(1,24)=0.143, p=.718]. Arsenic reduced the levels of miR-21 slightly in both males and females. At GD18 (Figure 4 right panel), again, there was no significant effect of treatment [F(1,24)= 1.330, p=.260], no effect of sex [F(1,24)=2.373, p=.137] and no treatment by sex interaction [F(1,24)=0.420, p=.523].
Figure 4.
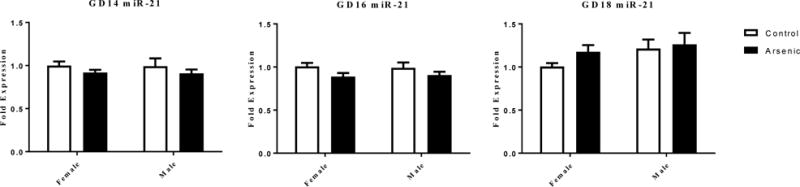
The effect of prenatal arsenic on miR-21 RNA expression assessed by qPCR in whole homogenate from GD14 (left panel), GD16 (center panel) and GD18 (right panel) in arsenic (filled bars) and control (open bars) male and female brains. Fold expression of miR-21 was acquired by the ΔΔCt (Comparative CT) method. Data are mean ± SEM, n=7.
Impact of arsenic on the nuclear localization of Gas5
GAS5 is present in both the cytoplasm and nucleus of cells [8,15,45,97]. The subcellular localization of lncRNAs has been reported to determine their function [14]. We assessed the levels of Gas5 in the unfractionated brain homogenate in Figure 1 to assess changes due to developmental age and arsenic exposure. In Figure 3 we measured total Gas5 levels in males and females in response to arsenic. Here we assessed the levels of Gas5 in the nuclear fraction to determine if there were differences in the nuclear availability of Gas5, which, in turn, could alter GR transcriptional regulation (Figure 5). In these studies, we assessed only GD14 and GD18, as these two developmental time points revealed the most robust sex differences in response to arsenic exposure.
Figure 5.
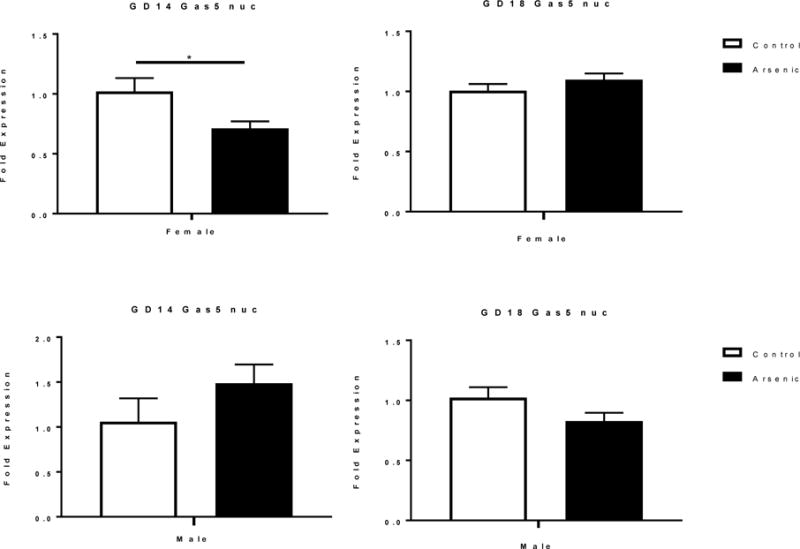
The effect of prenatal arsenic on nuclear levels of Gas5 expression assessed by qPCR in GD14 (left column) GD18 (right column) in arsenic (filled bars) and control (open bars) female (top row) and male (bottom row) nuclear fraction. Fold expression of nuclear Gas5 was acquired by the ΔΔCt (Comparative CT) method. Data are mean ± SEM, n=5–7, *p< .05.
At GD14 there was no overall treatment effect on the levels of nuclear Gas5 [F(1,12)=.114, p=.741] but there was an overall sex effect [F(1,12)= 5.236, p=.041] and a trend towards a treatment by sex interaction [F(1,12)= 4.291, p= .061]. Post hoc analysis showed a significant decrease in nuclear Gas5 levels among arsenic-exposed females. There was a non-significant trend of increased Gas5 levels in the arsenic-exposed male nuclear fraction.
At GD18 there was no significant treatment effect [F(1,20)= 0.525, p=.477] and no sex effect [F(1,20)= 3.22, p= .088], while the treatment by sex interaction approached significance [F(1,20)= 4.101, p=.056]. This was likely due to a trend toward decreasing nuclear Gas5 levels in the arsenic-exposed males.
RIP analysis of the Interaction between nuclear Gas5 and GR
Gas5 plays a key role in the regulation of GR-dependent transcription [39,45,80]. It is possible that arsenic regulates GR transcriptional activity by controlling the level of free (uncomplexed) nuclear GR. To explore this possibility, we preformed an RNA binding protein co-immunoprecipitation (RIP) assay (Figure 6) in which we immunopercipitated the GR and quantified the amount of Gas5 bound. The results from the nuclear Gas5 determinations (Figure 5) were critical to the interpretation of the RIP data, thus we only assessed GD14 and GD18 developmental time points.
Figure 6.
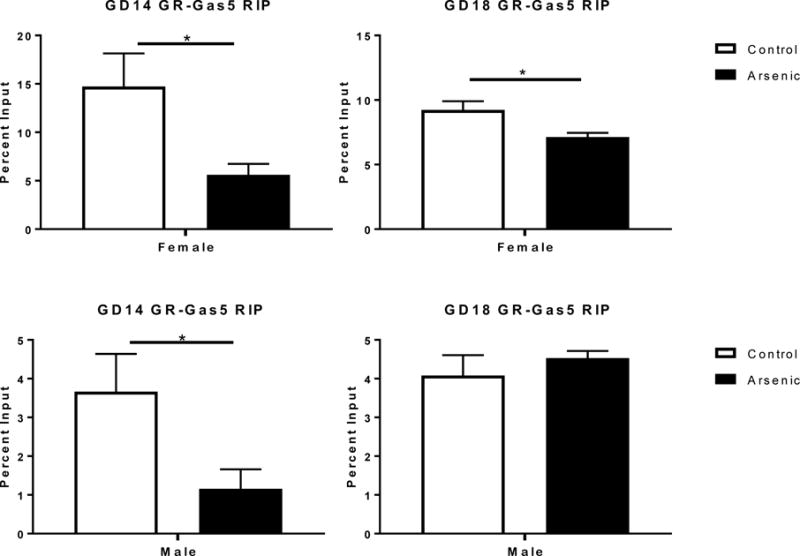
The effect of prenatal arsenic on the percent input levels of Gas5 bound to GR by qPCR following RNA immunoprecipitation from GD14 (left column) GD18 (right column) in arsenic (filled bars) and control (open bars) female (top row) and male (bottom row) in the nuclear fraction. Data are mean ± SEM, n=5–7, *p<.05.
A two-way ANOVA of the GD14 data revealed a significant overall effect of treatment [F(1,17)=10.471, p=.005], a significant effect of sex [F(1,17)=18.606, p=.0001] and no sex by treatment interaction [F(1,17)= 3.378, p=.084]. There was a significant decrease in Gas5-GR association following arsenic exposure in both male [t(9)= 2.41, p =.039] and female [t(8)= 2.53. p= .035] GD14 brains. Analysis of the GD18 data found no overall treatment effect [F(1,20)=3.150, p=.091], a significant effect of sex [F(1,20)= 69.492, p=.0001] and a significant sex by treatment interaction [F(1,20)= 7.504, p= .013]. The source of the significant sex by treatment interaction was a significant decrease in GR-associated Gas5 at GD 18 in the females [t(10)= 2.81, p=.019] exposed to arsenic, but not the males [t(10)= .801, p=.437]. The GD18 arsenic-exposed males had a trend towards an increase in bound Gas5. Finally, there was a 3-fold difference between the sexes at GD14 and a 2-fold difference at GD18 in the percentage of Gas5 input levels bound to the GR with females having a higher percentage of total Gas5 bound to GR.
Discussion
Males and females differ in their responses to a variety of both natural experiences and experimental manipulations across the lifespan. In general, the existing literature indicates that males are more sensitive to the effects of in utero experiences/exposures [17,18,21,23,62,73] and are more at risk for adverse pregnancy outcomes [20,78,87] than are females. However, there are reports that the effect of sex may be measure-specific [31,88] and that, for some exposures and outcomes, females are more sensitive than are males to alterations in the in utero environment [6,10,19,36,60]. In fact, a series of studies on the populations in Bangladesh found that in utero arsenic exposure produced significant associations of urinary arsenic and lower IQ and a reduction in physical growth in females but not in male school-aged children [32,72]. While our current studies do not assess cognitive function, in our previous work with the prenatal arsenic model we have found significant cognitive deficits in male offspring [52,53], and in our molecular work we have often found changes in males but not females exposed to prenatal arsenic [2,83,84], suggesting a protective effect of female sex in our low to moderate exposure mouse model. While the relationship between Gas5 and cognition is not known, our studies do demonstrate that, in fetal brain, prenatal exposure to arsenic selectively alters GR levels in males, while modifying total Gas5 and nuclear Gas5 association with GR in both males and females. Collectively, the data indicate that prenatal arsenic exposure was associated with reduced GR signaling potential in male fetal brain and increased GR signaling potential in female fetal brain (see below). Thus, we observed measure-specific, sex-dependent effects of prenatal arsenic exposure. In general, the results agree with our previous observations that glucocorticoid signaling is altered in male but not in female fetal placenta and brain [9].
In order to assess the implications of the findings from the RIP study, we applied a simplified model of reversible binding (interaction) of two molecules [43,93] with a stoichiometry of 1:1 for the binding of GR and Gas5 [39,45]. We assumed that arsenic exposure does not change the affinity (Kd, dissociation constant) of the GR•Gas5 complex. It is important to acknowledge that this is a limitation of our model and requires further research to support the assumption. The model is described by the equation:
Applying this model to the findings from the measurements of nuclear GR (Figure 2; GRtotal), nuclear Gas5 (Figure 5; Gas5total) and nuclear GR•Gas5 complex, we developed the following conclusions. In the GD14 females, the findings of unaltered nuclear GR and reduced nuclear Gas5 predicted a decreased interaction of GR and Gas5, which was found in the RIP assay (Figure 6). Because GRtotal was not changed by arsenic exposure, the decreased level of GR•Gas5 will increase the level of GRfree, thus potentially increasing GR signaling. DISCUSS EFFECTS ON NUCLEAR FREE Gas5 In the GD14 males, nuclear GR levels were reduced while Gas5 levels were unchanged, predicting a decreased interaction of GR and Gas5, which was found in the RIP study. The reduction in the level of the GR•Gas5 complex increases the fraction of the GR pool that is free (uncomplexed) in the arsenic-exposed males, since GRtotal = GRfree + GRbound. However, a relatively large percentage of nuclear GR would need to be bound to Gas5 in control animals for an arsenic-dependent increase in free GR to compensate for the ~40% decrease in total GR that was measured in the arsenic-exposed mice. Although the present studies did not assess the percentage of nuclear GRs bound to Gas5, it seems likely that, relative to GRfree levels in control mice, GRfree levels, and, consequently, nuclear GR signaling potential, are reduced in the GD14 arsenic-exposed male brain. DISCUSS EFFECTS ON NUCLEAR FREE Gas5 In the GD18 females, GRtotal and Gas5total levels were unaltered, predicting that GR binding to Gas5 was not changed. However, the reduced GR•Gas5 binding detected in the RIP assay indicates that a smaller percentage of the Gas5 pool is complexed with GR, effectively increasing the percentage of GR that is free. Combined with the trend (p=.095) toward an increased level of GR, the data indicate that nuclear GR signaling is increased in the GD18 female arsenic-exposed brain. DISCUSS EFFECTS ON NUCLEAR FREE Gas5 Finally, in the GD18 male, the reduced GRtotal detected in the presence of an unaltered level of Gas5 predicted a decreased in GR•Gas5 interaction in the RIP assay. However, we found no change in GR•Gas5 binding, indicating that GRfree is reduced in the arsenic-exposed mice. Thus, we conclude that nuclear GR signaling is reduced in the GD18 males. DISCUSS EFFECTS ON NUCLEAR FREE Gas5.
Alternatively, the RIP assay findings can be interpreted in light of the reported interaction of arsenic with the four cysteine (C4) zinc finger DNA-binding domain of the GR [3], which blocks the binding of the receptor to GREs in hormone-responsive genes [29]. If arsenic competes with Gas5 for binding to the GR in vivo, GR-associated Gas5 levels detected in RIP samples prepared from prenatal arsenic-exposed animals would be predicted to be reduced, as some GRs would be bound to arsenic rather than Gas5, assuming no compensatory changes in GR or Gas5 levels or the affinity of the GR•Gas5 interaction. This also assumes that arsenic is present in the nuclear lysates used in the RIP assay, thus maintaining the predicted reduction in GR•Gas5 binding during immunoprecipitation of the GR complex. Importantly, the observed reduction in the association of GR with Gas5 would not indicate that “free” (uncomplexed) GR levels are increased, as predicted by the kinetic model. In fact, “free” GR levels could be further decreased, if arsenic is bound to receptors in addition to those displaced from Gas5. While the findings in the E14 male, E14 female and E18 female fetal brain RIP assays are consistent with an arsenic-mediated reduction in GR-Gas5 association, the finding of unaltered GR-Gas5 association in E18 males is not. If increases in “free” (not bound to Gas5) GR predicted by the RIP assay for GD14 males do not represent GRs that are available for regulation of gene transcription, then the reduced level of nuclear GRs indicates that GR signaling potential is reduced in these animals. If in the E14 and E18 females, the predicted increase in non-Gas5-bound GR simply represents GRs bound to arsenic, rather than uncomplexed GRs, then GR signaling potential would not be altered in these animals relative to control, since nuclear total levels were not changed. In the E18 males, the reduction in nuclear total GR and lack of change in Gas5-associated GR, combined with the expectation that some GRs are bound to arsenic, indicates that uncomplexed GR available for signaling is reduced in these animals.
Based on the above considerations, we propose that nuclear GR signaling potential is decreased in GD14 and GD18 prenatal arsenic-exposed males, while it is increased or maintained at levels approaching normal in GD14 and GD18 prenatal arsenic-exposed females. It is worth noting that the increase in the level of GR that is not bound to Gas5 predicted by the kinetic model will act to compensate for GRs that are bound by arsenic.
It is likely that the opposite effects of prenatal arsenic exposure on GR signaling in male and female fetal brain exert opposite effects on numerous developmental processes. GRs play a critical role in developmental programming of the HPA axis [69]. Sex-dependent differences in in utero programming of the HPA axis have been reported [11,36,54,63,79,92]. Thus, future studies could assess the role of the proposed changes in development of the HPA feedback control.
Alterations in GR levels and distribution in the fetal brain may be the result of direct effects of arsenic on the fetus or secondary to effects of arsenic on the pregnant dam. We have previously reported that arsenic consumption does not alter maternal corticosterone levels [9]. Further, we have reported that placental 11β-hydroxysteroid dehydrogenase 2 (11β-HSD2), which converts biologically active corticosterone (cortisol in humans) into inactive 11-dehydrocorticosterone (cortisone in humans), levels are increased in the GD14, but not GD18, male placenta [9]. These previous findings suggest that the effects of arsenic on the fetal brain found in the present studies are unlikely to be mediated by effects of the toxin on transplacental passage of corticosterone, although there may be reduced passage of maternal corticosterone to the GD14 male fetus. The more likely case is that the effects of arsenic are due to direct effects on the fetus.
The mechanisms underlying the effects of arsenic on GR and Gas5 levels and interactions will be areas of future study. We have reported that perinatal exposure to arsenic is associated with epigenetic modifications in adult offspring. Sex-dependent and region-dependent changes in global histone methylation (histone 3 lysine 4 trimethyl) and acetylation (histone 3 lysine 9 acetyl), as well as the levels of enzymes (histone methyltranferase MLL, histone demethylase KDM5B, histone acetylases GCN5 and PCAF, and histone deacetylases HDAC1 and HDAC2) that control these histone modifications, were found in adult offspring exposed to arsenic perinatally [83]. GR/Nr3c1 levels have been reported to be regulated by histone modifications [5,89,90]. A recent study by Marsit’s group found arsenic, as well as other heavy metals, increased the methylation of NR3C1 (GR gene) in human placental tissues [4]. Thus, epigenetic mechanisms provide targets for studies aiming to identify the sex- and gestational age-dependent effects of arsenic on GRs that we report.
We have reported reduced levels of microRNA-9, -9* and -124 in GD14 male, but not female, brain during prenatal arsenic exposure; no effects of prenatal arsenic exposure were seen in GD18 male or female brain [84]. GR expression has been reported to regulated by miR-124 [46]. In addition to the regulation of Gas5 levels by miR-21 noted above, miR-222 also has been shown to inhibit Gas5 expression as the result of binding to Gas5 [96]. Finally, Gas5 levels are increased in response to growth arrest [25,48,80], likely as the result of inhibition of the degradation of Gas5 by the nonsense-mediated RNA decay pathway [40,59,80]. Thus, treatments that target miRs or nonsense-mediated RNA decay pathways would be predicted to modify cellular GR and Gas5 levels.
Conclusions
We report measure-specific and sex-dependent effects of prenatal arsenic exposure in GD14, GD16 and GD18 brain. The findings indicate that prenatal arsenic exposure reduces GR regulation of gene expression in GD14 and GD18 male brain, while increasing regulation of gene expression in GD14 and GD18 female brain. Defining the relationships between these findings and altered programming of the HPA axis and the long-term consequences of in utero exposure to arsenic are areas of active research in our laboratories.
Highlights.
Long noncoding RNA Growth arrest specific transcript (Gas5) binds to GR
Gas5 nuclear levels may regulate GR mediated gene transcription
Prenatal arsenic (50ppb) alters brain Gas5 levels in an age and sex-dependent manner.
Acknowledgments
This work was supported by a grant from the National Institute of Environmental Health Sciences 2RO1ES019583 to AMA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahmed S, Mahabbat-e Khoda S, Rekha RS, Gardner RM, Ameer SS, Moore S, Ekstrom EC, Vahter M, Raqib R. Arsenic-associated oxidative stress, inflammation, and immune disruption in human placenta and cord blood. Environ Health Perspect. 2011;119:258–64. doi: 10.1289/ehp.1002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allan AM, Hafez AK, Labrecque MT, Solomon ER, Shaikh MN, Zheng X, Ali A. Sex-Dependent effects of developmental arsenic exposure on methylation capacity and methylation regulation of the glucocorticoid receptor system in the embryonic mouse brain. Toxicol Rep. 2015;2:1376–1390. doi: 10.1016/j.toxrep.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anzellotti AI, Farrell NP. Zinc metalloproteins as medicinal targets. Chem Soc Rev. 2008;37:1629–51. doi: 10.1039/b617121b. [DOI] [PubMed] [Google Scholar]
- 4.Appleton AA, Jackson BP, Karagas M, Marsit CJ. Prenatal exposure to neurotoxic metals is associated with increased placental glucocorticoid receptor DNA methylation. Epigenetics. 2017:0. doi: 10.1080/15592294.2017.1320637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Begum G, Davies A, Stevens A, Oliver M, Jaquiery A, Challis J, Harding J, Bloomfield F, White A. Maternal undernutrition programs tissue-specific epigenetic changes in the glucocorticoid receptor in adult offspring. Endocrinology. 2013;154:4560–9. doi: 10.1210/en.2013-1693. [DOI] [PubMed] [Google Scholar]
- 6.Brunton PJ, Sullivan KM, Kerrigan D, Russell JA, Seckl JR, Drake AJ. Sex-specific effects of prenatal stress on glucose homoeostasis and peripheral metabolism in rats. J Endocrinol. 2013;217:161–73. doi: 10.1530/JOE-12-0540. [DOI] [PubMed] [Google Scholar]
- 7.Buckley CT, Caldwell KK. Two-layer antibody capture of enzymes on the surface of microtiter plates: application to the study of the regulation of phospholipase C-gamma1 catalytic activity. Anal Biochem. 2003;320:193–8. doi: 10.1016/s0003-2697(03)00394-4. [DOI] [PubMed] [Google Scholar]
- 8.Cabili MN, Dunagin MC, McClanahan PD, Biaesch A, Padovan-Merhar O, Regev A, Rinn JL, Raj A. Localization and abundance analysis of human lncRNAs at single-cell and single-molecule resolution. Genome Biol. 2015;16:20. doi: 10.1186/s13059-015-0586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caldwell KE, Labrecque MT, Solomon BR, Ali A, Allan AM. Prenatal arsenic exposure alters the programming of the glucocorticoid signaling system during embryonic development. Neurotoxicol Teratol. 2015;47:66–79. doi: 10.1016/j.ntt.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao-Lei L, de Rooij SR, King S, Matthews SG, Metz GAS, Roseboom TJ, Szyf M. Prenatal stress and epigenetics. Neurosci Biobehav Rev. 2017 doi: 10.1016/j.neubiorev.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Carpenter T, Grecian SM, Reynolds RM. Sex differences in early-life programming of the hypothalamic-pituitary-adrenal axis in humans suggest increased vulnerability in females: a systematic review. J Dev Orig Health Dis. 2017;8:244–255. doi: 10.1017/S204017441600074X. [DOI] [PubMed] [Google Scholar]
- 12.Carroll CR, Noonan C, Garroutte EM, Navas-Acien A, Verney SP, Buchwald D. Low-level inorganic arsenic exposure and neuropsychological functioning in American Indian elders. Environ Res. 2017;156:74–79. doi: 10.1016/j.envres.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J, Xue Y. Emerging roles of non-coding RNAs in epigenetic regulation. Sci China Life Sci. 2016;59:227–35. doi: 10.1007/s11427-016-5010-0. [DOI] [PubMed] [Google Scholar]
- 14.Chen LL. Linking Long Noncoding RNA Localization and Function. Trends Biochem Sci. 2016;41:761–72. doi: 10.1016/j.tibs.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Coccia EM, Cicala C, Charlesworth A, Ciccarelli C, Rossi GB, Philipson L, Sorrentino V. Regulation and expression of a growth arrest-specific gene (gas5) during growth, differentiation, and development. Mol Cell Biol. 1992;12:3514–21. doi: 10.1128/mcb.12.8.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen SM. Everything old is new again: (linc)RNAs make proteins! EMBO J. 2014;33:937–8. doi: 10.1002/embj.201488303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuffe JS, O’Sullivan L, Simmons DG, Anderson ST, Moritz KM. Maternal corticosterone exposure in the mouse has sex-specific effects on placental growth and mRNA expression. Endocrinology. 2012;153:5500–11. doi: 10.1210/en.2012-1479. [DOI] [PubMed] [Google Scholar]
- 18.Cuffe JS, Turton EL, Akison LK, Bielefeldt-Ohmann H, Moritz KM. Prenatal corticosterone exposure programs sex-specific adrenal adaptations in mouse offspring. J Endocrinol. 2017;232:37–48. doi: 10.1530/JOE-16-0417. [DOI] [PubMed] [Google Scholar]
- 19.Cuffe JS, Walton SL, Singh RR, Spiers JG, Bielefeldt-Ohmann H, Wilkinson L, Little MH, Moritz KM. Mid- to late term hypoxia in the mouse alters placental morphology, glucocorticoid regulatory pathways and nutrient transporters in a sex-specific manner. J Physiol. 2014;592:3127–41. doi: 10.1113/jphysiol.2014.272856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Renzo GC, Rosati A, Sarti RD, Cruciani L, Cutuli AM. Does fetal sex affect pregnancy outcome? Gend Med. 2007;4:19–30. doi: 10.1016/s1550-8579(07)80004-0. [DOI] [PubMed] [Google Scholar]
- 21.DiPietro JA, Voegtline KM. The gestational foundation of sex differences in development and vulnerability. Neuroscience. 2017;342:4–20. doi: 10.1016/j.neuroscience.2015.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards M, Hall J, Gong G, O’Bryant SE. Arsenic exposure, AS3MT polymorphism, and neuropsychological functioning among rural dwelling adults and elders: a cross-sectional study. Environ Health. 2014;13:15. doi: 10.1186/1476-069X-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJ. Boys live dangerously in the womb. Am J Hum Biol. 2010;22:330–5. doi: 10.1002/ajhb.20995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleming JV, Fontanier N, Harries DN, Rees WD. The growth arrest genes gas5, gas6, and CHOP-10 (gadd153) are expressed in the mouse preimplantation embryo. Mol Reprod Dev. 1997;48:310–6. doi: 10.1002/(SICI)1098-2795(199711)48:3<310::AID-MRD2>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 25.Fleming JV, Hay SM, Harries DN, Rees WD. Effects of nutrient deprivation and differentiation on the expression of growth-arrest genes (gas and gadd) in F9 embryonal carcinoma cells. Biochem J. 1998;330(Pt 1):573–9. doi: 10.1042/bj3300573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goggin SL, Labrecque MT, Allan AM. Perinatal exposure to 50 ppb sodium arsenate induces hypothalamic-pituitary-adrenal axis dysregulation in male C57BL/6 mice. Neurotoxicology. 2012;33:1338–45. doi: 10.1016/j.neuro.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong G, Hargrave KA, Hobson V, Spallholz J, Boylan M, Lefforge D, O’Bryant SE. Low-level groundwater arsenic exposure impacts cognition: a project FRONTIER study. J Environ Health. 2011;74:16–22. [PubMed] [Google Scholar]
- 28.Gonzalez H, Lema C, Kirken RA, Maldonado RA, Varela-Ramirez A, Aguilera RJ. Arsenic-exposed Keratinocytes Exhibit Differential microRNAs Expression Profile; Potential Implication of miR-21, miR-200a and miR-141 in Melanoma Pathway. Clin Cancer Drugs. 2015;2:138–147. doi: 10.2174/2212697X02666150629174704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gosse JA, Taylor VF, Jackson BP, Hamilton JW, Bodwell JE. Monomethylated trivalent arsenic species disrupt steroid receptor interactions with their DNA response elements at non-cytotoxic cellular concentrations. J Appl Toxicol. 2014;34:498–505. doi: 10.1002/jat.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo X, Deng K, Wang H, Xia J, Shan T, Liang Z, Yao L, Jin S. GAS5 Inhibits Gastric Cancer Cell Proliferation Partly by Modulating CDK6. Oncol Res Treat. 2015;38:362–6. doi: 10.1159/000433499. [DOI] [PubMed] [Google Scholar]
- 31.Haley DW, Handmaker NS, Lowe J. Infant stress reactivity and prenatal alcohol exposure. Alcohol Clin Exp Res. 2006;30:2055–64. doi: 10.1111/j.1530-0277.2006.00251.x. [DOI] [PubMed] [Google Scholar]
- 32.Hamadani JD, Tofail F, Nermell B, Gardner R, Shiraji S, Bottai M, Arifeen SE, Huda SN, Vahter M. Critical windows of exposure for arsenic-associated impairment of cognitive function in pre-school girls and boys: a population-based cohort study. Int J Epidemiol. 2011;40:1593–604. doi: 10.1093/ije/dyr176. [DOI] [PubMed] [Google Scholar]
- 33.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–31. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 34.He W, Greenwell RJ, Brooks DM, Calderon-Garciduenas L, Beall HD, Coffin JD. Arsenic exposure in pregnant mice disrupts placental vasculogenesis and causes spontaneous abortion. Toxicol Sci. 2007;99:244–53. doi: 10.1093/toxsci/kfm162. [DOI] [PubMed] [Google Scholar]
- 35.He X, Chen X, Zhang X, Duan X, Pan T, Hu Q, Zhang Y, Zhong F, Liu J, Zhang H, et al. An Lnc RNA (GAS5)/SnoRNA-derived piRNA induces activation of TRAIL gene by site-specifically recruiting MLL/COMPASS-like complexes. Nucleic Acids Res. 2015;43:3712–25. doi: 10.1093/nar/gkv214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hiroi R, Carbone DL, Zuloaga DG, Bimonte-Nelson HA, Handa RJ. Sex-dependent programming effects of prenatal glucocorticoid treatment on the developing serotonin system and stress-related behaviors in adulthood. Neuroscience. 2016;320:43–56. doi: 10.1016/j.neuroscience.2016.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsieh RL, Huang YL, Shiue HS, Huang SR, Lin MI, Mu SC, Chung CJ, Hsueh YM. Arsenic methylation capacity and developmental delay in preschool children in Taiwan. Int J Hyg Environ Health. 2014;217:678–86. doi: 10.1016/j.ijheh.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Huang CC, Shih MC, Hsu NC, Chien Y, Chung BC. Fetal glucocorticoid synthesis is required for development of fetal adrenal medulla and hypothalamus feedback suppression. Endocrinology. 2012;153:4749–56. doi: 10.1210/en.2012-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hudson WH, Pickard MR, de Vera IM, Kuiper EG, Mourtada-Maarabouni M, Conn GL, Kojetin DJ, Williams GT, Ortlund EA. Conserved sequence-specific lincRNA-steroid receptor interactions drive transcriptional repression and direct cell fate. Nat Commun. 2014;5:5395. doi: 10.1038/ncomms6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ideue T, Sasaki YT, Hagiwara M, Hirose T. Introns play an essential role in splicing-dependent formation of the exon junction complex. Genes Dev. 2007;21:1993–8. doi: 10.1101/gad.1557907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnsson P, Lipovich L, Grander D, Morris KV. Evolutionary conservation of long non-coding RNAs; sequence, structure, function. Biochim Biophys Acta. 2014;1840:1063–71. doi: 10.1016/j.bbagen.2013.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kapranov P, Cheng J, Dike S, Nix DA, Duttagupta R, Willingham AT, Stadler PF, Hertel J, Hackermuller J, Hofacker IL, et al. RNA maps reveal new RNA classes and a possible function for pervasive transcription. Science. 2007;316:1484–8. doi: 10.1126/science.1138341. [DOI] [PubMed] [Google Scholar]
- 43.Kastritis PL, Bonvin AM. On the binding affinity of macromolecular interactions: daring to ask why proteins interact. J R Soc Interface. 2013;10:20120835. doi: 10.1098/rsif.2012.0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kino T. Stress, glucocorticoid hormones, and hippocampal neural progenitor cells: implications to mood disorders. Front Physiol. 2015;6:230. doi: 10.3389/fphys.2015.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kino T, Hurt DE, Ichijo T, Nader N, Chrousos GP. Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci Signal. 2010;3:ra8. doi: 10.1126/scisignal.2000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ledderose C, Mohnle P, Limbeck E, Schutz S, Weis F, Rink J, Briegel J, Kreth S. Corticosteroid resistance in sepsis is influenced by microRNA-124–induced downregulation of glucocorticoid receptor-alpha. Crit Care Med. 2012;40:2745–53. doi: 10.1097/CCM.0b013e31825b8ebc. [DOI] [PubMed] [Google Scholar]
- 47.Li X, Shi Y, Wei Y, Ma X, Li Y, Li R. Altered expression profiles of microRNAs upon arsenic exposure of human umbilical vein endothelial cells. Environ Toxicol Pharmacol. 2012;34:381–387. doi: 10.1016/j.etap.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 48.Liu Y, Zhao J, Zhang W, Gan J, Hu C, Huang G, Zhang Y. lncRNA GAS5 enhances G1 cell cycle arrest via binding to YBX1 to regulate p21 expression in stomach cancer. Sci Rep. 2015;5:10159. doi: 10.1038/srep10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lucafo M, De Iudicibus S, Di Silvestre A, Pelin M, Candussio L, Martelossi S, Tommasini A, Piscianz E, Ventura A, Decorti G. Long noncoding RNA GAS5: a novel marker involved in glucocorticoid response. Curr Mol Med. 2015;15:94–9. doi: 10.2174/1566524015666150114122354. [DOI] [PubMed] [Google Scholar]
- 50.Maccari S, Krugers HJ, Morley-Fletcher S, Szyf M, Brunton PJ. The consequences of early-life adversity: neurobiological, behavioural and epigenetic adaptations. J Neuroendocrinol. 2014;26:707–23. doi: 10.1111/jne.12175. [DOI] [PubMed] [Google Scholar]
- 51.Makarewich CA, Olson EN. Mining for Micropeptides. Trends Cell Biol. 2017 doi: 10.1016/j.tcb.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martinez-Finley EJ, Ali AM, Allan AM. Learning deficits in C57BL/6J mice following perinatal arsenic exposure: consequence of lower corticosterone receptor levels? Pharmacol Biochem Behav. 2009;94:271–7. doi: 10.1016/j.pbb.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinez EJ, Kolb BL, Bell A, Savage DD, Allan AM. Moderate perinatal arsenic exposure alters neuroendocrine markers associated with depression and increases depressive-like behaviors in adult mouse offspring. Neurotoxicology. 2008;29:647–55. doi: 10.1016/j.neuro.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCormick CM, Smythe JW, Sharma S, Meaney MJ. Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain Res Dev Brain Res. 1995;84:55–61. doi: 10.1016/0165-3806(94)00153-q. [DOI] [PubMed] [Google Scholar]
- 55.Meier I, Fellini L, Jakovcevski M, Schachner M, Morellini F. Expression of the snoRNA host gene gas5 in the hippocampus is upregulated by age and psychogenic stress and correlates with reduced novelty-induced behavior in C57BL/6 mice. Hippocampus. 2010;20:1027–36. doi: 10.1002/hipo.20701. [DOI] [PubMed] [Google Scholar]
- 56.Mineno J, Okamoto S, Ando T, Sato M, Chono H, Izu H, Takayama M, Asada K, Mirochnitchenko O, Inouye M, et al. The expression profile of microRNAs in mouse embryos. Nucleic Acids Res. 2006;34:1765–71. doi: 10.1093/nar/gkl096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mourtada-Maarabouni M, Hedge VL, Kirkham L, Farzaneh F, Williams GT. Growth arrest in human T-cells is controlled by the non-coding RNA growth-arrest-specific transcript 5 (GAS5) J Cell Sci. 2008;121:939–46. doi: 10.1242/jcs.024646. [DOI] [PubMed] [Google Scholar]
- 58.Mourtada-Maarabouni M, Pickard MR, Hedge VL, Farzaneh F, Williams GT. GAS5, a non-protein-coding RNA, controls apoptosis and is downregulated in breast cancer. Oncogene. 2009;28:195–208. doi: 10.1038/onc.2008.373. [DOI] [PubMed] [Google Scholar]
- 59.Mourtada-Maarabouni M, Williams GT. Growth arrest on inhibition of nonsense-mediated decay is mediated by noncoding RNA GAS5. Biomed Res Int. 2013;2013:358015. doi: 10.1155/2013/358015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murphy VE, Gibson PG, Giles WB, Zakar T, Smith R, Bisits AM, Kessell CG, Clifton VL. Maternal asthma is associated with reduced female fetal growth. Am J Respir Crit Care Med. 2003;168:1317–23. doi: 10.1164/rccm.200303-374OC. [DOI] [PubMed] [Google Scholar]
- 61.Nemoda Z, Szyf M. Epigenetic Alterations and Prenatal Maternal Depression. Birth Defects Res. 2017;109:888–897. doi: 10.1002/bdr2.1081. [DOI] [PubMed] [Google Scholar]
- 62.O’Sullivan L, Cuffe JS, Koning A, Singh RR, Paravicini TM, Moritz KM. Excess prenatal corticosterone exposure results in albuminuria, sex-specific hypotension, and altered heart rate responses to restraint stress in aged adult mice. Am J Physiol Renal Physiol. 2015;308:F1065–73. doi: 10.1152/ajprenal.00676.2014. [DOI] [PubMed] [Google Scholar]
- 63.Ohkawa T, Rohde W, Takeshita S, Dorner G, Arai K, Okinaga S. Effect of an acute maternal stress on the fetal hypothalamo-pituitary-adrenal system in late gestational life of the rat. Exp Clin Endocrinol. 1991;98:123–9. doi: 10.1055/s-0029-1211108. [DOI] [PubMed] [Google Scholar]
- 64.Parajuli RP, Umezaki M, Fujiwara T, Watanabe C. Association of cord blood levels of lead, arsenic, and zinc and home environment with children neurodevelopment at 36 months living in Chitwan Valley, Nepal. PLoS One. 2015;10:e0120992. doi: 10.1371/journal.pone.0120992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Perrone-Bizzozero NI, Sower AC, Bird ED, Benowitz LI, Ivins KJ, Neve RL. Levels of the growth-associated protein GAP-43 are selectively increased in association cortices in schizophrenia. Proc Natl Acad Sci U S A. 1996;93:14182–7. doi: 10.1073/pnas.93.24.14182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pickard MR, Mourtada-Maarabouni M, Williams GT. Long non-coding RNA GAS5 regulates apoptosis in prostate cancer cell lines. Biochim Biophys Acta. 2013;1832:1613–23. doi: 10.1016/j.bbadis.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 67.Polajeva J, Swartling FJ, Jiang Y, Singh U, Pietras K, Uhrbom L, Westermark B, Roswall P. miRNA-21 is developmentally regulated in mouse brain and is co-expressed with SOX2 in glioma. BMC Cancer. 2012;12:378. doi: 10.1186/1471-2407-12-378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pratheeshkumar P, Son YO, Divya SP, Wang L, Zhang Z, Shi X. Oncogenic transformation of human lung bronchial epithelial cells induced by arsenic involves ROS-dependent activation of STAT3-miR-21-PDCD4 mechanism. Sci Rep. 2016;6:37227. doi: 10.1038/srep37227. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Reichardt HM, Schutz G. Feedback control of glucocorticoid production is established during fetal development. Mol Med. 1996;2:735–44. [PMC free article] [PubMed] [Google Scholar]
- 70.Rodrigues EG, Bellinger DC, Valeri L, Hasan MO, Quamruzzaman Q, Golam M, Kile ML, Christiani DC, Wright RO, Mazumdar M. Neurodevelopmental outcomes among 2- to 3-year-old children in Bangladesh with elevated blood lead and exposure to arsenic and manganese in drinking water. Environ Health. 2016;15:44. doi: 10.1186/s12940-016-0127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roy A, Kordas K, Lopez P, Rosado JL, Cebrian ME, Vargas GG, Ronquillo D, Stoltzfus RJ. Association between arsenic exposure and behavior among first-graders from Torreon, Mexico. Environ Res. 2011;111:670–6. doi: 10.1016/j.envres.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 72.Saha KK, Engstrom A, Hamadani JD, Tofail F, Rasmussen KM, Vahter M. Pre- and postnatal arsenic exposure and body size to 2 years of age: a cohort study in rural Bangladesh. Environ Health Perspect. 2012;120:1208–14. doi: 10.1289/ehp.1003378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sakamoto M, Nakano A, Akagi H. Declining Minamata male birth ratio associated with increased male fetal death due to heavy methylmercury pollution. Environ Res. 2001;87:92–8. doi: 10.1006/enrs.2001.4293. [DOI] [PubMed] [Google Scholar]
- 74.Sanchez Y, Simon GP, Calvino E, de Blas E, Aller P. Curcumin stimulates reactive oxygen species production and potentiates apoptosis induction by the antitumor drugs arsenic trioxide and lonidamine in human myeloid leukemia cell lines. J Pharmacol Exp Ther. 2010;335:114–23. doi: 10.1124/jpet.110.168344. [DOI] [PubMed] [Google Scholar]
- 75.Schneider C, King RM, Philipson L. Genes specifically expressed at growth arrest of mammalian cells. Cell. 1988;54:787–93. doi: 10.1016/s0092-8674(88)91065-3. [DOI] [PubMed] [Google Scholar]
- 76.Smith CM, Steitz JA. Classification of gas5 as a multi-small-nucleolar-RNA (snoRNA) host gene and a member of the 5′-terminal oligopyrimidine gene family reveals common features of snoRNA host genes. Mol Cell Biol. 1998;18:6897–909. doi: 10.1128/mcb.18.12.6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Speirs HJ, Seckl JR, Brown RW. Ontogeny of glucocorticoid receptor and 11beta-hydroxysteroid dehydrogenase type-1 gene expression identifies potential critical periods of glucocorticoid susceptibility during development. J Endocrinol. 2004;181:105–16. doi: 10.1677/joe.0.1810105. [DOI] [PubMed] [Google Scholar]
- 78.Stevenson DK, Verter J, Fanaroff AA, Oh W, Ehrenkranz RA, Shankaran S, Donovan EF, Wright LL, Lemons JA, Tyson JE, et al. Sex differences in outcomes of very low birthweight infants: the newborn male disadvantage. Arch Dis Child Fetal Neonatal Ed. 2000;83:F182–5. doi: 10.1136/fn.83.3.F182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Szuran TF, Pliska V, Pokorny J, Welzl H. Prenatal stress in rats: effects on plasma corticosterone, hippocampal glucocorticoid receptors, and maze performance. Physiol Behav. 2000;71:353–62. doi: 10.1016/s0031-9384(00)00351-6. [DOI] [PubMed] [Google Scholar]
- 80.Tani H, Torimura M, Akimitsu N. The RNA degradation pathway regulates the function of GAS5 a non-coding RNA in mammalian cells. PLoS One. 2013;8:e55684. doi: 10.1371/journal.pone.0055684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tyler CR, Allan AM. Adult hippocampal neurogenesis and mRNA expression are altered by perinatal arsenic exposure in mice and restored by brief exposure to enrichment. PLoS One. 2013;8:e73720. doi: 10.1371/journal.pone.0073720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tyler CR, Allan AM. The Effects of Arsenic Exposure on Neurological and Cognitive Dysfunction in Human and Rodent Studies: A Review. Curr Environ Health Rep. 2014;1:132–147. doi: 10.1007/s40572-014-0012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tyler CR, Hafez AK, Solomon ER, Allan AM. Developmental exposure to 50 parts-per-billion arsenic influences histone modifications and associated epigenetic machinery in a region- and sex-specific manner in the adult mouse brain. Toxicol Appl Pharmacol. 2015;288:40–51. doi: 10.1016/j.taap.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tyler CR, Labrecque MT, Solomon ER, Guo X, Allan AM. Prenatal arsenic exposure alters REST/NRSF and microRNA regulators of embryonic neural stem cell fate in a sex-dependent manner. Neurotoxicol Teratol. 2017;59:1–15. doi: 10.1016/j.ntt.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tyler CR, Solomon BR, Ulibarri AL, Allan AM. Fluoxetine treatment ameliorates depression induced by perinatal arsenic exposure via a neurogenic mechanism. Neurotoxicology. 2014;44:98–109. doi: 10.1016/j.neuro.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vahter M. Effects of arsenic on maternal and fetal health. Annu Rev Nutr. 2009;29:381–99. doi: 10.1146/annurev-nutr-080508-141102. [DOI] [PubMed] [Google Scholar]
- 87.Vatten LJ, Skjaerven R. Offspring sex and pregnancy outcome by length of gestation. Early Hum Dev. 2004;76:47–54. doi: 10.1016/j.earlhumdev.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 88.Verburg PE, Tucker G, Scheil W, Erwich JJ, Dekker GA, Roberts CT. Sexual Dimorphism in Adverse Pregnancy Outcomes - A Retrospective Australian Population Study 1981–2011. PLoS One. 2016;11:e0158807. doi: 10.1371/journal.pone.0158807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 90.Weaver IC, Diorio J, Seckl JR, Szyf M, Meaney MJ. Early environmental regulation of hippocampal glucocorticoid receptor gene expression: characterization of intracellular mediators and potential genomic target sites. Ann N Y Acad Sci. 2004;1024:182–212. doi: 10.1196/annals.1321.099. [DOI] [PubMed] [Google Scholar]
- 91.Webster JC, Huber RM, Hanson RL, Collier PM, Haws TF, Mills JK, Burn TC, Allegretto EA. Dexamethasone and tumor necrosis factor-alpha act together to induce the cellular inhibitor of apoptosis-2 gene and prevent apoptosis in a variety of cell types. Endocrinology. 2002;143:3866–74. doi: 10.1210/en.2002-220188. [DOI] [PubMed] [Google Scholar]
- 92.Weinstock M, Matlina E, Maor GI, Rosen H, McEwen BS. Prenatal stress selectively alters the reactivity of the hypothalamic-pituitary adrenal system in the female rat. Brain Res. 1992;595:195–200. doi: 10.1016/0006-8993(92)91049-k. [DOI] [PubMed] [Google Scholar]
- 93.Wilkinson KD. Quantitative analysis of protein-protein interactions. Methods Mol Biol. 2004;261:15–32. doi: 10.1385/1-59259-762-9:015. [DOI] [PubMed] [Google Scholar]
- 94.Wolf OT, Atsak P, de Quervain DJ, Roozendaal B, Wingenfeld K. Stress and Memory: A Selective Review on Recent Developments in the Understanding of Stress Hormone Effects on Memory and Their Clinical Relevance. J Neuroendocrinol. 2016;28 doi: 10.1111/jne.12353. [DOI] [PubMed] [Google Scholar]
- 95.Yorifuji T, Kato T, Ohta H, Bellinger DC, Matsuoka K, Grandjean P. Neurological and neuropsychological functions in adults with a history of developmental arsenic poisoning from contaminated milk powder. Neurotoxicol Teratol. 2016;53:75–80. doi: 10.1016/j.ntt.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 96.Yu F, Zheng J, Mao Y, Dong P, Lu Z, Li G, Guo C, Liu Z, Fan X. Long Non-coding RNA Growth Arrest-specific Transcript 5 (GAS5) Inhibits Liver Fibrogenesis through a Mechanism of Competing Endogenous RNA. J Biol Chem. 2015;290:28286–98. doi: 10.1074/jbc.M115.683813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang Z, Zhu Z, Watabe K, Zhang X, Bai C, Xu M, Wu F, Mo YY. Negative regulation of lncRNA GAS5 by miR-21. Cell Death Differ. 2013;20:1558–68. doi: 10.1038/cdd.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zierold KM, Knobeloch L, Anderson H. Prevalence of chronic diseases in adults exposed to arsenic-contaminated drinking water. Am J Public Health. 2004;94:1936–7. doi: 10.2105/ajph.94.11.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]


