Abstract
Dehydrophos is a tripeptide phosphonate antibiotic produced by Streptomyces luridus. Its biosynthetic pathway involves the use of aminoacyl-tRNA (aa-tRNA) for amide bond formation. The first amide bond during biosynthesis is formed by DhpH-C, a peptidyltransferase that utilizes Leu-tRNALeu. DhpH-C is a member of a burgeoning family of natural product biosynthetic enzymes that make use of aa-tRNA outside of canonical translation activities in the cell. Here we used site-directed mutagenesis of both DhpH-C and tRNALeu to investigate the enzyme mechanism and substrate specificity, respectively, and analyzed the substrate scope for the production of a set of dipeptides. DhpH-C appears to recognize both the amino acyl group on the tRNA and the tRNA acceptor stem, and the enzyme can accept other hydrophobic residues in addition to leucine. These results contribute to a better understanding of enzyme-aa-tRNA interactions and the growing exploration of aa-tRNA usage beyond translation.
Several recent studies have uncovered a role for aminoacyl-tRNA (aa-tRNA) in antibiotic biosynthesis.1–5 Biosynthetic enzymes that make use of aa-tRNA originate from at least three different protein folds to perform amide or ester bond ligations.6, 7 Examples include cyclodipeptide synthases (CDPSs) that are structural homologs of aa-tRNA synthetases, amide bond-forming ligases that are homologs of FemX peptidyltransferases from cell wall biosynthesis, and ribosomally synthesized and posttranslationally modified peptide dehydratases with a new protein fold.2–4
Enzymes known to interact with aa-tRNA share several common features, despite their strong variations in sequence. Studies of FemX and MprF (multiple peptide resistance factor), which transfer amino acids from aa-tRNA to UDP-MurNAc pentapeptide and phosphatidylglycerol, respectively, have revealed a Phe-Lys motif potentially important for the binding of the amino acyl group on the tRNA and the tRNA acceptor stem.8, 9 In fact, this Lys residue appears to be conserved across many of the biochemically validated aa-tRNA-dependent biosynthetic enzymes.6 Additionally, several studies have highlighted the importance of the tRNA acceptor stem for substrate recognition.10 For example, the activities of two unrelated enzymes, the CDPS AlbC and the lantibiotic dehydratase MibB, are both negatively affected if nucleotides at the top of the acceptor stems of their aa-tRNA substrates are changed.11, 12 In this study, we investigated whether these features are important for the function of an aa-tRNA-dependent enzyme involved in phosphonate natural product biosynthesis.
Currently, the biosyntheses of two characterized phosphonopeptides with antibacterial or antifungal properties are known to involve aa-tRNA-dependent enzymes.13, 14 This discovery was made through a comparison of enzymes encoded in the dehydrophos and fosfazinomycin biosynthetic gene clusters with FemX peptidyltransferases. We were intrigued by the use of aa-tRNA as a requirement for amide bond formation, given that previously investigated phosphonopeptides are produced by other amide bond-forming mechanisms. For instance, the biosynthetic pathway to the herbicide phosphinothricin tripeptide includes two non-ribosomal peptide synthetases, which activate amino acids by adenylation.15–17 In contrast, ATP-grasp enzymes activate amino acids by phosphorylation during the production of the antifungal rhizocticins.18, 19 In dehydrophos biosynthesis, a new mode of amino acid activation was uncovered for phosphonopeptides with the determination that leucine and glycine are transferred to the phosphonate warhead using aa-tRNA as a substrate (Figure 1a). The C-terminal domain of DhpH (DhpH-C) catalyzes the formation of an amide bond between (R)-(−)-1-aminoethylphosphonate (L-AlaP) and Leu using Leu-tRNALeu as substrate (Figure 1a). Dehydrophos is a ‘Trojan horse’ antibiotic in which the proteinogenic amino acids Gly and Leu help deliver the phosphonate warhead via peptide permeases.20, 21 Once the tripeptide enters the cell, internal peptidases release 1-aminovinylphosphonate, which spontaneously converts to methyl acetylphosphonate, a potent inhibitor of pyruvate dehydrogenase. Thus, the function of DhpH-C is linked to the antibacterial potential of dehydrophos. Here, we investigated the features of DhpH-C and aa-tRNA that are necessary for dipeptide formation. We performed site-directed mutagenesis on the putative active site of DhpH-C to determine residues important for activity, investigated the ability of DhpH-C to recognize other amino acids in addition to Leu, and analyzed the effects of mutating tRNALeu acceptor stems on product formation. These findings contribute to the growing understanding of aa-tRNA usage by secondary metabolite enzymes.
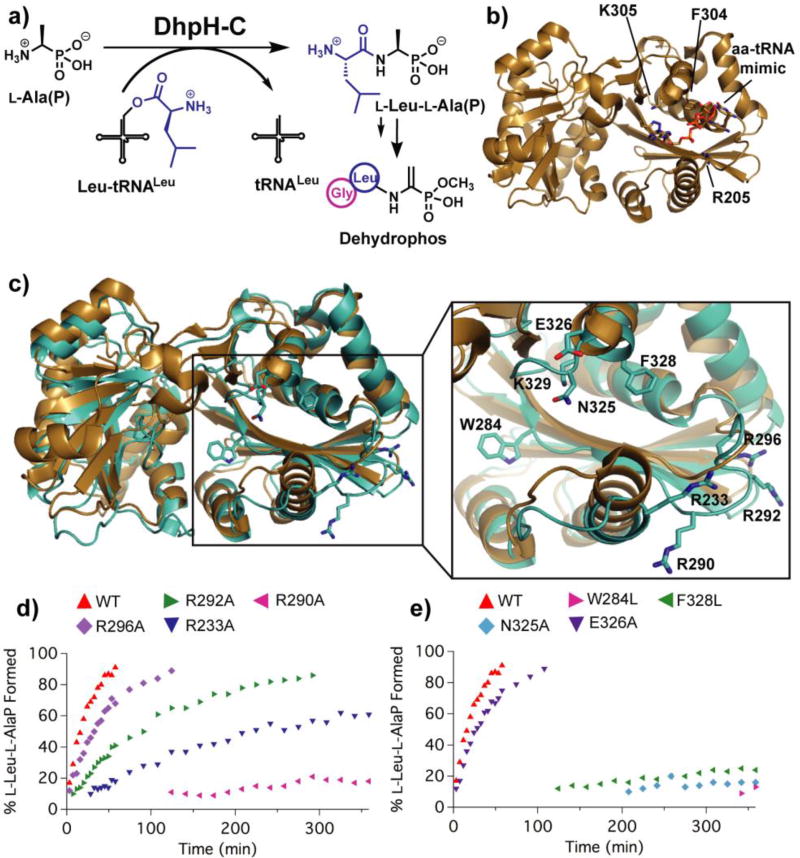
Figure 1.
a) The DhpH-C reaction and the final structure of dehydrophos. b) Crystal structure of FemX in complex with the CCA portion of the tRNA acceptor stem and a triazole ring to mimic the placement of aa-tRNA (PDB 4II9).8 Highlighted residues are predicted to be important for interaction with the tRNA acceptor stem and the amino acid. c) Overlay of FemX (tan) with an I-TASSER model of DhpH-C (cyan).8, 22 Highlighted residues of DhpH-C were targeted for site-directed mutagenesis. d–e) Percent product formed plotted against time of the DhpH-C reaction as measured by 31P NMR spectroscopy.
We first investigated which residues of DhpH-C may play a role in enzyme activity. A recent structure of FemX from Weissella viridicens8 co-crystallized with the last three nucleotides of the tRNAAla acceptor stem and an I-TASSER22 model of DhpH-C facilitated analysis of the putative DhpH-C active site (Figure 1b–c). Furthermore, previous work on AlbC, a cyclodipeptide synthase involved in the production of albonoursin, revealed an Arg-rich basic patch suitable for interacting with the negatively charged phosphate groups of aa-tRNA.11 Thus, Arg residues inside the potential aa-tRNA binding pocket of DhpH-C were identified, and site-directed mutagenesis to Ala was performed to determine the contribution of each residue to product formation (Figure 1c). Each Ala variant was then tested in a coupled enzyme assay based on a previously described method13 using E. coli leucyl-tRNA synthetase (LeuRS) to generate aa-tRNA in situ from E. coli total tRNA, L-Leu, and ATP. Product formation was monitored over the course of 6 h by 31P NMR spectroscopy (Figure 1d and S1). All Ala mutations negatively impacted product formation, with the most pronounced decline resulting from mutated residues Arg233 and Arg290 located in a helix and loop region lining the bottom of the putative active site, respectively (Figure 1c–d and Figure S2). These results imply that the use of Arg residues to bind aa-tRNA may be a shared feature of aa-tRNA-dependent enzymes regardless of protein structural fold, considering that the CDPS AlbC is not related by sequence or structure to DhpH-C and other FemX-like enzymes.
Next, we examined the consequences of mutating three residues of DhpH-C that structurally align with three residues important for FemX activity. In the I-TASSER model, Arg233 is structurally in a similar location as Arg205 in the crystal structure of FemX, which is proposed to be important for hydrogen-bonding to the phosphate group of the final nucleotide at the 3’ end of the tRNA acceptor stem.8 As discussed above, the R233A mutant had a pronounced negative effect on product formation over time. Residues Phe328 and Lys329 are structurally similar to Phe304 and Lys305 of FemX, possibly forming the Phe-Lys motif for tRNA interaction also seen in MprF.9 The DhpH-C-F328L mutant was still functional, however product formation was drastically lowered compared to wild type (Figure 1e). We also removed positive charge with a K329M mutant, or displaced the positive charge with a K329R mutant. Both DhpH-C K329M and K329R showed no observable activity after 12 h, indicating that the placement of the charge on Lys329 is critical for enzyme function (Figure S3). Finally, we chose to investigate the effects of mutating a set of residues (Trp284, Asn325, and Glu326) in the vicinity of the critical Lys329 residue (Figure 1c). The most striking effect on product formation came from the W284L and N325A mutants, which provides more evidence that this region of DhpH-C is critical for function (Figure 1e). These results lend support to the proposed pocket of DhpH-C for substrate interaction, and suggest that the importance of a conserved Lys residue in other aa-tRNA-dependent enzymes extends to Dhp-C.
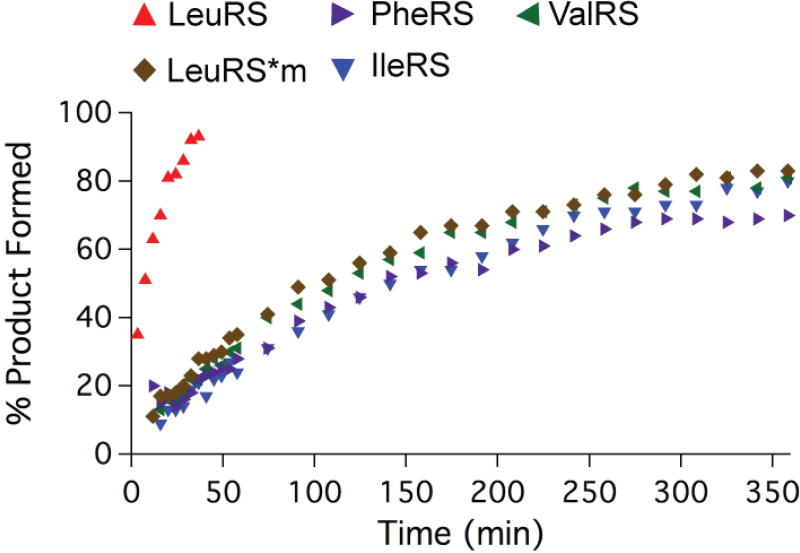
Our next goal was to determine how DhpH-C activity with Leu-tRNALeu compares with other hydrophobic aa-tRNA cognate pairs carrying Val, Ile, and Phe. Each amino acid and its corresponding aminoacyl-tRNA synthetase (aaRS) were used to substitute L-Leu and LeuRS in the coupled enzyme assay described above for DhpH-C activity. When the concentration of each aaRS was doubled, the reaction rate did not increase, indicating that the production of aa-tRNA was not the rate-limiting step under the assay conditions (Figure S4). Furthermore, the amount of total E. coli tRNA used, which has different amounts of the tRNAs for various amino acids, was normalized to produce equal amounts of each aa-tRNA substrate at the initiation of each experiment (Figures S5 and S6). Under these conditions, production of the dipeptides was observed (Figure S7) and was most efficient with Leu-tRNALeu as the substrate (Figure 2 and S8). When Ile-tRNAIle, Val-tRNAVal, or Phe-tRNAPhe was used, both the rate and extent of product formation decreased. Interestingly, similar rates and yields were observed for each of these alternative substrates. Because of the experimental set-up that regenerates the aa-tRNA, we cannot identify if the observed change is an effect on kcat or KM, but the overall strong decrease of activity is clear. To distinguish whether the effect on product formation was a result of the change in amino acid or the change in the tRNA sequence delivering the amino acid, we set up an assay with Ile-tRNALeu as a substrate. In other words, using the tRNA for the cognate amino acid, but with the non-cognate amino acid Ile attached. The mutant enzyme LeuRS-Y330A/D342A/D345A (LeuRS*m) has been shown previously to misacylate tRNALeu with Ile.23 When Ile-tRNALeu was the substrate for DhpH-C, similar levels of product formation were observed to the Ile-tRNAIle reaction, with both being significantly slower than the reaction containing Leu-tRNALeu (Figure 2). Thus, at minimum the identity of the amino acid is important for product formation by DhpH-C.
Figure 2.
Percent product formation as measured by 31P NMR spectroscopy plotted against reaction time for the DhpH-C coupled enzyme reaction with various amino acid/aaRS pairs. All reactions were conducted with total tRNA from E. coli.
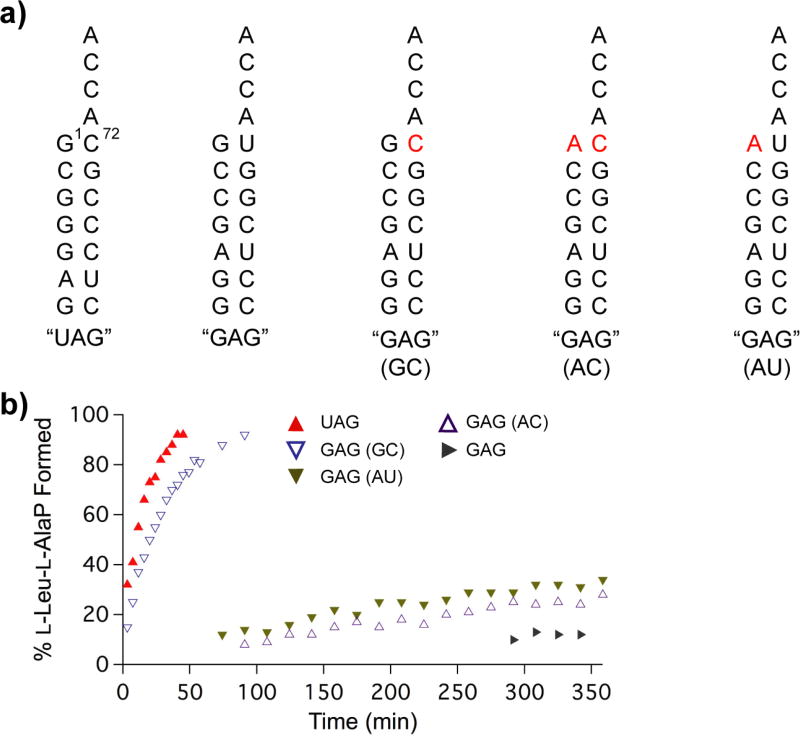
We investigated whether DhpH-C identifies recognition elements on its tRNALeu substrates using a different strategy. For this series of experiments, we kept Leu as the amino acid and determined whether DhpH-C has a preference for select isoacceptors of E. coli tRNALeu. Isoacceptor tRNAs were obtained by in vitro transcription and will be named herein based on their anticodon sequence. Previous studies on the CDPS AlbC showed that the enzyme prefers tRNA substrates with a G1-C72 base pair match in the acceptor stem.11 To investigate whether DhpH-C shares this feature, the tRNALeu (UAG) and tRNALeu (GAG) sequences were used as substrates, since their acceptor stems contain a G-C match and a G-U mismatch, respectively (Figure 3a). We observed that DhpH-C does display a clear preference, since the reaction was complete with nearly 100% product formation in about 1 h with the tRNALeu (UAG) isoacceptor, but less than 20% product was formed in 6 h with the tRNALeu (GAG) isoacceptor (Figure 3b). Importantly, generation of the tRNALeu (GAG) U72C isoacceptor mutant to introduce the G1-C72 base pair match resulted in the rescue of activity back to levels commensurate with the tRNALeu (UAG) substrate (Figure 3b). Such a drastic change in product formation levels with the mutation of just one nucleotide corroborates the hypothesis that the acceptor stem is important for recognition of the tRNA substrate,11, 12, 24 and that the anti-codon is not likely to be involved in substrate recognition. Interestingly, DhpH-C and AlbC are not related by structure or sequence, yet they have converged towards similar modes of tRNA substrate recognition.
Figure 3.
a) Sequences of E. coli tRNALeu acceptor stems named by the sequence of their anticodon. Mutations are shown in red. b) Percent product formation over time of DhpH-C WT with the various in vitro transcribed tRNALeu variants.
Finally, to further probe the G1-C72 recognition element of DhpH-C, we compared a series of variant tRNALeu (GAG) substrates: U72C (GC pair), G1A (AU pair), and G1A-U72C (AC pair). FemX has been shown to require C72 for substantial product formation, with a G1-C72 match being most effective.24, 25 For DhpH-C, none of the additional variants showed activity equal to the GAG (GC) substrate (Figure 3b). Thus, the presence of the G1-C72 base pair match on the acceptor stem is important for DhpH-C activity. It is possible that the enzyme makes specific contacts with G1 and C72, or that the strong base pair helps position the acceptor stem for enzyme interaction with the amino acid.
In conclusion, we have investigated the molecular basis of DhpH-C interaction with its aa-tRNA substrate by determining key residues important for enzyme activity and essential nucleotides of the tRNA acceptor stem that aid in enzyme recognition. Our substrate scope analysis with hydrophobic amino acids revealed that DhpH-C can produce multiple dipeptide phosphonates. This tolerance could potentially be leveraged to generate new phosphonate analogs chemoenzymatically. The strong preference displayed for Leu may explain why thus far no congeners of dehydrophos have been observed to be produced by S. luridus. Our results also highlight the similarities among aa-tRNA-dependent enzymes in natural product biosynthesis, despite the three distinct structural folds. Like the CDPS AlbC and dehydratases involved in modifying ribosomal peptide natural products,11, 12 DhpH-C recognizes nucleotides in the tRNA acceptor stem.
Methods
Activity Assays by 31P NMR Spectroscopy
NMR experiments were performed on an Agilent 600 MHz spectrometer equipped with an OneNMR probe. 31P spectra were recorded in 20% D2O at room temperature. A typical reaction contained 50 mM HEPES pH 7.5, 150 mM KCl, 15 mM MgCl2, 6 mM ATP, 6 mM L-Leu, 5 mM L-AlaP, 8 µM LeuRS, 10 µM DhpH-C WT and variants, 0.01 U/µL TIPP, and 3 mg/mL total E. coli tRNA (Sigma Aldrich/Roche) or 16 µM in vitro transcribed tRNALeu [8 µM for GAG (AU)] in 500 µL. Based on aminoacylation studies, the amount of total tRNA in each aaRS reaction was increased as follows: IleRS (1.7x), ValRS (1.3x), PheRS (3.2x), and LeuRSm* (1.8x). Reactions with various synthetases (8 µM) were incubated at room temperature with components needed for aminoacylation before the addition of 35 µM DhpH-C WT (10 µM for the LeuRS sample) and 5 mM L-AlaP was added to initiate the amide-forming reaction after 15 min (1 h for LeuRS*m). Product formation was followed by collecting spectra every 4 min for 6 h. Spectra were collected for 2 h for the in vitro transcribed tRNALeu (UAG) since the reaction had gone to completion within that amount of time. Spectra were analyzed using MestReNova version 8.0.0–10524 software. Percent product formation was calculated by integration of peak area. Stages of the reactions in which less than ~10% of the product was formed or substrate remained were difficult to analyze because of the low-signal-to-noise. As a result, data for experiments that ran to completion were not plotted after ~85–90% product formation. Replicate endpoint assays were quenched by treatment with Chelex® (Sigma Aldrich, 20 min of shaking at room temperature) and filtration through a 10 kDa molecular weight cut off Amicon filter (EMD Millipore). D2O (100 µL) was added before analysis. Results were plotted using Igor Pro version 6.32A.
Supplementary Material
Acknowledgments
Funding was provided by National Institutes of Health Grant P01 GM077596 (to W.A.v.d.D.). NMR spectra were collected on a 600 MHz NMR spectrometer that was funded by NIH grant number S10-RR028833 located in the Carl R. Woese Institute for Genomic Biology Core Facility. We thank Z. Li of the Metabolomics Center, Roy J. Carver Biotechnology Center at the University of Illinois at Urbana-Champaign for performing the LCMS analysis and K. A. Wang for preliminary LCMS testing. We thank S. Martinis (UIUC) for providing the plasmid for LeuRS and LeuRS Y330A/D342A/D345A, Z. Huang for preparation of pET-15b valRS, and M. Ortega for preparation of T7 RNA polymerase. In addition, we thank M. Saks and A. Banerjee for helpful discussions and I. Bothwell and K. A. Wang for critically reviewing the manuscript.
Footnotes
The supporting information is available free of charge via the internet at http://pubs.acs.org. Included in the supporting information are materials used in this study, additional experimental procedures, and supporting figures.
References
- 1.Garg RP, Qian XL, Alemany LB, Moran S, Parry RJ. Investigations of valanimycin biosynthesis: elucidation of the role of seryl-tRNA. Proc. Natl. Acad. Sci. U. S. A. 2008;105:6543–6547. doi: 10.1073/pnas.0708957105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gondry M, Sauguet L, Belin P, Thai R, Amouroux R, Tellier C, Tuphile K, Jacquet M, Braud S, Courcon M, Masson C, Dubois S, Lautru S, Lecoq A, Hashimoto S, Genet R, Pernodet JL. Cyclodipeptide synthases are a family of tRNA-dependent peptide bond-forming enzymes. Nat. Chem. Biol. 2009;5:414–420. doi: 10.1038/nchembio.175. [DOI] [PubMed] [Google Scholar]
- 3.Zhang W, Ntai I, Kelleher NL, Walsh CT. tRNA-dependent peptide bond formation by the transferase PacB in biosynthesis of the pacidamycin group of pentapeptidyl nucleoside antibiotics. Proc. Natl. Acad. Sci. U. S. A. 2011;108:12249–12253. doi: 10.1073/pnas.1109539108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ortega MA, Hao Y, Zhang Q, Walker MC, van der Donk WA, Nair SK. Structure and mechanism of the tRNA-dependent lantibiotic dehydratase NisB. Nature. 2015;517:509–512. doi: 10.1038/nature13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maruyama C, Niikura H, Izumikawa M, Hashimoto J, Shin-Ya K, Komatsu M, Ikeda H, Kuroda M, Sekizuka T, Ishikawa J, Hamano Y. tRNA-dependent aminoacylation of an amino sugar intermediate in the biosynthesis of a streptothricin-related antibiotic. Appl. Environ. Microbiol. 2016;82:3640–3648. doi: 10.1128/AEM.00725-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moutiez M, Belin P, Gondry M. Aminoacyl-tRNA-utilizing enzymes in natural product biosynthesis. Chem. Rev. 2017;117:5578–5618. doi: 10.1021/acs.chemrev.6b00523. [DOI] [PubMed] [Google Scholar]
- 7.Ulrich EC, van der Donk WA. Cameo appearances of aminoacyl-tRNA in natural product biosynthesis. Curr. Opin. Chem. Biol. 2016;35:29–36. doi: 10.1016/j.cbpa.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fonvielle M, Li de La Sierra-Gallay I, El-Sagheer AH, Lecerf M, Patin D, Mellal D, Mayer C, Blanot D, Gale N, Brown T, van Tilbeurgh H, Etheve-Quelquejeu M, Arthur M. The structure of FemX(Wv) in complex with a peptidyl-RNA conjugate: mechanism of aminoacyl transfer from Ala-tRNA(Ala) to peptidoglycan precursors. Angew. Chem. Int. Ed. 2013;52:7278–7281. doi: 10.1002/anie.201301411. [DOI] [PubMed] [Google Scholar]
- 9.Hebecker S, Krausze J, Hasenkampf T, Schneider J, Groenewold M, Reichelt J, Jahn D, Heinz DW, Moser J. Structures of two bacterial resistance factors mediating tRNA-dependent aminoacylation of phosphatidylglycerol with lysine or alanine. Proc. Natl. Acad. Sci. U. S. A. 2015;112:10691–10696. doi: 10.1073/pnas.1511167112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz A, Elgamal S, Rajkovic A, Ibba M. Non-canonical roles of tRNAs and tRNA mimics in bacterial cell biology. Mol. Microbiol. 2016;101:545–558. doi: 10.1111/mmi.13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moutiez M, Seguin J, Fonvielle M, Belin P, Jacques IB, Favry E, Arthur M, Gondry M. Specificity determinants for the two tRNA substrates of the cyclodipeptide synthase AlbC from Streptomyces noursei. Nucleic Acids Res. 2014;42:7247–7258. doi: 10.1093/nar/gku348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ortega MA, Hao Y, Walker MC, Donadio S, Sosio M, Nair SK, van der Donk WA. Structure and tRNA specificity of MibB, a lantibiotic dehydratase from Actinobacteria involved in NAI-107 biosynthesis. Cell Chem. Biol. 2016;23:370–380. doi: 10.1016/j.chembiol.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bougioukou DJ, Mukherjee S, van der Donk WA. Revisiting the biosynthesis of dehydrophos reveals a tRNA-dependent pathway. Proc. Natl. Acad. Sci. U. S. A. 2013;110:10952–10957. doi: 10.1073/pnas.1303568110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang Z, Wang KA, van der Donk WA. New insights into the biosynthesis of fosfazinomycin. Chem. Sci. 2016;7:5219–5223. doi: 10.1039/c6sc01389a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz D, Alijah R, Nussbaumer B, Pelzer S, Wohlleben W. The peptide synthetase gene phsA from Streptomyces viridochromogenes is not juxtaposed with other genes involved in nonribosomal biosynthesis of peptides. Appl. Environ. Microbiol. 1996;62:570–577. doi: 10.1128/aem.62.2.570-577.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz D, Grammel N, Heinzelmann E, Keller U, Wohlleben W. Phosphinothricin tripeptide synthetases in Streptomyces viridochromogenes Tü494. Antimicrob. Agents Chemother. 2005;49:4598–4607. doi: 10.1128/AAC.49.11.4598-4607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JH, Evans BS, Li G, Kelleher NL, van der Donk WA. In vitro characterization of a heterologously expressed nonribosomal peptide synthetase involved in phosphinothricin tripeptide biosynthesis. Biochemistry. 2009;48:5054–5056. doi: 10.1021/bi900164d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kino K, Kotanaka Y, Arai T, Yagasaki M. A novel L-amino acid ligase from Bacillus subtilis NBRC3134, a microorganism producing peptide-antibiotic rhizocticin. Biosci. Biotechnol. Biochem. 2009;73:901–907. doi: 10.1271/bbb.80842. [DOI] [PubMed] [Google Scholar]
- 19.Borisova SA, Circello BT, Zhang JK, van der Donk WA, Metcalf WW. Biosynthesis of rhizocticins, antifungal phosphonate oligopeptides produced by Bacillus subtilis ATCC6633. Chem. Biol. 2010;17:28–37. doi: 10.1016/j.chembiol.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Circello BT, Miller CG, Lee JH, van der Donk WA, Metcalf WW. The antibiotic dehydrophos is converted to a toxic pyruvate analog by peptide bond cleavage in Salmonella enterica. Antimicrob. Agents Chemother. 2011;55:3357–3362. doi: 10.1128/AAC.01483-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuemin M, van der Donk WA. Structure-activity relationships of the phosphonate antibiotic dehydrophos. Chem. Commun. 2010;46:7694–7696. doi: 10.1039/c0cc02958k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics. 2008;9:40. doi: 10.1186/1471-2105-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinis SA, Briggs JM, Mursinna RS, Lee KW, Lincecum TL, Williams AM, Zhai Y. Method and composition for leucyl-tRNA synthetases and derivatives thereof that activate and aminoacylate non-leucine amino acid to tRNA adaptor molecules. 7,785,827 B2. U. S. Patent. 2010 Aug 31;
- 24.Villet R, Fonvielle M, Busca P, Chemama M, Maillard AP, Hugonnet JE, Dubost L, Marie A, Josseaume N, Mesnage S, Mayer C, Valery JM, Etheve-Quelquejeu M, Arthur M. Idiosyncratic features in tRNAs participating in bacterial cell wall synthesis. Nucleic Acids Res. 2007;35:6870–6883. doi: 10.1093/nar/gkm778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fonvielle M, Chemama M, Villet R, Lecerf M, Bouhss A, Valery JM, Etheve-Quelquejeu M, Arthur M. Aminoacyl-tRNA recognition by the FemXWv transferase for bacterial cell wall synthesis. Nucleic Acids Res. 2009;37:1589–1601. doi: 10.1093/nar/gkn1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.