Abstract
Accumulating data have revealed that abnormal activity of the mTOR (mammalian target of rapamycin) pathway plays an important role in epileptogenesis triggered by various factors. We previously reported that pretreatment with perifosine, an inhibitor of Akt (also called protein kinase B), abolishes the rapamycin-induced paradoxical increase of S6 phosphorylation in a rat model induced by kainic acid (KA). Since Akt is an upstream target in the mTOR signaling pathway, we set out to determine whether perifosine has a preventive effect on epileptogenesis. Here, we explored the effect of perifosine on the model of temporal epilepsy induced by KA in rats and found that pretreatment with perifosine had no effect on the severity or duration of the KA-induced status epilepticus. However, perifosine almost completely inhibited the activation of p-Akt and p-S6 both acutely and chronically following the KA-induced status epilepticus. Perifosine pretreatment suppressed the KA-induced neuronal death and mossy fiber sprouting. The frequency of spontaneous seizures was markedly decreased in rats pretreated with perifosine. Accordingly, rats pretreated with perifosine showed mild impairment in cognitive functions. Collectively, this study provides novel evidence in a KA seizure model that perifosine may be a potential drug for use in anti-epileptogenic therapy.
Keywords: Akt-mTOR signaling, Perifosine, Kainic acid, Spontaneous seizure, Cognitive function
Introduction
Epilepsy is a chronic brain disorder characterized by recurrent seizures caused by abnormal neuronal activity; it has an incidence of 5–10 per 1000 people [1, 2]. Patients with epilepsy display a wide range of devastating medical, social, and economic disadvantages, which result in a significant burden to both patients and family [3, 4]. Although substantial progress has been made in the development of anti-epileptic drugs and understanding their underlying mechanisms, new anti-epilepsy approaches are still required.
The mammalian target of rapamycin (mTOR) is a serine/threonine kinase that controls protein synthesis related to cell growth and proliferation. The Akt (protein kinase B)-mTOR signaling pathway plays a significant role in the regulation of various neuronal functions, including synaptic plasticity, neurogenesis, and dendritic and axonal morphology [5, 6]. Accumulating data have revealed that abnormal activity of the mTOR pathway is involved in epileptogenesis since our first report in animal models of tuberous sclerosis complex (TSC) and kainic acid (KA) [7, 8]. Several mTOR inhibitors have provided a useful option for treating seizures in patients with TSC in clinical practice [9–11]. Among them, rapamycin, an FDA-approved mTOR inhibitor used clinically as an anti-cancer agent, has demonstrated anti-epileptogenic effects in various animal models such as the genetic disease TSC and acquired epilepsy [12, 13].
However, paradoxically, we have found that rapamycin exacerbates seizures during short-term administration, whereas the Akt inhibitor perifosine abolishes the rapamycin-induced increase of phosphorylation of S6 (Ser240/244) in the rat model induced by KA [14]. Perifosine is a novel Akt inhibitor administered orally as an anticancer drug in phase III clinical trials [15]. In addition to Akt inhibition, perifosine has been shown to inhibit other major components including mTOR, raptor, rictor, p70S6K, and 4E-BP-1 in the mTOR signaling pathway by promoting their degradation [16]. Based on these reports, we hypothesized that perifosine may have an effect similar to rapamycin in preventing epileptogenesis without causing paradoxical results. Thus, in the present study, we explored the effect of perifosine on the inhibition of mTOR signaling, neuronal cell death, mossy fiber sprouting, spontaneous seizures, and cognitive function in a model of temporal epilepsy induced by KA in rats.
Materials and Methods
Animals and Drug Treatment
Male Sprague-Dawley rats 5 weeks–6 weeks of age were purchased from Shanghai Slac Laboratory Animal Corporation (certificate: SCXK 2012-0002) and housed in a controlled environment with ad libitum access to food and water. All animal experiments were performed in accordance with the guidelines approved by the Animal Studies Committee at Zhejiang University School of Medicine (Permit number: ZJU2015-489-02). Perifosine (supplied by Keryx Biopharmaceuticals Inc, Boston, MA) was dissolved in saline and stored at −20 °C. Two different perifosine treatment paradigms were used.
Based on our previous studies on the effects of perifosine on phospho-S6 expression, rats were treated with perifosine (20 mg/kg, i.p.) or saline for 3 consecutive days prior to KA treatment. On day 4, both groups were injected with KA (12 mg/kg, i.p.; Nanocs, New York, NY) to induce acute status epilepticus. Seizure activity was monitored behaviorally and graded according to a previous report [12] by two trained investigators blinded to the experimental groups: stage 1, behavioral arrest with mouth/facial movements; stage 2, head nodding; stage 3, forelimb clonus; stage 4, rearing; stage 5, rearing and falling. The latency from KA injection to the onset of the first seizure, the total seizure duration, and the maximal stage severity were measured. Seizure scores were based on the stage of the most severe seizure recorded for each rat. Calculation of seizure duration was started once the rat exhibited seizure stage 4, and ended when it moved freely around the cage. Rats that had stage 4 or 5 seizures were used for subsequent experiments. Some rats were used for video-EEG to correlate behavioral and EEG seizure activity during KA-induced acute status epilepticus. All animals were monitored for the development of spontaneous seizures (see below for video-EEG recording methods). An additional group of saline-treated rats that did not receive KA was used as the control. For Western blotting analysis and histology, 6 rats were used in each group. For video-EEG recording and Y-maze testing, 10 rats were used in each group.
Western Blot Analysis
Rats were sacrificed at various time points (1 h, 3 h, 6 h, 1 day, 3 days, 1 week, and 3 weeks) following the onset of status epilepticus. Western blotting was performed as described previously [17]. In brief, proteins separated by SDS-PAGE were transferred to nitrocellulose membranes, which were incubated with the rabbit anti-phospho-Akt or phospho-S6 antibody (1:1000, Cell Signaling, Beverly, MA) followed by peroxidase-conjugated secondary antibody. After the signals were visualized with ECL reagent (Pierce, Rockford, IL), the membranes were re-probed and incubated with rabbit anti-Akt and anti-S6 antibody (1:1000, Cell Signaling). Signals were analyzed with ImageJ software and the ratios of phospho-Akt/Akt and phospho-S6K/S6 were normalized to the control group.
Fluoro-Jade B (FJB) Staining
Neuronal death was analyzed by FJB staining 7 days after KA-induced status epilepticus. Rats were transcardially perfused with PBS followed by 4% paraformaldehyde. The brain was removed, post-fixed with 4% paraformaldehyde overnight at 4 °C, and then dehydrated in 30% sucrose for at least 24 h. The brains were sectioned coronally at 20 μm on a microtome. Three sections selected from a one-in-six series were collected from each animal at the same level of the hippocampus. After rehydration in 100% ethanol for 5 min, 70% ethanol for 2 min, and distilled water for 2 min, the sections were oxidized in 0.06% potassium permanganate solution for 10 min followed by 0.0004% FJB for 20 min in the dark. Thereafter, the sections were washed, air dried, cleared, and cover-slipped. Images were acquired with a confocal microscope (Olympus ix2-UCB, Shinjuku, Tokyo, Japan). The numbers of FJB-positive cells per image field in hippocampal CA1, CA3, and hilus were counted in each of the three sections per animal by an examiner blind to the experiment.
Video-EEG Recording
Another cohort of rats was used for video-EEG recording from 1 to 6 weeks after KA-induced status epilepticus. Rats were implanted with 4 epidural electrodes using a stereotaxic frame. Bilateral anterior and posterior epidural cortical screw electrodes (−1.60 mm bregma, 1.80 mm lateral and −4.0 mm bregma, 3.0 mm lateral), reference (+2.0 mm bregma, 1.0 mm lateral) and ground (−10 mm bregma, 1.0 mm lateral) were inserted into the skull and secured with dental cement. Twenty-four hours after surgery, rats were acclimated in cylindrical 10-inch diameter acrylic cages for at least 1 day before monitoring with a digital video-EEG acquisition system (V-Amp 16, Brain Products GmbH, Gilching, Germany). Rats were monitored continuously for 2 days each week, and EEG data were analyzed off-line by two independent trained observers.
Y-Maze Test
After 6 weeks of EEG recording, rats were tested in a Y-maze that was 50 cm long and 16 cm wide with walls 14 cm high and a copper shock grid on the bottom of the maze. A signal lamp was placed at the end of each arm. The arm with the light on (bright arm) indicated a safe area without shock. The safe and unsafe arms were set randomly. A correct response was recorded when the rat directly ran to the bright arm without hesitating after changing the safe and unsafe arms. The rats were trained 20 times per day for 4 consecutive days. On day 5, the rats were tested 20 times and the percentage of correct choices and the latency to the safe area were recorded. All tests were carried out in a small, dark, and quiet room.
Timm Staining
After the Y-maze test, the rats were perfused for histological analysis of mossy fiber sprouting using Timm staining. Rats were transcardially perfused with 1% sodium sulfide solution. After coronal serial sections were cut, they were incubated in a solution containing 120 mL of 50% gum arabic, 20 mL of 2 mol/L citrate buffer, 60 mL of 0.5 mol/L hydroquinone, and 1 mL of 19% silver nitrate for ~120 min until the molecular layer was clearly stained. Mossy fiber sprouting was assessed under a Nikon light microscope (Tokyo, Japan). The degree of mossy fiber sprouting was rated using semi-quantitative analysis [18] as follows: (1) sparse Timm granules in the supragranular zone; (2) more numerous granules in a continuous distribution; (3) prominent granules and patches; (4) a dense laminar band in the supragranular layer; and (5) a dense laminar band extending to the inner molecular layer.
Statistical Analysis
Data are presented as mean ± SEM. Differences among experimental groups were analyzed by one-way ANOVA with the Student-Newman-Keuls-Q test for post-hoc multiple comparisons (SPSS ver. 16.0, SPSS Inc., Chicago, IL). In some cases, two-way ANOVA with a post hoc multiple-comparisons test was used to analyze differences at each time point among different groups. P < 0.05 was considered significant.
Results
Perifosine Inhibits the Activation of p-Akt and p-S6 Both Acutely and Chronically Following KA-Induced Status Epilepticus
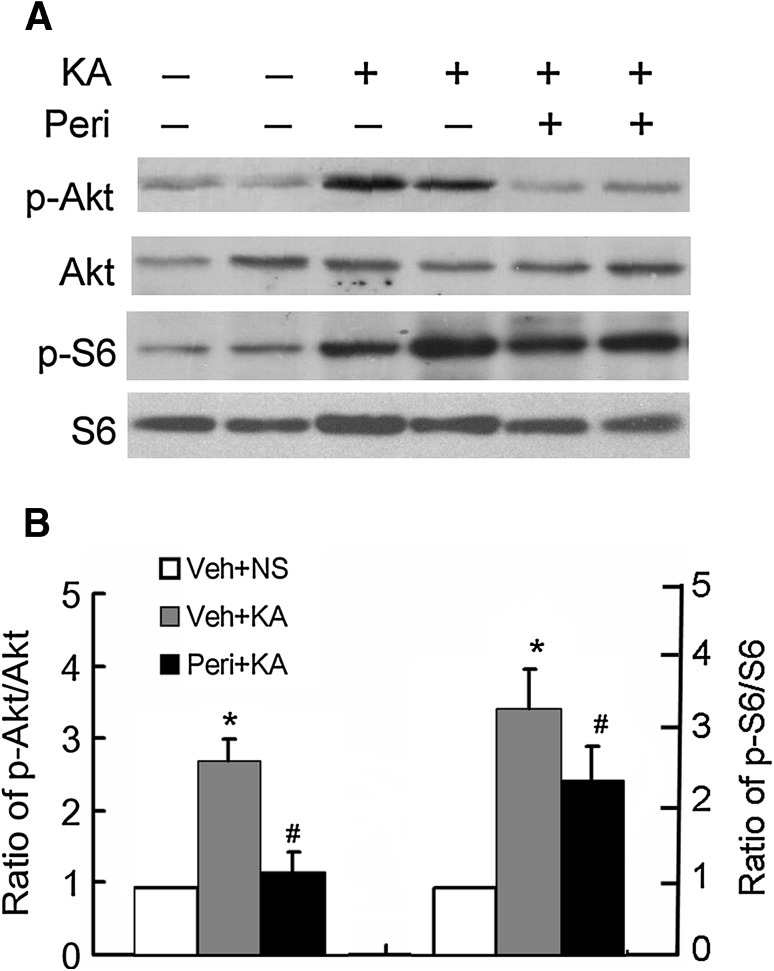
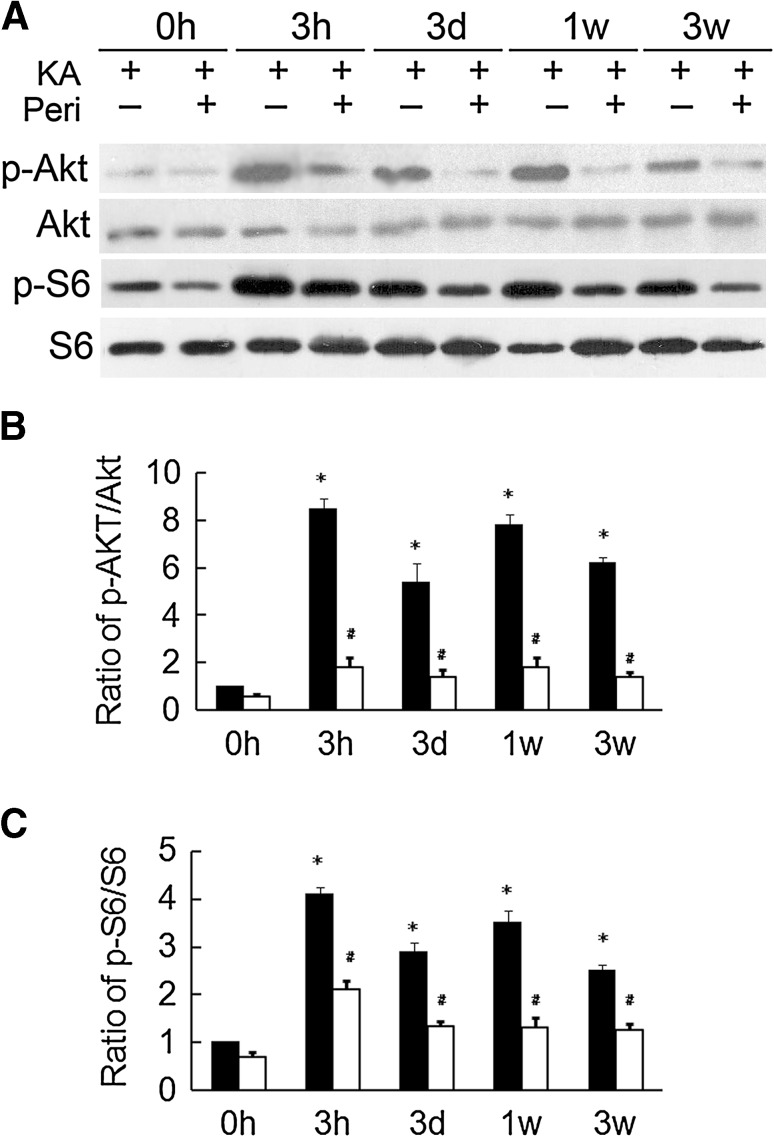
We previously reported that perifosine administration markedly decreases p-Akt from 10 min to 24 h, and subsequently, decreases p-S6 from 1 h to 24 h in normal rats. In this study, we performed a series of pilot experiments to test various doses and durations of perifosine treatment to block seizure-induced activation of the mTOR pathway. Pretreatment with perifosine had no effect on KA-induced status epilepticus, including seizure latency, severity, and duration (data not shown), indicating that perifosine has no anti-seizure effect in this rat model of acute seizures. Furthermore, no significant difference was noted in the percentage of rats that developed seizure stage 4 after KA administration (85.9% for vehicle versus 87.4% for perifosine-pretreatment). We found that pretreatment with perifosine at 20 mg/kg (i.p.) per day for 3 consecutive days prior to KA injection effectively blocked the KA-induced over-activation of the mTOR pathway, as reflected by Akt and S6 phosphorylation 1 h after seizure onset (Fig. 1). To assess its effectiveness at other time points, we also analyzed Akt and S6 phosphorylation at 3 h, 3 days, 1 week, and 3 weeks after KA-induced status epilepticus. Pretreatment with perifosine markedly decreased the KA-induced over-activation of Akt and S6 at all time points (Fig. 2), suggesting that such treatment blocks the over-activation of the mTOR signaling pathway induced by KA seizures.
Fig. 1.
Perifosine pretreatment decreases Akt and S6 activation after KA-induced acute seizures. A KA-induced seizures result in activation of the mTOR pathway. Representative western blots showing that phospho-Akt (p-Akt) and phospho-S6 (p-S6) expression in the hippocampus increased within 1 h of the onset of KA-induced seizures in perifosine-pretreated (Peri + KA) and vehicle-pretreated (Veh + KA) rats. Vehicle-treated rats that did not receive KA (Veh + KA) served as an additional control. B Quantitative summary of p-Akt/Akt and p-S6/S6 at 1 h (*P < 0.05, vs Veh + NS group, # P < 0.05 vs Veh + KA group, n = 6 rats/group).
Fig. 2.
Perifosine pretreatment decreases Akt and S6 activation long after KA-induced seizures. A Representative blots of Akt and S6 at different time intervals after KA-induced seizures. B Quantitative analysis showing that phosphorylation of Akt was increased from 3 h to 3 weeks after the onset of KA-induced seizures. There was a significant decrease of the p-Akt/Akt ratio compared to rats without perifosine pretreatment. C Quantitative analysis showing that phosphorylation of S6 was increased from 3 h to 3 weeks after the onset of KA-induced seizures. There was a significant decrease of the p-S6t/S6 ratio compared to rats without perifosine pretreatment. *P < 0.05, vs Veh + NS group, # P < 0.05 vs Veh + KA group, n = 6 rats/group.
Perifosine Pretreatment Suppresses KA Seizure-Induced Neuronal Death
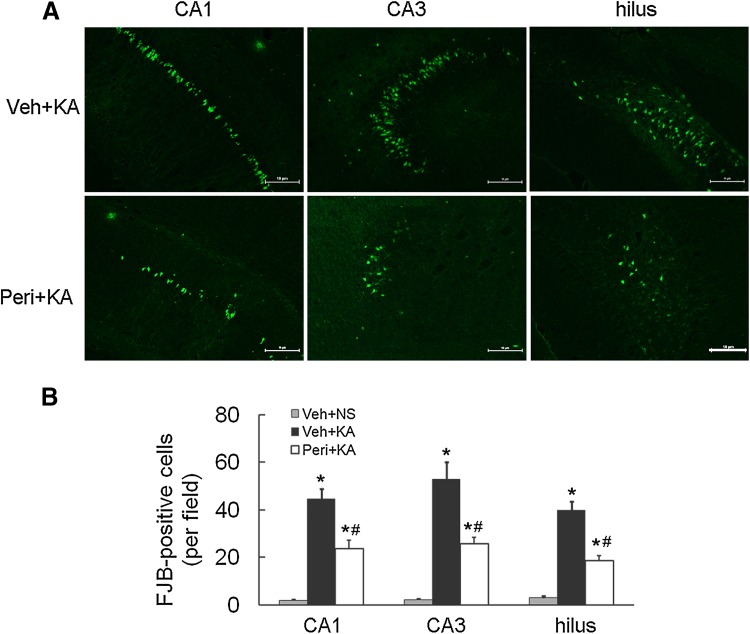
We next determined whether perifosine prevents the activation of cellular/molecular mechanisms implicated in the process of chronic epileptogenesis in the KA model, such as neuronal death and mossy fiber sprouting. FJB staining was performed to detect neuronal apoptosis 7 days after KA-induced status epilepticus. Parallel to our previous report, all vehicle-pretreated rats demonstrated robust neuronal cell death after KA-induced status epilepticus in hippocampal CA1 (44.7 ± 4.1 positive cells/mm3), CA3 (52.8 ± 7.2), and the hilus (39.8 ± 3.8) as assessed by FJB staining. In the group pretreated with perifosine, the numbers of FJB-positive neurons were markedly decreased in CA1 (23.6 ± 3.7), CA3 (25.8 ± 2.78), and the hilus (18.6 ± 2.2) (Fig. 3). These findings revealed that perifosine has a potential neuroprotective effect against KA-induced seizures.
Fig. 3.
Perifosine pretreatment suppresses kainic acid-induced neuronal death. A Representative images of FJB staining following KA-induced seizures in the vehicle-pretreated (Veh + KA) and the perifosine-pretreated (Peri + KA) groups (scale bars, 100 µm). B Quantitative analysis showing a decrease in FJB-positive neurons in the perifosine-pretreated rats (*P < 0.05, vs Veh + NS group, # P < 0.05 vs Veh + KA group, n = 6/group).
Perifosine Prominently Alleviates KA Seizure-Induced Mossy Fiber Sprouting
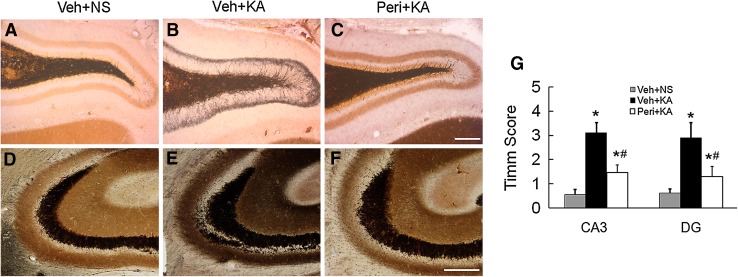
The Timm scores were also evaluated in different groups in CA3 and the dentate gyrus (DG). At 7 weeks after status epilepticus, most of the rats in the vehicle-pretreated group exhibited robust mossy fiber sprouting with a Timm score of 2.9 ± 0.63 in the DG, but only mild sprouting was detected in the perifosine-pretreated group with a Timm score of 1.3 ± 0.42. Similar results were found in CA3. No sprouting was evident in the control group (Fig. 4).
Fig. 4.
Perifosine pretreatment reduces mossy fiber sprouting after KA-induced seizures. A–F Representative Timm staining of mossy fiber sprouting in the DG (A–C) and CA3 (D–F) 6 weeks after KA-induced seizures (scale bars, 100 μm). G Quantitative analysis showing mild mossy fiber sprouting in perifosine-pretreated rats (*P < 0.05, vs Veh + NS group, # P < 0.05 vs Veh + KA group, n = 6/group).
Perifosine Pretreatment Decreases the Frequency of Spontaneous Seizures and the Impairment of Cognitive Function
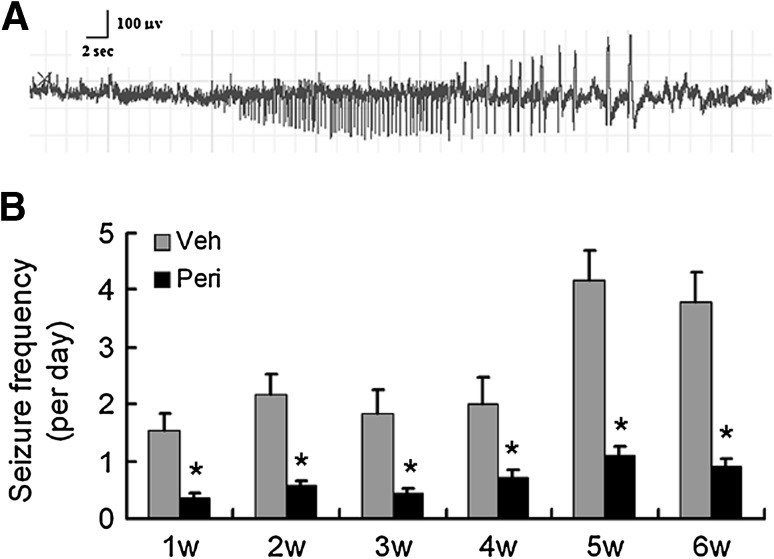
Rats pretreated with vehicle or perifosine were monitored for spontaneous seizures from week 1 to week 6 after KA-induced status epilepticus. A representative ictal seizure is shown in Fig. 5A. All the vehicle-treated rats developed spontaneous seizures, with an average of 1.5 ± 0.3 seizures/24 h in week 1 and 3.8 ± 0.5 in week 6. In contrast, the perifosine-pretreated rats had a low frequency of 0.5 ± 0.1 seizures/24 h from week 1 to week 6 (F = 3.124, P < 0.05, Fig. 5B). There was no evident difference in seizure duration and severity between the two groups (data not shown).
Fig. 5.
Perifosine pretreatment reduces seizure frequency in KA-induced epilepsy. Seizures started to develop within one week after KA administration and became more frequent in vehicle-pretreated rats, whereas only rare seizures occurred in perifosine-pretreated rats. A Representative electrographic seizure was shown. B Statistics for the number of spontaneous seizures per day in vehicle- and perifosine-pretreated rats following status epilepticus. *P < 0.05, two-way ANOVA (n = 10 rats per time point in each group).
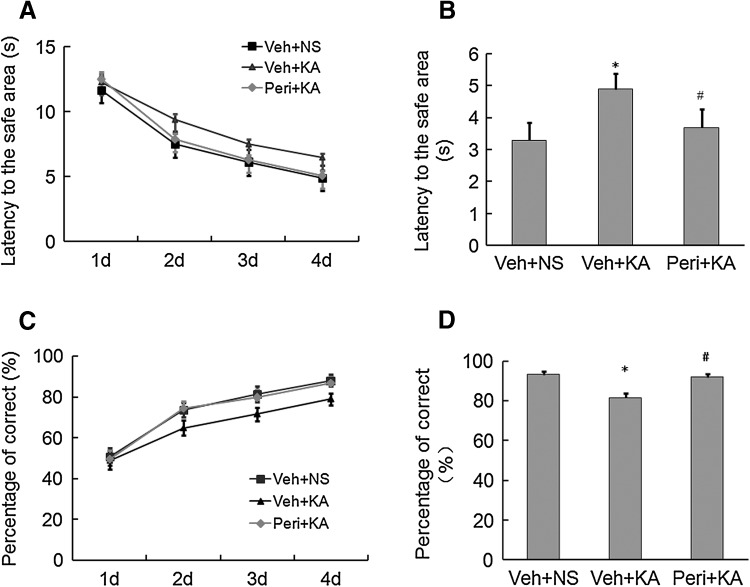
Since repeated seizures usually result in impairment of cognitive function, we also investigated whether perifosine had any effect on the KA seizure-induced cognitive impairment (Fig. 6). Seizures resulted in an increased latency to the safe area of the Y maze and a decrease in the percentage of correct choices, indicating cognitive impairment. Rats pretreated with perifosine exhibited a shorter latency to the safe area (3.7 ± 0.56 s versus 4.9 ± 0.48 s, P < 0.05, F = 2.438, n = 10/group) and an increased percentage of correct choices (91.4% ± 1.4% versus 81.6% ± 2.1%, P < 0.05, F = 3.877, n = 10/group) compared to vehicle-treated rats, indicating that perifosine protects against seizure-induced cognitive impairment.
Fig. 6.
Perifosine pretreatment alleviates cognitive impairment induced by KA seizures. A Latency to the safe area during training days 1–4. B Latency to the safe area on day 5. C Percentage of correct responses during training days 1–4. D Percentage of correct responses on day 5. *P < 0.05, vs Veh + NS group, # P < 0.05 vs Veh + KA group, two-way ANOVA (n = 10 rats per time point in each group).
Discussion
The present study is the first to investigate the effect and mechanism of action of perifosine on seizures in the KA rat model. Here, we revealed that perifosine effectively blocked seizure-induced over-activation of the mTOR signaling pathway. In addition, it suppressed the development of spontaneous seizures via inhibiting neuronal cell death and mossy fiber sprouting, which subsequently decreased the cognitive impairment.
Since we first reported a relationship between mTOR pathway activation and epilepsy in a mouse model of TSC [17], this pathway has also been implicated in several animal models of seizures [19–21]. mTOR inhibitors have been clinically evaluated in both genetic and acquired epilepsy syndromes [8, 22, 23], and the systemic administration of rapamycin has been shown to prevent epileptogenesis [24–26]. In addition to mossy fiber sprouting, neuronal death and neurogenesis may contribute to epileptogenesis and can be reversed by rapamycin under certain conditions, but this remains controversial [19, 27–29]. Decreased dendritic density and complexity have also been noted in seizure-free animals treated with rapamycin [30]. Other studies have raised concern about the chronic side-effects of rapamycin, as its effect in preventing epilepsy appears to be dependent on its long-term administration, starting at a very early age in genetic epilepsy [31]. We have previously reported paradoxical exacerbation of increased mTOR pathway activity reflected by S6 phosphorylation when rapamycin is administered shortly before KA injection. Since the mTOR pathway has complicated positive and negative feedback, the inhibitory effect of rapamycin on this pathway may be overcompensated. Fortunately, we have shown that perifosine can abolish the paradoxical increase of S6 phosphorylation [14]. Together with the present study, our results provide novel evidence that perifosine is most likely better than rapamycin in inhibiting overactivation of the mTOR pathway and subsequent epileptogenesis.
Perifosine is an orally bioavailable alkylphospholipid that inhibits the mTOR pathway and exhibits antitumor activity in various tumors, such as neuroblastoma, neuroendocrine tumors, and T-cell acute lymphoblastic leukemia [32–34]. Perifosine inhibits Akt activity mainly by interfering with the pleckstrin homology domain of Akt, thus preventing its membrane localization and phosphorylation [35]. However, the effect of perifosine on epilepsy has not been investigated. In the present study, perifosine showed a clear inhibitory effect on the mTOR pathway over several weeks in a rat model of epilepsy induced by KA. Upstream of Akt-mTOR signaling, Akt-induced activation of the mTOR pathway enables mRNA translation through the activation of P70S6 kinase and then the activation of S6. Thus, besides inhibition of Akt activity, perifosine pretreatment successfully blocked the overactivation of S6 over several weeks after KA-induced status epilepticus. The link between Akt and its down-regulated mTOR signaling means it was not surprising to find a reduction of mossy fiber sprouting and neuronal death in the perifosine pretreatment groups, finally resulting in fewer spontaneous seizures. Furthermore, cognitive impairment was also reversed by perifosine pretreatment. Since perifosine pretreatment had no effect on the parameters of the KA-induced acute seizures, the effect of perifosine on cognitive function most likely results from its inhibition of spontaneous seizures.
Even though many studies have provided convincing evidence that perifosine is an Akt inhibitor, its precise anti-epileptogenic actions are unclear. Recent studies have revealed that perifosine may inhibit the assembly of both the mTOR/raptor and mTOR/rictor complexes [16], which differs from the inhibition of mTOR signaling by classical mTOR inhibitors such as rapamycin. However, the multiple interactions and feedback loops certainly add a layer of complexity to the biology of these pathways [36–38]. Our results did not show compensatory over-activation of S6 after perifosine pretreatment, which is different from rapamycin. Thus, it is reasonable to suggest that inhibiting the over-activation of upstream signaling of mTOR is important for anti-epileptogenic strategies.
In conclusion, administration of perifosine is a novel therapeutic approach to the prevention of epilepsy. It suppresses epileptogenesis and avoids the paradoxical activation reaction induced by rapamycin. Additional research in this direction is essential, including optimization of the critical time interval and dosage.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81371429), the Public Welfare Technology Application Research Project of Zhejiang Province, China (2016C33211), and the Science and Technology Commission of Hangzhou Municipality, Zhejiang Province, China (20140633B37 and 20160533B73).
Footnotes
Feng Zhu and Jiejing Kai have contributed equally to this work.
References
- 1.Niemeyer B, Niemeyer R, Abdalla G, Marchiori E. Amyloid β-related angiitis of the central nervous system presenting with seizures and cognitive deficit. Eur Neurol. 2017;77:173–174. doi: 10.1159/000456713. [DOI] [PubMed] [Google Scholar]
- 2.Jin B, So NK, Wang S. Advances of intracranial electroencephalography in localizing the epileptogenic zone. Neurosci Bull. 2016;32:493–500. doi: 10.1007/s12264-016-0035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saada F, Wang ZS, Bautista RED. Infocus: the everyday lives of families of adult individuals with epilepsy. Epilepsy Behav. 2015;50:10–13. doi: 10.1016/j.yebeh.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 4.Schraegle WA, Titus JB. The relationship of seizure focus with depression, anxiety, and health-related quality of life in children and adolescents with epilepsy. Epilepsy Behave. 2017;68:115–122. doi: 10.1016/j.yebeh.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Wong M. Mammalian target of rapamycin (mtor) pathways in neurological diseases. Biomed J. 2013;36:40–50. doi: 10.4103/2319-4170.110365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zoncu R, Efeyan A, Sabatini DM. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jancic J, Duric V, Ivancevic N, Nikolic B, van den Anker JN, Samardzic J. Current use of mTOR inhibitors for the treatment of subependymal giant cell astrocytomas and epilepsy in patients with TSC. Curr Med Chem. 2016;23:4260–4269. doi: 10.2174/0929867323666161013091144. [DOI] [PubMed] [Google Scholar]
- 8.Citraro R, Leo A, Constanti A, Russo E, De Sarro G. mTOR pathway inhibition as a new therapeutic strategy in epilepsy and epileptogenesis. Pharmacol Res. 2016;107:333–343. doi: 10.1016/j.phrs.2016.03.039. [DOI] [PubMed] [Google Scholar]
- 9.Sadowski K, Kotulska-Jóźwiak K, Jóźwiak S. Role of mTOR inhibitors in epilepsy treatment. Pharmacol Rep. 2015;67:636–646. doi: 10.1016/j.pharep.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 10.Ebrahimi-Fakhari D, Müller CS, Meyer S, Flotats-Bastardas M, Vogt T, Pföhler C. Topical rapamycin for facial angiofibromas in a child with tuberous sclerosis complex (tsc): a case report and long-term follow-up. Dermatol Ther (Heidelb) 2017 doi: 10.1007/s13555-017-0174-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canpolat M, Per H, Gumus H, Yikilmaz A, Unal E, Patiroglu T, et al. Rapamycin has a beneficial effect on controlling epilepsy in children with tuberous sclerosis complex: results of 7 children from a cohort of 86. Childs Nerv Syst. 2014;30:227–240. doi: 10.1007/s00381-013-2185-6. [DOI] [PubMed] [Google Scholar]
- 12.Mazumder AG, Padwad YS, Singh D. Anticancer mammalian target of rapamycin (mTOR) signaling pathway inhibitors: current status, challenges and future prospects in management of epilepsy. CNS Neurol Disord Drug Targets. 2016;15:945–955. doi: 10.2174/1871527315666160615022203. [DOI] [PubMed] [Google Scholar]
- 13.Rensing N, Han L, Wong M. Intermittent dosing of rapamycin maintains antiepileptogenic effects in a mouse model of tuberous sclerosis complex. Epilepsia. 2015;56:1088–1097. doi: 10.1111/epi.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen L, Hu L, Dong JY, Ye Q, Hua N, Wong M, et al. Rapamycin has paradoxical effects on S6 phosphorylation in rats with and without seizures. Epilepsia. 2012;53:2026–2033. doi: 10.1111/epi.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson PG, Eng C, Kolesar J, Hideshima T, Anderson KC. Perifosine, an oral, anti-cancer agent and inhibitor of the Akt pathway: mechanistic actions, pharmacodynamics, pharmacokinetics, and clinical activity. Expert Opin Drug Metab Toxicol. 2012;8:623–633. doi: 10.1517/17425255.2012.681376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu L, Kim YA, Wang X, Wu X, Yue P, Lonial S, et al. Perifosine inhibits mTOR signaling through facilitating degradation of major components in the mTOR axis and induces autophagy. Cancer Res. 2009;69:8967–8976. doi: 10.1158/0008-5472.CAN-09-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng LH, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63:444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavazos JE, Golarai G, Sutula TP. Mossy fiber synaptic reorganization induced by kindling: time course of development, progression, and permanence. J Neurosci. 1991;11:2795–2803. doi: 10.1523/JNEUROSCI.11-09-02795.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin (mTOR) signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci. 2009;29:6964–6972. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang X, McMahon J, Huang Y. Rapamycin attenuates aggressive behavior in a rat model of pilocarpine-induced epilepsy. Neuroscience. 2012;215:90–97. doi: 10.1016/j.neuroscience.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Reeves C, Michalak Z, Coppola A, Diehl B, Sisodiya SM, et al. Evidence for mTOR pathway activation in a spectrum of epilepsy-associated pathologies. Acta Neuropathol Commun. 2014;2:71. doi: 10.1186/2051-5960-2-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ostendorf AP, Wong M. mTOR inhibition in epilepsy: rationale and clinical perspectives. CNS Drugs. 2015;29:91–99. doi: 10.1007/s40263-014-0223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.French JA, Lawson JA, Yapici Z, Ikeda H, Polster T, Nabbout R, et al. Adjunctive everolimus therapy for treatment-resistant focal-onset seizures associated with tuberous sclerosis (EXIST-3): a phase 3, randomised, double-blind, placebo-controlled study. Lancet. 2016;388:2153–2163. doi: 10.1016/S0140-6736(16)31419-2. [DOI] [PubMed] [Google Scholar]
- 24.Buckmaster PS, Ingram EA, Wen X. Inhibition of the mammalian target of rapamycin signaling pathway suppresses dentate granule cell axon sprouting in a rodent model of temporal lobe epilepsy. J Neurosci. 2009;29:8259–8269. doi: 10.1523/JNEUROSCI.4179-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berdichevsky Y, Dryer AM, Saponjian Y, Mahoney MM, Pimentel CA, Lucini CA, et al. PI3K-Akt signaling activates mTOR-mediated epileptogenesis in organotypic hippocampal culture model of post-traumatic epilepsy. J Neurosci. 2013;33:9056–9067. doi: 10.1523/JNEUROSCI.3870-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drion CM, Borm LE, Kooijman L, Aronica E, Wadman WJ, Hartog AF, et al. Effects of rapamycin and curcumin treatment on the development of epilepsy after electrically induced status epilepticus in rats. Epilepsia. 2016;57:688–697. doi: 10.1111/epi.13345. [DOI] [PubMed] [Google Scholar]
- 27.Chen LL, Feng HF, Mao XX, Ye Q, Zeng LH. Status epilepticus longer than one hour results in spontaneous motor seizures and memory deficits, and spontaneous seizures are likely associated with robust mossy fiber sprouting but not neuronal death. Neurosci Bull. 2013;29:295–302. doi: 10.1007/s12264-013-1310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sliwa A, Plucinska G, Bednarczyk J, Lukasiuk K. Post-treatment with rapamycin does not prevent epileptogenesis in the amygdala stimulation model of temporal lobe epilepsy. Neurosci Lett. 2012;509:105–109. doi: 10.1016/j.neulet.2011.12.051. [DOI] [PubMed] [Google Scholar]
- 29.Hester MS, Hosford BE, Santos VR, Singh SP, Rolle IJ, LaSarge CL, et al. Impact of rapamycin on status epilepticus induced hippocampal pathology and weight gain. Exp Neurol. 2016;280:1–12. doi: 10.1016/j.expneurol.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lasarge CL, Danzer SC. Mechanisms regulating neuronal excitability and seizure development following mTOR pathway hyperactivation. Front Mol Neurosci. 2014;7:18. doi: 10.3389/fnmol.2014.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu Z, Liu F, Chen L, Zhang H, Ding Y, Liu J, et al. Effect of chronic administration of low dose rapamycin on development and immunity in young rats. PLoS One. 2015;10:e0135256. doi: 10.1371/journal.pone.0135256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kushner BH, Cheung NV, Modak S, Becher OJ, Basu EM, Roberts SS, et al. A phase I/Ib trial targeting the Pi3k/Akt pathway using perifosine: Long-term progression-free survival of patients with resistant neuroblastoma. Int J Cancer. 2017;140:480–484. doi: 10.1002/ijc.30440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zitzmann K, Vlotides G, Brand S, Lahm H, Spöttl G, Göke B, et al. Perifosine-mediated Akt inhibition in neuroendocrine tumor cells: role of specific Akt isoforms. Endocr Relat Cancer. 2012;19:423–434. doi: 10.1530/ERC-12-0074. [DOI] [PubMed] [Google Scholar]
- 34.Cani A, Simioni C, Martelli AM, Zauli G, Tabellini G, Ultimo S, et al. Triple Akt inhibition as a new therapeutic strategy in T-cell acute lymphoblastic leukemia. Oncotarget. 2015;6:6597–6610. doi: 10.18632/oncotarget.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gills JJ, Dennis PA. Perifosine: update on a novel Akt inhibitor. Curr Oncol Rep. 2009;11:102–110. doi: 10.1007/s11912-009-0016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang K, Fingar DC. Growing knowledge of the mTOR signaling network. Semin Cell Dev Biol. 2014;36:79–90. doi: 10.1016/j.semcdb.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang G, Murashige DS, Humphrey SJ, James DE. A positive feedback loop between Akt and mTORC2 via SIN1 phosphorylation. Cell Rep. 2015;12:937–943. doi: 10.1016/j.celrep.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 38.Lukawski K, Gryta P, Luszczki J, Czuczwar SJ. Exploring the latest avenues for antiepileptic drug discovery and development. Expert Opin Drug Discov. 2016;11:369–382. doi: 10.1517/17460441.2016.1154840. [DOI] [PubMed] [Google Scholar]