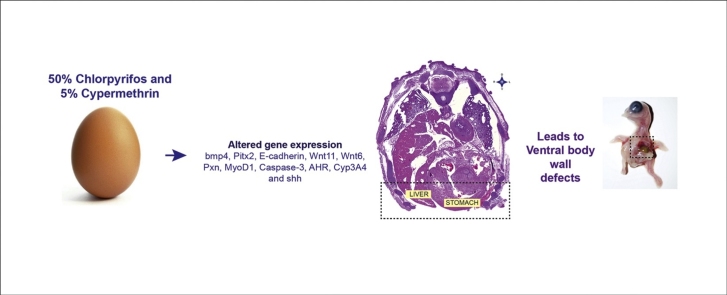
Graphical abstract
Keywords: Ventral body wall defect, Combination insecticide, Chick embryo
Highlights
-
•
Chlorpyrifos and cypermethrin treatment induced developmental anomalies in chicks.
-
•
Protrusion of visceral organs and microphthalmia were the major anomalies observed.
-
•
Treated embryos were conspicuous with incomplete ventral body wall and sternum.
-
•
Altered expression pattern of E-cadherin, Shh, bmp4, Wnt11 and Pitx2 were recorded.
-
•
Impairment of major regulators of development is suspected to induce VBWD.
Abstract
Pesticide exposure to the non target groups especially during embryonic development has quite often resulted in congenital malformations. A commercially available combination insecticide (Ci, 50% chlorpyrifos and 5% cypermethrin) is known to induce ventral body wall defects (VBWDs) wherein abdominal viscera protrude out of the ventral body wall. Herein, an attempt was made to understand the mechanistic insight into Ci induced VBWDs. For this, before incubation, the chick embryos were dosed with the test chemical and then at different developmental stages of incubation, they were monitored for the changes in the expression of certain genes, which are indispensable for the ventral body wall closure since they regulate the cell fate, proliferation and survival. Concurrently, histopathological changes during the embryonic development were examined to corroborate the above observations. The results of mRNA profiling revealed a significant downregulation of Shh on day 4 and upregulation on day 10, while bmp4, Pitx2, E-cadherin, Wnt11, Wnt6, Pxn, MyoD1, Caspase-3, AHR, Cyp3A4, showed a significant upregulation on day 4 and/or on day 10. N-cadherin, fgf8, bmp1 showed no significant changes. The possible means by which these skewed expression patterns of regulatory molecules culminated into the VBWD are discussed.
1. Introduction
Abdominal wall defects are the most commonly occurring congenital malformations classified as ventral body wall defects (VBWDs). This group includes deformities such as ectopia cordis, gastroschisis, omphalocele, bladder exstrophy etc [1]. Majority of these defects do not have a genetic basis, but are caused by a plethora of environmental insults. There are various hypotheses explaining how the ventral body wall defects arise and one of those theories which gained support was proposed by Duhamel [2]. The theory attributes this to teratogenic insult to the lateral body folds or during the process of organogenesis, which results in celosomia (hernia of abdominal wall). These folds arise from the parietal layer of lateral plate mesoderm (LPM) and continue to move ventrally towards the midline where they fuse [3]. This fusion process involves a well-orchestrated cell migration, cell proliferation and production of extracellular matrix [[4], [5]]. In addition to these folds, the potential forces that contribute to the movement of the body wall come from the somites. These blocks of cells in paraxial mesoderm further differentiate into sclerotome, myotome and dermatome regions. Some of the cells from these derivatives assist the parietal layer of LPM to form the lateral body wall folds [1]. Any alteration in above mentioned morphogenetic processes and organogenetic stage will produce an abnormal morphology [2].
Experimental studies carried out in mice and chicken relied on gene knockouts, surgery, irradiation and exposure to environmental chemicals to produce VBWDs [[6], [7], [8], [9]]. Among the environmental factors, the pesticide exposure has growing concerns over their potency of inflicting teratogenic effects. More so when there is a simultaneous multiple exposure [[10], [11]]. Previous work from our laboratory has shown that an exposure to a combination insecticide (Ci) causes a variety of birth defects like distorted cephalization, umbilical hernia, ectopic viscera, spina bifida and anophthalmia at sub-lethal doses in the developing chick embryo [[12], [13]]. Teratogenic potential of Ci was also reported to be passed onto the succeeding generation wherein only the parental generation received the dose of Ci (in-ovo, preincubation exposure) as reported by Khan et al. [14]. However, the precise molecular signalling which the Ci disrupts during the early embryogenesis and causes VBWD still stays unknown.
The events of embryonic development like cell migration, proliferation and production of extracellular matrix are all vital for the completion of organogenesis and therefore, for the closure of ventral abdominal body wall too. Here we hypothesized that Ci interrupts the expression patterns of certain early molecular signals which take up the function of embryonic events like cell proliferation, survival as well as patterning and hence might have led to the ventral body wall defects. Therefore, the overall objective of the current study was to understand the finer mechanisms by which Ci interrupts ventral body wall patterning during early embryogenesis of domestic hen.
2. Materials and methods
2.1. Test substance
A commercially available insecticide formulation retailed as Anaconda 505™ was used for the study. It constituted a combination of chlorpyrifos (50%) and cypermethrin (5%) and manufactured by AIMCO Pesticides Limited, Mumbai, India.
2.2. Egg procurement and insecticide injections
Freshly laid fertile eggs of Rhode Island Red breed of domestic chicken were obtained from the Intensive Poultry Development Unit, Vadodara, India. Eggs were wiped clean with 0.5% povidone iodine solution to remove any external contamination and were kept at 4 °C until use. The concentration of sub-lethal dose was decided from the previous work done in the lab [12]. The eggs were dosed by air sac method as devised by Blankenship et al. [15], on day “0” of incubation. The control group received olive oil (Figaro, India) and the treatment group was administered with 0.05 μg per egg of Ci with olive oil as vehicle, in a sterile environment in laminar air hood. The injection volume was kept 50 μl/egg. After dosing, the point of injection on the eggs was sealed by molten paraffin wax.
2.3. Incubation
Automated incubator (Scientific Equipment Works, New Delhi) was set at a temperature of 37.5 °C ± 0.5 and relative humidity of 70–75% for incubation. The eggs were kept with their broad ends facing upwards and were turned automatically every 1 h. The eggs were candled every two days and the dead ones were scrapped out. The experimental protocols were in full compliance with the guidelines of Drugs and Cosmetics rules 1945, Appendix-III animal care standard and were approved by the institutional animal ethics committee constituted as per the laid norms of the Indian regulatory authority (Protocol No. IAEC 84/08/2014-2).
2.4. Rate of mortality and malformations
After incubation, the embryos that had reached Hamilton-Hamburger stages 13 (Day 2), 24 (Day 4) and 36 (Day 10) were collected. The somites were observed in Day 2 embryos while rate of mortality and malformations were recorded in the day 4 and day 10 embryos. The experiment was performed thrice with n = 30 eggs each time.
2.5. Histological studies
Embryos were isolated, rinsed in PBS and then were fixed in 10% neutral buffer formalin. The tissue was further processed and paraffin wax blocks of the tissue samples were prepared. Longitudinal sections (5 μm) of day 4 embryos and cross sections of 5 μm thickness of Day 10 embryos were taken and stained with Harri’s haematoxylin and eosin. The histological details of the tissues on the slide were visualized using Leica DM2500 Microscope and pictures were captured using EC3 Camera (utilizing LAS EZ software).
2.6. RNA isolation and real time reverse transcription polymerase chain reaction
The chick embryos were quickly dissected to excise the abdominal tissue. From this sample, total RNA was isolated by TRIzol reagent (Applied Biosystems, USA) as per the recommended protocol. 1 μg of total RNA was reverse transcribed to cDNA using a one-step cDNA synthesis kit (Applied Biosystems, USA) as per manufacturer’s protocol. Primer sequences used in the study are given in Table 1. Quantitative real-time PCR was performed using LightCycler 96 (Roche Diagnostics, Switzerland). After initial denaturation step of 3 min at 95 °C, 42 cycles of amplification for each primer was carried out. Each cycle included a denaturation step for 10 s at 95 °C, an annealing step for 10 s at 60 °C and elongation for 10 s at 72 °C. Melt curve analysis was used to confirm specific product formation. Data was represented as mean Cq values normalized with 18S rRNA (endogenous control) levels and fold change in expression was calculated using the 2−ΔΔCq method of Livak and Schmittgen [16].
Table 1.
Primer sequences obtained from NCBI.
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Shh | TGCTAGGGATCGGTGGATAG | ACAAGTCAGCCCAGAGGAGA |
| Pitx2 | GCTACCCCGACATGTCCAC | TTCTTGAACCAAACCCGAACC |
| E-cadherin | GAAGACAGCCAAGGGCCTG | TCTGGTACCCCTACCCTCTTG |
| bmp4 | AGCCCACGGAGTTTGTAGTG | TTTGGTCCTTTTCTGAGGCCC |
| Caspase-3 | AAAGATGGACCACGCTCAGG | TGACAGTCCGGTATCTCGGT |
| vimentin | GACCAGCTGACCAACGACAA | GAGGCATTGTCAACATCCTGTC |
| fgf8 | GAGACCGACACCTTTGGGAG | TTGCCGTTACTCTTGCCGAT |
| N-cadherin | AGCCCACGGAGTTTGTAGTG | TTTGGTCCTTTTCTGAGGCCC |
| bmp1 | CCAGCAAAGTGTGTGTGTGG | GAGGCGCTTTTGATGTCGTC |
| pcna | TGTTCCTCTCGTTGTGGAGT | TCCCAGTGCAGTTAAGAGCC |
| Wnt11 | GACCTGGGTATCGATGGGGA | GGCTTTCAAGACCTGTCTCC |
| Wnt6 | TTGGTCATGGACCCCAACAG | CCTCGCTGACGATTTCTGGT |
| Pxn | TCTGACTTTAAGTTCATGGCACAG | TCGCTACCCCCAGTTTGTTC |
| MyoD1 | CGGAATCACCAAATGACCCAA | ATCTGGGCTCCACTGTCACT |
| AHR | ACCTGTGCAGAAAATAGTAAAGCC | GCTGAGCCTAAGCACAGACA |
| Cyp1A1 | ACCACGACGAGAAGATCTGG | AGATCAGCACCTTGTCAGCC |
| Cyp3A4 | AGTGCAATGGGACTCCTTCC | GGCCATATCCCATAGAGCACC |
| Cyp3A5 | TGGGTATGAGCCCACCAGTA | CATACGTGAGCGGAGCCTTA |
| 18S rRNA | GGCCGTTCTTAGTTGGTGGA | TCAATCTCGGGTGAAC |
2.7. Western blot
Tissue from the abdomen region was collected from 4 embryos of each experimental group and homogenized in Tris-SDS lysis buffer with protease inhibitor (Sigma Aldrich, USA). 10% homogenates were assayed for total protein content by Bradford method [17] Equal amount of total protein was loaded and separated by SDS-PAGE on 12% gels. Protein was transferred onto PVDF by semi-dry transfer at 100 mA for 20 min. The membrane was probed separately with anti-Shh, anti-β-Actin (IgG antibodies raised in Mouse) and anti-Caspase-3 (IgG antibodies raised in Rabbit). Alkaline phosphatase system was used for staining the protein of interest.
2.8. Statistical analysis
Abnormalities in somite number was subjected to Mann-Whitney U Test to compare differences between the groups. The rest of the data was analysed by two-tailed Student’s t-test using Prism v5.03 (GraphPad Software Inc., USA). Values were expressed as Mean ± SEM, and the differences between the control and treated groups were considered significant when the P value was less than or equal to 0.05.
3. Results
3.1. Rate of mortality and malformation
The pesticide dosed embryos were opened for examination on day 2 (stage 13), day 4 (stage 24) and day 10 (stage 36). The stage-13 control embryos showed the standard features i.e. presence of twenty somites, broad curves in cranial and cervical flexures and well-established stalk. The embryos treated with 0.05 μg of Ci after incubation of 48 h showed significantly reduced number of somites. 33% of the embryos showed abnormal somite disposition (Table 2). High rate of mortality and anomalies were observed in the treatment group. A steep rise (p ≤ 0.001) was observed in the mortality of embryos in pesticide dosed groups as compared to the control and it continued to increase as the embryo developed to day 10 (Table 3). As the study was focused on ventral body wall defects, the rate of occurrence of these malformations was checked. It was observed that 60.6% of the total live embryos observed on day 10 had defects in their abdominal wall closure (Table 4).
Table 2.
Reduction in somite numbers: Values are expressed as mode with range in parenthesis; n = 3 with 30 eggs per group per experiment; *p ≤ 0.05, **p ≤ 0.01.
| Group | Day 2 (Stage-13) |
|
|---|---|---|
| Somite number | % of embryos with abnormal somites | |
| Control | 18 (17, 19) | 6.0 ± 0.57 |
| Treated | 16 (15, 18)* | 33 ± 1.73** |
Table 3.
Mortality on day 2, day 4 and day 10 for sub-lethal dose Combination insecticide: The values represent mean ± standard error of mean; n = 3 with 30 eggs per group per experiment; **p ≤ 0.01, ***p ≤ 0.001.
| Group | Day 2 | Day 4 | Day 10 |
|---|---|---|---|
| Control | 3.66 ± 0.19 | 3 ± 0.28 | 3.66 ± 0.50 |
| Treated | 10.66 ± 0.35** | 12.33 ± 0.57*** | 14.66 ± 0.58*** |
Table 4.
Percent malformation on day 10 from live embryos: The values represent mean ± standard error of mean; n = 3 with 30 eggs per group per experiment; ***p ≤ 0.001.
| Group | Number of live embryos of Day 10 | % of embryos with malformations |
|---|---|---|
| Control | 26.33 ± 0.52 | 7.6 ± 0.52 |
| Treated | 15.33 ± 0.58*** | 60.6 ± 1.18*** |
3.2. Teratological malformations
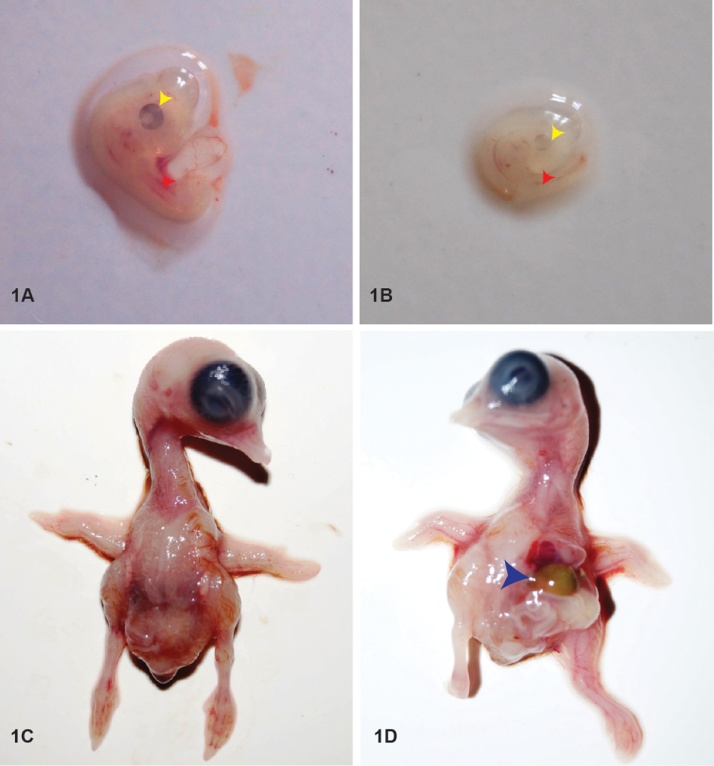
When the embryos were examined at stage 24 (96 h), the controls showed normal development with distinct eye pigmentation and limb buds (Fig. 1A). However, defects like microphthalmia, and stunted growth were found in 96 h incubated treated embryos (Fig. 1B). While the control embryos (Fig. 1C) examined on day 10 showed normal patterns of development of visceral organs and the abdominal wall, the treated embryos showed protrusion of heart, bowel and liver due to defect in midline fusion of abdominal wall (Fig1D). The other associated anomalies included anophthalmia, phocomelia, hematomas, and defect in ventral body wall closure in the thoracic region.
Fig. 1.
Ci induced structural anomalies: A) Control embryo of day4 with well-developed eye (yellow arrowhead) and limb buds (red arrowhead); B) Ci treated day 4 embryo with under developed eye (yellow arrowhead) and appendages (red arrowhead). C) and D) shows Day 10 − Control and Treated embryo respectively. The blue arrow head indicates protrusion of heart, liver and bowel without any covering sac in the treated embryo.
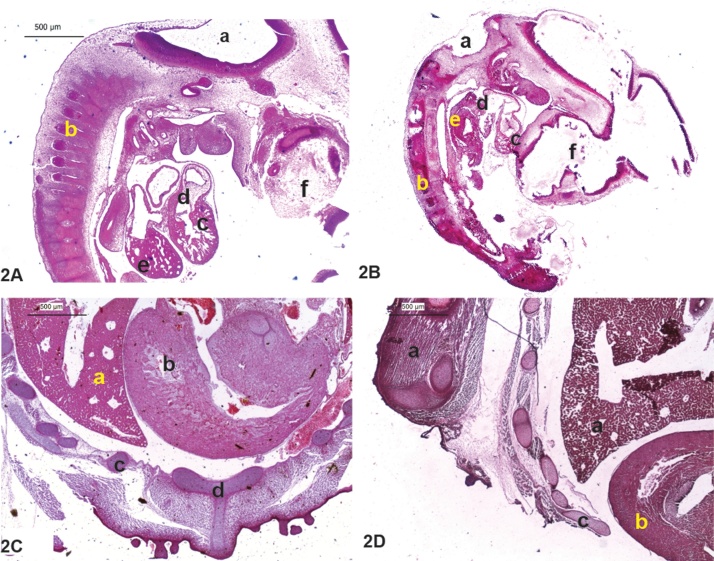
3.3. Histopathology
The extent of damage caused by the pesticide load in the tissue architecture was studied by differential staining in day 4 and day 10 chick embryos by using hematoxylin and eosin. The control embryos on day 4 showed properly arranged somites with well-developed myelencephalon and optic cup (Fig. 2A). The heart was well formed with clear demarcation between atrium and the ventricle. The treated embryo showed stunted growth, with a reduction in the somite numbers and distorted optic cup (Fig. 2B). The histological picture of day 10 treated embryos showed less developed musculature, and the internal organs were left uncovered. Absence of sternum in the treated embryo was a crucial observation (Fig. 2C and D).
Fig. 2.
Photomicrograph of Day4 and Day 10 chick embryo: longitudinal sections of A) Day 4 Control with well patterned structures; B) Day 4 Treated with reduced somites and distorted optic cup. Where, a: Myelencephalon; b: Somite; c: ventricle; d: Atrium; e: Liver-prominence, f: optic cup C) Cross section (insight) of abdominal region from day 10 control embryo shows well-formed abdominal wall; Figure D) Cross section (insight) Day 10 Treated embryo showing improper closure of the ventral part of the body. Where, a: liver; b: stomach; c: Inter coastal muscles; d: Sternum.
3.4. Relative mRNA expression levels
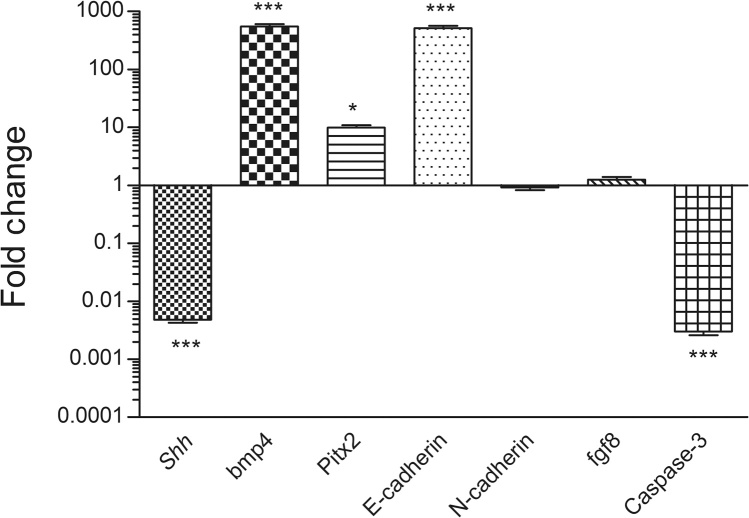
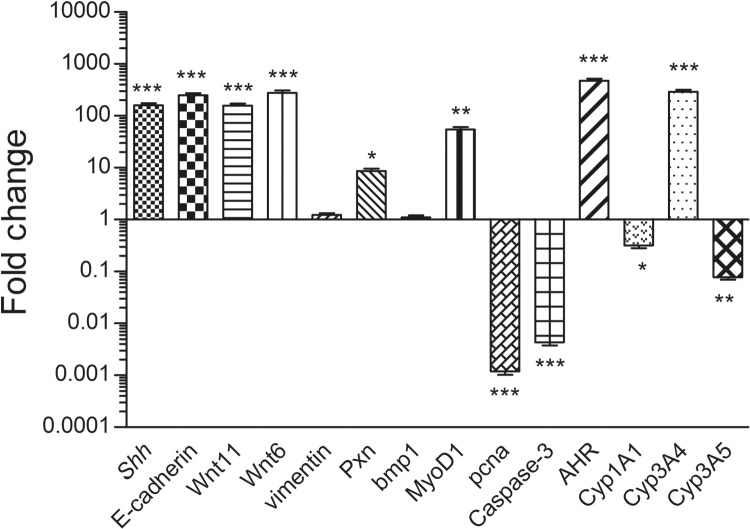
In day 4 embryos, the relative mRNA expression levels of Shh were found to be significantly lowered (p ≤ 0.001) in the treatment groups when compared to the controls, the bmp4 (p ≤ 0.001), Pitx2 (p ≤ 0.05), E-cadherin (p ≤ 0.001) and Caspase-3 (p ≤ 0.001) were found to be significantly increased in the treated group. No significant changes were seen in case of N-cadherin and fgf8 expression (Fig. 3).
Fig. 3.
Transcript levels for Ci treated Day 4 embryo: Fold change expression values for the genes. Fold change values for control embryo is 1.0 for all the genes, n = 10 eggs/group.
The qRT-PCR analysis in day 10 embryos showed that along with Shh, the E-cadherin, Wnt11 and Wnt6 expressions were significantly upregulated (p ≤ 0.001). The change in expression levels of vimentin were found statistically insignificant. Pxn (p ≤ 0.05) was found to show an increased expression. While the bmp1 supposedly remained unchanged, the levels of MyoD1 have significantly gone high (p ≤ 0.01) in the treated embryos. The pcna and Caspase-3 showed a highly significant (p ≤ 0.001) decrease and increase respectively. AHR (p ≤ 0.001) and Cyp3A4 (p ≤ 0.05) expression increased with significance, while Cyp1A1 (p ≤ 0.05) and Cyp3A5 (p ≤ 0.01) showed a downregulation (Fig. 4).
Fig. 4.
Transcript levels for Ci treated Day 10 embryo: Fold change expression values for the genes. Fold change values for control embryo is 1.0 for all the genes, n = 10 eggs/group.
3.5. Western blot analysis
In order to reaffirm the results of qRT-PCR analysis few representative proteins were quantified through western blot. Immunoblot analysis revealed reduced expression of Shh in the treated embryos on day 4 compared to that of in the control. This confirms that sonic hedgehog levels were reduced at both transcription and translation levels by pesticide intoxication. On day 10, E-cadherin and caspase levels were found to be increased and hence, validated the RT-PCR results (Fig. 5). β-actin was used as internal control.
Fig. 5.
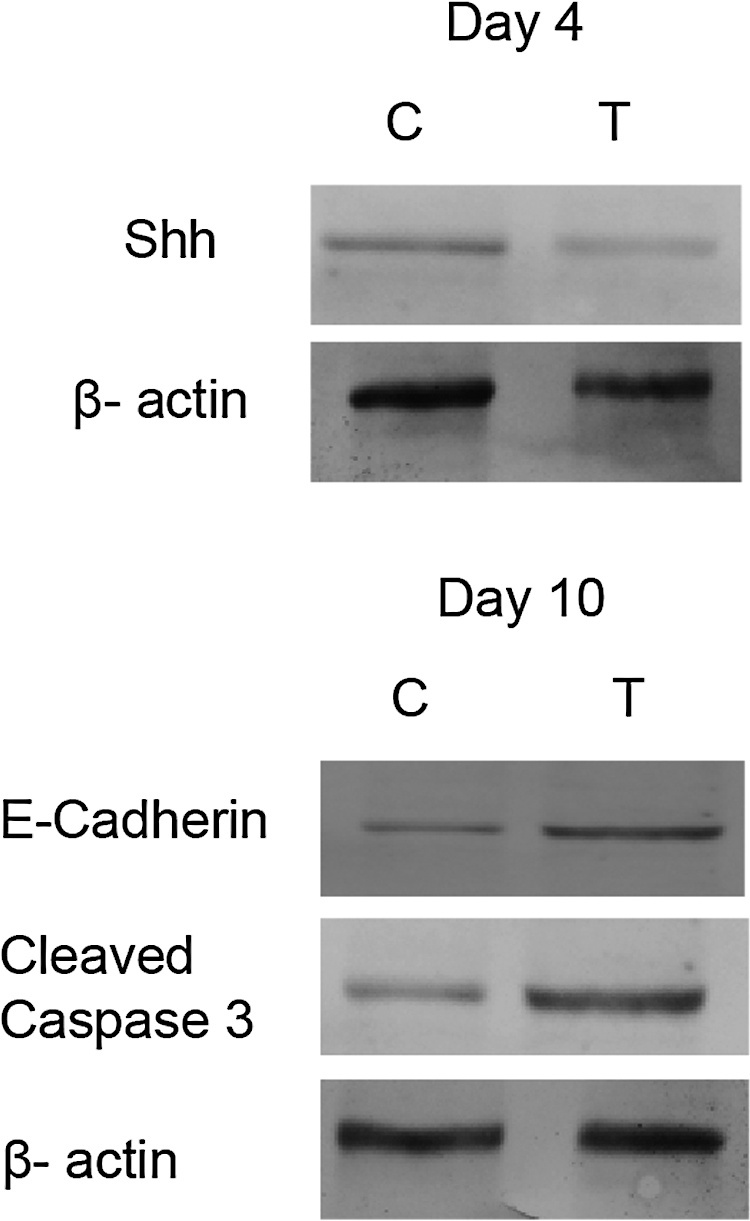
Western Blot images showing comparative expression of various proteins: Shh on Day 4; E-Cadherin and Cleaved caspase-3 on Day 10. β-actin was taken as loading control, n = 10 eggs/group.
4. Discussion
It is now largely accepted that the sporadic use of combination insecticide often affects the non-targeted organism through contaminated food and water. Moreover, whenever two or more chemicals are used in combination, they might exert synergistic, combined or competitive effect which may lead to multiple organ toxicity upon consumption of such contaminated food [10]. Apart from systemic toxicity, combination insecticides are also known to exert embryotoxicity [12]. Having known that toxicants like Ci are capable of inducing teratological manifestations in the developing embryos, the current study was designed to look for the underlying changes in the molecular mechanisms. The visual and microscopic observations of the treated embryos on day 2, 4 and 10 showed several teratological and histomorphological aberrations, amongst which the ventral body wall defects have been chosen for an elaborate study. The treated embryos which showed the VBD had an exposed viscera protruding out of ventral abdominal wall due to a failed midline closure.
The VBW formation can be traced back to its embryonic origin from the mesoderm formed during the gastrulation. Several studies were conducted in the past on different embryonic models, to understand the formation of the ventral body wall. The contribution of lateral plate mesoderm and paraxial or somatic mesoderm towards the formation of ventral body wall was discussed earlier by Sadler and Feldkamp [18]. The etiologies of failure in the formation of VBW were also investigated. These studies discussed about the embryonic dysplasia, vascular disruption, mechanical disruption, and also a disruption in the crosstalk between the epithelium and mesenchyme of the gastrulating embryos as the possible candidates leading to defects in VBW. The role of various pattern forming genes like Wnt, Pitx2, bmps, and cell adhesion molecules too were elaborated [[18], [19], [20], [21], [22]].
Shh, one of the early expressed pattern forming genes plays a significant role in most of the above discussed cellular processes. Shh and its synchronised activity with BMP4, regulates the patterning of the somites in developing embryos. Also, Shh controls the temporo-spatial expression of bmp4 signal by inhibiting it, and holding the LPM, from extending medially and ventrally [23]. The BMP is known to have a dual role, while expression of BMP4 in dorsal neural tube promotes muscle differentiation, its ectopic expression in the paraxial mesoderm results in inhibition of myotome formation. Consequently, a proper myotomal development would also require the BMP to be inhibited at the paraxial mesoderm in a specific temporal manner [24]. In the present study, the mRNA expression analysis on day 4 showed that the Ci treated embryos had a downregulation of Shh and upregulation of bmp4 when compared to the control embryos. This disregulation in the expression of Shh and bmp4 must have created the initial disturbances in the paraxial mesoderm, thereby disrupting the somite patterning and subsequently must have caused anomalies in the myotome differentiation too in the embryos.
Further, under normal conditions there is a differential expression of Pitx2 on the embryonic left and right sides resulting the axis formation, which is controlled upstream by selective inhibition of BMP4and fgf8. Though the changes in fgf8 expression remained insignificant, the increased expression of bmp4 and Pitx2 in the exposed embryo indicates a disparity. Contrary to our observations with regard to Pitx2, according to Kitamura et al. [19] deficiency of Pitx2 caused a failure of ventral body wall closure in mice, and they have also observed that the Pitx2 deficiency was influenced by a different genetic cascade other than fgf8-derived. In another study by Fung et al. [25] upregulated Pitx2 is indicated during oncogenic progression, where Pitx2 might be contributing towards the growth and migration of cells. Also, there are reports [26] indicating that an elevated Pitx2 ropes in the free radical scavenging by promoting the gene expression of antioxidants. Therefore, an increased expression of Pitx2 here might be an embryonic response to induce the formation of antioxidants against the stress induced by the Ci exposure. To further understand this response, we tried to look into some of the indicators of xenobiotic metabolizing mechanisms. The aryl hydrocarbon receptor (AHR), which is a ligand associated transcription factor acts like a xenosensor and has a role in upregulation of the cytochromes which subsequently work on metabolizing the xenobiotics [27]. Here, we observed a very significantly upregulated AHR and Cyp3A4 expressions in day 10 embryos, which is a clear indication of combat to overcome the stress laid by the Ci. However, an overburden of the extraneous agent when not efficiently dealt by the clearance pathways might culminate into dire consequences like renewal of teratogen targeted cell population by inducing apoptosis [28]. Our study shows a highly significant increase in the expression of Caspase-3 activity in both day 4 and day 10 embryos which can be correlated to the large-scale apoptosis occurring in the Ci treated embryos. At the same time the levels of pcna were found to be downregulated indicating that the cell proliferation was hampered. Also, activation in the Shh signalling pathways is seen under conditions of oxidative stress and could regulate cell proliferation and apoptosis [[29], [30]]. In concordance, we have observed an upregulated expression of the Shh in the day 10Ci treated embryos relative to the controls.
Furthermore, the somitogenesis, LPM formation and movement of lateral body folds are governed by cellular activities like programmed cell death, intercellular crosstalk, cell migration and cell proliferation [[4], [5]]. In the developing embryos, the migrating cell is highly polarized and is regulated by complex set of signals. The differentiating new cell types depend upon a finely balanced and coordinated regulation of gene expression and precise interaction amongst their neighbours. We therefore also sought to identify few such molecular signals that regulate the said processes. The expression of E-cadherins during embryonic development happens quite early where they play a role in adhesion and compaction of the blastomeres. It also signals the controlled epithelial-to-mesenchymal conversion and regulates developmental processes like cell migration and proliferation [[31], [32], [33], [34]]. The migration of mesodermal cell is favoured by downregulation of the E-cadherin and loss of cell adhesion [[35], [36]]. Our results have shown that levels of E- cadherins were found to be upregulated in both the day 4 as well as day 10Ci treated embryos, giving clear evidence that the cell migration was delayed and/or hampered. Further, the E −cadherin is known to be downstream target of Shh [37], which means that an inhibition of Shh would result in decreased E-cadherin expression. However, our results contrastingly have shown that the Shh was downregulated while E-cadherin was upregulated in the 4 day treated embryos, while in day 10 treated embryos, both were found to be upregulated. This indicates that E-cadherin though regulated by Shh, might as well be under the control of some other upstream signal. A negative correlation between the Shh and E-cadherin nevertheless, was reported during metastasis [[38], [39]]. Further, the Paxillin (Pxn), which is a multifunctional focal adhesion adaptor protein, was found to be upregulated here. Pxn expression has its significance not only during embryonic development and cell movement, but also elevated levels of Pxn are found in pathological conditions like oxidative stress and metastatic cancers [40]. An elevated Pxn observed in day 10 treated embryos could as well be related to the condition of oxidative stress caused by the Ci exposure. Earlier studies by Ray et al. [41] in neonatal rats has also shown that chlorpyrifos administration leads to differential expression of genes like Pxn involved in cell adhesion and migration.
Subsequently studies were extended to understand the pattern of Wnt expression. The Wnt signalling plays a significant role in abdominal myogenesis and formation of secondary ventral body wall. Also, Wnt11, a non-canonical Wnt member, has a role in cell adhesion and movement. The Wnt11 and Wnt6 are cited to be pivotal in maintaining the epithelial nature of the dorsomedial and ventro-lateral lips of dermomyotome and a deficit would lead to defects in the ventral musculature formation [42]. However, our study has shown a significant upregulation of Wnt11 and Wnt6 in the Ci treated embryos on day 10. Nevertheless, such aberrant upregulation of the Wnt signalling pathways has been reported after a chronic exposure to cadmium in mouse [43] and also in many cases of malignant human cancers [44].
A review by Wang et al., discusses the role of BMP4 in early developmental process, where, an absence of it leads to failure of mesoderm formation. And at a later stage, BMP1 deficiency would lead to the defects in ventral body wall closure. MyoD expression is a marker to the early myoblasts [42]. A relatively higher expression of the MyoD1 in the treated embryos could be an indication that the myoblasts remained so and failed to undergo further differentiation in the intoxicated embryos. Visual clues to this failure of muscle differentiation could be drawn from the histological sections of the embryos (Fig. 2B and D). Sonic hedgehog regulates MyoD expression to enhance the embryonic skeletal myogenesis [45]. Here an upregulation of MyoD1 would have been subsequent result of Shh upregulation, discussed earlier.
5. Conclusion
Visual as well as microscopic observations and the mRNA profiling of the molecular signals regulating the processes like epithelial-to-mesenchymal transition, cell proliferation, migration and survival or death were sought for, in the Ci treated chick embryos. We observed that the fate of these embryonic cells, which constantly rely on interpretation of the signals from their neighbours and surroundings, show a larger erroneous display in not just a single pathway, but in the intricate regulatory networks. The alterations came up in a way more complicated than do a single gene knockout studies that lead to the ventral body wall defects. It could be concluded that the developing embryonic mechanisms while metabolizing the toxin and clearing the oxidative burden, might face a grave consequence due to changes in signal strength and might have ultimately lost their potency to achieve the developmental milestones, the completion of ventral body wall formation being amongst them.
Acknowledgement
The authors are grateful to Gujarat State Biotechnology Mission (GSBTM) for financial assistance in the form of a major research project sanctioned to SB (Grant No. 1091 of FAP 2014).
References
- 1.Sadler T.W. The embryologic origin of ventral body wall defects. Semin. Pediatr. Surg. 2010;19(No. 3):209–214. doi: 10.1053/j.sempedsurg.2010.03.006. (WB Saunders) [DOI] [PubMed] [Google Scholar]
- 2.Duhamel B. Embryology of exomphalos and allied malformations. Arch. Dis. Child. 1963;38(198):142–147. doi: 10.1136/adc.38.198.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldkamp M.L., Carey J.C., Sadler T.W. Development of gastroschisis: review of hypotheses, a novel hypothesis, and implications for research. Am. J. Med. Genet. A. 2007;143(7):639–652. doi: 10.1002/ajmg.a.31578. [DOI] [PubMed] [Google Scholar]
- 4.Copp A., Cogram P., Fleming A., Gerrelli D., Henderson D., Hynes A., Kolatsi-Joannou M., Murdoch J., Ybot-Gonzalez P. Neurulation and neural tube closure defects. Dev. Biol. Protoc. 2000;II:135–160. doi: 10.1385/1-59259-065-9:135. [DOI] [PubMed] [Google Scholar]
- 5.Colas J.F., Schoenwolf G.C. Towards a cellular and molecular understanding of neurulation. Dev. Dyn. 2001;221(2):117–145. doi: 10.1002/dvdy.1144. [DOI] [PubMed] [Google Scholar]
- 6.Sitarek K. Embryolethal and teratogenic effects of carbendazim in rats. Teratog. Carcinog. Mutagen. 2001;21(5):335–340. [PubMed] [Google Scholar]
- 7.Thompson J., Bannigan J. Effects of cadmium on formation of the ventral body wall in chick embryos and their prevention by zinc pretreatment. Teratology. 2001;64(2):87–97. doi: 10.1002/tera.1050. [DOI] [PubMed] [Google Scholar]
- 8.Singh J. Gastroschisis is caused by the combination of carbon monoxide and protein-zinc deficiencies in mice. Birth Defects Res. B Dev. Reprod. Toxicol. 2003;68(4):355–362. doi: 10.1002/bdrb.10032. [DOI] [PubMed] [Google Scholar]
- 9.Williams T. August. Animal models of ventral body wall closure defects: a personal perspective on gastroschisis. Am. J. Med. Genet. C Semin. Med. Genet. 2008;148(No. 3):186–191. doi: 10.1002/ajmg.c.30179. Wiley Subscription Services, Inc., A Wiley Company. [DOI] [PubMed] [Google Scholar]
- 10.Tsatsakis A.M., Docea A.O., Tsitsimpikou C. New challenges in risk assessment of chemicals when simulating real exposure scenarios; simultaneous multi-chemicals' low dose exposure. Food Chem. Toxicol. 2016;96:174–176. doi: 10.1016/j.fct.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Tsatsakis A.M., Kouretas D., Tzatzarakis M.N., Stivaktakis P., Tsarouhas K., Golokhvast K.S., Rakitskii V.N., Tutelyan V.A., Hernandez A.F., Rezaee R., Chung G. Simulating real-life exposures to uncover possible risks to human health: a proposed consensus for a novel methodological approach. Hum. Exp. Toxicol. 2017;36(6):554–564. doi: 10.1177/0960327116681652. [DOI] [PubMed] [Google Scholar]
- 12.Uggini G.K., Patel P.V., Balakrishnan S. Embryotoxic and teratogenic effects of pesticides in chick embryos: a comparative study using two commercial formulations. Environ. Toxicol. 2012;27(3):166–174. doi: 10.1002/tox.20627. [DOI] [PubMed] [Google Scholar]
- 13.Uggini G.K., Suresh B. Genotoxic effects of two different classes of insecticide in developing chick embryos. Toxicol. Environ. Chem. 2013;95(6):992–1005. [Google Scholar]
- 14.Khan N.A., Uggini G.K., Patel S., Balakrishnan S. In-ovo treatment of chlorpyrifos and cypermethrin in combination altered the haematological parameters in two generations of domestic hen. Indian J. Fundam. Appl. Life Sci. 2015;5:120–126. [Google Scholar]
- 15.Blankenship A.L., Hilscherova K., Nie M., Coady K.K., Villalobos S.A., Kannan K., Powel D.C., Bursian S.J., Giesy J.P. Mechanisms of TCDD-induced abnormalities and embryo lethality in white leghorn chickens. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2003;136(1):47–62. doi: 10.1016/s1532-0456(03)00166-2. [DOI] [PubMed] [Google Scholar]
- 16.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-△△CT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72(1–2):248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 18.Sadler T.W., Feldkamp M.L. The embryology of body wall closure: relevance to gastroschisis and other ventral body wall defects. Am. J. Med. Genet. C Semin. Med. Genet. 2008;148(No. 3):180–185. doi: 10.1002/ajmg.c.30176. [DOI] [PubMed] [Google Scholar]
- 19.Kitamura K., Miura H., Miyagawa-Tomita S., Yanazawa M., Katoh-Fukui Y., Suzuki R., Ohuchi H., Suehiro A., Motegi Y., Nakahara Y., Kondo S. Mouse PITX2 deficiency leads to anomalies of the ventral body wall, heart, extra-and periocular mesoderm and right pulmonary isomerism. Development. 1999;126(24):5749–5758. doi: 10.1242/dev.126.24.5749. [DOI] [PubMed] [Google Scholar]
- 20.Brewer S., Williams T. Finally, a sense of closure? Animal models of human ventral body wall defects. Bioessays. 2004;26(12):1307–1321. doi: 10.1002/bies.20137. [DOI] [PubMed] [Google Scholar]
- 21.Matsumaru D., Haraguchi R., Miyagawa S., Motoyama J., Nakagata N., Meijlink F., Yamada G. Genetic analysis of Hedgehog signaling in ventral body wall development and the onset of omphalocele formation. PLoS One. 2011;6(1):e16260. doi: 10.1371/journal.pone.0016260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snowball J., Ambalavanan M., Cornett B., Lang R., Whitsett J., Sinner D. Mesenchymal Wnt signaling promotes formation of sternum and thoracic body wall. Dev. Biol. 2015;401(2):264–275. doi: 10.1016/j.ydbio.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilbert S.F. The morphogenesis of evolutionary developmental biology. Int. J. Dev. Biol. 2003;47(7–8):467. [PubMed] [Google Scholar]
- 24.Marcelle C., Stark M.R., Bronner-Fraser M. Coordinate actions of BMPs, Wnts, SHH and noggin mediate patterning of the dorsal somite. Development. 1997;124(20):3955–3963. doi: 10.1242/dev.124.20.3955. [DOI] [PubMed] [Google Scholar]
- 25.Fung F.K., Chan D.W., Liu V.W., Leung T.H., Cheung A.N., Ngan H.Y. Increased expression of PITX2 transcription factor contributes to ovarian cancer progression. PLoS One. 2012;7(5):e37076. doi: 10.1371/journal.pone.0037076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tao G., Kahr P.C., Morikawa Y., Zhang M., Rahmani M., Heallen T.R., Li L., Sun Z., Olson E.N., Amendt B.A., Martin J.F. Pitx2 promotes heart repair by activating the antioxidant response after cardiac injury. Nature. 2016;534(7605):119–123. doi: 10.1038/nature17959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens E.A., Mezrich J.D., Bradfield C.A. The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology. 2009;127(3):299–311. doi: 10.1111/j.1365-2567.2009.03054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torchinsky A., Fein A., Toder V. Teratogen-induced apoptotic cell death: does the apoptotic machinery act as a protector of embryos exposed to teratogens? Birth Defects Res. Part C: Embryo Today: Rev. 2005;75(4):353–361. doi: 10.1002/bdrc.20052. [DOI] [PubMed] [Google Scholar]
- 29.Heine V.M., Rowitch D.H. Hedgehog signaling has a protective effect in glucocorticoid-induced mouse neonatal brain injury through an 11βHSD2-dependent mechanism. J. Clin. Invest. 2009;119(2):267. doi: 10.1172/JCI36376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xia Y.P., Dai R.L., Li Y.N., Mao L., Xue Y.M., He Q.W., Huang M., Huang Y., Mei Y.W., Hu B. The protective effect of sonic hedgehog is mediated by the phosphoinositide [corrected] 3-kinase/AKT/Bcl-2 pathway in cultured rat astrocytes under oxidative stress. Neuroscience. 2012;209:1–11. doi: 10.1016/j.neuroscience.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 31.Fleming T.P., Javed Q., Hay M. Epithelial differentiation and intercellular junction formation in the mouse early embryo. Development. 1992;116(Suppl):105–112. [PubMed] [Google Scholar]
- 32.Barth A.I., Näthke I.S., Nelson W.J. Cadherins, catenins and APC protein: interplay between cytoskeletal complexes and signaling pathways. Curr. Opin. Cell Biol. 1997;9(5):683–690. doi: 10.1016/s0955-0674(97)80122-6. [DOI] [PubMed] [Google Scholar]
- 33.Kalluri R., Weinberg R.A. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 2009;119(6):1420. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bahm I., Barriga E.H., Frolov A., Theveneau E., Frankel P., Mayor R. PDGF controls contact inhibition of locomotion by regulating N-cadherin during neural crest migration. Development. 2017:dev-147926. doi: 10.1242/dev.147926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciruna B., Rossant J. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev. Cell. 2001;1(1):37–49. doi: 10.1016/s1534-5807(01)00017-x. [DOI] [PubMed] [Google Scholar]
- 36.Basson M.A. Signaling in cell differentiation and morphogenesis. Cold Spring Harbor Perspect. Biol. 2012;4(6):pa008151. doi: 10.1101/cshperspect.a008151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao C., Ogle S.A., Schumacher M.A., Schilling N., Tokhunts R.A., Orr-Asman M.A., Miller M.L., Robbins D.J., Hollande F., Zavros Y. Hedgehog signaling regulates E-cadherin expression for the maintenance of the actin cytoskeleton and tight junctions. Am. J. Physiol.-Gastrointest. Liver Physiol. 2010;299(6):G1252–G1265. doi: 10.1152/ajpgi.00512.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karhadkar S.S., Bova G.S., Abdallah N., Dhara S., Gardner D., Maitra A., Isaacs J.T., Berman D.M., Beachy P.A. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431(7009):707–712. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 39.Sun C., Zhang Z., He P., Zhou Y., Xie X. Involvement of PI3K/Akt pathway in the inhibition of hepatocarcinoma cell invasion and metastasis induced by SASH1 through downregulating Shh-Gli1 signaling. Int. J. Biochem. Cell Biol. 2017;89:95–100. doi: 10.1016/j.biocel.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 40.López-Colomé A.M., Lee-Rivera I., Benavides-Hidalgo R., López E. Paxillin: a crossroad in pathological cell migration. J. Hematol. Oncol. 2017;10(1):50. doi: 10.1186/s13045-017-0418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ray A., Liu J., Ayoubi P., Pope C. Dose-related gene expression changes in forebrain following acute, low-level chlorpyrifos exposure in neonatal rats. Toxicol. Appl. Pharmacol. 2010;248(2):144–155. doi: 10.1016/j.taap.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L., Li H., Yu J., Cao J., Chen H., Zhao H., Zhou R. Ectodermal Wnt signaling regulates abdominal myogenesis during ventral body wall development. Dev. Biol. 2014;387(1):64–72. doi: 10.1016/j.ydbio.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 43.Chakraborty P.K., Scharner B., Jurasovic J., Messner B., Bernhard D., Thévenod F. Chronic cadmium exposure induces transcriptional activation of the Wnt pathway and upregulation of epithelial-to-mesenchymal transition markers in mouse kidney. Toxicol. Lett. 2010;198(1):69–76. doi: 10.1016/j.toxlet.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 44.Loh Y.N., Hedditch E.L., Baker L.A., Jary E., Ward R.L., Ford C.E. The Wnt signalling pathway is upregulated in an in vitro model of acquired tamoxifen resistant breast cancer. BMC Cancer. 2013;13(1):174. doi: 10.1186/1471-2407-13-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voronova A., Coyne E., Al Madhoun A., Fair J.V., Bosiljcic N., St-Louis C., Li G., Thurig S., Wallace V.A., Wiper-Bergeron N., Skerjanc I.S. Hedgehog signaling regulates MyoD expression and activity. J. Biol. Chem. 2013;288(6):4389–4404. doi: 10.1074/jbc.M112.400184. [DOI] [PMC free article] [PubMed] [Google Scholar]