Abstract
Extracellular vesicles (EVs) are key mediators of intercellular communication that have been ignored for decades. Tumour cells benefit from the secretion of vesicles as they can influence the behaviour of neighbouring tumour cells within the tumour microenvironment. Several studies have shown that extracellular vesicles play an active role in pre-metastatic niche formation and importantly, they are involved in the metastatic organotropism of different tumour types. Tumour-derived EVs carry and transfer molecules to recipient cells, modifying their behaviour through a process known as “EV-driven education”. Several events that favour metastasis to sentinel lymph nodes and distal organs are reinforced by EV education, including angiogenesis, inflammation and lymphangiogenesis. Hence, in this review we will summarize the main mechanisms by which tumour-derived EVs regulate lymph node and distal organ metastasis. Moreover, since some cancers metastasize through the lymphatic system, we will discuss recent discoveries about the presence and function of tumour EVs in the lymph. Finally, we will address the potential value of tumour EVs as prognostic biomarkers in liquid biopsies, specially blood and lymphatic fluid, and the use of these tools as early detectors of metastases.
Keywords: Cancer, Exosomes, Extracellular vesicles, Metastasis
1. Introduction: Extracellular Vesicles as key vehicles of cell-cell communication
Multicellular organisms rely on cell-cell communication to guarantee tissue homeostasis. There are different ways that neighbouring cells can communicate, through cell-cell contacts, gap junctions, extracellular vesicles and tunnelling nanotubes (McMillen and Holley, 2015; Nawaz and Fatima, 2017). Of these, extracellular vesicles (EVs) have emerged as key messenguers for the intercellular communication that regulates both physiological and pathological processes (Becker et al., 2016; Maas et al., 2017). EVs are released by most cell types and they carry different molecules that influence the activity of the surrounding cells, including proteins, RNA, DNA, lipids and metabolites (Abels and Breakefield, 2016; Tkach and Thery, 2016; Valadi et al., 2007). EVs are a heterogeneous group of membrane vesicles mainly comprised of exosomes (40-100 nm diameter multivesicular vesicles of endocytic origin) and microvesicles (MVs, 100 to 1000 nm diameter that bud directly from the plasma membrane (Raposo and Stoorvogel, 2013). Nevertheless, recent studies suggest that smaller vesicles (<100 nm) derived from plasma membrane protrusions can be isolated together with exosomes (Colombo et al., 2014). Larger vesicles are also actively secreted by some cell types, like cytoplasts (Headley et al., 2016) and large oncosomes (Di Vizio et al., 2012), further demonstrating that EVs are a heterogeneous population of vesicles that influence different biological processes.
Different techniques can be used to isolate EVs, such as ultracentrifugation, filtration, size exclusion chromatography, immunoaffinity isolation and microfluidic approaches (Li et al., 2017). As such, the International Society for Extracellular Vesicles (ISEV) has published guidelines in order to standardize EV isolation methods across the research community (Witwer et al., 2013; Witwer et al., 2017). Ultracentrifugation is considered the gold-standard purification method to isolate exosomes and MVs, and it is one of the most commonly used techniques (Li et al., 2017). Polymeric precipitation has raised concerns in the EV research community due to the contamination with protein or soluble factors (Helwa et al., 2017; Lobb et al., 2015). The further development of protocols to isolate and characterize EVs, adapting microfluidic isolation (Gholizadeh et al., 2017), nanoplasmonic sensors (Im et al., 2014; Liang et al., 2017; Maiolo et al., 2015) or asymmetric flow fractionation (Petersen et al., 2014; Sitar et al., 2015), should shed light on their true complexity and heterogeneity (Kowal et al., 2016).
Exosome shedding is normally dependent on canonical pathways regulated by the Rab family of proteins, including Rab27, Rab11 and Rab35 (Hsu et al., 2010; Ostrowski et al., 2010; Savina et al., 2005; Savina et al., 2002). There are common molecules involved in the biogenesis of both MVs and exosomes, such as the VPS4 ATPase (Jackson et al., 2017). MVs are normally formed by direct budding of the plasma membrane (Muralidharan-Chari et al., 2010) and changes in Ca2+ seems to be critical for these membrane lipid rearrangements (Minciacchi et al., 2015). Although there has not been extensive research into the mechanisms that control MV release, the calcium dependent enzyme Calpain regulates MV biogenesis in platelets (Crespin et al., 2009) and malignant breast cancer cells, its inhibition blocking their secretion by the latter (Taylor et al., 2017). ADP-ribosylation factor 6 (ARF6) also control MV formation and membrane release (D’Souza-Schorey and Chavrier, 2006), and it has been implicated in regulating ERK-MLCK (myosin light-chain kinase) activation-dependent MV shedding from breast cancer cells (Muralidharan-Chari et al., 2009). In other human cancer cells, small GTPases like RhoA, Rac and Cdc42 are key players in MV biogenesis, not least because they regulate actin dynamics (Antonyak et al., 2012; Li et al., 2012). Through independent and non-redundant mechanisms, Ubiquitin ligase adaptors like the arrestin domain-containing proteins Arrdc1 and Arrdc4, influence the release and sorting of the EV cargo in HEK293 cells and gut explants (Mackenzie et al., 2016). It has also been hypothesized that the MV cargo is recruited to specific foci at the plasma membrane, increasing the local pressure at the membrane to force its curvature and posterior budding. Hence, the enrichment of the protein cargo at sites of future MV formation could be sufficient stimulus to generate extracellular MVs (Stachowiak et al., 2012). Interestingly, novel pathways of vesicle release have also been described, such as hyaluronan-coated EVs (Rilla et al., 2014). Due to this heterogeneity, it is sometimes not clear what kind of vesicles are referred to in the literature and therefore, in this review we will use the general term EV when the nomenclature is ambiguous or not defined.
The uptake of EVs by recipient cells has been little studied, although it is thought to involve two main mechanisms: direct membrane fusion or endocytosis (French et al., 2017). The most canonical and best characterized mechanism of EV uptake is endocytosis, an active process of engulfment that includes clathrin-mediated endocytosis, phagocytosis or macropinocytosis (French et al., 2017). However, it remains unclear whether this mechanism is dependent on specific receptors or proteins located on the EV surface that may target them to specific cell types. Interestingly, epithelial cells and astrocytes cannot normally internalize EVs from transformed cells, although they do internalize EVs when transformed through oncogenic Ras or c-Src expression (French et al., 2017). Thus, cellular transformation may reinforce EV uptake. The preferential interactions between EVs and certain cell types have also been observed in vivo, and melanoma-derived exosomes accumulate in the lungs and bone increasing the frequency of metastasis at these sites (Peinado et al., 2012). Similarly, integrins at the surface of exosomes and cells also influence exosome targeting to specific cell types, promoting their uptake and reinforcing organ specific metastasis (Hoshino et al., 2015). Exosomes from the lung-tropic 4175-LuT breast cancer cells contain α6β4 and α6β1 integrins, and they accumulate in regions of the lung rich in laminin (a ligand for these integrins), which favours lung metastases. Similarly, exosomes from the liver-tropic BxPC-3 pancreatic cancer cell line contain integrin αvβ5 and they preferentially accumulate in regions of the liver rich in the integrin αvβ5 ligand, fibronectin (Hoshino et al., 2015). Overall, these data suggest that EV localization in vivo is determined by adhesion molecules, such as integrins, and specific EV localization to these regions may be responsible for specific EV uptake. Futher studies will determine if these molecules are uniquely drivers of EV uptake or complementary to other receptors.
2. Tumour-derived EVs that remodel the tumour microenvironment at primary sites
Tumour cells release a wide variety of tumour-derived EVs (tEVs) that influence the behaviour of cells in the primary tumour microenvironment ((Bobrie and Thery, 2013; Thery et al., 2009). Pioneering studies showed that oncoproteins are shed and transferred from one tumour cell to another via tumour MVs (tMVs), referred to as oncosomes (Al-Nedawi et al., 2008; Rak and Guha, 2012). Thus, epidermal growth factor receptor variant III (EGFRvIII) can be packaged into MVs from EGFRvIII expressing glioma cells and transferred to EGFRvIII-negative cancer cells, activating mitogen-activated protein kinase (MAPK) and AKT signalling pathways in the recipient cells, and thereby enhancing their survival and tumour growth (Al-Nedawi et al., 2008). Similarly, human breast and colorectal cancer cells that harbour KRAS mutations secrete tumour exosomes (tExos) that are enriched in KRAS and EGFR ligands, and that enhance the invasiveness of neighbouring recipient cells (Demory Beckler et al., 2013; Higginbotham et al., 2011).
State-of-the-art technology has recently allowed the in vivo transfer of exosomes from highly to less metastatic cells to be visualized. For example, a Cre-LoxP system has been used in tExo-donor cells in association with GFP or Tomato genes to induce a colour switch in the recipient cells upon tExo uptake (Zomer et al., 2015). This approach made it possible to observe multiple non-tumour cells receiving tExos in both the tumour microenvironment and in peripheral tissues (e.g., lymph nodes, the lungs and spleen). These data highlight the ability of tExos to not only transfer information to neighbouring tumour cells but also, to stromal cells within the primary tumour microenvironment and to metastatic organs (Zomer et al., 2015). Endothelial cells have also been described as recipients of tEVs in glioblastoma and pancreatic cancer models, resulting in an activation of the angiogenesis that favours tumour growth and dissemination (Nazarenko et al., 2010; Skog et al., 2008). Fibroblasts can also be transformed into myofibroblasts following the uptake of transforming growth factor beta (TGFβ)-enriched prostate tExos (De Wever et al., 2014), and the tumour progression of these tExo-treated fibroblasts is favoured by vascularization, tumour growth and local invasion (De Wever et al., 2008; De Wever et al., 2010). Moreover, this myofibroblast phenotype is also observed in adipose tissue-derived mesenchymal stem cells when they receive breast cancer-derived tExos (Cho et al., 2013). Similarly, tEVs also help generate the immunosuppressive microenvironments that foster tumour growth, inducing a reprograming of macrophages towards a M2 tumour-supportive phenotype (de Vrij et al., 2015; Shinohara et al., 2017a), cytotoxic CD8+ T cell apoptosis (Wieckowski et al., 2009), a decrease in NK proliferation and a phenotypic shift of CD4+ cells to T regulatory lymphocytes (Whiteside, 2013). Myeloid-derived suppressor cells (MDSCs) can also be reprogramed through the transfer of glioma and carcinoma EV-mRNAs so that they elicit enhanced immunosuppression (Ridder et al., 2015).
Together, these data strongly support the role of tEVs as key vehicles driving information transfer between tumour and stromal cells within the tumour microenvironment, and as a relevant element in the progression of cancer. Indeed, attempts have been made to prevent this cell-cell communication by interfering with EV biogenesis in cancer cells. Inhibiting RAB27 expression and that of its effector proteins, the synaptotagmin-like protein 4 (Slp4) and synaptotagmin-like protein homologue lacking C2 domains b (Slac2b), reduced exosome secretion, indicating this is an important pathway in the regulation of exosome release (Hendrix and De Wever, 2013; Ostrowski et al., 2010). Interfering with RAB27 also reduces exosome release from different melanoma, breast, fibrosarcoma or prostate cancer cell lines, slowing down tumour progression, cell migration and metastasis (Tkach and Thery, 2016) and references therein). Other strategies that target MV release include blocking the small GTPasa RhoA and/or ADP-ribosylation factor 6 (Arf6) in cancer cells, similarly impairing tumour progression (Li et al., 2012; Muralidharan-Chari et al., 2009).
Impeding EV release has been successfully tested in several cell types, such as by inhibiting neutral sphingomyelinase (n-SMase), an enzyme regulating ceramide production (Trajkovic et al., 2008). There were fewer lung metastasis in a mouse model of Lewis lung carcinoma (LLC) treated with the n-SMase inhibitor GW4869, and part of this effect may be mediated by reduced exosome production (Fabbri et al., 2012). In addition, EV release is dampened by dimethyl amiloride (DMA), an inhibitor of Na+/H+ and Na+/Ca2+ exchangers (Vader et al., 2014). In three mouse tumour models, DMA effectively reduced EV release and restored anti-cancer immunity, as well as the effectiveness of the chemotherapeutic agent cyclophosphamide (Chalmin et al., 2010). In fact, many of the signals delivered to immune cells via cancer exosomes are involved in directing the immune system to specifically ignore cancer cells and hence, blocking cancer exosome release may be an effective adjuvant to current therapies. Another strategy might be to filter out or reduce the circulating tumour-derived exosomes in plasma using filtration or capture methods (Marleau et al., 2012), further evidence of the potential benefits of inhibiting EV release to treat of cancer. However, it remains unclear how to specifically interfere with these pathways in tumour cells without affecting the physiological function of these vesicles in normal cells. Indeed, it remains unclear how to differentiate tEVs from EVs secreted by hematopoietic or other stromal cells. There is currently no reliable method to identify and specifically eliminate tEVs, and thus, novel strategies must be developed to shed more light on the potential therapeutic benefits of specifically blocking or eradicating tEVs.
3. Circulating EVs
3.1. Tumour-derived EVs in plasma from cancer patients
Interestingly, tEVs are thought to be promising biomarkers of cancer diagnosis and/or prognosis, as well as novel targets for future therapies to combat cancer (Boukouris and Mathivanan, 2015; Ciardiello et al., 2016; Peinado et al., 2012). These EVs have been detected in the blood of patients with glioblastoma, breast, lung, ovary, prostate, colorectal, melanoma and gastric cancer (Costa-Silva et al., 2015; Hoshino et al., 2015; Peinado et al., 2012; Rosell et al., 2009; Silva et al., 2012; Skog et al., 2008; Sueta et al., 2017; Tavoosidana et al., 2011; Taylor and Gercel-Taylor, 2008). Whereas the size of tEVs is similar between healthy and cancer patients, particularly that of exosomes, the exosomal protein concentration is higher in cancer patients, which increases in a stage-dependent manner (Peinado et al., 2012).
Specific molecular signatures appear to be associated with tEVs, which has led to the identification of miRNAs with prognostic potential in different cancer types, such as colorectal cancer (Ogata-Kawata et al., 2014). In these patients, exosomal miR19a and miR17-92a expression was correlated with poor prognosis and increased recurrence (Matsumura et al., 2015). Similarly, miR141 and miR375 have been correlated with a metastatic disease in prostate cancer (Bryant et al., 2012; Ciardiello et al., 2016). In oesophageal cancer, exosomal miR21 expression was associated with recurrence and metastasis (Liao et al., 2016), while miR-21-3p was correlated with cisplatin resistance in ovarian cancer cells (Pink et al., 2015). By contrast, lower levels of miR125b were found in serum-derived EVs from advanced melanoma (Alegre et al., 2014), similar to the expression of miR718 or miR92a in exosomes of hepatocarcinoma (HCC), the levels of which are inversely correlated with tumour recurrence after liver transplantation and tumour progression (Masyuk et al., 2013; Wang et al., 2014).
Proteomics analysis of circulating EVs from the plasma or serum of cancer patients has also shed light on the specific protein signatures associated to tumour progression. There is more hepatocyte growth factor regulated tyrosine kinase substrate (HGS) in EVs, which is correlated with colorectal cancer (CRC) progression (Sun et al., 2016). Similarly, hepatocyte growth factor receptor (HGFR or c-MET) was also found in circulating exosomes of aggressive melanomas, together with VLA-4, HSP70, HSP90 and tyrosinase-related protein 2 (TYRP2: (Peinado et al., 2012). Circulating exosomal MIF has been proposed as a candidate prognostic marker of liver metastasis in pancreatic ductal adenocarcinoma (PDAC: (Costa-Silva et al., 2015), similar to exosomal EpCAM for ovarian cancer progression (Taylor and Gercel-Taylor, 2008). Other molecules have been identified as potential candidates in liquid biopsies of circulating breast cancer EVs, like Fibronectin, Survivin or Del-1 (Khan et al., 2014; Moon et al., 2016). Interestingly, exosomal integrins identify organotropic metastasis in different cancer subtypes (Hoshino et al., 2015), suggesting that identifying other markers within circulating EVs may also help predict future sites of metastasis.
Exosomal DNA is an additional source of material in the diagnosis and prognosis of cancer (Kalluri and LeBleu, 2016), and tEVs may contain single-stranded DNA (Balaj et al., 2011), mitochondrial DNA (Guescini et al., 2010a; Guescini et al., 2010b; Sansone et al., 2017) and double-stranded DNA (Kahlert et al., 2014; Thakur et al., 2014), protecting these nucleic acids from degradation (Jin et al., 2016). ExoDNA reflects the mutational status of the cell of origin, and BRAF (V600E) and EGFR mutations have been described in exoDNA isolated from various cancer cell lines (Thakur et al., 2014). Moreover, the entire gDNA mutational landscape could be represented in exoDNA. For example, KRAS, TP53, NOTCH1 and BRCA2 mutations have been detected in circulating exosomes from patients with pancreatic cancer (Kahlert et al., 2014; San Lucas et al., 2016; Yang et al., 2017), and gDNA mutations were also detected in prostate cancer-derived EVs (Lazaro-Ibanez et al., 2014). Interestingly, DNA sequences from glioma-derived EVs in the peripheral blood of patients could identify tumours able to cross the blood brain barrier (Garcia-Romero et al., 2017). However, the use of exoDNA is still limited in the clinical setting due to the isolation yields and sensitivity of detection.
The isolation of exoDNA and exoRNA in combination with circulating free DNA might offer a more reliable and sensitive screening tool to detect specific mutations. Thus, approaches now focus on developing new techniques that favour the sensitive detection of cancer-specific circulating EVs without the need for a purification step (Yoshioka et al., 2014). The development of kits to isolate RNA from circulating EVs has re-shaped the analysis of circulating mutations and RNAs in liquid biopsies, facilitating the analysis of larger patient cohorts (Enderle et al., 2015; Figueroa et al., 2017; McKiernan et al., 2016). Rencetly, next generation sequencing in nucleid acids from exosomes analyzing BRAF, KRAS, and EGFR mutations in advance cencer patients suggest that has higher sensitivity compared with clinical testing of archival tumor and testing of plasma cfDNA (Mohrmann et al., 2017).
Taken together, liquid biopsies of tumour-derived EVs offer notable advantages, since they represent a non-invasive method that can provide diagnostic and prognostic information prior to, during and after cancer treatment. RNA, proteins and DNA are promising biomarkers that are shed into tEVs, although most studies performed have analysed limited numbers of patients. The analysis of larger patient cohorts, combination of exosomal with circulating nucleid acids and the development of standardized protocols in clinical trials will better define the utility of these biomarkers in clinical settings.
3.2 EVs in Lymphatic fluid
Many cancer types use lymphatic spread to reach distal sites of metastasis, such as melanoma or breast cancer (van Akkooi et al., 2010). Since EVs mimic features of their tumours of origin and their size is within the range for lymphatic transport (5-100 nm: (Srinivasan et al., 2016), it is tempting to speculate that, besides soluble factors, tEVs could also use lymphatic pathways to establish pre-metastatic niches at both local (lymph nodes) or a distal sites (lungs)(Sleeman, 2015a, b). Given that EVs can be isolated from diverse body fluids other than blood, including urine, semen, saliva, breast milk, amniotic fluid, ascites fluid, cerebrospinal and bile fluids (Raposo and Stoorvogel, 2013), it should be possible to use tEVs from lymphatic fluid as a diagnostic and prognostic tools in cancer patients.
In vitro experiments show that human ovarian cancer-derived exosomes can be quickly and selectively transported through the lymphatic endothelium, suggesting that the lymphatic system actively transports tExos (Srinivasan et al., 2016). This process could be mediated by lymphatic endothelial cells (LECs: Figure 1a). In vivo transport of tExos through the lymphatic system has also been demonstrated in mouse models and, for example, tExos from human ovarian cancer cells (HEY) were detected in lymphatic vessels within minutes of being injected into the tip of the mouse’s tail. Indeed, the steady state value of tExos was reached 20 min after injection in both dominant and secondary draining lymphatic vessels (Srinivasan et al., 2016). Footpad injection of melanoma-derived EVs, which permits direct and exclusive entry of EVs into the lymphatic system, indicating that melanoma exosomes can home to lymph nodes ipsilateral to the injection site, in contrast to liposomes that distribute to both ipsilateral and contralateral lymph nodes (Hood et al., 2011). This is consistent with the efficient in vivo dissemination of tEVs via the lymphatic system to the lymph nodes (Pucci et al., 2016). These data not only confirm the ability of tExos to travel through the lymphatic system but also, to home selectively to the lymph nodes, suggesting a possible role of tEVs in the formation of a permissive microenvironment for tumour cells in the lymph nodes. This would be consistent with their already established functions in the formation of pre-metastatic niches (PMNs) at distal sites (Costa-Silva et al., 2015; Hoshino et al., 2015; Peinado et al., 2012).
Figure 1. Tumour-derived extracellular vesicles (tEVs) in the lymphatic system.
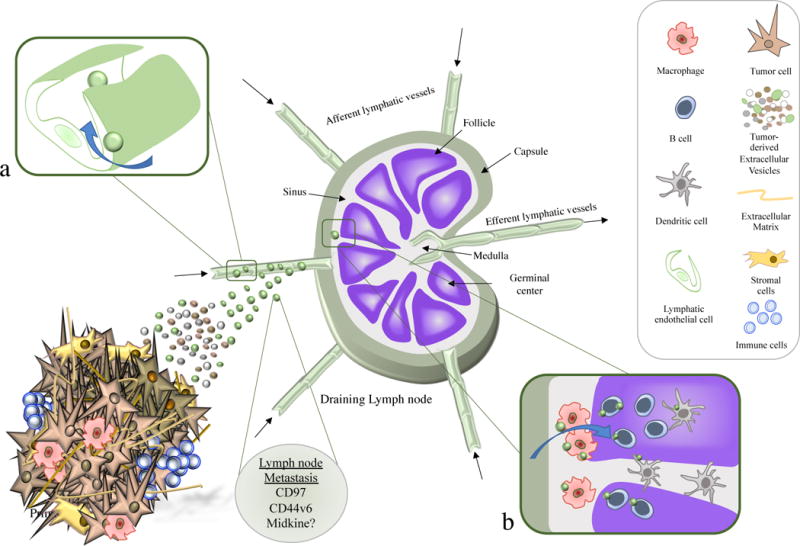
Tumour derived extracellular vesicles can disseminate quickly through the lymphatic vessels and reach the draining lymph nodes, a process favoured by lymphatic endothelial cells (a). Once in the lymph node, EVs are found in either the subscapular sinus (SCS) or in the follicular regions (b). At first, tEVs fuse with SCS macrophages but after crossing the macrophage barrier, they can also be taken up by B lymphocytes or Dendritic cells. The tEVs seem to condition the lymph node microenvironment to generate a pre-metastatic niche (PMN) that allows tumour cells to spread through the lymphatic system.
There is still no explanation as to why some tumours metastasize preferentially to lymph nodes, whereas other rarely use lymphatic spread to reach distal sites and intravasate directly into blood vessels, such as sarcomas (Leong et al., 2006). Transport via the blood and lymph is likely to result in different bio-distributions of exosomes. Indeed, intradermal injection preferentially targets the lymphatic pathway and ends with an accumulation of exosomes in the nodes and liver, whereas intravenous injection increases the EV load in the spleen and kidney (Saunderson et al., 2014; Srinivasan et al., 2016). Moreover, recent evidence from our laboratory suggests that the lymph is richer in tEVs than plasma (unpublished observations), which strongly supports the use of lymphatic fluid as suitable liquid biopsy tissue. However, using lymphatic fluid as a source of tEVs has only rarely been addressed (Maus et al., 2017; Pucci et al., 2016).
The number of metastatic lymph nodes directly correlates with decreased overall survival in clinical stage II (no lymph node metastases) and III (positive lymph node metastasis) patients. However, the exact relationship between lymph node metastasis and cancer dissemination remains unclear. Lymph node dissemination could merely represent a metastatic disease state in parallel with distant metastasis, or it could be a central hub necessary to establish future sites of dissemination (van Akkooi et al., 2010). The identification of biomarkers that help us predict distinct modes of metastasis could have an impact on patient diagnosis and treatment, with the source of tEVs possibly a powerful way to achieve this goal. Whereas blood-circulating exosomes could identify distal metastatic markers, specific signatures found in lymph-circulating exosomes could predict the dependence of metastasis on lymph nodes, helping to evaluate the benefits of invasive therapies like lymph node resection for patient survival. Analysing lymph-circulating EVs might shed light onto the mechanisms of metastasis. Nevertheless, one limitation of lymphatic fluid isolation compared to blood-based testing is that it normally requires invasive techniques like fine-needle aspiration or surgical intervention. Lymphatic drainage from the lymph node obtained after dissection has been used to profile disease markers in melanoma patients (Nowecki et al., 2008; Rutkowski et al., 2008; Wlodzimierz et al., 2004). Indeed, evaluating the presence of melanoma cell markers in lymphatic drainage after lymph node dissection facilitates a more reliable selection of patients at high and low risk of relapse when compared to blood. These findings suggest that melanoma marker detection in lymphatic drainage could be a promising approach with strong prognostic value (Nowecki et al., 2008; Rutkowski et al., 2008; Wlodzimierz et al., 2004). However, several limitations must first be overcome, including: 1) the standardization of the protocol in the clinic; 2) the poor quality of the samples obtained due to the storage time (normally overnight and at room temperature); and 3) the mix of the fractions of lymphatic fluid and plasma, sometimes obtaining a mixed fluid called seroma or lymphatic exudate. Development of state-of-the-art approaches allowing the isolation of this fluid and profiling secreted EVs would represent a significant advantage in the field.
4. EVs at local sites of Metastasis: lymph node reprograming
While melanoma and ovarian tEVs can be readily found in the lymph nodes (Hood et al., 2011; Pucci et al., 2016; Srinivasan et al., 2016), there is evidence that tEVs from other tumour origins also home to lymph nodes, such as breast or pancreatic cancer (Chow et al., 2014; Jung et al., 2009; Zomer et al., 2015). Importantly, while injected human ovarian cancer EVs are no longer detected in the lymphatic vessels after 24 hours, tEVs remain evident within the lymph nodes even 2 days later (Srinivasan et al., 2016). This persistence could reflect their fusion with recipient cells in the lymph nodes and support the positive role of tEVs in the formation of PMNs in the nodes. Similar results were obtained using live magnetic resonance imaging (MRI) to detect melanoma exosomes in the popliteal lymph nodes 1 and 48 hours after footpad injection (Hu et al., 2014). Melanoma-derived EVs can be captured by subcapsular sinus (SCS) macrophages (Figure 1b) and exosomes that interact with these macrophages prevent tEV fusion with B lymphocytes in the cortex (Pucci et al., 2016).
The association of tExos with macrophages has also been described in axillary lymph nodes of mice bearing xenografted human breast cancer (Chow et al., 2014). Interestingly, human ovarian cancer-derived exosomes more rapidly interact with CD11b+ cells in the nodes, whereas the association between exosomes and B cells is enhanced after 48 hours (Srinivasan et al., 2016), suggesting macrophages are the first barrier to exosome uptake. However, although CD11b+ is expressed by monocytes and macrophages in the SCS of the lymph nodes, the possibility that lymph node resident dendritic cells (DCs) also take-up tEVs cannot be ruled out, as they also express this receptor (Heath and Carbone, 2013). Moreover, melanoma EVs appear to influence DC maturation and they also promote PMN formation in tumour-draining lymph nodes (Maus et al., 2017).
Nevertheless, it is still not fully understood how melanoma-derived exosomes reach the sentinel lymph nodes (sLN), and how they affect the lymph node microenvironment during PMN formation, although there is evidence that tExos guide melanoma cells to exosome rich sites in draining lymph nodes (Hood et al., 2011; Peinado et al., 2012). Studies on gastric cancers also confirmed that tExos could promote metastatic behaviour in the lymph node, increasing human cancer gastric cell proliferation and invasion (Liu et al., 2016a). However, they also show that exosomes act in conjunction with other soluble factors to support metastatic spread, since conditioned medium containing both tExo and soluble factors enhanced the pro-metastatic effect of gastric cells (Liu et al., 2016a). These results are similar to those observed with CD44v6 conditioned media and rat pancreatic cancer cells (Jung et al., 2009). Hence, while regional or sentinel lymph nodes preconditioned by tEVs may play an active role in metastatic progression, little is known about the mechanisms underlying this process. For instance, do tEVs have a specific molecular signature that defines its lymph node predilection? What are the pre-metastatic changes they produce within the node to allow tumour cells to colonize them?
PMN formation is initiated by local changes, such as the induction of vascular leakiness, and a remodelling of the stroma and extracellular matrix (ECM), followed by dramatic systemic effects on the immune system (Peinado et al., 2017). Analysis of inguinal lymph nodes revealed that melanoma-derived exosomes produce significant changes in gene expression, including alterations to genes associated with tumour cell recruitment (Stabilin 1, Ephrin R b4 and integrin αv), modifications of the ECM (MapK14, urokinase plasminogen activator protease -uPA, Laminin 5, Collagen 18 α-1 and G-α13) and importantly, in vascular growth factors that are critical to promote tumour angiogenesis (TNF-α, VEGF-B, HIF1-a and Thbs1: (Hood et al., 2011). EVs containing CD97, a member of the GPRC family of adhesion molecules, seems to be essential to prepare the lymph node PMN for gastric cancer, upregulating their partners CD55 and α5β1 Integrin in the lymph nodes, and tumour metastatic factors like CD151, EpCAM or CD44v6 and CD31 that promote angiogenesis (Liu et al., 2016a). Interestingly, pancreatic cancer exosomes also induce CD31 expression in the lymph nodes (Jung et al., 2009). Moreover, CD44v6 expression in these EVs affects the profile of exosomal miRNA transfer to stromal cells in the lymph nodes, triggering cadherin-17 (Cdh17) downregulation and matrix metalloproteinase upregulation (Mmp2, Mmp3 and Mmp14:(Jung et al., 2009; Rana et al., 2013). Lymphangiogenesis is thought to be another key contributor to lymph node PMN formation, not only favouring local metastasis but also distal colonization (Mumprecht and Detmar, 2009; Olmeda et al., 2017). Interestingly, the heparin-binding factor Midkine (MDK) appears to be a systemic inducer of neo-lymphangiogenesis that defines patient prognosis (Olmeda et al., 2017). Despite being identified in exosomes, both exosomal or soluble MDK seems to underlie these pro-lymphangiogenic effects, highlighting the need for further studies to distinguish the specific contribution of exosomal MDK to the pre-metastatic transformation of the lymph node.
Since lymph nodes are key organs in orchestrating immune responses and several immune cells like macrophages, B cells and potentially DC cells are responsible for tEV uptake by the lymph node, it’s tempting to suggest that tEVs probably modulate immune responses in this organ, generating immune tolerance to support lymph node colonization. Thus, breast cancer cell-derived exosomes stimulate the activation of tumour-associated macrophages (TAMs), resulting in the secretion of pro-inflammatory IL6 and NF-kB activation (Chow et al., 2014). Moreover, breakdown of the SCS macrophage barrier in lymph nodes allows melanoma EVs to interact with B lymphocytes and initiate tumour-promoting humoral immunity (Pucci et al., 2016). Finally, the cargo of melanoma EVs significantly compromises DC maturation without affecting antigen presentation (Maus et al., 2017), further evidence that tEVs are key modulators of immune responses in the lymph node. However, although tEVs appear to regulate T, NK and myeloid cells in other settings (de Vrij et al., 2015; Ridder et al., 2015; Shinohara et al., 2017b; Whiteside, 2013; Wieckowski et al., 2009), it is still not fully understood how melanoma-derived exosomes influence immune responses in the sLN to promote tumour metastasis. Are tEVs passively or actively involved in PMN formation in lymph nodes? Are specific subpopulations of exosomes involved? Are PMNs in lymph nodes different to those at distal sites? Further studies will be required to resolve these questions.
5. EVs at distal sites of metastasis
Although several mechanisms have been proposed for tumour dissemination, homing, and metastatic organotropism (Obenauf and Massague, 2015), there is little information about the role of tEVs in these processes. How tumour cells reach distal organs and what are the mechanisms involved in metastatic organotropism are still key questions in metastasis biology. In this section, we will summarize the current information about the role of tEVs in PMN formation in four of the most common organs for metastasis: lungs, brain, bone, and liver.
5.1-Extracellular Vesicles and Lung Metastasis
The lungs are a common organ for metastasis, and lung metastases are usually correlated with poor prognosis and low overall survival. Lung metastases are frequently derived from cancers of the head and neck, melanoma, breast, stomach, pancreas, kidney, bladder, the male and female genitourinary tract, and sarcomas (Coleman, 2001; Herold et al., 1996). A role for EVs in lung metastasis was first proposed in 2005 (Janowska-Wieczorek et al., 2005). Intravenous injection of Lewis lung carcinoma cells covered with platelet-derived MVs (PMVs) increased lung metastasis (Janowska-Wieczorek et al., 2005) and mechanistically, PMV transferred the integrin α2β (CD41) to lung cancer cell lines, thereby stimulating proliferation and increasing tumour cell invasion (Janowska-Wieczorek et al., 2005). Similarly, CD105+ MVs from human renal cancer stem cells promote angiogenesis and the formation of PMNs in the lungs, reinforcing the metastasis of human renal carcinoma cells (Grange et al., 2011). Some of the events controlling the PMN have been described (Kaplan et al., 2005), indicating that “tumour-secreted humoral factors promote metastatic spread in specific distant organs”, although the nature of these factors was unclear at that time. Later, melanoma-secreted exosomes were shown to foster PMN formation in the lung by educating bone marrow-derived cells (BMDCs). Mechanistically, c-Met upregulation in bone marrow progenitor cells via tExos promoted pro-vasculogenic behaviour and the mobilization of BMDCs, reinforcing melanoma metastatic behaviour (Peinado et al., 2012). Specific integrin profiles of tExos target them to given organs, thereby driving metastatic organotropism (Hoshino et al., 2015). For example, expression of the α6β4 integrin heterodimer at the surface of tExos promotes their homing to lung PMNs. Two main types of stromal cell in the lung take-up tExos during this process, s100a4 positive cells (namely fibroblasts) and lung epithelial cells. However, lung fibroblasts are the main cell type involved in PMN formation after exosome uptake, upregulating s100 genes (Hoshino et al., 2015). The uptake of tExos by lung epithelial cells was also reported to promote neutrophil recruitment in the lung and metastasis (Liu et al., 2016b). In this model, transfer of non-coding snRNAs by tExos enhanced S100a8, S100a9 and fibronectin expression by lung epithelial cells and activated the toll-like receptor 3 (TLR3). TLR3 expression by epithelial cells promotes the recruitment of neutrophils to PMNs (Liu et al., 2016b).
EVs secreted by highly metastatic osteosarcoma are also preferentially detected in the lungs and these vesicles can induce metastatic behaviour in poorly metastatic clones (Macklin et al., 2016). Annexin II (AnxII), usually detected in exosomes, was recently proposed to be a key protein secreted in exosomes that promote lung metastasis. AnxII is expressed strongly by malignant breast cancer cells and it promotes tissue-type plasminogen activator dependent angiogenesis through its effects on immune cells. Indeed, exosomes carrying AnxII promote macrophage-mediated activation of the p38-MAPK pathway, and they increase IL-6 and TNF-α secretion, which participate in the pathogenesis of breast cancer. Significantly, treatment with AnxII-depleted exosomes reduces lung (2-fold) and brain (4-fold) metastasis (Maji et al., 2017). The importance of the lungs as a target organ for many metastatic primary tumours makes it hard to determine whether organotropic lung metastasis are regulated by specific EVs, or alternatively, if EVs drive systemic metastatic outgrowth of tumour cells, thereby promoting lung invasion concomitant with that of other organs.
5.2- Brain tumours and brain metastasis
Brain metastases most commonly arise in melanoma, lung and breast cancer patients. Although the incidence of brain metastasis should decrease due to improved primary tumour detection and treatment, the overall median survival time after diagnosis with current treatment regimens is still typically less than one year (Nieder et al., 2011). The brain is considered a “sanctuary site”, where the blood brain barrier (BBB, that blocks entry into the brain) protects it from the entry of tumour cells, although it also excludes most of the systemic therapeutic agents available. Thus, it is ultimately the ability of tumour cells to thrive in the parenchyma after crossing the BBB that determines their metastatic success. Several factors that promote the survival and outgrowth of brain metastases have been identified, including secreted proteins (Sevenich et al., 2014; Valiente et al., 2014) and microRNAs in tExos (Tominaga et al., 2015). In particular, transmigration of cancer cells across the BBB requires proteolytic processing of the junctional adhesion molecule B (JAMB -JAM2) by the cysteine cathepsin S secreted by tumour cells (Sevenich et al., 2014). Although the role of this protease in tExos has yet to be defined, it has been detected in microglia-derived exosomes (Potolicchio et al., 2005) suggesting that exosomes secreted by cells in the brain could play a role in BBB rupture. The capacity of tExos to disrupt BBB permeability has been studied, and breast cancer-derived exosomes can transfer miR105 to endothelial cells and alter their tight junctions (Zhou et al., 2014). MiR105 transfer enhances vascular permeability and reduces ZO1 expression, promoting BBB disruption followed by lung and brain metastases (Zhou et al., 2014). Similarly, miR-181c in breast cancer-derived exosomes promotes the BBB destruction through abnormal actin localization driven by the downregulation of its target gene in endothelial cells, PDPK1 (Tominaga et al., 2015). Brain metastasis is specifically reinforced in mice that receive breast cancer-derived exosomes through a mechanism dependent on miR-181c (Tominaga et al., 2015). High levels of miR-122 secreted by breast cancer cells also suppresses glucose uptake by niche cells in vitro and in vivo due to a downregulation of the glycolytic enzyme pyruvate kinase (PKM: (Zhang et al., 2015). Thus, breast tumour cells adapt to the metabolic environment in the PMN by increasing glucose available. In vivo inhibition of miR-122 restores glucose uptake in distant organs, including the brain and lungs, decreasing the incidence of metastasis (Fong et al., 2015). Downregulating PTEN in breast cancer cells is a mechanism by which astrocytes and stromal cells promote brain metastasis (Zhang et al., 2015). Interestingly, treatment with astrocyte-derived exosomes leads to a dose-dependent increase in miR-19a and an ensuing decrease in PTEN mRNA expression by brain metastatic breast cancer cells.
Common signatures have been described in exosomes derived from brain metastatic cell lines (mainly protein and miRNAs: (Camacho et al., 2013; Hoshino et al., 2015). MiR-210 was upregulated and miR-19/miR-29c downregulated in exosomes derived from brain metastatic breast cancer cells lines (Camacho et al., 2013). Similarly, there was an enrichment in proteins implicated in cell communication, the cell cycle, and in key cancer invasion and metastasis pathways, although the relevance of these molecules in brain metastasis remains elusive due to the absence of validated functional studies in vivo (Camacho et al., 2013). Quantitative mass spectrometry in a panel of exosomes derived from brain metastatic breast cancer and melanoma models showed that ITGβ3 was commonly present (Hoshino et al., 2015). The main stromal cell type that takes up these exosomes in the brain were endothelial cells. Thus, BBB rupture driven by tEVs could be considered as the first hallmark of PMN formation in the brain. In conjunction with the subsequent transfer of miRNAs between tumour and stromal cells, these may be the main mechanisms by which EVs favour brain metastasis. Future studies assessing the relevance of other cell types (e.g., microglia or immune cells) will further clarify the mechanisms involved in the selection and generation of brain metastatic clones.
5.3- Bone microenvironment, EVs and metastasis
Solid tumours frequently metastasize to bone, as occurs in approximately 70–80% of patients with breast or prostate cancer, and 30–40% of lung cancer patients (Kwakwa and Sterling, 2017). In patients with solid cancers (including breast and prostate cancer), the skeleton is the most frequent site for metastasis (Mundy, 1993; Paget, 1889). The formation of bone metastases alters bone homeostasis (the balance of the osteoclast degradation of the bone against the bone reconstruction by osteoblasts), a dynamic process that constantly changes the bone microenvironment and forces invading tumour cells to evolve with the milieu as it changes. Bone metastasis progresses from colonization to survival, dormancy and finally reactivation, interfering with physiological bone homeostasis (Croucher et al., 2016). Primary tumours modulate osteoblast activity and drive PMN formation by secreting factors like WNT, Bone Morphogenetic Proteins (BMPs), Fibroblast growth factors (FGF), insulin growth factors (IGF1 and IGF2), endothelin 1, Prostate specific antigen (PSA) or Vascular endothelial growth factor (VEGF-A: (Dai et al., 2005; Logothetis and Lin, 2005). Tumour cells also secrete signals that regulate osteoclast differentiation, modulating the production of receptor activator of nuclear factor-κB ligand (RANKL), a cytokine essential for osteoclast differentiation, and its antagonist osteoprotegerin (OPG). The RANK-RANKL interaction is a fundamental part of the osteoblast-osteoclast cycle, and OPG is a regulator of this cycle detected in EVs (Benito-Martin et al., 2013). Several carcinoma cells express RANK, including breast, prostate and murine melanoma cells, and RANKL promotes the motility of these cells in vitro, whereas inhibiting RANKL signalling with OPG prevents the establishment of bone metastasis after intracardiac injection of B16 melanoma cells (Jones et al., 2006). Non-small lung cell carcinoma exosomes that contain Amphiregulin (AREG) activate the EGFR pathway in pre-osteoclasts, in turn enhancing RANKL expression and triggering the vicious cycle in osteolytic bone metastasis (Taverna et al., 2017).
The tEVs derived from miR-192 overexpressing lung adenocarcinoma cells precondition the bone microenvironment, probably promoting osteolytic lesions and bone colonization by decreasing tumour-induced angiogenesis in vivo (Valencia et al., 2014). Bone tropic tExos express a limited integrin repertoire but they are capable of inducing vascular leakiness in the lung, despite not being taken up by the lung parenchyma (Hoshino et al., 2015). Interestingly, education with exosomes derived from lung tropic metastatic models redirects the metastasis of breast cancer cells with bone tropic behaviour to the lung (Hoshino et al., 2015). Hence, cell-extrinsic factors provided by tExos in PMNs appear to play an active role regulating metastatic organotropism.
5.4- EVs and LIVER metastasis
The role of EVs in metastasis to the liver has been assessed in the HCC context, suggesting that EVs may be involved in the intercellular communication within HCCs. Exosome-mediated miRNA transfer appears to be a means of auto-regulating miRNA expression by HCC cells, using Vps4A as a tumour suppressor. VpsA4 utilizes exosomes as mediators to regulate secretion and for the uptake of miRNAs by hepatoma cells (Wei et al., 2015). Exosomes secreted from pancreatic tumour cells execute the stepwise progression for PMN formation in the liver. Specifically, Kupffer cells, the resident macrophages in the liver, are the primary cell type that is activated following the uptake of exosomes derived from pancreatic cancer cells (Costa-Silva et al., 2015). Analysis of pancreatic ductal adenocarcinomas (PDAC)-derived exosomes revealed strong macrophage-inhibitory factor (MIF) expression, which when knocked-down in exosomes provoked a pronounced reduction in TGFβ, FN deposition, macrophage recruitment and heightened metastatic liver burden, without affecting the binding of exosomes to Kupffer cells. These findings suggest that MIF may serve as a potential prognostic marker for the development of PDAC liver metastasis. Recently, exosomes from highly metastatic pancreatic cancer cells were shown to induce liver PMN formation in naïve mice, as well as promoting primary tumour growth and liver metastasis in vivo (Yu et al., 2017).
Exosomes have also been proposed to promote colorectal cancer metastasis to the liver. The exosomes secreted from a highly liver metastatic colorectal cancer cell line (HT-29) increase the metastatic tumour burden and distribution of Caco-2 colorectal cancer cells in the mouse liver, cells that ordinarily exhibit poor liver metastatic potential. Colorectal cancer derived exosomes may promote colorectal cancer metastasis by inducing CXCR4-expressing stromal cells to develop a permissive metastatic microenvironment (Fong et al., 2015). The expression of the integrin αvβ5 heterodimer at the surface of tumour-derived exosomes promotes their homing to liver PMNs. Moreover, exosomes derived from liver-tropic cancer cells (BxPC-3, HPAF-II, Pan02) were taken up four times more efficiently by F4/80+ liver cells than non-liver tropic exosomes (Hoshino et al., 2015). In other studies assessing the liver organotropism driven by tumour exosomes, those secreted by gastric cancer cells carrying EGFR can be delivered into the liver. EGFR integrates into the plasma membrane of liver stromal cells and when it is translocated, it effectively activates hepatocyte growth factor (HGF) by suppressing miR-26a/b expression, contributing to PMN formation (Zhang et al., 2017).
6. Concluding remarks
Irrespective of the cancer type, metastatic disease is responsible for approximately 90% of cancer-associated deaths and thus, new strategies are required to prevent or detect metastasis at early stages. EVs represent promising biomarkers to assess the risk of tumour progression and the metastatic potential of primary tumours, and they have also been proposed as markers for other pathological situations (Karpman et al., 2017; Raghavan, 2017). A novel role for EVs in promoting metastasis has been proposed in recent years, as well as in helping to establish PMNs in target organs (Becker et al., 2016). From the early stages, to sentinel and draining lymph node invasion, to the invasion and successful colonization of highly protected organs, exosomes and secreted particles appear to play a determinant role in disease progression. EVs have been implicated in PMN formation in pre-clinical models, with evidence indicating that this process could also be crucial in human metastatic disease. Recent data suggest that metastasis could be happening earlier than thought before (Harper et al., 2016; Hosseini et al., 2016), therefore determining whether tEVs could condition metastatic lymph nodes and organs from the very begining in the tumor progression may help to interpret current theories of metastatic disease. TEVs play a crucial role in preparing the lymph nodes environment supporting processes such as lymphangiogenesis and extracellular matrix remodelling. Since lymph nodes adjacent to the primary tumor are often the first site of metastases, defining the mechanisms involved in lymph node PMN formation by tEVs could bring new clues to block the metastatic process from the beginning. The discovery of the exosomal integrin code and its role in determining organotropic metastasis has redefined our concept of the metastatic process (Hoshino et al., 2015). Defining if there are specific receptors for tEV uptake in sites of metastasis and the molecular mechanisms involved may define targeted therapies to block PMN formation and metastatic spread.
In the years to come, the use of EVs in clinical practice should become a reality, with circulating EVs in liquid biopsies serving as a novel and/or complementary tool to predict metastatic disease. Indeed, novel sources of EVs (e.g., lymphatic fluid) will open new avenues to explore the role of EVs in the formation of the PMN in lymph nodes and their repercussion on distal metastasis, and they could possibly be used to predict metastatic seeding in patients. Finally, identifying the specific molecules that define the release and uptake of the different types of EVs (including those related to their biogenesis, cargo or their implication in the metastatic process) could give an important impulse to the design of new therapies that block the communication between tumours and their metastatic microenvironments, or re-activate anti-tumor cell immunity which could put a brake on tumour dissemination.
Figure 2. Extracellular vesicles determine organotropic metastasis.
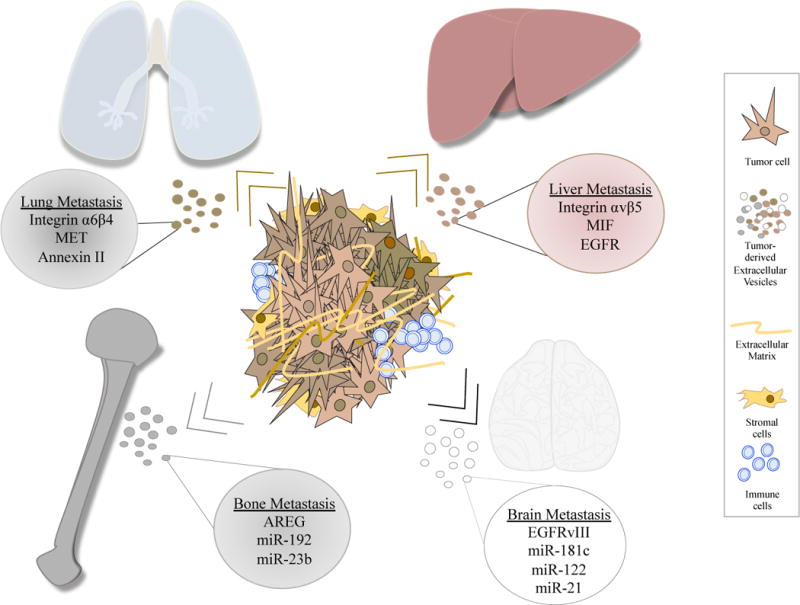
Extracellular vesicles contain proteins and nucleic acids that will differentially interact with target organs to promote metastasis. Exosomes secreted by the primary tumour will be selectively taken up by different cells in the lung, liver, bone and brain, transforming the microenvironment to facilitate pre-metastatic niche formation and the metastatic cascade.
Acknowledgments
We thank the members of Dr Peinado´s and Dr Lyden´s laboratory for helpful discussion of the scientific ideas, and we apologize to those authors not cited due to editorial limitations. The authors gratefully acknowledge the support of the following sources of funding: the US National Cancer Institute (CA169416), MINECO (SAF2014-54541-R), Asociación Española Contra el Cáncer, FERO foundation, WHRI Academy (People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme (FP7/2007-2013) under REA grant agreement n° 608765), Worldwide Cancer Research and Fundación Ramón Areces. We also thank the support of the MINECO-Red de Excelencia en Investigación e Innovación en Exosomas—REDiEX
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abels ER, Breakefield XO. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cellular and molecular neurobiology. 2016;36(3):301–312. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10(5):619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- Alegre E, Sanmamed MF, Rodriguez C, Carranza O, Martin-Algarra S, Gonzalez A. Study of circulating microRNA-125b levels in serum exosomes in advanced melanoma. Archives of pathology & laboratory medicine. 2014;138(6):828–832. doi: 10.5858/arpa.2013-0134-OA. [DOI] [PubMed] [Google Scholar]
- Antonyak MA, Wilson KF, Cerione RA. R(h)oads to microvesicles. Small GTPases. 2012;3(4):219–224. doi: 10.4161/sgtp.20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy SL, Breakefield XO, Skog J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nature communications. 2011;2:180. doi: 10.1038/ncomms1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell. 2016;30(6):836–848. doi: 10.1016/j.ccell.2016.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito-Martin A, Ucero AC, Zubiri I, Posada-Ayala M, Fernandez-Fernandez B, Cannata-Ortiz P, Sanchez-Nino MD, Ruiz-Ortega M, Egido J, Alvarez-Llamas G, Ortiz A. Osteoprotegerin in exosome-like vesicles from human cultured tubular cells and urine. PloS one. 2013;8(8):e72387. doi: 10.1371/journal.pone.0072387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrie A, Thery C. Unraveling the physiological functions of exosome secretion by tumors. Oncoimmunology. 2013;2(1):e22565. doi: 10.4161/onci.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukouris S, Mathivanan S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteomics Clinical applications. 2015;9(3–4):358–367. doi: 10.1002/prca.201400114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RJ, Pawlowski T, Catto JW, Marsden G, Vessella RL, Rhees B, Kuslich C, Visakorpi T, Hamdy FC. Changes in circulating microRNA levels associated with prostate cancer. British journal of cancer. 2012;106(4):768–774. doi: 10.1038/bjc.2011.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho L, Guerrero P, Marchetti D. MicroRNA and protein profiling of brain metastasis competent cell-derived exosomes. PloS one. 2013;8(9):e73790. doi: 10.1371/journal.pone.0073790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau D, De Thonel A, Multhoff G, Hamman A, Martin F, Chauffert B, Solary E, Zitvogel L, Garrido C, Ryffel B, Borg C, Apetoh L, Rebe C, Ghiringhelli F. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. The Journal of clinical investigation. 2010;120(2):457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YB, Lee WY, Park KJ, Kim M, Yoo HW, Yu CS. Autologous adipose tissue-derived stem cells for the treatment of Crohn’s fistula: a phase I clinical study. Cell transplantation. 2013;22(2):279–285. doi: 10.3727/096368912X656045. [DOI] [PubMed] [Google Scholar]
- Chow A, Zhou W, Liu L, Fong MY, Champer J, Van Haute D, Chin AR, Ren X, Gugiu BG, Meng Z, Huang W, Ngo V, Kortylewski M, Wang SE. Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-kappaB. Scientific reports. 2014;4:5750. doi: 10.1038/srep05750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardiello C, Cavallini L, Spinelli C, Yang J, Reis-Sobreiro M, de Candia P, Minciacchi VR, Di Vizio D. Focus on Extracellular Vesicles: New Frontiers of Cell-to-Cell Communication in Cancer. International journal of molecular sciences. 2016;17(2) doi: 10.3390/ijms17020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer treatment reviews. 2001;27(3):165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual review of cell and developmental biology. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, Garcia-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, Lyden D. Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol. 2015;17(6):816–826. doi: 10.1038/ncb3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespin M, Vidal C, Picard F, Lacombe C, Fontenay M. Activation of PAK1/2 during the shedding of platelet microvesicles. Blood coagulation & fibrinolysis: an international journal in haemostasis and thrombosis. 2009;20(1):63–70. doi: 10.1097/MBC.0b013e32831bc310. [DOI] [PubMed] [Google Scholar]
- Croucher PI, McDonald MM, Martin TJ. Bone metastasis: the importance of the neighbourhood. Nature reviews Cancer. 2016;16(6):373–386. doi: 10.1038/nrc.2016.44. [DOI] [PubMed] [Google Scholar]
- D’Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nature reviews Molecular cell biology. 2006;7(5):347–358. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- Dai J, Keller J, Zhang J, Lu Y, Yao Z, Keller ET. Bone morphogenetic protein-6 promotes osteoblastic prostate cancer bone metastases through a dual mechanism. Cancer research. 2005;65(18):8274–8285. doi: 10.1158/0008-5472.CAN-05-1891. [DOI] [PubMed] [Google Scholar]
- de Vrij J, Maas SL, Kwappenberg KM, Schnoor R, Kleijn A, Dekker L, Luider TM, de Witte LD, Litjens M, van Strien ME, Hol EM, Kroonen J, Robe PA, Lamfers ML, Schilham MW, Broekman ML. Glioblastoma-derived extracellular vesicles modify the phenotype of monocytic cells. International journal of cancer Journal international du cancer. 2015;137(7):1630–1642. doi: 10.1002/ijc.29521. [DOI] [PubMed] [Google Scholar]
- De Wever O, Demetter P, Mareel M, Bracke M. Stromal myofibroblasts are drivers of invasive cancer growth. International journal of cancer. Journal international du cancer. 2008;123(10):2229–2238. doi: 10.1002/ijc.23925. [DOI] [PubMed] [Google Scholar]
- De Wever O, Hendrix A, De Boeck A, Westbroek W, Braems G, Emami S, Sabbah M, Gespach C, Bracke M. Modeling and quantification of cancer cell invasion through collagen type I matrices. The International journal of developmental biology. 2010;54(5):887–896. doi: 10.1387/ijdb.092948ow. [DOI] [PubMed] [Google Scholar]
- De Wever O, Van Bockstal M, Mareel M, Hendrix A, Bracke M. Carcinoma-associated fibroblasts provide operational flexibility in metastasis. Seminars in cancer biology. 2014;25:33–46. doi: 10.1016/j.semcancer.2013.12.009. [DOI] [PubMed] [Google Scholar]
- Demory Beckler M, Higginbotham JN, Franklin JL, Ham AJ, Halvey PJ, Imasuen IE, Whitwell C, Li M, Liebler DC, Coffey RJ. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Molecular & cellular proteomics: MCP. 2013;12(2):343–355. doi: 10.1074/mcp.M112.022806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Vizio D, Morello M, Dudley AC, Schow PW, Adam RM, Morley S, Mulholland D, Rotinen M, Hager MH, Insabato L, Moses MA, Demichelis F, Lisanti MP, Wu H, Klagsbrun M, Bhowmick NA, Rubin MA, D’Souza-Schorey C, Freeman MR. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. The American journal of pathology. 2012;181(5):1573–1584. doi: 10.1016/j.ajpath.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enderle D, Spiel A, Coticchia CM, Berghoff E, Mueller R, Schlumpberger M, Sprenger-Haussels M, Shaffer JM, Lader E, Skog J, Noerholm M. Characterization of RNA from Exosomes and Other Extracellular Vesicles Isolated by a Novel Spin Column-Based Method. PloS one. 2015;10(8):e0136133. doi: 10.1371/journal.pone.0136133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ, Zanesi N, Crawford M, Ozer GH, Wernicke D, Alder H, Caligiuri MA, Nana-Sinkam P, Perrotti D, Croce CM. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(31):E2110–2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa JM, Skog J, Akers J, Li H, Komotar R, Jensen R, Ringel F, Yang I, Kalkanis S, Thompson R, LoGuidice L, Berghoff E, Parsa A, Liau L, Curry W, Cahill D, Bettegowda C, Lang FF, Chiocca EA, Henson J, Kim R, Breakefield X, Chen C, Messer K, Hochberg F, Carter BS. Detection of wtEGFR Amplification and EGFRvIII Mutation in CSF-Derived Extracellular Vesicles of Glioblastoma Patients. Neuro-oncology. 2017 doi: 10.1093/neuonc/nox085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong MY, Zhou W, Liu L, Alontaga AY, Chandra M, Ashby J, Chow A, O’Connor ST, Li S, Chin AR, Somlo G, Palomares M, Li Z, Tremblay JR, Tsuyada A, Sun G, Reid MA, Wu X, Swiderski P, Ren X, Shi Y, Kong M, Zhong W, Chen Y, Wang SE. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol. 2015;17(2):183–194. doi: 10.1038/ncb3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French KC, Antonyak MA, Cerione RA. Extracellular vesicle docking at the cellular port: Extracellular vesicle binding and uptake. Seminars in cell & developmental biology. 2017;67:48–55. doi: 10.1016/j.semcdb.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Romero N, Carrion-Navarro J, Esteban-Rubio S, Lazaro-Ibanez E, Peris-Celda M, Alonso MM, Guzman-De-Villoria J, Fernandez-Carballal C, de Mendivil AO, Garcia-Duque S, Escobedo-Lucea C, Prat-Acin R, Belda-Iniesta C, Ayuso-Sacido A. DNA sequences within glioma-derived extracellular vesicles can cross the intact blood-brain barrier and be detected in peripheral blood of patients. Oncotarget. 2017;8(1):1416–1428. doi: 10.18632/oncotarget.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholizadeh S, Shehata Draz M, Zarghooni M, Sanati-Nezhad A, Ghavami S, Shafiee H, Akbari M. Microfluidic approaches for isolation, detection, and characterization of extracellular vesicles: Current status and future directions. Biosensors & bioelectronics. 2017;91:588–605. doi: 10.1016/j.bios.2016.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, Tetta C, Bussolati B, Camussi G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer research. 2011;71(15):5346–5356. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- Guescini M, Genedani S, Stocchi V, Agnati LF. Astrocytes and Glioblastoma cells release exosomes carrying mtDNA. Journal of neural transmission. 2010a;117(1):1–4. doi: 10.1007/s00702-009-0288-8. [DOI] [PubMed] [Google Scholar]
- Guescini M, Guidolin D, Vallorani L, Casadei L, Gioacchini AM, Tibollo P, Battistelli M, Falcieri E, Battistin L, Agnati LF, Stocchi V. C2C12 myoblasts release micro-vesicles containing mtDNA and proteins involved in signal transduction. Experimental cell research. 2010b;316(12):1977–1984. doi: 10.1016/j.yexcr.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Harper KL, Sosa MS, Entenberg D, Hosseini H, Cheung JF, Nobre R, Avivar-Valderas A, Nagi C, Girnius N, Davis RJ, Farias EF, Condeelis J, Klein CA, Aguirre-Ghiso JA. Mechanism of early dissemination and metastasis in Her2+ mammary cancer. Nature. 2016 doi: 10.1038/nature20609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headley MB, Bins A, Nip A, Roberts EW, Looney MR, Gerard A, Krummel MF. Visualization of immediate immune responses to pioneer metastatic cells in the lung. Nature. 2016;531(7595):513–517. doi: 10.1038/nature16985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath WR, Carbone FR. The skin-resident and migratory immune system in steady state and memory: innate lymphocytes, dendritic cells and T cells. Nature immunology. 2013;14(10):978–985. doi: 10.1038/ni.2680. [DOI] [PubMed] [Google Scholar]
- Helwa I, Cai J, Drewry MD, Zimmerman A, Dinkins MB, Khaled ML, Seremwe M, Dismuke WM, Bieberich E, Stamer WD, Hamrick MW, Liu Y. A Comparative Study of Serum Exosome Isolation Using Differential Ultracentrifugation and Three Commercial Reagents. PloS one. 2017;12(1):e0170628. doi: 10.1371/journal.pone.0170628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix A, De Wever O. Rab27 GTPases distribute extracellular nanomaps for invasive growth and metastasis: implications for prognosis and treatment. International journal of molecular sciences. 2013;14(5):9883–9892. doi: 10.3390/ijms14059883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold CJ, Bankier AA, Fleischmann D. Lung metastases. European radiology. 1996;6(5):596–606. doi: 10.1007/BF00187656. [DOI] [PubMed] [Google Scholar]
- Higginbotham JN, Demory Beckler M, Gephart JD, Franklin JL, Bogatcheva G, Kremers GJ, Piston DW, Ayers GD, McConnell RE, Tyska MJ, Coffey RJ. Amphiregulin exosomes increase cancer cell invasion. Current biology: CB. 2011;21(9):779–786. doi: 10.1016/j.cub.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer research. 2011;71(11):3792–3801. doi: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S, Singh S, Williams C, Soplop N, Uryu K, Pharmer L, King T, Bojmar L, Davies AE, Ararso Y, Zhang T, Zhang H, Hernandez J, Weiss JM, Dumont-Cole VD, Kramer K, Wexler LH, Narendran A, Schwartz GK, Healey JH, Sandstrom P, Jorgen Labori K, Kure EH, Grandgenett PM, Hollingsworth MA, de Sousa M, Kaur S, Jain M, Mallya K, Batra SK, Jarnagin WR, Brady MS, Fodstad O, Muller V, Pantel K, Minn AJ, Bissell MJ, Garcia BA, Kang Y, Rajasekhar VK, Ghajar CM, Matei I, Peinado H, Bromberg J, Lyden D. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini H, Obradovic MM, Hoffmann M, Harper KL, Sosa MS, Werner-Klein M, Nanduri LK, Werno C, Ehrl C, Maneck M, Patwary N, Haunschild G, Guzvic M, Reimelt C, Grauvogl M, Eichner N, Weber F, Hartkopf AD, Taran FA, Brucker SY, Fehm T, Rack B, Buchholz S, Spang R, Meister G, Aguirre-Ghiso JA, Klein CA. Early dissemination seeds metastasis in breast cancer. Nature. 2016 doi: 10.1038/nature20785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, Bakhti M, Gronborg M, Mobius W, Rhee J, Barr FA, Simons M. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. The Journal of cell biology. 2010;189(2):223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Wickline SA, Hood JL. Magnetic resonance imaging of melanoma exosomes in lymph nodes. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2014 doi: 10.1002/mrm.25376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im H, Shao H, Park YI, Peterson VM, Castro CM, Weissleder R, Lee H. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nature biotechnology. 2014;32(5):490–495. doi: 10.1038/nbt.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson CE, Scruggs BS, Schaffer JE, Hanson PI. Effects of Inhibiting VPS4 Support a General Role for ESCRTs in Extracellular Vesicle Biogenesis. Biophysical journal. 2017 doi: 10.1016/j.bpj.2017.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, Ratajczak MZ. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. International journal of cancer Journal international du cancer. 2005;113(5):752–760. doi: 10.1002/ijc.20657. [DOI] [PubMed] [Google Scholar]
- Jin Y, Chen K, Wang Z, Wang Y, Liu J, Lin L, Shao Y, Gao L, Yin H, Cui C, Tan Z, Liu L, Zhao C, Zhang G, Jia R, Du L, Chen Y, Liu R, Xu J, Hu X, Wang Y. DNA in serum extracellular vesicles is stable under different storage conditions. BMC cancer. 2016;16(1):753. doi: 10.1186/s12885-016-2783-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DH, Nakashima T, Sanchez OH, Kozieradzki I, Komarova SV, Sarosi I, Morony S, Rubin E, Sarao R, Hojilla CV, Komnenovic V, Kong YY, Schreiber M, Dixon SJ, Sims SM, Khokha R, Wada T, Penninger JM. Regulation of cancer cell migration and bone metastasis by RANKL. Nature. 2006;440(7084):692–696. doi: 10.1038/nature04524. [DOI] [PubMed] [Google Scholar]
- Jung T, Castellana D, Klingbeil P, Cuesta Hernandez I, Vitacolonna M, Orlicky DJ, Roffler SR, Brodt P, Zoller M. CD44v6 dependence of premetastatic niche preparation by exosomes. Neoplasia. 2009;11(10):1093–1105. doi: 10.1593/neo.09822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlert C, Melo SA, Protopopov A, Tang J, Seth S, Koch M, Zhang J, Weitz J, Chin L, Futreal A, Kalluri R. Identification of double-stranded genomic DNA spanning all chromosomes with mutated KRAS and p53 DNA in the serum exosomes of patients with pancreatic cancer. The Journal of biological chemistry. 2014;289(7):3869–3875. doi: 10.1074/jbc.C113.532267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, LeBleu VS. Discovery of Double-Stranded Genomic DNA in Circulating Exosomes. Cold Spring Harbor symposia on quantitative biology. 2016;81:275–280. doi: 10.1101/sqb.2016.81.030932. [DOI] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438(7069):820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpman D, Stahl AL, Arvidsson I. Extracellular vesicles in renal disease. Nature reviews Nephrology. 2017 doi: 10.1038/nrneph.2017.98. [DOI] [PubMed] [Google Scholar]
- Khan S, Bennit HF, Turay D, Perez M, Mirshahidi S, Yuan Y, Wall NR. Early diagnostic value of survivin and its alternative splice variants in breast cancer. BMC cancer. 2014;14:176. doi: 10.1186/1471-2407-14-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Thery C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(8):E968–977. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwakwa KA, Sterling JA. Integrin alphavbeta3 Signaling in Tumor-Induced Bone Disease. Cancers. 2017;9(7) doi: 10.3390/cancers9070084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaro-Ibanez E, Sanz-Garcia A, Visakorpi T, Escobedo-Lucea C, Siljander P, Ayuso-Sacido A, Yliperttula M. Different gDNA content in the subpopulations of prostate cancer extracellular vesicles: apoptotic bodies, microvesicles, and exosomes. The Prostate. 2014;74(14):1379–1390. doi: 10.1002/pros.22853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong SP, Cady B, Jablons DM, Garcia-Aguilar J, Reintgen D, Jakub J, Pendas S, Duhaime L, Cassell R, Gardner M, Giuliano R, Archie V, Calvin D, Mensha L, Shivers S, Cox C, Werner JA, Kitagawa Y, Kitajima M. Clinical patterns of metastasis. Cancer Metastasis Rev. 2006;25(2):221–232. doi: 10.1007/s10555-006-8502-8. [DOI] [PubMed] [Google Scholar]
- Li B, Antonyak MA, Zhang J, Cerione RA. RhoA triggers a specific signaling pathway that generates transforming microvesicles in cancer cells. Oncogene. 2012;31(45):4740–4749. doi: 10.1038/onc.2011.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in Exosome Isolation Techniques. Theranostics. 2017;7(3):789–804. doi: 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K, Liu F, Fan J, Sun D, Liu C, Lyon CJ, Bernard DW, Li Y, Yokoi K, Katz MH, Koay EJ, Zhao Z, Hu Y. Nanoplasmonic Quantification of Tumor-derived Extracellular Vesicles in Plasma Microsamples for Diagnosis and Treatment Monitoring. Nature biomedical engineering. 2017;1 doi: 10.1038/s41551-016-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J, Liu R, Shi YJ, Yin LH, Pu YP. Exosome-shuttling microRNA-21 promotes cell migration and invasion-targeting PDCD4 in esophageal cancer. International journal of oncology. 2016;48(6):2567–2579. doi: 10.3892/ijo.2016.3453. [DOI] [PubMed] [Google Scholar]
- Liu D, Li C, Trojanowicz B, Li X, Shi D, Zhan C, Wang Z, Chen L. CD97 promotion of gastric carcinoma lymphatic metastasis is exosome dependent. Gastric cancer: official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association. 2016a;19(3):754–766. doi: 10.1007/s10120-015-0523-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Gu Y, Han Y, Zhang Q, Jiang Z, Zhang X, Huang B, Xu X, Zheng J, Cao X. Tumor Exosomal RNAs Promote Lung Pre-metastatic Niche Formation by Activating Alveolar Epithelial TLR3 to Recruit Neutrophils. Cancer Cell. 2016b;30(2):243–256. doi: 10.1016/j.ccell.2016.06.021. [DOI] [PubMed] [Google Scholar]
- Lobb RJ, Becker M, Wen SW, Wong CS, Wiegmans AP, Leimgruber A, Moller A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. Journal of extracellular vesicles. 2015;4:27031. doi: 10.3402/jev.v4.27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis CJ, Lin SH. Osteoblasts in prostate cancer metastasis to bone. Nature reviews Cancer. 2005;5(1):21–28. doi: 10.1038/nrc1528. [DOI] [PubMed] [Google Scholar]
- Maas SL, Breakefield XO, Weaver AM. Extracellular Vesicles: Unique Intercellular Delivery Vehicles. Trends in cell biology. 2017;27(3):172–188. doi: 10.1016/j.tcb.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie K, Foot NJ, Anand S, Dalton HE, Chaudhary N, Collins BM, Mathivanan S, Kumar S. Regulation of the divalent metal ion transporter via membrane budding. Cell discovery. 2016;2:16011. doi: 10.1038/celldisc.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklin R, Wang H, Loo D, Martin S, Cumming A, Cai N, Lane R, Ponce NS, Topkas E, Inder K, Saunders NA, Endo-Munoz L. Extracellular vesicles secreted by highly metastatic clonal variants of osteosarcoma preferentially localize to the lungs and induce metastatic behaviour in poorly metastatic clones. Oncotarget. 2016;7(28):43570–43587. doi: 10.18632/oncotarget.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiolo D, Paolini L, Di Noto G, Zendrini A, Berti D, Bergese P, Ricotta D. Colorimetric nanoplasmonic assay to determine purity and titrate extracellular vesicles. Analytical chemistry. 2015;87(8):4168–4176. doi: 10.1021/ac504861d. [DOI] [PubMed] [Google Scholar]
- Maji S, Chaudhary P, Akopova I, Nguyen PM, Hare RJ, Gryczynski I, Vishwanatha JK. Exosomal Annexin II Promotes Angiogenesis and Breast Cancer Metastasis. Molecular cancer research: MCR. 2017;15(1):93–105. doi: 10.1158/1541-7786.MCR-16-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marleau AM, Chen CS, Joyce JA, Tullis RH. Exosome removal as a therapeutic adjuvant in cancer. Journal of translational medicine. 2012;10:134. doi: 10.1186/1479-5876-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masyuk AI, Masyuk TV, Larusso NF. Exosomes in the pathogenesis, diagnostics and therapeutics of liver diseases. Journal of hepatology. 2013;59(3):621–625. doi: 10.1016/j.jhep.2013.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura T, Sugimachi K, Iinuma H, Takahashi Y, Kurashige J, Sawada G, Ueda M, Uchi R, Ueo H, Takano Y, Shinden Y, Eguchi H, Yamamoto H, Doki Y, Mori M, Ochiya T, Mimori K. Exosomal microRNA in serum is a novel biomarker of recurrence in human colorectal cancer. British journal of cancer. 2015;113(2):275–281. doi: 10.1038/bjc.2015.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maus RLG, Jakub JW, Nevala WK, Christensen TA, Noble-Orcutt K, Sachs Z, Hieken TJ, Markovic SN. Human Melanoma-Derived Extracellular Vesicles Regulate Dendritic Cell Maturation. Frontiers in immunology. 2017;8:358. doi: 10.3389/fimmu.2017.00358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan J, Donovan MJ, O’Neill V, Bentink S, Noerholm M, Belzer S, Skog J, Kattan MW, Partin A, Andriole G, Brown G, Wei JT, Thompson IM, Jr, Carroll P. A Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer at Initial Biopsy. JAMA oncology. 2016;2(7):882–889. doi: 10.1001/jamaoncol.2016.0097. [DOI] [PubMed] [Google Scholar]
- McMillen P, Holley SA. Integration of cell-cell and cell-ECM adhesion in vertebrate morphogenesis. Current opinion in cell biology. 2015;36:48–53. doi: 10.1016/j.ceb.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minciacchi VR, Freeman MR, Di Vizio D. Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Seminars in cell & developmental biology. 2015;40:41–51. doi: 10.1016/j.semcdb.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohrmann L, Huang H, Hong DS, Tsimberidou AM, Fu S, Piha-Paul S, Subbiah V, Karp DD, Naing A, Krug AK, Enderle D, Priewasser T, Noerholm M, Eitan E, Coticchia C, Stoll G, Jordan LM, Eng C, Kopetz S, Skog J, Meric-Bernstam F, Janku F. Liquid Biopsies Using Plasma Exosomal Nucleic Acids and Plasma Cell-Free DNA compared with Clinical Outcomes of Patients with Advanced Cancers. Clinical cancer research: an official journal of the American Association for Cancer Research. 2017 doi: 10.1158/1078-0432.CCR-17-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon PG, Lee JE, Cho YE, Lee SJ, Chae YS, Jung JH, Kim IS, Park HY, Baek MC. Fibronectin on circulating extracellular vesicles as a liquid biopsy to detect breast cancer. Oncotarget. 2016;7(26):40189–40199. doi: 10.18632/oncotarget.9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumprecht V, Detmar M. Lymphangiogenesis and cancer metastasis. Journal of cellular and molecular medicine. 2009;13(8A):1405–1416. doi: 10.1111/j.1582-4934.2009.00834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy GR. Cytokines and growth factors in the regulation of bone remodeling. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 1993;8(Suppl 2):S505–510. doi: 10.1002/jbmr.5650081315. [DOI] [PubMed] [Google Scholar]
- Muralidharan-Chari V, Clancy J, Plou C, Romao M, Chavrier P, Raposo G, D’Souza-Schorey C. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Current biology: CB. 2009;19(22):1875–1885. doi: 10.1016/j.cub.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan-Chari V, Clancy JW, Sedgwick A, D’Souza-Schorey C. Microvesicles: mediators of extracellular communication during cancer progression. Journal of cell science. 2010;123(Pt 10):1603–1611. doi: 10.1242/jcs.064386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawaz M, Fatima F. Extracellular Vesicles, Tunneling Nanotubes, and Cellular Interplay: Synergies and Missing Links. Frontiers in molecular biosciences. 2017;4:50. doi: 10.3389/fmolb.2017.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarenko I, Rana S, Baumann A, McAlear J, Hellwig A, Trendelenburg M, Lochnit G, Preissner KT, Zoller M. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer research. 2010;70(4):1668–1678. doi: 10.1158/0008-5472.CAN-09-2470. [DOI] [PubMed] [Google Scholar]
- Nieder C, Spanne O, Mehta MP, Grosu AL, Geinitz H. Presentation, patterns of care, and survival in patients with brain metastases: what has changed in the last 20 years? Cancer. 2011;117(11):2505–2512. doi: 10.1002/cncr.25707. [DOI] [PubMed] [Google Scholar]
- Nowecki ZI, Rutkowski P, Kulik J, Siedlecki JA, Ruka W. Molecular and biochemical testing in stage III melanoma: multimarker reverse transcriptase-polymerase chain reaction assay of lymph fluid after lymph node dissection and preoperative serum lactate dehydrogenase level. The British journal of dermatology. 2008;159(3):597–605. doi: 10.1111/j.1365-2133.2008.08710.x. [DOI] [PubMed] [Google Scholar]
- Obenauf AC, Massague J. Surviving at a distance: organ specific metastasis. Trends in cancer. 2015;1(1):76–91. doi: 10.1016/j.trecan.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata-Kawata H, Izumiya M, Kurioka D, Honma Y, Yamada Y, Furuta K, Gunji T, Ohta H, Okamoto H, Sonoda H, Watanabe M, Nakagama H, Yokota J, Kohno T, Tsuchiya N. Circulating exosomal microRNAs as biomarkers of colon cancer. PloS one. 2014;9(4):e92921. doi: 10.1371/journal.pone.0092921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmeda D, Cerezo-Wallis D, Riveiro-Falkenbach E, Pennacchi PC, Contreras-Alcalde M, Ibarz N, Cifdaloz M, Catena X, Calvo TG, Canon E, Alonso-Curbelo D, Suarez J, Osterloh L, Grana O, Mulero F, Megias D, Canamero M, Martinez-Torrecuadrada JL, Mondal C, Di Martino J, Lora D, Martinez-Corral I, Bravo-Cordero JJ, Munoz J, Puig S, Ortiz-Romero P, Rodriguez-Peralto JL, Ortega S, Soengas MS. Whole-body imaging of lymphovascular niches identifies pre-metastatic roles of midkine. Nature. 2017;546(7660):676–680. doi: 10.1038/nature22977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, Seabra MC, Darchen F, Amigorena S, Moita LF, Thery C. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(1):19–30. doi: 10.1038/ncb2000. sup pp 11-13. [DOI] [PubMed] [Google Scholar]
- Paget S. The Distribution Of Secondary Growths In Cancer Of The Breast. Lancet. 1889;133(3421):571–573. [PubMed] [Google Scholar]
- Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nature medicine. 2012;18(6):883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, Psaila B, Kaplan RN, Bromberg JF, Kang Y, Bissell MJ, Cox TR, Giaccia AJ, Erler JT, Hiratsuka S, Ghajar CM, Lyden D. Pre-metastatic niches: organ-specific homes for metastases. Nature reviews Cancer. 2017 doi: 10.1038/nrc.2017.6. [DOI] [PubMed] [Google Scholar]
- Petersen KE, Manangon E, Hood JL, Wickline SA, Fernandez DP, Johnson WP, Gale BK. A review of exosome separation techniques and characterization of B16-F10 mouse melanoma exosomes with AF4-UV-MALS-DLS-TEM. Analytical and bioanalytical chemistry. 2014;406(30):7855–7866. doi: 10.1007/s00216-014-8040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pink RC, Samuel P, Massa D, Caley DP, Brooks SA, Carter DR. The passenger strand, miR-21-3p, plays a role in mediating cisplatin resistance in ovarian cancer cells. Gynecologic oncology. 2015;137(1):143–151. doi: 10.1016/j.ygyno.2014.12.042. [DOI] [PubMed] [Google Scholar]
- Potolicchio I, Carven GJ, Xu X, Stipp C, Riese RJ, Stern LJ, Santambrogio L. Proteomic analysis of microglia-derived exosomes: metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. Journal of immunology. 2005;175(4):2237–2243. doi: 10.4049/jimmunol.175.4.2237. [DOI] [PubMed] [Google Scholar]
- Pucci F, Garris C, Lai CP, Newton A, Pfirschke C, Engblom C, Alvarez D, Sprachman M, Evavold C, Magnuson A, von Andrian UH, Glatz K, Breakefield XO, Mempel TR, Weissleder R, Pittet MJ. SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science. 2016;352(6282):242–246. doi: 10.1126/science.aaf1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan V. Role of exosomes in psychiatric disorders. Asian journal of psychiatry. 2017;28:78–79. doi: 10.1016/j.ajp.2017.03.032. [DOI] [PubMed] [Google Scholar]
- Rak J, Guha A. Extracellular vesicles–vehicles that spread cancer genes. BioEssays: news and reviews in molecular, cellular and developmental biology. 2012;34(6):489–497. doi: 10.1002/bies.201100169. [DOI] [PubMed] [Google Scholar]
- Rana S, Malinowska K, Zoller M. Exosomal tumor microRNA modulates premetastatic organ cells. Neoplasia. 2013;15(3):281–295. doi: 10.1593/neo.122010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. The Journal of cell biology. 2013;200(4):373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridder K, Sevko A, Heide J, Dams M, Rupp AK, Macas J, Starmann J, Tjwa M, Plate KH, Sultmann H, Altevogt P, Umansky V, Momma S. Extracellular vesicle-mediated transfer of functional RNA in the tumor microenvironment. Oncoimmunology. 2015;4(6):e1008371. doi: 10.1080/2162402X.2015.1008371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilla K, Siiskonen H, Tammi M, Tammi R. Hyaluronan-coated extracellular vesicles–a novel link between hyaluronan and cancer. Advances in cancer research. 2014;123:121–148. doi: 10.1016/B978-0-12-800092-2.00005-8. [DOI] [PubMed] [Google Scholar]
- Rosell R, Wei J, Taron M. Circulating MicroRNA Signatures of Tumor-Derived Exosomes for Early Diagnosis of Non-Small-Cell Lung Cancer. Clinical lung cancer. 2009;10(1):8–9. doi: 10.3816/CLC.2009.n.001. [DOI] [PubMed] [Google Scholar]
- Rutkowski P, Nowecki ZI, Kulik J, Ruka W, Siedlecki JA. Molecular staging by multimarker reverse transcriptase-polymerase chain reaction assay of lymphatic drainage and blood from melanoma patients after lymph node dissection. Melanoma research. 2008;18(4):246–252. doi: 10.1097/CMR.0b013e328307bf3f. [DOI] [PubMed] [Google Scholar]
- San Lucas FA, Allenson K, Bernard V, Castillo J, Kim DU, Ellis K, Ehli EA, Davies GE, Petersen JL, Li D, Wolff R, Katz M, Varadhachary G, Wistuba I, Maitra A, Alvarez H. Minimally invasive genomic and transcriptomic profiling of visceral cancers by next-generation sequencing of circulating exosomes. Annals of oncology: official journal of the European Society for Medical Oncology. 2016;27(4):635–641. doi: 10.1093/annonc/mdv604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansone P, Savini C, Kurelac I, Chang Q, Amato LB, Strillacci A, Stepanova A, Iommarini L, Mastroleo C, Daly L, Galkin A, Thakur BK, Soplop N, Uryu K, Hoshino A, Norton L, Bonafe M, Cricca M, Gasparre G, Lyden D, Bromberg J. Packaging and transfer of mitochondrial DNA via exosomes regulate escape from dormancy in hormonal therapy-resistant breast cancer. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(43):E9066–E9075. doi: 10.1073/pnas.1704862114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunderson SC, Dunn AC, Crocker PR, McLellan AD. CD169 mediates the capture of exosomes in spleen and lymph node. Blood. 2014;123(2):208–216. doi: 10.1182/blood-2013-03-489732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savina A, Fader CM, Damiani MT, Colombo MI. Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner. Traffic. 2005;6(2):131–143. doi: 10.1111/j.1600-0854.2004.00257.x. [DOI] [PubMed] [Google Scholar]
- Savina A, Vidal M, Colombo MI. The exosome pathway in K562 cells is regulated by Rab11. Journal of cell science. 2002;115(Pt 12):2505–2515. doi: 10.1242/jcs.115.12.2505. [DOI] [PubMed] [Google Scholar]
- Sevenich L, Bowman RL, Mason SD, Quail DF, Rapaport F, Elie BT, Brogi E, Brastianos PK, Hahn WC, Holsinger LJ, Massague J, Leslie CS, Joyce JA. Analysis of tumour- and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nat Cell Biol. 2014;16(9):876–888. doi: 10.1038/ncb3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara H, Kuranaga Y, Kumazaki M, Sugito N, Yoshikawa Y, Takai T, Taniguchi K, Ito Y, Akao Y. Regulated Polarization of Tumor-Associated Macrophages by miR-145 via Colorectal Cancer-Derived Extracellular Vesicles. Journal of immunology. 2017a doi: 10.4049/jimmunol.1700167. [DOI] [PubMed] [Google Scholar]
- Shinohara H, Kuranaga Y, Kumazaki M, Sugito N, Yoshikawa Y, Takai T, Taniguchi K, Ito Y, Akao Y. Regulated Polarization of Tumor-Associated Macrophages by miR-145 via Colorectal Cancer-Derived Extracellular Vesicles. Journal of immunology. 2017b;199(4):1505–1515. doi: 10.4049/jimmunol.1700167. [DOI] [PubMed] [Google Scholar]
- Silva J, Garcia V, Rodriguez M, Compte M, Cisneros E, Veguillas P, Garcia JM, Dominguez G, Campos-Martin Y, Cuevas J, Pena C, Herrera M, Diaz R, Mohammed N, Bonilla F. Analysis of exosome release and its prognostic value in human colorectal cancer. Genes, chromosomes & cancer. 2012;51(4):409–418. doi: 10.1002/gcc.21926. [DOI] [PubMed] [Google Scholar]
- Sitar S, Kejzar A, Pahovnik D, Kogej K, Tusek-Znidaric M, Lenassi M, Zagar E. Size characterization and quantification of exosomes by asymmetrical-flow field-flow fractionation. Analytical chemistry. 2015;87(18):9225–9233. doi: 10.1021/acs.analchem.5b01636. [DOI] [PubMed] [Google Scholar]
- Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleeman JP. The lymph node pre-metastatic niche. Journal of molecular medicine. 2015a;93(11):1173–1184. doi: 10.1007/s00109-015-1351-6. [DOI] [PubMed] [Google Scholar]
- Sleeman JP. Pre-metastatic conditioning of organ microenvironments by tumors: beyond preparing the soil. Journal of molecular medicine. 2015b;93(11):1171–1172. doi: 10.1007/s00109-015-1361-4. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Vannberg FO, Dixon JB. Lymphatic transport of exosomes as a rapid route of information dissemination to the lymph node. Scientific reports. 2016;6:24436. doi: 10.1038/srep24436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachowiak JC, Schmid EM, Ryan CJ, Ann HS, Sasaki DY, Sherman MB, Geissler PL, Fletcher DA, Hayden CC. Membrane bending by protein-protein crowding. Nat Cell Biol. 2012;14(9):944–949. doi: 10.1038/ncb2561. [DOI] [PubMed] [Google Scholar]
- Sueta A, Yamamoto Y, Takeshita MTT, Yamamoto-Ibusuki M, Iwase H. Differential expression of exosomal miRNAs between breast cancer patients with and without recurrence. Oncotarget. 2017 doi: 10.18632/oncotarget.19482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Zheng W, Guo Z, Ju Q, Zhu L, Gao J, Zhou L, Liu F, Xu Y, Zhan Q, Zhou Z, Sun W, Zhao X. A novel TP53 pathway influences the HGS-mediated exosome formation in colorectal cancer. Scientific reports. 2016;6:28083. doi: 10.1038/srep28083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna S, Pucci M, Giallombardo M, Di Bella MA, Santarpia M, Reclusa P, Gil-Bazo I, Rolfo C, Alessandro R. Amphiregulin contained in NSCLC-exosomes induces osteoclast differentiation through the activation of EGFR pathway. Scientific reports. 2017;7(1):3170. doi: 10.1038/s41598-017-03460-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavoosidana G, Ronquist G, Darmanis S, Yan J, Carlsson L, Wu D, Conze T, Ek P, Semjonow A, Eltze E, Larsson A, Landegren UD, Kamali-Moghaddam M. Multiple recognition assay reveals prostasomes as promising plasma biomarkers for prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(21):8809–8814. doi: 10.1073/pnas.1019330108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecologic oncology. 2008;110(1):13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Taylor J, Jaiswal R, Bebawy M. Calcium-calpain Dependent Pathways Regulate Vesiculation in Malignant Breast Cells. Current cancer drug targets. 2017;17(5):486–494. doi: 10.2174/1568009616666161026165736. [DOI] [PubMed] [Google Scholar]
- Thakur BK, Zhang H, Becker A, Matei I, Huang Y, Costa-Silva B, Zheng Y, Hoshino A, Brazier H, Xiang J, Williams C, Rodriguez-Barrueco R, Silva JM, Zhang W, Hearn S, Elemento O, Paknejad N, Manova-Todorova K, Welte K, Bromberg J, Peinado H, Lyden D. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24(6):766–769. doi: 10.1038/cr.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nature reviews Immunology. 2009;9(8):581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- Tkach M, Thery C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell. 2016;164(6):1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- Tominaga N, Kosaka N, Ono M, Katsuda T, Yoshioka Y, Tamura K, Lotvall J, Nakagama H, Ochiya T. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nature communications. 2015;6:6716. doi: 10.1038/ncomms7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- Vader P, Breakefield XO, Wood MJ. Extracellular vesicles: emerging targets for cancer therapy. Trends in molecular medicine. 2014;20(7):385–393. doi: 10.1016/j.molmed.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Valencia K, Luis-Ravelo D, Bovy N, Anton I, Martinez-Canarias S, Zandueta C, Ormazabal C, Struman I, Tabruyn S, Rebmann V, De Las Rivas J, Guruceaga E, Bandres E, Lecanda F. miRNA cargo within exosome-like vesicle transfer influences metastatic bone colonization. Molecular oncology. 2014;8(3):689–703. doi: 10.1016/j.molonc.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiente M, Obenauf AC, Jin X, Chen Q, Zhang XH, Lee DJ, Chaft JE, Kris MG, Huse JT, Brogi E, Massague J. Serpins promote cancer cell survival and vascular co-option in brain metastasis. Cell. 2014;156(5):1002–1016. doi: 10.1016/j.cell.2014.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Akkooi AC, Verhoef C, Eggermont AM. Importance of tumor load in the sentinel node in melanoma: clinical dilemmas. Nature reviews Clinical oncology. 2010;7(8):446–454. doi: 10.1038/nrclinonc.2010.100. [DOI] [PubMed] [Google Scholar]
- Wang H, Hou L, Li A, Duan Y, Gao H, Song X. Expression of serum exosomal microRNA-21 in human hepatocellular carcinoma. BioMed research international. 2014;2014:864894. doi: 10.1155/2014/864894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei JX, Lv LH, Wan YL, Cao Y, Li GL, Lin HM, Zhou R, Shang CZ, Cao J, He H, Han QF, Liu PQ, Zhou G, Min J. Vps4A functions as a tumor suppressor by regulating the secretion and uptake of exosomal microRNAs in human hepatoma cells. Hepatology. 2015;61(4):1284–1294. doi: 10.1002/hep.27660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside TL. Immune modulation of T-cell and NK (natural killer) cell activities by TEXs (tumour-derived exosomes) Biochemical Society transactions. 2013;41(1):245–251. doi: 10.1042/BST20120265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, Whiteside TL. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. Journal of immunology. 2009;183(6):3720–3730. doi: 10.4049/jimmunol.0900970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witwer KW, Buzas EI, Bemis LT, Bora A, Lasser C, Lotvall J, Nolte-’t Hoen EN, Piper MG, Sivaraman S, Skog J, Thery C, Wauben MH, Hochberg F. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. Journal of extracellular vesicles. 2013;2 doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witwer KW, Soekmadji C, Hill AF, Wauben MH, Di Vizio D, Falcon-Perez JM, Gardiner C, Hochberg F, Kurochkin IV, Lötvall J, Mathivanan S, Nieuwland R, Sahoo S, Tahara H, Torrecilhas AC, Weaver AM, Yin H, Zheng L, Song Gho Y, Quesenberry P, Thery C. Updating the MISEV minimal requirements for extracellular vesicle studies: building bridges to reproducibility. Journal of extracellular vesicles. 2017;6(1) doi: 10.1080/20013078.2017.1396823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodzimierz R, Rutkowski P, Nowecki ZI, Kulik J, Nasierowska-Guttmejer A, Siedlecki JA. Detection of melanoma cells in the lymphatic drainage after lymph node dissection in melanoma patients by using two-marker reverse transcriptase-polymerase chain reaction assay. Annals of surgical oncology. 2004;11(11):988–997. doi: 10.1245/ASO.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Yang S, Che SP, Kurywchak P, Tavormina JL, Gansmo LB, Correa de Sampaio P, Tachezy M, Bockhorn M, Gebauer F, Haltom AR, Melo SA, LeBleu VS, Kalluri R. Detection of mutant KRAS and TP53 DNA in circulating exosomes from healthy individuals and patients with pancreatic cancer. Cancer biology & therapy. 2017;18(3):158–165. doi: 10.1080/15384047.2017.1281499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka Y, Kosaka N, Konishi Y, Ohta H, Okamoto H, Sonoda H, Nonaka R, Yamamoto H, Ishii H, Mori M, Furuta K, Nakajima T, Hayashi H, Sugisaki H, Higashimoto H, Kato T, Takeshita F, Ochiya T. Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nature communications. 2014;5:3591. doi: 10.1038/ncomms4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Zhao S, Ren L, Wang L, Chen Z, Hoffman RM, Zhou J. Pancreatic cancer-derived exosomes promote tumor metastasis and liver pre-metastatic niche formation. Oncotarget. 2017 doi: 10.18632/oncotarget.18831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Deng T, Liu R, Bai M, Zhou L, Wang X, Li S, Wang X, Yang H, Li J, Ning T, Huang D, Li H, Zhang L, Ying G, Ba Y. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nature communications. 2017;8:15016. doi: 10.1038/ncomms15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang S, Yao J, Lowery FJ, Zhang Q, Huang WC, Li P, Li M, Wang X, Zhang C, Wang H, Ellis K, Cheerathodi M, McCarty JH, Palmieri D, Saunus J, Lakhani S, Huang S, Sahin AA, Aldape KD, Steeg PS, Yu D. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015;527(7576):100–104. doi: 10.1038/nature15376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Fong MY, Min Y, Somlo G, Liu L, Palomares MR, Yu Y, Chow A, O’Connor ST, Chin AR, Yen Y, Wang Y, Marcusson EG, Chu P, Wu J, Wu X, Li AX, Li Z, Gao H, Ren X, Boldin MP, Lin PC, Wang SE. Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell. 2014;25(4):501–515. doi: 10.1016/j.ccr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomer A, Maynard C, Verweij FJ, Kamermans A, Schafer R, Beerling E, Schiffelers RM, de Wit E, Berenguer J, Ellenbroek SI, Wurdinger T, Pegtel DM, van Rheenen J. In Vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell. 2015;161(5):1046–1057. doi: 10.1016/j.cell.2015.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]


