Abstract
Acute lymphoblastic leukemia (ALL) is the most common type of pediatric cancer, although about 4 of every 10 cases occur in adults. The enzyme drug L-asparaginase serves as a cornerstone of ALL therapy and exploits the asparagine-dependency of ALL cells. In addition to hydrolyzing the amino acid L-asparagine, all FDA-approved L-asparaginases also have significant L-glutaminase coactivity. Since several reports suggest that L-glutamine depletion correlates with many of the side effects of these drugs, enzyme variants with reduced L-glutaminase coactivity might be clinically beneficial if their anti-leukemic activity would be preserved. Here we show that novel low L-glutaminase variants developed on the backbone of the FDA-approved Erwinia chrysanthemi L-asparaginase were highly efficacious against both T and B cell ALL, while displaying reduced acute toxicity features. These results support the development of a new generation of safer L-asparaginases without L-glutaminase activity for the treatment of human ALL.
INTRODUCTION
Bacterial L-asparaginases are enzymes with dual activities. The predominant one, the L-asparaginase activity that gives these enzymes their name, is the ability to hydrolyze the amino acid L-asparagine (Asn) into L-aspartic acid (Asp) and ammonia. The secondary activity present in L-asparaginases is an L-glutaminase activity, which drives hydrolysis of L-glutamine (Gln) to L-glutamic acid (Glu) and ammonia. For the FDA-approved L-asparaginases (Escherichia coli (EcA) and Erwinia chrysanthemi (ErA), approved in 1978 and 2011, respectively), the L-glutaminase activity ranges from 2 to 10% of their primary L-asparaginase activity (1). The dual L-asparaginase and L-glutaminase property of EcA and ErA is expected to manifest itself in the depletion of both Asn and Gln in the patient’s blood, a notion that is supported by several studies (2–5).
The anticancer effect of L-asparaginase is believed to be predominantly due to the depletion of Asn from the blood. Indeed, leukemic blasts from ALL patients completely depend on scavenging Asn from the blood, as they lack or display very low levels of the asparagine synthetase (ASNS) enzyme (6–8). In contrast, the clinical importance of the L-glutaminase activity present in all FDA-approved versions of L-asparaginases is still under debate, with conflicting reports in the literature about its putative anti-leukemic effect (9,10). On one hand, pharmacodynamic analyses showed that deamination of Gln is critically required for optimal Asn deamination (2) and other more recent studies indicated contribution of L-glutaminase activity to the cytotoxicity of L-asparaginase on leukemic cells (10,11). In contrast, others have found that the L-glutaminase activity is not required for the drug’s in vitro anticancer effect, as long as the ALL cells lack ASNS (9).
Common side effects in patients treated with L-asparaginases, in addition to an immune response against the bacterial enzymes, include hepatotoxicity, hyperglycemia, dyslipidemia, perturbations in blood coagulation factors, and pancreatitis (12–14). Several clinical studies have documented the Gln depletion resulting from the L-glutaminase coactivity of current L-asparaginase preparations (4,15,16), and suggested that the aforementioned side effects can, at least in part, be attributed to this property of the drugs. For example, a link between the L-glutaminase activity and the immunosuppressive effects of these drugs have been reported (17,18), as well as its role in hepatotoxicity (19) which was proposed to be due to deleterious effects on Gln homeostasis (3). Likewise, Gln depletion could likely contribute significantly to the disrupted protein synthesis in the liver and spleen that is a cause of the coagulopathy aspects of drug toxicity (20). Moreover, hydrolysis of both Asn and Gln will produce ammonia as a byproduct of the reaction. However, given that Gln concentrations are much higher in the blood as compared to Asn, Gln hydrolysis will have a more profound effect on the eventual concentration of ammonia in the blood. Indeed, hyperammonemia has been observed in patients undergoing L-asparaginase treatment (16,21–25), which has been associated with neurotoxicity.
Additional information on the putative interplay between L-glutaminase activity and drug toxicity came from at least four clinical trials of L-asparaginases. First, in the early 1980s, a clinical trial, which examined an L-asparaginase from Acinetobacter with very high L-glutaminase activity, was forced to terminate early due to central nervous system toxicity (26). Second, between 2001–2008, the L-asparaginase from Wolinella succinogenes, which was initially thought to be a low L-glutaminase enzyme, was evaluated clinically through a US National Cancer Institute Rapid Access to Intervention Development (NCI RAID) grant. However, the enzyme produced via this program was found to be toxic in patients and we recently showed that it actually does contain significant L-glutaminase activity (27). Third, in 2008, a phase II clinical trial examining the FDA-approved EcA in ovarian cancer patients had to be terminated early due to excessive toxicities (28). Interestingly, while weight loss was reported as one of the main drug-related toxicities in the phase II ovarian cancer study, it is also a significant L-glutaminase-related toxicity indicator in our actual pre-clinical study. Finally and very recently, a clinical trial of eryaspase (red blood cell encapsulated EcA) showed that, for a yet unclear reason, the encapsulation process reduced the L-glutaminase activity (i.e. increased the selectivity for Asn hydrolysis over Gln hydrolysis), a factor pointed out as an explanation for the decrease in adverse events in the eryaspase clinical trial compared to naked EcA (29). Hence, these trials support the notion that certain side effects observed in patients undergoing L-asparaginase treatment might be associated with the level of L-glutaminase activity. Therefore, reducing the L-glutaminase activity of available L-asparaginases may be advantageous to lessen toxic side effects, but for now it is unclear whether this would be detrimental for the anti-leukemic efficacy of these drugs.
Previously, we engineered variants of ErA with decreased L-glutaminase activity while maintaining near wild-type L-asparaginase activity (30). Here we evaluated these novel ErA variants in vitro and in vivo for their ability to kill ALL cells, and compared them to their wild-type counterpart. It is important to appreciate the experimental complexity when comparing different L-asparaginases for their efficacy and toxicity, since in addition to the kinetic properties of the enzyme drugs, pharmacokinetics and immunogenicity (when tested in patients) play a major role in determining the outcome. To simplify the interpretation of the results, here we present the comparison of L-asparaginases that have similar L-asparaginase activities and that only differ by 1–3 residues, suggesting very similar pharmacokinetic properties, but that have vastly different L-glutaminase activity. Together, our results suggest that high L-glutaminase activity, as present in current FDA drugs, is not essential for efficient in vivo elimination of L-asparaginase sensitive ALL cells. Additionally, reduced toxicity was observed in the low L-glutaminase variants compared to the high L-glutaminase enzymes. This sets up the rationale for further evaluation of such low L-glutaminase variants, which are predicted to have fewer side effects, as alternatives to the current FDA-approved bacterial L-asparaginases for the treatment of ALL.
Materials and Methods
Expression and Purification of L-asparaginases
Enzymes used for kinetic, NMR, and cell culture studies were expressed and purified as previously reported in Nguyen et al. (30,31) for ErA-WT, ErA-E63Q, ErA-DM, and ErA-TM; and as in Schalk et al. (32) for EcA-WT.
Kinetic Assays
L-asparaginase and L-glutaminase activities were determined using a continuous spectroscopic enzyme-coupled assay as previously described (32,33).
Cell culture
The LOUCY cell line was established from the peripheral blood of a T-cell ALL patient (34). The luciferase-positive LOUCY cell line was generated as described previously (35). The SUP-B15 cell line was established from cells harvested from the bone marrow of a Philadelphia chromosome positive B-cell ALL patient (36). The luciferase-expressing SUP-B15 cell line was a kind gift from Dr. Michael Jensen, University of Washington School of Medicine. All cell lines were analyzed by STR (Short Tandem Repeat) and confirmed to match 100% to corresponding STR profile data from the Global Bioresource Center ATCC. All cell lines were verified to be mycoplasma free. The Alamar Blue assay for cell viability is described in Supplementary Methods. IC50 values were determined by GraphPad Prism 6.0 using sigmoidal interpolation model with 95% confidence intervals.
In vivo treatment of cell line xenografts with L-asparaginases
Non-obese diabetic/severe combined immune-deficient γ (NSG) mice (The Jackson Laboratory) were i.v. injected at 6 weeks of age with 150 µL DPBS containing 5×106 luciferase-positive LOUCY or SUP-B15 cells. At regular time points, the bioluminescence was measured using the IVIS Lumina II imaging system (PerkinElmer). After evidence of leukemic cell engraftment, the mice were randomly divided into different groups that were administered via i.p. injection at a dose of 50 IU/mouse daily for 14 days with either ErA-WT, ErA-E63Q, ErA-DM, ErA-TM or the same volume of DPBS. In another experiment, LOUCY-engrafted mice were treated with 25 IU/mouse at days 0, 2, 4, 7, 9, 11, 12, 13 and 14 via i.p. injection. The bioluminescent imaging (BLI) signal was measured every two to three days as indicated in Fig. 2; Fig. S1, S2 and S3. During the experiment, the mice were observed and weighed every day. The ethical committee on animal welfare at University of Illinois at Chicago approved this animal experiment.
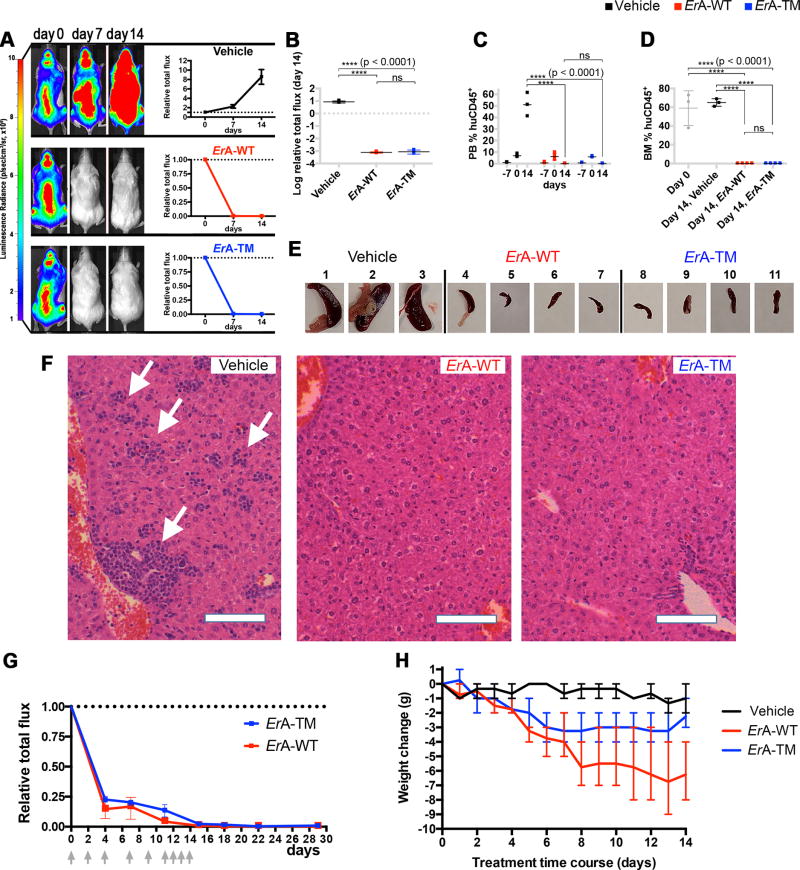
Fig. 2. The low L-glutaminase ErA-TM eliminates T-ALL LOUCY cells as effectively as the high L-glutaminase ErA-WT and with reduced toxicity.
(A) Female mice tail vein-injected with luciferase-expressing LOUCY cells four weeks prior were treated daily with vehicle (n=3); ErA-WT (n=4); and ErA-TM (n=4) for 14 days (drug dose 50 IU/mouse/day; i.p.). For each group, the representative animal shown had the highest BLI signal at day 0 of treatment. BLIs from all animals are presented in Supplementary Fig. 4 and 6. The average BLI signal of each group at day 7 and 14 relative to the value at day 0 (day 0 = 1) was plotted with mean and standard deviation (SD). See Supplementary Biostatistics on imaging for detailed standard error analysis. (B) Relative BLI flux at day 14 between the vehicle, ErA-WT, and ErA-TM groups. The flux for the vehicle mice increased 10-fold relative to day 0. For both treated groups, the flux decreased dramatically relative to vehicle control (p-value <0.0001), returning to background levels by day 14, with no significant (ns) difference between the treated groups. Mean with SD were plotted. See Supplementary Biostatistics on imaging for detailed standard error analysis. (C) PB %huDC45+ levels were determined one week prior to treatment initiation (day -7), at treatment start (day 0), and at end of treatment (day 14). At day 0, all animals were highly engrafted, as indicated by %huCD45+ >8%. By day 14, for the vehicle-treated mice, the %huDC45+ increased to 40–60%, whereas for both treatment groups, the %huCD45+ was undetectable (p-value <0.0001 between vehicle- and enzyme-treated groups; ns between the two enzyme-treated groups). Mean with SD were plotted. All tests were set at controlling for probability of Type I error of 0.05. See Supplementary Biostatistics for more details. (D) At day 0, assessment of BM %huDC45+ in 3 mice with similar BLI flux as the ones used for treatment revealed high engraftment (gray boxes). At day 14, BM %huDC45+ remained high in the vehicle-treated mice, but was undetectable in both enzyme-treated groups (p-value <0.0001 between vehicle- and enzyme-treated groups; ns between the two enzyme-treated groups). Mean with SD were plotted. All tests were set at controlling for probability of Type I error of 0.05. See Supplementary Biostatistics for more details. (E) Spleens from the vehicle-treated mice were highly enlarged, whereas spleens from the ErA-WT and ErA-TM groups resembled normal mouse spleens in size. (F) H&E-stained paraffin sections of livers from vehicle-, ErA-WT- and ErA-TM-treated mice. Vehicle-treated animals had livers filled with deposits of lymphoblastic leukemic cells (arrows). In contrast, livers of mice treated with ErA-WT or ErA-TM had no detectable leukemic cells present; bar = 10 µm. (G) Female mice tail vein-injected with luciferase-expressing LOUCY cells four weeks prior were treated i.p. with ErA-WT (n=3); and ErA-TM (n=3) for 14 days (a total of 9 drug doses of 25 IU/mouse on days indicated by gray arrows). The average BLI (+SD) signal of each group at day 0, 4, 7, 11, 15, 18, 22 and 29 relative to the value at day 0 (day 0 = 1) is plotted. (H) Correlation between L-glutaminase activity and toxicity of the ErA variants. Weight loss (in grams, relative to day 0), an indicator of toxicity, was monitored in mice treated with vehicle (black trace), ErA-WT (red trace, L-glutaminase@Gln500µM=15.87 sec−1) and ErA-TM (blue trace, Gln500µM=0.01 sec−1). The pronounced daily weight loss in the ErA-WT-treated group is ameliorated in the ErA-TM-treated group by 0.29 g/day, p-value <0.0001.
Acute toxicity study
The experimental design of this study incorporated a blinded strategy where the toxicologist was provided with samples labeled as #1 and #2, without knowing the identity of the enzymes (ErA-WT or ErA-TM). In this dose escalation study, 6 animals (3 males, 3 females) per dose group were administered the enzymes i.v. at a starting dose of 40 IU/g, increasing to 80 and finally 160 IU/g. Due to a shortage of the enzymes, ErA-TM group 6 was limited to 4 animals (3 females, 1 male), and a few animals did not receive the full intended dose (one animal of ErA-WT group 3 received 136 instead of the intended 160 IU/g, one animal of the ErA-TM group 5 received 60 instead of the intended 80 IU/g). The unexpected shortage of enzymes was due to higher than expected loss during filtration through a 0.22 µm filter and the adjustment needed for bigger body weight of the mice. After enzyme administration, the animals were monitored daily and clinical signs (hunched posture, decreased activity, sunken eyes, and rough coat) were noted if observed. None of the animals died during the 4-day observation period, and all the animals were euthanized at the end of day 4.
In vivo asparaginase activity determination
C57BL/6 mice of 7–10 weeks old were i.p. injected with two batches of 50 IU of ErA-WT or ErA-TM. 24 hours after the injection, peripheral blood was collected (5 animals per group) via cardiac puncture under anesthesia (5% isoflurane in oxygen). Shortly after collection, blood was centrifuged in heparin-coated tubes (2000 g, 10 min, 4°C) for plasma preparation. Plasma L-asparaginase activity was quantified by incubating the samples with an excess amount of L-aspartic acid β-hydroxamate (AHA) (Sigma-Aldrich A6508) at 37.0°C. L-asparaginase hydrolyses AHA to L-aspartic acid and hydroxylamine, which was detected at 690 nm with a SpectraMax M3 (Molecular Devices) spectrophotometer, after condensation with 8-hydroxyquinoline (Merck 8.20261) and oxidation to indooxine. A detailed procedure can be found in the Supplementary Data. The ethical committee on animal welfare at Ghent University Hospital approved the experiment.
In vivo pharmacodynamics of amino acid
C57BL/6 mice of 7–10 weeks old were i.p. injected with 50 IU of ErA-WT or ErA-TM. For the pharamacodynamic study, peripheral blood was collected at days 1, 3, 7, and 14 (5 animals per group) via cardiac puncture under anesthesia (5% isoflurane in oxygen). In addition, blood of seven untreated mice was collected to determine the baseline value (day 0). Shortly after collection, blood was centrifuged in heparin-coated tubes (2000 g, 10 min, 4°C) for plasma preparation. Plasma was diluted with equal volume of a 10% 5’-sulfosalicylic acid dihydrate solution in water and stored at −80°C for the determination of amino acid levels.
For the amino acid analysis, the plasma samples (50 µL) were deproteinized by adding 100 µL of a 10% sulfosalicylic acid solution containing 50 µM internal standards mix. After vortexing, 50 µL of UPLC-grade water was added. Centrifugation occurred for 10 minutes at 9960 g. Derivatization of 10 µL of supernatant was performed according to the manufacturer’s instructions of the AccQ-Tag® kit of Waters. The 4 amino acids (asparagine, aspartic acid, glutamine and glutamic acid) were measured on an Acquity UPLC with QDA detector of Waters and quantified based on a 5-points calibration curve. The ethical committee on animal welfare at Ghent University Hospital approved these experiments.
Patient-derived xenograft experiment
A xenograft of a pediatric primary human T-ALL sample was established in NSG mice. Upon establishment of disease, human leukemic cells were isolated from the spleen via Ficoll-Paque (GE Healthcare) density gradient centrifugation. Next, these cells were injected in the tail vein of 15 female NSG mice at 7 weeks of age. Each mouse received 150 µl PBS containing 1.2×106 cells. Engraftment of the cells was followed by measuring the percentage of human CD45 positive (%huCD45+) cells in the blood. Upon evidence of leukemic cell engraftment, mice were randomly divided into 3 groups (day 0), and treated daily via i.p. injection with 50 IU/mouse for 13 days of either ErA-WT, ErA-TM or the same volume of PBS. At day 0, 7 and 13, blood was collected via the tail vein. At day 13, all mice were sacrificed and the spleen and bone marrow were collected. The %huCD45+ cells in the blood, bone marrow and spleen was analyzed by staining with a phycoerythrin-labeled antibody for human CD45 (130–098-141; Miltenyi Biotec, Bergisch Gladbach, Germany), performing red blood cell lysis and measuring the percentage on a LSRII flow cytometer using FACSDiva software (BD Bioscience). During the experiment, mice were observed and weighed every day. The experiment was approved by the ethical committee on animal welfare at Ghent University Hospital.
qPCR experiments
Total RNA was isolated using the miRNeasy mini kit (Qiagen) and the RNAse-Free DNAse set (Qiagen). cDNA was synthesized with the iScript Advanced cDNA synthesis kit (Bio-Rad). The SsoAdvanced Universal SYBR Green Supermix (Bio-Rad) was used and the PCR reactions were run on the LightCycler 480 (Roche, model LC480). Every sample was analyzed in duplicate. qBasePLUS software (Biogazelle, Zwijnaarde, Belgium) was used for analysis. Gene expression was normalized against 3 reference genes (GAPDH, TBP, YWHAZ). ASNS primers: (F) 5’-CCCTGCACGCCCTCTATG-3’, (R) 5’-GGATCCTGAGGTTGTTCTTCACA-3’; GAPDH primers: (F) 5’-TGCACCACCAACTGCTTAGC-3’, (R) 5’- GGCATGGACTGTGGTCATGAG-3’; TBP primers: (F) 5’- CACGAACCACGGCACTGATT -3’, (R) 5’- TTTTCTTGCTGCCAGTCTGGAC -3’ and YWHAZ primers: (F) 5’-ACTTTTGGTACATTGTGGCTTCAA-3’, (R) 5’- CCGCCAGGACAAACCAGTAT-3’.
Statistical Methods
See Supplementary data file for details on Biostatistics.
RESULTS
Design and characterization of ErA variants with high L-asparaginase and low L-glutaminase activities
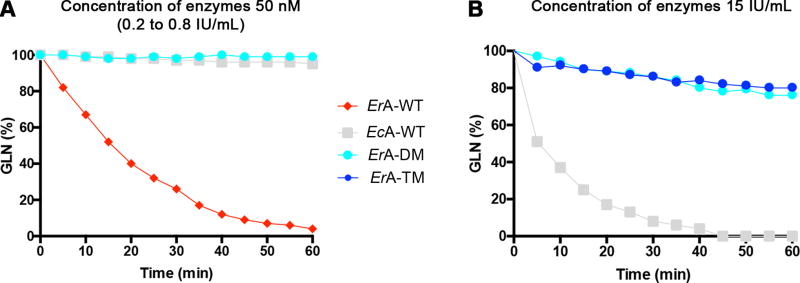
To determine whether L-asparaginase variants with low L-glutaminase activity may hold clinical potential, we investigated several L-glutaminase-deficient ErA variants (denoted as ErA-E63Q, ErA-DM (double mutant) and ErA-TM (triple mutant)) that retain most of their wild-type L-asparaginase activity (30). Comparisons of kinetic parameters between these ErA variants and the FDA-approved wild-type versions of ErA and EcA (denoted as ErA-WT and EcA-WT) are summarized in Table 1. We selected ErA-WT over EcA-WT as the backbone to develop low L-glutaminase variants because of its superior L-asparaginase activity (~2.5-fold higher rate than EcA-WT in hydrolyzing Asn at the physiological concentration of 50 µM). However, ErA-WT also has >70-fold higher L-glutaminase activity compared to EcA-WT at the physiological concentration of 500 µM Gln. Since both kcat (i.e. the rate at saturating substrate concentration) and Km influence the turnover of enzyme catalyzed reactions, the kcat/Km ratio is often taken as a measure of enzyme efficiency, and this ratio is shown in Table 1 for the L-asparaginase and L-glutaminase activities of the examined enzymes. However, to assess the specificities of the enzymes, we also calculated the ratio between the kcat/Km of the L-asparaginase reaction to the kcat/Km of the L-glutaminase reaction (the larger the number, the higher is the specificity for the L-asparaginase reaction). From this calculation, it is clear that ErA-TM and ErA-DM are much more L-asparaginase specific (ratio = 68,750 and 4,842, respectively) compared to the original ErA-WT enzyme (ratio = 58.6), but that only ErA-TM is significantly more specific than EcA-WT (ratio = 4,625). However, this analysis based on the ratios of the L-asparaginase and L-glutaminase kcat/Km values may not best reflect the physiological conditions. Therefore, we also calculated specificity ratios based on the observed rates (kobs) at physiological substrate concentrations (50 µM for Asn, 500 µM for Gln). These calculations show that our engineered ErA variants have significantly superior Asn:Gln specificity as compared to ErA-WT (Table 1). Using this calculation, even ErA-DM is about 2-fold more L-asparaginase specific compared to EcA-WT, with ErA-TM being 47-fold more specific. The reduced rate of L-glutaminase activity for each ErA mutant was also demonstrated through measuring changes to Gln concentrations over time by NMR spectroscopy (Fig. 1A & B). Notably, while EcA-WT completely hydrolyzed Gln in ~45 minutes, solutions with ErA-DM and ErA-TM contained >80% of the starting Gln after 1 hour, demonstrating their exceptionally low L-glutaminase activities (Fig. 1B).
Table 1.
Enzyme kinetic parameters
| Enzyme name | kcat (sec−1) |
Km (µM) |
kcat/Km (sec−1µM−1) |
kobs @50 µMa (sec−1) |
kobs @50 µMb (sec−1) |
|
|---|---|---|---|---|---|---|
| L-asparaginase activity | ErA-WT | 207.5 ± 3.6 | 47.5 ± 3.5 | 4.37 | 118.9 | 145.8 |
| ErA-TM | 261.2 ± 2.8 | 95.0 ± 3.5 | 2.75 | 79.6 | 56.4 | |
| ErA-DM | 169.8 ± 1.5 | 185.3 ± 5.5 | 0.92 | 22.4 | 23.3 | |
| ErA-E63Q | 186.8 ± 1.7 | 50.7 ± 2.0 | 3.68 | 112.7 | 135.9 | |
|
|
||||||
| EcA-WT | 44.4 ± 0.3 | 15.0 ± 0.5 | 2.96 | 41.3 | ||
|
| ||||||
| Enzyme name | kcat (sec−1) | Kmc(µM) | kcat/Kmc (sec−1µM−1) | kobs @0.5 mMa (sec−1) | kobs @0.5 mMb (sec−1) | |
|
|
||||||
| L-glutaminase activity | ErA-WT | 26.84 ± 0.26 | 360 ± 20 | 74.56 × 10−3 | 15.87 | 14.76 |
| ErA-TM | 1.84 ± 0.11 | 47,460 ± 695 | 0.04 × 10−3 | 0.01 | 0.04 | |
| ErA-DM | 2.93 ± 0.03 | 15,800 ± 300 | 0.19 × 10−3 | 0.11 | 0.09 | |
| ErA-E63Q | 8.33 ± 0.16 | 3,860 ± 230 | 3.68 × 10−3 | 0.74 | 1.05 | |
|
|
||||||
| EcA-WT | 0.89 ± 0.01 | 1,380 ± 90 | 0.64 × 10−3 | 0.22 | ||
|
| ||||||
| Enzyme name | kobs[Asnphs] / kobs[Glnphs]d | kcat/Km (Asn) / kcat/Km (Gln) | ||||
|
|
||||||
| Specificity | ErA-WT | 6.6 | 58.6 | |||
| ErA-TM | 8910 | 68,750 | ||||
| ErA-DM | 330 | 4,842 | ||||
| ErA-E63Q | 124.6 | 1,000 | ||||
|
|
||||||
| EcA-WT | 187.7 | 4,625 | ||||
kobs for enzymes without the SUMO tag
kobs for enzymes with the SUMO tag
Concentrations are given in µM to facilitate comparison with the L-asparaginase data
kobs for Asn@50µM, kobs for Gln@500µM
Fig. 1. Engineered ErA variants have reduced L-glutaminase activity.
(A) NMR spectroscopy was used to monitor the ability of various L-asparaginases to hydrolyze Gln. The starting Gln concentration (600 µM; labeled as 100%) was chosen to reflect physiological Gln levels. All enzymes were added to the same final concentration of 50 nM, which depending on molecular weight and L-asparaginase rate translates to 0.2 – 0.8 IU/ml. Under these conditions, ErA-WT fully hydrolyses the Gln in an hour (red trace). In contrast, ErA-DM exhibits negligible Gln hydrolysis (green trace), reduced even compared to EcA-WT (gray trace). (B) To further compare the L-glutaminase activity of the ErA variants relative to EcA-WT, an experiment was conducted at a higher enzyme concentration relative to that shown in panel A. Enzyme amounts were based on matched L-asparaginase activity level; with all enzymes at 15 IU/ml, EcA-WT completely depletes Gln within 45 minutes, whereas for both ErA-DM and ErA-TM, Gln levels are only reduced by 20% after 1 hour.
In vitro testing of ErA-WT and ErA mutants in ALL cell lines
The development of ErA mutants with comparable L-asparaginase but variable L-glutaminase activity (ErA-E63Q > ErA-DM > ErA-TM) allowed us to test whether the high intrinsic L-glutaminase activity of ErA-WT is truly required for its clinical efficacy. Notwithstanding the limitations of evaluating L-asparaginase in cell culture, we first validated the anti-proliferative effect of wild type and mutant L-asparaginases in vitro on the human leukemic cell lines, LOUCY (T-ALL) and SUP-B15 (B-ALL). Results indicated that both ALL cell lines were similarly sensitive to ErA-WT, EcA-WT, and to each of the L-glutaminase-deficient ErA mutants (Table 2 and Fig. S4). Since most cell lines depend on high Gln levels in culture, the slightly lower IC50 values for ErA-WT and EcA-WT compared to the L-glutaminase-deficient ErA mutants is not surprising.
Table 2.
Sensitivity of LOUCY and SUP-B15 cells to L-asparaginase
| Enzyme name | LOUCY IC50 (mIU/mL)a |
SUP-B15 IC50 (mIU/mL) |
|---|---|---|
| ErA-WT | 0.33 | 0.16 |
| ErA-E63Q | 0.55 | 0.24 |
| ErA-DM | 0.65 | 0.31 |
| ErA-TM | 0.61 | 0.27 |
|
| ||
| EcA-WT | 0.35 | 0.22 |
mIU; milli International unit.
The His-SUMO tag acts to stabilize the ErA variants in vivo
Given that in vitro studies cannot unambiguously clarify whether L-glutaminase activity is required for in vivo effectiveness of L-asparaginases, we subsequently used xenograft models of luciferase-positive LOUCY and SUP-B15 cells to perform in vivo drug treatment experiments. Engraftment of human leukemic cells in mice is often considered successful when the percentage of peripheral blood (PB) cells positive for the human CD45 antigen (%huCD45+) is ≥1–2% (37,38). Four weeks after NOD-scid IL2Rgammanull mice (NSG) received cell line injections, bioluminescence imaging (BLI) flux signals corresponding to a PB %huCD45+ greater than 8% confirmed successful engraftment and showcased the high level of disease burden in the examined animals (Fig. S1, S2 and S3; Fig. S5 reports the calibration between BLI flux and PB %huCD45+). With this level of engraftment, daily drug treatment with ErA-WT (intraperitoneal injection (i.p.) of 50 IU/day for 14 days) was initiated. Surprisingly, this FDA-approved L-asparaginase failed to reduce tumor cell growth in vivo. Of note, ErA-WT has a half-life of only 0.65 days in humans, compared to 1.24 days for EcA-WT (39). Furthermore, half-lives of these drugs are dramatically shortened in mice (40). Thus, we hypothesized that the short half-life of ErA-WT prevented therapeutic efficacy. To evaluate whether drug instability indeed hindered the anticancer effect, we retained the N-terminal SUMO tag, which was originally incorporated to increase stability and facilitate the heterologous expression of the enzymes in E. coli. The SUMO tag has only moderate impact on the enzymatic activity of the variants (see Table 1), and dosing of the drugs according to their activity (i.e. adjusted to deliver the same IU) largely accounts for the effect of the SUMO tag on activity. Notably, treatment of the LOUCY cells xenografted mice with this adapted SUMO-ErA-WT enzyme (50 IU/day i.p. for 12 days) resulted in a marked decrease in tumor burden (Fig. S1), suggesting that maintaining the SUMO tag increased the stability of the enzyme with no or minimal effects towards the therapeutic enzymatic properties of ErA-WT. Therefore, this stability tag was incorporated in the wild-type and mutant ErA enzymes used in subsequent in vivo drug treatment experiments.
In vivo testing of ErA-WT and ErA mutants in ALL cell line xenograft models
We next evaluated the in vivo efficacy of the low L-glutaminase variants listed in Table 1. ErA-E63Q and ErA-DM were as efficient as ErA-WT in reducing the BLI signal in mice engrafted with LOUCY (Fig. S1) or SUP-B15 cells (Fig. S2). For a more stringent evaluation of the requirement for the L-glutaminase coactivity for L-asparaginase efficacy against ALL in vivowe compared ErA-TM, the variant with the lowest L-glutaminase activity, with ErA-WT, which has inherently high L-glutaminase activity. Results indicated that both enzymes are indistinguishable in their ability to rapidly reduce leukemic burden (Fig. 2A; Fig. S3 and Fig. S6). After 14 days of consecutive treatment, both ErA-WT- and ErA-TM-treated mice displayed BLI signals diminished to background levels (Fig. 2B). In addition, analysis of huCD45+ cells in PB and bone marrow (BM) of ErA-WT- and ErA-TM-treated mice showed undetectable levels of leukemic cells at day 14 of treatment (Fig. 2, C and D). Smaller spleen size and reduced lymphoblastic invasion of liver tissue further confirmed the comparable anti-leukemic efficacy of ErA-WT and ErA-TM (Fig. 2, E and F).
Since the dosing regimen used for the above studies (50 IU/mouse daily) induced an abrupt decrease in the BLI signal for both ErA-WT and ErA-TM treated groups (BLI signal below 7% at day 3 and less than 1% relative to treatment start already by day 7, Fig. 2A, Fig. S3 and Fig. S6), we asked whether a less aggressive dosing regimen would reveal differences in efficacy between the two enzyme variants. Therefore, we conducted an additional efficacy study where the animals were treated with 25 IU/mouse (half the dose of the previous experiments). Since after the initial 6 drug treatments, which were administered on a Mon-Wed-Fri schedule, the BLI was not below background level for both groups (1 – 5% relative to day 0), we added three daily drug injections at the same dose (for a total of 9 drug administrations per group). The ALL burden, quantitated by the BLI signal, was monitored during the treatment period followed by an additional 15 days after the last treatment (Fig 2G). Even at this reduced dose, both enzymes rapidly decreased the BLI signal. Multiple t-tests were calculated for every single imaging data point revealing no statistically significant difference in BLI between the two treated groups (p-value >0.01). The BLI signal was below 0.5% at the last day of treatment and a week later (day 22), and remained below 1% even at day 29 (15 days post last treatment).
However, the residual BLI signal indicated that the cancer was not eradicated in both treated cohorts, suggesting that further drug dosing would be needed. Whereas the ErA-TM treated group could tolerate additional doses, this was not the case for the ErA-WT-treated group, a point clearly illustrated by the condition of the shredding toys (untouched by the ErA-WT-treated group, shredded by the ErA-TM-treated group), shown both at the last day of drug treatment and 10 days later (Fig. S7). Collectively, these results convincingly show that the ultra-low L-glutaminase ErA-TM enzyme maintains a very similar ability to combat ALL cells in vivo as compared to the high L-glutaminase ErA-WT enzyme but with an improved tolerability profile.
Indication for reduced toxicity of the low L-glutaminase ErA variants
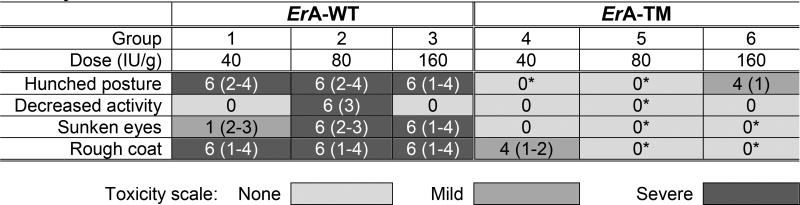
In addition to noting the reduced activity of the high L-glutaminase ErA-WT-treated mice, but not of the low L-glutaminase ErA-TM-treated mice, we also compared the impact of the drugs on the animals’ body weight. At the end of the experiment we observed 27% mean weight loss in ErA-WT-treated mice (Fig. 2H), which is consistent with results from previous studies (41,42). This effect was largely mitigated in the animals treated with ErA-TM, which only experienced 10% mean weight loss. Hence, by treatment day 14, the ErA-WT group lost on average an additional 4 grams of body weight (7 versus 3) compared to ErA-TM (p-value <0.0001). In addition, the correlation between percentage weight loss and level of enzyme L-glutaminase activity level was reproducible across several independent experiments (Fig. S8, A and B). Finally, we examined the acute toxicity of ErA-WT and ErA-TM in a blinded single dose-escalation study in CD-1 mice, using a concentration range of 40 to 160 IU/g. For comparison, the dose used to treat the ALL-bearing mice was ~2.5 IU/g. No fatalities occurred in either group, but clear physical and behavioral signs of toxicity were observed in the ErA-WT-treated group (Table 3). These signs were virtually absent in ErA-TM-challenged mice (Table 3). Together, these data show a clear correlation between reduced L-glutaminase activity and reduced drug toxicity. As previously noted, a common side effect of L-asparaginase treatment is hepatotoxicity, which presents histopathologically as macrovesicular hepatic steatosis (43), coupled with abnormally elevated serum levels of the liver enzymes alanine aminotransferase (ALT) (44) and aspartate aminotransferase (AST) (45). However, blood chemistry analysis did not display a clear difference in ALT or AST levels between ErA-WT- and ErA-TM-treated mice. The absence of a difference in serum liver enzyme levels in the studied animals may not be surprising since L-asparaginase induced hepatotoxicity in humans has been linked to fatty liver disease (46), elevated BMI (12,47), and increased age (48), whereas the mice studied did not present histologically with hepatic fat accumulation (Fig. 2F), were lean, and young.
Table 3.
Acute toxicity study showing correlation between L-glutaminase activity and toxicity of the ErA variants
CD-1 mice were subjected to an acute single dose-escalation toxicity study. Each group had 3 males and 3 females, except group 6 which had only 4 animals (3 females, 1 male) due to a shortage of ErA-TM. Animals administered ErA-WT presented significant physical and behavioral symptoms, whereas the ErA-TM administered animals were largely devoid of such symptoms. The number of animals (out of 6 total) presenting symptoms is indicated. In parenthesis, the day(s) on which the symptom was observed is noted. One male in group 3 was dosed with 136 IU/g, and one female in group 5 was dosed with 60 IU/g. *p < 0.05, analysis by Fisher’s exact test: (Group 1 vs. Group 4, Group 2 vs. Group 5, Group 3 vs. Group 6). *p < 0.05, analysis by Mann-Whitney U Test: (Group 1 vs. Group 4, Group 2 vs. Group 5, Group 3 vs. Group 6). Mean with SD were plotted. See Supplementary Biostatistics on imaging for detailed standard error analysis.
The differences in toxicity between ErA-WT and ErA-TM is not due to a difference in in vivo stability but correlates with the enzyme’s impact on the blood glutamine levels
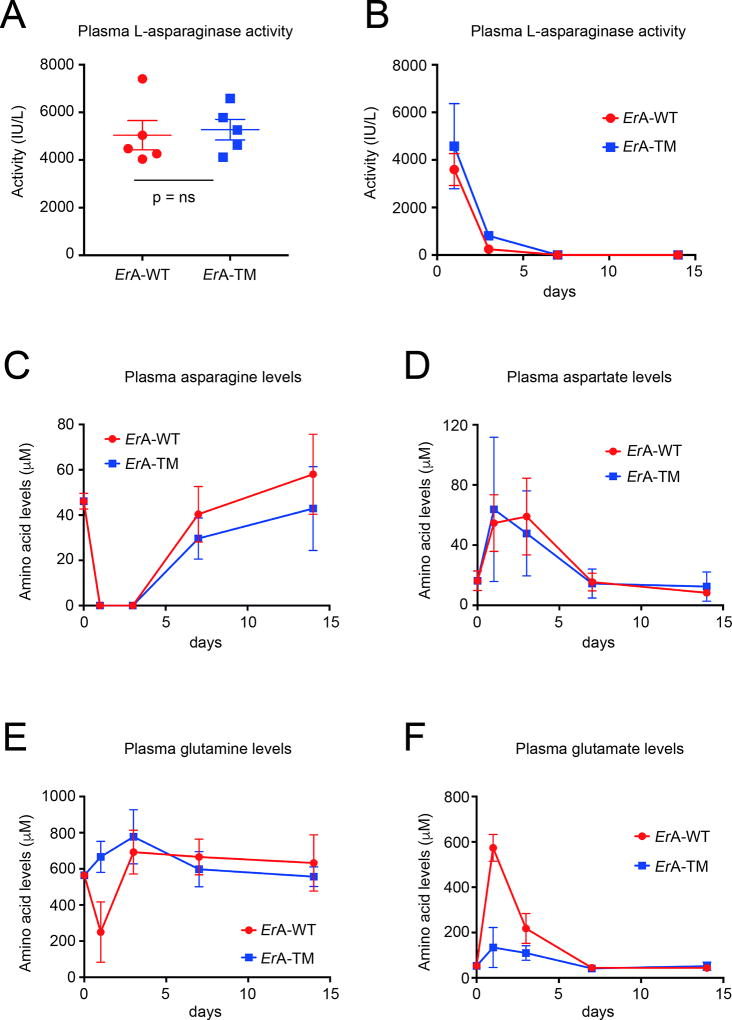
The similar anti-ALL power of ErA-WT and ErA-TM but dissimilar toxicity profile could be due to their different L-glutaminase activity, but could also be due to different pharmacokinetic properties. To investigate this possibility, mice were injected i.p. with 50 IU of ErA-WT or ErA-TM from two different batches - we chose this drug dose to be consistent with the majority of our efficacy studies. The L-asparaginase activity in blood plasma samples was measured 24 h after the initial injection (for both batches) and in addition at days 3, 7 and 14 for the second batch. We measured comparable L-asparaginase activity for both enzymes at all time points, indicating a similar in vivo stability (Fig. 3A and B). We also determined the levels of the amino acids asparagine, aspartate, glutamine, and glutamate prior to the treatment and at days 1, 3, 7 and 14 post administration of ErA-WT or ErA-TM. Both enzymes depleted the blood asparagine, which was below detection at day 1 and 3 and then slowly recovered (Fig. 3C). Consistent with this, the levels of aspartate increased at day 1 and 3, and then decreased to the pre-treatment level by day 7 (Fig. 3D).
Fig. 3. Similar in vivo stability for ErA-WT and ErA-TM and ability to deplete blood asparagine but dissimilar impact on glutamine homeostasis.
(A) L-asparaginase activity measurement in blood plasma samples of mice obtained 24 h after injection of 50 IU of ErA-WT or ErA-TM from batch #1. Mean with SEM (standard error of the mean) is shown for each group and non-parametric Mann-Whitney test was used for statistics. (B) L-asparaginase activity measurement in blood plasma samples of mice obtained 1, 3, 7 and 14 days after injection of 50 IU of ErA-WT or ErA-TM from batch #2. (C) Determination of asparagine levels in blood plasma samples of mice prior to the treatment and at days 1, 3, 7 and 14 post administration of ErA-WT or ErA-TM. For each time point, the mean and standard deviation are shown. (D) Determination of aspartate levels in blood serum samples of mice prior to the treatment and at days 1, 3, 7 and 14 post administration of ErA-WT or ErA-TM. For each time point, the mean and standard deviation are shown. (E) Determination of glutamine levels in blood plasma samples of mice prior to the treatment and at days 1, 3, 7 and 14 post administration of ErA-WT or ErA-TM. For each time point, the mean and standard deviation are shown. (F) Determination of glutamate levels in blood plasma samples of mice prior to the treatment and at days 1, 3, 7 and 14 post administration of ErA-WT or ErA-TM. For each time point, the mean and standard deviation are shown.
Notably, when we examined the effect of the enzymes on glutamine, we noted that ErA-WT reduced the glutamine level from ~600 µM pre-treatment to ~250 µM at day 1, which then promptly recovered by day 3. In contrast, ErA-TM did not reduce the glutamine levels (Fig. 3E). As expected from this, the glutamate levels increased for the ErA-WT-treated mice, but not for the ErA-TM-treated animals (Fig. 3F). These results are consistent with the predictions from the kinetic properties of these enzymes (Table 1) and the NMR experiments (Fig. 1), showing that the in vitro observed similarity in the L-asparaginase activity but differences in the L-glutaminase activity translates into the in vivo setting. Moreover, whereas the glutamine levels recovered by day 3, we would expect that the daily dosing schedule, as used in our efficacy studies, would continuously impact the glutamine levels.
In vivo evaluation of ErA-WT and ErA-TM using a patient derived T-ALL xenograft model
The previous experiments demonstrated the anti-ALL equivalence between ErA-WT and ErA-TM in both T- and B-ALL cell lines. To further probe the potential clinical relevance of ErA-TM, we compared these enzymes using a patient derived xenograft (PDX) model from a primary human T-ALL. After verifying leukemia engraftment (%huCD45+ range 1.8 – 7.5%), animals (n=5/group) were randomized into control, ErA-WT-, and ErA-TM- treated groups (50 IU/dose daily for 13 days). PB %huCD45+ increased dramatically in the control group (average 4% increasing to 78% at day 13; Fig. 4A). In contrast, both of the L-asparaginase treated groups displayed very low %huCD45+ in PB at day 13. These data were further recapitulated when examining %huCD45+ in BM after the animals were sacrificed at day 13 (Fig. 4B), and in spleens at that time point (Fig. 4C). Likewise, analysis of the spleen weights showed enlarged spleens for the controls but normal sized spleens for the treated animals (Fig. 4D). These data demonstrate that both ErA-WT and ErA-TM carry strong pre-clinical in vivo activity, while bigger cohorts might be essential to demonstrate whether or not differences in efficacy between the enzymes are present. The seemingly moderate increase in potency of ErA-WT comes with increased toxicity, as indicated by the amount of weight lost by the animals. Indeed, recapitulating the weight loss trend we observed before (Fig. 2H), mice treated with ErA-WT lost nearly twice as much weight compared to ErA-TM (30% versus 15% of starting body weight, p-value <0.05). (Fig. 4E). As in the previous experiment with the LOUCY cell line, we could not detect differences in ALT or AST levels between ErA-WT- and ErA-TM-treated mice.
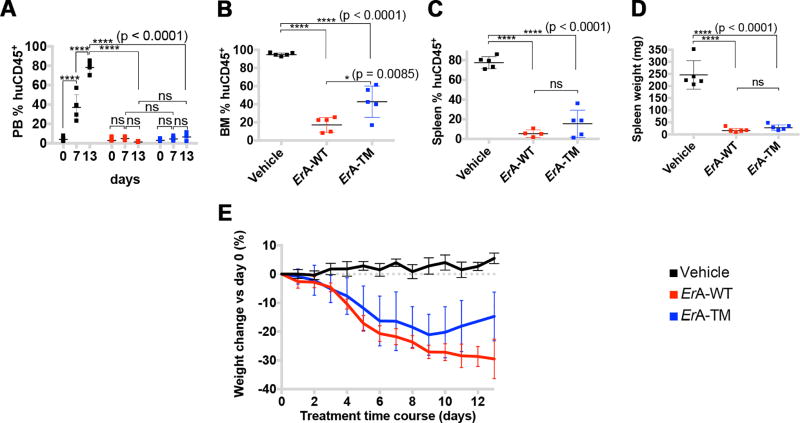
Fig. 4. In a T-ALL PDX model, ErA-TM displays similar cell killing combined with reduced toxicity compared to ErA-WT.
Female mice injected with primary T-ALL cells five weeks prior were treated daily with vehicle; ErA-WT or ErA-TM (n=5 for each group) for 13 days (drug dose 50 IU/mouse/day; i.p.). Cell debris and duplicates were gated out during flow cytometry data analysis. (A) PB %huCD45+ levels were determined once every week and at day 0, all animals were engrafted, as indicated by %huCD45+ ~4%. For the vehicle-treated mice, the %huCD45+ increased to ~40% by day 7 and ~80% by day 13, whereas for both treatment groups, the %huCD45+ stayed statistically indifferent to day 0 (p-value >0.2). No difference was detected between the two enzyme-treated groups at day 7 and day 13 (p-value >0.6). The %huCD45+ levels could not be analyzed for one mouse in the ErA-WT group and for one mouse treated with ErA-TM due to bad sample quality. (B) Similar to panel A, but for the BM at day 13. Whereas the BM of the vehicle group was full of cancer cells with %huCD45+ ~95%, the disease was largely controlled in the two enzyme-treated groups with %huCD45+ ~20 – 40%. (C) Similar to panel B, but for the spleen at day 13. The analysis of spleen sample of mouse 18 (ErA-WT-treated) was not included because of bad sample quality. Whereas the vehicle-treated group’s spleens were ~80% invaded with cancer cells, less than 20% of %huCD45+ was detected in the enzyme-treated groups. No significant difference was detected between the ErA-WT- and ErA-TM-treated groups (p-value ~0.2). (D) On sacrifice, spleens were harvested and weighed. Shown are the spleen weights for the 3 groups. Consistent with the cancer cell invasion data in panel C, the weight of spleens from the vehicle-treated group are significantly higher than the enzyme-treated groups, but no significant difference was detected between the two enzyme-treated groups (p-value >0.8). (E) Mice weight change, shown as % change relative to day 0, over the course of the 13-day treatment period is shown. On average, at day 13, ErA-WT- treated mice lost ~30% of body weight, whereas ErA-TM- treated mice lost ~15% (p-value <0.05). n.s. = not significant. In all panels, the color code depicting the vehicle-treated group, the ErA-WT- treated group and the ErA-TM- treated group is black, red and blue, respectively. Mean with SD were plotted.
Whereas treatment of mice engrafted with the T-ALL cell line LOUCY with ErA-WT or ErA-TM resulted in complete loss of the BLI signal and in undetectable %huCD45+ in the PB and BM (Fig. 2, A–D), treatment of mice engrafted with primary T-ALL cells resulted in lowered but still detectable leukemia burden (Fig. 4A–D). One possible explanation for the difference in efficacy between the human cell lines and the PDX could be the ASNS expression level. To probe this point, we used quantitative PCR to measure the ASNS mRNA levels in the LOUCY cell line, in the patient-derived cells used in the PDX experiment, and in 4 additional T-ALL patient samples. Indeed, the ASNS mRNA in the LOUCY cells is 20 to 30-fold lower than that measured in the patients’ sample (Fig. S9). However, we note that a growing body of evidence conclusively revealed no correlation between ASNS expression and sensitivity to L-asparaginase in patients (49–52). Hence, the genetic reason(s) that make ALL susceptible to L-asparaginase therapy is/are not fully understood. Based on the data presented here, it appears that patients with measurable ASNS levels will have similar responsiveness but better drug tolerance to the low L-glutaminase ErA-TM variant compared to the FDA-approved ErA-WT drug.
DISCUSSION
Identifying the L-asparaginase with the best clinical properties is a challenge. Scoring L-asparaginases based solely on cell culture data, where L-glutamine is indispensable, may be misleading, due to the significant L-glutaminase activity of these enzymes. Scoring L-asparaginases based on in vivo data is much more clinically relevant but also presents several challenges. The enzyme’s kinetic properties (kcat and Km values) for both Asn and Gln are important parameters to consider. Additionally, the drug’s persistence in circulation (i.e., half-life) will determine the duration of enzymatic Asn and Gln depletion, a parameter that can be fine-tuned by appropriate dose and frequency of drug administration. Ideally, one would like to combine a prolonged half-life as seen with the introduction of pegylated EcA, high L-asparaginase activity, and only low or absence of L-glutaminase activity, the latter predicted to be in part responsible for acute toxicities of the drug. Currently, a pegylated version of ErA-WT is being evaluated (53). While such a version is predicted to solve the problem of short in vivo persistence of native ErA, we caution that with the concomitant longer time for Asn depletion, such an enzyme will also have a longer duration of Gln depletion. Considering the very high L-glutaminase activity of ErA-WT, this would predict increased side effects.
Here we present ErA variants with significantly lower L-glutaminase but comparable L-asparaginase activities relative to ErA-WT and extended circulation time achieved by maintaining the SUMO tag. We demonstrated that our engineered low L-glutaminase Erwinia L-asparaginase variants have preserved cell killing properties, similar to ErA-WT. Comparable pharmacokinetic properties of the SUMO-tagged ErA-WT vs ErA-TM enzymes accompanied by similar Asn but remarkably different Gln depletion profiles convincingly discount the possibility that the observed anti-leukemic effect in ErA-TM was due to glutamine depletion. Moreover, the comparable serum persistence and anti-leukemic properties of the enzymes indicate that the significant difference in the toxicity profile is linked to the difference in impact on Gln levels, which suggests a superior tolerability for the low L-glutaminase variant ErA-TM.
Others have also recognized the potential advantages of L-asparaginases with low L-glutaminase activity. One in particular is the low L-glutaminase S121 variant of the Wolinella succinogenes L-asparaginase (WoA-S121) – for additional details about this enzyme see Supplementary material. The ratio of the L-asparaginase to L-glutaminase rates (measured at the physiological substrate concentrations of 50 µM for Asn and 500 µM for Gln) best conveys the clinically-relevant substrate specificity of these drugs (a high ratio describes a more specific L-asparaginase with low L-glutaminase). We recently investigated the properties of WoA-S121 and discovered that this ratio is 62 (27), which is actually inferior to that for EcA-WT (ratio = 188), but superior to ErA-WT (ratio = 6.6) – Table 1. However, this ratio for ErA-TM is ~9,000, showcasing the extremely high L-asparaginase preference of this variant.
In conclusion, this study convincingly shows that high L-glutaminase activity, as present in current FDA-approved L-asparaginase drugs, is not essential for efficient in vivo elimination of L-asparaginase sensitive ALL cells. Furthermore, our results suggest a decline in in vivo toxicity when the drug’s L-glutaminase activity is reduced. Since the debilitating side effects of current L-asparaginases often result in treatment cessation, an event associated with inferior event-free survival (48,54), L-asparaginases with diminished toxicity are highly pertinent to improving ALL treatment outcome. In line with this notion, a recent pilot study, (55) which evaluated an intensified L-asparaginase treatment in a population of high-risk pediatric ALL patients using pegylated EcA (the current standard of care in the USA with >20-fold higher L-glutaminase activity compared to ErA-TM), had to be aborted due to an unacceptable frequency of adverse-effects. However, since the patients were receiving additional chemotherapeutic drugs, the causative agent behind the increased toxicity cannot be directly linked to the more frequent dosing of the L-asparaginase. Notably, the ultra-low L-glutaminase ErA-TM variant, as presented in this study, now provides an alternative L-asparaginase that can directly probe this point.
Supplementary Material
Acknowledgments
We thank Dr. Michael Jensen (University of Washington School of Medicine) for the generous gift of the SUP-B15-luciferase cell line and Béatrice Lintermans for excellent technical assistance. The histology work was carried out by the UIC Research Resources Center’s Research Histology and Tissue Imaging Core.
Financial support
A. Lavie. Was supported in part by NIH grant RO1 EB013685 and by Merit Review Award I01BX001919 from the United States (U.S.) Department of Veterans Affairs Biomedical Laboratory Research and Development Service, and by the UIC Cancer Center. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
P. Van Vlierberghe was supported by the Fund for Scientific Research Flanders (FWO) grant 3G0C4713, by the Belgian Foundation Against Cancer grant 365W3415W and by Stand up to Cancer, the Flemish Cancer Society research grant 365Y9115W. S. Piers was support by doctoral and postdoc FWO grant FWO17/PDO/111.
Footnotes
Disclosure of Potential Conflicts of Interest
H.A. Nguyen, A.M. Schalk, Y. Su. and A. Lavie declare competing financial interest by being founders with equity stake in Enzyme by Design, Inc. a startup developing new L-asparaginases. Y. Saunthararajah is on the Scientific Advisory Board of Enzyme by Design, Inc. All other authors declare no competing financial interests.
References
- 1.Narta UK, Kanwar SS, Azmi W. Pharmacological and clinical evaluation of L-asparaginase in the treatment of leukemia. Critical reviews in oncology/hematology. 2007;61:208–21. doi: 10.1016/j.critrevonc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Panosyan EH, Grigoryan RS, Avramis IA, Seibel NL, Gaynon PS, Siegel SE, et al. Deamination of glutamine is a prerequisite for optimal asparagine deamination by asparaginases in vivo (CCG-1961) Anticancer research. 2004;24:1121–5. [PubMed] [Google Scholar]
- 3.Ollenschlager G, Roth E, Linkesch W, Jansen S, Simmel A, Modder B. Asparaginase-induced derangements of glutamine metabolism: the pathogenetic basis for some drug-related side-effects. European journal of clinical investigation. 1988;18:512–6. doi: 10.1111/j.1365-2362.1988.tb01049.x. [DOI] [PubMed] [Google Scholar]
- 4.Grigoryan RS, Panosyan EH, Seibel NL, Gaynon PS, Avramis IA, Avramis VI. Changes of amino acid serum levels in pediatric patients with higher-risk acute lymphoblastic leukemia (CCG-1961) In vivo. 2004;18:107–12. [PubMed] [Google Scholar]
- 5.Hawkins DS, Park JR, Thomson BG, Felgenhauer JL, Holcenberg JS, Panosyan EH, et al. Asparaginase pharmacokinetics after intensive polyethylene glycol-conjugated L-asparaginase therapy for children with relapsed acute lymphoblastic leukemia. Clin Cancer Res. 2004;10:5335–41. doi: 10.1158/1078-0432.CCR-04-0222. [DOI] [PubMed] [Google Scholar]
- 6.Broome JD. Studies on the mechanism of tumor inhibition by L-asparaginase. Effects of the enzyme on asparagine levels in the blood, normal tissues, and 6C3HED lymphomas of mice: differences in asparagine formation and utilization in asparaginase-sensitive and -resistant lymphoma cells. The Journal of experimental medicine. 1968;127:1055–72. doi: 10.1084/jem.127.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prager MD, Bachynsky N. Asparagine synthetase in normal and malignant tissues: correlation with tumor sensitivity to asparaginase. Archives of biochemistry and biophysics. 1968;127:645–54. doi: 10.1016/0003-9861(68)90273-7. [DOI] [PubMed] [Google Scholar]
- 8.Prager MD, Bachynsky N. Asparagine synthetase in asparaginase resistant and susceptible mouse lymphomas. Biochemical and biophysical research communications. 1968;31:43–7. doi: 10.1016/0006-291x(68)90028-4. [DOI] [PubMed] [Google Scholar]
- 9.Chan WK, Lorenzi PL, Anishkin A, Purwaha P, Rogers DM, Sukharev S, et al. The glutaminase activity of L-asparaginase is not required for anticancer activity against ASNS-negative cells. Blood. 2014;123:3596–606. doi: 10.1182/blood-2013-10-535112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parmentier JH, Maggi M, Tarasco E, Scotti C, Avramis VI, Mittelman SD. Glutaminase activity determines cytotoxicity of L-asparaginases on most leukemia cell lines. Leukemia research. 2015;39:757–62. doi: 10.1016/j.leukres.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Offman MN, Krol M, Patel N, Krishnan S, Liu J, Saha V, et al. Rational engineering of L-asparaginase reveals importance of dual activity for cancer cell toxicity. Blood. 2011;117:1614–21. doi: 10.1182/blood-2010-07-298422. [DOI] [PubMed] [Google Scholar]
- 12.Aldoss I, Douer D, Behrendt CE, Chaudhary P, Mohrbacher A, Vrona J, et al. Toxicity profile of repeated doses of PEG-asparaginase incorporated into a pediatric-type regimen for adult acute lymphoblastic leukemia. European journal of haematology. 2015 doi: 10.1111/ejh.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hijiya N, van der Sluis IM. Asparaginase-associated toxicity in children with acute lymphoblastic leukemia. Leukemia & lymphoma. 2016;57:748–57. doi: 10.3109/10428194.2015.1101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tong WH, Pieters R, de Groot-Kruseman HA, Hop WC, Boos J, Tissing WJ, et al. The toxicity of very prolonged courses of PEGasparaginase or Erwinia asparaginase in relation to asparaginase activity, with a special focus on dyslipidemia. Haematologica. 2014;99:1716–21. doi: 10.3324/haematol.2014.109413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avramis VI, Sencer S, Periclou AP, Sather H, Bostrom BC, Cohen LJ, et al. A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: a Children's Cancer Group study. Blood. 2002;99:1986–94. doi: 10.1182/blood.v99.6.1986. [DOI] [PubMed] [Google Scholar]
- 16.Heitink-Polle KM, Prinsen BH, de Koning TJ, van Hasselt PM, Bierings MB. High incidence of symptomatic hyperammonemia in children with acute lymphoblastic leukemia receiving pegylated asparaginase. JIMD reports. 2013;7:103–8. doi: 10.1007/8904_2012_156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durden DL, Distasio JA. Characterization of the effects of asparaginase from Escherichia coli and a glutaminase-free asparaginase from Vibrio succinogenes on specific ell-mediated cytotoxicity. International journal of cancer Journal international du cancer. 1981;27:59–65. doi: 10.1002/ijc.2910270110. [DOI] [PubMed] [Google Scholar]
- 18.Kafkewitz D, Bendich A. Enzyme-induced asparagine and glutamine depletion and immune system function. Am J Clin Nutr. 1983;37:1025–30. doi: 10.1093/ajcn/37.6.1025. [DOI] [PubMed] [Google Scholar]
- 19.Durden DL, Salazar AM, Distasio JA. Kinetic analysis of hepatotoxicity associated with antineoplastic asparaginases. Cancer Res. 1983;43:1602–5. [PubMed] [Google Scholar]
- 20.Reinert RB, Oberle LM, Wek SA, Bunpo P, Wang XP, Mileva I, et al. Role of glutamine depletion in directing tissue-specific nutrient stress responses to L-asparaginase. The Journal of biological chemistry. 2006;281:31222–33. doi: 10.1074/jbc.M604511200. [DOI] [PubMed] [Google Scholar]
- 21.Leonard JV, Kay JD. Acute encephalopathy and hyperammonaemia complicating treatment of acute lymphoblastic leukaemia with asparaginase. Lancet. 1986;1:162–3. doi: 10.1016/s0140-6736(86)92304-4. [DOI] [PubMed] [Google Scholar]
- 22.Alvarez OA, Zimmerman G. Pegaspargase-induced pancreatitis. Medical and pediatric oncology. 2000;34:200–5. doi: 10.1002/(sici)1096-911x(200003)34:3<200::aid-mpo7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 23.Laterza OF, Gerhardt G, Sokoll LJ. Measurement of plasma ammonia is affected in patients receiving asparaginase therapy. Clinical chemistry. 2003;49:1710–1. doi: 10.1373/49.10.1710. [DOI] [PubMed] [Google Scholar]
- 24.Jorck C, Kiess W, Weigel JF, Mutze U, Bierbach U, Beblo S. Transient hyperammonemia due to L-asparaginase therapy in children with acute lymphoblastic leukemia or non-Hodgkin lymphoma. Pediatric hematology and oncology. 2011;28:3–9. doi: 10.3109/08880018.2010.484852. [DOI] [PubMed] [Google Scholar]
- 25.Nussbaum V, Lubcke N, Findlay R. Hyperammonemia secondary to asparaginase: A case series. Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners. 2016;22:161–4. doi: 10.1177/1078155214551590. [DOI] [PubMed] [Google Scholar]
- 26.Warrell RP, Jr, Arlin ZA, Gee TS, Chou TC, Roberts J, Young CW. Clinical evaluation of succinylated Acinetobacter glutaminase-asparaginase in adult leukemia. Cancer Treat Rep. 1982;66:1479–85. [PubMed] [Google Scholar]
- 27.Nguyen HA, Durden DL, Lavie A. The differential ability of asparagine and glutamine in promoting the closed/active enzyme conformation rationalizes the Wolinella succinogenes L-asparaginase substrate specificity. Scientific reports. 2017;7:41643. doi: 10.1038/srep41643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hays JL, Kim G, Walker A, Annunziata CM, Lee JM, Squires J, et al. A phase II clinical trial of polyethylene glycol-conjugated L-asparaginase in patients with advanced ovarian cancer: Early closure for safety. Molecular and clinical oncology. 2013;1:565–9. doi: 10.3892/mco.2013.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorenzi PL, Horvath TD, Martin LA, Chan WK, Du D, Hawke DH, et al. Red Blood Cell-Encapsulation of L-Asparaginase Favorably Modulates Target Selectivity and Pharmacodynamics. American Society of Hematology 58th Annual Meeting; San Diego, CA. 2016. p https://ash.confex.com/ash/2016/webprogram/Paper97607.html. [Google Scholar]
- 30.Nguyen HA, Su Y, Lavie A. Design and Characterization of Erwinia Chrysanthemi l-Asparaginase Variants with Diminished l-Glutaminase Activity. The Journal of biological chemistry. 2016;291:17664–76. doi: 10.1074/jbc.M116.728485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen HA, Su Y, Lavie A. Structural Insight into Substrate Selectivity of Erwinia chrysanthemi l-Asparaginase. Biochemistry. 2016;55:1246–53. doi: 10.1021/acs.biochem.5b01351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schalk AM, Nguyen HA, Rigouin C, Lavie A. Identification and structural analysis of an L-asparaginase enzyme from guinea pig with putative tumor cell killing properties. The Journal of biological chemistry. 2014;289:33175–86. doi: 10.1074/jbc.M114.609552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandez CA, Cai X, Elozory A, Liu C, Panetta JC, Jeha S, et al. High-throughput asparaginase activity assay in serum of children with leukemia. International journal of clinical and experimental medicine. 2013;6:478–87. [PMC free article] [PubMed] [Google Scholar]
- 34.Ben-Bassat H, Shlomai Z, Kohn G, Prokocimer M. Establishment of a human T-acute lymphoblastic leukemia cell line with a (16;20) chromosome translocation. Cancer genetics and cytogenetics. 1990;49:241–8. doi: 10.1016/0165-4608(90)90148-4. [DOI] [PubMed] [Google Scholar]
- 35.Peirs S, Matthijssens F, Goossens S, Van de Walle I, Ruggero K, de Bock CE, et al. ABT-199 mediated inhibition of BCL-2 as a novel therapeutic strategy in T-cell acute lymphoblastic leukemia. Blood. 2014;124:3738–47. doi: 10.1182/blood-2014-05-574566. [DOI] [PubMed] [Google Scholar]
- 36.Fainstein E, Marcelle C, Rosner A, Canaani E, Gale RP, Dreazen O, et al. A new fused transcript in Philadelphia chromosome positive acute lymphocytic leukaemia. Nature. 1987;330:386–8. doi: 10.1038/330386a0. [DOI] [PubMed] [Google Scholar]
- 37.Szymanska B, Wilczynska-Kalak U, Kang MH, Liem NL, Carol H, Boehm I, et al. Pharmacokinetic modeling of an induction regimen for in vivo combined testing of novel drugs against pediatric acute lymphoblastic leukemia xenografts. PloS one. 2012;7:e33894. doi: 10.1371/journal.pone.0033894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan R, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur G, et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer discovery. 2014;4:362–75. doi: 10.1158/2159-8290.CD-13-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asselin BL, Whitin JC, Coppola DJ, Rupp IP, Sallan SE, Cohen HJ. Comparative pharmacokinetic studies of three asparaginase preparations. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1993;11:1780–6. doi: 10.1200/JCO.1993.11.9.1780. [DOI] [PubMed] [Google Scholar]
- 40.Reiff A, Zastrow M, Sun BC, Takei S, Mitsuhada H, Bernstein B, et al. Treatment of collagen induced arthritis in DBA/1 mice with L-asparaginase. Clinical and experimental rheumatology. 2001;19:639–46. [PubMed] [Google Scholar]
- 41.Viau AT, Abuchowski A, McCoy JR, Kazo GM, Davis FF. Toxicologic studies of a conjugate of asparaginase and polyethylene glycol in mice, rats, and dogs. American journal of veterinary research. 1986;47:1398–401. [PubMed] [Google Scholar]
- 42.Erwinase (L-asparaginase) Pharamcology Review. FDA Center for drug evaluation and research. 2011:1–44. [Google Scholar]
- 43.Bodmer M, Sulz M, Stadlmann S, Droll A, Terracciano L, Krahenbuhl S. Fatal liver failure in an adult patient with acute lymphoblastic leukemia following treatment with L-asparaginase. Digestion. 2006;74:28–32. doi: 10.1159/000095827. [DOI] [PubMed] [Google Scholar]
- 44.Bessho F, Kinumaki H, Yokota S, Hayashi Y, Kobayashi M, Kamoshita S. Liver function studies in children with acute lymphocytic leukemia after cessation of therapy. Medical and pediatric oncology. 1994;23:111–5. doi: 10.1002/mpo.2950230208. [DOI] [PubMed] [Google Scholar]
- 45.Cairo MS. Adverse reactions of L-asparaginase. The American journal of pediatric hematology/oncology. 1982;4:335–9. [PubMed] [Google Scholar]
- 46.Roesmann A, Afify M, Panse J, Eisert A, Steitz J, Tolba RH. L-carnitine ameliorates L-asparaginase-induced acute liver toxicity in steatotic rat livers. Chemotherapy. 2013;59:167–75. doi: 10.1159/000353402. [DOI] [PubMed] [Google Scholar]
- 47.Christ TN, Stock W, Knoebel RW. Incidence of asparaginase-related hepatotoxicity, pancreatitis, and thrombotic events in adults with acute lymphoblastic leukemia treated with a pediatric-inspired regimen. Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners. 2017 doi: 10.1177/1078155217701291. 1078155217701291. [DOI] [PubMed] [Google Scholar]
- 48.Stock W, Douer D, DeAngelo DJ, Arellano M, Advani A, Damon L, et al. Prevention and management of asparaginase/pegasparaginase-associated toxicities in adults and older adolescents: recommendations of an expert panel. Leukemia & lymphoma. 2011;52:2237–53. doi: 10.3109/10428194.2011.596963. [DOI] [PubMed] [Google Scholar]
- 49.Chen SH, Yang W, Fan Y, Stocco G, Crews KR, Yang JJ, et al. A genome-wide approach identifies that the aspartate metabolism pathway contributes to asparaginase sensitivity. Leukemia. 2011;25:66–74. doi: 10.1038/leu.2010.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fine BM, Kaspers GJ, Ho M, Loonen AH, Boxer LM. A genome-wide view of the in vitro response to l-asparaginase in acute lymphoblastic leukemia. Cancer Res. 2005;65:291–9. [PubMed] [Google Scholar]
- 51.Hermanova I, Zaliova M, Trka J, Starkova J. Low expression of asparagine synthetase in lymphoid blasts precludes its role in sensitivity to L-asparaginase. Experimental hematology. 2012;40:657–65. doi: 10.1016/j.exphem.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 52.Stams WA, den Boer ML, Beverloo HB, Meijerink JP, Stigter RL, van Wering ER, et al. Sensitivity to L-asparaginase is not associated with expression levels of asparagine synthetase in t(12;21)+ pediatric ALL. Blood. 2003;101:2743–7. doi: 10.1182/blood-2002-08-2446. [DOI] [PubMed] [Google Scholar]
- 53.Chien WW, Allas S, Rachinel N, Sahakian P, Julien M, Le Beux C, et al. Pharmacology, immunogenicity, and efficacy of a novel pegylated recombinant Erwinia chrysanthemi-derived L-asparaginase. Investigational new drugs. 2014;32:795–805. doi: 10.1007/s10637-014-0102-9. [DOI] [PubMed] [Google Scholar]
- 54.Silverman LB, Gelber RD, Dalton VK, Asselin BL, Barr RD, Clavell LA, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001;97:1211–8. doi: 10.1182/blood.v97.5.1211. [DOI] [PubMed] [Google Scholar]
- 55.Rodriguez V, Kairalla J, Salzer WL, Raetz EA, Loh ML, Carroll AJ, et al. A Pilot Study of Intensified PEG-Asparaginase in High-risk Acute Lymphoblastic Leukemia: Children's Oncology Group Study AALL08P1. Journal of pediatric hematology/oncology. 2016;38:409–17. doi: 10.1097/MPH.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.