Abstract
Background
The kinetics of the antibody response during severe influenza are not well documented.
Methods
Critically ill patients infected with 2009 pandemic influenza A(H1N1) virus (A[H1N1]pdm09), confirmed by reverse-transcription polymerase chain reaction analysis or seroconversion (defined as a ≥4-fold rise in titers), during 2009–2011 in Canada were prospectively studied. Antibody titers in serially collected sera were determined using hemagglutinin inhibition (HAI) and microneutralization assays. Average antibody curves were estimated using linear mixed-effects models and compared by patient outcome, age, and corticosteroid treatment.
Results
Of 47 patients with A(H1N1)pdm09 virus infection (median age, 47 years), 59% had baseline HAI titers of <40, and 68% had baseline neutralizing titers of <40. Antibody titers rose quickly after symptom onset, and, by day 14, 83% of patients had HAI titers of ≥40, and 80% had neutralizing titers ≥40. Baseline HAI titers were significantly higher in patients who died compared with patients who survived; however, the antibody kinetics were similar by patient outcome and corticosteroid treatment. Geometric mean titers over time in older patients were lower than those in younger patients.
Conclusions
Critically ill patients with influenza A(H1N1)pdm09 virus infection had strong HAI and neutralizing antibody responses during their illness. Antibody kinetics differed by age but were not associated with patient outcome.
Keywords: Influenza, critical illness, humoral immunity
Certain individuals, including those at the extremes of age or with underlying medical conditions, are at high risk of developing severe illness from seasonal influenza virus infection. It is unknown whether delayed or deficient antibody responses in individuals contribute to their risk of severe illness and death. Studies have suggested that convalescent plasma may be useful to treat severe influenza, providing some evidence that the humoral immune response may be associated with recovery [1, 2].
Antibodies against influenza viruses can block viral entry, neutralize virus, inhibit viral spread, and assist in cell-mediated viral clearance. Antibodies against the hemagglutinin protein of influenza viruses correlate with protection against influenza virus infection [3, 4], and a hemagglutinin inhibition (HAI) antibody titer of 40 has been shown to correlate with a 50% reduction in the risk of seasonal influenza virus infection in adults [4–6]. Most studies investigating the antibody response during influenza virus infection have focused on 2 time points of sera collection relative to symptom onset, but more specimen collection time points are needed to fully understand the kinetics of the antibody response during severe influenza and the impact of antibody titers on outcomes of infection. The few studies that have assessed antibodies from serial blood specimen collections suggest that low antibody titers early after influenza virus infection and slow increases in titers are predictive of death from fulminant illness [7, 8]. However, these studies are limited by their small sample size and unique clinical setting. Identification of immunological markers that can predict outcomes early after illness onset could be beneficial in influenza clinical management, but the strength of the evidence for using HAI or neutralizing antibody titers as markers of clinical severity is currently weak.
In this study, we analyzed the kinetics of the antibody responses in critically ill patients admitted to intensive care units (ICUs) with 2009 pandemic influenza A(H1N1) virus (A[H1N1]pdm09) infection during the 2009 pandemic and the 2010–2011 influenza season in Canada [9]. We further aimed to examine the association of antibody kinetics and clinical outcomes, patient age, and treatment with systemic corticosteroids.
METHODS
Patient Recruitment, Enrollment, and Data Collection
During the 2009 pandemic, Canadian ICU physicians designed and established a multicenter cohort of critically ill adolescent and adult patients hospitalized with confirmed, probable, or suspected influenza virus infection [9]. Patients were recruited into this cohort from multiple sites throughout Canada (see Acknowledgments) between April 2009 and April 2011 from both an observational study and an accompanying randomized trial of the effect of high-dose oseltamivir (225 mg twice daily) versus standard-dose oseltamivir (75 mg twice daily) on influenza viral clearance from the respiratory tract (clinical trials registration NCT01010087). Analyses from the current study were blinded to the treatment arm of the patients from the randomized trial.
Patients were enrolled into the observational cohort from among all patients admitted to the adult ICU with suspected or confirmed influenza. Nonpregnant individuals aged ≥12 years who were hospitalized with suspected or confirmed influenza and required ICU admission were eligible for the clinical trial. Regardless of study, patients provided informed consent for specimen collection and storing blood specimens for future analysis. Blood sample collection occurred on days 1, 2, 3, 5, 7, 10, 14, 21, and 28 of hospitalization and at ICU discharge if the patient was able to provide blood specimens. Patients could refuse specimen collection at any time.
The clinical teams collected information on baseline demographic and clinical features, date of symptom onset, use and dates of clinical interventions (including mechanical and pharmaceutical interventions), and dates of patient disposition, including discharge from the ICU, hospital discharge, or death, as described in the clinical trial protocol [9].
Ethical Approvals
Ethical approval to conduct the clinical trial was provided by each of the participating institutions. The use of sera for further testing was approved by the Centers for Disease Control and Prevention (CDC).
Laboratory Methods
Blood samples were collected and transported to a central processing laboratory in Canada, where they were centrifuged and serum was removed and frozen at −80°C prior to shipment to CDC.
HAI assays were performed at CDC as previously described, using 0.5% turkey erythrocytes [10]. Serum samples were treated with receptor-destroying enzyme to remove nonspecific inhibitors. Nonspecific agglutinins were removed by serum adsorption with packed turkey erythrocytes. Serial 2-fold dilutions of sera were made from an initial 1:10 dilution. The HAI antibody titer was defined as the reciprocal of the last dilution of serum that completely inhibited hemagglutination, with titers of <10 assigned a value of 5. Final titers were reported as the geometric mean of all replicates.
For microneutralization assays, serum samples were first inactivated by heating, and then serial 2-fold dilutions were made starting at an initial 1:10 dilution. Influenza virus (100 50% tissue culture infective doses) was added to serum dilutions, incubated at 37°C with 5% CO2 for 1 hour, and used to infect 1.5 × 104 Madin-Darby canine kidney cells per well. After over-night incubation, viral infection was quantified by an enzyme-linked immunosorbent assay, using monoclonal antibodies specific to the influenza A virus nucleoprotein. Neutralizing antibody titers were defined as the reciprocal of the highest dilution of serum that yielded at least 50% neutralization. Final titers were reported as described for the HAI assay. Egg-propagated wild-type A/Mexico/4108/2009 A(H1N1)pdm09 virus was used in the HAI and microneutralization assays.
Seroconversion was defined as a ≥4-fold rise in HAI or neutralizing antibody titers between any 2 serum specimens, where the second specimen had a titer of ≥40. Influenza A(H1N1) pdm09 virus infection was confirmed by detection of viral RNA in respiratory tract specimens, using reverse-transcription polymerase chain reaction (RT-PCR) analysis, according to local protocols for clinical diagnosis, or evidence of seroconversion by either HAI or neutralizing antibodies. A seropositive threshold was defined as an HAI or neutralizing antibody titer of 40.
Statistical Analysis
For analysis, corticosteroid doses were converted to prednisone-equivalent dosing by using an online calculator [11]. Prednisone-equivalent dosing of ≤50 mg/day was considered low-dose and >50 mg/day considered high-dose corticosteroid treatment.
Serum samples were considered to have been collected during the acute phase of infection if collected ≤10 days after illness onset. Geometric mean antibody titers (GMTs) were estimated for patients with blood specimens collected during the intervals of 0–4, 5–10, 11–14, 15–19, 20–29, 30–39, and ≥40 days after symptom onset. GMTs and 95% confidence intervals were plotted by interval and compared by patient age, receipt of corticosteroids (either none, low dose, or high dose), and patient outcome (survival or death).
Average antibody curves for log2-transformed HAI and neutralizing titers were estimated using linear mixed-effects models among survivors from whom the first blood specimen was collected in the acute phase and at least 3 blood specimens were collected during hospitalization. This subset included 25 patients with influenza A(H1N1)pdm09 virus infection confirmed by RT-PCR or serological analysis. The linear mixed-effects model included time as a linear, squared, and cubed term, with random error terms for the intercept and a linear term for each patient. This cubic model for antibody titers over time was selected after initial inspection of the data and with the understanding that antibody levels generally follow a cubic function after illness onset, with an initial rise to a plateau. Differences were visually assessed by overlaying observed patient data on the average curve, estimated from the linear mixed-effects model. Antibody curves were compared between patients who survived and died, by patient age, and by treatment with corticosteroids. The small sample size of the cohort precluded formal statistical comparison of antibody curves by patient outcome, age, and corticosteroid therapy.
Two-sided Wilcoxon rank sum tests were used to statistically compare median antibody titers by patient outcome, age, and corticosteroid therapy, and the Fisher exact test was used to compare frequencies. Data analyses were conducted using SAS, version 9.4. P values of <.05 were considered statistically significant.
RESULTS
Eighty-three hospitalized adolescents and adults in Canada with acute respiratory illness and suspected influenza were enrolled in either the clinical trial or observational cohort during April 2009–May 2010 and during October 2010–April 2011.
Of the 83 patients, 40 had influenza A(H1N1)pdm09 virus infection confirmed by RT-PCR analysis, of whom 25 (63%) also had evidence of seroconversion by the HAI or neutralizing assays. In addition, 7 patients with respiratory specimens that tested negative by RT-PCR analysis for influenza A(H1N1) pdm09 virus had evidence of seroconversion by either HAI or neutralizing assays. Therefore, 47 patients had laboratory-confirmed influenza A(H1N1)pdm09 virus infection, based on RT-PCR or serologic evidence of infection.
The median age of patients with laboratory-confirmed influenza A(H1N1)pdm09 virus infection was 47 years, 17% were aged ≥65 years, 34% were male, and 85% had at least 1 characteristic that put them at high risk for influenza-associated complications (Table 1). Four patients reported that they had been vaccinated against influenza within the last year; 3 of these patients were likely vaccinated with the 2008–2009 seasonal influenza vaccine, which did not include an influenza A(H1N1) pdm09–like virus antigen, and 1 patient was likely vaccinated with the 2010–2011 seasonal vaccine, which contained an influenza A(H1N1)pdm09–like virus antigen. Patients were hospitalized a median of 5 days after illness onset, and all were admitted to an ICU within 2 days of hospital admission (Table 1). Ninety-one percent of patients required invasive mechanical ventilation, and 1 patient underwent extracorporeal membrane oxygenation. Although data were blinded to the dosage given to individual patients, all patients received oseltamivir, and 9 patients received high-dose oseltamivir (225 mg twice daily). In addition, 52% (25 of 47) received corticosteroid therapy during their illness, of whom 60% (15 of 25) received high-dose corticosteroids (>50 mg prednisone-equivalent dose). The median time from illness onset to the start of any corticosteroid therapy was 5 days (interquartile range [IQR], 2–11 days). High-dose corticosteroid therapy was also started a median of 5 days (IQR, 2–9 days) after illness onset. The median time of high-dose corticosteroid therapy was 5 days (IQR, 3–8 days).
Table 1.
Demographic and Clinical Characteristics of 47 Critically Ill Adolescents and Adults Hospitalized With 2009 Pandemic Influenza A(H1N1) Virus Infection, by Patient Outcome—Canada, 2009–2011
| Variable | Total | Survived | Died |
|---|---|---|---|
| Patients | 47 (100) | 39 (83) | 8 (17) |
| Demographic characteristics | |||
| Influenza season of illness | |||
| Pandemic spring wave (Apr–Jul 2009) | 12 (26) | 10 (26) | 2 (25) |
| Pandemic fall wave (Aug 2009–May 2010) | 29 (62) | 23 (59) | 6 (75) |
| Oct 2010–Apr 2011 | 6 (13) | 6 (15) | 0 (0) |
| Age, y | 47 (17–80) | 48 (17–80) | 56 (17–74) |
| <50 | 26 (55) | 22 (56) | 4 (50) |
| 50–64 | 13 (28) | 13 (33) | 0 (0) |
| ≥65 | 8 (17) | 4 (10) | 4 (50) |
| Sex | |||
| Male | 16 (34) | 14 (36) | 2 (25) |
| Female | 31 (66) | 25 (64) | 6 (75) |
| Race | |||
| White | 29 (62) | 25 (64) | 4 (50) |
| Asian descent | 3 (6) | 2 (5) | 1 (13) |
| First Nations | 14 (30) | 11 (28) | 3 (38) |
| Other/unknown | 1 (2) | 1 (3) | 0 (0) |
| Received influenza vaccine | 4 (9) | 2 (5) | 2 (25) |
| High-risk conditions | |||
| Age ≥65 y | 8 (17) | 4 (10) | 4 (50) |
| Chronic lung condition | 19 (40) | 17 (44) | 2 (25) |
| Cardiovascular condition | 16 (34) | 11 (28) | 5 (63) |
| Renal condition | 9 (19) | 6 (15) | 3 (38) |
| Hepatic condition | 1 (2) | 1 (3) | 0 (0) |
| Immunosuppression | 9 (19) | 9 (23) | 0 (0) |
| Diabetes mellitus | 9 (19) | 6 (15) | 3 (38) |
| First Nations | 14 (30) | 11 (28) | 3 (38) |
| Obesity (BMI ≥40a) | 11 (23) | 10 (26) | 1 (13) |
| Pregnant | 3 (6) | 2 (5) | 1 (13) |
| Other | 7 (15) | 6 (15) | 1 (13) |
| Any of the above | 40 (85) | 31 (79) | 7 (88) |
| Clinical characteristics | |||
| Invasive mechanical ventilation use | 39 (91) | 32 (89) | 7 (100) |
| ECMO use | 1/39 (3) | 0/32 (0) | 1/7 (14) |
| Received influenza antiviral therapy | 47 (100) | 39 (100) | 8 (100) |
| Received corticosteroid therapy | |||
| Overall | 25 (53) | 21 (54) | 4 (50) |
| Low dose | |||
| Received (≤50 mg/d, prednisone equivalent) | 14/25 (56) | 13/21 (62) | 1/4 (25) |
| Duration, d | 5 (3–7) | 4 (3–6) | 8 (NA) |
| High dose | |||
| Received (>50 mg/d, prednisone equivalent) | 15/25 (60) | 13/21 (62) | 2/4 (50) |
| Duration, d | 5 (3–8) | 5 (3–8) | 9 (7–11) |
| Pneumonia | 13/28 (46) | 11/24 (46) | 2/4 (50) |
| Sepsis | 8 (17) | 7 (18) | 1 (13) |
| Shock | 19 (40) | 15 (38) | 4 (50) |
| Septic | 8 (42) | 7 (47) | 1 (25) |
| Nonseptic | 11 (58) | 8 (53) | 3 (75) |
| Clinical event duration, d | |||
| From symptom onset to hospitalization | 5 (3–8) | 5 (3–8) | 6 (2–8) |
| From hospitalization to ventilation | 1 (1–2) | 1 (1–2) | 3 (1–4) |
| From hospitalization to ICU admission | 1 (1–2) | 1 (1–2) | 2 (1–4) |
| From symptom onset to discharge or death | 28 (18–45) | 31 (18–52) | 24 (16–31) |
| ICU stayb | 14 (9–26) | 14 (9–26) | 17 (12–26) |
| Hospital stay | 21 (15–31) | 24 (16–51) | 17 (15–25) |
Data are no. or proportion (%) of patients or median value (interquartile range).
Abbreviations: ECMO, extracorporal membrane oxygenation; ICU, intensive care unit; NA, not available.
Body mass index (BMI) is calculated as the weight in kilograms divided by the height in meters squared.
One patient, a survivor, did not have a known date of ICU discharge.
The median time from onset of symptoms to collection of the first blood specimen was 7 days (IQR, 5–10 days), and a median of 5 blood samples (IQR, 4–7 samples) were collected from each patient during their illness. Of 37 patients with an acute-phase blood specimen collected (collected ≤10 days from illness onset), 62% had baseline HAI titers and 68% had baseline neutralizing titers of <40. Based on the average antibody curve, estimated among survivors with an acute-phase blood specimen collected and at least 3 blood specimens collected, both HAI and neutralizing titers rose quickly over the first 10 days after symptom onset and then began to plateau (Figure 1A and 1B). By day 7 after illness onset, 46% of patients (11 of 24 with blood samples collected) had an HAI titer of ≥40, and 42% (10 of 24) had neutralizing titers of ≥40. By day 14 after illness onset, 83% (34 of 41 with blood samples collected) and 80% (33 of 41 with blood samples collected) had HAI and neutralizing titers of ≥40, respectively.
Figure 1.
A, Hemagglutinin inhibition (HAI) antibody titers and B, neutralizing antibody titers over time for critically ill adolescents and adults hospitalized with influenza A(H1N1)pdm09 virus infection — Canada, 2009–2011. Average curve was estimated among 25 survivors with ≥3 blood draws and at least one acute phase draw, collected ≤10 days after illness onset, using an unadjusted cubic linear mixed model. Individual lines are shown for all 47 enrolled patients with laboratory-confirmed influenza A(H1N1) pdm09 virus infection.
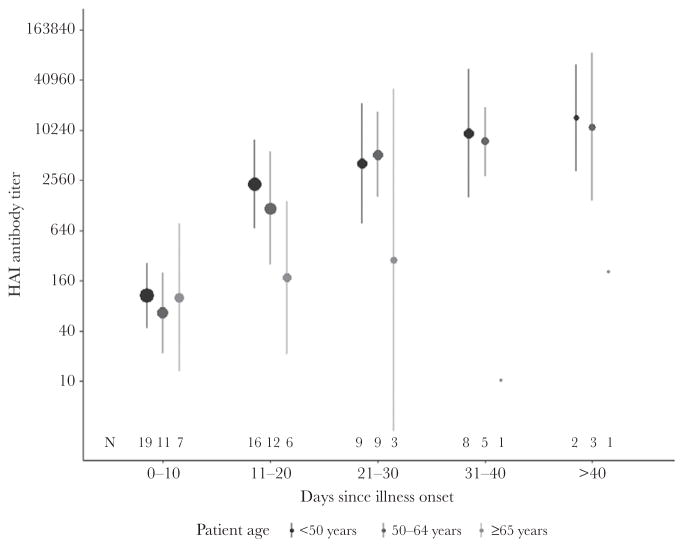
Time to collection of the acute-phase blood specimen was similar by patient age (Table 2). The frequency of seroconversion, by either HAI or microneutralization assay, was slightly lower (63%) among patients aged ≥65 years, compared with 68% of patients aged <50 years and 92% of patients aged 50–64 years, although this was not statistically significant (P = .24). The geometric mean HAI antibody titers in patients aged ≥65 years did not rise as high as in younger patients; however, the differences in GMTs by age group were not statistically significant (Figure 2).
Table 2.
Timing of First Blood Specimen Collection and Hemagglutinin Inhibition (HAI) and Neutralizing Antibody (Ab) Titers Among Critically Ill Adolescents and Adults Hospitalized With 2009 Pandemic Influenza A(H1N1) Virus Infection—Canada, 2009–2011
| Characteristic | Patients, No. | Time From Symptom Onset to Baseline Blood Specimen Collection, d
|
Baseline Acute-Phase HAI Ab Titera
|
Baseline Acute-Phase Neutralizing Ab Titera
|
|||
|---|---|---|---|---|---|---|---|
| Median (IQR) | Pb | Median (IQR) | Pb | Median (IQR) | Pb | ||
| Overall | 47 | 7 (5–10) | 20 (5–80) | 7 (5–40) | |||
|
| |||||||
| Age, y | .42 | .75 | .90 | ||||
|
| |||||||
| <50 | 26 | 8 (6–12) | 28 (5–80) | 5 (5–40) | |||
|
| |||||||
| 50–64 | 13 | 7 (5–9) | 14 (5–57) | 20 (3–48) | |||
|
| |||||||
| ≥65 | 8 | 5 (3–9) | 20 (5–160) | 5 (5–226) | |||
|
| |||||||
| Outcome | .83 | .027 | .59 | ||||
|
| |||||||
| Survived | 39 | 7 (5–10) | 12 (5–57) | 6 (5–40) | |||
|
| |||||||
| Died | 8 | 8 (5–9) | 63 (20–226) | 20 (5–160) | |||
Abbreviation: IQR, interquartile range.
Overall, 37 patients had a first, baseline blood specimen collected in the acute phase, ≤10 days after symptom onset. Nineteen patients aged <50 years, 11 patients aged 50–64 years, and 7 patients aged ≥65 years had a baseline blood specimen collected in the acute phase. Seven patients who died and 30 patients who survived had a baseline blood specimen collected in the acute phase.
By the 2-sided Kruskal-Wallis test, for differences by patient age, and the Wilcoxon rank sum test, for differences by patient outcome.
Figure 2.
Geometric mean hemagglutinin inhibition (HAI) antibody titers over time for 26 adolescents and adults aged <50 years, 13 adults aged 50–64 years, and 9 adults aged ≥65 years hospitalized with critical illness due to influenza A(H1N1)pdm09 virus infection — Canada, 2009–2011. Size of dot is proportionate to the number patients with a blood draw in the time interval. Wilcoxon rank sum test comparing the median antibody titer between patients aged <50 years and patients aged ≥65 years: P = .64 at 0–10 days, P = .09 at 11–20 days, P = .29 at 21–30 days, P = .21 at 31–40 days, and P = .60 at >40 days from illness onset.
Of the 20 patients who received any corticosteroid treatment, 5 received low-dose corticosteroid therapy, and 15 received high-dose corticosteroid therapy. Among those receiving low-dose therapy, 60% (3 of 5 with ≥2 blood specimens collected) experienced seroconversion by either HAI or neutralizing antibody titers, which was similar to the frequency in those who did not receive corticosteroids (75% of 20 with ≥2 blood specimens collected; P = .60). Thirteen patients (93% of 14 with ≥2 blood specimens collected) who received high-dose steroid therapy had evidence of seroconversion (P = .36, compared with patients with no corticosteroid therapy). There did not appear to be a difference in antibody kinetics whether patients were given low-dose or high-dose corticosteroid therapy as compared to no corticosteroid treatment (Supplementary Figure 1A and 1B).
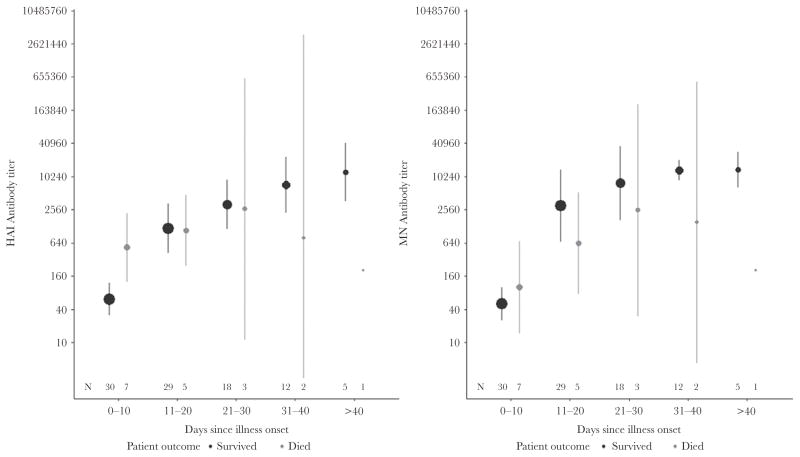
Eight patients (19%) with laboratory-confirmed influenza A(H1N1)pdm09 virus infection died. Death occurred a median of 19 days (IQR, 17–28 days) after symptom onset. Two deaths occurred during the spring pandemic wave (April–July 2009), and 7 deaths occurred during the fall pandemic wave (August 2009–May 2010). No deaths occurred among patients enrolled during the 2010–2011 influenza season (Table 3). Patients who died had significantly higher baseline HAI antibody titers but similar baseline neutralizing antibody titers, compared with patients who survived (Table 2). Otherwise, the shape of the antibody curves was similar between patients who died and patients who survived (Figure 3). All 8 patients who died and 85% of patients who survived reached the seropositive threshold for HAI antibody titers (P = .57); similarly, 88% of patients who died and 79% of patients who survived reached the seropositive threshold for neutralizing antibody titers (P = 1.00). For three patients (patients 2, 3, and 5 in Table 3), death appeared to be related to fulminant influenza because of the short time after symptom onset. For these individuals, HAI titers were ≥40, and neutralizing antibody titers were rising at the time of death for 2 (Supplementary Figures 2A, 2B, 3A, and 3B).
Table 3.
Demographic and Clinical Characteristics and Cause of Death for 9 Critically Ill Adolescents and Adults Who Died With 2009 Pandemic Influenza A(H1N1) Virus Infection—Canada, 2009–2011
| Patient Age, Index |
Time of Illness Onset |
Sex | Age, y | PCR-Confirmed Infection |
Seroconversion | Baseline Blood Sample | High-Risk Characteristic(s) | Time From Symptom Onset to Death, d |
Clinical Comment(s) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time From Symptom Onset to Collection |
HAI Ab Titer |
Neutralizing Ab Titer |
|||||||||
| <65 y | |||||||||||
| 1 | Jun 2009 | Female | 17 | Yes | No | 9 | 226 | 160 | Pregnant, First Nations race | 28 | Shock, ECMO use |
| 2 | Sep 2009 | Female | 40 | Yes | No | 3 | 160 | 5 | First Nations race | 17 | |
| 3 | Jun 2009 | Male | 42 | Yes | Yes | 7 | 28 | 5 | Renal condition | 16 | Septic shock, renal failure, pneumonia, acute tension pneumothorax |
| 4 | Nov 2009 | Female | 47 | No | Yes | 8 | 63 | 28 | Chronic lung condition, cardiovascular condition, renal condition, BMI ≥40,a diabetes mellitus, hematologic malignancy | 34 | Septic shock |
| ≥65 y | |||||||||||
| 5 | Nov 2009 | Female | 65 | No | Yes | 8 | 20 | 5 | Aged ≥65 y | 16 | Shock, pneumonia, acute lung injury |
| 6 | Nov 2009 | Male | 73 | Yes | No | 3 | 640 | 453 | Aged ≥65 y, First Nations race, chronic lung condition, cardiovascular condition | 28 | Pneumonia |
| 7 | Nov 2009 | Female | 73 | No | Yes | 29 | 5 | 10 | Aged ≥65 y | 45 | Malignancy |
| 8 | Nov 2009 | Female | 74 | Yes | Yes | 9 | 20 | 20 | Aged ≥65 y, cardiovascular condition, renal condition, diabetes mellitus | 19 | Intracranial hemorrhage |
Abbreviations: Ab, antibody; BMI, body mass index; ECMO, extracorporal membrane oxygenation; HAI, hemagglutinin inhibition; PCR, polymerase chain reaction.
Body mass index (BMI) is calculated as the weight in kilograms divided by the height in meters squared.
Figure 3.
Hemagglutinin inhibition (HAI) and neutralizing (MN) antibody titer average curves and changes over time for 8 critically ill adults who died with influenza A(H1N1)pdm09 virus infection — Canada, 2009–2011. Size of dot is proportionate to the number patients with a blood draw in the time interval.
DISCUSSION
In this study, patients critically ill with influenza A(H1N1) pdm09 virus infection during the 2009 pandemic and 2010– 2011 influenza season had strong HAI and neutralizing antibody responses against influenza A(H1N1)pdm09 virus during their illness. While the number of patients with fatal outcomes was low, we did not observe differences in the antibody responses of fatal cases versus survivors. Antibody titers against influenza A(H1N1)pdm09 virus initially increased rapidly, such that by 2 weeks after symptom onset 83% of patients had HAI antibody titers of ≥40. From the average curves, antibody titers appeared to plateau after the second week of illness.
The frequency of seroconversion in these critically ill patients was similar to the HAI antibody titers described in similar cohorts of critically ill patients and in cross-sectional serological studies of patients with influenza A(H1N1)pdm09 virus infection [7, 12–14]. In our study, the timing of neutralizing antibody titer rise during illness mirrored the rise in HAI antibody titers; however, compared with a study of neutralizing antibodies in serial blood specimens from patients infected with influenza A(H7N9) virus, there was a more rapid rise in neutralizing antibodies to influenza A(H1N1)pdm09 virus infection in our cohort [8]. This difference may be due to immunological priming due to pre-pandemic exposure to influenza A(H1N1) viruses among subjects included in this study. However, there were also methodologic and population differences between the cohorts that could have contributed to differences in measured antibody titers.
The majority of patients in our cohort demonstrated a rise in antibody titers within 2 weeks after illness onset; however, some patients did not have increases in antibody titers until several weeks later, and 17% and 21% never had HAI or neutralizing antibody titers, respectively, that met the seropositive threshold. While most patients with laboratory-confirmed influenza A(H1N1)pdm09 virus infection included in cross-sectional serological studies during the 2009 pandemic had HAI antibody titers of ≥40, 11%–24% did not have titers that met this seropositive threshold, even >22 days after illness onset [13, 14]. Among a retrospective cohort study of 11 survivors of critical illness from influenza A(H1N1)pdm09 virus infection, 2 patients did not have detectable antibodies at any point during follow-up, out to 40 days after illness onset [15]. Thus, it appears that a small fraction of people will not have a measureable level of strain-specific HAI antibodies despite sometimes severe infection and recovery.
The reasons for variation in antibody responses are not fully understood. We found that some of this variation may have been age-related as patients aged ≥65 years appeared to have lower peak antibody levels, which may reflect immunosenescence. We hypothesized that immunomodulatory therapy could also be a source of variability in antibody response. The use of corticosteroid treatment in the management of critically ill patients infected with influenza A(H1N1)pdm09 infection has been reported to be harmful in most but not all observational studies and remains controversial [16–20]. Although corticosteroids may be given as immunomodulatory therapy to reduce pulmonary inflammation produced by the innate immune response and cytokine dysregulation, immunosuppression from high-dose corticosteroid treatment can also result in prolonged influenza viral replication, nosocomial bacterial or fungal infection, ventilator-associated pneumonia, and a higher risk of mortality due to influenza [16, 18, 19]. However, we found no differences in the antibody responses in patients treated with low-dose or high-dose corticosteroids as compared to patients not treated with corticosteroids, suggesting that there may not be suppression of the humoral immune response to influenza A(H1N1)pdm09 virus infection, at least within the levels of corticosteroids used in these patients.
Antibody titers tend not to be used for clinical management of influenza; however, a French study of critically ill patients with influenza A(H1N1)pdm09 virus infection reported that the absence of a detectable HAI antibody in blood 4 days after illness onset could predict fatal outcome from fulminant disease [7]. Through further laboratory investigations, they suggested that the absence of antibodies in the blood was not related to B-cell deficiencies, but rather to antibody sequestration in pulmonary immune complexes [7]. In our study, only 10 individuals (21%) were admitted within 4 days of illness onset, making it challenging to evaluate antibody titers on day 4 as an outcome predictor. However, among 3 patients in our cohort who likely died from fulminant illness, there was not consistent evidence of low antibody titers in acute-phase sera. One of the other 2 patients who likely died from fulminant disease had antibody titers of ≥40 on day 3 but only had 2 blood specimens collected (on days 3 and 4 after symptom onset), which could not be used to determine change in titers. The third patient with fulminant illness had increases in HAI and neutralizing antibody titers from the time of first blood specimen collection until the time of death, 8 days later. Surprisingly, we did find that patients in our cohort who died had significantly higher baseline HAI antibody titers than those who survived. The implications of this finding are unclear, as there were small numbers of deaths and the contribution of influenza to death could not be fully determined on the basis of the data. However, this finding is generally consistent with findings by Monsalvo et al, who reported higher titers of influenza A(H1N1)pdm09 virus hemagglutinin–specific immunoglobulin G of relatively lower avidity in Brazilian patients with severe illness, compared with those having milder influenza A(H1N1)pdm09 virus disease [21].
We were able to detect evidence of seroconversion in 7 patients whose illness was not diagnosed on the basis of RT-PCR testing of respiratory specimens during hospitalization but who were suspected of having influenza A(H1N1) pdm09 virus infection. This suggests that influenza A(H1N1) pdm09 virus infection may have been underdetected in other critically ill patients. In studies of critically ill patients during the 2009 pandemic, the diagnosis of influenza A(H1N1)pdm09 virus infection was not always made because insensitive or nonspecific assays were used or because upper respiratory tract specimens were tested after viral shedding had declined [22, 23]. As we observed in this cohort, testing paired acute-phase and convalescent-phase sera can yield a retrospective diagnosis of influenza virus infection, which is useful for fully ascertaining case patients for clinical and epidemiologic studies.
This analysis and our conclusions are subject to limitations. First, because of the long duration between symptom onset and death, it is likely that many patients in the cohort died from complications of influenza A(H1N1)pdm09 virus infection and not from fulminant disease. This may cloud associations between antibody response and influenza-specific death, if they exists. Second, the cohort was limited in size, and the number of deaths was also few; therefore, we may be underpowered to detect differences for some comparisons. Third, our cohort was limited to patients who consented to provide serially collected blood specimens. It is possible that patients who died rapidly and likely as a direct result of influenza were not included. Fourth, in this study we only examined humoral immune responses. However, future analyses are planned to explore cell-mediated immune responses, as cytokine dysregulation and deficiencies in host innate and adaptive immune responses have been reported in critically ill patients with influenza A(H1N1)pdm09 virus infection [12, 24]. Characterization of the humoral response to older influenza A(H1N1) viruses, as well as to other influenza A virus subtypes, may help us explore whether immune priming affects severity or recovery from influenza virus infection. Finally, we lacked data on influenza A(H1N1)pdm09 viral load and duration of shedding in respiratory specimens to assess the impact of the antibody response.
In conclusion, critically ill adolescent and adult patients infected with influenza A(H1N1)pdm09 virus during the 2009 pandemic and first postpandemic season showed robust antibody responses against influenza A(H1N1)pdm09 virus. The neutralizing antibody response mirrored the HAI antibody response, with slight differences by age, but responses appeared not to differ on the basis of treatment with high-dose corticosteroids or among patients who died. Prospective serial collection of blood specimens from hospitalized patients can facilitate understanding of the humoral immune response, as well as the adaptive immune response, and should be assessed in future studies to help inform understanding of pathogenesis, effectiveness of interventions, and clinical management of seasonal influenza and novel influenza A virus infections.
Supplementary Material
Acknowledgments
We thank Vic Veguilla, Influenza Division, Centers for Disease Control and Prevention (Atlanta, GA); and members of the Canadian Severe Influenza (CSIS) and the Randomized, Double-Blinded Controlled Trial Comparing High vs Standard Dose Oseltamivir in Severe, Influenza Infection in ICU (ROSII) Study Groups: Gordon Wood, MD, Royal Jubilee Hospital and Victoria General Hospital (Victoria, Canada); Steve Reynolds, MD, Vancouver General Hospital, University of British Columbia (Vancouver Canada); Vinay Dhingra, MD, Vancouver General Hospital, University of British Columbia; Brent Winston, MD, Foothills Medical Centre and Peter Lougheed Hospital, University of Calgary (Calgary, Canada); Sean Bagshaw, MD, University of Alberta Hospital, University of Alberta (Edmonton, Canada); Jim Kutsogiannis, MD, Royal Alexandra Hospital, University of Alberta; William Anderson, MD, Thunder Bay Regional Health Sciences Centre, Northern Ontario School of Medicine (Thunder Bay, Canada); Michael Silverman, MD, Rouge Valley Hospital and Lakeridge Hospital (Ajax and Oshawa, Canada); Margaret Herridge, MD, Toronto General Hospital and Toronto Western Hospital, University of Toronto (Toronto, Canada); Alison McGeer, MD, Mount Sinai Hospital, University of Toronto; Mary-Anne Aarts, MD, St Michael’s Hospital, University of Toronto; John Marshall, MD, St Michael’s Hospital, University of Toronto; Deborah Cook, MD, St Joseph’s Healthcare, McMaster University (Hamilton, Canada); Lauralyn McIntyre, MD, Ottawa Hospital General Campus and, Civic Campus, University of Ottawa (Ottawa, Canada); Stephane Ahern, MD, Hopital Maissonneuve– Rosemont, Universite de Montreal (Montreal, Canada); Kosar Khwaja, MD, Montreal General Hospital and Royal Victoria Hospital, McGill University (Montreal); Natalie Bandrauk, MD, Health Sciences Centre and St Claire’s Mercy Hospital, Memorial University (St. John’s, Canada); Richard Hall, MD, Queen Elizabeth Health Sciences Centre–Halifax Infirmary and Victoria General Site, Dalhousie University (Halifax, Canada); and Jordi Rello, MD, Hospital Universitali Joan XXIII–CIBER Enfermedades Respiratorias, Universitat Rovira i Virgili (Tarragona, Spain).
Financial support. This work was supported by Hoffman-La Roche and the Public Health Agency of Canada.
Footnotes
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Potential conflicts of interest. J. M. K. reports 2 patents related to influenza vaccines. A. K. reports receiving funding from Roche Pharma and the Public Health Agency of Canada. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Beigel JH, Tebas P, Elie-Turenne MC, et al. IRC002 Study Team. Immune plasma for the treatment of severe influenza: an open-label, multicentre, phase 2 randomised study. Lancet Respir Med. 2017;5:500–11. doi: 10.1016/S2213-2600(17)30174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145:599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- 3.Coudeville L, Bailleux F, Riche B, Megas F, Andre P, Ecochard R. Relationship between haemagglutination-inhibiting antibody titres and clinical protection against influenza: development and application of a bayesian random-effects model. BMC Med Res Methodol. 2010;10:18. doi: 10.1186/1471-2288-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972;70:767–77. doi: 10.1017/s0022172400022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res. 2004;103:133–8. doi: 10.1016/j.virusres.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 6.Potter CW, Oxford JS. Determinants of immunity to influenza infection in man. Br Med Bull. 1979;35:69–75. doi: 10.1093/oxfordjournals.bmb.a071545. [DOI] [PubMed] [Google Scholar]
- 7.Guihot A, Luyt CE, Parrot A, et al. Low titers of serum antibodies inhibiting hemagglutination predict fatal fulminant influenza A(H1N1) 2009 infection. Am J Respir Crit Care Med. 2014;189:1240–9. doi: 10.1164/rccm.201311-2071OC. [DOI] [PubMed] [Google Scholar]
- 8.Zhang A, Huang Y, Tian D, et al. Kinetics of serological responses in influenza A(H7N9)-infected patients correlate with clinical outcome in China, 2013. Euro Surveill. 2013;18:20657. doi: 10.2807/1560-7917.es2013.18.50.20657. [DOI] [PubMed] [Google Scholar]
- 9.Kumar A, Zarychanski R, Pinto R, et al. Canadian Critical Care Trials Group H1N1 Collaborative. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–9. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 10.WHO Global Influenza Surveillance Network. Manual for the laboratory diagnosis and virological surveillance of influenza. Geneva, Switzerland: WHO; 2011. Serological diagnosis of influenza by haemagglutination inhibition testing; pp. 59–62. [Google Scholar]
- 11.Kane SP. [Accessed December 6 2016];ClinCalc.com: corticosteroid conversion calculator. http://clincalc.com/corticosteroids/
- 12.Bermejo-Martin JF, Martin-Loeches I, Rello J, et al. Host adaptive immunity deficiency in severe pandemic influenza. Crit Care. 2010;14:R167. doi: 10.1186/cc9259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet. 2010;375:1100–8. doi: 10.1016/S0140-6736(09)62126-7. [DOI] [PubMed] [Google Scholar]
- 14.Veguilla V, Hancock K, Schiffer J, et al. Sensitivity and specificity of serologic assays for detection of human infection with 2009 pandemic H1N1 virus in U.S. populations. J Clin Microbiol. 2011;49:2210–5. doi: 10.1128/JCM.00229-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grund S, Michel S, Barthuber C, Adams O. Serum and mucosal antibodies fail as prognostic markers during critical influenza A infection. J Clin Virol. 2016;74:32–6. doi: 10.1016/j.jcv.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Brun-Buisson C, Richard JC, Mercat A, Thiébaut AC, Brochard L REVA-SRLF A/H1N1v 2009 Registry Group. Early corticosteroids in severe influenza A/H1N1 pneumonia and acute respiratory distress syndrome. Am J Respir Crit Care Med. 2011;183:1200–6. doi: 10.1164/rccm.201101-0135OC. [DOI] [PubMed] [Google Scholar]
- 17.Delaney JW, Pinto R, Long J, et al. Canadian Critical Care Trials Group H1N1 Collaborative. The influence of corticosteroid treatment on the outcome of influenza A(H1N1pdm09)-related critical illness. Crit Care. 2016;20:75. doi: 10.1186/s13054-016-1230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SH, Hong SB, Yun SC, et al. Corticosteroid treatment in critically ill patients with pandemic influenza A/H1N1 2009 infection: analytic strategy using propensity scores. Am J Respir Crit Care Med. 2011;183:1207–14. doi: 10.1164/rccm.201101-0110OC. [DOI] [PubMed] [Google Scholar]
- 19.Martin-Loeches I, Lisboa T, Rhodes A, et al. ESICM H1N1 Registry Contributors. Use of early corticosteroid therapy on ICU admission in patients affected by severe pandemic (H1N1)v influenza A infection. Intensive Care Med. 2011;37:272–83. doi: 10.1007/s00134-010-2078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Yang SG, Gu L, et al. National Influenza A(H1N1) pdm09 Clinical Investigation Group of China. Effect of low-to-moderate-dose corticosteroids on mortality of hospitalized adolescents and adults with influenza A(H1N1)pdm09 viral pneumonia. Influenza Other Respir Viruses. 2017;11:345–54. doi: 10.1111/irv.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monsalvo AC, Batalle JP, Lopez MF, et al. Severe pandemic 2009 H1N1 influenza disease due to pathogenic immune complexes. Nat Med. 2011;17:195–9. doi: 10.1038/nm.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rello J, Rodríguez A, Ibañez P, et al. H1N1 SEMICYUC Working Group. Intensive care adult patients with severe respiratory failure caused by Influenza A (H1N1)v in Spain. Crit Care. 2009;13:R148. doi: 10.1186/cc8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice TW, Rubinson L, Uyeki TM, et al. Critical illness from 2009 pandemic influenza A virus and bacterial coinfection in the United States. Crit Care Med. 2012;40:1487–98. doi: 10.1097/CCM.0b013e3182416f23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao R, Bhatnagar J, Blau DM, et al. Cytokine and chemokine profiles in lung tissues from fatal cases of 2009 pandemic influenza A (H1N1): role of the host immune response in pathogenesis. Am J Pathol. 2013;183:1258–68. doi: 10.1016/j.ajpath.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.