Abstract
Background
The relatively short duration of effect of local anesthetics has been addressed by encapsulation in drug delivery systems. Co-delivery with a single compound that produces an adjuvant effect on nerve block but without intrinsic local anesthetic properties can further prolong the nerve block effect. Here, we investigated whether co-delivery of more than one encapsulated adjuvant compound can further enhance nerve blockade.
Methods
Liposomes loaded with bupivacaine (Bup), dexamethasone phosphate (DexP), or dexmedetomidine (DMED) were synthesized and their in vitro drug release profiles were determined. Animals (Sprague-Dawley rats) were injected with liposomal bupivacaine (Lipo-Bup) and adjuvants at the sciatic nerve and underwent a modified hot-plate test to assess the degree of nerve block. The duration of block was monitored and the tissue reaction was assessed.
Results
Co-injection of Lipo-Bup with liposomal dexamethasone phosphate (Lipo-DexP) and liposomal dexmedetomidine (Lipo-DMED) prolonged the duration of sciatic nerve block 2.9 fold compared to Lipo-Bup alone (95% CI: 1.9–3.9 fold). The duration of the block using this combination was significantly increased to 16.2 ± 3.5 h, compared to Lipo-Bup with a single liposomal adjuvant (8.7 ± 2.4 h with Lipo-DMED, P = 0.006 and 9.9 ± 5.9 h with Lipo-DexP, P = 0.008). The co-injection of Lipo-Bup with liposomal adjuvants decreased tissue inflammation (P = 0.014) but did not have a significant effect on myotoxicity when compared to Lipo-Bup alone. Co-injection of Lipo-Bup with un-encapsulated adjuvants prolonged the duration of nerve block as well (25.0 ± 6.3 h, P < 0.001) however was accompanied by systemic side effects.
Conclusions
Co-delivery of Lipo-DexP and Lipo-DMED enhanced the efficacy of Lipo-Bup. This benefit was also seen with co-delivery of both adjuvant molecules in the un-encapsulated state, but with marked systemic toxicity.
1. Introduction
Current pain treatments rely to a great degree on orally administered opioids 1. However, due to their limited effectiveness and significant side effects, alternative methods of pain control are of interest,2,3 such as local anesthesia. Bupivacaine (Bup) is a local anesthetic frequently utilized clinically in infiltration, regional and neuraxial blockade. Its use for post-operative pain – in the absence of delivery by a catheter - has been limited by its relatively brief duration of action 4,5. Placement and maintenance of such catheters, while necessary to achieve prolonged pain control, require specialized skilled personnel and an inpatient setting. Furthermore, the patient must be tethered to an external delivery system, presenting a potential portal for infection.
Previous studies have combined local anesthetics with adjuvants such as the α2-adrenergic agonist dexmedetomidine (DMED) in order to extend the duration of the nerve block while also reducing local inflammation 6. DMED could prolong the duration of nerve block though vasoconstriction7, by maintaining the local concentration of the local anesthetic, or by inhibition of the hyperpolarization-activated cation current (Ih current)8. The glucocorticoid agonist Dexamethasone (Dex) has also been used to prolonged nerve block from local anesthetics 9. Possible mechanisms by which Dex could prolong nerve block with local anesthetic solutions include vasoconstriction10, anti-inflammatory effects11, and the activation of inhibitory potassium channels on C-fibers12. However, these drugs tend to have a relatively modest effect on the duration of nerve block from local anesthetic solutions 6,13.
A wide variety of controlled release systems have been studied to further prolong the duration of local anesthetic-induced nerve block14. Such systems allow the drug concentration near the nerve to be maintained at therapeutic levels for a prolonged period while minimizing systemic absorption. Liposomes, because of their biocompatibility 15 and adoption into clinical practice, have been utilized to encapsulate Bup to provide prolonged duration of local anesthesia 16.
Encapsulating local anesthetics together with adjuvant molecules can markedly prolong the duration of effect. For example, while Bup microspheres injected at the sciatic nerve produce nerve block lasting a number of hours, co-encapsulation with Dex resulted in nerve block lasting days17. Similarly, rat sciatic nerve block from microspheres containing two local anesthetics (Bup and the site 1 sodium channel blocker tetrodotoxin) was greatly enhanced by addition of Dex (from 1.5 days to > 9 days). In these examples, the effect of drug delivery systems containing one or more local anesthetics was enhanced by co-encapsulation with a single adjuvant compound with no intrinsic local anesthetic activity.
Here we investigated whether co-delivery of more than one adjuvant molecule without intrinsic local anesthetic activity can enhance the duration of block from encapsulated local anesthetics. We used Bup as the local anesthetic, and DMED and dexamethasone phosphate (DexP) as the two adjuvants. Sprague-Dawley rats were used as the animal model, consistent with studies in the field18,19, to enable us to investigate in the effect of drug combinations with all other parameters kept constant.
2. Methods
2.1. Liposome Preparation and Characterization
Liposomes were prepared by the thin film hydration method 20–22. A lipid mixture of 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC; Avanti Polar Lipids, Alabaster, AL, USA) and cholesterol (Sigma, St. Louis, MO, USA) 2:1 molar ratio was dissolved in a 9:1 chloroform : methanol solution. The lipid mixture underwent vacuum evaporation to form a dry lipid film. T-butanol was used to dissolve the lipid thin film and freeze-dried to form a lipid cake. The lipid cake was hydrated with phosphate buffered saline (PBS), DMED solution (1 mg/mL PBS; Sigma, St. Louis, MO, USA), DexP solution (50 mg/mL PBS; Sigma, St. Louis, MO, USA), or 250 mM (NH4)2SO4. The liposomes then underwent ten freeze-thaw cycles followed by dialysis against PBS for 48 hours. Bup (Sigma, St. Louis, MO, USA) was loaded into liposomes using a previously reported remote loading technique 23. Briefly, after dialysis in PBS, the liposomes that were hydrated with 250 mM (NH4)2SO4 were loaded with Bup upon incubation with a 50 mg/mL Bup hydrochloride solution at 50°C for 1 h. The liposomes were then dialyzed against PBS for 48 hours.
Liposome size was determined by dynamic light scattering (Delsa Nano, Beckman Coulter, Brea, CA, USA). Drug loading was determined by HPLC after disrupting the liposomes with 100 mM octyl β-D-glucopyranoside (Sigma, St. Louis, MO, USA).
2.2. In Vitro Drug Release Kinetics
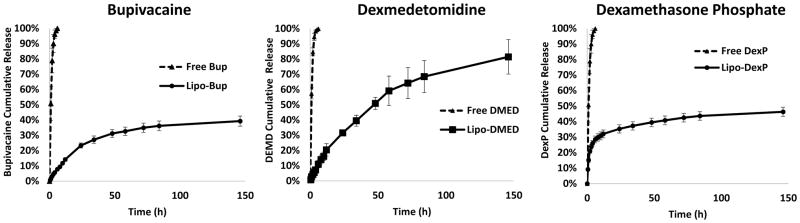
Drug release kinetics was performed by dialyzing 100 μL of liposome solution against 14 mL of PBS at 37°C in a Slide-A-Lyzer MINI dialysis device (Thermo Fisher, Waltham, MA, USA) with a 20 kDa MWCO. Samples were collected at predetermined time intervals and replaced with fresh PBS. The concentration of drug in each sample was determined by HPLC (Fig. 1).
Figure 1.
In vitro release kinetics from drug-loaded liposomes. Data are means ± standard deviation, N = 4.
2.3. Animal Studies
Animal studies were performed with protocol approved by the Boston Children’s Hospital Animal Care and Use Committee that conform to the requirements of the International Association for the Study of Pain 24. Starting at 6 AM, the rats were in light for 12 hours of each day. Male Sprague–Dawley rats (Charles River Laboratories) weighing 320g to 425g were used for this study.
The rats were randomly allocated to each experimental group and anesthetized with isoflurane-oxygen followed by injections of test materials at the sciatic nerve 21. The liposomal drug combinations were: 300 μL liposomal bupivacation (Lipo-Bup) with 100 μL liposomal DMED (Lipo-DMED) and/or 100 μL liposomal DexP (Lipo-DexP). We also injected the rats with a mixture of 300 μL of PBS-filled liposomes (Lipo-PBS) with 100 μL Lipo-DMED or 100 μL Lipo-DexP to serve as controls. The free solution drug combinations provided equal mass of drug as the liposomal solution: 300 μL of 15 mg/mL Bup hydrochloride (in citrate buffer) with 100 μL of 0.4 mg/mL DMED solution and/or 100 μL of 2.5 mg/mL DexP solution.
The duration of sensory nerve blocks were measured at pre-determined time intervals using a modified hotplate test 18,25. In brief, the rat’s hindpaw was placed on a hotplate at 56 °C, and the degree of nerve block was determined by measuring the time until the animal removed its hindpaw from the hotplate. 2 s was normal latency and indicative of no sensory nerve blockade. 12 s was the maximum amount of time the hindpaw was kept on the hotplate before being removed to prevent thermal injury. Duration of effective block was defined as the duration required for the thermal latency to return to 7 s, which was the midpoint between the maximum block (12 s) and baseline (2 s). The duration of local anesthesia from that successful block was calculated as the time for the latency to return to 7 s 22. The number of animals in each group were as follows: Lipo-Bup + Lipo-DMED, n = 5; Lipo-Bup + Lipo-DexP, n = 8; Lipo-Bup + Lipo-DexP + Lipo-DMED, n = 10; and N=4 for all other groups.
2.4. Histology
4 d after the injections of liposomes, the animals were euthanized with carbon dioxide. Tissue samples containing the sciatic nerve and adjacent muscle were collected and underwent standard hematoxylin and eosin (H&E) staining procedures. The H&E slides were scored for myotoxicity (0–6) and for inflammation (0–4) as previously reported 22,26. These scores were assigned by a researcher blinded to the status of each sample (A.Y.R.). Inflammation was scored subjectively to quantify severity; 0 was normal and 4 was severe inflammation. Myotoxicitiy was evaluated by the nuclear internalization and regeneration of myocytes, two representative characteristics of myotoxicity in local anesthesia. The scoring scale was as follows: 0 = normal; 1 = perifasicular internalization; 2 = deep internalization (> 5 cell layers); 3 = perifascicular regeneration; 4 = deep tissue regeneration (> 5 cell layers); 5 = hemifascicular regeneration; 6 = holofascicular regeneration.
2.5. Statistical Analysis
Myotoxicity and inflammation histological scores were analyzed by the nonparametric Mann-Whitney U-test due to their ordinal scales and reported as median scores and ranges for liposomal combinations compared to Lipo-Bup alone with a Bonferroni adjusted P<0.017 to account for three planned comparisons (the significance criterion was set as 0.05/m to maintain alpha of 0.05 for each outcome, where M is the number of tests within each hypothesis). Duration of sciatic block was compared using one-factor analysis of variance (ANOVA) with Dunnett post-hoc tests.26 Fieller’s theorem was used to construct a 95% confidence interval (CI) around the fold-difference of prolonged nerve block between co-injection of Lipo-Bup with Lipo-DMED and Lipo-DexP as compared to Lipo-Bup.27 Effect sizes regarding mean differences in block duration relative to Lipo-Bup+Lipo-DMED+Lipo_DexP were determined by ANOVA and shown graphically with 95% CIs. In this work, detection of large mean differences or effects (4–5 hours) in nerve block duration would be considered scientifically meaningful. The sample sizes used for groups for in vivo nerve block experiment (Figure 3) provided 80% power to detect effect sizes of 1.25 or larger, based on a noncentral F statistic in ANOVA (G*Power, Dussseldorf, Germany). Statistical analysis was performed using Stata software Release 15 (Stata Statistical Software, StataCorp LLC, College Station, TX).
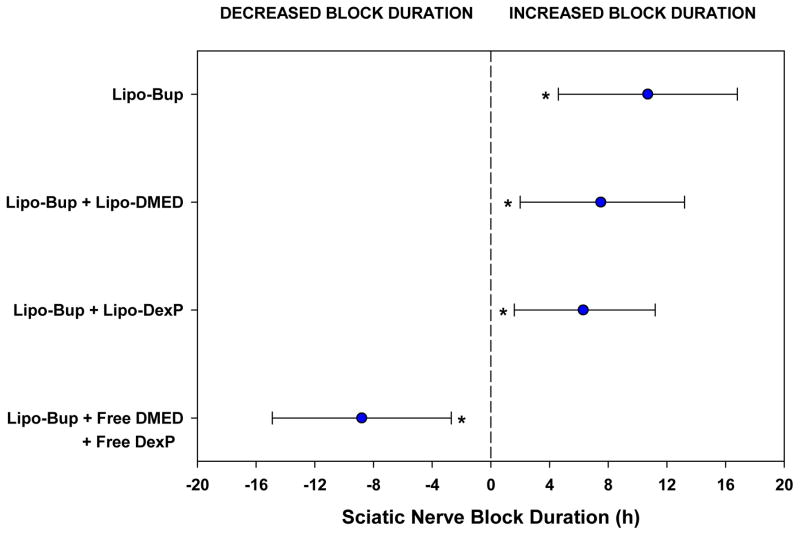
Figure 3.
Estimated effect sizes for sciatic nerve block duration are shown relative to the Lipo-Bup + Lipo-DMED + Lipo-DexP treatment group (n = 10) and shown by blue circles for each comparison. The precision of the effects are represented by 95% confidence intervals (CI) based on one-factor fixed effects ANOVA with Dunnett comparisons. The average increased block duration was 10.7 hours (95% CI: 4.6–16.8 h; P < 0.001) compared to Lipo-Bup (n = 4), 7.5 hours (95% CI: 2.0–13.2 h, P = 0.006) compared to Lipo-Bup + Lipo-DMED (n = 5), and 6.3 hours (95% CI: 1.6–11.2 h, P = 0.008) compared to Lipo-Bup + Lipo-DexP (n = 8). Block duration was decreased on average by 8.8 hours (95% CI: 2.7–14.9 h, P = 0.003) as compared with Lipo-Bup + Free DMED + Free DexP (n = 4). Asterisks denote significant differences in effects compared to Lipo-Bup + Lipo-DMED + Lipo-DexP.
3. Results
3.1. Liposomal Properties
Drug-loaded liposomes ranged in diameter between 2 and 5 μm (Table 1) as measured by dynamic light scattering. The average Bup, DMED, and DexP concentrations of the liposomal solutions were 14.7 mg/mL, 0.38 mg/mL, and 2.5 mg/mL respectively (Table 1). All liposomal solutions had an average lipid concentration of 62.5 mg/mL.
Table 1.
Characterization of drug-loaded liposomes
| Formulation | Size (μm) | Drug Concentration (mg/mL) | Loading Efficiency |
|---|---|---|---|
| Lipo-Bup | 2.5 ± 0.4 | 14.7 ± 1.0 | 29% ± 2% |
| Lipo-DMED | 4.7 ± 0.5 | 0.38 ± 0.03 | 38% ± 3% |
| Lipo-DexP | 4.0 ± 0.7 | 2.5 ± 0.2 | 5% ± 0.4% |
Data are means ± standard deviations. N =4.
3.2. In Vitro Drug Release
Liposomal drug release profiles were measured by dialyzing 100 μL of liposomal samples against 14 mL of phosphate buffered saline (PBS), with changes in solution at predetermined time-points, at which the drug content was measured (Figure 1). Liposomal bupivacaine (Lipo-Bup) showed relatively rapid release of Bup in the first 48 h followed by slower release (Figure 1); 39% of drug was released by the end of the release experiment (144 h). With liposomal dexmedetomidine (Lipo-DMED) the release rate of DMED was relatively constant throughout the 6 days, releasing 81% of drug in that time frame. Liposomal dexamethasone phosphate (Lipo-DexP) showed rapid release of DexP within the initial 12 h followed by a slower drug release rate; 46% of drug was released in 6 days. The free, unencapsulated, drugs were all released from the dialysis kits very quickly in the same experimental set-up, demonstrating the controlled release of each drug from its respective liposomal formulation.
3.3. Duration of Sciatic Nerve Blockade
Under oxygen-isoflurane anesthesia, animals were injected at the sciatic nerve with a combination of liposomal solutions including 300 μL Lipo-Bup, with or without 100 μL Lipo-DMED, and/or 100 μL Lipo-DexP, and/or 100–200 μL blank (no drug) liposomes (Lipo). Upon awakening, animals underwent neurobehavioral testing (modified hotplate test; see Methods). Lipo-DMED or Lipo-DexP with Lipo-PBS did not result in nerve block. These results also implied that free DMED and DexP did not have intrinsic peripheral nerve blocking activity, as has been reported elsewhere13,27.
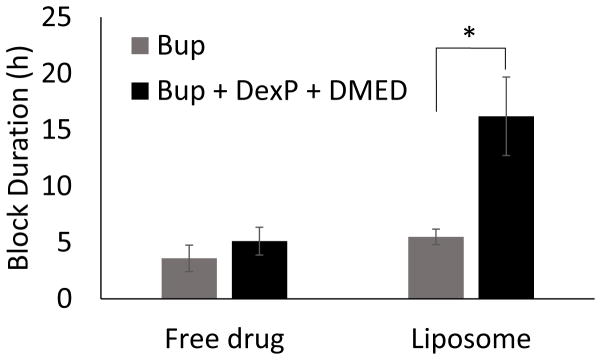
Injection of Lipo-Bup induced a sensory nerve blockade of 5.5 ± 0.7 h (Fig. 2; mean ± standard deviation). An equal volume and concentration of free Bup induced a nerve block duration of 3.6 ± 1.2 h (P = 0.03 in comparison with Lipo-Bup; Fig. 2). Co-injection of free Bup with DMED and DexP did not result in a longer duration of block than from free Bup alone (Fig. 2). Co-injection of Lipo-Bup, Lipo-DMED and Lipo-DexP (Lipo-Bup+Lipo-DMED+Lipo-DexP) greatly prolonged sensory nerve blockade 2.9 fold to 16.2 ± 3.5 h (Fig. 3, P < 0.01 compared to Lipo-Bup; the 95% confidence interval for the fold difference between the durations of nerve block for Lipo-Bup and for Lipo-Bup+Lipo-DMED+Lipo-DexP was determined by Fieller’s theorem to have a lower bound of 1.9 and upper bound of 3.9), significantly longer than block from co-injection of Lipo-Bup with Lipo-DMED (Lipo-Bup+Lipo-DMED) or Lipo-DexP (Lipo-Bup+Lipo-DexP) that resulted in nerve block durations of 8.7 ± 2.4 and 9.9 ± 5.9 h respectively (Fig. 3, P < 0.01). No contralateral nerve block was observed in any of the groups, suggesting a lack of systemic drug distribution or toxicity.
Figure 2.
Nerve block duration of Bup with and without DexP and DMED. Data are means ± standard deviation, N = 10 for the liposomal Bup + DexP + DMED group and N=4 for all other groups. * P<0.01.
In order to determine whether the enhancement of block from Lipo-Bup required that the adjuvant drug be encapsulates, we injected Lipo-Bup with free DexP and DMED, at the same final concentrations as with their liposomal formulations (see Methods). The resulting nerve block lasted 25.0 ± 2.6 h (Fig. 3). However, animals appeared to be heavily sedated in the first four hours post-injection, with little activity.
3.4 Histology
The sciatic nerve and adjacent muscle tissues were harvested 4 d after injection. Macroscopic examination showed a white material (the liposomes) surrounding the nerve (Fig. 4). Tissue samples were made into hematoxylin and eosin stained slides and scored for myotoxicity (0–6) and inflammation (0–4) (Table 2 and Fig. S1, see Methods for details). Lipo-Bup, Lipo-Bup+Lipo-DexP, and Lipo-Bup+Lipo-DMED produced comparable degrees of inflammation, with observable inflammation infiltrate that consisted of lymphocytes and neutrophils around the soft tissues surrounding the muscle layer (Table 2, Fig. S1). The inflammation infiltrate was also present in the perifascicular layers of the muscle tissue (Table 2, Fig. S1). Lipo-Bup+Lipo-DMED+Lipo-DexP had a statistically significantly reduced inflammation score compared to Lipo-Bup, with similar cell types (P = 0.014, Fig. 4). Foamy macrophages were observed in all groups, suggesting the ingestion of liposomes. The myotoxicity score for Lipo-Bup+Lipo-DMED+Lipo-DexP was similar to that of Lipo-Bup (P = 0.19), consisting of myocytes of nuclear internalization throughout the muscle tissue and occasionally showing myocyte regeneration.
Figure 4.
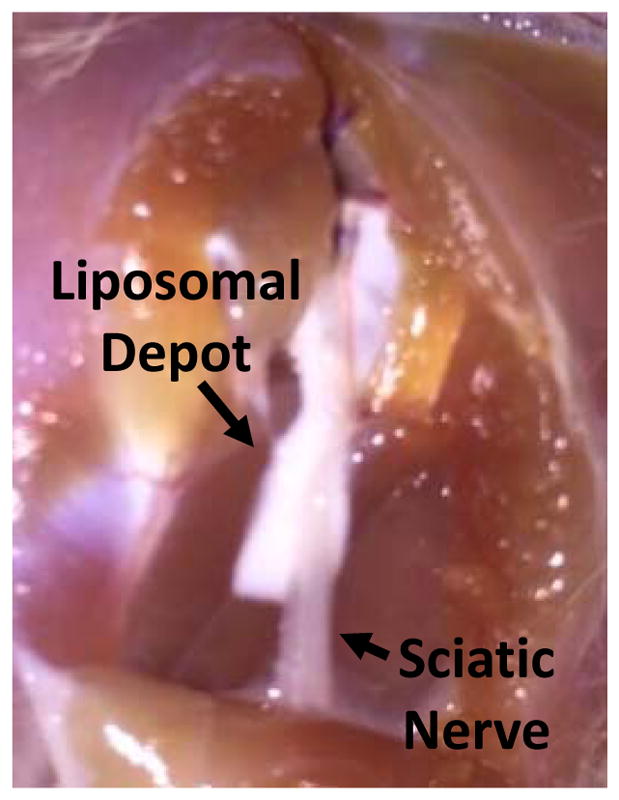
Representative photograph of liposomal residue at site of injection 4 days after administration
Table 2.
Tissue reaction 4 days after injection of liposomal formulations
| Formulation | Inflammation | Myotoxicity | N |
|---|---|---|---|
| Lipo-Bup | 2.5 (1–3) | 3 (2–3) | 4 |
| Lipo-Bup + Lipo-DMED | 2 (1–2) | 0 (0–1)** | 5 |
| Lipo-Bup + Lipo-DexP | 3 (1–3) | 3.5 (2–4) | 8 |
| Lipo-Bup + Lipo-DMED + Lipo-DexP | 1 (0–2)* | 1 (0–3) | 10 |
Data are median score (range). Liposomal combination groups were compared to Lipo-Bup alone using the nonparametric Mann-Whitney U-test, resulting in three planned comparisons (see Methods).
P = 0.014
P = 0.016
4. Discussion
Co-delivery of two adjuvant drugs increased the duration of effect of Lipo-Bup almost three-fold. The adjuvant molecules used here work by distinct but partially overlapping mechanisms. One possibility is that the activation of C-fiber glucocorticoid receptors 12 by DexP may block nerve signal transduction and be combined with the vasoconstriction effect of DMED 6,28 mediated by the α2-adrenergic receptor 27,29, or DMED’s direct action on the peripheral nerve, where the inhibition of the hyperpolarization-activated cation current (Ih current) blocks pain signal transduction 8, to produce such prolongation effects. The exact mechanism remains to be studied.
These results cannot allow us to assert that the effect of the two adjuvants are synergistic vs. additive, i.e. that a similar effect might not have been achieve by simply increasing the dosage of DexP or DMED. However, it is important to be aware that both adjuvant molecules can have potential side effects with increasing dose. This is clearly seen, for example, in the case of DMED, where excessive dosing can lead to systemic sedation30. The use of two adjuvants that have a common desired effect (enhancement of nerve blockade) but different toxicities could allow for longer blocks to be achieved safely. It also bears mentioning that the ability of adjuvant molecules to prolong nerve block does have a ceiling. For example, Dex does not prolong the duration of nerve block from Bup in polymeric microspheres beyond a w/w loading of 0.05%17. Co-delivery of adjuvants that act by different mechanisms could potentially help increase the peak effect.
Co-injection of the three drugs in free form did not significantly prolong the duration of block over that of free Bup, while co-inection of encapsulated forms did. This discrepancy between the effects of Dex on free and encapsulated local anesthetitics is consistent with previous reports with the rat sciatic nerve model 13,17. DMED has been reported to cause a modest (2.5 h to 4 h) prolongation of nerve block in rats 6; it may be that we did not see that effect here because we used a larger dose of Bup, resulting in a longer duration of nerve block of 3.6 h.
Unencaspulated, free, DMED and DexP were able to prolong the effect of Lipo-Bup. Previous work on the effects of vasoconstrictors such as DMED27 and epinephrine31 on local anesthetics suggest that vasoconstriction at the sciatic nerve lasts approximately three hours. While such relatively brief periods of vasoconstriction can greatly enhance the duration of nerve block from compounds such as tetrodotoxin, which have difficulty penetrating into nerves due to their hydrophilicity, they tend to have a modest effect on the duation of amphiphilic drugs such as Bup18. The mechanism by which the free adjuvants prolonged the duration of block of Lipo-Bup are not known to us. We postulate, however, that the relatively brief effects of free adjuvants would not be able to produce the multi-day nerve blocks that have been achieved with some formulations17,32,33.
Co-injection of Lipo-Bup with unencapsulated DMED and DexP provided a longer nerve block than its co-injection with encapsulated DMED and DexP. This unexpected effect may be due to the fact that the encapsulated drugs (DMED and DexP) were released at a much slower rate than the free drug initially (Fig. 1), and the rate of release of the encapsulated drugs decresed further over time. Thus, while the given dose of the encapsulated drugs may been released for over a longer period than the free compounds, their release above a therapeutic threshold for effectiveness may have been shorter.
Lipo-Bup induced mild to moderate myotoxicity, consistent with previous reports 4,16; it would be expected to resolve fully with time16,34. Such myotoxicity has not caused major clinical concerns, and Lipo-Bup has been commercialized35. The co-injection of Lipo-DMED and Lipo-DexP did not cause further tissue injury and was able to prolong the nerve block by approximately 3 fold. Moreover, all of the drugs and lipid material used in this study are FDA-approved for use in humans.
Further studies are needed to investigate the mechanisms by which DexP and DMED prolong block Lipo-Bup. Also, throughout this study a single concentration was used for each adjuvant to demonstrate proof-of-concept of the effect upon the co-injection of two encapsulated and unencapsulated adjuvants with Lipo-Bup. A more detailed study of the effects of various dosages is needed for minimizing side effects and maximizing the duration of prolonged nerve block from Lipo-Bup, as well as for establishing whether the effects of DexP and DMED are additive or synergistic.
Supplementary Material
Key Points.
Question: Would the co-administration of liposomal adjuvants enhance the nerve block duration of liposomal bupivacaine?
Findings: Co-injection of liposomal bupivacaine with liposomal dexamethasone phosphate and liposomal dexmedetomidine prolonged the duration of sciatic nerve block 2.9 fold compared to liposomal bupivacaine alone.
Meaning: Duration of nerve block from liposomal bupivacaine could be enhanced by the addition of encapsulated adjuvants and potentially improve patient care.
Acknowledgments
Financial support: Supported by National Institutes of Health grant R01 GM073626 and Foundation for Anesthesia Education and Research (FAER) 2016-MSARF-Sherburn
This work was supported by NIH Grant GM073626 (to D.S.K.). R.T.S. gratefully acknowledges the Foundation for Anesthesia Education and Research (FAER) and the Medical Student Anesthesia Research Fellowship program.
Footnotes
Conflicts of interest: None
Author contribution:
Alina Y. Rwei: This author has designed the experiments, performed the experiments, analyzed data, and wrote the paper.
Robert T. Sherburne: This author has performed the experiments, analyzed data, and wrote the paper.
David Zurakowsky: This author has analyzed data and wrote the paper.
Bruce Wang: This author has performed the experiments.
Daniel S. Kohane: This author has designed the experiments, analyzed the data, and wrote the paper
References
- 1.Turk DC. Clinical effectiveness and cost-effectiveness of treatments for patients with chronic pain. Clin J Pain. 2002;18:355–365. doi: 10.1097/00002508-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Lynch ME, Watson CP. The pharmacotherapy of chronic pain: a review. Pain Res Manag. 2006;11:11–38. doi: 10.1155/2006/642568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dolin SJ, Cashman JN, Bland JM. Effectiveness of acute postoperative pain management: I. Evidence from published data. Br J Anaesth. 2002;89:409–23. [PubMed] [Google Scholar]
- 4.Padera R, Bellas E, Tse JY, Hao D, Kohane DS. Local myotoxicity from sustained release of bupivacaine from microparticles. Anesthesiology. 2008;108:921–8. doi: 10.1097/ALN.0b013e31816c8a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pere P, Watanabe H, Pitkanen M, Wahlstrom T, Rosenberg PH. Local myotoxicity of bupivacaine in rabbits after continuous supraclavicular brachial plexus block. Reg Anesth. 1993;18:304–7. [PubMed] [Google Scholar]
- 6.Brummett CM, Norat MA, Palmisano JM, Lydic R. Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology. 2008;109:502–511. doi: 10.1097/ALN.0b013e318182c26b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yabuki A, Higuchi H, Yoshitomi T, Tomoyasu Y, Ishii-Maruhama M, Maeda S, Miyawaki T. Locally injected dexmedetomidine induces vasoconstriction via peripheral alpha-2A adrenoceptor subtype in guinea pigs. Reg Anesth Pain Med. 2014;39:133–136. doi: 10.1097/AAP.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 8.Brummett CM, Hong EK, Janda AM, Amodeo FS, Lydic R. Perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolongs the duration of analgesia by blocking the hyperpolarization-activated cation current. Anesthesiology. 2011;115:836–843. doi: 10.1097/ALN.0b013e318221fcc9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherif AA, Elsersy HE. Dexamethasone as adjuvant for femoral nerve block following knee arthroplasty: a randomized, controlled study. cta Anaesthesiologica Scandinavica. 2016;60:977–987. doi: 10.1111/aas.12750. [DOI] [PubMed] [Google Scholar]
- 10.Shishido H, Kikuchi S, Heckman H, Myers RR. Dexamethasone decreases blood flow in normal nerves and dorsal root ganglia. Spine. 2002;27:581–586. doi: 10.1097/00007632-200203150-00005. [DOI] [PubMed] [Google Scholar]
- 11.Drager C, Benziger D, Gao F, Berde CB. Prolonged intercostal nerve blockade in sheep using controlled-release of bupivacaine and dexamethasone from polymer microspheres. Anesthesiology. 1998;89:969–79. doi: 10.1097/00000542-199810000-00022. [DOI] [PubMed] [Google Scholar]
- 12.Johansson A, Hao J, Sjolund B. Local corticosteroid application blocks transmission in normal nociceptive C-fibers. Acta Anaesthesiologica Scandinavica. 1990;34:335–338. doi: 10.1111/j.1399-6576.1990.tb03097.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu Q, Zhan C, Barhoumi A, Wang W, Santamaria C, McAlvin JB, Kohane DS. A Supramolecular Shear-Thinning Anti-Inflammatory Steroid Hydrogel. Advanced Materials. 2016;28:6680–6686. doi: 10.1002/adma.201601147. [DOI] [PubMed] [Google Scholar]
- 14.Santamaria CM, Woodruff A, Yang R, Kohane DS. Drug delivery systems for prolonged duration local anesthesia. Materials Today. 2017;20:22–31. doi: 10.1016/j.mattod.2016.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mallick S, Choi JS. Liposomes: Versatile and Biocompatible Nanovesicles for Efficient Biomolecules Delivery. Journal of Nanoscience and Nanotechnology. 2014;14:755–765. doi: 10.1166/jnn.2014.9080. [DOI] [PubMed] [Google Scholar]
- 16.McAlvin JB, Padera RF, Shankarappa SA, Reznor G, Kwon AH, Chiang HH, Yang J, Kohane DS. Multivesicular liposomal bupivacaine at the sciatic nerve. Biomaterials. 2014;35:4557–64. doi: 10.1016/j.biomaterials.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castillo J, Curley J, Hotz J, Uezono M, Tigner J, Chasin M, Wilder R, Langer R, Berde C. Glucocorticoids prolong rat sciatic nerve blockade in vivo from bupivacaine microspheres. Anesthesiology. 1996;85:1157–1166. doi: 10.1097/00000542-199611000-00025. [DOI] [PubMed] [Google Scholar]
- 18.Kohane DS, Yieh J, Lu NT, Langer R, Strichartz GR, Berde CB. A re-examination of tetrodotoxin for prolonged duration local anesthesia. Anesthesiology. 1998;89:119–31. doi: 10.1097/00000542-199807000-00019. [DOI] [PubMed] [Google Scholar]
- 19.Thalhammer JG, Vladimirova M, Bershadsky B, Strichartz GR. Neurologic evaluation of the rat during sciatic nerve block with lidocaine. Anesthesiology. 1995;82:1013–1025. doi: 10.1097/00000542-199504000-00026. [DOI] [PubMed] [Google Scholar]
- 20.Epstein-Barash H, Shichor I, Kwon AH, Hall S, Lawlor MW, Langer R, Kohane DS. Prolonged duration local anesthesia with minimal toxicity. Proc Natl Acad Sci U S A. 2009;106:7125–30. doi: 10.1073/pnas.0900598106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shankarappa SA, Tsui JH, Kim KN, Reznor G, Dohlman JC, Langer R, Kohane DS. Prolonged nerve blockade delays the onset of neuropathic pain. Proc Natl Acad Sci U S A. 2012;109:17555–60. doi: 10.1073/pnas.1214634109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rwei AY, Lee JJ, Zhan C, Liu Q, Ok MT, Shankarappa SA, Langer R, Kohane DS. Repeatable and adjustable on-demand sciatic nerve block with phototriggerable liposomes. Proc Natl Acad Sci U S A. 2015;112:15719–24. doi: 10.1073/pnas.1518791112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grant GJ, Barenholz Y, Bolotin EM, Bansinath M, Turndorf H, Piskoun B, Davidson EM. A novel liposomal bupivacaine formulation to produce ultralong-acting analgesia. Anesthesiology. 2004;101:133–7. doi: 10.1097/00000542-200407000-00021. [DOI] [PubMed] [Google Scholar]
- 24.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 25.Thalhammer JG, Vladimirova M, Bershadsky B, Strichartz GR. Neurologic evaluation of the rat during sciatic nerve block with lidocaine. Anesthesiology. 1995;82:1013–25. doi: 10.1097/00000542-199504000-00026. [DOI] [PubMed] [Google Scholar]
- 26.Padera RF, Tse JY, Bellas E, Kohane DS. Tetrodotoxin for prolonged local anesthesia with minimal myotoxicity. Muscle Nerve. 2006;34:747–53. doi: 10.1002/mus.20618. [DOI] [PubMed] [Google Scholar]
- 27.Rwei AY, Zhan C, Wang B, Kohane DS. Multiply repeatable and adjustable on-demand phototriggered local anesthesia. Journal of Controlled Release. 2017;251:68–74. doi: 10.1016/j.jconrel.2017.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yektas A, Belli E. The effects of 2 microg and 4 microg doses of dexmedetomidine in combination with intrathecal hyperbaric bupivacaine on spinal anesthesia and its postoperative analgesic characteristics. Pain Res Manag. 2014;19:75–81. doi: 10.1155/2014/956825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yabuki A, Higuchi H, Yoshitomi T, Tomoyasu Y, Ishii-Maruhama M, Maeda S, Miyawaki T. Locally injected dexmedetomidine induces vasoconstriction via peripheral alpha-2A adrenoceptor subtype in guinea pigs. Regional Anesthesia and Pain Medicine. 2014;39:133–136. doi: 10.1097/AAP.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 30.McAlvin JB, Zhan C, Dohlman JC, Kolovou PE, Salvador-Culla B, Kohane DS. Corneal anesthesia with site 1 sodium channel blockers and dexmedetomidine. Invest Ophthalmol Vis Sci. 2015;56:3820–3826. doi: 10.1167/iovs.15-16591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohane DS, Lu NT, Cairns BE, Berde CB. Effects of adrenergic agonists and antagonists on tetrodotoxin-induced nerve block. Reg Anesth Pain Med. 2001;26:239–245. doi: 10.1053/rapm.2001.23215. [DOI] [PubMed] [Google Scholar]
- 32.Kohane DS, Smith SE, Louis DN, Colombo G, Ghoroghchian P, Hunfeld NG, Berde CB, Langer R. Prolonged duration local anesthesia from tetrodotoxin-enhanced local anesthetic microspheres. Pain. 2003;104:415–21. doi: 10.1016/s0304-3959(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 33.Epstein-Barash H, Shichor I, Kwon AH, Hall S, Lawlor MW, Langer R, Kohane DS. Prolonged duration local anesthesia with minimal toxicity. Proc Natl Acad Sci USA. 2009;106:7125–7130. doi: 10.1073/pnas.0900598106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohane DS, Lipp M, Kinney RC, Anthony DC, Louis DN, Lotan N, Langer R. Biocompatibility of lipid-protein-sugar particles containing bupivacaine in the epineurium. J Biomed Mater Res. 2002;59:450–459. doi: 10.1002/jbm.1261. [DOI] [PubMed] [Google Scholar]
- 35.Cohen SM. Extended pain relief trial utilizing infiltration of Exparel((R)), a long-acting multivesicular liposome formulation of bupivacaine: a Phase IV health economic trial in adult patients undergoing open colectomy. J Pain Res. 2012;5:567–72. doi: 10.2147/JPR.S38621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.