Abstract
Metastasis is the commonest bone tumors. The commonest primary metastasis to the bone are the breast, lung, prostate, kidney, and thyroid. The bone is the third common site of metastatic disease, only the lung and the liver have higher metastatic rate than skeleton. We have no epidemiological studies conducted to evaluate the various aspects of skeletal metastasis like age, sex distribution, presentation, common sites of primary and associated secondary metastases, and investigation from Indian subcontinent. Here we are presenting the first epidemiological study of skeletal metastasis from our region. We have conducted a prospective descriptive study in the Departments of Orthopedics and Radiotherapy, Government Medical College, Kozhikode, during the period of 2007 to 2009. One hundred eleven patients were included in the study above the age of 30 years. Clinical examination and investigation were done on these patients. Skeletal metastasis commonly occurs in the fifth decade of life with modest male preponderance. In most of the cases, the primary site of malignancy was undetected at the time of presentation. Pain was the commonest presented complaint of the patient. The spine is the commonest site affected and the lung was the common site of primary metastasis. Most of the cases were detected by radiogram and confirmation was obtained by minimally invasive technique like FNAC or CNB.
Keywords: Skeletal metastasis, Epidemiology, Bone metastasis
Introduction
Metastases are the commonest of bone tumors. With improved treatment of the primary malignancy, the incidence of bone tumors is on the increasing trend [1]. Any tumor can cause metastasis to the bone, but the commonest sites of primary that metastasis to the bone are the breast, lung, prostate, kidney, and thyroid [2]. These account for approximately 80% of the skeletal metastasis [3]. Approximately 20% of patients, who develop metastatic carcinoma, develop clinically evident bone metastasis during their life time [4]. Incidence of skeletal metastasis is greatest for breast and prostate cancer.
The bone is the third common site of metastatic disease, only the lung and the liver have higher metastatic rate than the skeleton [5]. Metastasis to this region is indicative of advanced lesion. Metastasis involves axial skeleton more than appendicular skeleton [6]. The most common sites for metastasis include the spine, ribs, pelvis, skull, and proximal femur [7, 8]. Metastasis to hand and feet is uncommon and is usually associated with carcinoma of the lung [9, 10]. Pain is the most common presentation of skeletal metastasis, especially during the night [11]. Lesion in flat bones such as the clavicle and the rib may be asymptomatic for a long time [12]. Lesion in weight-bearing bone can present as pathological fracture. About 25% of lesions may be asymptomatic and detected only after a bone scan [11, 12]. Metastasis to the spine can present as back pain or neurological deficit [13].
There are many mechanisms of tumor metastasis to the bone [14]. As per the current understanding, there are three mechanisms of skeletal metastasis. The first mechanism is the intrinsic properties of tumor cells. These properties potentiate the tumor cells’ ability to leave the site of primary neoplasm and take residence in distant skeletal environment. The second mechanism is the anatomic predisposition of host that allows seeding of the neoplastic cells in specific regions, as described by Batson. The last mechanism is the properties of skeletal host and its response to the migration of malignant cells from the site of primary tumor [15–19].
The diagnostic evaluation of metastatic bone tumors includes thorough history, physical examination, and a complete haemogramme including erythrocyte sedimentation rate. Serum tumor markers and chest X-rays must be included. Technitum 99 m MDP bone scanning is a vital component of screening for metastatic bone tumors [20, 21]. Plain radiograph of the involved area should be performed in every patient suspected of having skeletal metastasis. Radiologically, the lesions can be osteolytic, osteoblastic, or mixed [22]. Lung, kidney, and thyroid carcinomas are typically osteolytic, whereas prostate cancer most commonly appears osteoblastic. Breast, ovarian, cervical, testicular, and some lung carcinoma can appear mixed on plain radiographs [2, 23, 24]. MRI is the most useful imaging technique in detecting early skeletal metastasis because it allows direct visualization of the bone marrow and is very sensitive [25]. CT scan is also widely used for detecting osteolytic and osteoblastic bone lesions involving cortical bone [26]. We can also do guided biopsy with the help of computerized tomography. PET scan also be used to detect multiple metastasis but it is expensive [27]. Biopsy is the gold standard procedure in arriving final diagnosis [28].
A multidisciplinary approach to treatment of metastatic bone disease is of paramount importance [6, 9]. The treatment goals include improvement of patient’s general health, control of local symptoms, and treatment of primary disease. The multidisciplinary team includes a qualified orthopedic surgeon, medical oncologist, radiation oncologist, and musculoskeletal radiologist [29].
There are very few studies conducted to find out the epidemiology of skeletal metastasis. Here we are presenting the first epidemiological study of skeletal metastasis from North Kerala. This study was conducted in Government Medical College, Kozhikode, which caters patients mostly from the four districts of northern Kerala state which is a thickly populated region. We think that our data will be useful for planning and adopting strategies for the early detection and management of skeletal metastasis.
Materials and Method
After obtaining the approval from the Institutional Research and Ethical Committee of Government Medical College, Kozhikode, we have done a prospective observational study over the period of 24 months from October 2007 to October 2009. The study was carried out in the Departments of Orthopedics and Radiotherapy of Government Medical College, Kozhikode. The Medical College Hospital is a referral hospital and takes care of the population of northern Kerala. All patients with skeletal metastasis over the age of 30 were included in the study after getting informed consent. Patients with primary bone tumors, multiple myeloma, and other haemopoietic malignancy were excluded.
A total of 123 patients with skeletal lesions were identified during the study period, out of which 4 patients did not give consent and 5 patients were excluded because of multiple myeloma and other bony primary malignancy. We lost a follow-up of 3 patients. Finally, 111 patients with skeletal metastasis were included in the study.
Detailed history of the nature of onset, duration, progression, and risk factors for malignancy and history of malignant disease in the family were taken. Physical examination of the musculoskeletal system and other systems was done in all patients, with special emphasis on the examination of the breast, thyroid, prostate, abdomen, chest, and lymph nodes. Laboratory evaluation included complete haemogramme, ESR, serum calcium, phosphorus, and alkaline phosphatase. Tumor markers were also done. Radiogram of the involved region and the chest was taken. MRI and CT scans were included in indicated patients. Histopathological confirmation was done by FNAC, core needle biopsy (CNB), or open biopsy as required.
The data was entered and analyzed using Microsoft Excel version 2002 software. Statistical analysis was done using SPSS statistical package version 16 and significance was calculated using Pearson’s Chi-square test. Proportions and means of relevant variables such as demographic characters, causes, sites, and presentation were derived.
Results
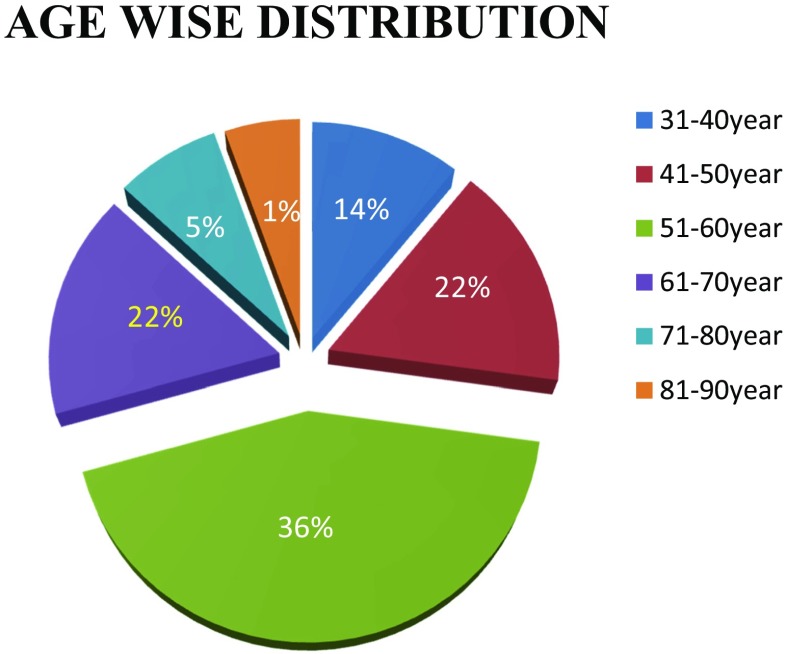
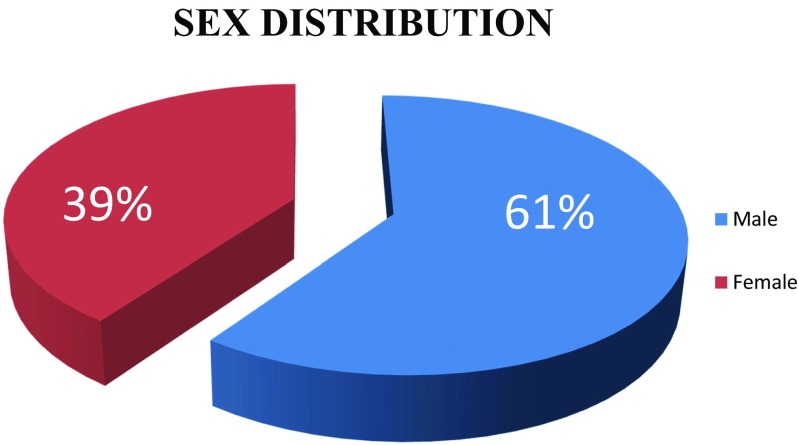
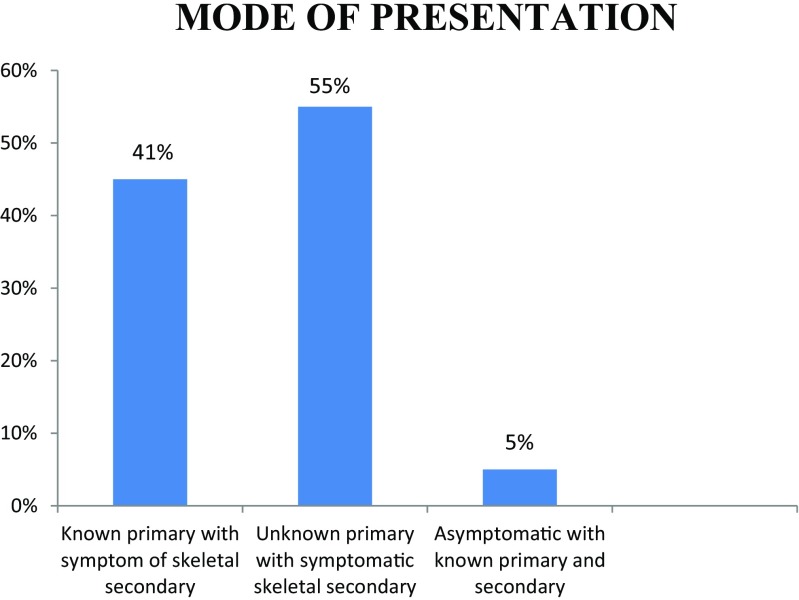
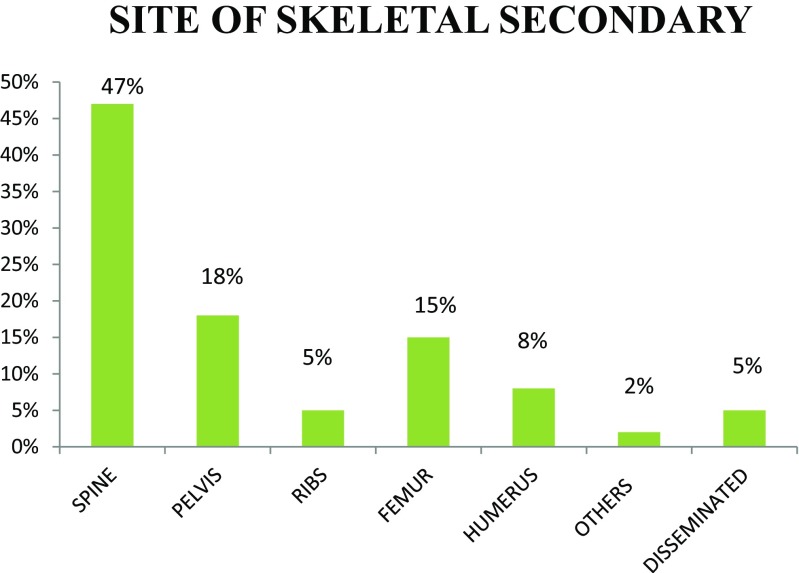
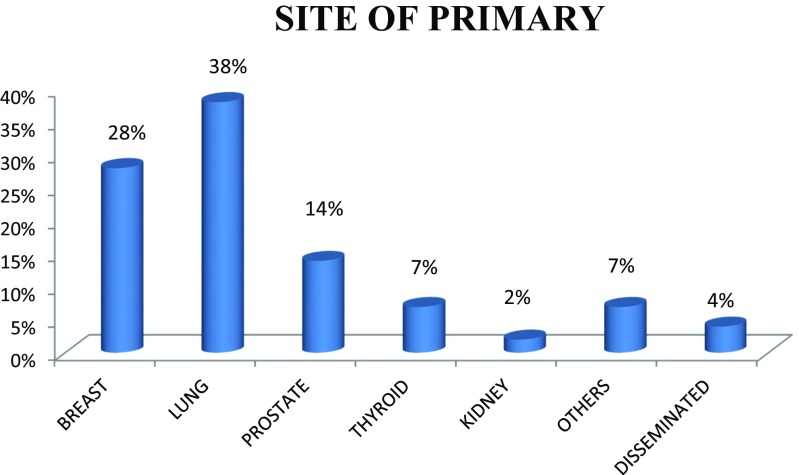
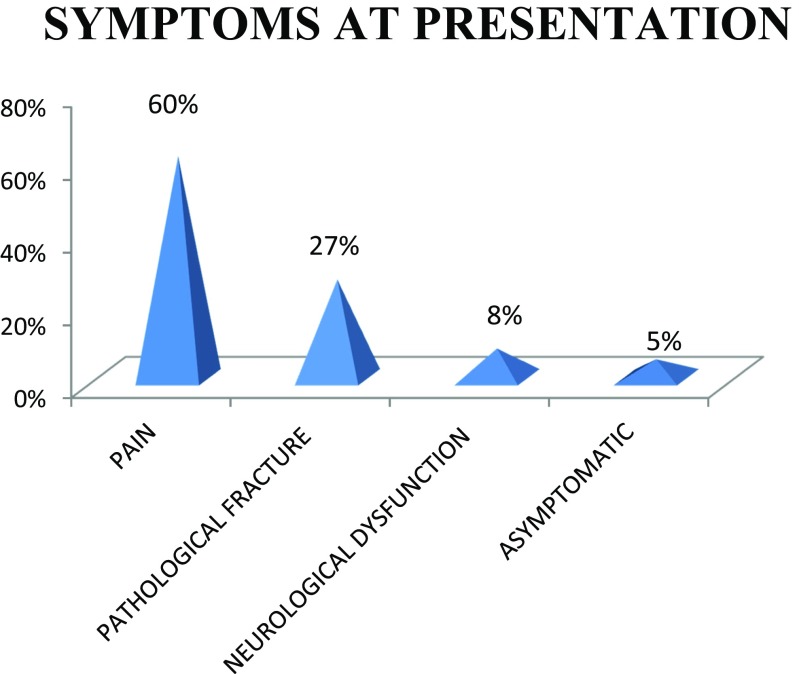
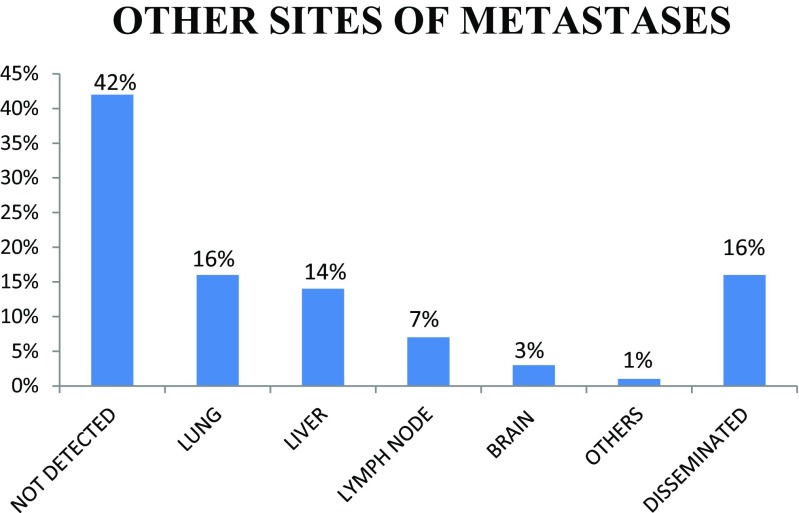
Out of the 111 patients, 61% are male with male to female ratio of 3:2. The patients were in the age group 31 to 81 years. Most of the cases present in the fifth decade (36%) and the least in the eighth decade, probably due to fewer number of patients in this age group (Figs. 1 and 2). Only 5% of the patients were asymptomatic with a known primary malignancy at the time of presentation. However, 55% of the patients presented with unknown primary malignancy and 41% were with known primary malignancy (Fig. 3). The most common occult primary site was the lung (38%). Sixty percent of the patients’ initial presentation was pain and 27% of the patients presented with pathological fractures. The most common site of metastasis was the spine (47%), followed by the pelvis (18%), and the proximal femur (15%) (Fig. 4). There were disseminated skeletal metastases in 6% of the cases. The most common site of primary malignancy was the lung (38%) and others included the breast (28%), prostate (14%), and thyroid (7%) (Fig. 5). There were other sites of metastasis along with skeletal metastasis such as the lung (16%) and the liver (14%) and another 16% of the patients had metastasis in more than two sites (Figs. 6 and 7). Sixteen percent had disseminated metastasis at the time of presentation. Forty two percent of the patients presented with isolated skeletal metastasis.
Fig. 1.
Age-wise distribution
Fig. 2.
Sex distribution
Fig. 3.
Mode of presentation
Fig. 4.
Site of skeletal secondary metastasis
Fig. 5.
Site of primary malignancy
Fig. 6.
Symptoms at presentation
Fig. 7.
Other sites of metastasis
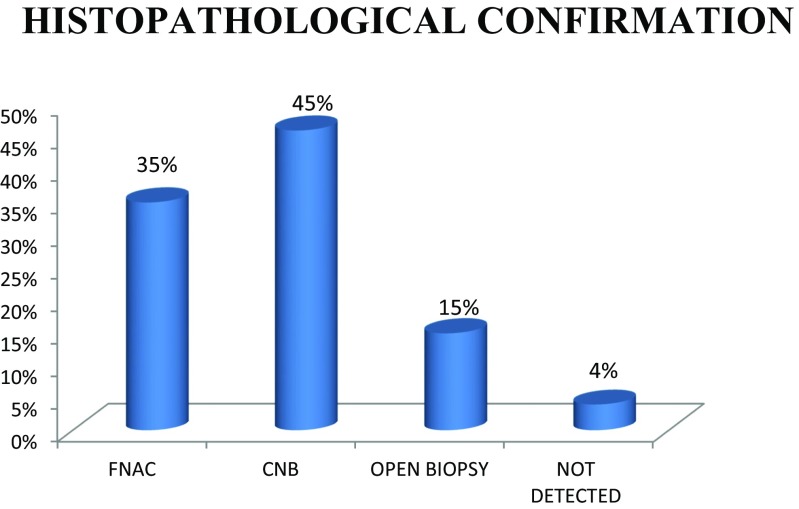
Further, 78% of the patients in the present study had an elevated ESR of more than 80 mm/h (Table 1). Alkaline phosphatase was elevated in 75% of the patients. High level of calcium was noted in 33% of the patients (Table 2). Seventy patients in our study showed roentgenographic evidence of metastasis, 18 patients were detected to have bone metastasis by bone scan. MRI was taken in 15 patients with spine involvement, and neurological deficit. Computerised tomography scan was considered in 5 patients especially for guided biopsy. We got histopathological confirmation of metastasis by FNAC or CNB in 81% of the cases (FNAC 35%, CNB 46%). Open biopsy was needed in 16% of the cases. In 4% of the cases, we were not able to locate the site of primary malignancy either by FNAC or CNB and open biopsy was deferred due to terminal illness of the patients (Fig. 8).
Table 1.
ESR more than 80 mm/h
| Frequency | Percent | |
|---|---|---|
| No | 25 | 22.5 |
| Yes | 86 | 77.5 |
| Total | 111 | 100 |
Table 2.
Calcium more than 11 g%
| Frequency | Percent | |
|---|---|---|
| No | 78 | 70.3 |
| Yes | 33 | 29.7 |
| Total | 111 | 100 |
Fig. 8.
Histopathological confirmation
Discussion
Metastasis is relatively rare in patients younger than 40 years. Males are more commonly affected than females [30]. In a study by Wegner G, the incidence of skeletal metastasis presenting without symptoms at presentation was 25%, but in our study it was only 5%. This may be due to fewer number of cases in our study and fewer number of lesions in flat bones such as the ribs and the clavicle, which can remain asymptomatic for a longer period [10]. The most common symptomatic site of primary malignancy in skeletal metastasis is renal cell carcinoma [30]. But in our study, we have only a very few cases of renal cell carcinoma. The lung was the most common asymptomatic primary producing skeletal metastasis which was attributed to the large site to which tumor in the lung can grow before symptoms are evident. Pain is the most common presentation of skeletal metastasis which was true with our study also [11]. According to Fidler, pathological fractures can occur in skeletal metastasis only when more than 50% of the cortex is destroyed [31]. Patients usually have pain before pathological fractures especially in the long bone, spine, and pelvis [32]. But pathological fractures without antecedent symptoms are common in the clavicle and the ribs. In our study, 27% of patients were presented with pathological fractures. Eight percent of our patients were having neurological dysfunction at the time of presentation. Mostly, back pain may be an antecedent symptom of pathological fracture and neurological deficit in spine metastasis. So it is preferable to attempt to diagnose the lesions at risk before fracture. The morbidity associated with operative treatment of pathological fractures of the spine can be avoided by early treatment of impending fractures [32].
Unlike primary bone tumors, metastasis usually involves axial skeleton and proximal segments of limb bones only. In extremely rare cases, metastasis can occur in distal to knee and elbow [9]. The same was true in our study also. As per the study by Coleman R E, the breast, bronchus, and prostate account for more 80% of metastatic bone disease [33]. According to BuckWalter JA and Brandser EA, five specific carcinomas—breast, lung, prostate, kidney and thyroid—account for approximately 80% of skeletal metastasis. According to them, skeletal metastasis is greatest for breast and prostate cancer [3]. Higher incidence of secondaries from lung cancer in our series may be due to greater prevalence of carcinoma lung in this area and small sample size. The bone is the third common site of metastatic disease, only the lung and the liver have higher metastatic rate than skeleton. In our study also, we have combined metastasis involving the liver and the lung along with skeletal metastasis.
A study by Rougraff et al. showed that only 17 patients out of 40 (42%) had elevation in ESR, but in our study, more than 70% of the cases have ESR of more than 80 mm/h [30]. Even though ESR is a nonspecific marker, it is worthwhile to suspect metastasis in patients above 40 years of age with elevated ESR of more than 80 mm/h. The same study also showed 10 percentages of patients having an elevated ALP, compared to 75% of the patients in our study (Table 3). Hypercalcemia is present in 8–20% of cancer patients [33]. Our study showed 33% of hypercalcemia in skeletal metastasis. Bone scan is one of the most cost effective investigations to find out the multiple metastatic lesions in the bone (Table 4) [34]. Patients with neurological symptoms and metastasis in the pelvis and spine can be better delineated by MRI or CT scan. It is very difficult to identify the site of primary carcinoma on the basis of biopsy alone, but it is an excellent method of confirmation of already suspecting site of primary malignancy [30]. In the present study, we have followed the same strategy; histopathological confirmation was obtained by minimally invasive techniques such as FNAC or CNB in 81% of the cases. A study by Ottolenghi et al. on aspiration biopsy of spine got 73% positive results, 13% doubtful, and 14% negative results [35]. For the evaluation of primary and metastatic bone tumors, the diagnostic accuracy of FNAC or CNB is 67 to 97%. Combining the two techniques may provide complementary information that increases the diagnostic accuracy further [36–38].
Table 3.
ALP more than 200 IU
| Frequency | Percent | |
|---|---|---|
| No | 36 | 32.4 |
| Yes | 75 | 67.6 |
| Total | 111 | 100 |
Table 4.
Radiological mode of detection
| Frequency | Percent | |
|---|---|---|
| X-ray | 71 | 64 |
| CT scan | 5 | 4.5 |
| MRI | 16 | 14.4 |
| Bone scan | 19 | 17.1 |
| Total | 111 | 100 |
Our study has certain limitations. As our institution is a referral center, an element of referral bias is possible; hence, complication and mortality appear higher than expected. Due to very poor general conditions and financial constraints, screening bone scan could be done only in 39 cases. Our study was conducted in a small number of patients; further multicenter study may be needed to extend the results for population benefit.
Conclusion
Skeletal metastasis commonly occurs in the fifth decade of life with modest male preponderance. In most of the cases, the primary site of malignancy was undetected at the time of presentation. Pain was the commonest presented complaint of the patient. The spine is the commonest site affected and the lung is the common site of primary malignancy. Most of the cases were detected by radiogram and confirmation was obtained by minimally invasive technique like FNAC or CNB.
Authors’ Contribution
All authors help in collecting data, analysis, statistics, writing, and editing the manuscript.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no conflict of interest.
Contributor Information
Balaji Zacharia, Phone: +919447667138, Email: balaji.zacharia@gmail.com.
Dhiyaneswaran Subramaniam, Email: dhiyane.mmc@gmail.com.
Jerin Joy, Email: drjerry.81@gmail.com.
References
- 1.Shimada H, Setoguchi T, Nakamura S, Yokouchi M, Ishidou Y, Tominaga H, Kawamura I, Nagano S, Komiya S. Evaluation of prognostic scoring systems for bone metastases using single-center data. Mol Clin Oncol. 2015;3(6):1361–1370. doi: 10.3892/mco.2015.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat RevCancer. 2002;2:584. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 3.Buckwalter JA, Brandser EA. Metastatic disease of the skeleton. Am Fam Physician. 1997;55(5):1761–1768. [PubMed] [Google Scholar]
- 4.Davies AM, Sundaram M, Steven JJ (eds) Imaging of bone tumors and tumor-like lesions: techniques and applications. Medical radiology and diagnostic imaging. Springer, Berlin Heidelberg
- 5.Piccioli A, Maccauro G, Spinelli MS, Biagini R, Rossi B. Bone metastases of unknown origin: epidemiology and principles of management. J Orthop Traumatol. 2015;16(2):81–86. doi: 10.1007/s10195-015-0344-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devita, Hellman, and Rosenberg: Cancer, principles and practice of oncology ... Lawrence, Theodore S;Rosenberg, Steven A. Philadelphia:Wolters Kluwer, [2016]....Principles and practice of oncology review: Govindan, Ramaswamy. 3rd ed
- 7.Ugras N, Yalcinkaya U, Akesen B, Kanat O. Solitary bone metastases of unknown origin. Acta Orthop Belg. 2014;80(1):139–143. [PubMed] [Google Scholar]
- 8.Khan MN, Sharfuzzaman A, Mostafa MG. Spinal cord compression as initial presentation of metastaticoccult follicular thyroid carcinoma. J Neurosci Rural Pract. 2014;5(2):155–159. doi: 10.4103/0976-3147.131661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Healey JH, Turnbull AD, Miedema B, Lane JM. Acrometastases: a study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68(5):743–746. doi: 10.2106/00004623-198668050-00017. [DOI] [PubMed] [Google Scholar]
- 10.Libson E, Bloom RA, Husband JE (1987) Husband: metastatic tumors of bones of the hand and foot: a comparative review and report of 43 additional cases affiliated with ….Department of Radiology, Royal Marsden Hospital, Dennis J. Stoker Skeletal Radiology 16(5):387–392 [DOI] [PubMed]
- 11.Hage WD, Aboulafia AJ, Aboulafia DM (2000) Aboulafia: incidence, location and diagonostic evaluation of metastatic bone disease. Orthop Clin North Am 31(4):515–528 [DOI] [PubMed]
- 12.Wagner G. Frequency of pain in patients with cancer. Recent Results Cancer Res. 1984;89:64–71. doi: 10.1007/978-3-642-82028-1_7. [DOI] [PubMed] [Google Scholar]
- 13.Weinstein JN. Differential diagnosis and surgical treatment of pathologic spine fractures. Instr Course Lect. 1992;41:301–315. [PubMed] [Google Scholar]
- 14.Springfield DS. Mechanism of metastasis. Clin Orthop. 1982;169:15. [PubMed] [Google Scholar]
- 15.Ali IU, et al. Reduction to homozygosity of genes on chromosomes 11 in human breast neoplasia. Science. 1987;238(4824):185–188. doi: 10.1126/science.3659909. [DOI] [PubMed] [Google Scholar]
- 16.Arguello F, Baggs RB, Frantz CN. A murine model of experimental metastasis to bone and bone marrow. Cancer Res. 1988;48(23):6876–6881. [PubMed] [Google Scholar]
- 17.Cher ML, Bova GS, Moore DH, Small EJ, Carroll PR, Pin SS, Epstein JI, Isaacs WB, Jensen RH. Genetic alterations in untreated metastases and androgen-independent prostate cancer detected by comparative genomic hybridization and allelotyping. Cancer Res. 1996;56(13):3091–3102. [PubMed] [Google Scholar]
- 18.Harris M, Howell A, Chrissohou M, Swindell RI, Hudson M, Sellwood RA. A comparison of the metastatic pattern of infiltrating lobular carcinoma and infiltrating ductcarcinoma of the breast. Br J Cancer. 1984;50(1):23–30. doi: 10.1038/bjc.1984.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mundy GR. Mechanisms of bone metastasis. Cancer. 1997;80(8 Suppl):1546–1545. doi: 10.1002/(SICI)1097-0142(19971015)80:8+<1546::AID-CNCR4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 20.Bates SE. Clinical applications of serum tumor markers. Ann Intern Med. 1991;115(8):623–638. doi: 10.7326/0003-4819-115-8-623. [DOI] [PubMed] [Google Scholar]
- 21.Rosenthal D. Radiologic diagnosis of bone metastases. Cancer. 1997;80(8 Suppl):1595–1607. doi: 10.1002/(SICI)1097-0142(19971015)80:8+<1595::AID-CNCR10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 22.Ortiz A, Lin SH. Osteolytic and osteoblastic bone metastases: two extremes of the same spectrum? Recent Results Cancer Res. 2012;192:225–233. doi: 10.1007/978-3-642-21892-7_11. [DOI] [PubMed] [Google Scholar]
- 23.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 24.Roudier MP, Vesselle H, True LD, et al. Bone histology at autopsy and matched bone scintigraphy findings in patients with hormone refractory prostate cancer: the effect of bisphosphonate therapy on bone scintigraphy results. Clin Exp Metastasis. 2003;20:171–180. doi: 10.1023/A:1022627421000. [DOI] [PubMed] [Google Scholar]
- 25.Hanna SL, Fletcher BD, Fairclough DL, et al. Magnetic resonance imaging of disseminated bone marrow disease in patients treated for malignancy. Skelet Radiol. 1991;20:79. doi: 10.1007/BF00193815. [DOI] [PubMed] [Google Scholar]
- 26.Yang HL, Liu T, Wang XM, Xu Y, Deng SM. Diagnosis of bone metastases: a meta-analysis comparing 18FDG PET, CT, MRI and bone scintigraphy. Eur Radiol. 2011;21(12):2604–2617. doi: 10.1007/s00330-011-2221-4. [DOI] [PubMed] [Google Scholar]
- 27.Hamaoka T, Madewell JE, Podoloff DA, Hortobagyi GN, Ueno NT. Bone imaging in metastatic breast cancer. J Clin Oncol. 2004;22(14):2942–2953. doi: 10.1200/JCO.2004.08.181. [DOI] [PubMed] [Google Scholar]
- 28.Traina F, et al. Current concepts in the biopsy of musculoskeletal tumors: AAOS exhibit selection. J Bone Joint Surg Am. 2015;97(2):e7. doi: 10.2106/JBJS.N.00661. [DOI] [PubMed] [Google Scholar]
- 29.Blum RH, Shasha D, Fleishman SB The multidisciplinary approach to bone metastases Review Article | May 31, 2003 | Bone Metastases, Cancer Complications, Palliative and Supportive Care, Oncology Journal [PubMed]
- 30.Rougraff BT, Kneisl JS, Simon MA. Skeletal metastases of unknown origin. A prospective study of a diagnostic strategy. J Bone Joint Surg Am. 1993;75(9):1276–1281. doi: 10.2106/00004623-199309000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Fidler M (1986) Anterior decompression and stabilization of metastatic spinal fracture. J Bone J Surg[Br] (68):83–90 [DOI] [PubMed]
- 32.Alan M, Levine AJA (ed) Pathologic fractures. Browner skeletal trauma, 4th ed
- 33.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 Pt 2):6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 34.Saifuddin DJSA, chapter 48, Bone tumors(2): Malignant lesion in Adam: Grainger and Allison’s diagnostic radiology,5th ed
- 35.Ottolenghi CE. Aspiration biopsy of the spine. J Bone Joint Surg Am. 1969;51:1531–1544. doi: 10.2106/00004623-196951080-00004. [DOI] [PubMed] [Google Scholar]
- 36.Kilpatrick SE, Ward WG, Cappellari JO, Bos GD. Fine-needle aspiration biopsy of soft tissue sarcomas: a cytomorphologic analysis with emphasis on histologic subtyping, grading, and therapeutic significance. Am J ClinPathol. 1999;112:179–188. doi: 10.1093/ajcp/112.2.179. [DOI] [PubMed] [Google Scholar]
- 37.Schweitzer ME, Gannon FH, Deely DM, O’Hara BJ, Juneja V. Percutaneous skeletal aspiration and core biopsy: complementary techniques. AJR Am J Roentgenol. 1996;166(2):415–418. doi: 10.2214/ajr.166.2.8553958. [DOI] [PubMed] [Google Scholar]
- 38.Bommer KK, Ramzy I, Mody D. Fine-needle aspiration biopsy in the diagnosis and management of bone lesions: a study of 450 cases. Cancer. 1997;81(3):148–156. doi: 10.1002/(SICI)1097-0142(19970625)81:3<148::AID-CNCR4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]