Abstract
Two catalyst systems are described, which together provide mild and general conditions for the Pd-catalyzed C–O cross-coupling of primary alcohols. For activated substrates, such as electron-deficient aryl halides, the commercially available ligand L2 promotes efficient coupling for a variety of alcohol nucleophiles. In the case of unactivated electrophiles, such as electron-rich aryl halides, the new ligand L8 was developed to improve these challenging C–O bond-forming reactions.
Graphical abstract
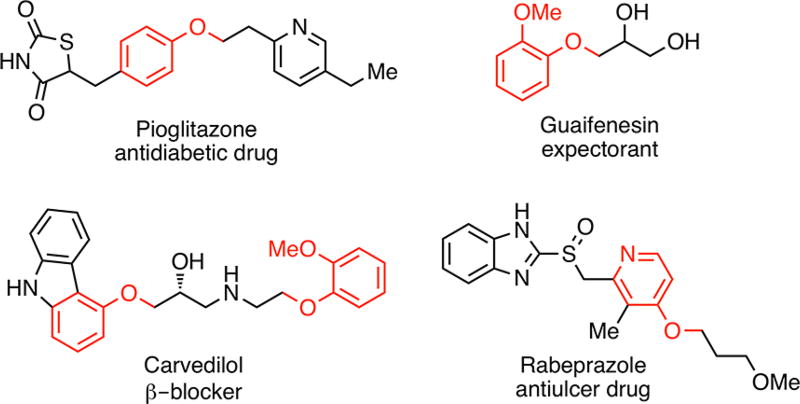
Ethers are important structural components that are prevalent in many natural products,1 pharmaceuticals,2 and agrochemicals.3 In particular, alkyl aryl ethers are featured in approximately 20% of the top 200 pharmaceuticals in 2017 (Figure 1).2a
Figure 1.
Examples of pharmaceutical molecules containing alkyl aryl ethers.
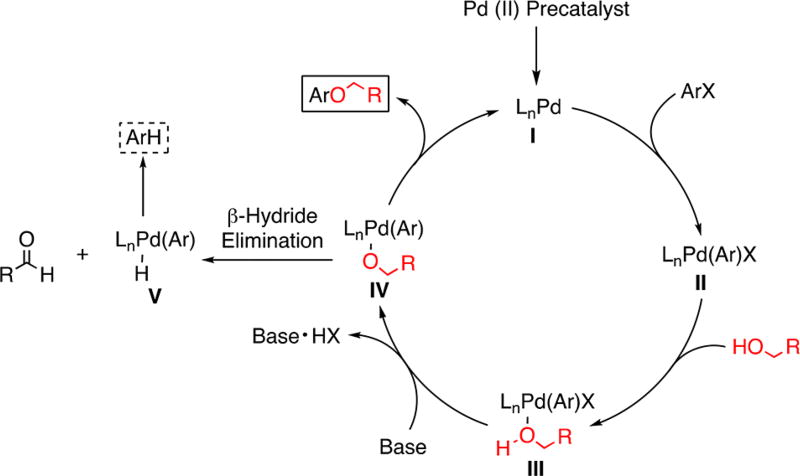
Significant efforts have been devoted to the development of mild and general syntheses of alkyl aryl ethers. Traditional approaches, such as the Williamson ether synthesis,4 the Mitsunobu reaction,5 and nucleophilic aromatic substitution,6 often provide limited functional group compatibilities and restricted substrate scope. In recent years, transition-metalcatalyzed7–9 or metal-free10 transformations have been invented to address these limitations. In particular, Pd-catalyzed O-arylation of aliphatic alcohols has become the subject of significant research activity.8 Unfortunately, the best results are often only realized with activated (i.e., electron-deficient) aryl halides, and in many instances, elevated temperatures are required. The most challenging Pd-catalyzed C–O cross-coupling reactions typically involve unactivated (i.e., electron-rich) aryl halides and alcohols bearing β-hydrogens, including primary alcohols. The generally accepted catalytic cycle for the Pd-catalyzed coupling reactions of aryl halides and alcohols is shown in Scheme 1. Reductive elimination from the intermediate [LnPdII(Ar) (alkoxide)] (IV) is slow in these reactions compared with the analogous process in Pd-catalyzed C–N cross-coupling reactions.11 Thus, competitive β-hydride elimination can occur, leading to the overall reduction of the aryl halide. Despite progress, new and more effective ligands for Pd-catalyzed C–O bond-forming processes are needed.
Scheme 1.
General Catalytic Cycle for Pd-Catalyzed C–O Cross-Coupling of Aryl Halides and Primary Alcohols
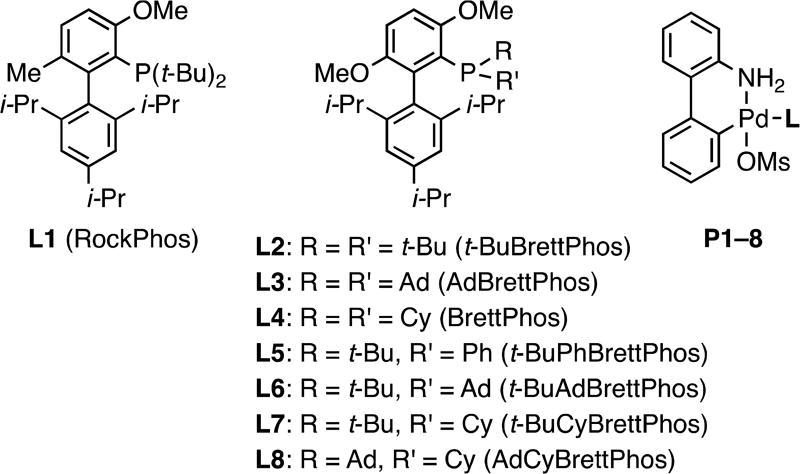
We previously reported RockPhos (L1) (Figure 2) for the coupling of primary and secondary alcohols with aryl halides.8g It was proposed that the conformational rigidity of complex IV, afforded by the biaryl backbone, led to enhanced rates of reductive elimination, thus diminishing the amount of the undesired arene byproduct formed. The use of L1 allowed the preparation of alkyl aryl ethers from an array of (hetero)aryl halides. However, electron-rich aryl chlorides remained challenging and more difficult than the corresponding aryl bromides for this catalyst system. This indicated that reductive elimination was not the only factor controlling the success of the transformation. More recently, our group reported the use of palladacycle precatalyst P2 for the synthesis of short-chain alkyl aryl ethers.8d With the commercially available t-BuBrettPhos (L2), a wide range of (hetero)aryl halides could be converted to the corresponding methyl ethers with 5 equiv of MeOH. Herein we report an expanded scope of compatible alcohol partners using L2, without necessitating the use of a large excess of the alcohol, as well as the development of a new ligand L8 to effect the more challenging coupling processes involving unactivated aryl chlorides.
Figure 2.
Biaryl phosphine ligands and their palladacycle precatalysts.
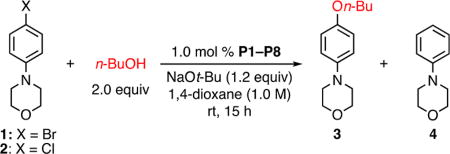
Initially, electron-rich aryl halides containing a p-morpholino substituent (1, 2) and n-BuOH were chosen as model coupling partners. Under conditions similar to those employed in our previous report,8d we compared the reactivities of L1 and L2 in the form of their palladacycle precatalysts P1 and P2. For aryl bromide 1, P2 provided a better ratio of product to reduction byproduct (Table 1, entries 1 and 2). However, neither ligand afforded more than 40% conversion in the case of aryl chloride 2 (Table 1, entries 3 and 4), consistent with previous observations of the lower reactivity of aryl chlorides compared with aryl bromides.
Table 1.
Ligand Evaluation for Pd-Catalyzed C–O Cross-Coupling of Electron-Rich Aryl Halides with n-BuOHa
| |||||
|---|---|---|---|---|---|
|
| |||||
| entry | X | ligand | conversion (%)b | yield of 3 (%)c | yield of 4 (%)c |
| 1 | Br | L1 | 100 | 84 | 13 |
| 2 | Br | L2 | 100 | 96 | 3 |
| 3 | Cl | L1 | 36 | 29 | 7 |
| 4 | Cl | L2 | 33 | 25 | 3 |
| 5 | Cl | L3 | 24 | 22 | 3 |
| 6 | Cl | L4 | 5 | – | 4 |
| 7 | Cl | L5 | 20 | – | 9 |
| 8 | Cl | L6 | 27 | 19 | 7 |
| 9 | Cl | L7 | 56 | 38 | 12 |
| 10 | Cl | L8 | 88 | 80 | 3 |
| 11d | Cl | L8 | 100 | 97 | – |
Reaction conditions: ArX (0.5 mmol), n-BuOH (1.0 mmol), NaOt-Bu (0.6 mmol), P1–P8 (1.0 mol %), 1,4-dioxane (0.5 mL, 1.0 M), rt, 15 h.
Determined by GC using an internal standard.
Determined by 1H NMR spectroscopy using an internal standard.
P8 (1.2 mol %).
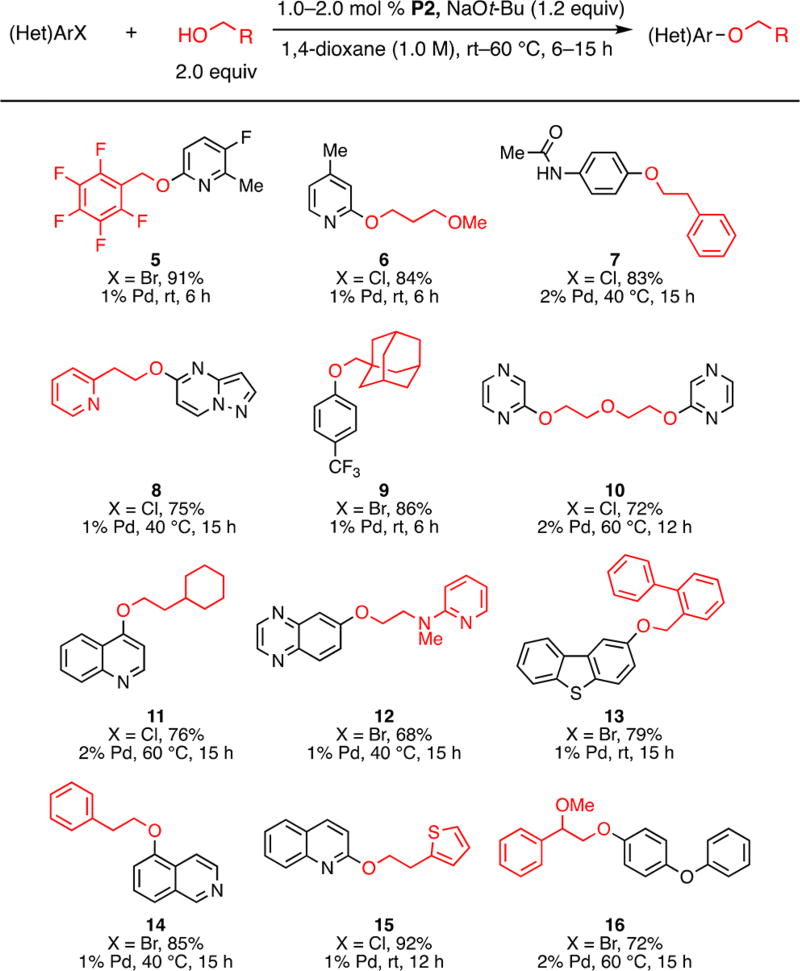
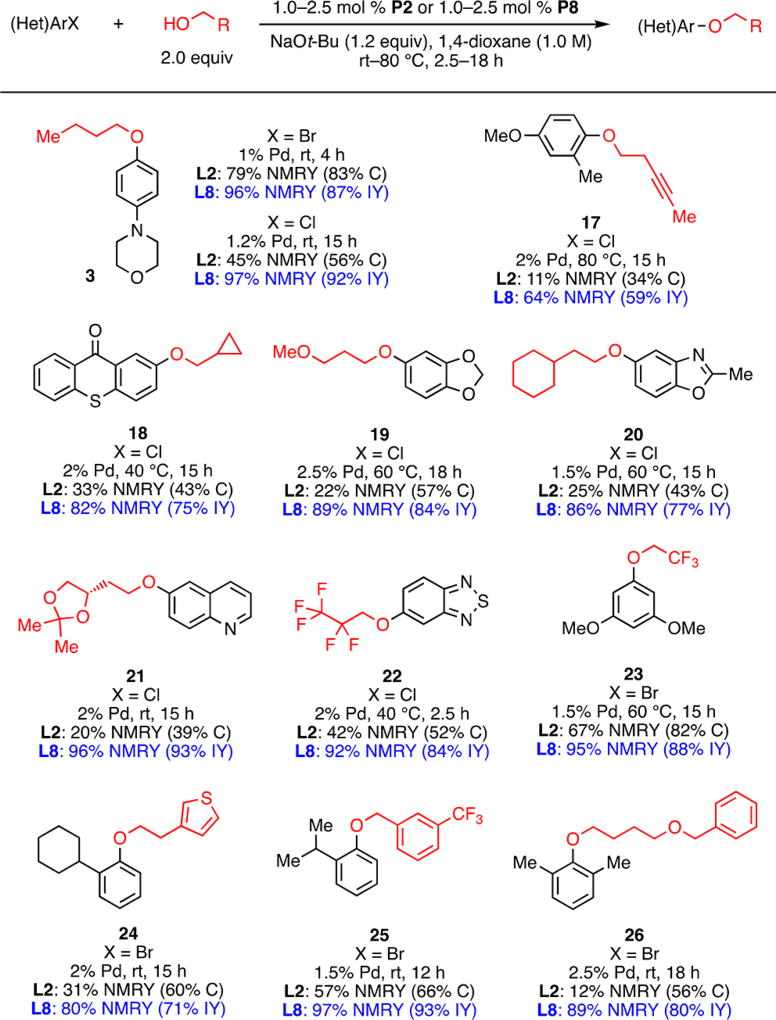
Although further ligand development was clearly necessary to address the reactions of the most challenging unactivated aryl chlorides, we noted that the commercially available ligand L2 was already highly effective in coupling reactions of activated aryl halides with a more diverse range of alcohols than previously reported (Scheme 2). Moreover, these couplings took place under mild conditions (rt–60 °C) and in many instances necessitated the use of only 1 mol % catalyst. Pharmaceutically relevant alcohols, such as fluorinated benzyl alcohol (to prepare 5), 3-methoxyl-1-propanol (to prepare 6), and 1-adamantanemethanol (to prepare 9), readily underwent C–O bond formation. Further, efficient cross-coupling was achieved for a variety of (hetero)aryl halides. Heterocycles, such as pyridines (5, 6, 8, 12), quinolines (11, 15), an isoquinoline (14), a thiophene (15), a pyrazine (10), a quinoxaline (12), a dibenzothiophene (13) and a pyrazolopyrimidine (8), were tolerated either in the aryl halide or in the alcohol substrate. Compounds 8 and 14, which were previously prepared by Maligres and co-workers at Merck,8e can be obtained in similar yields under significantly improved conditions: the catalyst loading was reduced from 10 to 1 mol %, and the temperature was reduced from 80 to 40 °C.
Scheme 2.
Pd-Catalyzed C–O Cross-Coupling of Activated Aryl Halides with Primary Alcoholsa,b
aReaction conditions: ArX (1.0 mmol), n-BuOH (2.0 mmol), NaOt-Bu (1.2 mmol), P2 (1.0–2.0 mol %), 1,4-dioxane (1.0 mL, 1.0 M), rt–60 °C, 6–15 h. bThe reported isolated yields are average results of two runs.
Next, since efficient coupling of certain (hetero)aryl chlorides could not be achieved with L2 (see Scheme 3 for examples), we considered potential ligand modifications to achieve improvement in these cases.
Scheme 3.
Pd-Catalyzed C–O Cross-Coupling of Unactivated Substratesa,b,c,d
aReaction conditions: ArX (1.0 mmol), n-BuOH (2.0 mmol), NaOt-Bu (1.2 mmol), P2/P8 (1.0–2.5 mol %), 1,4-dioxane (1.0 mL, 1.0 M), rt–80 °C, 2.5–18 h. bNMRY: yields determined by 1H NMR spectroscopy using an internal standard. cC: conversions determined by GC using an internal standard. dIY: average isolated yields from two runs.
As previously mentioned, successful Pd-catalyzed C–O cross-coupling reactions require that C–O reductive elimination be fast relative to competing β-hydride elimination. Our group12 and others13 have demonstrated that increasing the steric bulk around the palladium center facilitates reductive elimination processes. Thus, we envisioned that congeners of L2 bearing larger –P(R)R′ units might exhibit improved performance. In particular, the bulky ligand L3 containing two adamantyl groups is effective in Pd-catalyzed C–N cross-coupling of five-membered heterocyclic halides12a as well as in Pd-catalyzed C–F cross-coupling,14 two cases where reductive elimination is known to be difficult. However, employing L3 (in the form of its precatalyst) in our reaction resulted in only 24% conversion and 22% yield of the desired product (Table 1, entry 5). Likewise, with the smaller ligand BrettPhos (L4), poor conversion (5%) was observed (Table 1, entry 6). These results, combined with our recent work on the arylation of hindered, α-trisubstituted primary amines using a hybrid CPhos-based ligand,15 suggested to us that finer tuning of the steric and electronic environment near the palladium center may be necessary. Indeed, testing different combinations of R and R′ units with varying sizes and electronic properties (Table 1, entries 7–10) revealed that the use of L8 significantly improved the cross-coupling of 2 with n-BuOH (Table 1, entry 10). Increasing the loading of P8 to 1.2 mol % led to full conversion of the aryl halide and a 97% yield of the product in 15 h when the reaction was carried out at room temperature (Table 1, entry 11). These results further demonstrated that a successful ligand must be able to faciliate reductive elimination, as indicated by the poor performance of the small ligand L4. Meanwhile, the observation that the medium-sized ligand L8 provided the most efficient C–O bond formation suggested that reductive elimination should not be the sole consideration for ligand design.
The substrate scope of Pd-catalyzed C–O cross-coupling between unactivated aryl halides and alcohols was explored using L8, with a comparison to L2 under identical conditions (Scheme 3). In general, the use of L8 provided an improvement in both conversions and yields. Notably, coupling reactions of electron-rich aryl chlorides (3, 17, 19) and challenging heterocyclic substrates (18, 20–22) all proceeded to full conversion with L8 and exhibited up to a 76% increase (21) in the yield (by 1H NMR analysis) compared with the same reactions using L2. A small rate enhancement was also observed for the electron-rich aryl bromide (3). In addition, the use of L8 proved to be superior to L2 for ortho-substituted hindered substrates (24–26). Lastly, fluorinated primary alcohols, which have decreased nucleophilicity, were also arylated more efficiently with L8 (22, 23).
In conclusion, a mild and general procedure for the Pd-catalyzed C–O cross-coupling of primary alcohols has been developed. The commercial ligand L2 was effective in the O-arylation of several pharmaceutically relevant and heterocycle-containing alcohols with activated (hetero)aryl halides. Additionally, a new hybrid biaryl phosphine ligand, L8, was effective for the more challenging coupling of unactivated (hetero)aryl halides, including many instances where employing L2 failed to deliver synthetically useful conversions and yields. We are undertaking mechanistic studies to rationalize more precisely the better performance of L8 and gain general insight into the mechanism.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institutes of Health (GM-58160 and GM-122483). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Heath. The Varian 500 MHz instrument used for portions of this work was supported by the National Science Foundation (Grant CHE 9808061). We acknowledge Sigma-Aldrich for the generous donations of t-BuBrettPhos (L2), BrettPhos (L4), 1-adamantylzinc bromide solution, and 2-iodo-2′,4′,6′-triisopropyl-3,6-dimethoxybiphenyl used in this work. Dr. Nayouki Hoshiya (MIT) was the first to synthesize L5 and L8. We thank Jeffrey Yang (MIT), Dr. Kurt Armbrust (MIT), and Dr. Nicholas White (MIT) for helpful discussions. We thank Richard Liu (MIT), Dr. Andy Thomas (MIT), and Dr. Christine Nguyen (MIT) for their assistance with the preparation of this article.
Footnotes
ASSOCIATED CONTENT
- Experimental procedures and characterization data for new compounds (PDF)
The authors declare the following competing financial interest(s): MIT has or has filed patents on ligands/precatalysts that are described in the paper from which S.L.B. and former co-workers receive royalty payments.
References
- 1.(a) Fuwa H. Toxins and Biologically Active Compounds from Microalgae. Vol. 1. CRC Press; Boca Raton, FL: 2014. Total Synthesis of Marine Polycyclic Ether Natural Products; pp. 348–412. [Google Scholar]; (b) Pitsinos EN, Vidali VP, Couladouros EA. Eur. J. Org. Chem. 2011;2011:1207. [Google Scholar]; (c) Nicolaou KC, Boddy CNC, Bräse S, Winssinger N. Angew. Chem. Int. Ed. 1999;38:2096. doi: 10.1002/(sici)1521-3773(19990802)38:15<2096::aid-anie2096>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 2.(a) [accessed Nov 9, 2017];Top Drugs. http://www.pacific.edu/Academics/Schools-and-Colleges/Thomas-J-Long-School-of-Pharmacy-and-Health-Sciences/Academics/Doctor-of-Pharmacy/Curriculum-and-Courses/Top-Drugs.html.; (b) Roughley SD, Jordan AM. J. Med. Chem. 2011;54:3451. doi: 10.1021/jm200187y. [DOI] [PubMed] [Google Scholar]
- 3.(a) Stevenson TM, Reddy RP. WO2017011288A1. 2017 [Google Scholar]; (b) Wang X, Liu A, Cao L, Lei M, Ren Y, Zhou B, Huang L, Gao D, Chen H, Cheng L. CN105985246A. 2016 [Google Scholar]
- 4.Wang Z. Comprehensive Organic Name Reactions and Reagents. John Wiley & Sons; Hoboken, NJ: 2010. Williamson Ether Synthesis; pp. 3026–3030. [Google Scholar]
- 5.Swamy KCK, Kumar NNB, Balaraman E, Kumar KVPP. Chem. Rev. 2009;109:2551. doi: 10.1021/cr800278z. [DOI] [PubMed] [Google Scholar]
- 6.Caron S, Ghosh A. Practical Synthetic Organic Chemistry. John Wiley & Sons; Hoboken, NJ: 2011. Nucleophilic Aromatic Substitution; pp. 237–253. [Google Scholar]
- 7.Keerthi Krishnan K, Ujwaldev SM, Sindhu KS, Anilkumar G. Tetrahedron. 2016;72:7393. [Google Scholar]
- 8.For examples of Pd-catalyzed C–O cross-coupling see: Sawatzky RS, Hargreaves BKV, Stradiotto M. Eur. J. Org. Chem. 2016;2016:2444.Rangarajan TM, Brahma R, Ayushee, Prasad AK, Verma AK, Singh RP. Tetrahedron Lett. 2015;56:2234.Rangarajan TM, Singh R, Brahma R, Devi K, Singh RP, Singh RP, Prasad AK. Chem. - Eur. J. 2014;20:14218. doi: 10.1002/chem.201404121.Cheung CW, Buchwald SL. Org. Lett. 2013;15:3998. doi: 10.1021/ol401796v.Maligres PE, Li J, Krska SW, Schreier JD, Raheem IT. Angew. Chem. Int. Ed. 2012;51:9071. doi: 10.1002/anie.201203460.Gowrisankar S, Neumann H, Beller M. Chem. - Eur. J. 2012;18:2498. doi: 10.1002/chem.201103347.Wu X, Fors BP, Buchwald SL. Angew. Chem. Int. Ed. 2011;50:9943. doi: 10.1002/anie.201104361.Gowrisankar S, Sergeev AG, Anbarasan P, Spannenberg A, Neumann H, Beller M. J. Am. Chem. Soc. 2010;132:11592. doi: 10.1021/ja103248d.Milton EJ, Fuentes JA, Clarke ML. Org. Biomol. Chem. 2009;7:2645. doi: 10.1039/b907784g.Withbroe GJ, Singer RA, Sieser JE. Org. Process Res. Dev. 2008;12:480.Vorogushin AV, Huang X, Buchwald SL. J. Am. Chem. Soc. 2005;127:8146. doi: 10.1021/ja050471r.Torraca KE, Huang X, Parrish CA, Buchwald SL. J. Am. Chem. Soc. 2001;123:10770. doi: 10.1021/ja016863p.Palucki M, Wolfe JP, Buchwald SL. J. Am. Chem. Soc. 1997;119:3395.Mann G, Hartwig JF. J. Org. Chem. 1997;62:5413.Mann G, Hartwig JF. J. Am. Chem. Soc. 1996;118:13109.
- 9.For examples of Cu-catalyzed C–O cross-coupling, see: Lam PYS. Synthetic Methods in Drug Discovery. Vol. 1. Chapter 7. Royal Society of Chemistry; Cambridge, U.K: 2016. Chan–Lam Coupling Reaction: Copper-Promoted C–Element Bond Oxidative Coupling Reaction with Boronic Acids; pp. 242–273.Lin H, Sun D. Org. Prep. Proced. Int. 2013;45:341. doi: 10.1080/00304948.2013.816208.Niu J, Zhou H, Li Z, Xu J, Hu S. J. Org. Chem. 2008;73:7814. doi: 10.1021/jo801002c.Altman RA, Shafir A, Choi A, Lichtor PA, Buchwald SL. J. Org. Chem. 2008;73:284. doi: 10.1021/jo702024p.Zhang H, Ma D, Cao W. Synlett. 2007;2007:0243.Wolter M, Nordmann G, Job GE, Buchwald SL. Org. Lett. 2002;4:973. doi: 10.1021/ol025548k.
- 10.For examples of metal-free C–O cross-coupling, see: Sundalam SK, Stuart DR. J. Org. Chem. 2015;80:6456. doi: 10.1021/acs.joc.5b00907.Shen X, Neumann CN, Kleinlein C, Goldberg NW, Ritter T. Angew. Chem. Int. Ed. 2015;54:5662. doi: 10.1002/anie.201500902.Ghosh R, Lindstedt E, Jalalian N, Olofsson B. ChemistryOpen. 2014;3:54. doi: 10.1002/open.201402006.Liu Q, Lu Z, Ren W, Shen K, Wang Y, Xu Q. Chin. J. Chem. 2013;31:764.Lindstedt E, Ghosh R, Olofsson B. Org. Lett. 2013;15:6070. doi: 10.1021/ol402960f.Sach WN, Richter DT, Cripps S, Tran-Dubé M, Zhu H, Huang B, Cui J, Sutton SC. Org. Lett. 2012;14:3886. doi: 10.1021/ol301615z.Merritt EA, Olofsson B. Angew. Chem. Int. Ed. 2009;48:9052. doi: 10.1002/anie.200904689.
- 11.(a) Hartwig JF. Inorg. Chem. 2007;46:1936. doi: 10.1021/ic061926w. [DOI] [PubMed] [Google Scholar]; (b) Widenhoefer RA, Buchwald SL. J. Am. Chem. Soc. 1998;120:6504. [Google Scholar]
- 12.(a) Su M, Buchwald SL. Angew. Chem. Int. Ed. 2012;51:4710. doi: 10.1002/anie.201201244. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Aranyos A, Old DW, Kiyomori A, Wolfe JP, Sadighi JP, Buchwald SL. J. Am. Chem. Soc. 1999;121:4369. [Google Scholar]
- 13.(a) Yamashita M, Hartwig JF. J. Am. Chem. Soc. 2004;126:5344. doi: 10.1021/ja0315107. [DOI] [PubMed] [Google Scholar]; (b) Mann G, Shelby Q, Roy AH, Hartwig JF. Organometallics. 2003;22:2775. [Google Scholar]
- 14.Lee HG, Milner PJ, Buchwald SL. Org. Lett. 2013;15:5602. doi: 10.1021/ol402859k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruiz-Castillo P, Blackmond DG, Buchwald SL. J. Am. Chem. Soc. 2015;137:3085. doi: 10.1021/ja512903g. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.