Abstract
Extracellular vesicles (EVs) are membrane derived nanometer-sized vesicles. EVs are released by normal, diseased, and transformed cells in vitro and in vivo, and carry lipids, proteins, mRNAs, non-coding RNAs, and even DNA out of cells. Transferring biological information via EVs to neighboring cells and inter-cellular communication not only maintain physiological functions, but also involve in the pathogenesis of several diseases, including cancer. The aim of this review is to discuss the emerging role of EVs in viral hepatitis, non-alcoholic or alcoholic liver disease and liver cancers. We summarize what is known about exosome biogenesis, and role in liver disease progression, and discuss the potential clinical applications of EVs as predictive biomarkers and therapeutic modalities.
Keywords: Extracellular vesicles, Exosomes, Liver, NAFLD, NASH, HCC
1. Introduction
In the past decade, emerging evidences observed the interest in the role of extracellular vesicles (EVs), especially exosomes, for intercellular communication. The important role of EVs for intercellular transport of trophic materials was first reported in 1980 (Trams et al., 1981). Since then, increasing evidences in the field of extracellular vesicle research has implicated the role of EVs as novel mediators of intercellular communication for both short and longer-range signaling events (Raposo and Stoorvogel, 2013; Simons and Raposo, 2009; Balaj et al., 2011; Cossetti et al., 2014; Mass et al., 2017).
EVs contain different cytosolic proteins derived from the parent cell. These proteins are particularly enriched in integrins, MHC molecules, and cytoskeletal proteins, and also express a selection of relatively vesicle-specific proteins often used as EV markers such as the tetraspanins TSG10 or CD63 (Huang-Doran et al., 2017). For EV isolation, these markers are used in immune-affinity-based techniques or for assessing the purity of the molecules after isolating using other techniques such as ultracentrifugation, density gradient separation, and polymer-based precipitation methods.
All EVs bear surface molecules that allow them to be targeted to recipient cells. Once attached to a target cell, EVs can induce signaling via receptor-ligand interaction or can be internalized by endocytosis and/or phagocytosis or even fuse with the target cell’s membrane to deliver their content into its cytosol, thereby modifying the physiological state of the recipient cell. EVs can be isolated from many biological fluids, including blood, milk, saliva, malignant ascites, amniotic fluid and urine (Thery et al., 2006; Keller et al., 2011; Lässer et al., 2011). Cells can secrete different types of EVs that have been classified according to their sub-cellular origin (Colombo et al., 2014).
Liver is a multicellular organ and comprised of parenchymal (hepatocytes) and non-parenchymal cells such as Kupffer cells, hepatic stellate cells, liver endothelial cells and intrahepatic lymphocytes including T cells, natural killer T (NKT) cells, and natural killer (NK) cells (Crispe, 2009). All these cellular populations need an intercellular communication for coordination of their behaviors to function properly. More evidences suggested the role of secreted extracellular vesicles in the intracellular signaling within the liver, besides autocrine-paracrine and cell-cell contacts.
1.1. Classification of extracellular vesicles
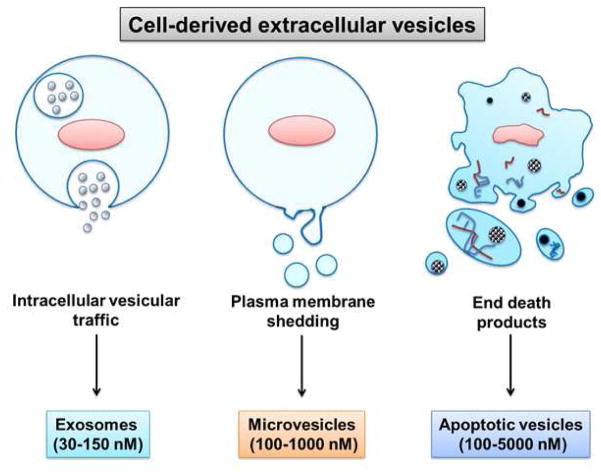
EVs carry a battery of bioactive cargo of soluble and membrane-bound protein, lipids, metabolites, DNA, and RNA (mRNA, miRNAs, and other small regulatory RNAs), and represent a nonidentical subset of the contents of the parent cell of origin (Tkach & Thery, 2016; Mass et al., 2017). EVs can be divided into three categories based on the current state of knowledge of their biogenesis (Huang-Doran et al, 2017). Discrete biogenesis pathways result in subsets of EVs namely: (i) exosomes, (ii) microvesicles, and (iii) apoptotic bodies, as schematically depicted in figure 1.
Figure 1. Classification of extracellular vesicles according to the mechanism of generation.
Exosomes are generated intracellularly from multivesicular bodies. Microvesicles are produced by budding from the extracellular membrane. Apoptotic vesicles are released upon cell fragmentation during apoptotic cell death. Representative sizes are shown at the bottom.
Exosomes are complex (30–150 nm) vesicles formed by the interior budding of endosomal membranes to form large multivesicular bodies (MVBs). Exosomes play an important role in not only cellular homeostasis, but also in the pathogenesis of major human diseases. More evidence suggested that exosomes carry material from one cell to other cell for initiation of disease. Further, exosomes have been implicated for a promising source of disease-associated biomarkers, and may eventually be used as delivery vehicle for targeted biological therapies. Extracellular vesicles may be produced by budding from the extracellular membrane yielding particles from 100 to 1,000 nm known as microvesicles, shedding microvesicles or microparticles. Apoptotic vesicles are formed by large-scale plasma membrane blebbing, released during apoptotic cell death and are generally larger (100–5,000 nm in diameter) (Van der Pol et al., 2012). Apoptotic bodies are not the focus of this review wherein we focus on EVs released under sublethal pathophysiologic conditions. Recently, a larger size EV population (1–10 μm diameter) was identified from highly migratory cancer cells termed oncosomes (Wendler et al., 2016).
1.2. Biogenesis and cellular release of extracellular vesicles
The process of distinguishing exosomes and microvesicles are based on their biogenesis and release into extracellular milieu. However, this process is still not well understood and many studies suggest that the mechanisms of exosome biogenesis can be cell specific and pathological or physiological condition of the cells, the stimuli triggering their release, and the different pathways of EV biogenesis (Van der Pol et al., 2012; Kourembanas, 2015). Exosomes are mainly secreted by two different mechanisms, constitutive release via the trans-Golgi network and inducible release (Perez-Hernandez et al., 2013, Record et al., 2014). In the vesicle generation process, the coordination of endosomal sorting complex proteins (ESCRTs) plays important roles. ESCRT0 ubiquitinates proteins for MVB delivery and recruits ESCRTI to endosomal membrane, which in turn recruits ESCRTII and ESCRTIII. Polymeric filaments formation are mediated by ESCRTIII which leads to membrane invaginations and eventually results in intraluminal vesicle (ILV) formation (Katzmann et al., 2001; Babst et al., 2002; Wollert et al., 2009; Tamai et al., 2010; Kowal et al., 2014). The presence of ESCRT components in exosomes was identified using high throughput protein analysis methods. Downregulation of key components of ESCRT system abrogates ILV formation and release of exosomes (Tamai et al., 2010).
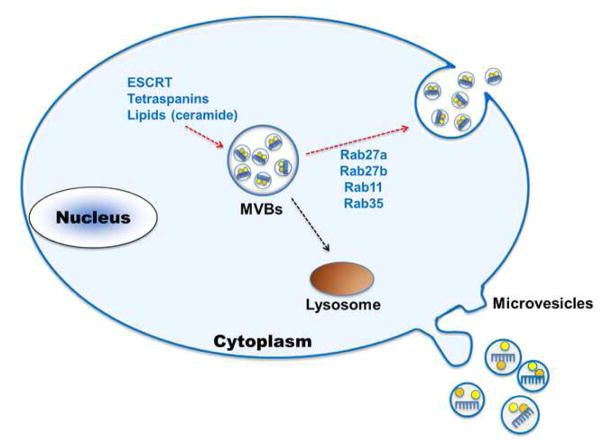
Exosome release is also controlled by Rab guanosine triphosphatases. For example, Rab11 associates with vesicles derived from the trans-Golgi network, promoting MVB formation and subsequent plasma membrane fusion (Hirsova et al., 2016). We observed that knockdown of Rab27a inhibits generation of intracellular and extracellular infectious hepatitis C virus particles (Shrivastava et al., 2015). Further, silencing of Rab27a decreases exosome release in the culture supernatant without altering the exosome protein content (Ostrowski et al., 2010; Shrivastava et al., 2015). The process of biogenesis and exosome secretion is described elsewhere (Kowal et al., 2014; Hirsova et al., 2016; Mass et al., 2017) and summarized in figure 2.
Figure 2. Schematic representation of exosome biogenesis by eukaryotic cells.
Exosomes are formed as ILVs by budding into early endosomes and MVBs. Several molecules are involved in the biogenesis of ILVs, such as the ESCRT machinery, lipids (such as ceramide) and the tetraspanins. The fate of MVBs can be either fusion with lysosomes or fusion with the PM, which allows the release of their content to the extracellular milieu. Several RAB proteins (RAB11, RAB27 and RAB35) have been shown to be involved in the transport of MVBs to the PM and in exosome secretion. Other types of secreted vesicles bud directly from the plasma membrane, and are often called microvesicles.
Methods of exosomes isolation are summarized below. Culture supernatants from control or virus infected hepatocytes were collected and centrifuged at 300 x g at 4 °C for 5 min. Without disturbing the cell pellet, supernatants were transferred to new tube. Supernatants then sequentially centrifuged at 2,000 x g for 10 min at 4 °C, 26,500 x g for 30 min at 4 °C followed by at 110,000 x g for 90 min at 4 °C. After discarding the supernatant, and the exosome pellet was washed two times with PBS by centrifugation at 110,000 x g for 60 min at 4 °C. The final pellet was suspended in PBS to 1/10 of the original volume of culture supernatant for analysis. Alternatively, culture supernatants were centrifuged at 3,000 x g at 4 °C for 15 min. ExoQuick reagent from System Bioscience (Palo Alto, CA) at a ratio of 5:1 was added, mixed well and incubated overnight at 4 °C. Mixer was centrifuged at 2,000 x g for 30 min at 4 °C. Residual supernatant was removed and pellet was suspended in PBS for analysis. Other methods are discussed in a rodent review (Szabo and Momen-Heravi, 2017). The size distribution of exosomes was checked by dynamic light scattering (DLS) using a Zetasizer Nano (Malvern Instruments).
Extracellular vesicles alterations in liver diseases
EVs have been the topic of great interest in recent years in medical research. In particular, EVs are of great interest in liver pathology because they regulate cell-cell communications and a number of pathophysiological events in various types of cells via horizontal transfer of their cargo which can be transferred from donor cells to recipient cells and can activate or regulate cell activities such as protein expression, cell proliferation and differentiation or antiviral responses.
1.3. The role of extracellular vesicles in viral hepatitis
Extracellular vesicles, especially exosomes, are involved in viral spread, immune regulation and antiviral response during viral infection (Chahar et al., 2015; Kouwaki et al., 2017). EVs are important players in the pathogenesis of viral hepatitis, including viral spread, antiviral innate immune response, and initiation of disease progression. Hepatitis A virus (HAV) is a hepatotropic positive-strand RNA virus. HAV infection occurs sporadically and in epidemics worldwide, and causes moderate to severe acute liver inflammation but is unable to establish persistent infection. It has been shown that exosomally packaged virions, termed eHAV, are responsible for plasmacytoid dendritic cell (pDC) activation and spread of infection (Feng et al., 2013). eHAVs may be egressed via an exosome-like mechanism involving endosomal budding of HAV capsids into MVBs (McKnight et al., 2017)
Hepatitis B virus (HBV) infection is one of the major causes of hepatocellular carcinoma. Interferon-α (IFN-α) induced antiviral response can be spread from liver nonparenchymal cells (LNPCs) to HBV-infected hepatocytes via exosomes (Li et al., 2013). Exosomes isolated from chronic hepatitis B (CHB) patients’ sera contained HBV viral components, which induces active HBV infection in naive hepatocytes and also into NK cells (Yang et al., 2017). Exposure to HBV in NK cells causes NK-cell dysfunction, and impairment of innate immune response.
Hepatitis C virus (HCV), a blood borne human pathogen, is a major player for end-stage liver disease. EVs, especially exosomes, isolated from HCV infected hepatocytes transmit HCV infection in vitro to naive human hepatoma cells and establishing a productive infection (Ramakrishnaiah et al., 2013; Longatti et al., 2015; Shrivastava et al., 2015). Exosomes isolated from HCV-infected patients’ sera contain HCV RNA and elevated levels of Ago2 and HSP90 proteins helping viral receptor-mediated transmission to hepatocytes (Bukong et al., 2014). Liver-resident plasmacytoid dendritic cells (pDCs) produce type I interferon when exposed to exosomes isolated from infected hepatocytes (Dreux et al., 2012). Exosomes isolated from liver endothelial cells stimulated by either type I or III IFNs can suppress HCV replication in infected hepatocytes (Giugliano et al., 2015).
Hepatitis E virus (HEV) causes acute and chronic hepatitis in humans. HEV particles are transferred and released through the MVBs (Nagashima et al., 2014). Treatment of exosome blocker or silencing Rab27A or Hrs significantly decreases HEV particles released from the cells.
Circulating EVs are heterogeneous and derived from diverse cell types. Therefore, it is possible that immune cell derived EVs from patients with chronic hepatitis C could be differentiated from patients with nonalcoholic steatohepatitis (NASH) and may be used as markers (Kornek M et al, 2012). These findings suggest that both viral infection and antiviral response are mediated by cell-cell communication through exosomes depending on the type of pathogen and target cells. Thus, exosomes can be exploited as candidate for antiviral or vaccine (Devhare and Ray, 2017). Further, identification and delivery of specific antiviral molecules through EVs is a potential therapeutic strategy for chronic hepatitis B and C.
1.4. The role of extracellular vesicles in non-alcoholic steatohepatitis
Hepatocyte cellular dysfunction and death induced by lipids and macrophage-associated inflammation are characteristics of NASH. Lipid induced EVs derived from hepatocytes contain tumor necrosis factor-related apoptosis-inducing ligand and induce inflammatory phenotype in mouse bone marrow-derived macrophages (Hirsova et al., 2016). EVs are released from hepatocytes in response to lipotoxic fatty acids and internalized by stellate cells leading to their fibrogenic activation (Ban et al., 2016). Lipotoxic hepatocytes also release ceramide enriched EVs which activates macrophage chemotaxis, a potential mechanism for the recruitment of macrophages to the liver under lipotoxic conditions (Kakazu et al., 2016). EVs released from lipotoxic hepatocytes activate hepatic stellate cells (HSCs) (Povero et al., 2015). MiR-128-3p carried via EVs suppresses proliferator-activated receptor-γ (PPAR-γ), a critical modulator of stellate cell activation. Patients with non-alcoholic fatty liver disease (NAFLD) or NASH secrete increased levels of microvesicles derived from macrophages and natural killer T cells (Kornek et al., 2012). Involvement of circulating EVs in the innate immune response with steatosis suggests their role in the progression from early NAFLD to NASH. NAFLD is implicated as a major driver of hepatic fibrosis. This is important from physiological perspective because encapsulation of certain molecules can prevent enzymatic degradation, especially in an unpredictable disease environment. On the other hand, these protected molecules may lead to stimulate inflammatory cells, resulting in exacerbation of tissue injury.
1.5. The role of extracellular vesicles in alcoholic hepatitis
EVs play a role in alcoholic hepatitis. Both hepatocyte-derived and monocyte-derived EVs have been suggested to regulate macrophage phenotype, resulting in inflammation in alcoholic hepatitis. Human monocytes exposed to ethanol release significant amounts of exosomes and these exosomes can stimulate naïve monocytes to polarize and differentiate into M2-macrophages. Monocytes exposed to alcohol also secrete exosomes containing increased levels of miR-27a leading to increased cytokine secretion, followed by activation and polarization of other monocytes (Saha et al., 2015 and 2016). EVs derived from ethanol exposed hepatocytes carry proteins for macrophage activation. CD40 ligand carried through EV cargo promotes macrophage activation in experimental models of alcoholic hepatitis (Verma et al., 2016). These findings suggest that cells under disease conditions secrete elevated level of EVs or exosomes containing unique molecules, and trigger various pathophysiological events. Several studies suggested that EVs are increased in patients with alcoholic hepatitis and even in patients consuming excess ethanol (Momen-Heravi et al., 2015; Saha et al., 2016). Indeed, further studies are necessary to understand whether number of EVs or molecules carried via EVs can serve as a diagnostic and/or prognostic biomarker for alcoholic hepatitis.
1.6. The role of extracellular vesicles in liver fibrosis
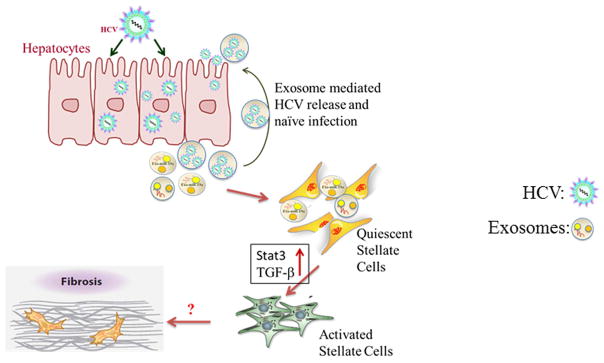
Hepatic fibrosis occurs from deregulation of wound healing with the accumulation of extracellular matrix (ECM), including collagen Type I, leading to scar formation. Different cell types in the liver make a network and relate to hepatic fibrogenesis regulation (Guo and Friedman, 2007). Hepatic stellate cells (HSCs) are involved in the pathogenesis of liver fibrosis. EVs carry several molecules, suggesting their role in cell-to-cell communication, and support the idea that exosomes might constitute an exquisite mechanism for local and systemic intercellular transfer not only of proteins but also of genetic information in the form of RNA. Exosomes mediated intercellular shuttling of miR-214 was reported to regulate connective tissue growth factor 2 (CCN2) dependent fibrogenesis in HSCs (Chen et al., 2014). CCN2 is over-expressed during liver fibrosis (Huang and Brigstock, 2012), carried by exosomes within HSCs and promotes fibrogenic activation (Charrier et al., 2014). Liver cell derived microparticles are shown to have angiogenic properties on liver endothelial cells (Witek et al., 2009; Povero et al., 2013). Recently, we evaluated exosome mediated intercellular communication between HCV infected hepatocytes and hepatic stellate cells (Devhare et al., 2017). We observed that the miR-19a carried through the exosomes from HCV-infected hepatocytes activates HSC by modulating SOCS-STAT3-TGFβ1 signaling axis (Fig. 3).
Figure 3. Schematic representation showing role of exosomes in HCV mediated liver disease.
Exosomes released from HCV infected hepatocytes carry microRNA including miR-19a (Exo-miR-19a). Exo-miR-19a enters in quiescent hepatic stellate cells for fibrogenic activation. Circulatory miR-19a, miR-20a and miR-92a were upregulated in HCV-infected fibrosis patients’ sera.
Endothelial cell-derived exosomes regulate pathological HSC activation by triggering sphingosine 1-phosphate dependent migration (Wang et al., 2015), suggesting paracrine crosstalk between endothelial cell and HSC. Therefore, EVs may be key mediators in fibrosis by enhancing HSC activation.
1.7. The role of extracellular vesicles in hepatocellular carcinoma
Emerging body of literature suggested the role of EVs in progression of hepatocellular carcinoma (HCC). For example, exosomes secreted from HCC cells contain a variety of miRNAs, which modulated the transforming growth factor β activated kinase-1 (TAK1) pathway in the other recipient HCC cells. As a result, anchorage independent growth of HCC cell lines is increased dramatically with HCC cell-derived exosomes (Kogure et al., 2011). HCC cell-derived exosomes also promoted the cell growth, migration, and invasion of HCC cells and had the ability to shuttle miRNAs to recipient cells (Wei et al., 2015). Exosomes derived from HCC cell lines stimulated recipient hepatocytes to secrete matrix metalloproteinase-2 and -9 that facilitated the invasion of HCC cells (He et al., 2015), suggesting their participation in tumor microenvironment. Furthermore a key regulator of exosome biogenesis, Vps4A, is downregulated in HCC tissues, which is associated with tumor progression and metastasis (Wei et al., 2015). This study showed that Vps4A utilizes exosomes as mediators to regulate the secretion and uptake of tumor suppressor miRNAs in hepatoma cells; unveiling a new mechanism of HCC development. Several non-coding RNAs such as, miRNAs and lncRNAs are involved in HCC process. Selected exosomal miRNAs (e.g., miR-584, miR-517c, miR-378, miR-520f, miR142-5p, miR-451, miR-518d, miR-215, miR-376a, miR-133b, and miR-367 are released from HCC cell lines and HCC developed in rodents (Santangelo et al., 2017). A few lncRNAs such as VLDLR, ROR, and TUC339 are found in circulating EVs. Exosomes released by CD90+ liver cancer stem like cells promote angiogenic phenotype and cell-to-cell adhesion (Conigliaro et al., 2015). Furthermore, the authors suggested the lncRNA H19, carried via exosomes, as a possible mediator of angiogenic effects. Therefore, these studies suggested an important role of EVs in the development of HCC. We have summarized the possible role of non-coding RNAs in liver diseases in Table 1.
Table 1.
Extracellular vesicles associated non-coding RNAs in liver disease
| Non-coding RNA based biomarkers [expression] | Biological samples | Species | Type of liver disease | Reference |
|---|---|---|---|---|
| Micro-RNA (miR) | ||||
| miR19a [↑] | Serum and cell line | Human | HCV infected patient and liver fibrosis | Devhare PB, 2017 |
| miR 122 [↑] | Serum | Human, mice | Alcohol induced hepatitis, viral hepatitis, HCC | Yu X, 2016; Szabo G, 2017; Maji S, 2017 |
| miR 122, miR 155 [↑] | Serum | mice | Alcohol induced hepatitis | Bala S, 2012; |
| miR 718 [↓] | Serum | Human | HCC | Szabo G, 2017; Sugimachi K, 2015 |
| miR21 [↑] | Serum | Human | HCC, Hepatoblastoma | Szabo G, 2017; Liu W, 2016; Wang H, 2014 |
| miRNA-34a, miRNA-34b and miRNA-34c [↓] | Serum | Human | Hepatoblastoma | Jiao C, 2017 |
| miR214 [↑] | Serum | Mice | Liver fibrosis | Szabo G, 2017; |
| miRNA-192, miRNA-122, and miRNA-30a [↑] | Serum | Mice, human | Alcohol induced hepatitis, fatty liver disease | Momen-Heravi F, 2015; Maji S, 2017; Povero D, 2014 |
| miR 122, miR134, miR424-3p, miR629-5p [↑] | Serum | Human | HCV hepatitis | Zhang S, 2015; |
| miR 198 [↓] | Serum | Human | HCC | Yu X, 2016 |
| miR-939, miR- 595, and miR- 519d | Serum | Human | differentially expressed in cirrhotic patients with and without HCC | Fornari F, 2015 |
| miR-18a, miR- 221, miR-222, and miR-224 [↑] | Serum | Human | High in patients with chronic hepatitis B virus (HBV)- related HCC than in those with either HBV alone or liver cirrhosis | Sohn W, 2015 |
| miR-101, miR-106b, miR-122, and miR-195 | Serum | Human | low in patients with HCC than in patients with HBV | Sohn W, 2015 |
| miR320 [↓] | Xenograft tumor | Mice | HCC | Zhang Z, 2017 |
| miR-519d, miR- 21, miR-221 and miR-1228 [differential expression] | Serum | Human | Cirrhotic patients with and without HCC | Fornari F, 2015 |
| miR-18a, miR-221, miR-222 and miR-224 [↑] | Serum | Human | HCC | Sohn W, 2015 |
| miR-221, let-7a, and miR-26a | Serum | Human | HCC | Li Y, 2015 |
| Long-non coding RNA (lncRNA) | ||||
| lncRNA-ROR [↑] | Tumor | Human | HCC | aTakahashi K, 2014; bTakahashi K, 2014 |
| lncRNA- VLDLR [↑] | Tumor | Human | HCC | cTakahashi K, 2014 |
| lncRNA- TUC339 [↑] | Tumor | Human | HCC | Kogure T, 2013 |
| lncRNA H19 [↑] | CD90+ cancer cells | Human | HCC | Conigliaro A, 2015 |
1.8. Extracellular vesicles as Biomarkers
HCC is a silent disease and identification of a potential diagnostic biomarker is a thrust area of research in the field. Since EVs carry unique molecules such as proteins, mRNAs and miRNAs, the potential of EVs to use as a biomarker is quite high. Moreover, blood EVs can serve as a “liquid biopsy” and is minimally invasive, and can reduce the use of invasive liver biopsy for diagnosis of liver disease severity.
Expression profile of miRNAs in serum exosomes between patients with and without HCC recurrence after liver transplantation found that miR-718 is significantly decreased in exosomes of patients with HCC recurrence (Sugimachi et al., 2015). MiR-21 was found to be enriched in serum exosomes from patients with HCC which may serve as a potential biomarker for HCC diagnosis (Wang et al., 2014). Another study demonstrated the higher levels of serum exosomal miR-18a, miR-221, miR-222 and miR-224 and lower levels of miR-101, miR-106b, miR-122 and miR-195 in patients with HCC, suggesting the potential role of serum exosomal microRNAs as novel serological biomarkers for HCC (Sohn et al., 2015). We also have demonstrated the role of exosomal miR-19a in fibrogenic activation of HSCs as well as the higher expression of serum derived exosomal miR-19a and miR-20a in patients with HCV mediated liver fibrosis (Shrivastava et al., 2013; Devhare et al., 2017) implicating their role as predictive biomarkers of HCV mediated liver disease. The expression profile of miRNAs in circulating vesicles of fibrotic patients with early stage of HBV and HCV induced liver fibrosis suggested the potential use of these vesicle-associated miRNAs as markers for early stages of liver fibrosis (Lambrecht et al., 2017).
Hepatocyte derived EVs contain mRNAs (Herrera et al., 2010) and liver specific mRNAs are found in bloodstream of galactosamine-induced liver damage in animal models. For detecting liver damage, amplification and quantification of these nucleic acids in blood samples have proved to be more sensitive than traditional transaminase activity quantification (Wetmore et al., 2010). The effect of CCl4-injured hepatocytes on the differentiation of the non-adherent fraction of bone marrow-derived mononuclear cells was investigated. Hepatocyte-like characteristics were observed in the non-adherent bone marrow-derived mononuclear cells after 24h of co-culture with injured hepatocytes. Microvesicles derived from differentiated cells revealed the presence of hepatocyte-specific mRNAs, including albumin, coagulation factor V, alpha-fetoprotein, and cytokeratin 18, suggesting a possible role in the induction of cell plasticity (Simon et al., 2015). Circulating miRNAs in serum exosomes have potential as novel biomarkers for predicting HCC recurrence.
Recently, lncRNAs are found to be upregulated in HCC patient sera. lncRNA PVT1 and uc002mbe.2 showed potential as a diagnostic marker for HCC (Yu et al., 2016). These lncRNAs display association with clinical parameters including tumor size, Barcelona Clinic Liver Cancer (BCLC) stage, and serum bilirubin. Together, this information suggested that further work is needed to establish EVs as a potential diagnostic biomarker for liver disease.
1.9. Extracellular vesicles role in therapeutic approaches
Emerging studies demonstrated the importance of EVs in disease progression, suggesting they can be potential target for therapeutic intervention. EV-mediated pathological processes can be interrupted by inhibiting EV release, specific cargo carried by the vesicles or modulating the EV-target cell interaction. For example, inhibition of multiple proteins from the RAB guanosine triphosphatase family can prevent EV release. The effect of EVs can be disrupted by inhibiting a specific signaling molecule into EVs. Mixed lineage kinase 3 inhibitors can prevent a lipotoxicity-induced enrichment of C-X-C motif ligand 10 (CXCL10) in hepatocyte-derived EVs and decrease immune cell infiltration of the liver during NASH (Ibrahim et al., 2016). Blocking specific EV components or their interacting counterparts on the acceptor cells can inhibit the interaction between EV and target cell. Blockade of the fibronectin-integrin interaction attenuates exosome-induced HSC AKT phosphorylation and migration (Wang et al., 2015).
There are several advantages using EV-based therapy. EVs show low immunogenicity, toxicity and are stable in tissues and in circulation. EVs, especially exosomes, are used for anti-tumor vaccine delivery in clinical trials in non-small cell lung cancer patients (Lener et al., 2015). EVs can also be used as a vehicle for drug delivery. Both unmodified and engineered EVs can deliver nucleic acids, proteins, lipids, peptides, and chemotherapeutics to the target cells (Hirsova et al., 2016). Tumor-derived exosomes potently carry HCC antigens are reported to elicit a strong DC-mediated immune response for educating HCC microenvironment and tumor suppression in preclinical model (Rao et al., 2016). Together this information suggests that EVs have a great translational potential as a therapeutics or delivery vehicles for targeted therapy.
2. Summary and future prospects
Recent years have witnessed a renewed research interest in EVs, especially exosomes, and advances in our understanding of their assembly, release, and uptake and also the functions. Since EVs carry several molecules such as, DNA, protein, miRNAs, mRNAs, lncRNAs, they help in intra-cellular as well as inter-cellular communication. For liver cancer, early diagnosis is extremely important. It is conceivable that EVs carry specific elements (like distinct noncoding RNAs- miRNAs and lncRNAs) which can be considered as predictive biomarker. We and others have shown that HCV can release via exosomes and blocking exosome release can lower the HCV titer in cell culture system (Ramakrishnaiah et al., 2013; Shrivastava et al., 2015). EVs isolated from HCV infected hepatocytes carry noncoding RNAs which activates hepatic stellate cells towards fibrosis (Devhare et al., 2017). Emerging evidences suggest that EVs secreted from tumor cells either promote antitumor immune responses for tumor growth or attenuates anti-tumor immunity. The role of other liver diseases such as liver injury or cholangiopathies are discussed elsewhere (Hirsova et al., 2016). There are several outstanding challenges and questions remain for EVs. Isolation and purification of EVs especially exosomes are coming to an agreement, however due to diverse size of these molecules, it should be rigorously monitored to avoid over-interpreting tantalizing observations. Uncovering the role of EVs for cell-cell communication may deliver new tools to further improve therapeutics and diagnosis.
Supplementary Material
Acknowledgments
Funding
This work was supported by research grants R01 DK081817 and R21CA188472 from the National Institutes of Health.
We thank Subhayan Sur and Reina Sasaki for their comments for this manuscript. We tried to cover several current citations, however it is impossible to cover all. We apologize in advance if we miss them.
Footnotes
Conflicts of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Babst M, Katzmann DJ, Snyder WB, Wendland B, Emr SD. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev Cell. 2002;3:283–289. doi: 10.1016/s1534-5807(02)00219-8. [DOI] [PubMed] [Google Scholar]
- 2.Bala S, Petrasek J, Mundkur S, Catalano D, Levin I, Ward J, Alao H, Kodys K, Szabo G. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology. 2012;56(5):1946–57. doi: 10.1002/hep.25873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balaj L, Lessard R, Dai L, Cho Y-J, Pomeroy SL, Breakefield XO, Skog J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat Commun. 2011;2:180. doi: 10.1038/ncomms1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ban LA, Shackel NA, McLennan SV. Extracellular Vesicles: A New Frontier in Biomarker Discovery for Non-Alcoholic Fatty Liver Disease. Int J Mol Sci. 2016;17(3):376. doi: 10.3390/ijms17030376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukong TN, Momen-Heravi F, Kodys K, Bala S, Szabo G. Exosomes from hepatitis C infected patients transmit HCV infection and contain replication competent viral RNA in complex with Ago2-miR122-HSP90. PLoS Pathog. 2014;10:e1004424. doi: 10.1371/journal.ppat.1004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chahar HS, Bao X, Casola A. Exosomes and their role in the life cycle and pathogenesis of RNA viruses. Viruses. 2015;7(6):3204–3225. doi: 10.3390/v7062770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charrier A, Chen R, Chen L, Kemper S, Hattori T, Takigawa M, et al. Exosomes mediate intercellular transfer of pro-fibrogenic connective tissue growth factor (CCN2) between hepatic stellate cells, the principal fibrotic cells in the liver. Surgery. 2014;156:548–555. doi: 10.1016/j.surg.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L, Charrier A, Zhou Y, Chen R, Yu B, Agarwal K, et al. Epigenetic regulation of connective tissue growth factor by microRNA-214 delivery in exosomes from mouse or human hepatic stellate cells. Hepatology. 2014;59:1118–1129. doi: 10.1002/hep.26768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 10.Conigliaro A, Costa V, Lo Dico A, Saieva L, Buccheri S, Dieli F, Manno M, Raccosta S, Mancone C, Tripodi M, De Leo G, Alessandro R. CD90+ liver cancer cells modulate endothelial cell phenotype through the release of exosomes containing H19 lncRNA. Mol Cancer. 2015;14:155. doi: 10.1186/s12943-015-0426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cossetti C, Iraci N, Mercer TR, Leonardi T, Alpi E, Drago D, Alfaro-Cervello C, Saini HK, Davis MP, Schaeffer J. Extracellular vesicles from neural stem cells transfer IFN-γ via Ifngr1 to activate Stat1 signaling in target cells. Mol Cell. 2014;56:193–204. doi: 10.1016/j.molcel.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crispe IN. The liver as a lymphoid organ. Annu Rev Immunol. 2009;27:147–163. doi: 10.1146/annurev.immunol.021908.132629. [DOI] [PubMed] [Google Scholar]
- 13.Devhare PB, Ray RB. A novel role of exosomes in the vaccination approach. Ann Transl Med. 2017;5(1):23. doi: 10.21037/atm.2016.12.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devhare PB, Sasaki R, Shrivastava S, Di Bisceglie AM, Ray R, Ray RB. Exosome-Mediated Intercellular Communication between Hepatitis C Virus-Infected Hepatocytes and Hepatic Stellate Cells. J Virol. 2017;91(6) doi: 10.1128/JVI.02225-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dreux M, Garaigorta U, Boyd B, Décembre E, Chung J, Whitten-Bauer C, Wieland S, Chisari FV. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe. 2012;12(4):558–70. doi: 10.1016/j.chom.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng Z, Hensley L, McKnight KL, Hu F, Madden V, Ping L, Jeong SH, Walker C, Lanford RE, Lemon SM. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature. 2013;496(7445):367–71. doi: 10.1038/nature12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fornari F, Ferracin M, Trerè D, Milazzo M, Marinelli S, Galassi M, Venerandi L, Pollutri D, Patrizi C, Borghi A, Foschi FG, Stefanini GF, Negrini M, Bolondi L, Gramantieri L. Circulating microRNAs, miR-939, miR-595, miR-519d and miR-494, Identify Cirrhotic Patients with HCC. PLoS One. 2015;10(10):e0141448. doi: 10.1371/journal.pone.0141448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giugliano S, Kriss M, Golden-Mason L, Dobrinskikh E, Stone AE, Soto-Gutierrez A, et al. Hepatitis C virus infection induces autocrine interferon signaling by human liver endothelial cells and release of exosomes, which inhibits viral replication. Gastroenterology. 2015;148:e313. doi: 10.1053/j.gastro.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo J, Friedman SL. Hepatic fibrogenesis. Semin Liver Dis. 2007;27(4):413–26. doi: 10.1055/s-2007-991517. [DOI] [PubMed] [Google Scholar]
- 20.He M, Qin H, Poon TC, Sze SC, Ding X, Co NN, et al. Hepatocellular carcinoma-derived exosomes promote motility of immortalized hepatocyte through transfer of oncogenic proteins and RNAs. Carcinogenesis. 2015;36:1008–1018. doi: 10.1093/carcin/bgv081. [DOI] [PubMed] [Google Scholar]
- 21.Herrera MB, Fonsato V, Gatti S, Deregibus MC, Sordi A, Cantarella D, et al. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med. 2010;14:1605–18. doi: 10.1111/j.1582-4934.2009.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirsova P, Ibrahim SH, Verma VK, Morton LA, Shah VH, LaRusso NF, Gores GJ, Malhi H. Extracellular vesicles in liver pathobiology: Small particles with big impact. Hepatology. 2016;64(6):2219–2233. doi: 10.1002/hep.28814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang G, Brigstock DR. Regulation of hepatic stellate cells by connective tissue growth factor. Front Biosci. 2012;17:2495–507. doi: 10.2741/4067. [DOI] [PubMed] [Google Scholar]
- 24.Huang-Doran I, Zhang CY, Vidal-Puig A. Extracellular Vesicles: Novel Mediators of Cell Communication in Metabolic Disease. Trends Endocrinol Metab. 2017;28(1):3–18. doi: 10.1016/j.tem.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim SH, Hirsova P, Tomita K, Bronk SF, Werneburg NW, Harrison SA, Goodfellow VS, Malhi H, Gores GJ. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology. 2016;63(3):731–44. doi: 10.1002/hep.28252. Epub 2015 Dec 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiao C, Jiao X, Zhu A, Ge J, Xu X. Exosomal miR-34s panel as potential novel diagnostic and prognostic biomarker in patients with hepatoblastoma. J Pediatr Surg. 2017;52(4):618–624. doi: 10.1016/j.jpedsurg.2016.09.070. [DOI] [PubMed] [Google Scholar]
- 27.Kakazu E, Mauer AS, Yin M, Malhi H. Hepatocytes release ceramide-enriched pro-inflammatory extracellular vesicles in an IRE1α-dependent manner. J Lipid Res. 2016;57(2):233–45. doi: 10.1194/jlr.M063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katzmann DJ, Babst M, Emr SD. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–155. doi: 10.1016/s0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- 29.Keller S, Ridinger J, Rupp A-K, Janssen J, Altevogt P. Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med. 2011;9 doi: 10.1186/1479-5876-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kogure T, Lin WL, Yan IK, Braconi C, Patel T. Intercellular nanovesicle-mediated microRNA transfer: a mechanism of environmental modulation of hepatocellular cancer cell growth. Hepatology. 2011;54(4):1237–48. doi: 10.1002/hep.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kogure T, Yan IK, Lin WL, Patel T. Extracellular Vesicle-Mediated Transfer of a Novel Long Noncoding RNA TUC339: A Mechanism of Intercellular Signaling in Human Hepatocellular Cancer. Genes Cancer. 2013;4(7–8):261–72. doi: 10.1177/1947601913499020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kornek M, Lynch M, Mehta SH, Lai M, Exley M, Afdhal NH, et al. Circulating microparticles as disease-specific biomarkers of severity of inflammation in patients with hepatitis C or nonalcoholic steatohepatitis. Gastroenterology. 2012;143:448–458. doi: 10.1053/j.gastro.2012.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu Rev Physiol. 2015;77:13–27. doi: 10.1146/annurev-physiol-021014-071641. [DOI] [PubMed] [Google Scholar]
- 34.Kouwaki T, Okamoto M, Tsukamoto H, Fukushima Y, Oshiumi H. Extracellular Vesicles Deliver Host and Virus RNA and Regulate Innate Immune Response. Int J Mol Sci. 2017;18:666. doi: 10.3390/ijms18030666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kowal J, Tkach M, Théry C. Biogenesis and secretion of exosomes. Curr Opin Cell Biol. 2014;29:116–25. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Lambrecht J, Jan Poortmans P, Verhulst S, Reynaert H, Mannaerts I, et al. Circulating ECV-Associated miRNAs as Potential Clinical Biomarkers in Early Stage HBV and HCV Induced Liver Fibrosis. Front Pharmacol. 2017;9(8):56. doi: 10.3389/fphar.2017.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lässer C, Alikhani VS, Ekström K, Eldh M, Paredes PT, Bossios A, Sjöstrand M, Gabrielsson S, Lötvall J, Valadi H. Human saliva, plasma and breast milk exosomes contain RNA: Uptake by macrophages. J Transl Med. 2011:9. doi: 10.1186/1479-5876-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lener T, Gimona M, Aigner L, Bõrger V, Buzas E, Camussi G, et al. Applying extracellular vesicles based therapeutics in clinical trials – an ISEV position paper. J Extracell Vesicles. 2015;4:30087. doi: 10.3402/jev.v4.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Liu K, Liu Y, Xu Y, Zhang F, Yang H, Liu J, Pan T, Chen J, Wu M, Zhou X, Yuan Z. Exosomes mediate the cell-to-cell transmission of IFN-α-induced antiviral activity. Nat Immunol. 2013;14(8):793–803. doi: 10.1038/ni.2647. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Xiang GM, Liu LL, Liu C, Liu F, Jiang DN, Pu XY. Assessment of endogenous reference gene suitability for serum exosomal microRNA expression analysis in liver carcinoma resection studies. Mol Med Rep. 2015;12(3):4683–91. doi: 10.3892/mmr.2015.3919. [DOI] [PubMed] [Google Scholar]
- 41.Liu W, Chen S, Liu B. Diagnostic and prognostic values of serum exosomal microRNA-21 in children with hepatoblastoma: a Chinese population-based study. Pediatr Surg Int. 2016;32(11):1059–1065. doi: 10.1007/s00383-016-3960-8. [DOI] [PubMed] [Google Scholar]
- 42.Longatti A, Boyd B, Chisari FV. Virion-independent transfer of replication-competent hepatitis C virus RNA between permissive cells. J Virol. 2015;89(5):2956–61. doi: 10.1128/JVI.02721-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maas SLN, Breakefield XO, Weaver AM. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends Cell Biol. 2017;27(3):172–188. doi: 10.1016/j.tcb.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maji S, Matsuda A, Yan IK, Parasramka M, Patel T. Extracellular vesicles in liver diseases. Am J Physiol Gastrointest Liver Physiol. 2017;312(3):G194–G200. doi: 10.1152/ajpgi.00216.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McKnight KL, Xie L, González-López O, Rivera-Serrano EE, Chen X, Lemon SM. Protein composition of the hepatitis A virus quasi-envelope. Proc Natl Acad Sci U S A. 2017;114(25):6587–6592. doi: 10.1073/pnas.1619519114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Momen-Heravi F, Saha B, Kodys K, Catalano D, Satishchandran A, Szabo G. Increased number of circulating exosomes and their microRNA cargos are potential novel biomarkers in alcoholic hepatitis. J Transl Med. 2015;13:261. doi: 10.1186/s12967-015-0623-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nagashima S, Jirintai S, Takahashi M, Kobayashi T, Tanggis Nishizawa T, Kouki T, Yashiro T, Okamoto H. Hepatitis E virus egress depends on the exosomal pathway, with secretory exosomes derived from multivesicular bodies. J Gen Virol. 2014;95(Pt 10):2166–75. doi: 10.1099/vir.0.066910-0. [DOI] [PubMed] [Google Scholar]
- 48.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, Seabra MC, Darchen F, Amigorena S, Moita LF, Thery C. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30. doi: 10.1038/ncb2000. sup pp 1–13. [DOI] [PubMed] [Google Scholar]
- 49.Perez-Hernandez D, Gutierrez-Vazquez C, Jorge I, Lopez-Martin S, Ursa A, Sanchez-Madrid F, Vazquez J, Yanez-Mo M. The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes. J Biol Chem. 2013;288:11649–11661. doi: 10.1074/jbc.M112.445304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Povero D, Eguchi A, Li H, Johnson CD, Papouchado BG, Wree A, Messer K, Feldstein AE. Circulating extracellular vesicles with specific proteome and liver microRNAs are potential biomarkers for liver injury in experimental fatty liver disease. PLoS One. 2014;9(12):e113651. doi: 10.1371/journal.pone.0113651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Povero D, Eguchi A, Niesman IR, Andronikou N, de Mollerat du Jeu X, Mulya A, et al. Lipid-induced toxicity stimulates hepatocytes to release angiogenic microparticles that require Vanin-1 for uptake by endothelial cells. Sci Signal. 2013;6(296):ra88. doi: 10.1126/scisignal.2004512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Povero D, Panera N, Eguchi A. Lipid-induced hepatocyte-derived extracellular vesicles regulate hepatic stellate cells via microRNA targeting peroxisome proliferator-activated receptor-γ. Cell Mol Gastroenterol Hepatol. 2015;1(6):646–663. doi: 10.1016/j.jcmgh.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ramakrishnaiah V, Thumann C, Fofana I, Habersetzer F, Pan, et al. Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc Natl Acad Sci U S A. 2013;110(32):13109–13113. doi: 10.1073/pnas.1221899110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rao Q, Zuo B, Lu Z, Gao X, You A, Wu C, Du Z, Yin H. Tumor-derived exosomes elicit tumor suppression in murine hepatocellular carcinoma models and humans in vitro. Hepatology. 2016;64(2):456–72. doi: 10.1002/hep.28549. [DOI] [PubMed] [Google Scholar]
- 55.Raposo G, Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Record M, Carayon K, Poirot M, Silvente-Poirot S. Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. BiochimBiophysActa. 2014;1841:108–120. doi: 10.1016/j.bbalip.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 57.Saha B, Bruneau JC, Kodys K, Szabo G. Alcohol-induced miR-27a regulates differentiation and M2 macrophage polarization of normal human monocytes. J Immunol. 2015;194:3079–3087. doi: 10.4049/jimmunol.1402190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saha B, Momen-Heravi F, Kodys K, Szabo G. MicroRNA cargo of extracellular vesicles from alcohol-exposed monocytes signals naive monocytes to differentiate into M2 macrophages. J Biol Chem. 2016;291:149–159. doi: 10.1074/jbc.M115.694133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Santangelo L, Battistelli C, Montaldo C, Citarella F, Strippoli R, Cicchini C. Functional Roles and Therapeutic Applications of Exosomes in Hepatocellular Carcinoma. Biomed Res Int. 2017;2017:2931813. doi: 10.1155/2017/2931813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shrivastava S, Devhare P, Sujijantarat N, Steele R, Kwon Y-C, Ray R, Ray RB. Knockdown of Autophagy Inhibits Infectious Hepatitis C Virus Release by the Exosomal Pathway. J Virol. 2015;90(3):1387–96. doi: 10.1128/JVI.02383-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shrivastava S, Petrone J, Steele R, Lauer GM, Di Bisceglie AM, Ray RB. Upregulation of Circulating miR-20a is correlated with Hepatitis C Virus Mediated Liver Disease Progression. Hepatology. 2013;58(3):863–71. doi: 10.1002/hep.26296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Simon L, López M, Uribe-Cruz C, Vergara DF, Silla L, Matte U. Injured hepatocyte-released microvesicles induce bone marrow-derived mononuclear cells differentiation. Differentiation. 2015;90(1–3):40–7. doi: 10.1016/j.diff.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 63.Simons M, Raposo G. Exosomes-vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 64.Sohn W, Kim J, Kang SH, Yang SR, Cho JY, Cho HC, Shim SG, Paik YH. Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma. Exp Mol Med. 2015;47:e184. doi: 10.1038/emm.2015.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sugimachi K, Matsumura T, Hirata H, Uchi R, Ueda M, Ueo H, Shinden Y, Iguchi T, Eguchi H, Shirabe K, Ochiya T, Maehara Y, Mimori K. Identification of a bona fide microRNA biomarker in serum exosomes that predicts hepatocellular carcinoma recurrence after liver transplantation. Br J Cancer. 2015;112(3):532–8. doi: 10.1038/bjc.2014.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Szabo G, Momen-Heravi F. Extracellular vesicles in liver disease and potential as biomarkers and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2017;14(8):455–466. doi: 10.1038/nrgastro.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tamai K, Tanaka N, Nakano T, Kakazu E, Kondo Y, Inoue J, Shiina M, Fukushima K, Hoshino T, Sano K, et al. Exosome secretion of dendritic cells is regulated by Hrs, an ESCRT-0 protein. Biochem Biophys Res Commun. 2010;399:384–390. doi: 10.1016/j.bbrc.2010.07.083. [DOI] [PubMed] [Google Scholar]
- 68.Takahashi K, Yan IK, Kogure T, Haga H, Patel T. Extracellular vesicle-mediated transfer of long non-coding RNA ROR modulates chemosensitivity in human hepatocellular cancer. FEBS Open Bio. 2014;4:458–67. doi: 10.1016/j.fob.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takahashi K, Yan IK, Haga H, Patel T. Modulation of hypoxia-signaling pathways by extracellular linc-RoR. J Cell Sci. 2014;127(Pt 7):1585–94. doi: 10.1242/jcs.141069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takahashi K, Yan IK, Wood J, Haga H, Patel T. Involvement of extracellular vesicle long noncoding RNA (linc-VLDLR) in tumor cell responses to chemotherapy. Mol Cancer Res. 2014;12(10):1377–87. doi: 10.1158/1541-7786.MCR-13-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;3:22.1–3.22.29. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 72.Tkach M, Théry C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell. 2016;164:1226–32. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 73.Trams EG, Lauter CJ, Salem N, Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645:63–70. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- 74.Van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64(3):676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- 75.Verma VK, Li H, Wang R, Hirsova P, Mushref M, Liu Y, Cao S, Contreras PC, Malhi H, Kamath PS, Gores GJ, Shah VH. Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles. J Hepatol. 2016;64(3):651–60. doi: 10.1016/j.jhep.2015.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang H, Hou L, Li A, Duan Y, Gao H, Song X. Expression of serum exosomal microRNA-21 in human hepatocellular carcinoma. Biomed Res Int. 2014;2014:864894. doi: 10.1155/2014/864894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang R, Ding Q, Yaqoob U, de Assuncao TM, Verma VK, et al. Exosome Adherence and Internalization by Hepatic Stellate Cells Triggers Sphingosine 1-Phosphate-dependent Migration. J Biol Chem. 2015;290(52):30684–96. doi: 10.1074/jbc.M115.671735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei JX, Lv LH, Wan YL, Cao Y, Li GL, Lin HM, Zhou R, Shang CZ, Cao J, He H, Han QF, Liu PQ, Zhou G, Min J. Vps4A functions as a tumor suppressor by regulating the secretion and uptake of exosomal microRNAs in human hepatoma cells. Hepatology. 2015;61(4):1284–94. doi: 10.1002/hep.27660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wendler F, Stamp GW, Giamas G. Tumor-Stromal Cell Communication: Small Vesicles Signal Big Changes. Trends Cancer. 2016;2(7):326–329. doi: 10.1016/j.trecan.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 80.Wetmore BA, Brees DJ, Singh R, Watkins PB, Andersen ME, Loy J, et al. Quantitative analyses and transcriptomic profiling of circulating messenger RNAs as biomarkers of rat liver injury. Hepatology. 2010;51(6):2127–39. doi: 10.1002/hep.23574. [DOI] [PubMed] [Google Scholar]
- 81.Witek RP, Yang L, Liu R, Jung Y, Omenetti A, Syn WK, et al. Liver cell-derived microparticles activate hedgehog signaling and alter gene expression in hepatic endothelial cells. Gastroenterology. 2009;136:320–330. doi: 10.1053/j.gastro.2008.09.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wollert T, Wunder C, Lippincott-Schwartz J, Hurley JH. Membrane scission by the ESCRT-III complex. Nature. 2009;458:172–177. doi: 10.1038/nature07836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang Y, Han Q, Hou Z, Zhang C, Tian Z, Zhang J. Exosomes mediate hepatitis B virus (HBV) transmission and NK-cell dysfunction. Cell Mol Immunol. 2017;14(5):465–475. doi: 10.1038/cmi.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu J, Han J, Zhang J, Li G, Liu H, Cui X, Xu Y, Li T, Liu J, Wang C. The long noncoding RNAs PVT1 and uc002mbe.2 in sera provide a new supplementary method for hepatocellular carcinoma diagnosis. Medicine (Baltimore) 2016;95(31):e4436. doi: 10.1097/MD.0000000000004436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu X, Odenthal M, Fries JW. Exosomes as miRNA Carriers: Formation-Function-Future. Int J Mol Sci. 2016;17(12) doi: 10.3390/ijms17122028. pii: E2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang S, Ouyang X, Jiang X, Gu D, Lin Y, Kong SK, Xie W. Dysregulated Serum MicroRNA Expression Profile and Potential Biomarkers in Hepatitis C Virus-infected Patients. Int J Med Sci. 2015;12(7):590–8. doi: 10.7150/ijms.11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang Z, Li X, Sun W, Yue S, Yang J, Li J, Ma B, Wang J, Yang X, Pu M, Ruan B, Zhao G, Huang Q, Wang L, Tao K, Dou K. Loss of exosomal miR-320a from cancer-associated fibroblasts contributes to HCC proliferation and metastasis. Cancer Lett. 2017;397:33–42. doi: 10.1016/j.canlet.2017.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.