Abstract
Periprosthetic joint infection (PJI) is a serious complication in total knee arthroplasty (TKA) and represents one of the most common causes of revision. The challenge for surgeons treating an infected TKA is to quickly obtain an infection-free joint in order to re-implant, when possible, a new TKA. Recent literature confirms the role of local antibiotic-loaded beads as a strong bactericidal, allowing higher antibiotic elution when compared with antibiotic loaded spacers only. Unfortunately, classical Polymethylmethacrylate (PMMA) beads might allow bacteria adhesion, secondary development of antibiotic resistance and eventually surgical removal once antibiotics have eluted. This article describes a novel surgical technique using static, custom-made antibiotic loaded spacers augmented by calcium sulphate antibiotic-impregnated beads to improve the success rate of revision TKA in a setting of PJI. The use of calcium sulphate beads has several potential benefits, including a longer sustained local antibiotic release when compared with classical PMMA beads and, being resorbable, not requiring accessory surgical interventions.
Keywords: Total knee arthroplasty, Periprosthetic joint infection, Beads, DAPRI, Static cement-spacer, Local antibiotic therapy
1. Introduction
Periprosthetic joint infection (PJI) is a serious complication of total knee arthroplasty (TKA) with devastating effects on the local knee anatomy and on the general health of patients. Currently, infection in TKA represents one of the most common reasons for revision, causing 25% of overall failures1: the incidence is reported nearly 2% within 20 years from primary TKA (41% of these occurring in the first 2 years) and between 8% and 12% from revision TKA.2, 3.
Incidence is growing up rapidly: the demand for TKA increases over time and Parvizi et al.4 predicted an annual rate of PJIs between 38 000 and 270 000 in the United States by the year 2030. Furthermore, PJIs represent an important economic load on the health care system: an average cost for hospitalization of knee patients with PJI of $25 300 (CI, $22 500–$28 100) in 2001 and $24 200 (CI, $22 800–$25 600) in 2009 has been previously reported and an increasing annual cost from $ 566 millions in 2009 to 1,62 billions in the 2020 is expected in the United States.5
Diagnosis a PJI is a challenge for surgeons but it is mandatory to distinguish between an aseptic and a septic loosening in case of a painful TKA: infections jeopardize the general health status of patients, requiring prolonged hospitalizations and repeated surgical treatments. In some cases, these treatments can result in loss of implant, leading to limb deformity and reduction of autonomy during daily living activities.6
Physical examination, symptoms and an evaluation of risk factor and comorbidities are mandatory to determinate how likely or unlikely a PJI may occur. First, a “without doubt” diagnosis of PJI is still challenging at our days: there is not a universally recognized definition of deep periprosthetic infection and which variables participate in making a final call are still subjects of debate. In 2011, the workgroup of the musculoskeletal infection society7 tried to produce a “gold standard” definition for PJI to be universally adopted by physicians: several criteria were proposed, including the presence of a sinus tract communicating directly with the prosthesis and the identification of the pathogen is at least two samples of tissue or articular fluid obtained from the affected joint.
Second, surgeons are used to perform expensive and often useless multidisciplinary diagnostic tests trying to identify an infected TKA; unfortunately, the current literature showed no effective diagnostic tests for periprosthetic infections and proposed algorithms in case of suspected infection remain unclear. A clinical practice guideline has been adopted in 2010 by the American Academy of Orthopaedic Surgeons to facilitate diagnosis in suspected PJI: the initial screening of patients with a painful TKA includes the measurement of the levels of several systemic markers of inflammation, such as C-Reactive Protein (CRP) and Erythrocyte Sedimentation Rate (ESR), followed by an arthrocentesis performed for synovial fluid analysis to detect leukocyte counts and differentials and culture and sensitivity in those patients with elevated levels of CRP and ESR.
Third, CRP and ESR usually remain elevated for 3–8 weeks post-operative as markers of normal inflammation resulting after surgery; this might interfere with interpretation of the results in the diagnosis of early PJI.8 Furthermore, as showed by McArthur et al.9 in their study, almost 4% of patient with PJIs are seronegative showing normal values of ESR and CRP. Seronegative PJIs are associated with a lower aspirate cell count and lower incidence of staphylococcus Aureus infection.
Joint aspiration has become the “gold standard” in diagnosis of periprosthetic infections and it could also be repeated in case of discrepancy between clinic presentation and initial aspiration culture results. Fluid obtained from the joint should be sent for analysis of synovial fluid white blood cell count, percentage of neutrophils and culture for aerobic and anaerobic organism.10 Culture of aerobic and anaerobic organism usually require several days to verify presence or absence of germs; thus, interpretation of synovial fluid leukocyte count is faster and easier. Trampuz et al.11 in their study concluded that a synovial fluid leukocyte of >65% neutrophils had a sensitivity of 97% and a specificity of 98% detecting PJI while a leukocyte count of >1,7 × 103/μl had a sensitivity and a specificity of 94% and 88% respectively.
Currently, synovial biomarkers such as leukocyte esterase, Interleukine 6 and alpha defensin are showing promising results improving diagnostic accuracy12 while nuclear imaging is nowadays weakly recommended in several guidelines, showing benefit only in case of strong discrepancy between clinical presentation and laboratory exams and establishing a lower o higher probability of infection.10
Conventionally, there are three different surgical options to treat an infected TKA: debridement, antibiotics and implant retention (DAIR), one stage exchange arthroplasty and a two-stage exchange implant using dynamic or static cement spacer. Arthrodesis or amputations are also two drastic options reserved for patients who have persistent infected TKA after a failed subsequent two-stage revision arthroplasty.
The DAIR Procedure is particularly indicated for acute (within 3 weeks from the original surgery) or hematogenous PJI, having the goal to reduce microorganism loads before of bacterial biofilm formation. This surgical procedure includes removal of skin margins and eventual sinuses, a radical, “tumor-like” synovectomy and exchange of polyethylene insert. Choi et al.13 have shown that leaving the original tibial insert is an important risk factor for failure.
Promising results have been shown comparing the success rate of DAIR for early versus chronic knee PJIs: treating acute infection showed a success rate between 31% and 100%, while a success rate between 28% and 62% is reported when treating chronic TKA infection.14 Furthermore, Vilchez et al.15 concluded that using debridement with implant retention treating Hematogenous PJI due to S. Aureus showed worse results than early post-surgical infections.
The current literature shows the current “gold standard” being a surgical procedure characterized by a total exchange of all components; however, which is the optimum management to treat infected TKA between one or two-stage revision is still unclear.16
One stage revision is a procedure where the removal of prosthetic components and debridement is immediately followed by the re-implantation, while, in two stage procedures, the re-implantation is performed after a period of systemic antibiotic treatment combined with an intra-articular antibiotic loaded cement spacer, static or dynamic, to fill the intra-articular defect left by the removed components and to increase the local elution of antibiotics.17
Traditionally, the debate on one or two-stage revision has favoured two stage procedures; however, some studies suggest one-stage exchange arthroplasty may provide superior outcomes, including lower re-infection rates and superior function in selected patients. Interestingly, articles supporting one stage revisions have been published after 2000; before that time, no significant differences in reinfection rate between the two procedures were reported.18, 19
Historically, the surgical treatment has been combined by the use of local and systemic antibiotic therapy: different systemic antibiotic therapy protocols have been chosen, depending upon several factors like bacterial properties, metabolic activity and related antibiotic resistance. Unfortunately, the literature on the role of adjunctive local antibiotic therapy is sparse: the current study proposes a novel surgical technique for double-stage revision TKA using a custom-made static antibiotic spacer combined with calcium sulphate antibiotic impregnated beads aiming to increase the success rate in revision TKA for PJI.
2. Surgical technique for revision TKA using calcium sulphate antibiotic impregnated beads
At the senior author institution, in a PJI setting, we routinely use two different surgical techniques according to the PJI staging.7 The first technique is represented by Debridement, Antibiotic Pearls and Retention of the Implant (DAPRI); the second technique is a standard two stages technique modified by utilizing a static antibiotic spacer plus Calcium sulphate antibiotic impregnated beads to treat the deep periprosthetic infection of the knee. Both surgical techniques characterized by the use of calcium sulphate antibiotics impregnated beads were recently implemented by the other current authors to target patients affected by PJIs following a clinical diagnosis established by elevated values of systemic markers of inflammation, such as high CRP and ESR concentration and confirmed by a pre-operative synovial fluid analysis showing elevated white blood cells count and elevated percentage of neutrophils.6
These Calcium sulphate antibiotic impregnated beads (Stimulan, Biocomposites Ltd., Keele, UK) are a biocompatible and dissolvable antibiotic loaded intra-articular system to allow for an intraarticular, continuous delivery of antibiotics in the infected joint. They are composed by hydrophilic crystals, initially soft after hydration, which usually disappear on radiologic examination in a four to six weeks timeframe after being used as intra-articular devices.
At the senior author Institution, before surgery, all patients undergo standard antero-posterior, lateral and Merchant View of patella20 in order to exactly detect the septic loosening of the implant. A CT study is often required in order to quantify the amount of bone loss following PJI and in all cases of painful TKA to evaluate eventual components malalignment. Patients are scheduled after blood and synovial testing confirming an acute or chronic infection of the implant. In the case of an acute or haematogenous infection7 with identification of the responsible organism and no sinus-tract, the current authors suggest a DAPRI procedure: this procedure include an aggressive tumor-like intra articular synovectomy and capsulotomy, a three minutes diluted povidone iodine bath, abundant irrigation with antibiotic-containing solution, exchange of the polyethylene insert and final addiction of calcium sulphate antibiotic-impregnated (according to the culture and sensitivity test) custom made beads.
In the case of a delayed or chronic PJI,7 a two-stage revision is recommended. This second surgical technique deeply described here, includes a standard median parapatellar capsulotomy, following a skin incision routinely placed on the previous surgical scar: 3 soft tissues samples are intraoperatively obtained and sent for standard bacteriological exams. Following this, an aggressive synovectomy is performed: the goal of this synovectomy is to remove all the soft tissue which has been in contact with the intraarticular space. The supra-patellar pouches are freed from any scar formations and adhesions and the patellar tendon is freed from the scar which usually includes the fat pad: at the same time, an aggressive peeling on the antero-medial compartment of the proximal tibia is performed too. This usually allows an easier, osteotome guided, removal of the polyethylene insert. The femoral and tibial components are then removed with the subsequent use of osteotomies, oscillating and reciprocating saws: the patellar component is finally removed using an oscillating saw. Particular attention is then paid to the removal of all cement mantle, including from the canals.
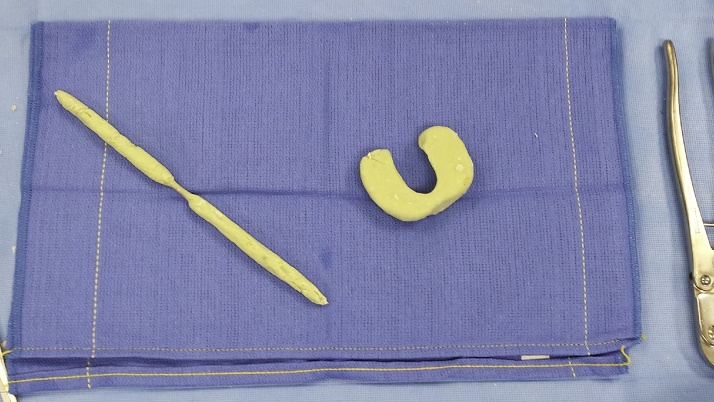
Abundant pulse irrigation, using 3 l of bacitracin added saline solution, is then performed. At this point, an “horse shoe” shaped static spacer is built on the side table: at the same time a Stainmann pin covered by antibiotic–loaded cement to be used as an intramedullary antibiotic delivery system [Fig. 1].
Fig. 1.
The “horse shoe” shaped static spacer and a Stainmann pin covered by antibiotic–loaded are built and used as an intramedullary delivery system.
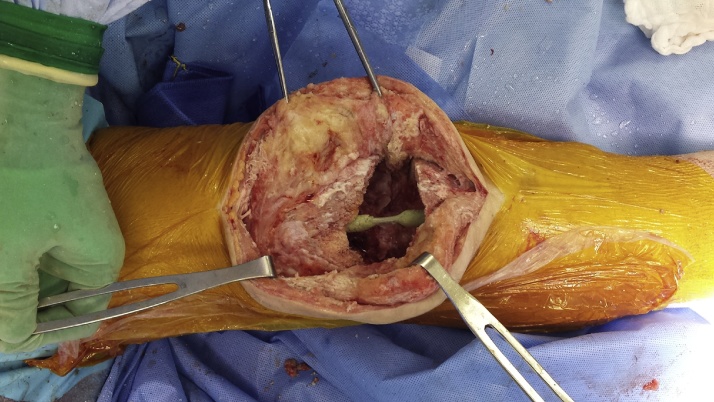
The Stainmann pin routinely used is 5 mm in diameter and has a 25-cm length. The antibiotic loaded cement is prepared mixing 7,2 g of tobramycin solution and 2 g of Vancomycin diluted into 80 g of Palacos LV+G cement (Zimmer Biomet, Warsaw, USA). The current authors choose these specific amounts of antibiotics according to the current literature.21 The dimensions of the “horse shoe” shaped spacer are decided according to the original polyethylene component thickness: this particular shape allows to place the spacer around the Stainmann pin which is first placed into the intramedullary tibial and femoral canals, bridging the distal femur and proximal tibia and to fill the intra-articular space at the same time [Fig. 2].
Fig. 2.
The Stainmann pin is first placed into the intramedullary tibial and femoral canals, bridging the distal femur and proximal tibia.
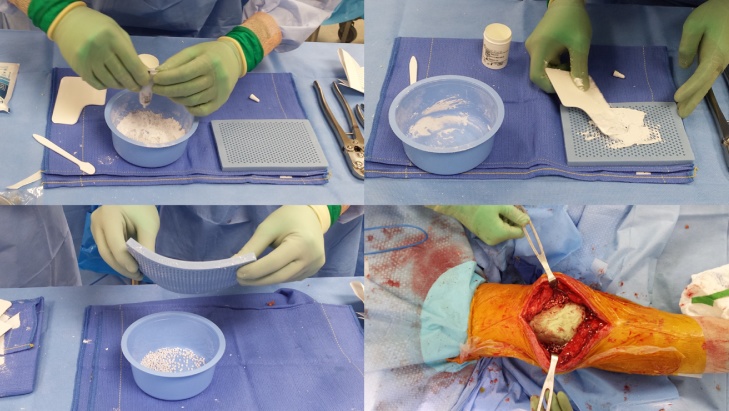
On a different and sterile work station, calcium sulphate antibiotic-impregnated beads are prepared: a 10-ml kit of PG-CSH (Stimulan; Biocomposites Ltd., United Kingdom) is mixed with 1000 mg vancomycin hydrochloride powder plus 6 ml of a 40-mg/ml tobramycin solution; a smooth paste is first formed mixing all components for 60 s and pressed into 4.8-mm-diameter hemispherical cavities in a flexible mold [Fig. 3]. The beads usually become hard and ready for implantation after resting between 30 and 60 min [22]. Usually, beads are placed along the supra-patellar pouches and around the tibia and femur in order to achieve an high concentration of antibiotic in the intra-articular space and surrounding soft tissues. The capsule is then close with re-absorbable suture and the skin with naylon. The knee is then placed into a knee immobilizer lock in extension.
Fig. 3.
Calcium sulphate antibiotic-impregnated beads are prepared mixing a 10-ml kit of PG-CSH with 1000 mg vancomycin hydrochloride powder plus 6 ml of a 40-mg/ml tobramycin solution.
At the senior author Institution, the postoperative recovery is mainly driven by the Infectious Diseases consultant. First, particular attention is paid to detect an eventual renal impairment or an abnormal high level of serum calcium, performing daily hematic tests. In fact, antibiotics like gentamicin and tobramycin are frequently used in orthopaedic infections because their wide spectrum of activity despite a potential otovestibular toxicity and nephrotoxicity23; furthermore, hypercalcaemia is described as a possible complication following the use of antibiotic-eluting absorbable calcium sulphate beads in revision arthroplasty for PJI.24 Secondarily, the surgical treatment is always followed by a systemic antibiotic therapy, which is intravenously administrated during hospitalization and in the first six weeks following the index procedure. The chosen antibiotic therapy depends on the resistance and/or sensibility of the isolated organism after deep tissue bacterial cultures according to our consultant microbiologist. The intravenous antibiotical therapy is usually stopped and substituted by oral antibiotical therapy at 6 weeks from the index procedure: the total length of the antibiotic therapy depends upon the normalization of the laboratory tests and the duration of the clinical presentation. The time of re-implantation varies according to the time required for the eradication of the PJI.
3. Discussion
The addiction of local antibacterial agents to support systemic antibiotic therapy in the treatment of PJIs has the goal to elevate the intra-articular antibiotic level and to increase the overall success rate in TKA revision surgery.25 Different surgical treatments have been historically proposed as an adjuvant for bone and soft tissue healing in the scenario of an infected total knee replacement: antibiotic-loaded bone cement (in a static or dynamic configuration), implants antibacterial coating and local antibiotic-loaded beads (in a resorbable or not configuration) represent the most used techniques.
Polymethylmethacrylate (PMMA) beads represent a non-biodegradable tool which has been used in revision TKA for PJIs: few studies demonstrated an important reduction in re-infection rates using this technology in the knee as well as in the hip.26 PMMA beads impregnated with gentamicin act as a local and temporary (1–2 weeks) antibiotic delivery system: by that time 20%–70% of the antibiotic incorporated in the beads is usually released into the body and the local gentamicin concentrations has decreased dramatically.27
Rationale using beads is that maximum level of concentration depends on the surface of the carrier and on the volume of the hematoma or cavities where they have been used28: small gentamicin beads release seven times more of their gentamicin content than large beads, resulting in a release of 93% versus 24% of their content in 2 weeks. For the same reason, the larger surface of beads compared with spacer surface explain an higher concentration of antibiotic in wound exudate.29
Janssen et al.30 evaluated a large series of 120 PJIs (95 total hip replacement and 25 total knee replacement) treated with explant of the prosthetic components followed by one or more debridement with systemic antibiotic and implantation of gentamicin-loaded PMMA beads; successful treatment of the infections was achieved for 105 of 120 prostheses (88%) confirming the role of beads as highly bactericidal tool used locally to achieve infection healing: 21 of 25 TKA infections healed and re-implantation was performed in 18 on 25 knees.
Hsieh et al.31 evaluated 128 consecutive patients treated with two-stage revision hip arthroplasty for infection, comparing results of patients treated with the interim use of antibiotic-loaded cement beads (n = 70) with those of patients treated with the interim use of an antibiotic-loaded cement prosthesis. (n = 58). They concluded there was no evidence of recurrent infection in 122 patients (95.3%) and the infection-free rates in both groups were similar, but an higher hip score, a shorter hospital stay, and better walking capacity in the interim period was associated with the use of spacer prosthesis; furthermore, a decreased operative time, less blood loss, and a lower transfusion requirement were showed at the time of re-implantation in those patients.
In 2002 Taggart et al.32 reported the results of 33 arthroplasties (26 hips and 7 knees) which had performed a two stage revision procedure implemented by the use of vancomycin impregnated cement beads for infection caused by different organisms; after a mean follow-up of 67 months, 32 patient remained clinically and radiologically free from infection. The authors concluded that vancomycin played a major role in the management of infection after arthroplasties.
Most recently, Chen et al.33 demonstrated good results using a protocol of aggressive surgical debridement, local antibiotic-loaded cement beads, combined parenteral and oral antibiotic therapy and re-implantation after normalization of ESR and CRP levels.: forty-six out of forty-eight (96%) hips treated following this protocol and using interim antibiotic-impregnated cement beads were free of recurrent infection, at least according to the clinical examination and laboratory tests at their latest follow up; thirty-five patients (74%) achieved excellent or good results.
Despite their antibiotic release capability, classic PMMA antibiotic added beads act as a biomaterial surface to which bacteria preferentially adhere, grow and potentially develop antibiotic resistance; moreover, PMMA beads require surgical removal once antibiotics have eluted.34
Different studies confirmed promising results using biodegradable beads because of several advantages: first, calcium sulphate beads showed an “in vitro” excellent elution profile. Howlin et al.22 observed a much longer elution of antibiotic using calcium sulphate beads when compared with classical PMMA beads: on the other side, a gradual absorption results in a sustained local antibiotic release avoiding the potential toxicity of intravenous antibiotics. Udomkusonsri et al.35 also showed that the elution capability of the antibiotic (cefazolin) they added to their calcium sulphate beads was significantly higher than from PMMA beads both at day 1 as well s at day 11.
Second, the use of PMMA beads requires a second surgery to remove these intra-articular devices; this characteristic represents an important limitation of PMMA beads in single stage procedures. Calcium sulphate beads might be also useful both in single stage revision surgery for infected joint arthroplasties as well as prophylactic treatment in case of revision for aseptic loosening of the prosthetic components in a patient characterized by a high risk of re-infection.
Unfortunately, an high rate of complications is reported in several clinical trials showing outcomes of the use of calcium-derivate beads in the treatment of PJS and chronic osteomyelitis: wound drainage is reported as the main problem encountered during the post-operative period, with a reported complication rate between 25 and 30%.36 This percentage is higher in cases where the volume of beads used was ≥30 cc and several explanations existing for this occurrence37: it might be caused by an excessive stretching of the deep soft tissue or by an hyperosmotic effect as calcium sulphate absorption results in joint distention and serous wound leakage. Furthermore, a toxic reactive synovitis, due to high local levels of antibiotics or calcium, could explain the serous drainage following an abundant fluid production.
The formation of heterotopic Ossification (HO) is another potential complication following the use of calcium-derivate beads. McPherson et al.36 evaluating 342 THA and TKA revisions, including aseptic revisions or two stage septic revision treated with pure calcium sulphate beads, reported a 1,2% overall incidence of HO. However, those authors concluded that the formed heterotopic bone was easily removed at the time of re-implantation.
In the two-stage surgical technique suggested by the current authors, an hypothetic longer local antibiotic release when compared with classical PMMA beads and not requiring accessory surgical interventions, represent potential benefits; the authors still acknowledge several limitations of this technique. First, this surgical technique has always been applied in a two-stage TKA revision in a delayed or chronic infection scenario: theoretically, once the chronic PJI responsible organism has been isolated, the use of calcium sulphate beads loaded with a specific antibiotic targeted versus a specific bacteriological profile might allow a single-stage re-implant. Second, this technique requires the use of a static antibiotic-loaded cement spacer: a dynamic spacer might be more appropriate in selected clinical scenarios. Third, despite our initial observations using bio-absorbable beads are promising, the authors recognize that, adding this technology, increases the final cost of the TKA revision procedure: a future, retrospective study might be useful to evaluate the direct and indirect costs of adding this procedure and to evaluate its ability to influence the overall costs of care, including prolonged hospitalization, long-term pharmaceutical intervention and the need for multi-disciplinar consultations.
4. Conclusion
Healing after a total knee arthroplasty infection is the main target of the surgical technique proposed in this study; it is mandatory that revision surgery must be performed when infection healing is completed. In the current literature, the use of local antibiotics with gentamicin-impregnated PMMA beads has been showed to be helpful when associated with antibiotic spacers in order to elevate the local concentration of antibiotics and to maintain better future joint function and bone stock preservation (either mobile or static spacer). Few studies showed that antibiotic added beads have higher elution characteristics than spacers alone because an increased contact area. The inferior elution properties of spacers emphasize the importance of additional systemic antibiotics for this PJI procedure during a prolonged postoperative period. Sulphate antibiotic-impregnated beads seems to be a good option with the advantage that they do not require to be surgically removed; on the other side, they can be used in aseptic loosening in high risk patients and in all one-stage revisions.
Further studies are necessary to evaluate the appropriate type, the exact dose and the effective elution of the local antibiotic to be included in the beads; on the other side, further studies are needed to highlight if the length and the overall final dose of the systemic antibiotic therapy might be reduced in the presence of an effective intra-articular antibiotic elution leading to a faster and complete infection remission.
Conflicts of interest
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Bozic K.J., Kurtz S.M., Lau E. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468:45–51. doi: 10.1007/s11999-009-0945-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berbari E.F., Hanssen A.D., Duffy M.C. Risk factors for prosthetic joint infection: case–control study. Clin Infect Dis. 1998;27(5):1247–1254. doi: 10.1086/514991. [DOI] [PubMed] [Google Scholar]
- 3.Della Valle C.J., Zuckerman J.D., Di Cesare P.E. Periprosthetic sepsis. Clin Orthop Relat Res. 2004;420:26–31. doi: 10.1097/00003086-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Parvizi J., Shohat N., Gehrke T. Prevention of periprosthetic joint infection: new guidelines. Bone Jt J. 2017;99-B(April (4 Suppl. B)):3–10. doi: 10.1302/0301-620X.99B4.BJJ-2016-1212.R1. [DOI] [PubMed] [Google Scholar]
- 5.Kurtz S.M., Lau E., Watson H., Schmier J.K., Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27:61–65. doi: 10.1016/j.arth.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 6.Volpe L., Indelli P.F., Latella L., Poli P., Yakupoglu J., Marcucci M. Periprosthetic joint infections: a clinical practice algorithm. Joints. 2015;2(February (4)):169–174. eCollection 2014 Oct–Dec. [PMC free article] [PubMed] [Google Scholar]
- 7.Parvizi J., Zmistowski B., Berbari E.F. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthop Relat Res. 2011;469(November (11)):2992–2994. doi: 10.1007/s11999-011-2102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alijnipour P., Bakhshi H., Parvizi J. Diagnosis of perprosthetic joint infection: the threshold for serological markers. Clin Orthop Relat Res. 2013;471(October (10)):3186–3195. doi: 10.1007/s11999-013-3070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McArthur B.A., Abdel M.P., Taunton M.J. Seronegative infections in hip and knee arthroplasty: periprosthetic infections with normal erythrocyte sedimentation rate and C-reactive protein level. Bone Jt J. 2015;97-B(July (7)):939–944. doi: 10.1302/0301-620X.97B7.35500. [DOI] [PubMed] [Google Scholar]
- 10.Della Valle C., Parvizi J., Bauer T.W. Diagnosis of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18(December (12)):760–770. doi: 10.5435/00124635-201012000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Trampuz A., Hanssen A.D., Osmon D.R. Synovial fluid leukocyte and differential for the diagnosis of prosthetic knee infection. Am J Med. 2004;117(8):556–562. doi: 10.1016/j.amjmed.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 12.Deirmengian C., Kardos K., Kilmartin P. Combined measurement of synovial fluid alpha-defensin and C-reactive protein levels: highly accurate for diagnosing periprosthetic joint infection. J Bone Jt Surg Am. 2014;96:1439–1445. doi: 10.2106/JBJS.M.01316. [DOI] [PubMed] [Google Scholar]
- 13.Choi H.R., Von Knoch F., Zurakowski D. Can implant retention be recommended for treatment of infected TKA? Clin Orthop Relat Res. 2011;469(April (4)):961–969. doi: 10.1007/s11999-010-1679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quasim S.N., Swann A., Ashford R. The DAIR(debridement, antibiotics and implant retention) procedure for infected total knee replacement – a literature review. SICOT J. 2017;3:2. doi: 10.1051/sicotj/2016038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vilchez F., Martinez-Pastor J.C., Garcia-Ramiro S. Efficacy of debridement in Hematogenous and early post-surgical prosthetic joint infecions. Int Artif Organs. 2011;34(September (9)):863–869. doi: 10.5301/ijao.5000029. [DOI] [PubMed] [Google Scholar]
- 16.Teeny S.M., Dorr L., Murata G. Treatment of infected total knee arthoplasty. Irrigation and debridement versus two-stage reimplantation. J Arthroplasty. 1990;5(March (1)):35–39. doi: 10.1016/s0883-5403(06)80007-0. [DOI] [PubMed] [Google Scholar]
- 17.Insall J.N., Thompson F.M., Brause B.D. Two-stage reimplantation for the salvage of infected total knee arthroplasty. J Bone Jt Surg Am. 1983;65:1087–1098. [PubMed] [Google Scholar]
- 18.Nagra N.S., Hamilton T.W., Ganatra S. One-stage versus two-stage exchange arthroplasty for infected total knee arthroplasty: a systematic review. Knee Surg Sport Traumatol Arthrosc. 2016;24(October (10)):3106–3114. doi: 10.1007/s00167-015-3780-8. [DOI] [PubMed] [Google Scholar]
- 19.Haddad F.S., Sukeik M., Alazzawi S. Is single-stage revision according to a strict protocol effective in treatment of chronic knee arthroplasty infections? Clin Orthop Relat Res. 2014;473:8–14. doi: 10.1007/s11999-014-3721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merchant A.C., Mercer R.L., Jacobsen R.H. Roentgenographic analysis of patellofemoral congruence. J Bone Jt Surg Am. 1974;56:1391–1396. [PubMed] [Google Scholar]
- 21.Iarikov D., Demian H., Rubin D., Alexander J., Nambiar S. Choice and doses of antibacterial agents for cement spacers in treatment of prosthetic joint infections: review of published studies. Clin Infect Dis. 2012;55(December (11)):1474–1480. doi: 10.1093/cid/cis735. [DOI] [PubMed] [Google Scholar]
- 22.Howlin R.P., Brayford M.J., Webb J.S. Antibiotic-loaded synthetic calcium sulfate beads for prevention of bacterial colonization and biofilm formation in periprosthetic infections. Antimicrob Agents Chemother. 2015;59(January (1)):111–120. doi: 10.1128/AAC.03676-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Raaij T.M., Visser L.E., Vulto A.G., Verhaar J.A. Acute renal failure after local gentamicin treatment in an infected total knee arthroplasty. J Arthroplasty. 2002 Oct;17(October (7)):948–950. doi: 10.1054/arth.2002.34525. [DOI] [PubMed] [Google Scholar]
- 24.Kallala R., Haddad F.S. Hypercalcaemia following the use of antibiotic-eluting absorbable calcium sulphate beads in revision arthroplasty for infection. Bone Jt J. 2015;97-B(September (9)):1237–1241. doi: 10.1302/0301-620X.97B9.34532. [DOI] [PubMed] [Google Scholar]
- 25.Hanssen A.D., Rand J.A., Osmon D.R. Treatment of the infected total knee arthroplasty with insertion of another prosthesis. The effect of antibiotic-impregnated bone cement. Clin Orthop Relat Res. 1994;309(December):44–55. [PubMed] [Google Scholar]
- 26.Parvizi J., Saleh K.J., Ragland P.S. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop. 2008;79:335–341. doi: 10.1080/17453670710015229. [DOI] [PubMed] [Google Scholar]
- 27.Walenkamp G.H., Vree T.B., Van Rens T.J. Gentamicin-PMMA beads. Pharmacokinetic and nephrotoxicological study. Clin Orthop. 1986;205:171–183. [PubMed] [Google Scholar]
- 28.Walenkamp G. Small PMMA beads improve gentamicin release. Acta Orthop Scand. 1989;60(December (6)):668–669. doi: 10.3109/17453678909149599. [DOI] [PubMed] [Google Scholar]
- 29.Walenkamp G.H.I.M. Antibiotic loaded cement: from research to clinical evidence. In: Meani E., Romano C., Crosby L., Hofmann G., editors. Infection and local treatment in orthopaedic surgery. Springer; Berlin: 2007. pp. 170–178. [Google Scholar]
- 30.Janssen D.M., Geurts J.A., Jütten L.M., Walenkamp G.H. 2-stage revision of 120 deep infected hip and knee prostheses using gentamicin-PMMA beads. Acta Orthop. 2016;87(August (4)):324–332. doi: 10.3109/17453674.2016.1142305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsieh P.H., Shih C.H., Chang Y.H., Lee M.S., Shih H.N., Yang W.E. Two-stage revision hip arthroplasty for infection: comparison between the interim use of antibiotic-loaded cement beads and a spacer prosthesis. J Bone Jt Surg Am. 2004;86-A(September (9)):1989–1997. [PubMed] [Google Scholar]
- 32.Taggart T., Kerry R.M., Norman P., Stockley I. The use of vancomycin-impregnated cement beads in the management of infection of prosthetic joints. J Bone Jt Surg Br. 2002;84-B(1):70–72. doi: 10.1302/0301-620x.84b1.11948. [DOI] [PubMed] [Google Scholar]
- 33.Chen W.S., Fu T.H., Wang J.W. Two-stage reimplantation of infected hip arthroplasties. Chang Gung Med J. 2009;32(March–April (2)):188–197. [PubMed] [Google Scholar]
- 34.Neut D., van de Belt H., van Horn J.R. Residual gentamicin-release from antibiotic-loaded polymethylmethacrylate beads after 5 years of implantation. Biomaterials. 2003;24(May (10)):1829–1831. doi: 10.1016/s0142-9612(02)00614-2. [DOI] [PubMed] [Google Scholar]
- 35.Udomkusonsri P., Kaewmokul Santi, Arthitvong Surapomg. Elution profiles of cefazolin from PMMA and calcium sulfate beads prepared from commercial cefazolin formulations. J Vet Med Sci. 2012;74(3):301–305. doi: 10.1292/jvms.11-0095. [DOI] [PubMed] [Google Scholar]
- 36.Lee G.H., Khoury J.G., Bell J.E. Adverse reactions to osteoset bone graft substitute, the incidence in a consecutive series. Iowa Orthop J. 2002;22:35–38. [PMC free article] [PubMed] [Google Scholar]
- 37.McPherson E.J., Dipane M.V., Sherif S.M. Dissolvable antibiotic beads in treatment of preiprosthetic joint infection – the use of synthetic pure calcium sulfate (Stimulan®) impregnated with vancomycin and tobramycin. Reconstr Rev. 2013:32. Joint Implant Surgery & Research Foundation. [Google Scholar]