Abstract
Background
Cannula and pump positions are associated with clinical outcomes such as device thrombosis in patients with HeartMate II; however, clinical implications of HVAD (HeartWare International, Framingham, Massachusetts) cannula position are unknown. This study aims to assess the relationship among cannula position, left ventricular (LV) unloading, and patient prognosis.
Methods and results
Twenty-seven HVAD patients (60.0 ± 12.6 years of age and 19 males [70%]) underwent ramp test. Device position was quantified from chest X-ray parameters obtained at the time of the hemodyamic ramp test: (1) cannula coronal angle, (2) pump depth, (3) cannula sagittal angle, and (4) pump area. Lower cannula coronal angle was associated with LV unloading (as measured by smaller LV diastolic dimension and lower pulmonary capillary wedge pressure). Smaller pump area was associated with LV dynamic unloading, as assessed by steeper negative slopes of LV diastolic dimension and pulmonary capillary wedge pressure during incremental rotational speed change. Cannula coronal angle ≤65° was associated with reduced heart failure readmission rate (hazard ratio, 10.33; P = .007 by log-rank test).
Conclusion
HVAD cannula and pump positions are associated with LV unloading and improved clinical outcomes. Prospective studies evaluating surgical techniques to ensure optimal device positioning and its effects on clinical outcomes are warranted.
Keywords: Ramp, hemodynamics, HVAD
Graphical abstract
Widely used left ventricular assist devices (LVADs) such as HVAD (HeartWare International, Framingham, Massachusetts) and HeartMate II (St Jude, Pleasanton, California) have improved survival rates in patients with stage D heart failure (HF).1,2 However, recurrent decompensated HF resulting from insufficient left ventricular (LV) unloading remains a common cause of readmission,3 despite adjustment of rotational speed setting and appropriate medical therapy.4
There has been recent focus on cannula and pump position as 1 of the major causes of device thrombosis with HeartMate II support,5–7 and a proper device insertion protocol with optimal positioning of cannula, pump and outflow graft have been proposed for better clinical outcomes.8,9
However, there are no studies demonstrating the clinical implications of device positioning of HVAD. Sufficient LV unloading is the key to preventing HF recurrence,10 and optimal device position may result in better LV unloading and improved patient prognosis. In this study, we investigate the clinical significance of device position on LV unloading in patients who underwent ramp test with HVAD support.
Methods
Patient Selection
HVAD patients enrolled into a prospective echocardiographic and hemodynamics database were screened. Patients with complete posteroanterior and lateral chest X-rays (CXRs) were enrolled. The study protocol was approved by the Ethics Committee at our institution. Written informed consent was obtained before enrollment. All patients received guideline-directed standard medical therapy during the study period, and rotational speed was optimized at the end of the ramp test.11 Patients suspicious for device malfunction were excluded.
Ramp Test Protocol
Hemodynamic and echocardiographic ramp tests were performed per the previously described protocol as part of routine clinical care.12 Serial measurements were performed starting with the patient’s baseline rotational speed, and again after the device was turned down to a minimal acceptable speed. Measurements were taken at progressively increased speeds of 100 rpm to a maximal value.
At the conclusion of the test, the attending cardiologist reviewed the results and the LVAD was adjusted to a set speed that best approached hemodynamic optimization (central venous pressure <12 mmHg and pulmonary capillary wedge pressure [PCWP] < 18 mmHg).
Radiographic Assessments
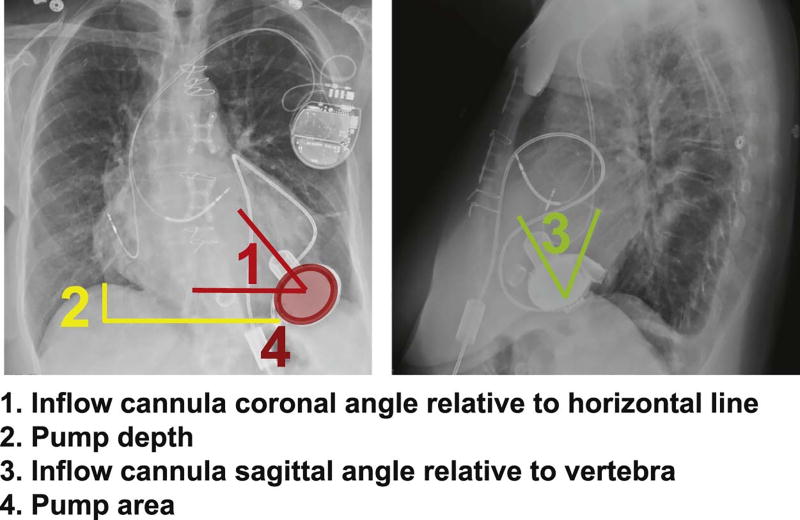
We used posteroanterior and lateral CXRs obtained after LVAD implantation and at the ramp test to measure cannula and pump positions. All CXRs were reviewed by 2 blinded experts and the measurements were averaged. Measurements taken for the study are summarized in Fig. 1. All radiographs were obtained in standing position, and confirmation of sternal wires directly overlying the vertebra in all cases was performed beforehand.
Fig. 1.
Definition of device position parameters measured in this study.
The cannula coronal angle was measured relative to a horizontal line in the posteroanterior view. The pump depth was measured from the dome of the right hemidiaphragm to the bottom of the pump body. The cannula sagittal angle was measured relative to the vertebral line in the lateral view. Ventral and dorsal angles were adopted equally. The pump area was calculated from the measurement of the lengths of the semiminor and semimajor axes of the pump in the posteroanterior view of the CXR by using a following formula: (semiminor axis length) · (semimajor axis length) · π.
Other Variables Evaluated
At the ramp test, background characteristics, and pump, hemodynamic, and echocardiographic data were obtained per protocol. Slopes parameters during rotational speed change were calculated by linear regression model.13 A positive slope indicates an increasing trend in the measured variable at incremental rotational speeds. All patients were followed at the set speed, and HF readmission was censored over a 1-year study period after the ramp test. The observational duration was counted from the time of the ramp test. HF events were defined as hospitalizations to treat volume overloaded and pulmonary congestion with intravenous diuretics. Isolated right HF was defined as a clinically assessed volume overload with a normal or reduced PCWP (<18 mmHg) and elevated central venous pressure (>12 mmHg). Other clinical outcomes including sucking, stroke, pump thrombosis, gastrointestinal bleeding, and driveline infection that needed admission were also counted. All events were counted and adjudicated by 2 independent researchers.
Statistical Analyses
All statistical analyses were performed using SPSS Statistics 22 (SPSS Inc, Chicago, Illinois). Continuous variables were expressed as mean ± standard deviation, unless otherwise indicated, and compared using the unpaired t test or Mann Whitney U test as appropriate. Categorical variables were compared using the chi-square test or Fisher exact test as appropriate. Interobserver variability in device position parameters was assessed by the Ebel intraclass correlation coefficient. Distribution of normality was assessed by the Shapiro-Wilk test. Variables that distribute non-normally were expressed as median and interquartile range. Device position parameters after operation and the ramp test were compared by Wilcoxon signed-rank test or paired t test as appropriate. Comparison of data among 3 groups was performed by the Tukey test when analyses of variance approached significance.
The endpoint of sufficient LV unloading was PCWP <18 mmHg at the set speed. Univariate logistic regression analyses were used for this endpoint among device position parameters. The endpoint of prognosis was HF readmission. Prognostic impact of device position parameters was analyzed by univariate Cox proportional hazards ratio regression analysis. In both analyses, continuous variables with P < .05 were transformed into dichotomous variables with cutoff values calculated by receiver operating characteristic analyses with the endpoint of patient prognosis. In both regression analyses, we did not perform multivariate analyses. Patient prognosis stratified by the cutoff value was assessed by Kaplan-Meier analyses and compared by log-rank test.
Results
Baseline Characteristics
Twenty-nine HVAD patients underwent ramp test. After exclusion of 2 patients because of a lack of CXR, 27 HVAD patients were enrolled (Table 1). Patients were 60.0 ± 12.6 years of age and 19 (70%) were male. The majority of patients were implanted as destination therapy (63%) and 16 (59%) had an ischemic etiology of their cardiomyopathy. The mean duration between time of HVAD implantation and ramp test was 479.0 ± 585.3 days.
Table 1.
Baseline Characteristics
| N = 27 | |
|---|---|
| Demographics | |
| Age, y | 60.0 ± 12.6 |
| Race, Caucasian | 19 (70%) |
| Body surface area, m2 | 1.91 ± 0.23 |
| Sex, male | 19 (70%) |
| Destination therapy | 17 (63%) |
| Ischemic etiology | 16 (59%) |
| Hypertension | 10 (37%) |
| Diabetes mellitus | 10 (37%) |
| Atrial fibrillation | 14 (52%) |
| History of ventricular tachyarrhythmia | 7 (26%) |
| COPD | 4 (15%) |
| Chronic kidney disease | 5 (19%) |
| Pump parameters at set speed | |
| Rotational speed, rpm | 2731.1 ± 168.2 |
| Power, watts | 4.10 ± 0.82 |
| Flow, L/min | 3.78 ± 0.92 |
| Pulsatility, L/min | 3.26 ± 1.02 |
| Echo and hemodynamic parameters at set speed | |
| LVDd, cm | 5.94 ± 0.76 |
| LVEF, % | 18.2 ± 8.3 |
| CVP, mmHg | 7.3 ± 4.3 |
| Mean PAP, mmHg | 24.5 ± 6.8 |
| PCWP, mmHg | 12.8 ± 5.7 |
| CI, L/min/m2 | 2.65 ± 0.42 |
| Medications at ramp test | |
| Beta-blocker | 15 (56%) |
| ACE or ARB | 17 (63%) |
| Diuretics | 21 (78%) |
| Aldosterone antagonist | 16 (59%) |
ACE, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CI, cardiac index; COPD, chronic obstructive pulmonary disorder; CVP, central venous pressure; LVDd, left ventricular diastolic diameter; LVEF, left ventricular ejection fraction; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure.
Device Position Parameters
Interrater reliabilities of device parameters were 0.999 for coronal angle, 0.998 for pump depth, 0.997 for sagittal angle, and 0.979 for pump area. Distributions of these 4 position parameters are shown in Supplementary Figure S1A–D. All parameters are non-normally distributed except for pump depth. Cannula coronal angle was 49.3 ± 32.2° and ranged between 6 and 104°. No patients had a negative coronal angle, whereas 1 had a >90° coronal angle. All patients were stratified into 3 groups by using tertiles of each parameter (Supplementary Figure S1A–D).
There was no significant difference in device position parameters between postoperation and ramp test CXRs (Supplemental Table S1; P > .05 for all).
Device Position and LV Unloading at Set Speed
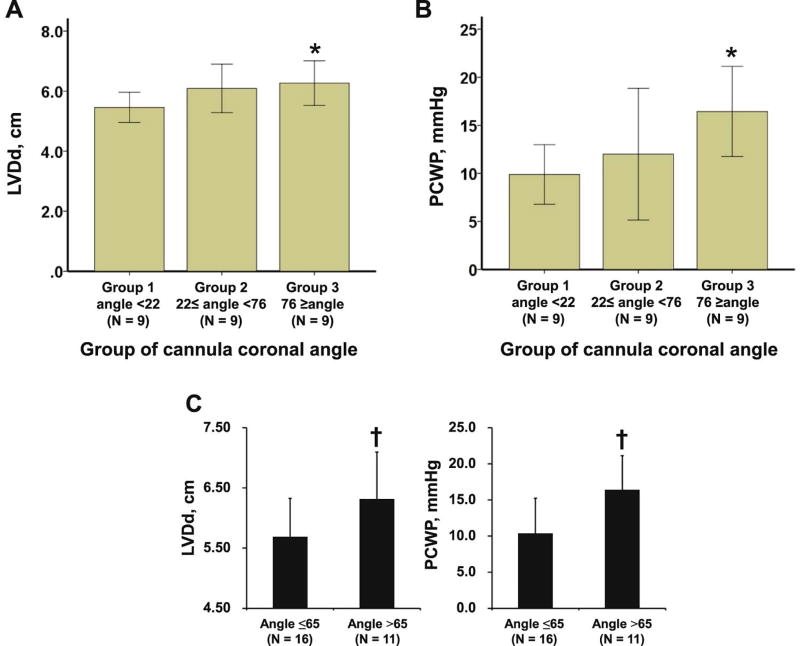
Rotational speed was set at 2794.6 ± 187.4 rpm. The relationship between device position and LV unloading, which was assessed by hemodynamic variables (eg, LV diastolic diameter [LVDd], PCWP) at set speed, was analyzed. Patients with the highest cannula coronal angle (group 3) had significantly larger LVDd and higher PCWP compared with group 1 (Fig. 2A,B; P < .05 for both), signifying less LV unloading. There was no significant association between hemodynamic variables and other device positions except for higher pump depth for larger LVDd.
Fig. 2.
Comparison of LVDd and PCWP among 3 groups stratified by cannula coronal angle (A, B). Comparison of PCWP and LVDd at set speed stratified by cannula coronal angle of 65° (C). *P < .05 compared with group 1 by Tukey test when analysis of variance approved significance. †P < .05 by unpaired t test. LVDd, left ventricular diastolic diameter; PCWP, pulmonary capillary wedge pressure.
Twenty-one patients had sufficient unloading with PCWP <18 mmHg, and cannula coronal angle ≤65° was a significant predictor for this endpoint (Table 2; odds ratio 12.50). Patients with cannula coronal angle ≤65° had significantly smaller LVDd and lower PCWP (Fig. 2C). There were no differences in prescribed HF medications between those with a cannula coronal angle ≤65° and those without (data not shown; P > .05 for all).
Table 2.
Univariate Logistic Regression Analysis for Prediction of Sufficient LV Unloading at Set Speed
| P Value | OR (95% CI) | |
|---|---|---|
| Continuous variables | ||
| Cannula coronal angle relative to horizontal line, ° | .039* | 0.942 (0.891–0.997) |
| Pump depth, mm | .16 | 0.980 (0.953–1.008) |
| Cannula sagittal angle relative to vertebra, ° | .43 | 1.020 (0.971–1.071) |
| Pump area, mm2 | .93 | 1.000 (0.998–1.003) |
| Dichotomous variables | ||
| Cannula coronal angle ≤65° | .035* | 12.50 (1.196–130.6) |
CI, confidence interval; HR, hazard ratio; LV, left ventricular.
P < .05 by logistic regression analysis.
Device Position and Dynamic LV Unloading
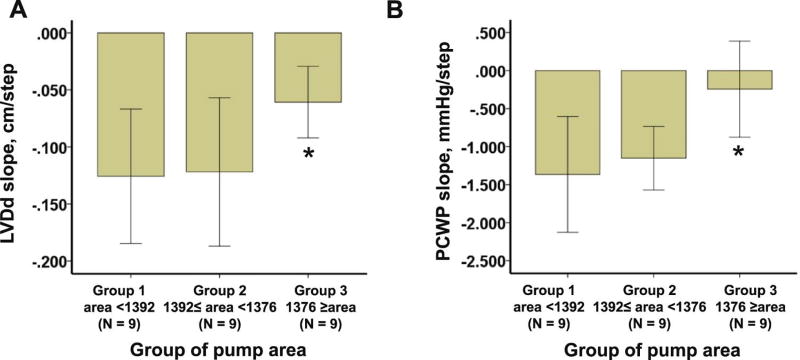
The relationship between device position and dynamic LV unloading, which was assessed by slope parameters (ie, LVDd and PCWP slope), was analyzed.With regard to pump area, group 3 (largest area group) had a significantly shallower negative slope of LVDd and PCWP compared with group 1 (Fig. 3A,B; P < .05 for all), signifying less LV unloading. In contrast to the set speed, cannula coronal angle was not associated with dynamic unloading parameters. There was no significant association between slope parameters and other device positions except for higher cannula sagittal angle for lower PCWP slope.
Fig. 3.
Comparison of LVDd and PCWP slope among 3 groups stratified by pump area (A, B). *P < .05 compared with group 1 by Tukey test when analysis of variance approved significance. LVDd, left ventricular diastolic diameter; PCWP, pulmonary capillary wedge pressure.
Device Position and Clinical Outcome
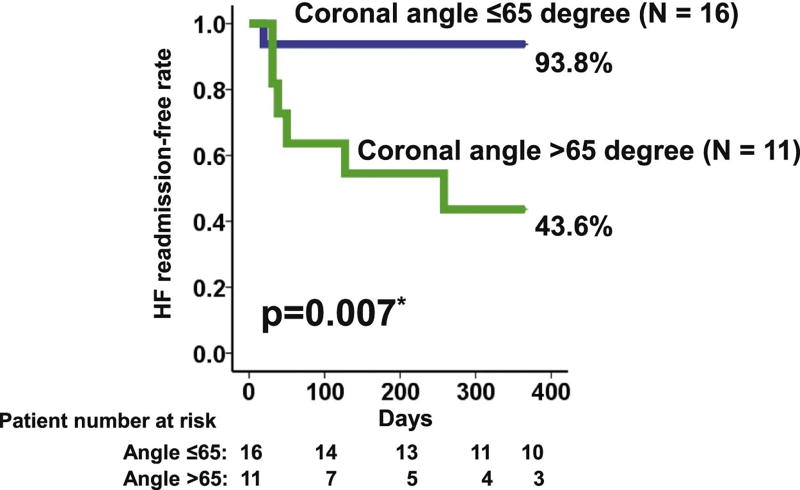
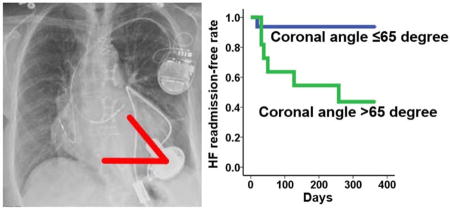
During a 1-year observational period, 7 patients (26%) experienced HF readmission. Cannula coronal angle >65° was a significant predictor of HF readmission (Table 3; hazard ratio 10.33). Patients with cannula coronal angle >65° had a significantly lower 1-year HF readmission-free rate compared with those with ≤65° (Fig. 4; 43.6% vs 93.8%, P = .007).
Table 3.
Univariate Cox Proportional Hazards Regression Analysis for Prediction of HF Rehospitalization
| P Value | HR (95% CI) | |
|---|---|---|
| Continuous variables | ||
| Cannula coronal angle relative to horizontal line, ° | .032* | 1.046 (1.004–1.090) |
| Pump depth, mm | .009* | 1.030 (1.007–1.054) |
| Cannula sagittal angle relative to vertebra, ° | .33 | 1.016 (0.984–1.050) |
| Pump area, mm2 | .66 | 1.000 (0.999–1.002) |
| Dichotomous variables | ||
| Cannula coronal angle >65° | .031* | 10.33 (1.237–86.26) |
| Pump depth >35 mm | .34 | 32.96 (0.025–18254) |
HF, heart failure; other abbreviations as in Table 2.
P < .05 by Cox proportional hazards regression analysis.
Fig. 4.
HF readmission-free rate stratified by cannula coronal angle. *P < .05 by log-rank test. HF, heart failure.
The frequencies of each clinical event were compared between the angle >65° and the angle ≤65° groups (Table 4). Nobody experienced pump thrombosis or isolated right ventricular failure. Two patients experienced ventricular tachyarrhythmia despite normal PCWPs (11 and 16 mmHg), and both had cannula coronal angle >65° (73 and 81°).
Table 4.
Comparison of Clinical Parameters and Outcomes Stratified By Cannula Coronal Angle
| Coronal Angle >65° (N = 11) |
Coronal Angle ≤65° (N = 16) |
P Value | |
|---|---|---|---|
| Age, y | 57.9 ± 10.5 | 61.4 ± 14.1 | .49 |
| Body surface area, m2 | 1.85 ± 0.26 | 1.96 ± 0.20 | .23 |
| Sex, male | 7 (64) | 9 (56) | .70 |
| Ischemic etiology | 6 (55) | 10 (63) | .68 |
| History of cardiotomy | 5 (45) | 5 (31) | .45 |
| Atrial fibrillation | 8 (73) | 6 (38) | .072 |
| History of ventricular tachyarrhythmia | 3 (27) | 4 (25) | .90 |
| Chronic obstructive lung disease | 3 (27) | 1 (6) | .13 |
| Chronic kidney disease | 2 (18) | 3 (19) | .97 |
| LVDd, cm | 7.33 ± 0.96 | 7.01 ± 0.73 | .34 |
| Pump parameters at set speed | |||
| 2794.6 ± 187.4 | 2687.5 ± 143.6 | .11 | |
| Power, watts | 4.31 ± 0.92 | 3.96 ± 0.74 | .28 |
| Flow, L/min | 3.73 ± 0.93 | 3.96 ± 0.90 | .57 |
| Pulsatility, L/min | 2.90 ± 0.76 | 3.54 ± 1.14 | .13 |
| Echo and hemodynamic parameters at set speed | |||
| 6.31 ± 0.78 | 5.68 ± 0.65 | .032* | |
| LVEF, % | 19.2 ± 9.2 | 17.4 ± 7.8 | .59 |
| CVP, mmHg | 8.6 ± 4.4 | 6.4 ± 4.1 | .19 |
| Mean PAP, mmHg | 26.2 ± 5.8 | 23.3 ± 7.4 | .29 |
| PCWP, mmHg | 16.4 ± 4.8 | 10.3 ± 4.9 | .004* |
| CI, L/min/m2 | 2.61 ± 0.48 | 2.67 ± 0.39 | .73 |
| Clinical outcomes | |||
| Death | 3 (27) | 1 (6) | .13 |
| 6 (55) | 1 (6) | .005† | |
| Ventricular tachyarrhythmia | 2 (18) | 0 (0) | .076 |
| Stroke | 2 (18) | 2 (13) | .68 |
| Gastrointestinal bleeding | 3 (27) | 1 (6) | .13 |
| Pump thrombosis | 0 (0) | 0 (0) | - |
| Isolated right ventricular failure | 0 (0) | 0 (0) | - |
| Driveline infection | 2 (18) | 0 (0) | .076 |
Clinical parameters were also stratified by the coronal angle of 65° and compared (Table 4). There were no significant differences between angle >65° and angle ≤65° groups, including LVAD set speeds and other pump parameters except for LVDd and PCWP.
Discussion
We analyzed the clinical implication of device position on LV unloading and patient prognosis during HVAD support. The main finding is that device position was associated with not only LV unloading, but also HF readmission-free rate. Specifically, lower cannula coronal angle was associated with better LV unloading and a higher HF readmission-free rate. Smaller pump area, which may indicate fewer component of longitudinal cannula direction, was associated with better dynamic LV unloading but not with better prognosis. Last, there were no changes in device positions during LVAD support. To the best of our knowledge, this is the first report discussing the clinical implication of HVAD positioning.
How to Assess Device Position
Several device position measurements have been proposed in HeartMate II management,6 whereas none has been proposed for the HVAD thus far. We measured cannula coronal angle and pump depth in the same manner as previous HeartMate II reports.7 The angle between inflow cannula and pump is key in clinical outcomes of HeartMate II support,6 but there are no such defined angles with the HVAD. Instead, we tried to estimate the longitudinal direction of cannula position by measuring pump area and cannula sagittal angle (eg, smaller pump area indicates that a cannula has fewer component of longitudinal direction).
Device position parameters were widely distributed. The cannula coronal angle was not affected by background characteristics, such as body composition or history of cardiotomy. Even preoperative LV size was not a determinant of cannula coronal angle. Device position may largely be dependent on surgical technique at time of HVAD implantation.
Patients supported with HeartMate II experience gradual contraction of the pump pocket, leading to device position changes over time.6,7 In contrast, we report in this HVAD study that there are no significant changes in all position-related parameters during HVAD support. The stability of the HVAD position is probably related to the intrapericardial position with the lack of a device pocket.
Device Position and LV Unloading
Lower cannula coronal angle was associated with better LV unloading at optimized rotational speed (set speed) irrespective of their HF medication regimen. To achieve sufficient LV unloading (ie, PCWP <18 mmHg), an appropriate target angle would be ≤65°. Such an angle indicates cannula direction toward the mitral valve and may be an optimal cannula position for sustainable and reliable blood removal from the LV cavity. In contrast, an enhanced turbulence within the LV cavity from inadequate LV unloading when the cannula is positioned in a vertical direction may limit LV reverse remodeling.
Another explanation is an intentional decrease in LVAD speed because of suction events in patients with higher cannula coronal angle. However, overall, LVAD speeds were comparable between high- and low-angle groups, and there was no difference in pulsatility at the set speed.
Lower pump depth was also associated with smaller LVDd. Considering that pump depth had a correlation with cannula coronal angle (P = .004, r = 0.538), deep pump positioning (inferior positioning) may not be recommended, although it may not be possible to control given the nature of the HVAD and the surgical techniques for its implantation.
The optimal position of the inflow cannula may not be the same as in HeartMate II. With the HeartMate II, a low coronal angle of the inflow cannula leads to an acute angle between the inflow cannula and pump, which could result in obstruction of blood flow.6,8
We also assessed dynamic LV unloading by using slope parameters during rotational speed changes.12 A smaller pump area in posteroanterior CXR, which indicates a smaller longitudinal component in the actual 3-dimensional cannula direction, was associated with better dynamic unloading. When the cannula is positioned along the longitudinal axis of the heart, which yields a large pump area, the tip may be near the LV wall (anterior or posterior), and may have a disadvantage during progressive unloading with incremental increases of rotational speed because of partial suction events.14 In contrast, the degree of dynamic LV unloading was comparable irrespective of coronal angle, probably because there is sufficient space between the cannula and the LV wall at any angle.
Device Position and Clinical Outcome
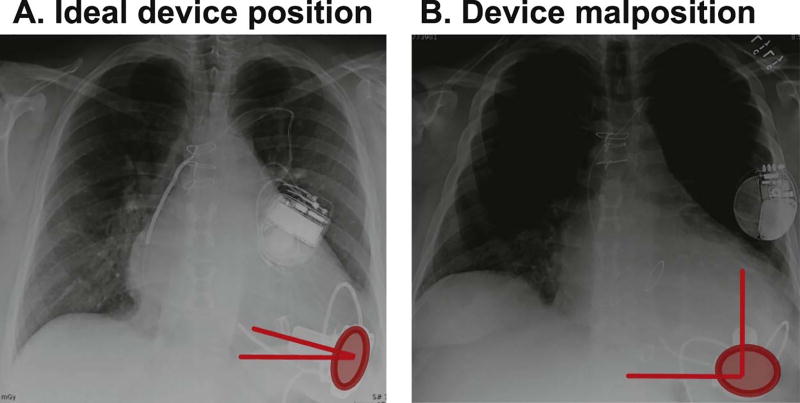
The cannula coronal angle was not only associated with LV unloading, but also was a strong predictor of HF readmission. The link between cannula coronal angle and clinical outcomes was strengthened by the result that there were no other differences in baseline demographics between high and low cannula coronal angle groups. It is evident that better LV unloading prevents recurrence of HF.15 HVAD implantation with the cannula positioned in a perpendicular direction is not advised (see example in Fig. 5).
Fig. 5.
Examples of ideal device position (A) and malposition (B). (A) Narrow cannula coronal angle and small pump area; (B) wide cannula coronal angle and large pump area.
Pump area was associated with dynamic LV unloading but not with patient prognosis. Jung et al showed that decreases in PCWP and increases in cardiac index at incremental LVAD speed were associated with better functional capacity and quality of life, but not with survival.16 A cannula position with smaller pump area may enhance exercise capacity because of better hemodynamic response during increased hemodynamic demands, although this was not demonstrated in this study. Insufficient decreases in LVDd during the ramp test and device malposition are associated with device thrombosis during HeartMate II support.6,17 Device malposition with large pump area may result in device thrombosis.
Future Direction and Study Limitations
This study has several limitations. Device position parameters remained unchanged over the study period, but this result cannot be extrapolated across multiple years of HVAD support. We used CXR to evaluate position based on its seas of acquisition, noninvasiveness, cost-effectiveness, and objectiveness (consistent high interrater reliability of measured device parameters). However, other device position parameters seen on echocardiography or cardiac computed tomography may have clinical implications. For example, we did not assess position of the outflow graft because of its transparency on CXR, but this may have clinical utility.18
This study was performed in a small number of patients at a single center. Accordingly, we could not perform meaningful multivariate analyses. We did not consider interinstitutional differences in surgical technique and patient management. Clinically stable outpatients received the ramp test at varying times after LVAD implantation; this may have affected the results. The benefits of targeted device positioning should be confirmed in a multicenter prospective study using meaningful multivariate analyses, similar to the Prevention of HeartMate II Pump Thrombosis Through Clinical Management study.9 Given that patient background characteristics and LVAD support duration did not affect device position, we anticipate optimal device positioning determined at HVAD implantation would result in a favorable clinical outcome. We did not observe any events of isolated right ventricular failure events; in any event, isolated right heart failure would unlikely be associated with device position in the setting. However, our findings may not be applicable in such situations.
Quantitative hydrodynamic analysis between cannula position and unloading should also be conducted in the Mock Circulatory System to confirm our results.19 Furthermore, innovation of the system with post-LVAD adjustment of cannula position may strengthen the clinical utility of our result.
Conclusion
HVAD cannula and pump positions are associated with LV unloading and improved clinical outcomes. Prospective studies evaluating surgical techniques to ensure optimal device positioning and its effects on clinical outcomes are warranted.
Supplementary Material
Acknowledgments
Teruhiko Imamura receives financial funding from Fukuda Foundation for Medical Technology and Postdoctoral Fellowship for Research Abroad of Japan Society for the Promotion of Science.
Conflict of Interest
Nir Uriel receives consultant fees and grant support from Abbott and Medtronic. Valluvan Jeevanandam receives consultant fees from Abbott. Daniel Burkhoff receives consultant fees from Medtronic, Corvia Medical, Sensible Medical, Impulse Dynamics, and Cardiac Implants, and educational grant support from Abiomed.
Footnotes
Supplementary data related to this article can be found at doi:10.1016/j.cardfail.2017.09.013.
References
- 1.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34:1495–504. doi: 10.1016/j.healun.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Magruder JT, Grimm JC, Crawford TC, Tedford RJ, Russell SD, Sciortino CM, et al. Survival After orthotopic heart transplantation in patients undergoing bridge to transplantation with the heartware HVAD Versus the heartmate II. Ann Thorac Surg. 2016 doi: 10.1016/j.athoracsur.2016.08.060. [DOI] [PubMed] [Google Scholar]
- 3.Hasin T, Marmor Y, Kremers W, Topilsky Y, Severson CJ, Schirger JA, et al. Readmissions after implantation of axial flow left ventricular assist device. J Am Coll Cardiol. 2013;61:153–63. doi: 10.1016/j.jacc.2012.09.041. [DOI] [PubMed] [Google Scholar]
- 4.Feldman D, Pamboukian SV, Teuteberg JJ, Birks E, Lietz K, Moore SA, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant. 2013;32:157–87. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Uriel N, Han J, Morrison KA, Nahumi N, Yuzefpolskaya M, Garan AR, et al. Device thrombosis in HeartMate II continuous-flow left ventricular assist devices: a multifactorial phenomenon. J Heart Lung Transplant. 2014;33:51–9. doi: 10.1016/j.healun.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Taghavi S, Ward C, Jayarajan SN, Gaughan J, Wilson LM, Mangi AA. Surgical technique influences HeartMate II left ventricular assist device thrombosis. Ann Thorac Surg. 2013;96:1259–65. doi: 10.1016/j.athoracsur.2013.05.081. [DOI] [PubMed] [Google Scholar]
- 7.Adamson RM, Bower BL, Sundareswaran KS, Farrar DJ, Dembitsky WP. Radiologic assessment of HeartMate II position: minimal pump migration after long-term support. J Heart Lung Transplant. 2015;34:1617–23. doi: 10.1016/j.healun.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Adamson RM, Mangi AA, Kormos RL, Farrar DJ, Dembitsky WP. Principles of HeartMate II implantation to avoid pump malposition and migration. J Card Surg. 2015;30:296–9. doi: 10.1111/jocs.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maltais S, Kilic A, Nathan S, Keebler M, Emani S, Ransom J, et al. PREVENtion of HeartMate II pump thrombosis through clinical management: the PREVENT multi-center study. J Heart Lung Transplant. 2017;36:1–12. doi: 10.1016/j.healun.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Al-Sarie M, Rauf A, Kfoury AG, Catino A, Wever-Pinzon J, Bonios M, et al. Myocardial structural and functional response after long-term mechanical unloading with continuous flow left ventricular assist device: axial versus centrifugal flow. JACC Heart Fail. 2016;4:570–6. doi: 10.1016/j.jchf.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 11.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Colvin MM, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2016;134:e282–93. doi: 10.1161/CIR.0000000000000435. [DOI] [PubMed] [Google Scholar]
- 12.Uriel N, Sayer G, Addetia K, Fedson S, Kim GH, Rodgers D, et al. Hemodynamic ramp tests in patients with left ventricular assist devices. JACC Heart Fail. 2016;4:208–17. doi: 10.1016/j.jchf.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Nahumi N, Jorde U, Uriel N. Slope calculation for the LVAD ramp test. J Am Coll Cardiol. 2013;62:2149–50. doi: 10.1016/j.jacc.2013.05.098. [DOI] [PubMed] [Google Scholar]
- 14.Saeed D, Maxhera B, Lichtenberg A, Albert A. Intraventricular pledgetted sutures to prevent suction events in patients with the heartware left ventricular assist device. Ann Thorac Surg. 2014;98:746–7. doi: 10.1016/j.athoracsur.2013.12.080. [DOI] [PubMed] [Google Scholar]
- 15.Muthiah K, Humphreys DT, Robson D, Dhital K, Spratt P, Jansz P, et al. Longitudinal structural, functional, and cellular myocardial alterations with chronic centrifugal continuous-flow left ventricular assist device support. J Heart Lung Transplant. 2017;36:722–31. doi: 10.1016/j.healun.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 16.Jung MH, Gustafsson F, Houston B, Russell SD. Ramp study hemodynamics, functional capacity, and outcome in heart failure patients with continuous-flow left ventricular assist devices. ASAIO J. 2016;62:442–6. doi: 10.1097/MAT.0000000000000387. [DOI] [PubMed] [Google Scholar]
- 17.Uriel N, Morrison KA, Garan AR, Kato TS, Yuzefpolskaya M, Latif F, et al. Development of a novel echocardiography ramp test for speed optimization and diagnosis of device thrombosis in continuous-flow left ventricular assist devices: the Columbia ramp study. J Am Coll Cardiol. 2012;60:1764–75. doi: 10.1016/j.jacc.2012.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neidlin M, Corsini C, Sonntag SJ, Schulte-Eistrup S, Schmitz-Rode T, Steinseifer U, et al. Hemodynamic analysis of outflow grafting positions of a ventricular assist device using closed-loop multiscale CFD simulations: preliminary results. J Biomech. 2016;49:2718–25. doi: 10.1016/j.jbiomech.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Prisco AR, Aliseda A, Beckman JA, Mokadam NA, Mahr C, Garcia GJ. Impact of LVAD implantation site on ventricular blood stagnation. ASAIO J. 2017;63:392–400. doi: 10.1097/MAT.0000000000000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.