Abstract
We conducted this study to evaluate the demography, clinical presentation, management and outcomes of medullary thyroid carcinoma (MTC) from the Indian context. This was a retrospective study of patients with MTC managed between January 2008 and December 2016. All pertinent data was collected and the results were analysed using STATA (v.13.1). MTC accounted for 90/2022 (4.45%) patients managed with thyroid cancer during the study period. The mean age of presentation was 40 years (range 14–70 years) with 47 males and 43 females. The most common presentation included goitre with cervical lymphadenopathy seen in 60 patients (66.7%). There were 11 patients (12.2%) with systemic metastasis at presentation. Rearranged during transfection (RET) testing was performed in 71 patients and was positive in 25 (35.2%). The mutations among these patients were seen in the following codons: 634 (12), 804 (8), 790 (3) and 618 (2). Persistent hypercalcitoninemia (calcitonin > 50 pg/ml) was observed in 62/80 (77.5%) patients. Forty patients underwent a meta-iodo-benzyl-guanidine (MIBG) scan in the postoperative period, 10 were positive. The mean duration of follow-up was 32 months and 10 patients defaulted from follow-up. Sixteen patients developed metastasis during the period of follow-up while eight patients expired. The mean survival was 85.75 months (95% CI 78.7–92.7). MTC accounted for 4.5% of thyroid carcinomas in this cohort among which 35% were hereditary. Persistent hypercalcitoninemia following surgery is seen in more than 70% of patients but this does not affect survival. RET screening should be performed for all patients with MTC as curative surgery can be offered for mutation positive offspring.
Keywords: Thyroid carcinoma, Medullary thyroid carcinoma, RET, Calcitonin
Introduction
Medullary carcinoma thyroid is a rare form of differentiated thyroid carcinoma accounting for 1–2% of all thyroid carcinomas in the USA [1]. They may be either sporadic (70–75%) or hereditary (25–30%) [2, 3]. The hereditary forms are linked to a germ line mutation in the rearranged during transfection (RET) oncogene. These mutations are transmitted as an autosomal dominant trait with close to a 100% penetrance of the disease. The demographic profile, presentation and prognosis differ not only between the two forms of medullary thyroid carcinoma (MTC) but also between the various subtypes of hereditary MTC. Hence MTC is a unique form of thyroid cancer with challenges in its management.
We conducted this study to analyse the clinical profile, histopathology, surgical, adjuvant therapies and outcomes of patients with MTC.
Methods
This was a retrospective analysis of patients with MTC managed at a tertiary care hospital between January 2008 and December 2015. The follow-up data were collected up to December 2016. The data of all patients diagnosed and treated for MTC was collected from a password-protected computerised hospital information system. Patients who had not reviewed in the outpatient department following their surgical management were contacted telephonically for follow-up details. The diagnosis of MTC was based on fine needle aspiration cytology (FNAC) or an elevated serum calcitonin value (normal range, 0–50 pg/ml) or the histopathological report following thyroidectomy. All patients who were diagnosed with MTC were offered the RET gene analysis. The method used for RET gene analysis was similar to that previously reported by Pai et al. [4]. Patients were stratified as either sporadic MTC or hereditary MTC based on the results of RET analysis. Those with hereditary MTC were further classified as MEN 2a, MEN 2b or familial MTC based on the revised American Thyroid Association (ATA) Guidelines for the management of MTC 2015 [1]. The family members of these patients were counselled regarding screening for RET mutation and those who were positive were advised prophylactic surgery.
Preoperatively, the demographic profile, presenting complaint, FNAC report, calcitonin value, presence of systemic metastasis at presentation and site, findings of the preoperative contrast enhanced computerised tomogram (CECT) and the operation performed were documented. Postoperatively, the tumour size and focality, calcitonin value, adjuvant treatment received, lymph nodal recurrence, new systemic metastasis, follow-up duration and mortality were accounted for. Patients were defined as being disease free following surgery if their postoperative calcitonin levels were below 50 pg/ml on follow-up.
The complications following surgery were also categorised. Temporary hypocalcaemia was defined as the postoperative serum-corrected calcium < 8 mg/dl or PTH < 8 pg/dl which normalised within 6 months of operation. Those in whom these two biochemical parameters remained subnormal 6 months following surgery were defined as having permanent hypocalcaemia. All patients undergoing a neck dissection and those with a fixed goitre had a preoperative vocal cord assessment. Patients who developed voice change following surgery were evaluated with nasopharyngo-laryngoscopy (NPL scopy) to assess their vocal cord function. A repeat NPL scopy was performed 6 months later in patients who had vocal cord palsy. Persistence of vocal cord dysfunction 6 months after operation was defined as permanent palsy.
Data was summarised using mean ± SD/median (min, max) for continuous variables and frequency with percentage for categorical variables. Survival was analysed using Kaplan-Meier estimates with mortality considered as the event. All the data was analysed using STATA IC/13.1.
Results
Demography and Presentation
There were 2022 patients with thyroid carcinoma managed during the study period. Among them, 90 patients (4.45%) were diagnosed with MTC. The mean age at presentation was 40 years (range 14–70 years) with 47 males and 43 females. Sixty (66.7%) patients presented with a goitre and palpable cervical lymph nodes, while 11 (12.2%) had systemic metastasis at presentation. The most common site of distant metastasis was the lung (seven patients) followed by bone and liver (three patients each) while two patients had metastasis to multiple sites (Table 1).
Table 1.
Depicting the demographic and clinicopathological profile of patients with MTC
| Variable | No. of patients (%) |
|---|---|
| 1. Age in years: mean (range) | 40.08 (14–70) |
| 2. Gender (M/F) | 47:43 |
| 3. Presenting complaint | |
| a. Goitre only | 24 (26.7%) |
| b. Goitre + lymph node | 60 (66.7%) |
| c. Pheochromocytoma | 3 (3.3%) |
| d. Screen detected | 3 (3.3%) |
| 4. Systemic metastasis at presentation | 11 (12.2%) |
| 5. Site of metastasis: | |
| a. Lung | 7 (63.6%) |
| b. Bone | 3 (27.3%) |
| c. Liver | 3 (27.3%) |
| d. Multiple sites (≥ 2) | 2 (18.2%) |
Diagnosis
FNAC could be performed in 71 patients and it was diagnostic of MTC in 45 patients [sensitivity of 63.4% (95% CI 51.10–74.5)]. Preoperative calcitonin levels were elevated in 75/76 (98.7%) patients. Preoperative calcitonin level more than 200 pg/ml was associated with cervical lymph node metastasis (p < 0.01).
Treatment
All patients underwent surgical intervention as the primary modality of treatment. Seventy-two patients had their primary surgery at our institution. In 66/72 patients, the primary operation included total thyroidectomy + central compartment lymph node dissection (CCLND) +/− selective neck dissection (SLND). In the remaining six patients, MTC was incidentally diagnosed on the biopsy specimen and hence the primary surgery was quite often incomplete. Two of them subsequently required CCLND and SLND, one required only CCLND, while the other three did not require further operations. Two of them were sporadic tumours with normal calcitonin levels on follow-up, one patient did not return for follow-up. Eighteen patients had their primary operation at another centre. Twelve patients had a complete operation upfront, while six underwent a two-staged procedure. Hence, the most common operation performed was total thyroidectomy + CCLND + SLND in 69/90 (76.6%) patients. Eighteen patients (20%) underwent total thyroidectomy + CCLND alone. This included three patients who underwent prophylactic thyroidectomy. Three patients received no further treatment after total thyroidectomy (Table 2).
Table 2.
Depicting the operation performed
| Operation performed | Number (%) |
|---|---|
| A. Upfront complete | 78 (86.7%) |
| 1. Total Thyroidectomy + CCLND + SLND | 61 (67.8%) |
| 2. Total Thyroidectomy +CCLND | 17 (18.9%) |
| B. Upfront incomplete | 12 (13.3%) |
| 1. Hemithyroidectomy followed by completion thyroidectomy + SLND | 4 (4.4%) |
| 2. Total thyroidectomy followed by CCLND + SLND | 4 (4.4%) |
| 3. Total thyroidectomy followed by CCLND | 1 (1.1%) |
| 4. Total thyroidectomy alone | 3 (3.3%) |
The histopathological examination of the specimen from patients who underwent prophylactic thyroidectomy revealed micro MTC (2 and 3 mm tumour size) in two and C cell hyperplasia in one. The overall mean tumour size was 3.5 cm (range, 0.2–9 cm). A coalition tumour—having both papillary carcinoma (PTC) and MTC—was present in 6/90 (6.7%). This included follicular variant of PTC (FVPTC) in one, mico-FVPTC in three and classic micro-PTC in two patients.
Complications
Thirty-two patients (35.5%) developed temporary hypocalcaemia while eight (8.9%) developed permanent hypocalcaemia. Temporary recurrent laryngeal nerve (RLN) palsy occurred in three patients (1.7%), seven patients (3.9%) developed permanent palsy, wherein the denominator is nerves at risk. Among the patients with permanent vocal cord palsy, in four the nerve was infiltrated by tumour and had to be shaved free during surgery, in one the nerve was inadvertently injured during surgery and in the remaining two no intraoperative difficulties were noted. Seven patients (10.3%) developed chyle leak, five of them responded to conservative management but two of them required surgical intervention to control the leak.
Postoperative Calcitonin
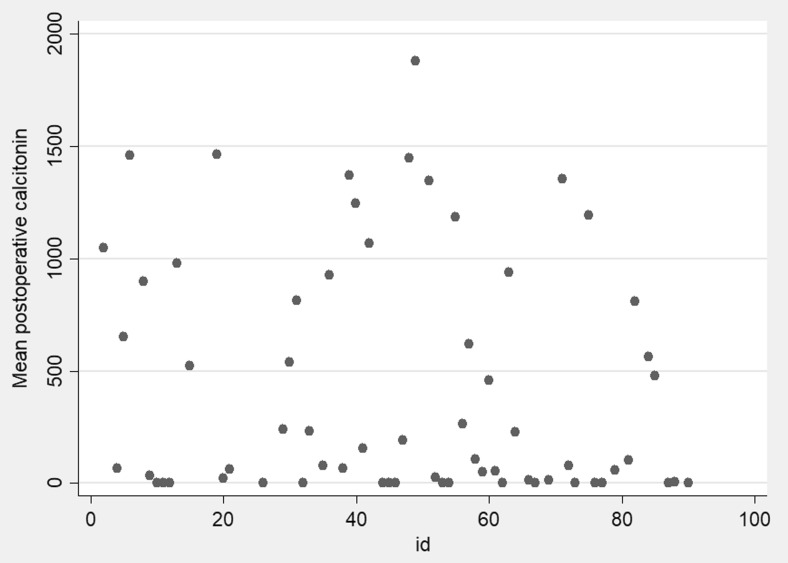
Calcitonin was serially monitored in 80 patients who came back for follow-up. Persistent hypercalcitoninemia (calcitonin > 50 pg/ml) was observed in 62 patients (77.5%). Figure 1 depicts the scatter plot of postoperative calcitonin values.
Fig. 1.
Scatter plot depicting the postoperative calcitonin values
Adjuvant Therapy
Meta-iodo-benzyl-guanidine (MIBG) scan was performed in 40 patients. Ten of them showed a positive uptake, seven in thyroid bed and three in metastatic sites (liver and bone). The three patients with distant metastasis to the bone and liver received MIBG therapy. 68Ga DOTA scintigraphy was performed in nine patients including seven in whom the MIBG scan was negative. Seven of these scans were positive. Positive uptake was seen in the lung, liver and bone in four patients, mediastinal lymph nodes in two and in the thyroid bed in one. Three patients with distant metastasis received Lutetium therapy. Fourteen patients received external beam radiotherapy (EBRT), 13 to the thyroid bed and 1 to a spinal metastasis causing paraparesis. The indication for EBRT, among these patients, was the presence of gross residual disease in 3 and increased risk of local recurrence in 10 patients. Twelve patients with metastatic disease received tyrosine kinase inhibitors (TKI). The other modalities of adjuvant treatment offered include 131I ablation (nine patients) and thalidomide (one patient). Sixty patients did not require adjuvant treatment. Fourteen patients needed more than one modality of adjuvant therapy.
Cervical Lymph Node Recurrence
On follow-up, 18 patients (26.5%) came back with cervical lymph nodal recurrence. Twelve of them (66.6%) required re-excision of the nodes, one was managed with alcohol ablation and five others (27.8%) did not need any intervention. Only one patient had recurrence in the central compartment. Following re-excision, the calcitonin levels remained static in three patients, came down in three and there was a twofold increase in calcitonin levels due to wide spread metastatic disease in five others. One patient was lost to follow-up.
Systemic Metastasis
Sixteen patients developed systemic metastasis on follow-up. The sites involved included the liver (13 patients), lung (10 patients), bone (7 patients) and multiple organs in (12 patients) (Table 3).
Table 3.
Depicting the sites of distant metastasis
| Sites of metastasis | Metastasis at presentation 11 (12.2%) |
New metastasis on follow-up 16 (17.8%) |
Total 27 (30%) |
|---|---|---|---|
| Lung | 7 | 10 | 17 |
| Liver | 3 | 13 | 16 |
| Bone | 3 | 7 | 10 |
| Multiple (≥ 2 sites) | 2 | 12 | 14 |
Follow-up
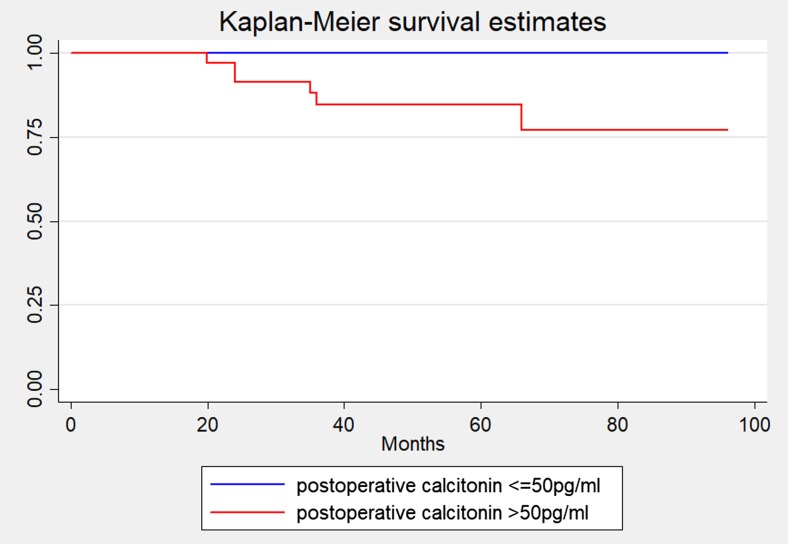
The mean duration of follow-up was 32 months with 10 defaulters. There were eight deaths during the study period. The 5-year survival rate and overall survival rate was 86.3% and 81.2%, respectively, with a mean survival of 87.5 months (95% CI 78.8–92.7). The mean disease-free survival was 15.2 months (95% CI 11.7, 18.8) with a disease-free survival rate of 9.3% at 24 months. There was no difference in the survival rate between patients with hypercalcitoninemia and those without hypercalcitoninemia (Fig. 2).
Fig. 2.
Comparing the overall survival analysis between normal postop calcitonin and postoperative hypercalcitoninemia
Hereditary MTC
Testing for RET gene mutations could be done in 71 patients. Twenty-five of them (35.2%) had an underlying mutation in the RET gene. The mutations involved codon 634 (12 patients), 804 (8 patients), 790 (3 patients) and 618 (2 patients). All the hereditary forms were those with MEN 2A.
Discussion
According to the Surveillance, Epidemiology and End Result (SEER) data report, it has been observed that over the last three decades there is a decreased incidence of MTCs (from 3 to 5% to 1–2%) due to progressive increased incidence of PTCs [1]. The incidence of MTCs at our institution remains at 3–5% as has been previously reported [5–8]. A referral bias to a quaternary care institution may account for the higher incidence of MTC seen in our series. The mean age at diagnosis was 40 years as compared to 43–52 years in other reports and there was no female preponderance as was reported in other studies [6, 9–13]. Majority (66%) of patients at presentation had lateral cervical lymph node metastasis demonstrating the aggressive nature of the disease. The reported incidence of cervical lymph node metastasis at presentation ranges from 50 to 80% [14, 15]. The incidence of distant metastasis (12.2%) at the time of presentation was similar to what was observed in other reports [11, 16].
An FNAC of the thyroid forms an integral part of the evaluation of any thyroid nodule but FNACs in patients with MTC has a low sensitivity [17, 18]. We report a similar finding (63.4% sensitivity). The preoperative calcitonin levels were elevated in 75 patients. Four patients had normal calcitonin levels. Three of them did not have a goitre, but were picked up on family screening for RET gene mutations. Only one patient had a normal calcitonin level (34.5 pg/ml) despite having a goitre and palpable cervical lymph nodes. In this patient, the preoperative CECT also revealed mediastinal lymph node metastasis and multiple bone metastasis. He underwent a total thyroidectomy with CCLND and bilateral SLND, the histopathology showed multifocal MTC. The postoperative calcitonin dropped to 3.6 pg/ml. The false negative preoperative calcitonin level in this patient could be attributed to the hook effect, but this was not confirmed as the calcitonin levels were not repeated in dilution [1]. Calcitonin levels were elevated in almost all patients (75/76) who presented with a goitre. Majority of patients (76.6%) underwent total thyroidectomy with CCLND and SLND. The rates of complications like hypocalcaemia [temporary (35.5%) and permanent (8.9%)] and chyle leak (10.3%) were higher than those reported for patients with well-differentiated thyroid carcinoma [19, 20]. This may be due to locally advanced disease requiring extensive surgery. Additionally, 18 patients underwent redo surgeries after their primary operation at other centres, this could have also contributed to the higher complication rates witnessed.
The permanent hypocalcaemia rate in this series was 8.9%. This rate is high when compared to permanent hypocalcaemia rates following total thyroidectomy for all thyroid pathologies taken together 0–3% [19]. Literature on post-thyroidectomy permanent hypocalcaemia in patients with MTC is limited. Reported rates range from 6.7 to 20% [21–24]. Sousa et al. have depicted that the histology of the gland predicts rates of permanent hypocalcaemia, showing rates of 1.1, 8.8 and 20% in patients with colloid goitre, papillary carcinoma and MTC, respectively [23]. Hence compared to surgery for benign thyroid pathologies and papillary carcinoma thyroid, the rates for post-thyroidectomy permanent hypocalcaemia in patients with MTC has been shown to be higher. Therefore, our rate of 8.9% seems to be acceptable.
Possible reasons for higher rates of permanent hypocalcaemia in MTC include the following:
The more aggressive nature of MTC resulting in patients presenting with locally advanced disease requiring an extensive surgery [23].
Prophylactic thyroidectomy performed for hereditary forms of MTC in children. A trend of higher rate of hypocalcaemia with younger age of patient (0–6 years) has been documented [22, 24].
Another reason for the high rates we have witnessed could be the varied definitions used for permanent hypocalcaemia in literature [19].
Rarely, PTC may occur concurrently with MTC. The prevalence of such coalition tumours ranges from 3.6 to 19% [25–27]. In this cohort, 6.7% patients had coalition tumours. Among them, one had a 2.5-cm FVPTC while the others had micro carcinomas. The existence of two malignancies in one organ is believed to be coincidental [1].
Serum calcitonin is a tumour marker for MTC and calcitonin levels are monitored for all patients on periodic follow-up. Persistent hyper calcitoninemia after surgery is quite common and is seen in more than 50% of patients with MTC [2, 28, 29]. Persistently elevated levels 3 months after surgery indicates the presence of either microscopic or macroscopic metastasis. This poses a problem for clinicians as attempts to identify these foci are often not rewarding. The protocol at our institution is to perform an ultra-sonogram (USG) of the neck to look for cervical node metastasis in patient’s postoperative calcitonin levels > 50 pg/ml. Eighteen patients (26.5%) came back with cervical nodal recurrence, 12 (66.6%) required re-excision of the affected lymph nodes. Only one of them had normal calcitonin levels after reoperation. Others have reported normalisation of calcitonin levels in 1/3rd of their patients after re-excision of cervical lymph nodes [30–32]. In five patients, the calcitonin levels continued to increase due to the presence of distant metastasis.
131I MIBG scan was performed in patients with postoperative calcitonin levels more than 150 pg/ml. If there was a positive uptake, 131I MIBG ablation therapy was offered. 131I MIBG scan was positive in 10/40 (25%) patients, this is similar to what has been reported in other studies [33–35]. In seven patients, the 131I MIBG uptake was in the thyroid bed, and in three there was evidence of distant metastasis. More recently, 68Ga DOTA scintigraphy has been used for evaluation of patients with persistent hypercalcitoninemia. Nine patients in our cohort underwent 68Ga DOTA scintigraphy, this included six patients who had negative 131I MIBG scans. All these six patients were picked up on the 68Ga DOTA scan, three others had negative scans. The positivity of DOTA scan in identifying the source of persistent hypercalcitoninemia was 66.7%. Three patients received Lutetium therapy, one patient expired, one has stable disease and the other was lost to follow-up. EBRT was offered for patients with gross residual disease in the neck, or for those at a high risk of local recurrence. EBRT is effective in preventing local recurrence in the neck but it does not improve the overall survival rates [36–38]. Fourteen patients in our study received EBRT. Recurrence of cervical lymphadenopathy following EBRT was seen in only two patients. Patients with significant tumour burden, those with symptoms and those with progressive metastatic disease were candidates for targeted therapy with TKI. Twelve patients with metastatic disease received TKI. Two of them expired and two have defaulted follow-up. Of the remaining eight patients, only three patients had stable disease while the other five continued to progress. Previous reports of the benefits of 131I ablation therapy due to the “bystander effect” led to nine patients receiving 131I ablation. More recently, studies have shown no benefit from 131I therapy and so this modality of treatment has been discontinued [1]. Though postoperative hypercalcitoninemia occurs in a majority of patients, we have observed that it did not affect the overall survival. Distant metastasis in MTC tends to affect multiple organs, they are present in 15% of patients at presentation, and another 15% can develop distant metastasis on follow-up [11, 13, 39, 40]. Our patient cohort witnessed similar findings.
Hereditary MTC is reported to occur in 20–30% of patients [2, 3, 41]. The actual incidence may be higher, close to 35%, as observed in our series and other reports [42]. All our patients with hereditary MTC had underlying MEN2a. Mutation of codon 634 was the most common (48%); this was followed by codon 804 (32%), codon 790 (12%) and codon 618 (8%). The frequency of mutations in codon 634 and codon 790 was similar to that reported by Machens and Dralle from their experience in Germany (41 and 12%, respectively), this being much higher than reports from other centres. Higher rate of mutations in codon 804 and codon 618 witnessed has also been previously reported [43–45]. These findings are probably a result of the smaller number of patients that have been analysed compared to that of other series. All three patients who underwent a prophylactic thyroidectomy had normal postoperative calcitonin levels on follow-up. This reiterates the importance of RET testing especially for relatives of the index patient.
Conclusion
MTC accounts for 4.5% of all thyroid cancers in this cohort, among which 35% are hereditary. As a diagnostic investigation, FNAC has low sensitivity (63.4%) while serum calcitonin serves as a good diagnostic marker. Persistent hypercalcitoninemia following surgery is observed in the majority of patients (77.5%) but this does not seem to affect survival. Postoperative 131I MIBG has a low rate of detection for metastatic disease and hence its role for treating residual disease seems to be limited. RET screening should be offered for all first-degree relatives of patients with MTC as prophylactic surgery is curative. In view of these unique characteristics and complicated nature of the disease, all patients with MTC should be referred and managed in a centre with special interest in neuroendocrine tumours.
Compliance with Ethical Standards
Conflict of Interest
All authors declare that they have no conflict of interest.
Human Rights and Inform Consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
For this type of study formal consent is not required.
References
- 1.Wells SA, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid Off J Am Thyroid Assoc. 2015;25(6):567–610. doi: 10.1089/thy.2014.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen SM, Bodenner D, Suen JY, Richter GT. Diagnostic and surgical dilemmas in hereditary medullary thyroid carcinoma. Laryngoscope. 2009;119(7):1303–1311. doi: 10.1002/lary.20299. [DOI] [PubMed] [Google Scholar]
- 3.Rowland KJ, Moley JF. Hereditary thyroid cancer syndromes and genetic testing. J Surg Oncol. 2015;111(1):51–60. doi: 10.1002/jso.23769. [DOI] [PubMed] [Google Scholar]
- 4.Pai R, Nehru GA, Samuel P, Paul MJ, Thomas N, Premkumar JA, Hephzibah J, Shanthly N, Oommen R, Nair A, Seshadri MS, Rajaratnam S. Mutational analysis of RET proto-oncogene among patients with medullary thyroid carcinoma and ‘at risk’ carriers from India. Clin Endocrinol. 2011;75(4):571–572. doi: 10.1111/j.1365-2265.2011.04069.x. [DOI] [PubMed] [Google Scholar]
- 5.Nosé V. Familial thyroid cancer: a review. Mod Pathol. 2011;24:S19–S33. doi: 10.1038/modpathol.2010.147. [DOI] [PubMed] [Google Scholar]
- 6.Calvo J, Torrealba G, Sáenz A, Santamaría C, Morera E, Alvarado S, et al. Genetic and clinical features of medullary thyroid carcinoma: the experience of a single center in Costa Rica. J Cancer Epidemiol [Internet]. 2016 [cited 2017 Jan 5]; 2016. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC5149694/ [DOI] [PMC free article] [PubMed]
- 7.Randolph GW, Maniar D. Medullary carcinoma of the thyroid. Cancer Control. 2000;7(3):253–253. doi: 10.1177/107327480000700305. [DOI] [PubMed] [Google Scholar]
- 8.Sakorafas GH, Friess H, Peros G. The genetic basis of hereditary medullary thyroid cancer: clinical implications for the surgeon, with a particular emphasis on the role of prophylactic thyroidectomy. Endocr Relat Cancer. 2008;15(4):871–884. doi: 10.1677/ERC-08-0098. [DOI] [PubMed] [Google Scholar]
- 9.Correia-Deur JEM, Toledo RA, Imazawa AT, Lourenco DM, Jr, Ezabella MC, et al. Sporadic medullary thyroid carcinoma: clinical data from a university hospital. Clinics. 2009;64(5):379–386. doi: 10.1590/S1807-59322009000500002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ukkat J, Gimm O, Brauckhoff M, Bilkenroth U, Dralle H. Single center experience in primary surgery for medullary thyroid carcinoma. World J Surg. 2004;28(12):1271–1274. doi: 10.1007/s00268-004-7608-9. [DOI] [PubMed] [Google Scholar]
- 11.Simões-Pereira J, Bugalho MJ, Limbert E, Leite V. Retrospective analysis of 140 cases of medullary thyroid carcinoma followed-up in a single institution. Oncol Lett. 2016;11(6):3870–3874. doi: 10.3892/ol.2016.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee CR, Lee S, Son H, Ban E, Kang S-W, Lee J, Jeong JJ, Nam KH, Chung WY, Park CS. Medullary thyroid carcinoma: a 30-year experience at one institution in Korea. Ann Surg Treat Res. 2016;91(6):278–287. doi: 10.4174/astr.2016.91.6.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cupisti K, Wolf A, Raffel A, Schott M, Miersch D, Yang Q, Eisenberger CF, Röher HD, Knoefel WT. Long-term clinical and biochemical follow-up in medullary thyroid carcinoma: a single institution’s experience over 20 years. Ann Surg. 2007;246(5):815–821. doi: 10.1097/SLA.0b013e31813e66b9. [DOI] [PubMed] [Google Scholar]
- 14.Moley JF, DeBenedetti MK. Patterns of nodal metastases in palpable medullary thyroid carcinoma. Ann Surg. 1999;229(6):880. doi: 10.1097/00000658-199906000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber T, Schilling T, Frank-Raue K, Colombo-Benkmann M, Hinz U, Ziegler R, Klar E. Impact of modified radical neck dissection on biochemical cure in medullary thyroid carcinomas. Surgery. 2001;130(6):1044–1049. doi: 10.1067/msy.2001.118380a. [DOI] [PubMed] [Google Scholar]
- 16.Moley JF. Medullary thyroid carcinoma: management of lymph node metastases. J Natl Compr Cancer Netw. 2010;8(5):549–556. doi: 10.6004/jnccn.2010.0042. [DOI] [PubMed] [Google Scholar]
- 17.Bugalho MJM, Santos JR, Sobrinho L. Preoperative diagnosis of medullary thyroid carcinoma: fine needle aspiration cytology as compared with serum calcitonin measurement. J Surg Oncol. 2005;91(1):56–60. doi: 10.1002/jso.20269. [DOI] [PubMed] [Google Scholar]
- 18.de Crea C, Raffaelli M, Maccora D, Carrozza C, Canu G, Fadda G, Bellantone R, Lombardi CP. Calcitonin measurement in fine-needle aspirate washouts vs. cytologic examination for diagnosis of primary or metastatic medullary thyroid carcinoma. Acta Otorhinolaryngol Ital. 2014;34(6):399–405. [PMC free article] [PubMed] [Google Scholar]
- 19.Lorente-Poch L, Sancho JJ, Muñoz-Nova JL, Sánchez-Velázquez P, Sitges-Serra A. Defining the syndromes of parathyroid failure after total thyroidectomy. Gland Surg. 2015;4(1):82–90. doi: 10.3978/j.issn.2227-684X.2014.12.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brennan PA, Blythe JN, Herd MK, Habib A, Anand R. The contemporary management of chyle leak following cervical thoracic duct damage. Br J Oral Maxillofac Surg. 2012;50(3):197–201. doi: 10.1016/j.bjoms.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Dralle H, Gimm O, Simon D, Frank-Raue K, Görtz G, Niederle B et al (1998) Prophylactic thyroidectomy in 75 children and adolescents with hereditary medullary thyroid carcinoma: German and Austrian experience. World J Surg 22(7):744–751 [DOI] [PubMed]
- 22.Schreinemakers JMJ, Vriens MR, Valk GD, de Groot J-WB, Plukker JT, Bax KMA et al (2010) Factors predicting outcome of total thyroidectomy in young patients with multiple endocrine neoplasia type 2: a nationwide long-term follow-up study. World J Surg 34(4):852–860 [DOI] [PMC free article] [PubMed]
- 23.Sousa A de A, Salles JMP, Soares JMA, Moraes GM de, Carvalho JR, Savassi-Rocha PR (2012) Predictors factors for post-thyroidectomy hypocalcaemia. Rev Col Bras Cir 39(6):476–482 [DOI] [PubMed]
- 24.Kluijfhout WP, van Beek D-J, Verrijn Stuart AA, Lodewijk L, Valk GD, van der Zee DC et al (2015) Postoperative complications after prophylactic thyroidectomy for very young patients with multiple endocrine neoplasia type 2. Medicine (Baltimore) 94(29):e1108 [DOI] [PMC free article] [PubMed]
- 25.Machens A, Dralle H (2012) Simultaneous medullary and papillary thyroid cancer: a novel entity? Ann Surg Oncol 19(1):37–44 [DOI] [PubMed]
- 26.Biscolla RP, Ugolini C, Sculli M, Bottici V, Castagna MG, Romei C et al (2004) Medullary and papillary tumors are frequently associated in the same thyroid gland without evidence of reciprocal influence in their biologic behavior. Thyroid 14(11):946–952 [DOI] [PubMed]
- 27.Kim WG, Gong G, Kim EY, Kim TY, Hong SJ, Kim WB et al (2010) Concurrent occurrence of medullary thyroid carcinoma and papillary thyroid carcinoma in the same thyroid should be considered as coincidental. Clin Endocrinol (Oxf) 72(2):256–263 [DOI] [PubMed]
- 28.Finny P, Jacob JJ, Thomas N, Philip J, Rajarathnam S, Oommen R et al (2007) Medullary thyroid carcinoma: a 20-year experience from a centre in south India. ANZ J Surg 77(3):130–134 [DOI] [PubMed]
- 29.Van Veelen W, Groot D, B JW, Acton DS, Hofstra RMW, Höppener JWM et al (2009) Medullary thyroid carcinoma and biomarkers: past, present and future. J Intern Med 266(1):126–140 [DOI] [PubMed]
- 30.Fialkowski E, DeBenedetti M, Moley J (2008) Long-term outcome of reoperations for medullary thyroid carcinoma. World J Surg 32(5):754–765 [DOI] [PubMed]
- 31.Kebebew E, Kikuchi S, Duh QY, Clark OH (2000) Long-term results of reoperation and localizing studies in patients with persistent or recurrent medullary thyroid cancer. Arch Surg 135(8):895–901 [DOI] [PubMed]
- 32.Tisell LE, Hansson G, Jansson S, Salander H (1986) Reoperation in the treatment of asymptomatic metastasizing medullary thyroid carcinoma. Surgery 99(1):60–66 [PubMed]
- 33.Castellani MR, Seregni E, Maccauro M, Chiesa C, Aliberti G, Orunesu E et al (2008) MIBG for diagnosis and therapy of medullary thyroid carcinoma: is there still a role? Q J Nucl Med Mol Imaging 52(4):430–440 [PubMed]
- 34.Ilias I, Divgi C, Pacak K (2011) Current role of MIBG in the diagnosis of pheochromocytoma and medullary thyroid cancer. Semin Nucl Med 41(5):364–368 [DOI] [PMC free article] [PubMed]
- 35.Rufini V, Salvatori M, Garganese MC, Di Giuda D, Lodovica Maussier M, Troncone L (2000) Role of nuclear medicine in the diagnosis and therapy of medullary thyroid carcinoma. Rays 25(2):273–282 [PubMed]
- 36.Terezakis SA, Lee NY (2010) The role of radiation therapy in the treatment of medullary thyroid cancer. J Natl Compr Cancer Netw 8(5):532–540 [DOI] [PubMed]
- 37.Martinez SR, Beal SH, Chen A, Chen SL, Schneider PD (2010) Adjuvant external beam radiation for medullary thyroid carcinoma. J Surg Oncol 102(2):175–178 [DOI] [PMC free article] [PubMed]
- 38.Brierley JD (2011) Update on external beam radiation therapy in thyroid cancer. J Clin Endocrinol Metab 96(8):2289–2295 [DOI] [PubMed]
- 39.Sippel RS, Kunnimalaiyaan M, Chen H (2008) Current management of medullary thyroid cancer. The Oncologist 13(5):539–547 [DOI] [PubMed]
- 40.Pacini F, Castagna MG, Cipri C, Schlumberger M (2010) Medullary thyroid carcinoma. Clin Oncol (R Coll Radiol) 22(6):475–485 [DOI] [PubMed]
- 41.A T, F S, G P, M B (2011) Genetic alterations in medullary thyroid cancer: diagnostic and prognostic markers. Curr Genomics 12(8):618–625 [DOI] [PMC free article] [PubMed]
- 42.Jiménez C, Hu MI-N, Gagel RF (2008) Management of medullary thyroid carcinoma. Endocrinol Metab Clin North Am 37(2):481–496 [DOI] [PubMed]
- 43.Machens A, Dralle H (2008) Familial prevalence and age of RET germline mutations: implications for screening. Clin Endocrinol (Oxf) 69(1):81–87 [DOI] [PubMed]
- 44.Romei C, Mariotti S, Fugazzola L, Taccaliti A, Pacini F, Opocher G, Mian C, Castellano M, degli Uberti E, Ceccherini I, Cremonini N, Seregni E, Orlandi F, Ferolla P, Puxeddu E, Giorgino F, Colao A, Loli P, Bondi F, Cosci B, Bottici V, Cappai A, Pinna G, Persani L, Uberta V, Boscaro M, Castagna MG, Cappelli C, Zatelli MC, Faggiano A, Francia G, Brandi ML, Falchetti A, Pinchera A, Elisei R (2010) Multiple endocrine neoplasia type 2 syndromes (MEN 2): results from the ItaMEN network analysis on the prevalence of different genotypes and phenotypes. Eur J Endocrinol 163(6):963–963 [DOI] [PubMed]
- 45.Romei C, Tacito A, Molinaro E, Agate L, Bottici V, Viola D, Matrone A, Biagini A, Casella F, Ciampi R, Materazzi G, Miccoli P, Torregrossa L, Ugolini C, Basolo F, Vitti P, Elisei R (2015) Twenty years of lesson learning: how does the genetic screening test impact the clinical management of medullary thyroid cancer? Clin Endocrinol 82(6):892–899 [DOI] [PubMed]