Abstract
Age-related declines in cognitive function can impair working memory, reduce speed of processing, and alter attentional resources. In particular, menopausal women may show an acceleration in the rate of cognitive decline as well as an increased vulnerability to brain diseases as estrogens may play a neuroprotective and neurotrophic role in the brain. To treat menopausal symptoms, many women turn to botanical estrogens that are promoted as a safe and natural alternative to traditional hormone replacement therapy. However, the majority of these compounds have not been systematically evaluated for efficacy and safety. The current study investigated the efficacy of the commercially available botanical estrogenic compound isoliquiritigenin (ISL) to alter performance on an operant working memory task, delayed spatial alternation (DSA). ISL is a compound found in licorice root that has been shown to have a wide range of effects on different biological systems, including estrogenic properties. This botanical is currently being used in over the counter dietary supplements. Middle-aged (12-month old) Long-Evans female rats were ovariectomized and orally dosed with either 0 mg, 6 mg, 12 mg or 24 mg of ISL 60 minutes before testing on the DSA task. The DSA task required the rat to alternate its responses between two retractable levers in order to earn food rewards. Random delays of 0, 3, 6, 9 or 18 seconds were imposed between opportunities to press. ISL treatment failed to alter DSA performance. Previous work from our research group has found that estrogenic compounds, including 17β-estradiol and the botanical estrogen genistein impair performance on the DSA task. The goal of our botanical estrogens research is to find compounds that offer some of the beneficial effects of estrogen supplementation, without the harmful effects. This work suggests that ISL may not carry the cognitive risks associated with most other estrogenic compounds tested to date.
Keywords: Isoliquiritigenin, Phytoestrogens, Aging, Prefrontal, Working memory
1. Introduction
Botanical estrogens are non-steroidal plant compounds that can mimic estrogen in the body (Glazier and Bowman, 2001). These compounds are widely sold as dietary supplements despite a dearth of research on their effects (Mahady et al., 2003). Many over the counter botanical supplements contain licorice root powder (Geller and Studee, 2005). Isoliquiritigenin (ISL), and its active metabolite liquiritigenin (LIQ) are two of the primary bioactive compounds in licorice root and both compounds have been demonstrated to have estrogenic activity both in vivo and in vitro (Maggiolini et al 2002; Mersereau et al, 2008; Miksicek et al 1993).
There is a large body of research indicating that estrogens can affect cognition (Engler-Chiurazzi et al., 2016; McEwen, 2001). However, the effects of estrogens on cognition can vary widely depending on a variety of factors including the cognitive task and the brain areas engaged during completion of the task. Specifically, the prefrontal cortex and the hippocampus are known to play important roles in working memory tasks (D’Esposito, 2007; Floresco et al, 1997). Most rodent tests of working memory have utilized maze paradigms, and these tests often involve the use of cue configurations together with relatively long delays between testing trials. Both of these factors likely engage the hippocampus during performance of the task (Maruki et al, 2001; Sloan et al, 2006; Wang and Cai, 2006). In contrast, operant based working memory tasks such as delayed matching to sample or delayed alternation that use short inter-trial delays of less than 20 seconds do not appear to require the hippocampus (Maruki et al, 2001; Sloan et al, 2006). Most work on the effects of estrogens on learning and memory has focused on hippocampus-sensitive tasks, while relatively little work has focused on tasks that rely on the prefrontal cortex. Thus, we have utilized a delayed spatial alternation (DSA) operant task to investigate the effects of estrogens on a prefrontal cortex mediated aspect of learning and memory.
We have previously reported that ovariectomized (OVX) young (3 month old), middle-aged (12 month old) or old (18 month old) rats given physiological levels of 17β-estradiol were impaired on an operant DSA task relative to OVX vehicle control treated rats (Wang et al., 2008; Wang et al, 2009). Wang et al. (2008) tested young (3 month old) OVX rats on the operant DSA task. Rats were implanted with a silastic capsule containing 17β-estradiol (serum level of 20.82 ± 3.41 pg/ml: Wang et al., 2009) or one containing cholesterol. This 17β-estradiol dose was chosen to mimic a high physiological level. We found that chronic 17β-estradiol supplementation resulted in a deficit in DSA performance compared to rats given cholesterol vehicle only. In a follow up study, Wang et al. (2009) tested young (3 month old), middle-aged (12 month old) and old (18 month old) OVX rats on the DSA task. Rats were implanted with a silastic capsule containing 10% 17β-estradiol or cholesterol. We found that chronic 17β-estradiol supplementation resulted in a deficit in DSA performance in all the tested age groups compared to age matched rats given cholesterol vehicle only. In order to further investigate the role of estrogens in modulating performance on the DSA task, middle aged (12 month old) rats were OVX and given either the ERα agonist propyl pyrazole triol (PPT), the ERβ agonist diarylpropionitrile (DPN) (0.02, 0.08, or 0.20 mg/kg/day for both agonists) or oil control (Neese et al., 2010a). Another group received 10% 17β-estradiol in a silastic capsule to serve as a positive control. This study replicated the estradiol results from Wang et al. (2009), finding that the 17β-estradiol group was impaired on the DSA task compared to age-matched oil control groups. This study also revealed that a low dose of the ERβ agonist DPN impaired performance, but that the ERα agonist PPT did not, suggesting that the 17β-estradiol induced deficit in DSA performance may be ERβ mediated (Neese et al., 2010a).
We have also investigated the effects of the botanical estrogens genistein and S-equol on DSA performance. Genistein is an ERβ selective isoflavone found in soy. S-equol is a metabolite of the soy isoflavone daidzein and is also ERβ selective (Muthyala et al., 2004). Neese et al. (2010b) tested the effects of oral genistein exposure in young (7 month old), middle-aged (16 month old) and old (22 month old) Long-Evans OVX rats on the DSA task. Rats were given genistein orally once daily at either a low dose (162 μg/kg/day) or a higher dose (323 μg/kg/day). We found that the old rats receiving the higher dose of genistein performed worse than both the young and middle-aged groups given that dose. Neese et al. (2012) investigated the effects of three daily exposures to genistein (3.4 mg/kg each time) on DSA and DRL performance in middle-aged (14 month old) OVX rats. This exposure paradigm was designed to keep serum genistein levels in the range of those found in humans consuming commercially available soy isoflavone supplements. We found that there was a trend for genistein treated rats to perform worse than sucrose control rats overall in DSA performance. Genistein treatment did not affect performance on the DRL task. Neese et al. (2014) also tested middle-aged (12–13 month old) OVX rats on the DSA task after treatment with S-equol. S-equol binds selectively to ERβ with an affinity similar to that of genistein but has lower transcriptional potency than genistein (Muthyala et al., 2004). S-equol was given at 10,000 or 19,000 μg/kg/day orally. We found that S-equol did not affect performance on the DSA task.
In the present study, we investigated the effects of ISL on the same operant DSA task used to assess other estrogens in these previous studies. Both ISL and LIQ can bind to estrogen receptors and LIQ has a twenty-fold higher affinity for ERβ over ERα (Mersereau et al, 2008). However, ISL and LIQ readily convert back and forth, and serum levels of the two compounds rapidly come to equilibrium after treatment with either compound (Simmler et al., 2013). Both compounds are lipophilic and have relatively low molecular weight and likely cross into the brain through the blood brain barrier (Srihari et al, 2012).
We chose to treat the rats with ISL, which is more readily available commercially. Similar to other estrogens, we hypothesized that treating OVX middle-aged (12 month old) rats with ISL would result in impairment on the operant DSA task. Dietary supplements containing ISL are already available for consumers to buy with no pre-market regulation. These supplements are marketed primarily to peri- and post-menopausal women, thus we used middle-aged OVX rats in order to model this population of consumers
2. Materials and methods
2.1 Animals and botanical exposure
A total of 64 middle-aged female Long-Evans rats were obtained from Harlan (Indianapolis, IN). The rats were received in one cohort. All animals were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). Rats were housed in a temperature and humidity controlled room (22 °C, 40–55% humidity) on a 12-h reverse light–dark cycle (lights off at 7:00 am). All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Illinois at Urbana-Champaign and were in accordance with the guidelines of the Public Health Service Policy on Humane Care and Use of Laboratory Animals and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (NIH, 1986; Van Sluyters and Obernier, 2004).
Rats were 12 month old retired breeders, and were assigned to one of four exposure groups (0,6,12 or 24 mg of ISL). The body weights of the rats ranged from 272 to 373 grams (M=316, SD=22). Thus, the doses of ISL correspond to approximately 0, 19, 38, or 76 mg/kg body weight. There were 16 rats per group. Rats were pair-housed in standard plastic cages (45 × 24 × 20 cm) with corncob bedding, and were allowed to acclimate to the vivarium for two weeks before ovariectomy. For the ovariectomy surgery, rats were anesthetized with isoflurane gas. Following removal of the ovaries, muscle and fat layers were individually sutured with silk suture thread (Fisher Scientific). The incision in the skin was then closed with stainless steel wound clips. A postsurgical injection of carprofen (5 mg/kg, s.c.) was administered for pain management. All animals were maintained on an AIN-93G soy-free diet (Harlan-Teklad, Madison, WI) after surgery to avoid exposure to dietary estrogens. Water was available ad libitum. Beginning nine days after the OVX surgery, rats were food restricted to reduce them to 85% of their free-fed body weights. Each animal was weighed daily and given a ration of food 30 minutes after testing. The amount of food given was adjusted daily based on current weight in comparison to free feeding weight. Twelve days after food restriction began, ISL treatment and operant testing began. Rats were tested once per day, 6 days per week during the dark phase of the light cycle in a darkened testing room. One hour prior to testing, rats were given fruit punch flavored pellets (Test Diet, Richmond, IN) containing doses of roughly 0mg/kg/day, 19mg/kg/day, 38mg/kg/day, or 76mg/kg/day of ISL. Rats were dosed 7 days per week, including on days they were not tested.
2.2 Serum ISL and LIQ concentration analysis
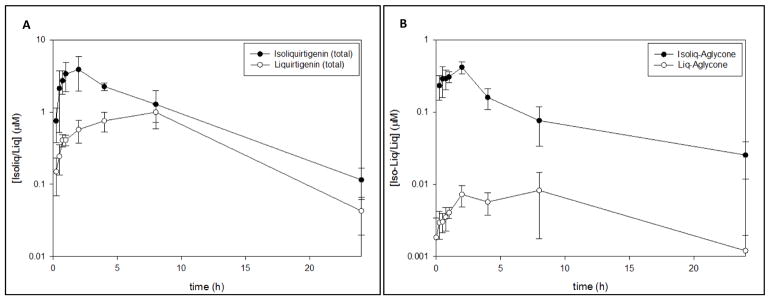
Serum ISL and LIQ levels were measured in a pilot study prior to the current study (Figure 1). Six Long-Evans rats aged 9–12 months were OVX and fed an AIN soy-free diet for 4–5 weeks. Rats were fed a dosing pellet containing 10mg of ISL and blood was drawn from a tail vein prior to dosing and again at 15 min, 30 min, 45 min, 1 hour, 2 hours, 4 hours, 8 hours and 24 hours after dosing. The mean dose of ISL used in the pilot study was between the lowest (6mg) and middle dose (12mg) used in the current study. This resulted in peak blood levels of total ISL and LIQ (conjugated + unconjugated forms) of approximately 5μM and 1μM respectively (Figure 1A). The peak aglycone levels of ISL and LIQ were approximately 0.5 μM and 0.01 μM respectively (Figure 1B). Dosing in the current study was timed so that rats had peak blood levels of ISL at the time of testing. Thus, at the time of DSA testing, total blood levels of ISL were likely between 3μM and 10μM, depending on the dose group. Levels of total ILQ and LIQ were determined using LC/MS/MS following quantitative hydrolysis of conjugates by β-glucuronidese/arylsulfatase (H. pomatia; Sigma H-1, 2 units/assay) sing procedures previously described (Madak-Erdogan et al., 2016). Levels of aglycone ILQ and LIQ were determined without the enzymatic hydrolysis step.
Figure 1.
Serum ISL and LIQ levels from a pilot study prior to the current study in female rats (n=6) provided 1 pellet containing 10 mg ISL (dose of ~32 mg/kg body weight. A) Total serum levels of ISL and LIQ across a 24 hour period. B.) Aglycone serum levels of ISL and LIQ across a 24 hour period.
2.3 Organ collection
At the time of sacrifice, liver and uterine horn weights were taken. The liver weight was divided by the body weight of the animal to derive a liver/body weight ratio. A higher liver/body weight ratio implies increased induction of liver enzymes, which is suggestive of hepatotoxicity. It is important to look at measures of toxicity when testing new compounds. The uterine horn was also weighed. This was done because uterine proliferation is suggestive of estrogenic activity, especially activity at estrogen receptor alpha (ERα). The horn was removed and proximal fat and vasculature were trimmed off to yield accurate weight of the horn. The uterine horn was also measured in length using a ruler. The weight of the uterine horn was divided by the length to yield a weight/length ratio (as described in Pisani et al, 2015).
2.4 Operant testing
2.4.1 Apparatus
Rats were behaviorally tested in standard automated operant chambers housed in sound-attenuated wooden boxes (Med Associates Inc., St. Albans, VT). The test chambers were 21.6 cm tall with a 29.2 cm × 24.8 cm stainless-steel grid floor that rested just above a tray filled with corn cob bedding. A pellet dispenser centered 2.5 cm above the floor on the operant panel dispensed 45 mg soy-free purified food pellets (Test Diet, Richmond, IN). Retractable response levers were located symmetrically on both sides of the pellet dispenser with a stimulus cue light above each lever. The levers were 5.7 cm from midline and 7.0 cm above the floor and the cue lights were located 5.7 cm above the levers. Each chamber also contained a Sonalert tone generator, a white noise generator, and a house light located on the back wall that remained off during testing.
Rats were fed a measured amount of food, sufficient to maintain 85% free-fed weight, 30 min after behavioral testing was completed each day. This feeding schedule was necessary to ensure that the animals were motivated to work for the food rewards used in the DSA test.
2.4.2 Response shaping
An autoshaping program was used to train rats to press the response levers (Widholm et al., 2004). At the beginning of the session, both response levers were extended into the operant chamber with white noise played continuously to mask extraneous noise. The cue light above the right response lever was illuminated for 15 seconds every 3 minutes according to a fixed-time 3-minute schedule (FT-3). After the cue light was turned off, a tone was played for 40ms and a single food pellet was provided at the same time. Presses to either lever at any time during the autoshaping session resulted in immediate reinforcement. The FT-3 schedule terminated when the animal had pressed a total of 10 times on either response lever during a session, at which point reinforcer delivery became contingent only on lever presses. Autoshaping test sessions terminated after 60 minutes elapsed or 100 reinforcers were delivered, whichever occurred first. Criterion for this condition was set at 100 lever presses within a single session.
2.4.3 Lever press training
After autoshaping, all animals went through a continuous reinforcement schedule in which the reinforced lever alternated after the delivery of every fifth reinforcer. This was done to strengthen the lever-press response the animal learned during autoshaping and to prevent the animals from developing a preference for a particular side or lever. At the start of each session, either the right or left lever was extended into the chamber while the cue light above that lever lit up. Pressing the extended lever resulted in reinforcement in the form of a food pellet. After the animal had pressed the extended lever five times, the cue light was extinguished and the lever was retracted. This repeated with the opposite lever. This cycle of alternating levers terminated after 100 reinforcers were received or 60 minutes had elapsed. Criterion for this condition was 100 reinforced lever presses for two or more consecutive sessions.
2.4.4 Cued alternation (CA)
Following lever press training, the rats were trained on a delayed spatial alternation (DSA) task with a variable delay, as detailed in Widholm et al (2004). DSA was preceded by two training tasks. In the first, cued alternation (CA), the cue light indicated the correct lever on each trial, and the cue light alternated between the right and left levers. Rats were reinforced for pressing the lever opposite the one pressed on the previous trial, regardless of whether that trial was correct or incorrect. The rat’s response on the first trial of a session was always correct. Each correct lever press resulted in reinforcement in the form of a food pellet, which was paired with a 40ms tone. Pressing the incorrect lever resulted in the retraction of the levers and the extinguishing of the cue-light. In between each trial, both levers were retracted and then immediately re-extended at the same time. There was no inter trial delay and no time limit to press a lever. Criterion for this condition was at least 60% correct cued lever presses within a session.
2.4.5 Non-cued alternation (NCA)
After CA, a non-cued spatial alternation task followed. In this condition, the cue light no longer indicated the correct lever, but correct responses still consisted of alternating between the left and right levers. Rats were reinforced for pressing the lever opposite the one pressed on the previous trial, regardless of whether that trial was correct or incorrect. The first trial of a session was always correct. Reinforcement was delivered in the form of a food pellet, which was paired with a 40ms tone. Pressing the incorrect lever resulted in the retraction of the levers and immediate simultaneous re-extension of the levers to begin a new trial. There was no time limit to press a lever. Each animal was tested for 10 sessions in this condition.
2.4.6 Delayed spatial alternation (DSA)
The DSA task was the final phase of testing. In this condition, delays of 0, 3, 6, 9 or 18 seconds were imposed semi-randomly between alternation trials, with the stipulation that no particular delay was presented on more than three consecutive trials. There were 40 trials at each delay and a total of 200 trials per session. Rats were reinforced for pressing the lever opposite the one pressed on the previous trial, regardless of whether that trial was correct or incorrect. Reinforcement was delivered in the form of a food pellet, which was paired with a 40ms tone. Pressing the incorrect lever resulted in the retraction of the levers and the immediate beginning of the delay period. In between each trial, both levers were retracted and then either immediately (0 second delay) or after 3, 6, 9 or 18 seconds they were re-extended. Both levers were retracted and then extended at the same time after the delay. Levers were not available during the delay. There was no time limit to press a lever. Each animal was tested for 25 sessions.
2.5 Statistical analysis
The data were analyzed via ANOVA using Systat for Windows Version 13.1 (systatsoftware.com/products/systat/). Exposure was included as a between-subjects factor and significance was set at p<0.05. For autoshaping, the days to reach criterion served as the measure for learning. The data for autoshaping were analyzed using a one-way ANOVA with exposure as a between-subjects factor. For CA, the number of errors that occurred across all sessions and days to reach criterion both served as measures of learning. The data were analyzed using a one-way ANOVA with exposure as a between-subjects factor for both measures. For NCA, the proportion correct across the ten testing sessions was used as the measure of learning. The data were analyzed using a 4 (exposure) × 10 (day) mixed ANOVA with day as a repeated measures factor. For DSA, the proportion correct was first combined into blocks of five sessions by averaging the proportion correct across sessions within each block. The proportion correct was then analyzed using a mixed 4 (exposure) × 5 (block) × 5 (delay) repeated measures ANOVA with block (1–5) and delay (0, 3, 6, 9, and 18 s) serving as repeated measures factors. The latency to respond before a correct response, an incorrect response, and latency to respond overall was also analyzed. This was done using a repeated measures ANOVA with block as a repeated measures factor and exposure as a between-subjects factor. The organ weights were analyzed via one-way ANOVA with exposure as the between subjects factor.
3. Results
3.1 Autoshaping
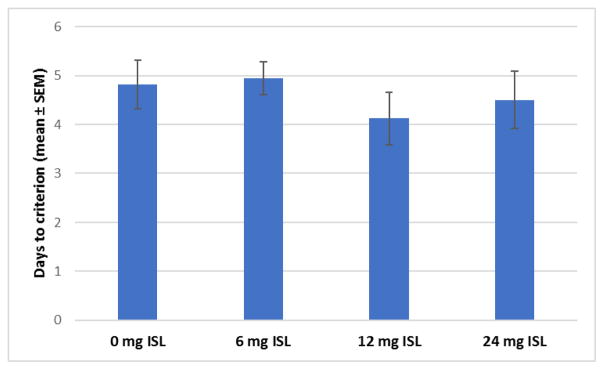
The days to reach criterion was used as the measure of learning during this phase. There were no main effects of exposure on the days to reach criterion (F[3,60]=0.532, p=0.662, Fig 2).
Figure 2.
There was no main effect of exposure on the days to criterion in the autoshaping phase of training (n=16).
3.2 Cued alternation (CA)
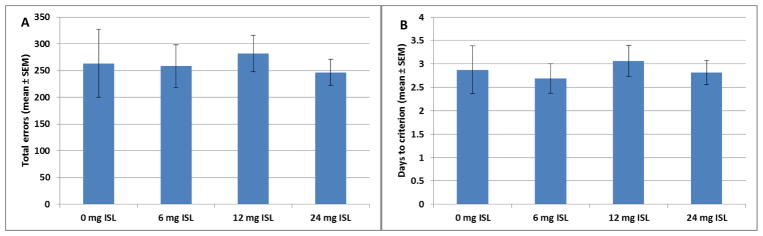
Both the number of total errors as well as the days to reach criterion were used as measures of learning during this phase. There were no main effects of exposure on either the total errors (F[3,60]=0.115, p=0.951, Fig 3A) or the number of days to reach criterion (F[3,60]=0.182, p=0.908, Fig 3B). There was no main effect of exposure on the latency to respond during the CA phase (F[3,59]=1.917, p=0.137). There was also no effect of exposure on the latency to respond before a correct response (F[3,59]=1.662, p=0.185) or an incorrect response (F[3,59]=1.794, p=0.158).
Figure 3.
A) There was no main effect of exposure on the total number of errors during the cued alternation phase of training (n=16). B) There was no main effect of exposure on the days to reach criterion on the cued alternation phase of training (n=16).
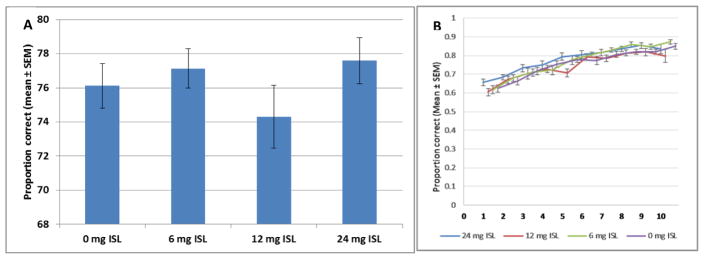
3.3 Non-cued alternation (NCA)
The proportion correct was used as a measure of learning in the NCA phase. There was no main effect of exposure on proportion correct during NCA (F[3,60]=1.284, p=0.288, Fig 4), and no interaction of exposure and day (F[19.262, 385.243]= 1.303, p=0.176). The analysis did reveal a significant effect of day as expected (F[6.421, 385.243]=103.423, p<0.001), showing that all exposure groups increased their proportion correct across days, an indication that learning occurred. There was no main effect of exposure on the latency to respond overall during the NCA phase (F[3,60]=2.045, p=0.117). There was also no effect of exposure on the latency to respond before a correct response (F[3,60]=2.154, p=0.103) or an incorrect response (F[3,60]=1.033, p=0.384).
Figure 4.
A) There was no main effect of exposure on the proportion correct during the non-cued alternation phase of training (n=16). B) All groups increased in their proportion correct across sessions of NCA, but did not differ significantly from each other (n=16).
3.4 Delayed spatial alternation (DSA)
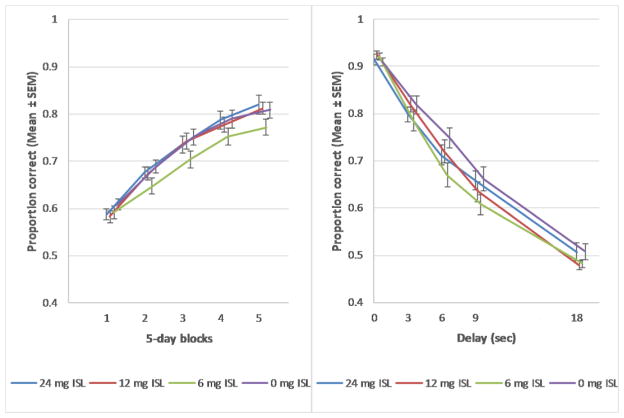
The proportion correct was used as a measure of learning in the DSA task. There was no main effect of exposure on the proportion correct (F[3,60]=1.149, p=0.337, Fig 3A). There were trends for interactions between exposure and testing block (F[72.000, 117.000]=1.341, p=0.079) and exposure and delay (F[12, 177]=1.720, p=0.066), (Figure 5A and 5B). The lowest dose group (6mg ISL) had the lowest proportion correct across blocks of testing, although this was not a statistically significant difference. Across delays, all exposure groups were below controls with this difference being clearest in the lowest dose group. However, simple main effects at each block as well as at each delay did not approach significance. There was no significant interaction between exposure, block and delay (F[54.673, 1093.458]=1.181, p=0.177). The analysis did reveal a significant effect of block (F[25.482, 37.00]=25.482, p<0.001) indicating that all exposure groups increased their proportion correct across block, an indication that learning occurred. There was also a significant effect of delay (F[4, 57]=908.8, p<0.001) indicating that all groups had lower proportions correct as the length of the delay increased (Figure 5B).
Figure 5.
A) (Left panel) There was no main effect of exposure on the proportion correct in the DSA task. There was also no interaction between exposure and block, indicating that all groups performed similarly within testing blocks (n=16). B) (Right panel) There was no interaction between exposure and delay in the DSA task, indicating that all groups performed similarly within each delay (n=16)
3.5 Latency to respond during DSA
There was no main effect of exposure on the latency to respond overall during the DSA task (F[3,60]=1.916, p=0.137). There was a significant effect of block on the latency to respond overall showing that the latency to respond tended to decrease across blocks of testing (F[4,240]=19.359, p<0.001). In addition, there was also no effect of exposure on the latency to respond following a correct (F[3,60]=1.980, p=0.127) or an incorrect response (F[3,60]=1.497, p=0.224).
3.6 Organ weights
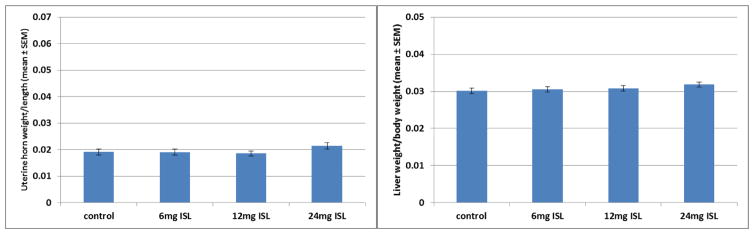
There was no significant effect of exposure on the size of the uterine horn (F[3,60]=1.324, p=0.275, Figure 6A). None of the doses of ISL altered the size of the uterine horn significantly from those of the OVX vehicle control animals.
Figure 6.
A) (Left panel) There was no main effect of exposure on the size of the uterine horn (n=16). B) (Right panel) There was no main effect of exposure on the liver weight to body weight ratio (n=16).
There was no significant effect of exposure on the liver to body weight ratio (F[3,59]=0.963, p=0.416, Figure 6B). None of the doses of ISL altered the size of the liver relative to the body weight significantly from those of the OVX vehicle control group.
4. Discussion
The present study investigated the effects of oral exposure to isoliquiritigenin (ISL) on performance of an operant delayed spatial alternation (DSA) task in middle-aged rats. Daily exposure to 19, 38, or 76 mg/kg body weight of ISL had no measurable effect on DSA performance. Exposure to ISL also did not affect learning in the cued alternation (CA) or non-cued alternation (NCA) training phases prior to DSA testing, and did not affect latency to respond in the DSA task.
Previous work from our research group has shown that treatment with 17β-estradiol tends to impair performance on the DSA task in young (3 month old), middle-aged (12 month old), as well as old (18 month old) OVX rats (Neese et al., 2010a; Wang et al., 2008; Wang et al., 2009). Additionally, we have shown that genistein, an ERβ selective soy isoflavone impairs performance on the DSA task in middle-aged (14 or 16 month old) and old (22 month old) OVX rats (Neese et al., 2010b; Neese et al., 2012). We have also shown that the ERβ agonist DPN impairs performance on the DSA task, but that the ERα agonist PPT does not, suggesting that the 17β-estradiol induced deficit in DSA performance may be ERβ mediated (Neese et al., 2010a).
In the present study, we hypothesized based on these previous studies that ISL, which has been shown to have estrogenic activity both in vitro and in vivo (Maggiolini et al 2002; Miksicek et al, 1993), would also impair performance on this task. LIQ, the active metabolite of ISL, has a twenty-fold higher affinity for ERβ over ERα (Mersereau et al, 2008). Contrary to our hypothesis, we found that ISL at the doses tested had no effect on DSA performance. One possibility is that the levels of ISL in the serum were not high enough to elicit an estrogen-like response. While serum levels of ISL were not measured in this study, based on a pilot study conducted in our lab we estimate that the peak serum levels of aglycone ISL+LIQ in the animals in the present study were between 0.5 and 1 μM. It is useful to look at combined ISL+LIQ levels because both compounds bind to the estrogen receptor and because the two compounds convert back and forth readily in vivo (Simmler et al., 2013). Miksicek et al (1993) found that ISL binds directly to the estrogen receptor in vitro and competes with estradiol for binding. They found that a range of 0.1–1μM of ISL is needed for a half-maximal response, compared to 0.2nM of 17β-estradiol. While Miksicek et al. (1993) used an in vitro model and we dosed animals with ISL in vivo, given their results it seems likely that the serum levels of ISL in our animals were high enough to have significant binding affinity at the estrogen receptor. Additionally, while the binding affinities of ISL and LIQ for the estrogen receptors are substantially lower than that of estradiol, LIQ appears to have greater potency and efficacy through ERβ in gene regulation than expected from its ERβ-binding affinity (Jiang et al., 2013). In an in vitro culture of MCF-7 cells containing ERα or ERβ receptors, a 1μM concentration of LIQ was able to stimulate both PgR and OTUB2 gene expression respectively, indicating that LIQ was able to induce transcriptional activity through both estrogen receptors at that concentration (Jiang et al., 2013). These results suggest that the levels of ISL+LIQ found in the present study were likely high enough to be able to affect transcriptional activity through the estrogen receptors. Additionally, the time course pilot study conducted in our lab indicates that levels of aglycone (bioactive) ISL and LIQ remain elevated in the serum for several hours after oral dosing, indicating that the lack of cognitive effects seen in this study were likely not due to rapid metabolism of ISL or LIQ (Figure 1). This is in contrast to an investigation of S-equol done previously in our lab in which the low transcriptional potency of S-equol as well as its rapid metabolism could have led to its lack of cognitive effects (Neese et al., 2014). Importantly, in the present study, ISL was administered orally, thus making the route of exposure relevant to human exposure. In addition, in another recent study we found that treatment with ISL, in the absence of other dietary manipulations, improved performance on a hippocampus sensitive task in OVX rats and the serum ISL+LIQ levels measured in that study were similar to those found in the current study (Kundu et al., 2016). This suggests that the levels of ISL+LIQ in the present study were high enough to have effects in the brain, but that ISL, unlike most other estrogens tested to date, does not impair performance on this operant version of the DSA task. There is evidence that ISL and LIQ cross the blood brain barrier (Srihari et al, 2012). However, it is a limitation of the current study that we do not have measurements of these compounds in the relevant brain areas.
It should be noted that while no significant effects of ISL exposure were observed in the current study, there was a trend for the lowest dose ISL group to have the lowest proportion correct across blocks of testing. Additionally, across delays, all exposure groups were below controls with this difference being clearest in the lowest dose group. While these effects were not statistically significant, they are in line with previous research from our group on the ERβ agonist DPN. Neese et al. (2010a) found that while a low dose of DPN impaired performance on the DSA task in OVX middle-aged (12 month old) rats, the two higher doses of DPN did not. Non-monotonic dose curves like that seen in Neese et al. (2010a) are common for compounds that act on hormone receptors (Vandenberg, 2014). Thus, future studies should expand the dose range to include both lower and higher doses of ISL in order to confirm whether a non-monotonic dose–response curve exists for ISL on the DSA task.
4.1 Organ weights
At the time of sacrifice, uterine horns and livers of all animals were weighed. Animals treated with 19, 38, or 76 mg/kg body weight of ISL had no differences in the size of the uterine horn compared with OVX animals given sucrose pellets only. ISL has the potential to act through estrogen pathways. However, the estrogen-mediated uterotrophic effect is largely an ERα-mediated effect, and since these botanicals are ERβ-selective, the lack of an effect on uterine weight is not surprising and is consistent with the lack of a uterotrophic effect from ISL treatment of adult OVX C57BL6 mice (Malak-Erdogan et al., 2016). Additionally, while ISL and LIQ have some potential to affect ERα-mediated gene expression, in addition to the lower binding affinity for the ERα receptor, these compounds fail to form the stable ER-coregulator complexes that are required for stimulation of reproductive tissues (Malak-Erdogan et al., 2016).
The liver to body weight ratio of animals treated with ISL also did not differ from those of sucrose controls. An increase in liver size is suggestive of increased hepatic enzyme induction, which is a sign of possible toxicity (Maronpot et al, 2010). Licorice root does contain glycyrrhizin, which has been implicated for its hepatotoxic effects at high doses (Isbrucker and Burdock, 2006), however, the pure compound ISL does not. Thus, exposure to ISL did not have any overt clinical effects on uterine proliferation or hepatic toxicity as measured in the current study.
5. Summary and Conclusions
In the present study we found that daily exposure to 19, 38, or 76 mg/kg body weight of isoliquiritigenin (ISL) had no effect on performance on the prefrontal cortex-sensitive delayed spatial alternation (DSA) task in middle-aged (12 month old) OVX rats. Exposure to ISL also did not alter learning in the cued alternation (CA) or non-cued alternation (NCA) training phases prior to DSA testing. This is in contrast to previous studies from our group showing that exposure to other estrogens, including 17β-estradiol, genistein, and the ERβ agonist DPN, impairs performance on this DSA task in OVX rats. Specifically, previous work from our group suggests that the deficit in DSA performance seen with administration of estrogens may be ERβ mediated. Thus, given that LIQ, the active metabolite of ISL, has a twenty-fold higher affinity for ERβ over ERα and did not cause a deficit in DSA performance in this study is significant. These results suggest that ISL may not carry the same cognitive risks as most other estrogens tested to date. However, further studies are needed to determine whether there are any negative effects of ISL on other prefrontally mediated tasks or on tasks mediated by other brain regions. In addition, a broader range of ISL doses spanning the dose range that humans could conceivably be exposed to from dietary supplements should be investigated for their biological effects to ensure that there are not negative effects at doses outside the range assessed in this study.
Highlights.
Botanical estrogens are marketed to peri-menopausal women to aide healthy aging
Isoliquiritigenin (ISL) is a botanical estrogen found in licorice root
Middle-aged ovariectomized (OVX) rats were treated with ISL
The efficacy of ISL to alter working memory was assessed
ISL treatment did not alter working memory in this task
Acknowledgments
This project was supported by NIH grant 5P50AT006268-05.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- D’Esposito M. From cognitive to neural models of working memory. Philosophical Transactions of the Royal Society B: Biological Sciences. 2007;362(1481):761–772. doi: 10.1098/rstb.2007.2086. https://doi.org/10.1098/rstb.2007.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler-Chiurazzi EB, Brown CM, Povroznik JM, Simpkins JW. Estrogens as neuroprotectants: Estrogenic actions in the context of cognitive aging and brain injury. Progress in neurobiology. 2016 doi: 10.1016/j.pneurobio.2015.12.008. https://doi.org/10.1016/j.pneurobio.2015.12.008. [DOI] [PMC free article] [PubMed]
- Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. Journal of Neuroscience. 1997;17(5):1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasbarri A, Pompili A, Clotilde Tavares M, Tomaz C. Estrogen and cognitive functions. Expert review of endocrinology & metabolism. 2009;4(5):507–520. doi: 10.1586/eem.09.30. http://dx.doi.org/10.1586/eem.09.30. [DOI] [PubMed] [Google Scholar]
- Geller SE, Studee L. Botanical and dietary supplements for menopausal symptoms: what works, what does not. Journal of women’s health. 2005;14(7):634–649. doi: 10.1089/jwh.2005.14.634. https://doi.org/10.1089/jwh.2005.14.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazier MG, Bowman MA. A review of the evidence for the use of phytoestrogens as a replacement for traditional estrogen replacement therapy. Archives of internal medicine. 2001;161(9):1161–1172. doi: 10.1001/archinte.161.9.1161. http://dx.doi.org/10.1001/archinte.161.9.1161. [DOI] [PubMed] [Google Scholar]
- Isbrucker RA, Burdock GA. Risk and safety assessment on the consumption of Licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regulatory Toxicology and Pharmacology. 2006;46(3):167–192. doi: 10.1016/j.yrtph.2006.06.002. https://doi.org/10.1016/j.yrtph.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Gong P, Madak-Erdogan Z, Martin T, Jeyakumar M, Carlson K, … Helferich WG. Mechanisms enforcing the estrogen receptor β selectivity of botanical estrogens. The FASEB Journal. 2013;27(11):4406–4418. doi: 10.1096/fj.13-234617. https://doi.org/10.1096/fj.13-234617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P, Tunur T, Korol D, Bandara S, Monaikul S, Helferich WG, Schantz S. The effects of estrogenic components of licorice root on a hippocampus-sensitive task. Presented at Society for Neuroscience 2016; November 12–16th; San Diego, California. 2016. [Google Scholar]
- Madak-Erdogan Z, Gong P, Zhao YC, Xu L, Wrobel KU, Hartman JA, … Twaddle NC. Dietary licorice root supplementation reduces diet-induced weight gain, lipid deposition, and hepatic steatosis in ovariectomized mice without stimulating reproductive tissues and mammary gland. Molecular nutrition & food research. 2016;60(2):369–380. doi: 10.1002/mnfr.201500445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggiolini M, Statti G, Vivacqua A, Gabriele S, Rago V, Loizzo M, … Amdò S. Estrogenic and antiproliferative activities of isoliquiritigenin in MCF7 breast cancer cells. The Journal of steroid biochemistry and molecular biology. 2002;82(4):315–322. doi: 10.1016/s0960-0760(02)00230-3. https://doi.org/10.1016/S0960-0760(02)00230-3. [DOI] [PubMed] [Google Scholar]
- Mahady GB, Parrot J, Lee C, Yun GS, Dan A. Botanical dietary supplement use in peri-and postmenopausal women. Menopause. 2003;10(1):65–72. doi: 10.1097/00042192-200310010-00011. https://doi.org/10.1080/13697130802666251. [DOI] [PubMed] [Google Scholar]
- Maronpot RR, Yoshizawa K, Nyska A, Harada T, Flake G, Mueller G, … Ward JM. Hepatic enzyme induction: histopathology. Toxicologic pathology. 2010;38(5):776–795. doi: 10.1177/0192623310373778. https://doi.org/10.1177/0192623310373778. [DOI] [PubMed] [Google Scholar]
- Maruki K, Izaki Y, Hori K, Nomura M, Yamauchi T. Effects of rat ventral and dorsal hippocampus temporal inactivation on delayed alternation task. Brain research. 2001;895(1):273–276. doi: 10.1016/s0006-8993(01)02084-4. https://doi.org/10.1016/S0006-8993(01)02084-4. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Invited review: Estrogens effects on the brain: multiple sites and molecular mechanisms. Journal of applied physiology. 2001;91(6):2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- Mersereau JE, Levy N, Staub RE, Baggett S, Zogric T, Chow S, … Leitman DC. Liquiritigenin is a plant-derived highly selective estrogen receptor β agonist. Molecular and cellular endocrinology. 2008;283(1):49–57. doi: 10.1016/j.mce.2007.11.020. https://doi.org/10.1016/j.mce.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miksicek RJ. Commonly occurring plant flavonoids have estrogenic activity. Molecular Pharmacology. 1993;44(1):37–43. [PubMed] [Google Scholar]
- Muthyala RS, Ju YH, Sheng S, Williams LD, Doerge DR, Katzenellenbogen BS, … Katzenellenbogen JA. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R-and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorganic & medicinal chemistry. 2004;12(6):1559–1567. doi: 10.1016/j.bmc.2003.11.035. https://doi.org/10.1016/j.bmc.2003.11.035. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health (US). Office for Protection from Research Risks. Public Health Service policy on humane care and use of laboratory animals. Office for Protection from Research Risks, National Institutes of Health; 1986. [Google Scholar]
- Neese SL, Korol DL, Katzenellenbogen JA, Schantz SL. Impact of estrogen receptor alpha and beta agonists on delayed alternation in middle-aged rats. Hormones and behavior. 2010a;58(5):878–890. doi: 10.1016/j.yhbeh.2010.08.017. https://doi.org/10.1016/j.yhbeh.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neese SL, Wang VC, Doerge DR, Woodling KA, Andrade JE, Helferich WG, #x02026; &, Schantz SL. Impact of dietary genistein and aging on executive function in rats. Neurotoxicology and teratology. 2010b;32(2):200–211. doi: 10.1016/j.ntt.2009.11.003. https://doi.org/10.1016/j.ntt.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neese SL, Bandara SB, Doerge DR, Helferich WG, Korol DL, Schantz SL. Effects of multiple daily genistein treatments on delayed alternation and a differential reinforcement of low rates of responding task in middle-aged rats. Neurotoxicology and teratology. 2012;34(1):187–195. doi: 10.1016/j.ntt.2011.09.002. https://doi.org/10.1016/j.ntt.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neese SL, Pisani SL, Doerge DR, Helferich WG, Sepehr E, Chittiboyina AG, … Schantz SL. The effects of dietary treatment with S-equol on learning and memory processes in middle-aged ovariectomized rats. Neurotoxicology and teratology. 2014;41:80–88. doi: 10.1016/j.ntt.2013.12.004. https://doi.org/10.1016/j.ntt.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani SL, Neese SL, Katzenellenbogen JA, Schantz SL, Korol DL. Estrogen receptor-selective agonists modulate learning in female rats in a dose-and task-specific manner. Endocrinology. 2016;157(1):292–303. doi: 10.1210/en.2015-1616. https://doi.org/10.1210/en.2015-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler C, Hajirahimkhan A, Lankin DC, Bolton JL, Jones T, Soejarto DD, … Pauli GF. Dynamic residual complexity of the isoliquiritigenin–liquiritigenin interconversion during bioassay. Journal of agricultural and food chemistry. 2013;61(9):2146–2157. doi: 10.1021/jf304445p. https://doi.org/10.1021/jf304445p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan HL, Good M, Dunnett SB. Double dissociation between hippocampal and prefrontal lesions on an operant delayed matching task and a water maze reference memory task. Behavioural brain research. 2006;171(1):116–126. doi: 10.1016/j.bbr.2006.03.030. https://doi.org/10.1016/j.bbr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Srihari S, Shanthibhai PK, Rajagopala S. Efficacy of Glycyrrhiza Glabra Linn (Yastimadhu) in learning, memory and cognitive activity- current findings and future avenues. Global Journal of Research on Medicinal Plants & Indigenous Medicine. 2012;1(6):247. [Google Scholar]
- RC, Obernier JA. Guidelines for the care and use of mammals in neuroscience and behavioral research. Contemporary topics in laboratory animal science. 2004:43.2. [PubMed] [Google Scholar]
- Vandenberg LN. Non-monotonic dose responses in studies of endocrine disrupting chemicals: bisphenol a as a case study. Dose-response. 2014;12(2) doi: 10.2203/dose-response.13-020.Vandenberg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GW, Cai JX. Disconnection of the hippocampal–prefrontal cortical circuits impairs spatial working memory performance in rats. Behavioural brain research. 2006;175(2):329–336. doi: 10.1016/j.bbr.2006.09.002. https://doi.org/10.1016/j.bbr.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Wang VC, Sable HJ, Ju YH, Allred CD, Helferich WG, Korol DL, Schantz SL. Effects of chronic estradiol treatment on delayed spatial alternation and differential reinforcement of low rates of responding. Behavioral neuroscience. 2008;122(4):794. doi: 10.1037/a0012513. http://dx.doi.org/10.1037/a0012513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang VC, Neese SL, Korol DL, Schantz SL. Chronic estradiol replacement impairs performance on an operant delayed spatial alternation task in young, middle-aged, and old rats. Hormones and behavior. 2009;56(4):382–390. doi: 10.1016/j.yhbeh.2009.07.005. https://doi.org/10.1016/j.yhbeh.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widholm JJ, Villareal S, Seegal RF, Schantz SL. Spatial alternation deficits following developmental exposure to Aroclor 1254 and/or methylmercury in rats. Toxicological sciences. 2004;82(2):577–589. doi: 10.1093/toxsci/kfh290. https://doi.org/10.1093/toxsci/kfh290. [DOI] [PubMed] [Google Scholar]