Abstract
The pathogenesis of the second major neurodegenerative disorder, Parkinson’s disease (PD), is closely associated with the dysfunction of potassium (K+) channels. Therefore, PD is also considered to be an ion channel disease or neuronal channelopathy. Mounting evidence has shown that K+ channels play crucial roles in the regulations of neurotransmitter release, neuronal excitability, and cell volume. Inhibition of K+ channels enhances the spontaneous firing frequency of nigral dopamine (DA) neurons, induces a transition from tonic firing to burst discharge, and promotes the release of DA in the striatum. Recently, three K+ channels have been identified to protect DA neurons and to improve the motor and non-motor symptoms in PD animal models: small conductance (SK) channels, A-type K+ channels, and KV7/KCNQ channels. In this review, we summarize the physiological and pharmacological effects of the three K+ channels. We also describe in detail the laboratory investigations regarding K+ channels as a potential therapeutic target for PD.
Keywords: Parkinson’s disease, A-type K+ channels, SK channels, KV7/KCNQ channels, Dopamine
Introduction
Parkinson’s disease (PD) is the second most common and debilitating age-associated human neurodegenerative disorder, characterized by cardinal motor symptoms such as static tremor, bradykinesia, and muscle rigidity. PD patients also exhibit non-motor symptoms in the early stage of the disease. The neuropathological feature of PD is the degenerative lesion of dopamine (DA) neurons in the mesencephalic substantia nigra (SN)–striatum pathway, leading to the loss of SN DA neurons and the formation of Lewy bodies. However, the etiology of PD has yet to be fully uncovered. Many factors such as apoptosis, mitochondrial dysfunction, nigral iron accumulation, and oxidative stress are thought to be involved in the process of neurodegeneration [1]. Therefore, it is difficult to pin-point a single therapeutic target and evaluate its potential application. Currently available therapeutic methods include pharmacotherapy, functional neurosurgery, gene therapy, and cell-based therapy [2]. However, the most commonly used drug in clinical practice is levodopa. Since many side-effects occur after prolonged treatment with levodopa, it is urgent to explore new targets for pharmacological intervention.
Recently, potassium (K+) channels have been proposed as possible new therapeutic targets in PD. The K+ ion is indispensable to life. It establishes the cell resting membrane potential, regulates neurotransmitter release and neuronal excitability, and maintains cellular homeostasis and cell volume [3]. According to their structure, firing characteristics, and physiological and pathological effects, K+ channels are divided into voltage-gated K+ channels (Kv: Kv1–Kv12 or KCNA–KCND, KCNF–KCNH, KCNQ, KCNS), Ca2+-activated K+ channels (KCa: KCa1–KCa5 or KCNM–KCNN, or big, medium, and small conductance (SK) channels), inwardly rectifying K+ channels (Kir: Kir1–Kir7 or KCNJ), and background/leak or tandem 2-pore K+ channels (K2P: K2P1–K2P7, K2P9–K2P10, K2P12–K2P13, K2P15–K2P18 or KCNK) [4]. Almost all the K+ channels exist in the SN–striatum system. Based on their central positions in metabolic and signaling pathways, K+ channels are increasingly recognized as potential targets for pharmacological intervention in PD [5]. For example, our recent finding has shown that the activation of ATP-sensitive K+ (KATP) channels by diazoxide results in hyperpolarization of the membrane potential and increases iron uptake in the DAergic SK-N-SH cells, leading to an increase in intracellular iron levels and a subsequent decrease in the mitochondrial membrane potential and increase in reactive oxygen species production [6]. The activation of G-protein-gated inward-rectifying K+(GIRK) channels can lead to a hyperpolarizing effect of somatodendritic D2-subtype autoreceptors (D2-ARs) in DA neurons to inhibit the negative feedback loop through which DA itself binds with D2-ARs to decrease cell firing and somatodendritic DA release [7]. There are several excellent reviews on the potential therapeutic roles of KATP channels and GIRK channels in PD [8–11]. Recent studies have also focused on the roles of both SK and Kv channels in the treatment or prevention of PD [12–14]. Therefore, in this review, we mainly focus on the most favorable K+ channels, SK channels, A-type K+ channels, and KV7/KCNQ channels in the pathogenesis of PD, and whether modulation of these channels can provide a new therapeutic target in PD treatment.
Roles of SK Channels in PD
SK channels are macromolecular protein complexes with a special pore-like structure. They are activated when the cytoplasmic concentration of Ca2+ is increased. However, there is no Ca2+-binding domain in SK channels; calmodulin constitutively binds to the proximal part of the C-terminus and acts as the Ca2+ sensor [15]. Recent studies have also demonstrated that the sensitivity of SK channels to Ca2+ concentration is influenced by the phosphorylation state of calmodulin [15, 16]. After Ca2+ binds to calmodulin, the SK channels are activated, leading to hyperpolarization of the cell membrane potential and a subsequent reduction in excitability [17]. SK channels play an essential role in the regulation of midbrain DAergic neuronal activity patterns, as well as the excitability of other types of neurons in the basal ganglia. The activation of SK channels in nigral DAergic neurons produces a medium afterhyperpolarization following a single action potential, regulating spike frequency adaptation and the frequency and precision of pacemaker activity [18–20]. The blockade of SK channels promotes N-methyl-D-aspartate-induced irregular burst firing in DAergic neurons and activates Ca2+-dependent signaling pathways, which enhance the release of DA in the striatum and relieve the symptoms of PD [21–23].
The subfamily of SK channels includes SK1, SK2, SK3, and SK4 [24]. The SK1-3 members are expressed differentially in excitable tissues of the brain and in peripheral tissues, whereas the SK4 channel only exists in non-excitable tissues, such as placenta and lung [25]. In the brain, the SK1 and SK2 channels are highly expressed in cortex and hippocampus, while the SK3 channel is particularly abundant in subcortical areas, especially in the monoamine cell group regions, such as the SN, dorsal raphe, and locus coeruleus [26]. A recent study has reported that the expression of SK channels in the basal ganglia is modified in parkinsonian rats. In situ hybridization of SK2 and SK3 mRNA was performed at 1, 8 or 21 days after surgery in sham rats and lesioned rats with bilateral infusions of 6-hydroxydopamine (6-OHDA) into the striatum. A significant decrease of SK3 channel expression was found in the SN pars compacta (SNc) of lesioned rats at three time points, with no change of SK2 channel expression. However, an upregulation of SK2 mRNA was found in the subthalamic nucleus at 21 days after the lesion [27].
Currently, there is no consensus on the role of SK channel blockade and activation in the treatment of PD. Many studies have found that blocking SK channels with bee venom (BV) and its specific component, apamine, improves the symptoms of PD both in vivo and in vitro. However, activation of SK channels by their agonists has also been reported to attenuate neurotoxin-induced DAergic neuronal death (Table 1). For example, in the acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of PD, bilateral acupoint stimulation of the lower hindlimbs with BV prevents the loss of tyrosine hydroxylase (TH) immunoreactivity in the SN and striatum through the inhibition of Jun activation [28]. Intraperitoneal injection of BV into the chronic MPTP mouse model and hemi-parkinsonian 6-OHDA lesion model also has protective effects on nigral DAergic neurons and improves motor performance [12, 29]. Similarly, subcutaneous injection of BV antagonizes MPTP-induced TH cell loss and microglial activation [30]. In addition, by in vivo electrophysiological recordings and analyzing the neuronal responses evoked by motor cortex stimulation, Maurice et al. showed that BV reverses the 6-OHDA- and neuroleptic-induced biases in basal ganglia sub-circuits [12]. However, a recent clinical randomized double-blind study did not show any clear symptomatic or disease-modifying effect of monthly BV injections over a 11-month period compared to placebo. But this study suggested that a higher administration frequency and possibly higher individual doses of BV may reveal its potency in treating PD [31].
Table 1.
Overview of the roles of blockers and openers of SK channels in PD.
| Blockers/openers of SK channels | Experimental model | Main function | References |
|---|---|---|---|
| BV | MPTP PD mice | BV acupuncture prevented the MPTP-induced loss of TH immunoreactivity and phospho-Jun immunoreactivity in the SN | [28] |
| BV | MPTP PD mice | Intraperitoneal injection of BV prevented the MPTP-induced DA cell loss in the SN, decreased striatal DA levels, and decreased striatal TNF-α levels | [29] |
| BV | 6-OHDA PD rats | Intraperitoneal injection of BV significantly alleviated contralateral forelimb akinesia and apomorphine-induced rotation, and reversed haloperidol-induced catalepsy. BV reversed the 6-OHDA-induced biases in the influence of the direct inhibitory and indirect excitatory striatonigral circuits | [12] |
| BV | MPTP PD mice | Subcutaneous injection of BV prevented the MPTP-induced DA cell loss and the enhanced expression of the inflammation markers MAC-1 and iNOS in the SNc | [30] |
| BV | PD patients | Subcutaneous monthly injection of BV for 11 months did not show symptomatic or disease-modifying effects | [31] |
| Apamin | DA neurons from embryonic rat mesencephalon undergo spontaneous and selective degeneration as they develop in culture | Apamin caused a small increase of DAergic neuronal excitability, and a moderate but persistent elevation in cytosolic Ca2+, which led to the activation of a Ca2+-dependent signaling pathway required to prevent apoptosis | [32] |
| Apamin | 6-OHDA PD rats | Intraperitoneal injection of apamin counteracted the depression, anxiety-like behaviors, social recognition, and spatial memory deficits produced by partial 6-OHDA lesions. Apamin also reduced asymmetric motor deficits in circling behavior and postural adjustments. Apamin attenuated the striatal DA loss after 6-OHDA lesions | [19] |
| Apamin | 6-OHDA PD rats | Intraperitoneal injection of apamin reversed haloperidol-induced catalepsy | [12] |
| Apamin | MPTP PD mice | Intraperitoneal injection of apamin prevented the MPTP-induced DA cell loss and decreased striatal DA levels. In the rotarod test, mice treated with MPTP/probenecid and receiving apamin spent significantly less time on the spindle than MPTP/saline-treated animals | [29] |
| 1-EBIO | Normal mice | Infusion of SK channel agonist 1-ethyl-2-benzimidazolone(1-EBIO) into the left SNc in normal mice increased the number of TH+ cells accompanied by an equal but opposite decrease in the number of TH- cells | [34] |
| 1-EBIO | Long-term cultures of SNc organotypic slices treated with 6-OHDA | 1-EBIO attenuated 6-OHDA-induced DAergic neuronal firing irregularity and subsequent neuronal death | [36] |
| NS309 | Human dopaminergic LUHMES cells treated with rotenone | Activation of SK channels by NS309 prevented rotenone-induced neuronal cell death and neuronal network degradation | [37] |
Apamin is a crucial component of BV, and has a much higher ability to penetrate the blood-brain barrier. In a model system of midbrain culture that mimics the selective demise of DAergic neurons in PD, apamin was first demonstrated to protect DAergic neurons [32]. In this model, blockade of SK channels causes a small increase in the excitability of DAergic neurons and a moderate but persistent elevation of cytosolic Ca2+, which leads to the activation of the Ca2+-dependent signaling pathway required to prevent apoptosis [32, 33]. Later, apamine was shown to protect against MPTP/6-OHDA-induced DAergic neuronal loss and increase the striatal DA levels [21, 29]. In addition, systemic administration of apamin reverses the depression, anxiety-like behaviors, and cognitive deficits caused by 6-OHDA [21].
Interestingly, SK channels have contradictory effects on the DA phenotype [25, 34]. Aumann et al. have compared the effects of an SK channel agonist and antagonist on the number of TH-positive (TH+) neurons. In this test, infusion of the SK channel agonist 1-EBIO into the left SNc in normal mice increased the number of TH+ cells, accompanied by an equal but opposite decrease in the number of TH- cells. Likewise, SK channel inhibition by apamine caused an apparent phenotype shift the other way, from TH+ to TH- in normal mice. Acute SK channel inhibition also shifted the electrophysiological phenotype of TH+ neurons to “intermediate TH+” neurons, or toward the TH- phenotype, embodied in the higher discharge rate and the shortened time of action potentials and afterhyperpolarization [34]. Indeed, previous studies have shown that facilitation of SK channels shifts the electrophysiological phenotype in the opposite direction [20, 35]. Recent findings have also demonstrated that activation of the SK channel by its agonist 1-EBIO attenuates 6-OHDA-induced DAergic neuronal firing irregularity and the subsequent neuronal death [36]. Dolga et al. have also shown that the pharmacological SK channel activator 6,7-dichloro-1H-indole-2,3-dione 3-oxime (NS309), a derivative of 1-EBIO, provides neuroprotection to human dopaminergic LUHMES cells in a model of rotenone toxicity. More importantly, they first reported that the SK2 channel primarily resides in the mitochondrial membrane. Therefore, NS309 might target SK channels in both the plasma membrane and mitochondria to reduce DAergic neuronal vulnerability triggered by mitochondrial dysfunction after rotenone challenge [37].
The possible mechanisms of the neuroprotective effects of SK channel activation involve the inhibition of NMDA receptor-mediated excitotoxicity [37–39]. Over-activation of NMDA receptors enhances burst firing and produces an excessive increase of DA that spontaneously oxidizes or is deaminated by monoamine oxidase to yield large amounts of toxic products; meanwhile, the activation of SK channels may provide a more permissive environment for increased DA (TH) synthesis in SNc neurons [10, 34, 40]. On the other hand, SK channels may directly regulate NADPH oxidase or the mitochondrial matrix, thus inhibiting the injury due to reactive oxygen species [41].
Roles of A-Type K+ Channels in PD
Kv4 generates the A-type K+ current and includes four α-subunits that have six transmembrane domains (S1–S6). S5 and S6 of the four α-subunits form a pore domain (P-loop) in Kv4 channels, allowing selective ion permeability; S1-S4 of the four α-subunits form the voltage-sensor domain [42]. Auxiliary Kv channel-interacting proteins (KChIPs) co-assemble with the pore-forming Kv4-subunits to form a native K+ channel complex and regulate the expression and gating properties of Kv4 currents. The Kv4 family in mammals is composed of three distinct genes: Kv4.1, Kv4.2, and Kv4.3. Kv4.2 and Kv4.3 are expressed abundantly in the brain, especially in midbrain DAergic neurons [43]. Recent findings have reported changes of Kv4.3 expression in both PD animal models and PD patients. Immunohistochemistry has shown a selective increase of Kv4.3 expression in the SNc in 7–8 month-old A53T mice [44]. What is more, cell-specific RT-qPCR analysis has identified elevated mRNA levels of Kv4.3 in the remaining TH+ SN DAergic neurons from PD patients [45].
Kv4.3 interacts with KChip3 to generate the A-type K+ current, the rapidly inactivating transient component of the outward K+ current present in SNc DAergic neurons [46, 47]. Functional studies have also revealed an A-type K+ channel in DAergic neurons of the SN that contributes to pacemaker control of their tonic activity [46]. Inhibition of A-type K+ channels results in depolarization and an increase in excitability converting the neuronal tonic firing mode to burst firing, thus enhancing the release of DA [48, 49].
Kv4.2 mRNA abundance is linearly related to A-type K+ current amplitude in striatal medium spiny neurons (MSNs) [50]. This may integrate a variety of intracellular signaling cascades into a coordinated output that dynamically modulates membrane excitability [42]. Activation of Kv4.2 induces the generation of somatodendritic A-type K+ channels in MSNs [50, 51]. Nigrostriatal DA neurodegeneration in PD causes a loss of spinal and glutamatergic synapses in the striatal MSNs. Adaptive responses, a form of homeostatic plasticity, to decreases of A-type K+ channels can enhance the intrinsic excitability of MSNs in order to compensate for the loss of glutamatergic synapses [52, 53].
AmmTX3, a member of the scorpion toxin α-KTX15 family, is a selective blocker of Kv4 channels [53, 54]. Striatal infusion of AmmTX3 reduces motor deficits, decreases anxiety, and restores short-term social and spatial memory in 6-OHDA-treated rats [55]. In addition, 4-aminopyridine, another powerful blocker that inhibits A-type K+ channels, also attenuates functional asymmetry in the apomorphine-induced rotational test in 6-OHDA-induced rats [48, 56, 57]. Furthermore, glial cell-derived neurotrophic factor, which regulates the development and function of the nervous system, rapidly and reversibly inhibits A-type K+ channels in midbrain DAergic neurons by activating mitogen-activated protein kinase, which also suggests the A-type K+ channel as a potential therapeutic target in PD [58].
Roles of Kv7/KCNQ Channels in PD
M-current is a subthreshold voltage-gated K+ current generated by Kv7/KCNQ channels. The KCNQ family consists of five members (KCNQ1–KCNQ5 or Kv7.1–Kv7.5), which are mainly expressed in the central and peripheral nervous systems. KCNQ2 and KCNQ4 are mainly confined to certain areas of the brain, such as the SN and ventral tegmental area of the midbrain [59–62]. In the striatum, GABAergic medium spiny projection neurons express KCNQ 2, 3,and 5 [63, 64]. And DAergic striatal nerve terminals express KCNQ2 and KCNQ3 [62].
M-current can modulate the firing frequency of mesencephalic DAergic neurons. The activator of KCNQ channels induces hyperpolarization of DAergic neurons and inhibits spontaneous or synaptically-induced excitatory activity [59, 65]. It has been shown that Kv7 channels located at both pre- and postsynaptic sites within the striatum may be targeted by the opener retigabine; this provides an effective molecular mechanism to counteract the strong excitatory effect of DA D2 receptor inhibition in vivo [66]. Blockade of KCNQ channels by XE991 promotes action potentials in DAergic neurons and potentiates burst firing, thus increasing their excitability [67, 68]. Moreover, XE991 also enhances suprathreshold synaptic responses and promotes the depolarization of GABAergic striatal projection neurons, which may affect the behavior of the whole striatal microcircuit [14].
Since the excitability of nigral DA neurons that supply the striatum with DA determines the function of the nigrostriatal system for motor coordination, one practical approach for alleviating PD symptoms is to enhance the excitability of surviving DAergic neurons in the SNc. Our group first investigated the neuroprotective effect of XE991 in a haloperidol-induced PD rat model. In this study, intraperitoneal injection of haloperidol was used to induce symptoms of catalepsy with characteristics of rigidity and akinetic posturing. Administration of XE991 into the SNc attenuated the catalepsy elicited by systemic administration of haloperidol [67]. In addition, our latest data also indicated that XE991 has neuroprotective effects against 6-OHDA-induced degeneration of the nigrostriatal DA system and attenuates motor dysfunction (unpublished data).
The KCNQ channel opener retigabine has also been used to attenuate the levodopa-induced dyskinesias in 6-OHDA-lesioned rats. As enhanced activity of GABAergic striatal projection neurons is responsible for the pathology of motor fluctuations and dyskinesia, in long-term treatment with levodopa, compounds which reduce the activity of striatal MSNs might have anti-dyskinetic effects [69].
Conclusion
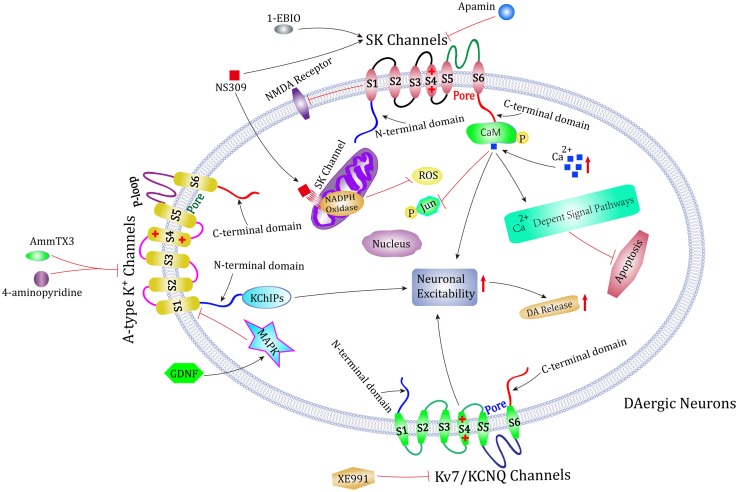
Clinical drugs are used to treat PD either by boosting the levels of DA in the striatum or mimicking the effects of DA. Symptomatic treatment is efficacious, but currently no drugs can slow the disease progression. Therefore, it is urgent to explore new treatment methods. In this review, we comprehensively describe three potential targets for PD treatment: SK channels, A-type K+ channels, and Kv7/KCNQ channels (Fig. 1). Activators or blockers of these three K+ channels can modulate the firing patterns in surviving SNc DA neurons and the excitability of striatal projection neurons to powerfully correct the motor symptoms in PD rat models. However, there is still a long way to go before we achieve a better performance of drugs that affect K+ channels in clinical trials. Whether potential pharmacological interventions in laboratory tests can be translated into clinical therapy still needs further confirmation.
Fig. 1.
Possible role of K+ channels in the treatment of PD. Both animal and cell models of PD show that SK channels, A-type K+ channels, and Kv7/KCNQ channels are three potential targets for PD treatment. Blockers of these three K+ channels or activators of SK channels can protect dopaminergic neurons in SNc, modulate neuronal excitability, influence dopamine release, and attenuate motor symptoms.
Acknowledgements
This review was supported by the National Natural Science Foundation of China (31671054 and 81430024), the Postdoctoral Science Foundation of China (2017M610412), and the Bureau of Science and Technology of Qingdao Municipality, China (17-1-1-44-jch).
Footnotes
Xiaoyan Chen and Bao Xue have contributed equally to this review.
Contributor Information
Limin Shi, Email: slm0532@163.com.
Junxia Xie, Email: jxiaxie@public.qd.sd.cn.
References
- 1.Przedborski S. The two-century journey of Parkinson disease research. Nat Rev Neurosci. 2017;18:251–259. doi: 10.1038/nrn.2017.25. [DOI] [PubMed] [Google Scholar]
- 2.Pires AO, Teixeira FG, Mendes-Pinheiro B, Serra SC, Sousa N, Salgado AJ. Old and new challenges in Parkinson’s disease therapeutics. Prog Neurobiol. 2017;156:69–89. doi: 10.1016/j.pneurobio.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Lawson K, McKay NG. Modulation of potassium channels as a therapeutic approach. Curr Pharm Des. 2006;12:459–470. doi: 10.2174/138161206775474477. [DOI] [PubMed] [Google Scholar]
- 4.Doyle DA, Cabral JM, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Yang PL, Tang JF, Lin JF, Cai XH, Wang XT, et al. Potassium channels: possible new therapeutic targets in Parkinson’s disease. Med Hypotheses. 2008;71:546–550. doi: 10.1016/j.mehy.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Du X, Xu H, Shi L, Jiang Z, Song N, Jiang H, et al. Activation of ATP-sensitive potassium channels enhances DMT1-mediated iron uptake in SK-N-SH cells in vitro. Sci Rep. 2016;6:33674. doi: 10.1038/srep33674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martel P, Leo D, Fulton S, Berard M, Trudeau LE. Role of Kv1 potassium channels in regulating dopamine release and presynaptic D2 receptor function. PLoS One. 2011;6:e20402. doi: 10.1371/journal.pone.0020402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lüscher C, Slesinger PA. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci. 2010;11:301–315. doi: 10.1038/nrn2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayfield J, Blednov YA, Harris RA. Behavioral and genetic evidence for GIRK channels in the CNS: role in physiology, pathophysiology, and drug addiction. Int Rev Neurobiol. 2015;123:279–313. doi: 10.1016/bs.irn.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duda J, Pötschke C, Liss B. Converging roles of ion channels, calcium, metabolic stress, and activity pattern of Substantia nigra dopaminergic neurons in health and Parkinson’s disease. J Neurochem. 2016;139:156–178. doi: 10.1111/jnc.13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du XX, Qin K, Jiao Q, Xie JX, Jiang H. Advances in the association of ATP-sensitive potassium channels and Parkinson’s disease. Sheng Li Xue Bao. 2016;68:644–648. [PubMed] [Google Scholar]
- 12.Maurice N, Deltheil T, Melon C, Degos B, Mourre C, Amalric M, et al. Bee venom alleviates motor deficits and modulates the transfer of cortical information through the basal ganglia in rat models of Parkinson’s disease. PLoS One. 2015;10:e0142838. doi: 10.1371/journal.pone.0142838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aidi-Knani S, Regaya I, Amalric M, Mourre C. Kv4 channel blockade reduces motor and neuropsychiatric symptoms in rodent models of Parkinson’s disease. Behav Pharmacol. 2015;26:91–100. doi: 10.1097/FBP.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 14.Pérez-Ramírez MB, Laville A, Tapia D, Duhne M, Lara-González E, Bargas J, et al. KV7 channels regulate firing during synaptic integration in GABAergic striatal neurons. Neural Plast 2015, 2015: 472676. [DOI] [PMC free article] [PubMed]
- 15.Sørensen US, Strøbæk D, Christophersen P, Hougaard C, Jensen ML, Nielsen EØ, et al. Synthesis and structure−activity relationship studies of 2-(N-substituted)-aminobenzimidazoles as potent negative gating modulators of small conductance Ca2+-activated K+ channels. J Med Chem. 2008;51:7625–7634. doi: 10.1021/jm800809f. [DOI] [PubMed] [Google Scholar]
- 16.Lam J, Coleman N, Garing ALA, Wulff H. The therapeutic potential of small-conductance KCa2 channels in neurodegenerative and psychiatric diseases. Expert Opin Ther Targets. 2013;17:1203–1220. doi: 10.1517/14728222.2013.823161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bond CT, Herson PS, Strassmaier T, Hammond R, Stackman R, Maylie J, et al. Small conductance Ca2+-activated K+ channel knock-out mice reveal the identity of calcium-dependent afterhyperpolarization currents. J Neurosci. 2004;24:5301–5306. doi: 10.1523/JNEUROSCI.0182-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deignan J, Luján R, Bond C, Riegel A, Watanabe M, Williams JT, et al. SK2 and SK3 expression differentially affect firing frequency and precision in dopamine neurons. Neuroscience. 2012;217:67–76. doi: 10.1016/j.neuroscience.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallworth NE, Wilson CJ, Bevan MD. Apamin-sensitive small conductance calcium-activated potassium channels, through their selective coupling to voltage-gated calcium channels, are critical determinants of the precision, pace, and pattern of action potential generation in rat subthalamic nucleus neurons in vitro. J Neurosci. 2003;23:7525–7542. doi: 10.1523/JNEUROSCI.23-20-07525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolfart J, Neuhoff H, Franz O, Roeper J. Differential expression of the small-conductance, calcium-activated potassium channel SK3 is critical for pacemaker control in dopaminergic midbrain neurons. J Neurosci. 2001;21:3443–3456. doi: 10.1523/JNEUROSCI.21-10-03443.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen L, Deltheil T, Turle-Lorenzo N, Liberge M, Rosier C, Watabe I, et al. SK channel blockade reverses cognitive and motor deficits induced by nigrostriatal dopamine lesions in rats. Int J Neuropsychopharmacol. 2014;17:1295–1306. doi: 10.1017/S1461145714000236. [DOI] [PubMed] [Google Scholar]
- 22.Waroux O, Massotte L, Alleva L, Graulich A, Thomas E, Liégeois JF, et al. SK channels control the firing pattern of midbrain dopaminergic neurons in vivo. Eur J Neurosci. 2005;22:3111–3121. doi: 10.1111/j.1460-9568.2005.04484.x. [DOI] [PubMed] [Google Scholar]
- 23.Liegeois J, Mercier F, Graulich A, Graulich-Lorge F, Scuvée-Moreau J, Seutin V. Modulation of small conductance calcium-activated potassium (SK) channels: a new challenge in medicinal chemistry. Curr Med Chem. 2003;10:625–647. doi: 10.2174/0929867033457908. [DOI] [PubMed] [Google Scholar]
- 24.Adelman JP, Maylie J, Sah P. Small-conductance Ca2+-activated K+ channels: form and function. Annu Rev Physiol. 2012;74:245–269. doi: 10.1146/annurev-physiol-020911-153336. [DOI] [PubMed] [Google Scholar]
- 25.Liu XK, Wang G, Chen SD. Modulation of the activity of dopaminergic neurons by SK channels: a potential target for the treatment of Parkinson’s disease? Neurosci Bull. 2010;26:265–271. doi: 10.1007/s12264-010-1217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarpal D, Koenig JI, Adelman JP, Brady D, Prendeville LC, Shepard PD. Regional distribution of SK3 mRNA-containing neurons in the adult and adolescent rat ventral midbrain and their relationship to dopamine-containing cells. Synapse. 2004;53:104–113. doi: 10.1002/syn.20042. [DOI] [PubMed] [Google Scholar]
- 27.Mourre C, Manrique C, Camon J, Aidi-Knani S, Deltheil T, Turle-Lorenzo N, et al. Changes in SK channel expression in the basal ganglia after partial nigrostriatal dopamine lesions in rats: Functional consequences. Neuropharmacology. 2017;113:519–532. doi: 10.1016/j.neuropharm.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Doo A-R, Kim S-T, Kim S-N, Moon W, Yin CS, Chae Y, et al. Neuroprotective effects of bee venom pharmaceutical acupuncture in acute 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced mouse model of Parkinson’s disease. Neurol Res. 2010;32:88–91. doi: 10.1179/016164109X12537002794282. [DOI] [PubMed] [Google Scholar]
- 29.Alvarez-Fischer D, Noelker C, Vulinović F, Grünewald A, Chevarin C, Klein C, et al. Bee venom and its component apamin as neuroprotective agents in a Parkinson disease mouse model. PLoS One. 2013;8:e61700. doi: 10.1371/journal.pone.0061700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim J-I, Yang EJ, Lee MS, Kim Y-S, Huh Y, Cho I-H, et al. Bee venom reduces neuroinflammation in the MPTP-induced model of Parkinson’s disease. Int J Neurosci. 2011;121:209–217. doi: 10.3109/00207454.2010.548613. [DOI] [PubMed] [Google Scholar]
- 31.Hartmann A, Müllner J, Meier N, Hesekamp H, Van Meerbeeck P, Habert M-O, et al. Bee venom for the treatment of Parkinson disease–a randomized controlled clinical trial. PLoS One. 2016;11:e0158235. doi: 10.1371/journal.pone.0158235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salthun-Lassalle B, Hirsch EC, Wolfart J, Ruberg M, Michel PP. Rescue of mesencephalic dopaminergic neurons in culture by low-level stimulation of voltage-gated sodium channels. J Neurosci. 2004;24:5922–5930. doi: 10.1523/JNEUROSCI.5668-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolfart J, Roeper J. Selective coupling of T-type calcium channels to SK potassium channels prevents intrinsic bursting in dopaminergic midbrain neurons. J Neurosci. 2002;22:3404–3413. doi: 10.1523/JNEUROSCI.22-09-03404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aumann T, Gantois I, Egan K, Vais A, Tomas D, Drago J, et al. SK channel function regulates the dopamine phenotype of neurons in the substantia nigra pars compacta. Exp Neurol. 2008;213:419–430. doi: 10.1016/j.expneurol.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Ji H, Shepard P. SK Ca2+-activated K+ channel ligands alter the firing pattern of dopamine-containing neurons in vivo. Neuroscience. 2006;140:623–633. doi: 10.1016/j.neuroscience.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Qu L, Wang X-L, Gao L, Li Z-Z, Gao G-D, et al. Firing pattern modulation through SK channel current increase underlies neuronal survival in an organotypic slice model of Parkinson’s disease. Mol Neurobiol. 2015;51:424–436. doi: 10.1007/s12035-014-8728-3. [DOI] [PubMed] [Google Scholar]
- 37.Dolga A, De Andrade A, Meissner L, Knaus H, Höllerhage M, Christophersen P, et al. Subcellular expression and neuroprotective effects of SK channels in human dopaminergic neurons. Cell Death Dis. 2014;5:e999. doi: 10.1038/cddis.2013.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gold M. Modulation mikroglialer Zellen in der Alzheimer-assoziierten Neuroinflammation (http://dx.doi.org/10.17192/z2013.0643). Philipps-Universität Marburg.
- 39.Dolga AM, Culmsee C. Protective roles for potassium SK/KCa2 channels in microglia and neurons. Front Pharmacol. 2012;3:196. doi: 10.3389/fphar.2012.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Dawson VL, Dawson TM. Oxidative stress and genetics in the pathogenesis of Parkinson’s disease. Neurobiol Dis. 2000;7:240–250. doi: 10.1006/nbdi.2000.0319. [DOI] [PubMed] [Google Scholar]
- 41.Sapolsky RM. Cellular defenses against excitotoxic insults. J Neurochem. 2001;76:1601–1611. doi: 10.1046/j.1471-4159.2001.00203.x. [DOI] [PubMed] [Google Scholar]
- 42.Birnbaum SG, Varga AW, Yuan LL, Anderson AE, Sweatt JD, Schrader LA. Structure and function of Kv4-family transient potassium channels. Physiol Rev. 2004;84:803–833. doi: 10.1152/physrev.00039.2003. [DOI] [PubMed] [Google Scholar]
- 43.Serôdio P, Rudy B. Differential expression of Kv4 K+ channel subunits mediating subthreshold transient K+ (A-type) currents in rat brain. J Neurophysiol. 1998;79:1081–1091. doi: 10.1152/jn.1998.79.2.1081. [DOI] [PubMed] [Google Scholar]
- 44.Subramaniam M, Althof D, Gispert S, Schwenk J, Auburger G, Kulik A, et al. Mutant α-synuclein enhances firing frequencies in dopamine substantia nigra neurons by oxidative impairment of A-type potassium channels. J Neurosci. 2014;34:13586–13599. doi: 10.1523/JNEUROSCI.5069-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dragicevic E, Schiemann J, Liss B. Dopamine midbrain neurons in health and Parkinson’s disease: emerging roles of voltage-gated calcium channels and ATP-sensitive potassium channels. Neuroscience. 2015;284:798–814. doi: 10.1016/j.neuroscience.2014.10.037. [DOI] [PubMed] [Google Scholar]
- 46.Liss B, Franz O, Sewing S, Bruns R, Neuhoff H, Roeper J. Tuning pacemaker frequency of individual dopaminergic neurons by Kv4. 3L and KChip3. 1 transcription. EMBO J. 2001;20:5715–5724. doi: 10.1093/emboj/20.20.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dufour MA, Woodhouse A, Goaillard JM. Somatodendritic ion channel expression in substantia nigra pars compacta dopaminergic neurons across postnatal development. J Neurosci Res. 2014;92:981–999. doi: 10.1002/jnr.23382. [DOI] [PubMed] [Google Scholar]
- 48.Haghdoost-Yazdi H, Faraji A, Fraidouni N, Movahedi M, Hadibeygi E, Vaezi F. Significant effects of 4-aminopyridine and tetraethylammonium in the treatment of 6-hydroxydopamine-induced Parkinson’s disease. Behav Brain Res. 2011;223:70–74. doi: 10.1016/j.bbr.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 49.Sun W, Smith D, Fu Y, Cheng JX, Bryn S, Borgens R, et al. Novel potassium channel blocker, 4-AP-3-MeOH, inhibits fast potassium channels and restores axonal conduction in injured guinea pig spinal cord white matter. J Neurophysiol. 2010;103:469–478. doi: 10.1152/jn.00154.2009. [DOI] [PubMed] [Google Scholar]
- 50.Tkatch T, Baranauskas G, Surmeier DJ. Kv4. 2 mRNA abundance and A-type K+ current amplitude are linearly related in basal ganglia and basal forebrain neurons. J Neurosci. 2000;20:579–588. doi: 10.1523/JNEUROSCI.20-02-00579.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Falk T, Zhang S, Erbe EL, Sherman SJ. Neurochemical and electrophysiological characteristics of rat striatal neurons in primary culture. J Comp Neurol. 2006;494:275–289. doi: 10.1002/cne.20819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Day M, Wokosin D, Plotkin JL, Tian X, Surmeier DJ. Differential excitability and modulation of striatal medium spiny neuron dendrites. J Neurosci. 2008;28:11603–11614. doi: 10.1523/JNEUROSCI.1840-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maffie JK, Dvoretskova E, Bougis PE, Martin-Eauclaire MF, Rudy B. Dipeptidyl-peptidase-like-proteins confer high sensitivity to the scorpion toxin AmmTX3 to Kv4-mediated A-type K+ channels. J Physiol. 2013;591:2419–2427. doi: 10.1113/jphysiol.2012.248831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vacher H, Alami M, Crest M, Possani LD, Bougis PE, Martin-Eauclaire MF. Expanding the scorpion toxin α-KTX 15 family with AmmTX3 from Androctonus mauretanicus. FEBS J. 2002;269:6037–6041. doi: 10.1046/j.1432-1033.2002.03294.x. [DOI] [PubMed] [Google Scholar]
- 55.Aidi-Knani S, Regaya I, Amalric M, Mourre C. Kv4 channel blockade reduces motor and neuropsychiatric symptoms in rodent models of Parkinson’s disease. Behav Pharmacol. 2015;26:91–100. doi: 10.1097/FBP.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 56.Haghdoost-Yazdi H, Piri H, Najafipour R, Faraji A, Fraidouni N, Dargahi T, et al. Blockade of fast A-type and TEA-sensitive potassium channels provide an antiparkinsonian effect in a 6-OHDA animal model. Neurosciences (Riyadh) 2017;22:44. doi: 10.17712/nsj.2017.1.20160266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taherian R, Ahmadi MA. 4-aminopyridine decreases MPTP-induced behavioral disturbances in animal model of Parkinson’s disease. Int Clin Neurosci J. 2016;2:142–146. [Google Scholar]
- 58.Yang F, Feng L, Zheng F, Johnson SW, Du J, Shen L, et al. GDNF acutely modulates excitability and A-type K+ channels in midbrain dopaminergic neurons. Nat Neurosci. 2001;4:1071–1078. doi: 10.1038/nn734. [DOI] [PubMed] [Google Scholar]
- 59.Hansen HH, Ebbesen C, Mathiesen C, Weikop P, Rønn LC, Waroux O, et al. The KCNQ channel opener retigabine inhibits the activity of mesencephalic dopaminergic systems of the rat. J Pharmacol Exp Ther. 2006;318:1006–1019. doi: 10.1124/jpet.106.106757. [DOI] [PubMed] [Google Scholar]
- 60.Cooper EC, Harrington E, Jan YN, Jan LY. M channel KCNQ2 subunits are localized to key sites for control of neuronal network oscillations and synchronization in mouse brain. J Neurosci. 2001;21:9529–9540. doi: 10.1523/JNEUROSCI.21-24-09529.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weber YG, Geiger J, Kämpchen K, Landwehrmeyer B, Sommer C, Lerche H. Immunohistochemical analysis of KCNQ2 potassium channels in adult and developing mouse brain. Brain Res. 2006;1077:1–6. doi: 10.1016/j.brainres.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 62.Martire M, D’Amico M, Panza E, Miceli F, Viggiano D, Lavergata F, et al. Involvement of KCNQ2 subunits in [3H] dopamine release triggered by depolarization and pre-synaptic muscarinic receptor activation from rat striatal synaptosomes. J Neurochem. 2007;102:179–193. doi: 10.1111/j.1471-4159.2007.04562.x. [DOI] [PubMed] [Google Scholar]
- 63.Saganich M, Machado E, Rudy B. Differential expression of genes encoding subthreshold-operating voltage-gated K+ channels in brain. J Neurosci. 2001;21:4609–4624. doi: 10.1523/JNEUROSCI.21-13-04609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shen W, Hamilton SE, Nathanson NM, Surmeier DJ. Cholinergic suppression of KCNQ channel currents enhances excitability of striatal medium spiny neurons. J Neurosci. 2005;25:7449–7458. doi: 10.1523/JNEUROSCI.1381-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peretz A, Sheinin A, Yue C, Degani-Katzav N, Gibor G, Nachman R, et al. Pre-and postsynaptic activation of M-channels by a novel opener dampens neuronal firing and transmitter release. J Neurophysiol. 2007;97:283–295. doi: 10.1152/jn.00634.2006. [DOI] [PubMed] [Google Scholar]
- 66.Hansen HH, Weikop P, Mikkelsen MD, Rode F, Mikkelsen JD. The pan-Kv7 (KCNQ) channel opener retigabine inhibits striatal excitability by direct action on striatal neurons in vivo. Basic Clin Pharmacol Toxicol. 2017;120:46–51. doi: 10.1111/bcpt.12636. [DOI] [PubMed] [Google Scholar]
- 67.Shi L, Bian X, Qu Z, Ma Z, Zhou Y, Wang K, et al. Peptide hormone ghrelin enhances neuronal excitability by inhibition of Kv7/KCNQ channels. Nat Commun. 2013;4:1435. doi: 10.1038/ncomms2439. [DOI] [PubMed] [Google Scholar]
- 68.Drion G, Bonjean M, Waroux O, Scuvée-Moreau J, Liégeois JF, Sejnowski TJ, et al. M-type channels selectively control bursting in rat dopaminergic neurons. Eur J Neurosci. 2010;31:827–835. doi: 10.1111/j.1460-9568.2010.07107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sander S, Lemm C, Lange N, Hamann M, Richter A. Retigabine, a KV 7 (KCNQ) potassium channel opener, attenuates l-DOPA-induced dyskinesias in 6-OHDA-lesioned rats. Neuropharmacology. 2012;62:1052–1061. doi: 10.1016/j.neuropharm.2011.10.016. [DOI] [PubMed] [Google Scholar]