Abstract
Bronchopulmonary dysplasia (BPD) is potentially one of the most devastating conditions in premature infants with longstanding consequences involving multiple organ systems including adverse effects on pulmonary function and neurodevelopmental outcome. Here we review recent studies in the field to summarize the progress made in understanding in the pathophysiology, prognosis, prevention, and treatment of BPD in the last decade. The work reviewed includes the progress in understanding its pathobiology, genomic studies, ventilatory strategies, outcomes, and therapeutic interventions. We expect that this review will help guide clinicians to treat premature infants at risk for BPD better and lead researchers to initiate further studies in the field.
Introduction
In 1967, Northway first described Bronchopulmonary Dysplasia (BPD) as a lung disease in premature infants, who required prolonged mechanical ventilation [1]. Since then, there has been an unrelenting effort to understand its pathophysiology and develop effective methods to prevent and treat BPD. Since its first description, the definition of BPD has evolved; currently, the National Institute of Child Health and Human Development (NICHD) severity and postmenstrual age (PMA)-based definition is used most widely. However, there are attempts to further refine this definition, e.g., recently, it has been suggested that oxygen/respiratory support at 40 weeks PMA might be a better predictor of chronic respiratory insufficiency and neurosensory morbidity than the traditional use of 36 week PMA [2]. The “Old BPD” was characterized by cystic changes and heterogeneous aeration in the involved lungs, whereas the “New BPD” in the antenatal steroid, post-surfactant, and gentler modes of ventilatory support era is characterized by more uniform inflation, less fibrosis, and the absence of airway epithelial metaplasia, and smooth muscle hypertrophy. The “New BPD” includes pathological evidence of larger simplified alveoli and dysmorphic pulmonary vasculature [3].
Epidemiology
BPD affects premature low birth weight infants (LBWI), occurring slightly more frequently in Caucasian males, and genetic heritability plays an important role in its pathogenesis [4]. Although advances in perinatal care continue to improve the survival rates of premature infants, this has not consistently resulted in decreased BPD. However, health care practices clearly influence its incidence. For example, in 501–1500 gram birth weight (BW) infants, the incidence of BPD decreased from 47% to 21% by avoiding intubation, adopting new pulse oximeter limits, and transitioning to Continuous Positive Airway Pressure (CPAP) early [5]. There was a wide variability in the incidence of BPD in ten regions of Europe, suggesting that different care practices affect the incidence of BPD [6]. In the U.S., the incidence of BPD decreased between 1993 to 2006, likely related to the increased use of non-invasive ventilator support [7].
Pathobiology (See Figure)
Figure 1.
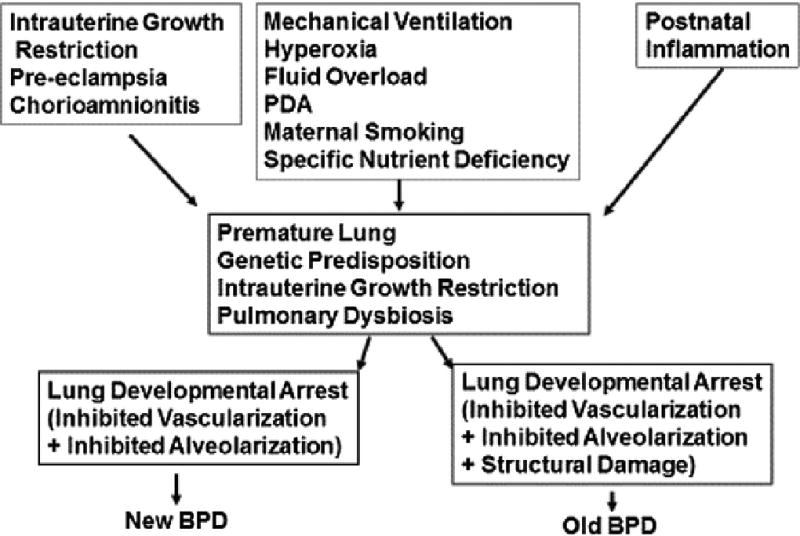
Bronchopulmonary Dysplasia: Pathogenesis
Bronchopulmonary dysplasia primarily occurs in infants born at a stage when their lungs are still transitioning from canalicular to saccular stage. Premature delivery truncates lung development in these stages and is frequently compounded by prenatal events such as intrauterine growth restriction (IUGR) and exposure to inflammation and postnatal events related to initial resuscitation, oxygen administration, mechanical ventilation, and pulmonary and systemic infections, which can all lead to arrested pulmonary vascular and alveolar development. It remains unclear if prematurity itself causes BPD or factors that contribute to prematurity are its direct cause, or if there are other yet unrecognized factors that lead to BPD. For example, the role of lung microbiome in lung and host-immune development is an emerging field of interest. It has become clear that decreased diversity of lung microbiome, in particular, decreased lactobacilli abundance, at the time of birth in preterm infants is strongly correlated with the development of BPD [8] [9]. Understanding how an imbalanced microbiome (~ dysbiosis) affects pulmonary macrophage activation and the expression of genes critical for normal lung development is key to exploit lung microbiome modulation in BPD prevention and treatment. Since in BPD pathogenesis, multiple events affect the complex well-orchestrated molecular processes involved in the developing lung, it is unlikely that there is a single pathophysiological basis for BPD. Nevertheless, in a variety of cellular and animal models, a breakdown in alveolar epithelial-mesenchymal signaling, leading to the transdifferentiation of pulmonary alveolar lipofibroblasts to myofibroblasts has been demonstrated on exposure to hyperoxia, infection, volutrauma, and other insults that lead to BPD [10]. Transdifferentiated lipofibroblasts are unable to maintain pulmonary epithelial cell growth and differentiation, resulting in failed alveolarization, seen characteristically in BPD. This serves the basis of some the experimental interventions (reviewed below) that aim to maintain lipofibroblast phenotype in an attempt to prevent/treat BPD.
Risk Factors
Apart from premature delivery, amongst the many prenatal factors, smoking, preeclampsia, lower socioeconomic status, and birth weight z- score, used as a marker for fetal growth restriction are most related to BPD development after adjustment for a variety of other perinatal characteristics [11]. However, apart from birth weight, prematurity, and IUGR, other risk factors must be studied further to determine their definitive significance.
Genetic Susceptibility
Much work has been done to identify heritable factors linking certain genes, pathways, and molecules to the development of BPD. For example, over-transmission of the A allele of rs4351 in the angiotensin-converting enzyme gene from parents was observed in infants with BPD. Moreover, a marker downstream of surfactant protein D gene, rs1923537, was found to be overtransmitted from parents to preterm infants who had a diagnosis of respiratory distress syndrome, which increases the risk of developing BPD [12]. In a genome-wide association study, SPOCK2 gene was associated with BPD [13]. Molecular pathways such as miR-219, a pathway involved in acute inflammation resolution, and CD44, a hyaluronic acid cell surface receptor involved in leukocyte trafficking and alveolar septation, were associated with BPD [14]. In additions, heritability for BPD is suggested by several studies on twins [15]. However, at present, the clinical application of genetic susceptibility findings is difficult to ascertain with certainty.
Inflammation
Although a role of inflammation in BPD has been validated in numerous studies, in preterm infants born in the setting of maternal inflammation (chorioamnionitis) and/or fetal inflammation (umbilical vasculitis), both increased and reduced odds of developing BPD have been reported [16]. In contrast, neonatal sepsis is consistently associated with BPD, but the type of infection is important [17]. It is also clear that both early- and late-onset sepsis increase the risk of BPD [18]. Overall, irrespective of the timing, the inflammatory exposure from sepsis plays an important role in BPD development. A significant association between Ureaplasma and BPD, likely via the suppression of the innate immune system and/or by increasing the ventilator-induced lung injury, has also been reported [19].
Transfusion
Multiple studies have linked blood transfusion to the development or worsening of BPD [20]. Possible mechanisms include increased oxidative injury, increased non-transferrin bound iron, and inflammatory mediators present in stored blood products. In a retrospective review of infants who received blood transfusions, an association between the number or volume of transfusions and BPD was seen at 28 days of age, but not at 36 weeks corrected gestational age (GA) [21]. However, no study clearly distinguishes an association of blood transfusions with BPD from its causation.
Prognosis
Pulmonary outcome
Children with a history of BPD continue to have significant abnormalities with airflow limitation on lung function tests [22]. In an 11-year-follow-up study, preterm infants continued to have impaired lung function and increased respiratory morbidity, especially among those with BPD [23]. Overall, multiple studies show that BPD not only decreases pulmonary function during infancy but also has a considerable impact on pulmonary function later in childhood in addition to increasing the chance of developing asthma [24].
Neurodevelopmental outcomes with BPD
Given the hostile extrauterine environment for brain development, compared to the natural in-utero setting, preterm infants in general are predisposed to poor neurodevelopment outcomes. In addition to other predetermined factors, BPD adversely affects preterm infants’ neurologic outcomes including a lower head circumference, cerebral palsy, and lower cognitive and language skills. Prolonged positive pressure ventilatory support, grade III–IV intraventricular hemorrhage, and discharge at > 43 weeks PMA have been found to be predictors of impaired neurodevelopment [25].
Cardiac outcome
Infants with BPD are at a higher risk for developing pulmonary hypertension (PH) and cardiac dysfunction. Patients with BPD and PH have substantially higher morbidity and mortality compared to patients with BPD without PH; for example, BPD with PH entails a mortality of up to 50% [26]. Although infants with BPD and BPD with PH share common risk factors, such as low GA at birth, IUGR, maternal preeclampsia, perinatal maternal inflammation, and prolonged durations of mechanical ventilation and oxygen supplementation, these risk factors are not unique only to BPD and BPD with PH. Only a few studies have evaluated the long-term cardiac function in infants in BPD, which suggest that almost 50% of infants with moderate/severe BPD at 36 weeks PMA have a lower right ventricular function on echocardiography compared to infants with no/mild BPD. Abnormal left ventricular myocardial performance also correlates with the severity of BPD [27]. Therefore, a high index of suspicion, active screening, and effective management of cardiac dysfunction are recommended in infants with moderate/severe BPD [28].
Prevention/Treatment
Anti-inflammatory therapy
Rationalizing inflammation as the final common pathway in the evolution of BPD, many studies have been conducted to decrease inflammation to prevent and/or treat BPD. Macrolide antibiotics have been tried for both antimicrobial and anti-inflammatory effects, i.e., via an inhibition of IL-6 expression and NF-kB activation. Though earlier studies demonstrated that LBWI treated with azithromycin required a shorter duration of mechanical ventilation [29], other studies have found no demonstrable difference between the azithromycin and placebo groups in the incidence of BPD/death; however, the Ureaplasma positive subgroup that received azithromycin had a lower incidence of BPD [30]. Nonetheless, the conclusive evidence from larger prospective antibiotic trials aimed to eradicate Ureaplasma respiratory colonization in preventing BPD is still awaited.
Hypothesizing vitamin D (VD)’s anti-inflammatory effects, a recent study demonstrated improvement in oxygenation and survival in a rat model following antenatal VD treatment [31]. In another study, rats exposed to inflammation/hyperoxia were treated with an IL-1 receptor antagonist, which decreased the incidence of severe BPD-like lung disease in the 65% O2 exposed group, but not in the 85% O2 exposed group [32]. Currently, more studies are in progress to test other anti-inflammatory approaches to treat and/or prevent BPD.
Postnatal Steroid Therapy
Postnatal steroid therapy, mainly dexamethasone therapy, during the first week of life has been tried as a preventive strategy against BPD. Although lower rates of failure to extubate and BPD are reported, adverse effects such as hyperglycemia, hypertension, hypertrophic cardiomyopathy, and growth failure preclude the routine use of this strategy to prevent BPD [33]. Similarly, reduction in BPD and facilitation of extubation have been observed with late postnatal (after 1st week) corticosteroid treatment. However, in view of the increased risk for infection and gastrointestinal bleeding with this approach, again, benefits do not outweigh the adverse effects [34]. Interestingly, as dexamethasone use has decreased over time, BPD rates have increased. Different dexamethasone doses are used at different institutions, which in fact also vary between clinicians at the same institution. Based on sixteen randomized controlled trials (RCTs), dexamethasone effect is most significant with a cumulative dose of ≥4 mg/kg, and when infants are treated moderately early, defined as 7–14 day of life [35]. However, multiple studies also show that postnatal steroids can result in a smaller brain volume and neurodevelopmental delays [36]. To avoid the systemic side effects, both inhaled and intratracheally administered steroids have been tried to prevent BPD. In a recent study, early intratracheal instillation of budesonide along with surfactant facilitated early extubation and improved pulmonary outcomes without significant adverse effects [37]. Though intratracheal administration of budesonide-surfactant combination appears to be a promising intervention to prevent BPD, there is a need for larger trials before this modality can be recommended as standard of care [38]. In addition, a recent RCT in extremely low birth weight infants (ELBWI) showed that prophylactic low-dose hydrocortisone during the first 10 days of life significantly decreased the incidence of BPD [39]. This effect was most pronounced in females and in infants exposed to chorioamnionitis before birth. Moreover, in contrast to studies suggesting adverse neurodevelopmental outcome with postnatal dexamethasone administration for preventing BPD, early low-dose hydrocortisone was not associated with a significant difference in neurodevelopment outcome at 2 years of age [40]. However, the long-term risk/benefit ratio for treatment with postnatal steroids still needs to be clearly defined, and there is no consensus on the type, dose, or the administration route of postnatal corticosteroids for preventing BPD.
Ventilatory Strategies
Multiple studies comparing high frequency ventilation with conventional mechanical ventilation have not demonstrated a clear benefit of one approach over the other. On the other hand, several studies reveal a significant reduction in the incidence of death or BPD with gentler ventilation strategies, which include avoiding endotracheal intubation altogether, whenever possible, routinely using CPAP or non-invasive intermittent positive pressure ventilation (IPPV) starting in the delivery room, and timely surfactant administration, using less invasive modes of administration. If endotracheal ventilation is required, it is prudent to use the lowest required ventilator settings, accept permissive hypercapnia, and aggressively wean ventilator support to extubate as soon as possible [41]. Although recent data suggest decreased intubation rates following nasal IPPV institution in the delivery room [42], in a previous study, comparing nasal IPPV to nasal CPAP, no difference was observed in BPD incidence [43]. A large meta-analysis (nine trials) comparing volume-targeted ventilation to pressure-limited ventilation in preterm infants demonstrated a shorter duration of mechanical ventilation and a lower incidence of BPD with volume ventilation strategy [44]. More recently, the use of neurally adjusted ventilator assist (NAVA) to deliver synchronized breaths suggested improved pulmonary outcomes [45]. Clearly, more studies are needed to support the benefits of volume ventilation and NAVA.
Target Oxygen Saturations
In a multicenter, randomized trial, lower target oxygen saturation parameters (85–89% vs. 91–95%) showed a trend towards decreased BPD or death at 36 weeks PMA [46]. Another study implied that targeting the functional oxygen saturation in 95–98% range rather than in 91–94% range improved growth and neurodevelopment in preterm infants, but this was at the expense of an increased risk of BPD and a higher mortality from pulmonary causes [47]. Though unresolved issues remain, in infants with GA<28 weeks until 36 weeks' PMA, it is reasonable to aim for a SaO2 in the 90–95% range [48].
Surfactant Therapy
Surfactant therapy and timing of its administration have been debatable and have varied widely. In a RCT, early surfactant therapy in the delivery room without mandatory ventilation decreased the need for mechanical ventilation [49]. Another RCT demonstrated lower oxygen requirements at 24 hours after surfactant therapy, but there was no evidence of decreased incidence of BPD in the surfactant treated group [50]. Surfactant therapy may decrease the need for mandatory ventilation; however, it does not decrease the rate of BPD. Cumulative data from studies using prophylactic surfactant followed by rapid extubation (INSURE or INtubate, SURfactant, Extubate) versus those randomized to routine intubation point to a decreased risk of BPD with the INSURE approach [51]. However, routine institution of CPAP in the delivery room reduces the risk of neonatal death and BPD, and in these infants selective administration of surfactant when significant respiratory distress manifests, is more beneficial [52]. Moreover, it is important to point out that now endotracheal intubation is no longer the preferred method for surfactant administration, since alternative modes of administration such as the aerosolized delivery and the less invasive surfactant administration (LISA) modes are used frequently in unstable preterm newborns, in whom the endotracheal intubation procedure still poses technical and ethical challenges [53]. It is also likely that newer and innovative synthetic surfactants, some of which are more resistant to inactivation and even have anti-inflammatory properties, might prove to be more effective in preventing BPD than what has been achieved so far [54].
Inhaled Nitric Oxide (iNO)
By decreasing pulmonary artery pressure and improving lung compliance, iNO was postulated to prevent BPD; however, in a multicenter RCT, no benefit in BPD reduction was noted [55]. Similarly, in iNO for Preventing Chronic Lung Disease trial, no benefit for the routine early use of iNO in preterm infants was observed. In 2010, the National Institutes of Health (NIH) Consensus Development Conference Statement concluded that the routine use of iNO was not recommended [56]. On the other hand, ELBWI who receive mechanical ventilation and iNO tend to receive less outpatient respiratory treatment including bronchodilators, inhaled or systemic corticosteroids, diuretics, and supplemental oxygen, but without any significant differences in re-hospitalizations or wheezing episodes, as reported by parents [57]. There are also data to suggest that some subpopulations of preterm infants might be more responsive and appropriate to consider for iNO treatment, e.g., infants with preterm premature rupture of membranes [58].
PDA ligation
Persistent patent ductus arteriosus (PDA) has been historically implicated in the development of BPD, with medical or surgical closure being the standard of care. However, recently, this approach has been questioned. Clyman et al. demonstrate that prophylactic surgical ligation of PDA significantly increased the incidence of BPD compared to the control group even though the control group had a higher incidence of necrotizing enterocolitis [59]. Surgical ligation of the PDA led to longer durations of mechanical ventilation and oxygen requirement compared to the pharmacologic closure group, indicating that there may be possible beneficial effects of medical closure [60]. In multiple studies, prophylactic surgical ligation of PDA has failed to show a consistent decrease in the incidence of BPD [61]. In a recent study comparing mandatory closure vs. a noninterventional approach to manage hemodynamically significant PDA in ELBWI, despite longer PDA exposure, the noninterventional approach was associated with significantly less BPD [62]. Additional studies are warranted to determine the benefits and risks of nonintervention for the hemodynamically significant PDA in ELBWI.
Vitamin A
Vitamin A is stored in the septal cells of the alveoli, and its deficiency can result in disruption of epithelial cell integrity and diminished alveolar septation. A NICHD Neonatal Network study indicated that supplementation with vitamin A decreases BPD or death at 36 weeks [63]. A meta-analysis on vitamin A use for BPD prevention also showed a borderline reduction in BPD [64]. However, possibly, due to the required invasive intramuscular dosing in ELBWI, who have limited muscle mass, fragile skin, and pain associated with injections, only about 20–30% centers routinely administer vitamin A supplementation. It is important to note that no significant differences were reported between the vitamin A-supplemented and control groups at 18–22 months of age [65]. A recent study also indicated that routine intramuscular injections of vitamin A were associated with an increased risk of sepsis in patients weighing > 1 kg [66]. Lastly, a retrospective study comparing vitamin A administration alone to vitamin A+iNO administration in extremely preterm infants revealed a lower incidence of BPD and improved neurocognitive outcomes at 1 year in infants who had received combined therapy [67].
Caffeine
In a relatively large study, early caffeine therapy (before 3 days of life) was associated with a lower incidence of BPD (23% vs. 30%), a shorter duration of mechanical ventilation, and less need for PDA treatment; however, this was associated with a slightly higher mortality (4.5% vs. 3.7%) [68]. Institution of caffeine administration immediately after birth, i.e., starting in the delivery room has shown to decrease the need for invasive ventilation in ELBWI [69]. On the other hand, a small observational study on infants with GA < 30 weeks showed that 24 hours after the caffeine load, IL-10 levels decreased significantly, and at one week from the initial load, caffeine levels directly correlated with TNF, IL-1β, and IL -6 levels, but inversely correlated with IL-10 when caffeine levels were > 20 mg/L. This implies caffeine’s pro-inflammatory effect when its levels are out of the therapeutic range [70]. Therefore, though multiple studies suggest beneficial effects of caffeine on BPD, caution is warranted since outside of the therapeutic range caffeine might be associated with a pro-inflammatory pulmonary profile.
Diuretics
Diuretics are widely used “off-label” in many units to prevent or treat BPD, and their frequency and specific regimens vary greatly. A meta-analysis of treatment effects of loop diuretics on infants with BPD demonstrated short-term benefits by improving pulmonary mechanics and oxygenation; however, long-term sequelae need to be studied further to determine benefit vs. harm of diuretics in the prevention and treatment of BPD [71].
Stem Cell Therapy
Based on novel understanding of the role of both resident lung and circulating stem cells in alveolar microvasculature formation, injury repair, and tissue homeostasis, there is an enormous interest in exploiting this knowledge and study stem cells to prevent BPD. Multiple promising preclinical studies convincingly show the reparative potential of stem/progenitor cells for lung growth and in preventing lung injury [72]. In animal models, using a variety of progenitor cells, protection against lipopolysaccharide and hyperoxia-induced neonatal lung injuries has been demonstrated [73]. Cell-based therapies primarily function by their paracrine effect rather than by stem cells directly constituting the repaired tissue [74]. More likely, stem cells can modulate innate and adaptive immune responses, decrease inflammation, enhance injury repair, and cause anti-apoptotic effects by producing paracrine factors [75]. In 2014, a phase I dose-escalation study examined the safety and feasibility of a single intratracheal transplantation of allogeneic human umbilical cord blood (hUCB)-derived mesenchymal stem cells (MSCs) in nine ELBWI at high risk for BPD. There was decreased severity of BPD in the transplanted group vs. the control group without any adverse outcomes [76]. Although this study suggested hUCB-MSC to be safe and a feasible therapy in preterm infants, further work is required before stem cells themselves or their conditioned medium can be safely used in clinical settings.
Vitamin D (VD)
VD, in addition to its anti-inflammatory role, as alluded to above, also mediates key alveolar signaling pathways, promoting perinatal lung maturation [77]. In animal models, VD administration protects against hyperoxia-induced neonatal lung injury as well as the development of a lung asthma phenotype associated with perinatal VD deficiency [78]. These experimental data provide an exciting opportunity to test VD’s role in preventing BPD in premature infants.
Peroxisome Proliferator-Activated Receptor (PPAR) γ Agonists
The nuclear transcription factor PPARγ plays an important role in mediating alveolar homeostasis and injury repair [79]. Multiple PPARγ agonists have been shown to promote alveolar maturation, and effectively block myofibroblast differentiation, a key event in BPD pathogenesis. In a neonatal rat model, both systemically and locally administered PPARγ agonists including curcumin have been shown to enhance neonatal lung maturation and block hyperoxia- and lipopolysaccharide-induced neonatal lung injuries, protecting both lung structure and function [80]. Therefore, PPARγ agonists offer a compelling rational approach to prevent/treat BPD; however, detailed pharmacodynamics, pharmacokinetics, and safety studies are needed before translating this approach to humans.
Phosphodiesterase Inhibitors (PIs)
Sildenafil, a selective cGMP-specific PI, has been extensively studied in the management of persistent PH in term neonates; however, there are only few studies examining its role in preventing BPD-induced PH. In experimental models, it preserves lung angiogenesis and alveolar growth, decreases pulmonary vascular resistance, right ventricular hypertrophy (RVH), decreases pulmonary inflammatory response, and improves survival [81], but in small retrospective clinical studies, the benefits have been only modest [82]. To assess sildenafil as a standard clinical therapy in BPD, larger prospective RCTs are needed.
Recombinant Human Clara Cell 10 Protein (rhCC10)
Clara Cell 10 Protein (CC10) inhibits phospholipase A2 and possesses potent anti-inflammatory and immunomodulatory properties. It is normally abundant in the respiratory tract but is deficient in premature infants [83]. In some animal models, CC10 has been shown to reduce lung inflammation and injury, improve pulmonary function, and up-regulate surfactant protein and vascular endothelial growth factor (VEGF) expression, rendering intratracheally administered CC10 protein a promising agent for preventing BPD [83] [84]. Currently, a RCT assessing its efficacy in preventing respiratory morbidity in infants 24–29 weeks GA is under way [85].
In addition to the above reviewed anti-inflammatory agents, many other agents have been tested for treating or preventing BPD [86]; despite this, there are only a few agents, e.g., vitamin A [64], caffeine [68], and postnatal steroids [33] [34], that have demonstrated a reduction in BPD. It appears that despite much promising experimental data, we still are not close to finding an effective pharmacologic intervention to prevent or treat BPD in the clinical setting any time soon.
Conclusion
As BPD has evolved since its first description by Northway five decades back, its definition, epidemiology, pathophysiology, prevention, and management have continued to evolve. This review summarizes the current information on pathophysiology, prevention, and treatment based on studies published mostly in the last decade. The knowledge on its pathophysiology is now poised to move forward rapidly, which is likely to open further avenues for effective BPD prevention and management. The majority of the interventions tried so far have not proven to be beneficial in rigorous meta-analyses of eligible studies. Currently, it is important that we focus on strategies that have been shown to help, namely, ensuring mothers at risk of preterm delivery get antenatal steroids; to avoid endotracheal intubation altogether, whenever possible; routinely initiate CPAP or IPPV in the delivery room; and when surfactant administration is required, to administer it early, using less invasive modes for its administration. If endotracheal ventilation is required, it is prudent to use the lowest required ventilator settings, accept permissive hypercapnia, and aggressively wean ventilator support to extubation at the earliest. Moreover, patients with moderately severe BPD need to be actively screened for PH and cardiac dysfunction. It is likely that these measures along with novel, innovative and targeted molecular interventions, and stem cell-based approaches will significantly lessen the burden of BPD within the next decade.
Acknowledgments
Funding support: Funding for the study was provided by NIH (HD51857, HL107118, HD071731, and HL127237) and TRDRP (17RT-0170 and 23RT-0018)
Footnotes
Conflict of interest: Dr. Jung Hwang and Dr. Virender Rehan declare that they have no conflict of interest.
Research involving human participants and/or animal: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed for the animal studies performed by the authors. This chapter does not contain any studies with human participants performed by any of the authors.
Informed consent: Not applicable
References
- 1.Northway WH, Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease: bronchopulmonary dysplasia. New England Journal of Medicine. 1967;276(7):357–368. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- 2.Isayama T, Lee SK, Yang J, Lee D, Daspal S, Dunn M, Shah PS. Revisiting the Definition of Bronchopulmonary Dysplasia: Effect of Changing Panoply of Respiratory Support for Preterm Neonates. JAMA Pediatrics. 2017;171(3):271–279. doi: 10.1001/jamapediatrics.2016.4141. [DOI] [PubMed] [Google Scholar]
- 3.Baraldi E, Filippone M. Chronic lung disease after premature birth. New England Journal of Medicine. 2007;357(19):1946–1955. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 4.Bhandari V, Bizzarro MJ, Shetty A, Zhong X, Page GP, Zhang H, Ment LR, Gruen JR. Familial and genetic susceptibility to major neonatal morbidities in preterm twins. Pediatrics. 2006;117(6):1901–1906. doi: 10.1542/peds.2005-1414. [DOI] [PubMed] [Google Scholar]
- 5.Birenbaum HJ, Dentry A, Cirelli J, Helou S, Pane MA, Star K, Melick CF, Updegraff L, Arnold C, Tamayo A, Torres V, Gungon N, Liverman S. Reduction in the incidence of chronic lung disease in very low birth weight infants: results of a quality improvement process in a tertiary level neonatal intensive care unit. Pediatrics. 2009;123(1):44–50. doi: 10.1542/peds.2007-2872. [DOI] [PubMed] [Google Scholar]
- 6.Kollee L, Cuttini M, Delmas D, Papiernik E, den Ouden A, Agostino R, Boerch K, Bréart G, Chabernaud J, Draper E, Gortner L. Obstetric interventions for babies born before 28 weeks of gestation in Europe: results of the MOSAIC study. BJOG: An International Journal of Obstetrics & Gynaecology. 2009;116(11):1481–1491. doi: 10.1111/j.1471-0528.2009.02235.x. [DOI] [PubMed] [Google Scholar]
- 7.Stroustrup A, Trasande L. Epidemiological Characteristics and Resource Use in Neonates With Bronchopulmonary Dysplasia: 1993–2006. Pediatrics. 2010;126(2):291–297. doi: 10.1542/peds.2009-3456. [DOI] [PubMed] [Google Scholar]
- 8.Lohmann P, Luna RA, Hollister EB, Devaraj S, Mistretta TA, Welty SE, Versalovic J. The airway microbiome of intubated premature infants: characteristics and changes that predict the development of bronchopulmonary dysplasia. Pediatric research. 2014;76(3):294–301. doi: 10.1038/pr.2014.85. [DOI] [PubMed] [Google Scholar]
- 9.Lal CV, Travers C, Aghai ZH, Jilling T, Halloran B, Carlo WA, Keeley J, Rezonzew G, Kumar R, Morrow C, Bhandari V, Ambalavanan N. The airway microbiome at birth. Scientific reports. 2016;6:31023. doi: 10.1038/srep31023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rehan VK, Torday JS. The lung alveolar lipofibroblast: an evolutionary strategy against neonatal hyperoxic lung injury. Antioxidants & redox signaling. 2014;21(13):1893–1904. doi: 10.1089/ars.2013.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bose C, Marter LJ, Laughon M, O'Shea TM, Allred EN, Karna P, Ehrenkranz RA, Boggess K, Leviton A. Fetal growth restriction and chronic lung disease among infants born before the 28th week of gestation. Pediatrics. 2009;124(3):e450–e458. doi: 10.1542/peds.2008-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryckman KK, Dagle JM, Kelsey K, Momany AM, Murray JC. Genetic associations of surfactant protein D and angiotensin-converting enzyme with lung disease in preterm neonate. Journal of Perinatology. 2012;32(5):349–355. doi: 10.1038/jp.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hadchouel A, Durrmeyer X, Bouzigon E, Incitti R, Huusko J, Jarreau PH, Lenclen R, Demenais F, Franco-Montoya M-L, Layouni I, Patkai J. Identification of SPOCK2 As a Susceptibility Gene for Bronchopulmonary Dysplasia. American journal of respiratory and critical care medicine. 2011;184(10):1164–1170. doi: 10.1164/rccm.201103-0548OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambalavanan N, Cotten CM, Page GP, Carlo WA, Murray JC, Bhattacharya S, Mariani TJ, Cuna AC, Faye-Petersen OM, Kelly D, Higgins RD. Integrated genomic analyses in bronchopulmonary dysplasia. The Journal of pediatrics. 2015;166(3):531–537. doi: 10.1016/j.jpeds.2014.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lavoie PM, Pham C, Jang KL. Heritability of bronchopulmonary dysplasia, defined according to the consensus statement of the national institutes of health. Pediatrics. 2008;122(3):479–485. doi: 10.1542/peds.2007-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Been JV, Rours IG, Kornelisse RF, Jonkers F, Krijger RRd, Zimmermann LJ. Chorioamnionitis alters the response to surfactant in preterm infants. The Journal of pediatrics. 2010;156(1):10–15. doi: 10.1016/j.jpeds.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 17.de Haan TR, Beckers L, Jonge RC, Spanjaard L, Toledo L, Pajkrt D, Wassenaer-Leemhuis AG, Lee JHvd. Neonatal Gram Negative and Candida Sepsis Survival and Neurodevelopmental Outcome at the Corrected Age of 24 Months. PloS one. 2013;8(3):e59214. doi: 10.1371/journal.pone.0059214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eriksson L, Haglund B, Odlind V, Altman M, Ewald U, Kieler H. Perinatal conditions related to growth restriction and inflammation are associated with an increased risk of bronchopulmonary dysplasia. Acta Paediatrica. 2015;104(3):259–263. doi: 10.1111/apa.12888. [DOI] [PubMed] [Google Scholar]
- 19.Lowe J, Watkins WJ, Edwards MO, Spiller OB, Jacqz-Aigrain E, Kotecha SJ, Kotecha S. Association between pulmonary ureaplasma colonization and bronchopulmonary dysplasia in preterm infants: updated systematic review and meta-analysis. The Pediatric infectious disease journal. 2014;33(7):697–702. doi: 10.1097/INF.0000000000000239. [DOI] [PubMed] [Google Scholar]
- 20.Collard KJ. Is there a causal relationship between the receipt of blood transfusions and the development of chronic lung disease of prematurity? Medical hypotheses. 2006;66(2):355–364. doi: 10.1016/j.mehy.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 21.Valieva OA, Strandjord TP, Mayock DE, Juul SE. Effects of Transfusions in Extremely Low Birth Weight Infants: A Retrospective Study. The Journal of pediatrics. 2009;155(3):331–337. doi: 10.1016/j.jpeds.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fakhoury KF, Sellers C, Smith E, Rama JA, Fan LL. Serial measurements of lung function in a cohort of young children with bronchopulmonary dysplasia. Pediatrics. 2010;125(6):e1441–e1447. doi: 10.1542/peds.2009-0668. [DOI] [PubMed] [Google Scholar]
- 23.Fawke J, Lum S, Kirkby J, Hennessy E, Marlow N, Rowell V, Thomas S, Stocks J. Lung function and respiratory symptoms at 11 years in children born extremely preterm: the EPICure study. American journal of respiratory and critical care medicine. 2010;182(2):237–245. doi: 10.1164/rccm.200912-1806OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davidson LM, Berkelhamer SK. Bronchopulmonary Dysplasia: Chronic Lung Disease of Infancy and Long-Term Pulmonary Outcomes. Journal of Clinical Medicine. 2017;6(1):4. doi: 10.3390/jcm6010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trittmann JK, Nelin LD, Klebanoff MA. Bronchopulmonary dysplasia and neurodevelopmental outcome in extremely preterm neonates. European journal of pediatrics. 2013;172(9):1173–1180. doi: 10.1007/s00431-013-2016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bui CB, Pang MA, Sehgal A, Theda C, Lao JC, Berger PJ, Nold MF, Nold-Petry CA. Pulmonary hypertension associated with bronchopulmonary dysplasia in preterm infants. Journal of reproductive immunology. 2017;124:21–29. doi: 10.1016/j.jri.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 27.Levy PT, Dioneda B, Holland MR, Sekarski TJ, Lee CK, Mathur A, Cade WT, Cahill AG, Hamvas A, Singh GK. Right ventricular function in preterm and term neonates: reference values for right ventricle areas and fractional area of change. Journal of the American Society of Echocardiography. 2015;28(5):559–69. doi: 10.1016/j.echo.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abman SH, Hansmann G, Archer SL, Ivy DD, Adatia I, Chung WK, Hanna BD, Rosenzweig EB, Raj JU, Cornfield D, Stenmark KR, Steinhorn R, Thébaud B, Fineman JR, Kuehne T, Feinstein JA, Kuehne T, Feinstein JA, Friedberg MK, Earing M, Barst RJ, Keller RL, Kinsella JP, Mullen M, Deterding R, Kulik T, Mallory G, Humpl T, Wessel DL. Pediatric Pulmonary Hypertension: Guidelines From the American Heart Association and American Thoracic Society. Circulation. 2015;132(21):2037–2099. doi: 10.1161/CIR.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 29.Ballard HO, Anstead MI, Shook LA. Azithromycin in the extremely low birth weight infant for the prevention of Bronchopulmonary Dysplasia: a pilot study. Respiratory research. 2007;8(1):41. doi: 10.1186/1465-9921-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ballard HO, Shook LA, Bernard P, Anstead MI, Kuhn R, Whitehead V, Grider D, Crawford TN, Hayes D. Use of azithromycin for the prevention of bronchopulmonary dysplasia in preterm infants: a randomized, double-blind, placebo controlled trial. Pediatric pulmonology. 2011;46(2):111–118. doi: 10.1002/ppul.21352. [DOI] [PubMed] [Google Scholar]
- 31.Mandell E, Seedorf G, Gien J, Abman SH. Vitamin D treatment improves survival and infant lung structure after intra-amniotic endotoxin exposure in rats: potential role for the prevention of bronchopulmonary dysplasia. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2014;306(5):L420–L428. doi: 10.1152/ajplung.00344.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nold MF, Mangan NE, Rudloff I, Cho SX, Shariatian N, Samarasinghe TD, Skuza EM, Pedersen J, Veldman A, Berger PJ, Nold-Petry CA. Interleukin-1 receptor antagonist prevents murine bronchopulmonary dysplasia induced by perinatal inflammation and hyperoxia. Proceedings of the National Academy of Sciences. 2013;110(35):14384–14389. doi: 10.1073/pnas.1306859110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doyle LW, Ehrenkranz RA, Halliday HL. Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database of Systematic Reviews. 2014;(5):CD001146. doi: 10.1002/14651858.CD001146.pub4. [DOI] [PubMed] [Google Scholar]
- 34.Doyle LW, Ehrenkranz RA, Halliday HL. Late (>7 days) postnatal corticosteroids for chronic lung disease in preterm infants. Cochrane Database of Systematic Reviews. 2014;(5):CD001145. doi: 10.1002/14651858.CD001145.pub3. [DOI] [PubMed] [Google Scholar]
- 35.Onland W, Offringa M, Jaegere APD, Kaam AHv. Finding the Optimal Postnatal Dexamethasone Regimen for Preterm Infants at Risk of Bronchopulmonary Dysplasia: A Systematic Review of Placebo-Controlled Trials. Pediatrics. 2009;123(1):367–377. doi: 10.1542/peds.2008-0016. [DOI] [PubMed] [Google Scholar]
- 36.Parikh NA, Lasky RE, Kennedy KA, Moya FR, Hochhauser L, Romo S, Tyson JE. Postnatal dexamethasone therapy and cerebral tissue volumes in extremely low birth weight infants. Pediatrics. 2007;119(2):265–272. doi: 10.1542/peds.2006-1354. [DOI] [PubMed] [Google Scholar]
- 37.Yeh TF, Lin HC, Chang CH, Wu TS, Su BH, Li TC, Pyati S, Tsai CH. Early Intratracheal Instillation of Budesonide Using Surfactant as a Vehicle to Prevent Chronic Lung Disease in Preterm Infants: A Pilot Study. Pediatrics. 2008;121(5):e1310–e1318. doi: 10.1542/peds.2007-1973. [DOI] [PubMed] [Google Scholar]
- 38.Venkataraman R, Kamaluddeen M, Hasan SU, Robertson HL, Lodha A. Intratracheal Administration of Budesonide-Surfactant in Prevention of Bronchopulmonary Dysplasia in Very Low Birth Weight Infants: A Systematic Review and Meta-Analysis. Pediatric Pulmonology. 2017;52(7):968–975. doi: 10.1002/ppul.23680. [DOI] [PubMed] [Google Scholar]
- 39.Baud O, Maury L, Lebail F, Ramful D, Moussawi FE, Nicaise C, Zupan-Simunek V, Coursol A, Beuchée A, Bolot P, Andrini P, Mohamed D, Alberti C. Effect of early low-dose hydrocortisone on survival without bronchopulmonary dysplasia in extremely preterm infants (PREMILOC): a double-blind, placebo-controlled, multicentre, randomised trial. Lancet. 2016;387(10030):1827–1836. doi: 10.1016/S0140-6736(16)00202-6. [DOI] [PubMed] [Google Scholar]
- 40.Baud O, Trousson C, Biran V, Leroy E, Mohamed D, Alberti C. Association Between Early Low-Dose Hydrocortisone Therapy in Extremely Preterm Neonates and Neurodevelopmental Outcomes at 2 Years of Age. JAMA. 2017;317(13):1329–1337. doi: 10.1001/jama.2017.2692. [DOI] [PubMed] [Google Scholar]
- 41.Fischer HS, Bührer C. Avoiding endotracheal ventilation to prevent bronchopulmonary dysplasia: a meta-analysis. Pediatrics. 2013;132(5):e1351–e1360. doi: 10.1542/peds.2013-1880. [DOI] [PubMed] [Google Scholar]
- 42.Biniwale M, Wertheimer F. Decrease in delivery room intubation rates after use of nasal intermittent positive pressure ventilation in the delivery room for resuscitation of very low birth weight infants. Resuscitation. 2017;116:33–38. doi: 10.1016/j.resuscitation.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Kugelman A, Feferkorn I, Riskin A, Chistyakov I, Kaufman B, Bader D. Nasal intermittent mandatory ventilation versus nasal continuous positive airway pressure for respiratory distress syndrome: a randomized, controlled, prospective study. The Journal of pediatrics. 2007;150(5):521–526. doi: 10.1016/j.jpeds.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 44.Wheeler KI, Klingenberg C, Morley CJ, Davis PG. Volume-targeted versus pressure-limited ventilation for preterm infants: a systematic review and meta-analysis. Neonatology. 2011;100(3):219–227. doi: 10.1159/000326080. [DOI] [PubMed] [Google Scholar]
- 45.Bhandari V, Finer NN, Ehrenkranz RA, Saha S, Das A, Walsh MC, Engle WA, VanMeurs KP. Synchronized nasal intermittent positive-pressure ventilation and neonatal outcomes. Pediatrics. 2009;124(2):517–526. doi: 10.1542/peds.2008-1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network. Target ranges of oxygen saturation in extremely preterm infants. The New England Journal of Medicine. 2010;362(21):1959–1969. doi: 10.1056/NEJMoa0911781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Askie LM, Henderson-Smart DJ, Irwig L, Simpson JM. Oxygen-saturation targets and outcomes in extremely preterm infants. New England Journal of Medicine. 2003;349(10):959–967. doi: 10.1056/NEJMoa023080. [DOI] [PubMed] [Google Scholar]
- 48.Saugstad OD, Aune D. Optimal oxygenation of extremely low birth weight infants: a meta-analysis and systematic review of the oxygen saturation target studies. Neonatology. 2013;105(1):55–63. doi: 10.1159/000356561. [DOI] [PubMed] [Google Scholar]
- 49.Rojas MA, Lozano JM, Rojas MX, Laughon M, Bose CL, Rondon MA, Charry L, Bastidas JA, Perez LA, Rojas C, Ovalle O, Celis LA, Garcia-Harker J, Jaramillo ML. Very early surfactant without mandatory ventilation in premature infants treated with early continuous positive airway pressure: a randomized, controlled trial. Pediatrics. 2009;123(1):137–142. doi: 10.1542/peds.2007-3501. [DOI] [PubMed] [Google Scholar]
- 50.Laughon M, Bose C, Moya F, Aschner J, Donn SM, Morabito C, Cummings JJ, Segal R, Guardia C, Liu G. A pilot randomized, controlled trial of later treatment with a peptide-containing, synthetic surfactant for the prevention of bronchopulmonary dysplasia. Pediatrics. 2009;123(1):89–96. doi: 10.1542/peds.2007-2680. [DOI] [PubMed] [Google Scholar]
- 51.Stevens TP, Blennow M, Myers EH, Soll R. Early surfactant administration with brief ventilation vs. selective surfactant and continued mechanical ventilation for preterm infants with or at risk for respiratory distress syndrome. Cochrane Database Systemic Review. 2007;(4):CD003063. doi: 10.1002/14651858.CD003063.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rojas-Reyes MX, Morley CJ, Soll R. Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database of Systematic Reviews. 2012;(3):CD000510. doi: 10.1002/14651858.CD000510.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lopez E, Gascoin G, Flamant C, Merhi M, Tourneux P, Baud O. Exogenous surfactant therapy in 2013: what is next? Who, when and how should we treat newborn infants in the future? BMC Pediatrics. 2013;13(1):165. doi: 10.1186/1471-2431-13-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sato A, Ikegami M. SP-B and SP-C Containing New Synthetic Surfactant for Treatment of Extremely Immature Lamb Lung. PLoS One. 2012;7(7):e39392. doi: 10.1371/journal.pone.0039392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Meurs KP, Wright LL, Ehrenkranz RA, Lemons JA, Ball MB, Poole WK, Perritt R, Higgins RD, Oh W, Hudak ML, Laptook AR, Shankaran S, Finer NN, Carlo WA, Kennedy KA, Fridriksson JH, Steinhorn RH, Sokol GM, Konduri G, Aschner JL, Stoll BJ, D'Angio CT, Stevenson DK. Inhaled nitric oxide for premature infants with severe respiratory failure. New England Journal of Medicine. 2005;353(1):13–22. doi: 10.1056/NEJMoa043927. [DOI] [PubMed] [Google Scholar]
- 56.Cole FS, Alleyne C, Barks JD, Boyle RJ, Carroll JL, Dokken D, Edwards WH, Georgieff M, Gregory K, Johnston MV, Kramer M, Mitchell C, Neu J, Pursley DM, Robinson WM, Rowitch DH. NIH consensus development conference: inhaled nitric oxide therapy for premature infants. NIH consensus and state-of-the-science statements. Pediatrics. 2011;127(2):363–369. doi: 10.1542/peds.2010-3507. [DOI] [PubMed] [Google Scholar]
- 57.Hibbs AM, Walsh MC, Martin RJ, Truog WE, Lorch SA, Alessandrini E, Cnaan A, Palermo L, Wadlinger SR, Coburn CE, Ballard PL, Ballard RA. One-year respiratory outcomes of preterm infants enrolled in the Nitric Oxide (to prevent) Chronic Lung Disease trial. The Journal of pediatrics. 2008;153(4):525–529. doi: 10.1016/j.jpeds.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ellsworth MA, Harris MN, Carey WA, Spitzer AR, Clark RH. Off-label use of inhaled nitric oxide after release of NIH consensus statement. Pediatrics. 2015;135(4):643–648. doi: 10.1542/peds.2014-3290. [DOI] [PubMed] [Google Scholar]
- 59.Clyman R, Cassady G, Kirklin JK, Collins M, Philips JB. The role of patent ductus arteriosus ligation in bronchopulmonary dysplasia: reexamining a randomized controlled trial. The Journal of pediatrics. 2009;154(6):873–876. doi: 10.1016/j.jpeds.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Youn Y, Lee J-Y, Lee JH, Kim S-Y, Sung IK, Lee JY. Impact of patient selection on outcomes of PDA in very low birth weight infants. Early human development. 2013;89(3):175–179. doi: 10.1016/j.earlhumdev.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 61.Jhaveri N, Moon-Grady A, Clyman RI. Early surgical ligation versus a conservative approach for management of patent ductus arteriosus that fails to close after indomethacin treatment. The Journal of pediatrics. 2010;157(3):381–387. doi: 10.1016/j.jpeds.2010.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sung SI, Chang YS, Chun JY, Yoon SA, Yoo HS, Ahn SY, Park WS. Mandatory closure versus nonintervention for patent ductus arteriosus in very preterm infants. The Journal of pediatrics. 2016;182:66–71. doi: 10.1016/j.jpeds.2016.06.046. [DOI] [PubMed] [Google Scholar]
- 63.Tyson JE, Wright LL, Oh W, Kennedy KA, Mele L, Ehrenkranz RA, Stoll BJ, Stevenson JALK, Bauer CR, Korones SB, Donovan EF, Carlo WA, Shankaran S, Stark AR, Papile L-A, Jobe A, Stacewicz-Sapuntzakis M, Verter J, Fanaroff AA. Vitamin A supplementation for extremely-low-birthweight infants. New England Journal of Medicine. 1999;340(25):1962–196. doi: 10.1056/NEJM199906243402505. [DOI] [PubMed] [Google Scholar]
- 64.Darlow BA, Graham PJ. Vitamin A supplementation to prevent mortality and short and long-term morbidity in very low birthweight infants. Cochrane Database of Systematic Reviews. 2011;(10):CD000501. doi: 10.1002/14651858.CD000501.pub3. [DOI] [PubMed] [Google Scholar]
- 65.Ambalavanan N, Tyson JE, Kennedy KA, Hansen NI, Vohr BR, Wright LL, Carlo WA. Vitamin A Supplementation for Extremely Low Birth Weight Infants: Outcome at 18 to 22 Months. Pediatrics. 2005;115(3):e249–e254. doi: 10.1542/peds.2004-1812. [DOI] [PubMed] [Google Scholar]
- 66.Uberos JM, Miras-Baldo M, Jerez-Calero A, Narbona-López E. Effectiveness of Vitamin A in the Prevention of Complications of Prematurity. Pediatrics & Neonatology. 2014;55(5):358–362. doi: 10.1016/j.pedneo.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 67.Gadhia MM, Cutter GR, Abman SH, Kinsella JP. Effects of early inhaled nitric oxide therapy and vitamin A supplementation on the risk for bronchopulmonary dysplasia in premature newborns with respiratory failure. The Journal of pediatrics. 2014;164(4):744–748. doi: 10.1016/j.jpeds.2013.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dobson NR, Patel RM, Smith PB, Kuehn DR, Clark J, Vyas-Read S, Herring A, Laughon MM, Carlton D, Hunt CE. Trends in caffeine use and association between clinical outcomes and timing of therapy in very low birth weight infants. The Journal of pediatrics. 2014;164(5):992–998. doi: 10.1016/j.jpeds.2013.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dekker J, Hooper SB, Vonderen JJv, Witlox RS, Lopriore E, Pas ABt. Caffeine to improve breathing effort of preterm infants at birth: a randomized controlled trial. Pediatric Research. 2017;82:290–296. doi: 10.1038/pr.2017.45. [DOI] [PubMed] [Google Scholar]
- 70.Valdez RC, Ahlawat R, Wills-Karp M, Nathan A, Ezell T, Gauda EB. Correlation between serum caffeine levels and changes in cytokine profile in a cohort of preterm infants. The Journal of pediatrics. 2011;158(1):57–64. doi: 10.1016/j.jpeds.2010.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stewart A, Brion LP. Intravenous or enteral loop diuretics for preterm infants with (or developing) chronic lung disease. Cochrane Database of Systematic Reviews. 2011;(9):CD001453. doi: 10.1002/14651858.CD001453.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pierro M, Thébaud B, Soll R. Mesenchymal stem cells for the prevention and treatment of bronchopulmonary dysplasia in preterm infants. Cochrane Database of Systematic Reviews. 2017;(11):CD011932. doi: 10.1002/14651858.CD011932.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.O'Reilly M, Thébaud B. Using Cell-Based Strategies to Break the Link between Bronchopulmonary Dysplasia and the Development of Chronic Lung Disease in Later. Pulmonary medicine. 2013:874161. doi: 10.1155/2013/874161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fung ME, Thébaud B. Stem cell-based therapy for neonatal lung disease: it is in the juice. Pediatric research. 2013;75(1):2–7. doi: 10.1038/pr.2013.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee JW, Fang X, Krasnodembskaya A, Howard JP, Matthay MA. Concise Review: Mesenchymal Stem Cells for Acute Lung Injury: Role of Paracrine Soluble Factors. Stem Cells. 2011;29(6):913–919. doi: 10.1002/stem.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chang YS, Ahn SY, Yoo HS, Sung SI, Choi SJ, Oh WI, Park WS. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. The Journal of pediatrics. 2014;164(5):966–972. doi: 10.1016/j.jpeds.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 77.Sakurai R, Shin E, Fonseca S, Sakurai T, Litonjua AA, Weiss ST, Torday JS, Rehan VK. 1α, 25 (OH) 2D3 and its 3-epimer promote rat lung alveolar epithelial-mesenchymal interactions and inhibit lipofibroblast apoptosis. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2009;297(3):L496–L505. doi: 10.1152/ajplung.90539.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yurt M, Liu J, Sakurai R, Gong M, Husain SM, Siddiqui MA, Husain M, Villarreal P, Akcay F, Torday JS, Rehan VK. Vitamin D supplementation blocks pulmonary structural and functional changes in a rat model of perinatal vitamin D deficiency. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2014;307(11):L859–L867. doi: 10.1152/ajplung.00032.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cerny L, Torday JS, Rehan VK. Prevention and treatment of bronchopulmonary dysplasia: contemporary status and future outlook. Lung. 2008;186(2):75–89. doi: 10.1007/s00408-007-9069-z. [DOI] [PubMed] [Google Scholar]
- 80.Rehan VK, Torday JS. PPARγ Signaling Mediates the Evolution, Development, Homeostasis, and Repair of the Lung. PPAR research. 2012:289867. doi: 10.1155/2012/289867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Visser YP, Walther FJ, Laghmani EH, Boersma H, Laarse AVd, Wagenaar GT. Sildenafil attenuates pulmonary inflammation and fibrin deposition, mortality and right ventricular hypertrophy in neonatal hyperoxic lung injury. Respiratory research. 2009;10(1):30. doi: 10.1186/1465-9921-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tan K, Krishnamurthy MB, O’Heney JL, Paul E, Sehgal A. Sildenafil therapy in bronchopulmonary dysplasia-associated pulmonary hypertension: a retrospective study of efficacy and safety. European journal of pediatrics. 2015;174(8):1109–1115. doi: 10.1007/s00431-015-2515-7. [DOI] [PubMed] [Google Scholar]
- 83.Wolfson MR, Funanage VL, Kirwin SM, Pilon AL, Shashikant BN, Miller TL, Shaffer TH. Recombinant human Clara cell secretory protein treatment increases lung mRNA expression of surfactant proteins and vascular endothelial growth factor in a premature lamb model of respiratory distress syndrome. American journal of perinatology. 2008;25(10):637–645. doi: 10.1055/s-0028-1090587. [DOI] [PubMed] [Google Scholar]
- 84.Miller TL, Shashikant BN, Melby JM, Pilon AL, Shaffer TH, Wolfson MR. Recombinant human Clara cell secretory protein in acute lung injury of the rabbit: effect of route of administration. Pediatric Critical Care Medicine. 2005;6(6):698–706. doi: 10.1097/01.pcc.0000165565.96773.08. [DOI] [PubMed] [Google Scholar]
- 85.Davis J, Parad R. Safety, pharmacokinetics, and anti-inflammatory effects of intratracheal recombinant human Clara cell protein in premature infants with respiratory distress syndrome. 2013 doi: 10.1203/01.PDR.0000156371.89952.35. ClinicalTrials.gov Identifier NCT01941745. [DOI] [PubMed]
- 86.Taylor S, Rehan VK. Bronchopulmonary Dysplasia. Springer International Publishing; 2016. Anti-inflammatory Agents for the Prevention of Bronchopulmonary Dysplasia; pp. 325–344. [Google Scholar]


