Abstract
Autism spectrum disorder (ASD) has complex neurodevelopmental impairments and origins that are linked to both genetic and environmental factors. Hence, there is an urgency to establish animal models with ASD-like characteristics to understand the underlying mechanisms of ASD. Prenatal exposure to valproic acid (VPA) has been shown to cause ASD-like symptoms in humans, rats, and recently zebrafish. The present study investigated the use of VPA exposure to create an ASD model in zebrafish that was verified through observation of ASD-like phenotypes in brain development and behavioral changes in embryonic and larval zebrafish. Our findings revealed that treating zebrafish embryos with VPA starting at 8 hours post fertilization (hpf) resulted in significant: increase in the ASD macrocephalic phenotype; hyperactivity of embryo/larvae movement behaviors; and increases of ASD-like larval social behaviors. Further analysis showed increases in cell proliferation, the proportion of mature newborn neurons, and neural stem cell proliferation in the brain region, which may contribute to the brain overgrowth and macrocephaly observed following VPA exposure. Our study demonstrated that VPA exposure can generate ASD-like phenotypes and behaviors, showing the validity of zebrafish as an alternative model for ASD and underlying mechanism research.
Keywords: zebrafish, VPA, head size, behaviors, neural cells expression
1. Introduction
Autism is a neurodevelopmental disorder that is diagnosable by the age of 3and is one of five disorders classified collectively as autism spectrum disorder (ASD) (Marisela Huerta et al., 2012). It is characterized by pervasive impairments in social interactions, deficits in verbal and nonverbal communication, and stereotyped, repetitive patterns of behaviors and interests. The observed behavioral disturbances also include self-injury, hyperactivity, and aberrant sensitivity to sensory stimulation. Prevalence of autism has increased since the 1990’s with variability based on ethnicity, sex, and geographical location (Christensen et al., 2016; Lauritsen et al., 2014). The etiology of autism is not known but it has a strong genetic (Ebert and Greenberg, 2013; Woods et al., 2012) and environmental (Kardas et al., 2015; Lam et al., 2011; Modabbernia et al., 2017) component. Exposure to at least two teratogens appear to be a risk factor for the disorder: thalidomide(Strömland et al., 1994; Teitelbaum, 2003), and valproic acid (VPA) (Schneider and Przewlocki, 2004).
VPA is a pharmaceutical used for treatment of seizures and migraines but prenatal exposure to the compound may cause several fetal deformities and neurobehavioral alterations associated with ASD (Jentink et al., 2010). Animal models using VPA exposures are being developed to study ASD-like behavioral alterations, and rodent data has shown anatomical, pathological similarities (Kim et al., 2014; Moore et al., 2000; Narita et al., 2010; Schneider and Przewlocki, 2004; Williams et al., 2001; Williams and Hersh, 1997). Now that the murine ASD model has been established, effort into alternative animal models for ASD-like symptoms has begun.
Zebrafish (Danio rerio) have been used extensively to elucidate basic mechanisms underlying neurobehavioral toxicology (Bailey et al., 2013). As a highly social and genetically tractable organism, there is great potential to study the pathogenesis of complex brain disorders and model a variety of deficits relevant to ASD (Meshalkina et al.; Stewart et al., 2014). Importantly, zebrafish and mammals display parallel social behaviors governed by similar underlying mechanisms (Oliveira, 2013). VPA exposure has previously been used to induce behavioral changes in zebrafish to characterize ASD-like responses. Zebrafish exposed to VPA during the first 48 h of development exhibit deficits in social interaction, anxiety, and hyperactivity at different developmental periods (Zimmermann et al., 2015). However, the social interactions and aggressive behaviors were mainly examined in older zebrafish (70 and 120 dpf) at a single VPA concentration (48 μM) (Zimmermann et al., 2015). While it was thought that social behavior in zebrafish was not fully developed between 6 and 30 dpf (Buske and Gerlai, 2011), using specific size dependent apparatuses may alleviate logistical concerns and allows for the study of the early, sensitive developmental state, a core stage for ASD screening. In addition, the early stage of the embryonic zebrafish can represent the early stage of the human infant, because the zebrafish embryos can be exposed to external chemicals directly (Lam et al., 2011; Roberts et al., 2013).
In the present study, the non-teratogenic doses of VPA were used to screen and build an ASD risk evaluation system in embryonic and larval zebrafish. In detail, the embryos were exposed to VPA from 8 hpf to 4.5 dpf, and the evaluations were carried out during 1-13 dpf. Specifically, larval head size, embryo/larval movement and social behaviors were evaluated. We hypothesized that VPA exposure will produce ASD-like physical and behavioral characteristics in embryonic and larval stages of zebrafish. And our study will also help establish the zebrafish as an ASD model for use in future related mechanistic studies.
2. Materials and methods
2.1. Fish husbandry and embryo collection
Wild-type AB line zebrafish were used in the present study. Zebrafish care and use were conducted according to established guidelines approved by the Institutional Animal Care and Use Committee of Wenzhou Medical University. Adult zebrafish were raised and kept at standard laboratory conditions of 28°C on a 14:10 (dark: light) photoperiod in a recirculation system according to standard zebrafish breeding protocols(Westerfield, 1995). Water supplied to the system was filtered by reverse osmosis (pH 7.0-7.5), and Instant Ocean® salt was added to raise the water conductivity to 450-1000 μS/cm (system water). The adult fish were fed twice daily with a zebrafish diet (Zeigler, Aquatic Habitats, Apopka Florida) and live artemia (Jiahong Feed Co., Tianjin, China).
Zebrafish embryos were obtained from spawning adults with a sex ratio of 1:1. Embryos were collected within 0.5 h of spawning, and rinsed in embryo medium(EM: 0.137M NaCl, 5.4mMKCl, 0.25mM Na2HPO4, 0.44mM KH2PO4,1.3mM CaCl2, 1.0mM MgSO4 and 4.2mM NaHCO3) (Westerfield, 1995). Fertilized embryos with normal morphology were staged under a dissecting microscope SMZ1500 (Nikon, Japan) according to the standard methods(Kimmel et al., 1995).
2.2. VPA stock solutions and exposure protocols
VPA [valproic acid sodium salt, CAS# 1069-66-5, purity>98%, sigma] stock solution (500mM) was prepared by dissolving VPA in ultrapure water that was then stored at −20°C. The working solution was prepared by dilutingthe stock solution immediately prior to experimental use. First, control and gradient concentrations of VPA (0, 5, 50, 500, 1000, and 1500 μM) were continuously exposed to zebrafish embryos from 8-120 hours post fertilization (hpf), with dosing based on a previous study (Zimmermann et al., 2015). Developmental endpoints included the malformations and mortality at 120 hpf. The embryos were exposed in 6-well plates, with 5 ml solution and 20 embryos per well. There were three biological triplicates with 20 embryos of one well considered one replicate.
Based on the developmental dose-effect results above, a subset of VPA concentrations (0, 5, 50, and 500 μM) were used for embryo exposures from 8 hpf to 4.5 dpf, the embryos were then moved to clean fish water for further behavioral evaluations. At 4.5 dpf, the head size evaluation were evaluated and the movement behavior evaluation on the next day. Following VPA exposure, the embryos were washed with EM three times and incubated in fish water (conductivity to 800 μS/cm) for continuous development under standard care procedures. At specific developmental stages, the ASD-related assays of head size, embryo/larval movement, social behaviors, and neural cells expression were evaluated (summarized in Fig.1) with the methods detailed below. In order to avoid the mechanical damage during each larval behavior assay, which may affect the second behavior assay, enough embryos were exposed at the beginning and part of the treated embryos was used for each behavior assay.
Fig. 1.
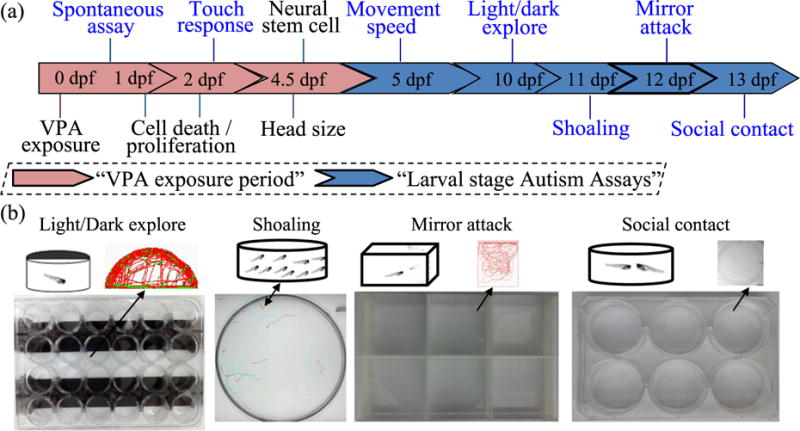
Schematic diagram of VPA exposures in zebrafish embryos and ASD-like head size, behaviors and neural cells expression assays. VPA exposures began at 8 hour post-fertilization (hpf) and ended at 4.5 days post fertilization (dpf). Evaluations occurred throughout the developmental stages from 1-13dpf. Exposure and evaluation times are displayed (a). The main social behavioral apparatuses and assays performed across time following VPA exposure are shown (b).
2.3. Head size evaluation using alcian blue staining
After VPA exposures (0 – 500 μM, from 8 – 108 hpf), 4.5 dpf larvae were euthanized with 0.025% MS-222, fixed with 4% paraformaldehyde (PFA), and stained in alcian blue (0.1% w/v in 0.37% HCl/70%EtOH) at room temperature (RT), overnight. Then, the larvae were washed in 0.37% HCl/70%EtOH, rehydrated, and digested with trypsin for 2 h at 37°C. Pigment was bleached in 0.75% H2O2 in 0.5% KOH for 30 min, and larvae were stored in 100% glycerol at 4°C. Following imaging, we measured body length (BL), intraocular distance (ID), lower jaw length (LJL), and certohyal cartilage length (CCL) in 30 larvae per group using the Image J software (National Institutes of Health, Bethesda, MD, USA) .
2.4. Embryos/larval movement behaviors
Spontaneous movement
Spontaneous movement of embryos (alternating tail bending or coiling) at 24 hpf was recorded for 1 min via a CCD camera (Nikon, Japan) mounted on a dissection microscope. All spontaneous movement recordings started after an adaptation period on the recording station of 5 min. Throughout all evaluations, the room temperature was maintained at 27 ± 0.5°C. The recording time from the first to the last well was less than 10 min. A total of 40 embryos in each treatment group from three replicate experiments were used for data analysis using the same method outlined in our previous work (Chen et al., 2011).
Touch response
Touch response at 48 hpf was evaluated with embryos that were manually dechorionated. Following a 10 min adaptation period in 24-well plates, response was evoked by touching the dorsal tail region with an eyelash probe. The distance moved after touching was scored manually with one perimeter of the well as 1 mm, and a semiquantitative assessment of one to three quarters of the well as 0.25, 0.5 and 0.75 mm. There were a total of 30 embryos in each treatment group from three biological repeats that were used for data analysis with methods previously described in detail (Wang, X. et al., 2013; Yang et al., 2011).
Light versus dark swim speed
Morphologically normal 5dpf larvae were transferred to a 24-well plate (1 larva and 2ml fish water /well) to assess free swimming behavior in a ZebraLab behavior monitoring station (ViewPoint Life Sciences, Inc., Montreal, Canada). Larvae were adapted for 20 min before recording swimming for a 10minlight (visible light) period, followed by a 10min dark (infrared light) period. The basic parameters for the assay were the same as our previous studies (Chen et al., 2013; Chen et al., 2011). The average larval movement speed was evaluated during the light and dark periods, respectively. Three biological repeats (embryos collected in separate days) were used to confirm the behavior response.
2.5. Larval social behaviors
In contrast to the older stage zebrafish social behavior apparatus used in the previous literature (Zimmermann et al., 2015), we selected a small, high-throughput detection apparatus to test larval zebrafish social behaviors. Four high throughput larval social behaviors were recorded in this study with a single animal only undergoing one behavioral test. All custom-made apparatuses were fit with the commercial ZebraLab behavior monitoring station (ViewPoint Life Sciences, Inc., Montreal, Canada), and the basic tracking setting was used for the larval movement tests detailed above.
Light/dark background preference behavior
After the exposure period (8 hpf-4.5 dpf), the larvae were incubated in fish water (conductivity to 800 μS/cm), in a petri dish (50ml, 40 larvae), until 10 dpf for the light/dark background preference analysis, between 11:00 am and 1:30 pm. The apparatus was a 24-well plate, with half of each well painted dark on the sides and bottom to achieve equal light and dark areas (Fig. 1). The larvae were transferred to the apparatus with one larva per well and 2 ml fish water per well. Software motion tracking was performed to identify the number of larvae swimming in the light area, and the number of times larva crossed between the light and dark areas with data collected every 60s for 6 min. Data included the average number of times that the light/dark area was crossed, and the average percentage of time in the light area. Each treatment group included 24 larvae, and the test was repeated three times.
Shoaling behavior
A shoaling test was carried out at 11 dpf using a glass petri dish (9 cm diameter) with 25 ml fish water and 10 larvae per dish, between 11:00am and 1:30 pm. The shoaling behavior assay parameters included the nearest neighbor distance (NND) and the inter-individual distance (IID) among a group of zebrafish. Specifically, for each group we tracked movement of 10 larvae for 8 min, and collected data every 15s. The first 2 min was used for acclimation, the remaining 6 min for analysis. Two trials were conducted for each group, trials were combined (20 larvae per group) and analyzed, the assay was repeated three times.
Mirror attack behavior
At 12 dpf, a mirror attack test was carried out using a 6-well plate made with ground glass (to inhibit fish from seeing each other, well size: 11.2 cm length×9 cm width×2 cm height) with each well consisting of a one sided mirror. Individual larvae were transferred to the apparatus with 10 ml fish water/well. Larval movement was recorded for 6 min with data collection data every 60s. Data included incidences of attack (lunges, biting) at the mirror and the percent time spent in the mirror zone (≤ 1.0 cm away from the mirror). Each treatment group included 24 larvae for the data analysis. And the assay was repeated three times to confirm the result.
Social contact
At 13 dpf, assessment of social contact behavior was carried out using a 6-well plate. The larvae were transferred to a 6-well plate with 5 ml fish water and 2 larvae /well. We tracked movement for 6 min, and collected data every 60s. Results included the average contact times (with social contact defined as when the distance between two larvae was ≤ 1 body length) and the contact duration time. Each treatment group included 24 larvae for the data analysis. And the assay was repeated three times.
2.6. Cell death and proliferation assays
The embryos were exposed to phenylthiourea (Sigma) at a final concentration of 0.0045% at 24 hpf to inhibit the formation of pigmentation. We manually dechorionated 48 hpf embryos, and used for terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick and labeling (TUNEL) analysis in whole mount zebrafish with an in situ Cell Death Detection Kit (Roche, USA) to visualize embryo cell death. TUNEL staining used the same method previously described (X. Chen et al., 2012). 48 hpf embryos were dechorionated manually and fixed in 4% PFA overnight at 4°C to visualize embryo cell proliferation. Then, we incubated the embryos in the first antibody solution, 1:750 anti-histone H3 (ser10)-R (sc-8656-R, HH3, Santa Cruz), and the second antibody solution 1:1000 Alex488. The HH3 staining used the same method described previously (Golzio et al., 2012). A fluorescence microscope (Nikon ECLIPSE Ti) captured the images, and ImageJ software counted AO/HH3 positive cells in the head region. In order to minimize sampling/observer bias, all the examined samples from the four different treatment groups were blinded to the researcher. For each test, one sample without primary antibody was used as negative control to identify the non-specific staining. Representative photographs are shown.
2.7. Sectioning and immunohistochemistry
After overnight fixation at 4 °C with 4% PFA, 4.5dpf larvae were transferred to 100% methanol and stored at −80 °C. The embryo heads were dissected under the SMZ 1500 (Nikon, Japan) dissecting microscope using the sharp scissor, and embedded with OCT (4583; SAKURA), snap frozen, and kept at −80 °C for future use. We sectioned the embedded larvae at 7 μm of three adjacent slides in the cerebrum position with a cryostat microtome (Leica CM1950). Protein-binding sites were unmasked via antigen retrieval with a citrate buffer [10 mM sodium citrate (pH 6.0) + 0.5% Tween-20] by boiling for 20 min. Following one 10min wash with PBST, and a 30min permeabilization step with PBST, slides were incubated with a blocking buffer consisting of 10% normal goat serum (S1000; Vector Laboratories) in PBT for 1 hr at RT. For the mature newborn neurons assay, slides were incubated with the primary antibodies of HuC/D (1:1000; host: mouse) and NEUN (1:500; host: rabbit) at 4°C overnight; and then incubated with secondary antibodies of Alex488 (1:1000; host: goat-mouse) and Alex568 (1:1000; host: goat-rabbit) at RT for 1hr. For the neural stem cell proliferation assay, slides were incubated with the primary antibodies of PCNA (1:1000; host: mouse) and GFAP (1:1000; host: goat) at 4°C overnight, then incubated with secondary antibodies of Alex488 (1:1000; host: donkey-mouse) and Alex568 (1:1000; host: donkey-goat) at RT for 1hr. The slides, after four 10min washes with PBT, were treated with DAPI (D1308; Molecular Probes; 1:1,000) for 5 min, and then washed three times with PBST. And fluorescent images were obtained using a confocal microscope (Nikon, A1R), and quantitative analysis performed using Image J software. Similar quality control methods were also used with the cell death/proliferation assay. And for the mature newborn neurons assay, the mean florescent density per unit area of the whole cerebrum was detected by the software automatically, then the ratio of the HuC/D+ and NEUN+ expression of the same slide was calculated. To minimize the bias of the cerebrum position, three adjacent slides of the sample were all used for statistical analysis. And for the neural stem cell proliferation assay, both the PCNA+ and GFAP+ positive staining (brown color staining) were counted blind from different samples. For the gut intestinal neurons expression, the mean positive cells per area in the gut was used for statistical analysis.
2.9. Statistical analysis
One-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test was used to compare differences between the treatment and the control groups. For neural cell expression analysis, an unpaired t-test with 5% FDR was performed. All data are reported as means ± standard error unless otherwise stated, and P < 0.05 was set as the significance level.
3. Results
3.1. Concentration screening for VPA produced developmental effects
VPA exposure induced malformations, including uninflated swim bladder, pericardial edema, and yolk sac edema (Fig. 2a). Occurrence of malformations trended with VPA exposure with significance from controls at concentrations including and above 500 μM (P < 0.001) with 100 % of larvae displaying malformations at 1500 μM (Fig. 2b). Mortality was not observed up to 120 hpf for all the exposure groups (Fig. 2b).
Fig. 2.
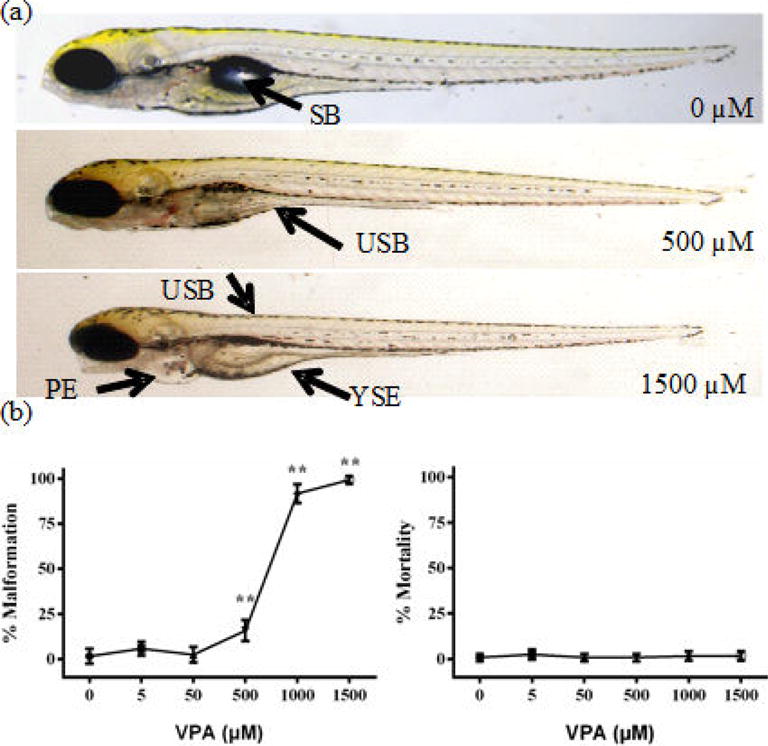
VPA exposure impacted hatch rate, malformations, and mortality. Zebrafish embryos were exposed to different doses of VPA (0 – 1500 μM) from 8-120 hpf. Representative malformation (SB = swim bladder, USB = uninflated swim bladder, PE = pericardial edema, YSE = yolk sac edema) images for the control, medium concentration (500 μM), and high concentration (1500 μM) are shown (a).The percent of malformations and mortality at 120 hpf are displayed (b). N = 60 (20 animals in triplicate). Values are plotted as mean ± SD. * P<0.05 and ** P<0.001 indicate significance compared to the vehicle control (0.1 % DMSO).
3.2. VPA exposure produced macrocephalic phenotype
The ASD-like phenotypic assay of head size was evaluated using alcian blue staining at 4.5 dpf, and representative images of each group are shown (Fig. 3a). Body length did not change for any of the VPA exposure groups when compared with the control (data not shown). For the intraocular distance (ID), only the lowest dose of VPA (5 μM, P < 0.05) was significantly increased, while the higher doses of 50 and 500 μM showed no difference when compared with the control (Fig. 3b). For the lower jaw length (LJL) and certohyal cartilage length (CCL), all VPA exposure groups (P < 0.05 for 50 and 500 μM; P < 0.001 for 5 μM) were significantly increased when compared with the control (Fig. 3b).
Fig. 3.
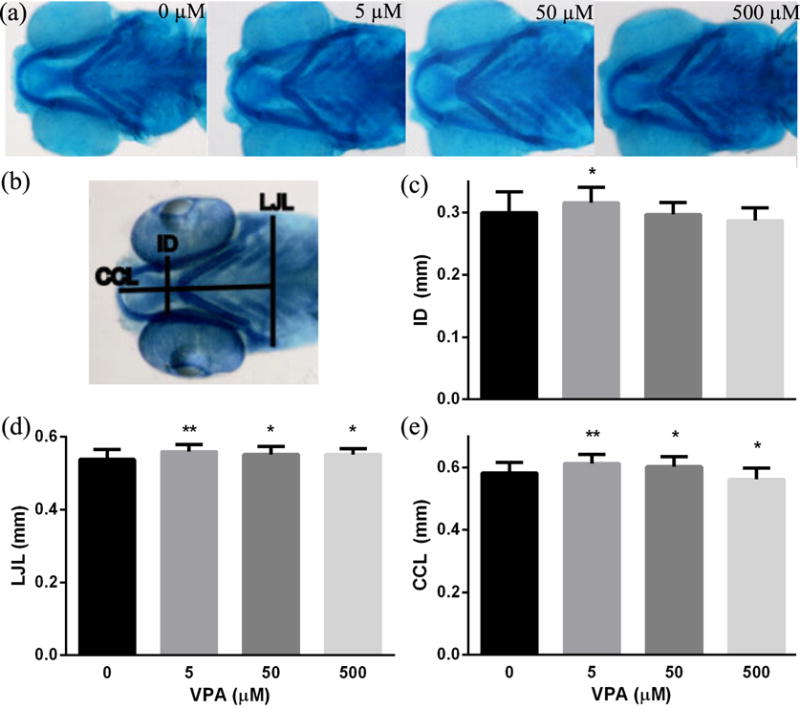
VPA exposure impacted larval head size. Zebrafish embryos were exposed to different concentrations of VPA (0 – 500 μM) from 8 hpf until staining with alcian blue at 4.5 dpf. (a) Representative staining images for each group and (b) the labeled measurements of intraocular distance (ID), lower jaw length (LJL) and certohyal cartilage length (CCL) are displayed. Quantified ID (c), LJL (d), and CCL (e) are displayed for all treatments and controls (N= 37-50). Values are plotted as mean ± SD. * P<0.05 and ** P<0.001 indicates significance from vehicle control (0.1 % DMSO).
3.3. VPA exposure produced hyperactive movement behavior
For spontaneous movement at 24 hpf, both 5 μM (P < 0.001) and 50 μM (P < 0.05) groups had a significant increase in tail bends when compared with the control (Fig. 4a). For touch response at 48 hpf, both 5 μM (P < 0.001) and 50 μM (P < 0.05) groups significantly increased the distance moved following touch on the dorsal tail region when compared with the control (Fig. 4b). For movement speed at 5 dpf, VPA exposure groups showed increased speed in both light (5, 500 μM, P < 0.05; 500 μM, P <0.001, respectively) and dark (5μM, P < 0.05; 50, 500 μM, P <0.001, respectively) (Fig. 4c, d).
Fig. 4.
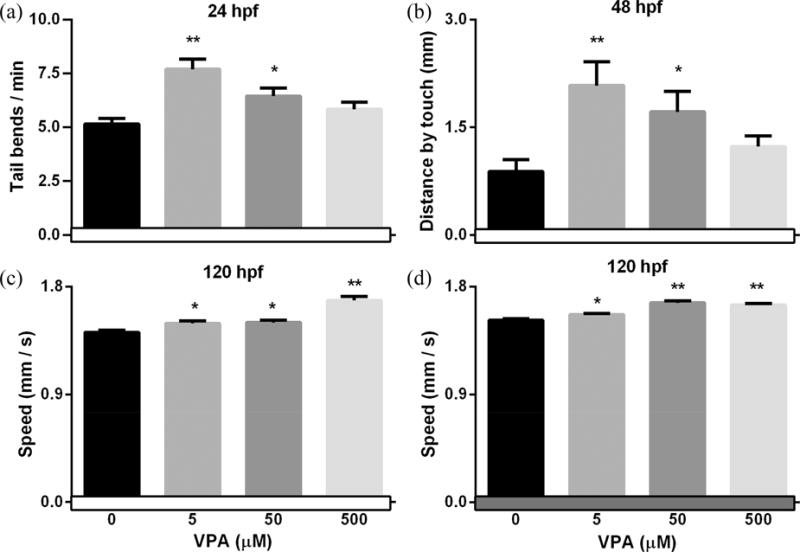
VPA exposure impacted embryo/larval movement behaviors. Zebrafish embryos were exposed to different concentrations of VPA (0 – 500 μM) from 8hpf to 4.5 dpf and behaviors were evaluated at different stages. Spontaneous movement at 24 hpf (a) and touch response at 48 hpf (b) was measured in all embryos. At 5 dpf, larval movement speed was measured during a 10 min light period (c) followed by a 10 min dark period (d). Values are plotted as mean ± SD. * P<0.05 and ** P<0.001 indicates significant differences from the vehicle control (0.1 % DMSO).
3.4. VPA exposure produced deficient social behavior
For the light/dark background exploration at 10 dpf, all VPA exposure groups had significant decreases in the number of light/dark crosses (Fig. 5a, at least P < 0.05) and a significant increase in the amount of time spent in the light area (Fig. 5b, at least P < 0.05) when compared with the control. For shoaling at 11 dpf, the nearest neighbor distance (NND) and the inter-individual distance (IID) were significantly increased for all VPA exposure groups (at least P < 0.05) when compared with the control (Fig. 5c, d). Mirror attacks at 12 dpf significantly decreased (Fig. 6a, at least P < 0.05) as did the amount of time spent in the mirror zone (Fig. 6b, at least P < 0.05) for all VPA exposure groups when compared with the control. For the social contact at 13 dpf: the low VPA concentration (5 μM) resulted in significantly decreased frequency of contact (Fig. 6c, P < 0.05) and the amount of time in contact (Fig. 6d, P < 0.05) when compared with control; the medium VPA concentration (50 μM) had a similar trend to the low concentration group, but was only significant for the frequency of contacts (P < 0.05) when compared with the control (Fig. 6c, d); and for the high VPA concentration (500 μM) group, there was no difference in either social contact measurement when compared with the control (Fig. 6c, d).
Fig. 5.
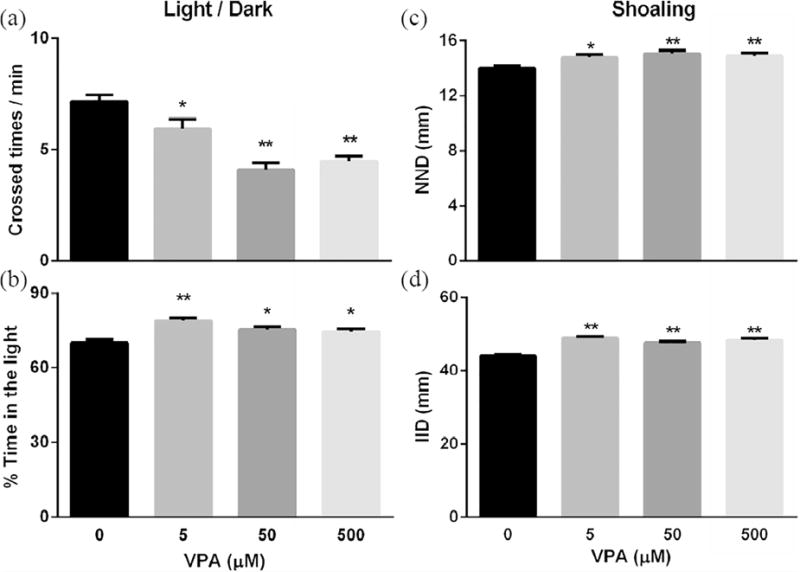
VPA exposure impacted larval light/dark exploration and shoaling. Zebrafish embryos were exposed to varying concentrations of VPA (0 – 500 μM) from 8hpf to 4.5 dpf and then washed with EM three times before incubation in EM under standard care procedures. Then larvae at 10 and 11 dpf were used to assess light/dark exploration (a, b) and shoaling behaviors (c, d), respectively. Values are plotted as mean ± SD. * P<0.05 and ** P<0.001 indicates significance from the vehicle control (0.1 % DMSO).
Fig. 6.
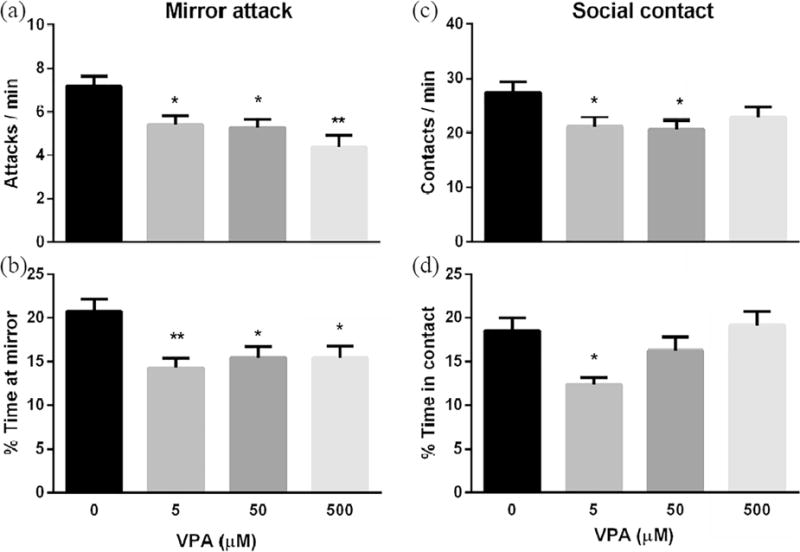
VPA exposure impacted larval mirror attack and social contact. Zebrafish embryos were exposed to varying concentrations of VPA (0 – 500 μM) from 8hpf to 4.5 dpf and then washed with EM three times before incubation in EM under standard care procedures. Then larvae at 12 and 13 dpf were used for the mirror attack (a, b) and social contact (c, d) assays, respectively. Values are plotted as mean ± SD. * P<0.05 and ** P<0.001 indicates significance from the vehicle control (0.1 % DMSO).
3.5. VPA exposure affected neural cell proliferation
To explore the potential mechanism of VPA induced ASD-like head size changes and behavioral alterations, we performed assays for cell death/proliferation, mature newborn neuron proportion, and neural stem cell proliferation at the low concentration of VPA exposure (5μM). Cell death was assessed by TUNEL staining at 48 hpf and there was not a significant difference in positive TUNEL cells between the VPA treatment groups and control (Fig. 7). Cell proliferation was assessed by HH3 staining at 48 hpf and positive HH3 cells in the VPA exposure group were significantly increased when compared with the control (P<0.001, Fig. 7). The proportion of mature newborn neurons was assessed through the expression of the newborn neurons marker, HuC/D+, divided by the mature neurons marker, NEUN+, at 4.5 dpf. The HuC/D+/NEUN+ expression ratio following VPA exposure was significantly increased compared to controls (P<0.05, Fig. 7). Neural stem cell proliferation was measured at 4.5 dpf using co-expression of the proliferation neural cell marker, PCNA+, and stem cell marker, GFAP+, with significantly increased PCNA+GFAP+ co-expression following VPA exposure when compared with the control (P<0.05, Fig. 7).
Fig. 7.
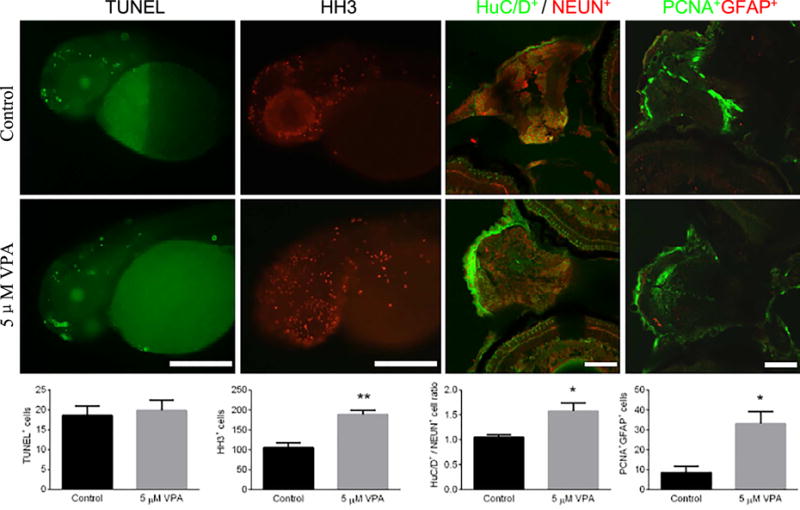
Measurement of cell death in larval neural cells by expression of TUNEL at 48 hpf, cell proliferation by HH3 staining at 48 hpf, proportion of mature newborn neurons(HuC/D+ / NEUN+ staining) at 4.5 dpf, and neural stem cell proliferation (PCNA+GFAP+ staining) at 4.5 dpf. Embryos underwent a waterborne exposure to 0.1% DMSO (control) or 5 μM VPA in 0.1% DMSO from 8hpf to 4.5 dpf. The top panel consists of representative images of each staining in controls and treatments. The bottom panel displays quantified positive cells expressions corresponding to the imagines and stains of the upper panel. n = 7-12. Values plotted are mean ± SEM. * P<0.05 and ** P<0.001 indicates significance from the vehicle control (0.1% DMSO).AC: apoptotic cells; HC: hyperplasia cells; MN: mature neuron; MAN: mitosis anaphase neuron and NSC: neural stem cells.
4. Discussion
In the present study, the non-teratogenic doses of VPA embryonic exposure resulted in macrocephalic phenotypes in larval zebrafish. And VPA exposure resulted in hyperactivity in embryos/larvae and impaired social behavior in larval zebrafish. These phenotypic and behavioral alterations may partially originate from the abundance of cell proliferation, mature newborn neuron proportion, and neural stem cell proliferation in the developmental brain. Our study demonstrated that embryonic VPA exposure can generate ASD-like physical characteristics and behaviors in zebrafish, positioning the zebrafish as an alternative model for studying ASD and the underlying mechanisms.
Characteristics associated with ASD include increased head circumference and volume (Sacco et al., 2015) as well as increased intraocular distance (ID) (Hazlett et al., 2017). These phenotypes have been recapitulated in animal models using various methods including embryonic VPA exposure in zebrafish (Zimmermann et al., 2015), and prenatal exposure in rats (Schneider and Przewlocki, 2004). In the present study, we tested a range of VPA concentrations (5-500 μM) in zebrafish starting at 8 hpf and observed a significant increase in malformations at concentrations above 500 μM. Importantly, at concentrations without significant malformations we detected increases in larval head size, with significant increases in ID, LJL, and CCL at 5 μM VPA. These results demonstrate the ability, at relatively low concentrations of VPA, to induce ASD-like phenotypes and are consistent with ASD rodent models and human characteristics (Kim et al., 2014; Narita et al., 2010; Schneider and Przewlocki, 2004).
The core ASD behavior symptoms include: deficits in social interaction, hyperactivity, anxiety, repetitive and stereotyped behaviors (Sacco et al., 2015; Zimmermann et al., 2015). Animal models with similar behavioral changes have been developed using VPA exposures in rodents (Schneider and Przewlocki, 2004)and embryonic zebrafish(Zimmermann et al., 2015). In previous zebrafish VPA exposure studies, ASD-like behaviors were identified including: hyperactivity through movements, lower exploratory activity, and decreased social contact numbers(Zimmermann et al., 2015). In the present study, lower VPA concentrations and earlier behavior assays were performed in order to quickly identify ASD-like behaviors. Exposure to 5 μM of VPA resulted in increased spontaneous movement at 24 hpf, touch response at 48 hpf, and free swimming speed in both the light and dark periods at 120 hpf. The early stage movement behaviors shown here are consistent with ASD-like behaviors and have also been replicated in rodent models of hyperactivity (Spencer et al., 2005).
Several social behaviors were evaluated respectively, in the larval zebrafish following the embryonic VPA exposure. In the light/dark background exploration assay, juvenile zebrafish that were exposed to VPA showed significantly decreased transition between light stages and increased time percent in the light area which is inconsistent with ASD rodent models that had increased transitions between light/dark compartments and no difference for the time percent in the light (Bernardet and Crusio, 2006). The reasons included difference in methodologies across laboratories (for more details see Ref. (Steenbergen et al., 2011), differences during ontogeny (Maximino et al., 2010), and ecological adaptive response, where diurnal species like zebrafish rely on vision and lit environments to capture prey and avoid predators (Burgess and Granato, 2007; Emran et al., 2008; MacPhail et al., 2009).In the shoaling assay, the VPA exposed larvae showed significantly increased IID and NND. The unconsolidated group’s fish shoaling pattern implied less sociality, which was consistent with ASD symptoms. In the mirror attacks assay, VPA exposed larvae showed significantly decreased attacks on the mirror and the percent time spent in the mirror area, which is consistent with previous rodent (Spencer et al., 2005) and zebrafish (Pham et al., 2012; Stewart et al., 2014) ASD studies. In the social contact assay, only the low concentration VPA group had significantly decreased contact times and contact duration, which was consistent with the autistic behaviors of less sociality in rodents prenatally exposed to VPA(Schneider and Przewlocki, 2004).
The lowest dose VPA group showed more significant changes compared to higher doses under these ASD-like behavioral assays, which may be due to the elevated VPA doses having greater potential for toxic effects. An additional reason may be the non-monotonic neurobehavioral effects which have previously been observed for many classes of compounds, such as endocrine disruptors (Lagarde et al., 2015), or dopaminergic compounds (Wang, J.-H. et al., 2013). The reason may arise from numerous molecular mechanisms such as opposing effects, receptor desensitization, negative feedback with increasing dose, or dose-dependent metabolism modulation.
Deficits in movement and social behaviors may involve dysfunction of the neural system (Levin et al., 2009). We monitored expression of several neural-related cells to explore the underlying mechanisms for the behavioral changes observed in the assays performed. The balance of apoptosis and proliferation is a critical aspect of normal development and is susceptible to perturbation by toxicant exposure (Ahmadi et al., 2003; Cole and Ross, 2001). For example, from the ASD model study, potassium channel tetramerization domain containing 13 (kctd13) knockdown resulted in a significant increase in cell proliferation (HH3 staining) but not cell death (TUNEL staining) in embryonic zebrafish (Golzio et al., 2012). In consistent, using TUNEL and HH3 staining, cell death was not altered in the VPA exposure group, but cell proliferation in the head region was significantly increased. HuC/D was a general marker of the late mitosis neurons and NEUN, a mature neuron marker (Grandel et al., 2006), were used to calculate the HuC/D+/ NEUN+ ratio as a measure of mature newborn neurons proportion. From this assay, we found HuC/D+/NEUN+ in the brain region was significantly increased in the VPA group. Antibodies of GFAP, a neural stem cells unique glial fibers acidic protein, and the proliferating cell nuclear antigen (PCNA) were used to measure PCNA+GFAP+ positive neural stem cell proliferation (Shimizu et al., 2015). We found that the VPA group exhibited these markers of increased neural cell proliferation in the brain region. The neuroanatomical and clinical outcome in early childhood with autism indicated a sudden and excessive increase in head size between 1 to 2 months and 6 to 14 months (Courchesne et al., 2003). So during this key developmental stage, the abnormal proliferation of neural cells may contribute to the occurrence of ASD. In addition, from the embryonic zebrafish ASD model study, kctd13 knockdown resulted in a significant increase of the nuclei number in the telencephalon, diencephalon and mesencephalon areas, as well as the HuC+/D+ cell numbers in the head region (Golzio et al., 2012). ASD-like responses in embryonic/larval zebrafish results from previous research (Golzio et al., 2012) and ours may imply the changes of the rodents or human fetus period life stages. Over all, VPA exposed zebrafish display ASD-like behaviors and head size may be partially due to neural cell over-proliferation.
A recent study of VPA exposure in zebrafish, after 25 μM from 10 to 24 hpf, at later developmental stage, impaired sociability and behavior, as well as a reduced number of histaminergic neurons and low histamine were found (Baronio et al., 2017). The impaired sociability was consistent with our results. Basal locomotion of the 5 dpf larval in the light period was decreased and increased under the dark stimulus periods in compared to controls, while in our study, VPA exposure resulted in hyperactivity in treated embryos and larvae (both the light and the dark periods). The decreased histaminergic neurons were not consistent with our study about the neuroal cells expressions. We detected VPA exposed resulted in more proliferation cells, mature newborn neurons, and the neural stem cell proliferation in the head regions. Since our measurements were based the total neural cells, which are difference from the specific histaminergic neurons. The difference also indicated that the complex of molecular mechanism under ASD occurrence, which involved the animal developmental stages, chemical treatment doses and duration.
The rationale for using zebrafish for neural related disease studies derives from the consensus that fundamental processes and mechanisms of neurodevelopment are remarkably conserved across species (Lein et al., 2005). Alternative animal models to study ASD exist, including rodent models which have been extensively used to ASD study. However, as a viviparous animal model, the exposure for the rodent fetus period may be influenced by the complex toxicokinetics from maternal exposure. While, zebrafish embryos developed externally, eliminating maternal toxicokinetics. And zebrafish are optically transparent during early developmental stage, which allows for the use of microscopic techniques to resolve individual neuronal cells in vivo. Additional advantages include their small size, rapid embryonic development, and short life cycle, which can assistant for the high throughput behaviors assays.
In conclusion, this research expanded the information available for the VPA-induced ASD zebrafish model by examining multiple concentrations and time points for behavioral changes. Following embryonic exposure to VPA, zebrafish displayed ASD-like phenotypes (macrocephaly) and behaviors (hyperactivity and decreased social interactions) in larvae. In addition to the ASD-like characteristics, there was an observed overgrowth of mature newborn neurons and neural stem cells in the developing brain after VPA exposure. These findings may bring more attention to a possible involvement of cell differentiation in outcomes related to neuropsychiatric disorders. And the assays utilized have the potential to serve as a robust screen for ASD-like characteristics in the early stages of zebrafish using VPA as a potential positive control. Furthermore, our study also supports zebrafish as a tool to investigate mechanisms underlying ASD-related diseases.
Highlights.
VPA embryonic exposure resulted in macrocephalic phenotypes.
VPA exposure resulted in hyperactivity and impaired social behavior.
Cell differentiation problem involved in VPA induced ASD phenotypes.
Acknowledgments
This work was supported in part by funding from the National Natural Science Foundation of China (No. 21477089 and 21307096). And the Talent Scientific Research Projects of Wenzhou Medical University (No. QTJ16025).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
We declare that there are no conflicts of interest.
References
- Ahmadi FA, Linseman DA, Grammatopoulos TN, Jones SM, Bouchard RJ, Freed CR, Heidenreich KA, Zawada WM. The pesticide rotenone induces caspase-3-mediated apoptosis in ventral mesencephalic dopaminergic neurons. J Neurochem. 2003;87(4):914–921. doi: 10.1046/j.1471-4159.2003.02068.x. [DOI] [PubMed] [Google Scholar]
- Bailey J, Oliveri A, Levin ED. Zebrafish Model Systems for Developmental Neurobehavioral Toxicology. Birth defects research. Part C, Embryo today: reviews. 2013;99(1):14–23. doi: 10.1002/bdrc.21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baronio D, Puttonen HAJ, Sundvik M, Semenova S, Lehtonen E, Panula P. Embryonic exposure to valproic acid affects the histaminergic system and the social behavior of adult zebrafish (Danio rerio) British Journal of Pharmacology, n/a-n/a. 2017 doi: 10.1111/bph.14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardet M, Crusio W. Fmr1 KO mice as a possible model of autistic features. Scientific World Journal. 2006;6:1164–1176. doi: 10.1100/tsw.2006.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HA, Granato M. Modulation of locomotor activity in larval zebrafish during light adaptation. Journal of Experimental Biology. 2007;210(14):2526–2539. doi: 10.1242/jeb.003939. [DOI] [PubMed] [Google Scholar]
- Buske C, Gerlai R. Early embryonic ethanol exposure impairs shoaling and the dopaminergic and serotoninergic systems in adult zebrafish. Neurotoxicology and Teratology. 2011;33(6):698–707. doi: 10.1016/j.ntt.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Das SR, La Du J, Corvi MM, Bai C, Chen Y, Liu X, Zhu G, Tanguay RL, Dong Q, Huang C. Chronic PFOS exposures induce life stage-specific behavioral deficits in adult zebrafish and produce malformation and behavioral deficits in F1 offspring. Environmental Toxicology and Chemistry. 2013;32(1):201–206. doi: 10.1002/etc.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Huang C, Zheng L, Simonich M, Bai C, Tanguay R, Dong Q. Trimethyltin chloride (TMT) neurobehavioral toxicity in embryonic zebrafish. Neurotoxicology and teratology. 2011;33(6):721–726. doi: 10.1016/j.ntt.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen D, Baio J, Braun K, et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Surveill Summ. 2016;65(SS-3):1–23. doi: 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole LK, Ross LS. Apoptosis in the Developing Zebrafish Embryo. Dev Biol. 2001;240(1):123–142. doi: 10.1006/dbio.2001.0432. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Carper R, Akshoomoff N. Evidence of brain overgrowth in the first year of life in autism. JAMA. 2003;290(3):337–344. doi: 10.1001/jama.290.3.337. [DOI] [PubMed] [Google Scholar]
- Ebert DH, Greenberg ME. Activity-dependent neuronal signalling and autism spectrum disorder. Nature. 2013;493(7432):327–337. doi: 10.1038/nature11860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emran F, Rihel J, Dowling JE. A Behavioral Assay to Measure Responsiveness of Zebrafish to Changes in Light Intensities. Journal of Visualized Experiments : JoVE. 2008;(20):923. doi: 10.3791/923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golzio C, Willer J, Talkowski ME, Oh EC, Taniguchi Y, Jacquemont S, Reymond A, Sun M, Sawa A, Gusella JF, Kamiya A, Beckmann JS, Katsanis N. KCTD13 is a major driver of mirrored neuroanatomical phenotypes associated with the 16p11.2 CNV. Nature. 2012;485(7398):363–367. doi: 10.1038/nature11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandel H, Kaslin J, Ganz J, Wenzel I, Brand M. Neural stem cells and neurogenesis in the adult zebrafish brain: Origin, proliferation dynamics, migration and cell fate. Developmental biology. 2006;295(1):263–277. doi: 10.1016/j.ydbio.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Gu H, Munsell BC, Kim SH, Styner M, Wolff JJ, Elison JT, Swanson MR, Zhu H, Botteron KN, Collins DL, Constantino JN, Dager SR, Estes AM, Evans AC, Fonov VS, Gerig G, Kostopoulos P, McKinstry RC, Pandey J, Paterson S, Pruett JR, Schultz RT, Shaw DW, Zwaigenbaum L, Piven J, The I.N. Early brain development in infants at high risk for autism spectrum disorder. Nature. 2017;542(7641):348–351. doi: 10.1038/nature21369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentink J, Loane MA, Dolk H, Barisic I, Garne E, Morris JK, de Jong-van den Berg LTW. Valproic Acid Monotherapy in Pregnancy and Major Congenital Malformations. New England Journal of Medicine. 2010;362(23):2185–2193. doi: 10.1056/NEJMoa0907328. [DOI] [PubMed] [Google Scholar]
- Kardas F, Bayram AK, Demirci E, Akin L, Ozmen S, Kendirci M, Canpolat M, Oztop DB, Narin F, Gumus H, Kumandas S, Per H. Increased Serum Phthalates (MEHP, DEHP) and Bisphenol A Concentrations in Children With Autism Spectrum Disorder. Journal of Child Neurology. 2015;31(5):629–635. doi: 10.1177/0883073815609150. [DOI] [PubMed] [Google Scholar]
- Kim KC, Lee DK, Go HS, Kim P, Choi CS, Kim JW, Jeon SJ, Song MR, Shin CY. Pax6-Dependent Cortical Glutamatergic Neuronal Differentiation Regulates Autism-Like Behavior in Prenatally Valproic Acid-Exposed Rat Offspring. Molecular Neurobiology. 2014;49(1):512–528. doi: 10.1007/s12035-013-8535-2. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203(3):253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Lagarde F, Beausoleil C, Belcher SM, Belzunces LP, Emond C, Guerbet M, Rousselle C. Non-monotonic dose-response relationships and endocrine disruptors: a qualitative method of assessment. Environmental Health. 2015;14(1):13. doi: 10.1186/1476-069X-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SH, Hlaing MM, Zhang X, Yan C, Duan Z, Zhu L, Ung CY, Mathavan S, Ong CN, Gong Z. Toxicogenomic and Phenotypic Analyses of Bisphenol-A Early-Life Exposure Toxicity in Zebrafish. PLoS ONE. 2011;6(12):e28273. doi: 10.1371/journal.pone.0028273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritsen MB, Astrup A, Pedersen CB, Obel C, Schendel DE, Schieve L, Yeargin-Allsopp M, Parner ET. Urbanicity and Autism Spectrum Disorders. Journal of autism and developmental disorders. 2014;44(2):394–404. doi: 10.1007/s10803-013-1875-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein P, Silbergeld E, Locke P, Goldberg AM. In vitro and other alternative approaches to developmental neurotoxicity testing (DNT) Environmental Toxicology and Pharmacology. 2005;19(3):735–744. doi: 10.1016/j.etap.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Levin ED, Aschner M, Heberlein U, Ruden D, Welsh-Bohmer KA, Bartlett S, Berger K, Chen L, Corl AB, Eddins D, French R, Hayden KM, Helmcke K, Hirsch HVB, Linney E, Lnenicka G, Page GP, Possidente D, Possidente B, Kirshner A. Genetic aspects of behavioral neurotoxicology. Neurotoxicology. 2009;30(5):741–753. doi: 10.1016/j.neuro.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPhail RC, Brooks J, Hunter DL, Padnos B, Irons TD, Padilla S. Locomotion in larval zebrafish: Influence of time of day, lighting and ethanol. Neurotoxicology. 2009;30(1):52–58. doi: 10.1016/j.neuro.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Huerta Marisela, Bishop Somer L, Duncan Amie, Hus Vanessa, Catherine Lord. Application of DSM-5 Criteria for Autism Spectrum Disorder to Three Samples of Children With DSM-IV Diagnoses of Pervasive Developmental Disorders. American Journal of Psychiatry. 2012;169(10):1056–1064. doi: 10.1176/appi.ajp.2012.12020276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximino C, Marques de Brito T, Dias CAGDM, Gouveia A, Morato S. Scototaxis as anxiety-like behavior in fish. Nat Protocols. 2010;5(2):209–216. doi: 10.1038/nprot.2009.225. [DOI] [PubMed] [Google Scholar]
- Meshalkina DA, Kizlyk NM, Kysil VE, Collier AD, Echevarria DJ, Abreu MS, Barcellos LJG, Song C, Warnick JE, Kyzar EJ, Kalueff AV. Zebrafish models of autism spectrum disorder. Experimental Neurology. doi: 10.1016/j.expneurol.2017.02.004. [DOI] [PubMed] [Google Scholar]
- Modabbernia A, Velthorst E, Reichenberg A. Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Molecular Autism. 2017;8:13. doi: 10.1186/s13229-017-0121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S, Turnpenny P, Quinn A, Glover S, Lloyd D, Montgomery T, Dean J. A clinical study of 57 children with fetal anticonvulsant syndromes. Journal of Medical Genetics. 2000;37(7):489–497. doi: 10.1136/jmg.37.7.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Oyabu A, Imura Y, Kamada N, Yokoyama T, Tano K, Uchida A, Narita N. Nonexploratory movement and behavioral alterations in a thalidomide or valproic acid-induced autism model rat. Neuroscience Research. 2010;66(1):2–6. doi: 10.1016/j.neures.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Oliveira RF. Mind the fish: zebrafish as a model in cognitive social neuroscience. Frontiers in Neural Circuits. 2013;7:131. doi: 10.3389/fncir.2013.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham M, Raymond J, Hester J, Kyzar E, Gaikwad S, Bruce I, Fryar C, Chanin S, Enriquez J, Bagawandoss S, Zapolsky I, Green J, Stewart AM, Robison BD, Kalueff AV. Assessing Social Behavior Phenotypes in Adult Zebrafish: Shoaling, Social Preference, and Mirror Biting Tests. In: Kalueff AV, Stewart AM, editors. Zebrafish Protocols for Neurobehavioral Research. Humana Press; Totowa, NJ: 2012. pp. 231–246. [Google Scholar]
- Roberts AL, Lyall K, Hart JE, Laden F, Just AC, Bobb JF, Koenen KC, Ascherio A, Weisskopf MG. Perinatal Air Pollutant Exposures and Autism Spectrum Disorder in the Children of Nurses’ Health Study II Participants. Environmental Health Perspectives. 2013;121(8):978–984. doi: 10.1289/ehp.1206187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco R, Gabriele S, Persico AM. Head circumference and brain size in autism spectrum disorder: A systematic review and meta-analysis. Psychiatry Research: Neuroimaging. 2015;234(2):239–251. doi: 10.1016/j.pscychresns.2015.08.016. [DOI] [PubMed] [Google Scholar]
- Schneider T, Przewlocki R. Behavioral Alterations in Rats Prenatally Exposed to Valproic Acid: Animal Model of Autism. Neuropsychopharmacology. 2004;30(1):80–89. doi: 10.1038/sj.npp.1300518. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Ito Y, Tanaka H, Ohshima T. Radial glial cell-specific ablation in the adult Zebrafish brain. genesis. 2015;53(7):431–439. doi: 10.1002/dvg.22865. [DOI] [PubMed] [Google Scholar]
- Spencer CM, Alekseyenko O, Serysheva E, Yuva-Paylor LA, Paylor R. Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome. Genes, Brain and Behavior. 2005;4(7):420–430. doi: 10.1111/j.1601-183X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- Steenbergen PJ, Richardson MK, Champagne DL. The use of the zebrafish model in stress research. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35(6):1432–1451. doi: 10.1016/j.pnpbp.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Stewart AM, Nguyen M, Wong K, Poudel MK, Kalueff AV. Developing zebrafish models of autism spectrum disorder (ASD) Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2014;50:27–36. doi: 10.1016/j.pnpbp.2013.11.014. [DOI] [PubMed] [Google Scholar]
- Strömland K, Nordin V, Miller M, Akerström B, Gillberg C. Autism in thalidomide embryopathy: a popolation study. Developmental Medicine & Child Neurology. 1994;36(4):351–356. doi: 10.1111/j.1469-8749.1994.tb11856.x. [DOI] [PubMed] [Google Scholar]
- Teitelbaum P. A proposed primate animal model of autism. European Child & Adolescent Psychiatry. 2003;12(1):48–49. doi: 10.1007/s00787-003-0306-6. [DOI] [PubMed] [Google Scholar]
- Wang JH, Rizak JD, Chen YM, Li L, Hu XT, Ma YY. Interactive effects of morphine and dopaminergic compounds on spatial working memory in rhesus monkeys. Neuroscience Bulletin. 2013;29(1):37–46. doi: 10.1007/s12264-013-1305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Dong Q, Chen Y, Jiang H, Xiao Q, Wang Y, Li W, Bai C, Huang C, Yang D. Bisphenol A affects axonal growth, musculature and motor behavior in developing zebrafish. Aquatic Toxicology. 2013;142:104–113. doi: 10.1016/j.aquatox.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book: a guide for the laboratory use of zebrafish (Brachydanio rerio) University of Oregon Press; 1995. [Google Scholar]
- Williams G, King J, Cunningham M, Stephan M, Kerr B, Hersh JH. Fetal valproate syndrome and autism: additional evidence of an association. Developmental Medicine & Child Neurology. 2001;43(3):202–206. [PubMed] [Google Scholar]
- Williams PG, Hersh JH. A male with fetal valproate syndrome and autism. Developmental Medicine & Child Neurology. 1997;39(9):632–634. doi: 10.1111/j.1469-8749.1997.tb07500.x. [DOI] [PubMed] [Google Scholar]
- Woods R, Vallero RO, Golub MS, Suarez JK, Ta TA, Yasui DH, Chi LH, Kostyniak PJ, Pessah IN, Berman RF, LaSalle JM. Long-lived epigenetic interactions between perinatal PBDE exposure and Mecp2(308) mutation. Human Molecular Genetics. 2012;21(11):2399–2411. doi: 10.1093/hmg/dds046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Lauridsen H, Buels K, Chi LH, La Du J, Bruun DA, Olson JR, Tanguay RL, Lein PJ. Chlorpyrifos-Oxon Disrupts Zebrafish Axonal Growth and Motor Behavior. Toxicological Sciences. 2011;121(1):146–159. doi: 10.1093/toxsci/kfr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann FF, Gaspary KV, Leite CE, De Paula Cognato G, Bonan CD. Embryological exposure to valproic acid induces social interaction deficits in zebrafish (Danio rerio): A developmental behavior analysis. Neurotoxicology and Teratology. 2015;52(Part A):36–41. doi: 10.1016/j.ntt.2015.10.002. [DOI] [PubMed] [Google Scholar]


