Abstract
In this study, the effects of Radio Electric Asymmetric Conveyer (REAC), a non-invasive physical treatment, on neuroinflammatory responses in a mouse model of parkinsonism induced by intoxication with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), were investigated in vivo. We found that the REAC tissue optimization treatment specific for neuro-regenerative purposes (REAC TO-RGN-N) attenuated the inflammatory picture evoked by MPTP-induced nigro-striatal damage in mice, decreasing the levels of pro-inflammatory molecules and increasing anti-inflammatory mediators. Besides, there was a significant reduction of both astrocyte and microglial activation in MPTP-treated mice exposed to REAC TO-RGN-N. These results indicated that REAC TO-RGN-N treatment modulates the pro-inflammatory responses and reduces neuronal damage in MPTP-induced parkinsonism.
Keywords: Parkinson’s disease, Neurodegeneration, Neuroinflammation, REAC TO-RGN-N treatment
Introduction
The innate immune response has been reported to be involved in the progression of several neurodegenerative disorders, including Alzheimer’s disease (AD), Parkinson’s disease (PD), Creutzfeld-Jacob disease, HIV-associated dementia, stroke, and multiple sclerosis [1–4]. In the healthy brain, microglia are responsible for active monitoring of the central nervous system environment, and are activated by any minor disturbance in the microenvironment, providing the first defense against injury and disease [5, 6]. In neuroinflammatory and neurodegenerative disorders, pro-inflammatory microglia become neurotoxic by secreting reactive oxygen species and cytokines in response to various environmental stimuli, causing neuronal injury and death if this response is prolonged. Microglia are involved in the inflammatory responses in the early stages of many neurodegenerative disorders [7, 8]. In particular, recent evidence has suggested that free radicals and oxidative stress play key roles in the pathogenesis of PD [9]. The widely-used methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of PD is characterized by substantial gliosis linked to activated astroglia and microglia [10]. Inflammation has been shown to play an important role in MPTP-induced neurotoxicity [11, 12]. Many studies have demonstrated the beneficial effects of the Radio Electric Asymmetric Conveyer (REAC) in neuro-stimulation [13–16], regenerative medicine [17, 18], cognitive disorders [19–25], and AD [26–28], as well as stem cell vitality during aging [29].
The REAC technology combines low-intensity electromagnetic fields and electrical fields and is based on the production of a flow of micro-currents in the human body during treatment. The current flow can be focused, as required, on specific areas through an (asymmetric) single probe-conveyor, specific to REAC technology. In this study, we have utilized a specific treatment for neuro-regenerative purposes (REAC TO-RGN-N) as previously described [28].
It has been demonstrated that, in PC12 neuronal cells exposed to a REAC TO-RGN-N protocol, the number of cells committed toward the neurogenic phenotype is significantly increased as indicated by specific neurogenic genes, such as neurogenin-1, beta3-tubulin, nerve growth factor, and tyrosine hydroxylase, suggesting that REAC TO-RGN-N is able to up-regulate the expression of catecholaminergic phenotype genes [30, 31].
The impact of REAC treatment has been demonstrated in a Tg2576 mouse model of AD, in which it modifies pathological neuroinflammation and mitigates part of the changes in complex motor behavior [28].
In this study, we investigated the effect of this non-invasive physical treatment on microglial activation in a well-established MPTP mouse model of PD, and explored the modulation of neuroinflammatory responses by REAC.
Materials and Methods
Description of REAC Technology
REAC is a novel technology for bio- and neuro-modulation. REAC technology concentrates the micro-currents produced by ion fluxes through an asymmetric conveyer probe (ACP), in order to optimize the molecular mechanisms driving cellular asymmetry and polarization. The radio-electric fields interact with all structures that contain electrical charges, such as the human body, and induce currents in them. These currents vary according to the molecular characteristics of the tissues. The REAC technology generates a radio-electric field of very low intensity. The peculiarity of REAC technology is not the emission itself, but the particular physical link between the device and the patient’s body. The ACP is this link. This is the innovation of REAC technology. REAC is an asymmetric technology, because there is only one single physical pole (asymmetrical circuit) instead of one positive and one negative (symmetrical circuit). This pole becomes the attractor (Asymmetric Conveyer) for the currents induced in the body by the radio-electric emission [14–16, 30–32]. The REAC technology is independent of the radio frequency emission used. REAC devices use only two frequencies (2.4 and 5.8 GHz), the most widely-used and permitted at the international level. Based on our clinical and scientific experience, 2.4 GHz was chosen because it better interacts with tissues, cell cultures, and the nervous system [13, 33, 34].
The REAC protocol used in this study was tissue optimization regenerative treatment type N as previously described [28]. The REAC device was set for the REAC TO-RGN-N protocol in order to generate a 2.4 GHz radiofrequency emission of very low intensity (0.1 V/m at a distance of 3 m from the emitter), with on/off waveform modulation (750 ms on/1500 ms off). The ACP was the aluminum cage floor, while the stimulation points were the mouse paws. Postural position has no influence on the effects of REAC treatment, so there was no need to control mouse position during treatment [17]. The REAC TO-RGN-N was administered for 7 days without interruption. The REAC model device used in this study was B.E.N.E. (Bio-Enhancer Neuro-Enhancer, ASMED, Florence, Italy).
Animals and Treatments
Sixty adult male 129SV mice (22–26 g, 8–10 weeks of age) were purchased from Harlan (Correzzana, Italy) and housed under a 12-h light/12-h dark cycle in a climate-controlled room with food and water ad libitum. All mouse experiments were performed following protocols approved by the Institutional Animal Committee and in accordance with EU Directive 2010/63/EU for animal experiments. All efforts were made to minimize animal suffering and to reduce the number of animals used. The treatment protocol details have been previously described [35, 36]. Briefly, a group of animals received four i.p. injections of the neurotoxin MPTP (20 mg/kg) (Sigma, Milan, Italy), in a total of four doses over an 8 h period and another group received sterile saline solution. Half of each group was daily subjected to REAC TO-RGN-N treatment starting from MPTP treatment day for 7 consecutive days, and were then sacrificed, since this is the optimal time for 80%–90% loss of dopamine in the striatum [35].
Immunohistochemistry
Twenty animals (five for each experimental condition) were sacrificed by CO2 inhalation at the end of the REAC TO-RGN-N treatment, 7 days after the MPTP or saline injection and then were perfused with 4% paraformaldehyde in 0.1 mol/L phosphate buffered saline (PBS) (pH 7.4). The brains were removed and placed in the same fixative for 1 h and transferred to 30% sucrose in 0.l mol/L PBS until they sank, then frozen. Brain sections (25 μm coronal sections) were cut on a cryomicrotome.
Immunohistochemistry was performed on free-floating sections following a standard avidin-biotin complex procedure. Briefly, the sections were incubated with 0.5% H2O2 in 0.1 mol/L PBS for 1 h, followed by 3% bovine serum albumin in 0.1 mol/L PBS for 1 h, and overnight at 4 °C with the mouse primary monoclonal antibodies anti-tyrosine-hydroxylase (TH) clone 2/40/15 (1:1500; Millipore, Milan, Italy), anti-glial fibrillary acidic protein (GFAP) clone GA5 (Millipore; 1:1000), or anti-CD11β clone M1/70 (1:20; Developmental Studies Hybridoma Bank, Iowa City, IA), and then with an anti-mouse biotinylated secondary antibody (1:1000; Dako, Milan, Italy) for 1 h at room temperature and for 1 h with extravidin peroxidase (1:2000; Sigma, Milan, Italy). The immunocomplexes were visualized using 3,3′-diaminobenzidine/H2O2 as chromogen.
Immunoblotting Assays
To obtain protein extracts, 20 mice were sacrificed by cervical dislocation 7 days after the last MPTP or saline injection, and the substantia nigra pars compacta (SNpc) was rapidly dissected according to Jackson-Lewis and Przedborski [35]. Sample preparation has been described by Panaro et al. [36]. Twenty-five micrograms of protein from each sample was size-fractionated on a denaturing, reducing 10% polyacrylamide minigel and electrophoretically transferred onto a nitrocellulose membrane.
Specific proteins were detected with optimal concentrations of the following specific antibodies: mouse anti-TH (Sigma-Aldrich), mouse anti-GFAP (Millipore), goat polyclonal antibody (pAb) anti-IL-1β (sc-1252), rabbit pAb anti-IL-1βRI (sc-25775), goat pAb anti-TNF-α (sc-1350), rabbit pAb anti-TNF-αRI (sc-7895), goat pAb anti-IL-6 (sc-1266), rabbit pAb anti-IL-6Rα (sc-660), rabbit anti-COX-2 (ab15191), rabbit anti-iNOS (sc-7271), goat pAb anti-SOCS-1 (sc-9021), rabbit anti-TLR4 (sc-10741) (Santa Cruz Biotechnology, Inc., Milan, Italy), and rabbit pAb anti-CD11β clone M1/70 (Abcam, Cambridge, UK). β-actin was used as a loading control. Membranes were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG and HRP-conjugated goat anti-mouse IgG (Bethyl Laboratories) for 60 min at room temperature in the dark on a shaker. Finally, bands were visualized by chemiluminescence (Bio-Rad, Milan, Italy), and subjected to densitometric analysis using ID image analysis software (Kodak Digital Science). The relative optical density was measured.
Real-Time PCR
The real-time PCR procedures have been described elsewhere [7]. Briefly, cDNA was amplified by real-time PCR where a target cDNA (GFAP, CD11β, IL-1β, IL-6, TNF-α, IL-1βRI, IL-6Rα and TNF-αRI, iNOS, TLR-4, COX-2, IL-10, SOCS-1, and TH) and a reference cDNA (glyceraldehyde-3-phosphate dehydrogenase, GAPDH) were amplified simultaneously using an oligonucleotide probe with a 5′ fluorescent reporter dye (6-FAM) and a 3′ quencher dye (NFQ). Fluorescence was determined on an ABI PRISM 7300-sequence detection system (Applied Biosystems, CA). Data were analyzed using the comparative threshold cycle (Ct) method and results are expressed as fold difference.
Statistical Analysis
All numerical data are expressed as the mean ± SD. Analysis of variance (ANOVA) was used to analyze all experimental data and the significance of the differences between results was determined by Scheffe’s F-test as a post-hoc test. P < 0.05 was considered to be statistically significant.
Results
Astroglial Activation
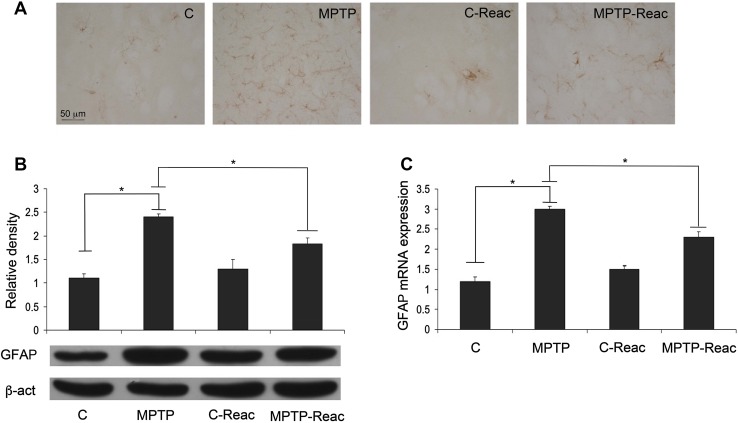
In the SNpc, GFAP immunoreactivity dramatically increased following MPTP treatment (Fig. 1A), suggesting astrocyte activation. However, mice given MPTP and treated with the REAC TO-RGN-N protocol showed a less intense GFAP immunoreaction. Western blot analysis in SNpc lysates revealed that GFAP expression was augmented after MPTP administration, while REAC TO-RGN-N treatment caused a significant reduction of this protein in MPTP-treated mice. Mice exposed to REAC TO-RGN-N alone showed a GFAP expression level comparable to controls (Fig. 1B). These data were further confirmed by GFAP mRNA expression analysis (Fig. 1C).
Fig. 1.
GFAP expression in SNpc. A GFAP immunoreactivity in the control (C), MPTP, C-Reac, and MPTP plus Reac (MPTP-Reac) groups of mice (scale bar, 50 µm). B Western blots and values of relative optic density after normalization against β-actin expression for GFAP expression levels in the SNpc of untreated (C), MPTP, C-Reac, and MPTP plus Reac groups of mice. C Real-time PCR analysis of GFAP mRNA extracted from SNpc of untreated (C), MPTP, C-Reac and MPTP plus Reac groups of mice (fold-change relative to GAPDH; mean ± SD; n = 5 per group, 5 replicates; *P < 0.05).
Microglial Activation
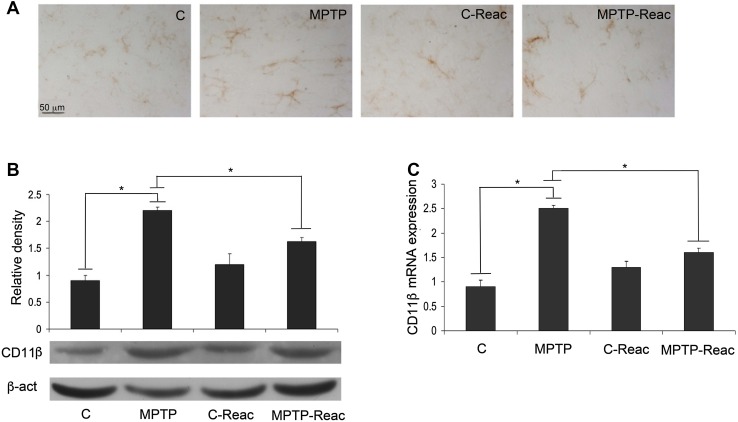
CD11β was evaluated as a microglial marker in the SNpc of treated mice. The intensity of CD11β-immunoreactivity was augmented following MPTP administration in comparison to untreated animals. The increase of CD11β-immunoreactivity was reversed by REAC TO-RGN-N treatment. However, REAC TO-RGN-N treatment alone did not significantly alter CD11β-immunoreactivity relative to controls.
Immunoblotting analysis at the SNpc also showed a significant reduction of the CD11β level in MPTP-treated mice after exposure to REAC TO-RGN-N treatment (Fig. 2B). Quantitative analysis of transcripts further confirmed that the CD11β mRNA level was significantly higher in MPTP-treated mice than in controls, whereas REAC TO-RGN-N exposure of MPTP-treated mice reduced the mRNA level (Fig. 2C).
Fig. 2.
CD11β expression in the SNpc. A Representative microphotographs of CD11β immunostaining in control (C), MPTP, C-Reac, and MPTP plus Reac (MPTP-Reac) groups (scale bar, 50 μm). B Western blots showing CD11β protein levels in the SNpc of untreated (C), MPTP, C-Reac, and MPTP plus Reac groups (relative optical density after normalization against β-actin). C Real-time PCR analysis of CD11β mRNA extracted from SNpc of untreated (C), MPTP, C-Reac, and MPTP plus Reac groups of mice (fold-change relative to GAPDH). All values are expressed as mean ± SD (n = 5/group, 5 replicates; *P < 0.05).
Analysis of Transcripts of Pro-inflammatory Cytokines
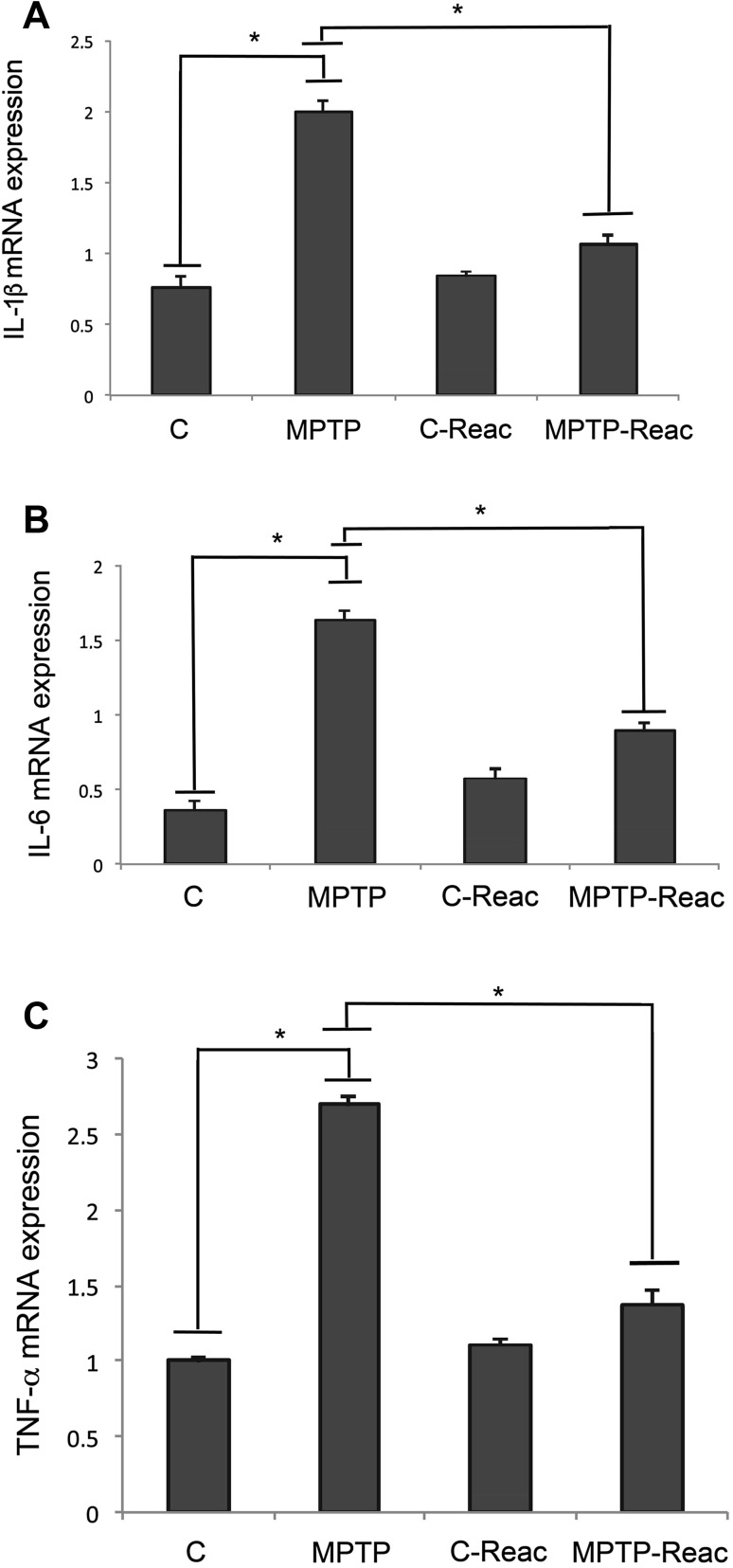
We also analyzed the expression of pro-inflammatory cytokines by quantitative RT-PCR. In the MPTP-treated mice, an increase of mRNA transcripts of IL-1β was detected (Fig. 3A). Interestingly, we found that in MPTP mice exposed to REAC TO-RGN-N, IL-1β mRNA levels were significantly lower than in mice exposed to MPTP alone. No effects on the pro-inflammatory cytokine mRNA levels were detected in mice treated with REAC TO-RGN-N alone. Similar results were also obtained for IL-6 and TNF-α expression (Fig. 3B and C).
Fig. 3.
Analysis of pro-inflammatory cytokine expression. A Real-time PCR analysis of IL-1β mRNA expression levels in SNpc of untreated (C), MPTP, C-Reac, and MPTP plus Reac (MPTP-Reac) groups. B Real-time PCR analysis of IL-6 mRNA expression levels in SNpc of control (C), MPTP, C-Reac, and MPTP-Reac groups. C Real-time PCR analysis of TNF-α mRNA expression levels in SNpc of untreated (C), MPTP, C-Reac, and MPTP-Reac groups. Values represent fold-changes relative to GAPDH and are expressed as mean ± SD (n = 5/group, 5 replicates; *P < 0.05).
Analysis of Pro-inflammatory Cytokine Receptors
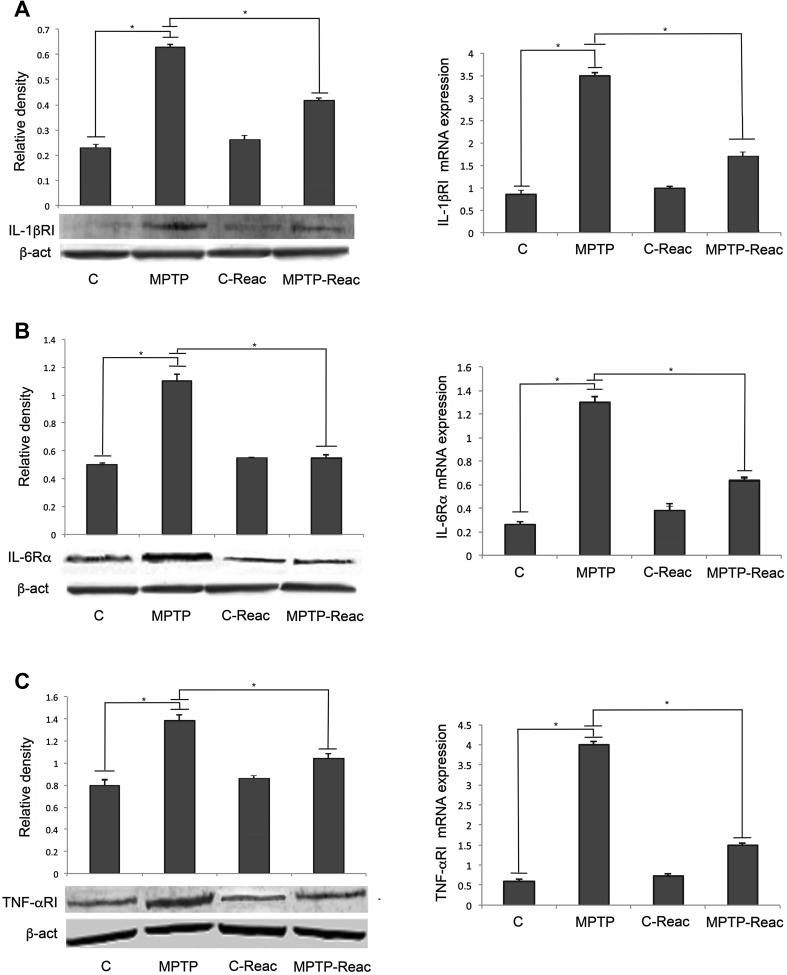
IL-1βRI, IL-6Rα, and TNF-αRI expression at the level of the SNpc was assayed by immunoblotting and quantitative RT-PCR (Fig. 4A, B and C, left). Densitometric analysis of western blots revealed that REAC TO-RGN-N in MPTP-treated animals induced a significant reduction of pro-inflammatory cytokine receptors, in comparison to MPTP-treated mice not exposed to REAC TO-RGN-N. Quantitative analysis of mRNA transcripts (Fig. 4A, B and C, right) confirmed these results.
Fig. 4.
Analysis of pro-inflammatory cytokine receptors in the SNpc. A Left: densitometric analysis of IL-1βRI protein level in the control (C), MPTP, C-Reac, and MPTP-Reac groups (relative optical density after normalization against β-actin). Right: real-time PCR analysis of IL-1βRI mRNA expression levels in untreated (C), MPTP, C-Reac, and MPTP-Reac groups (fold change relative to GAPDH). B Left: densitometric analysis of IL-6R protein level in control (C), MPTP, C-Reac, and MPTP-Reac groups (relative optical density after normalization against β-actin). Right: real-time PCR analysis of IL-6R mRNA level in untreated (C), MPTP, C-Reac, and MPTP-Reac groups (fold-change relative to GAPDH). C Left: densitometric analysis of TNF-αRI protein level in control (C), MPTP, C-Reac, and MPTP-Reac groups (relative optical density after normalization against β-actin). Right: real-time PCR analysis of TNF-αRI mRNA expression in untreated (C), MPTP, C-Reac, and MPTP-Reac groups (fold-change relative to GAPDH). All values are expressed as mean ± SD (n = 5/group, 5 replicates; *P < 0.05).
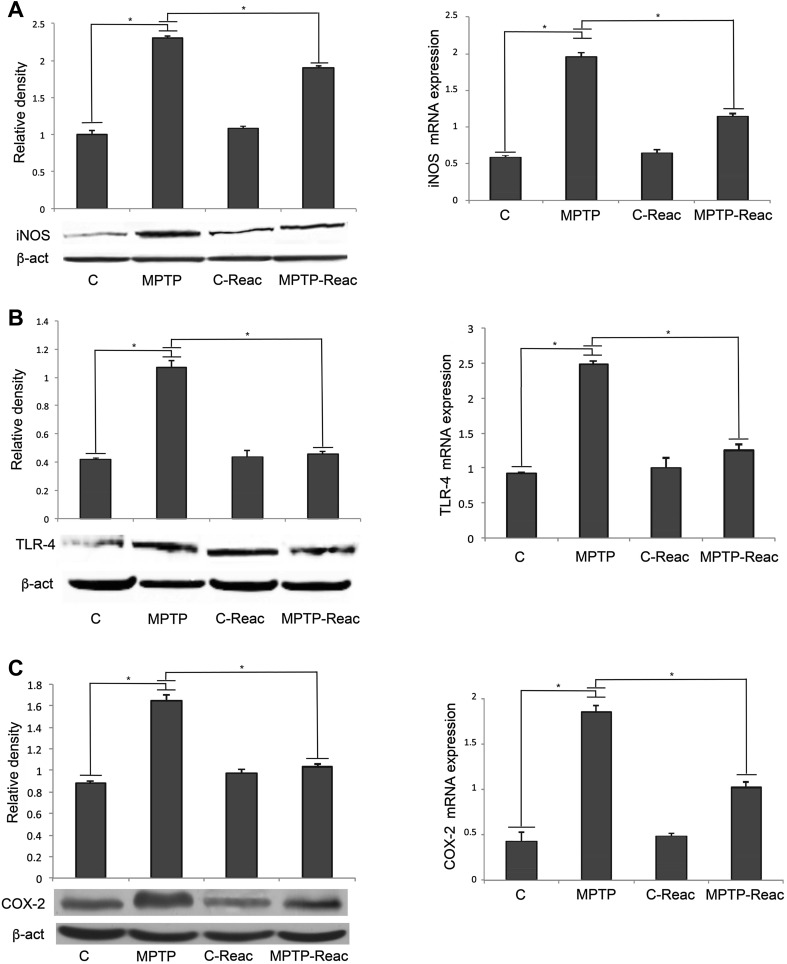
TLR-4, iNOS, and COX-2 Expression
We also analyzed the expression levels of other well-known pro-inflammatory mediators, TLR-4, iNOS, and COX-2 in the SNpc by Western blotting and qRT-PCR. Results concerning the analysis of iNOS expression showed that in MPTP-treated mice, the levels of this protein were much higher than in untreated mice (Fig. 5A, left). REAC TO-RGN-N induced a significant reduction of iNOS in MPTP-treated mice relative to animals only given MPTP. These findings were confirmed by qPCR analysis (Fig. 5A, right).
Fig. 5.
iNOS, TLR-4, and COX-2 expression analysis in the SNpc. A Left: densitometric analysis of iNOS protein level in control (C), MPTP, C-Reac, and MPTP-Reac groups (relative optical density after normalization against β-actin). Right: real-time PCR analysis of iNOS mRNA expression in untreated (C), MPTP, C-Reac, and MPTP-Reac groups (fold-changes relative to GAPDH). B Left: densitometric analysis of TLR-4 protein level in control (C), MPTP, C-Reac, and MPTP-Reac groups (relative optical density after normalization against β-actin). Right: real-time PCR analysis of TLR-4 mRNA expression in untreated (C), MPTP, C-Reac, and MPTP-Reac groups (fold-change relative to GAPDH). C Left: densitometric analysis of COX-2 protein level in control (C), MPTP, C-Reac, and MPTP-Reac groups (relative optical density after normalization against β-actin). Right: real-time PCR analysis of COX-2 mRNA expression in untreated (C), MPTP, C-Reac, and MPTP-Reac groups (fold-change relative to GAPDH). All values are expressed as mean ± SD (n = 5/group, 5 replicates; *P < 0.05).
Results of the analysis of TLR-4 expression (Fig. 5B, left) evidenced that in MPTP-treated mice, the protein levels were much higher than in control animals. Meaningfully, the protein levels from densitometric analysis demonstrated that the amount of this receptor was significantly reduced in MPTP-treated mice after exposure to REAC TO-RGN-N in comparison to animals with MPTP alone. The qPCR analysis also confirmed these results for the TLR-4 mRNA levels (Fig. 5B, right). Similar results were obtained for COX-2 expression (Fig. 5C, left and right).
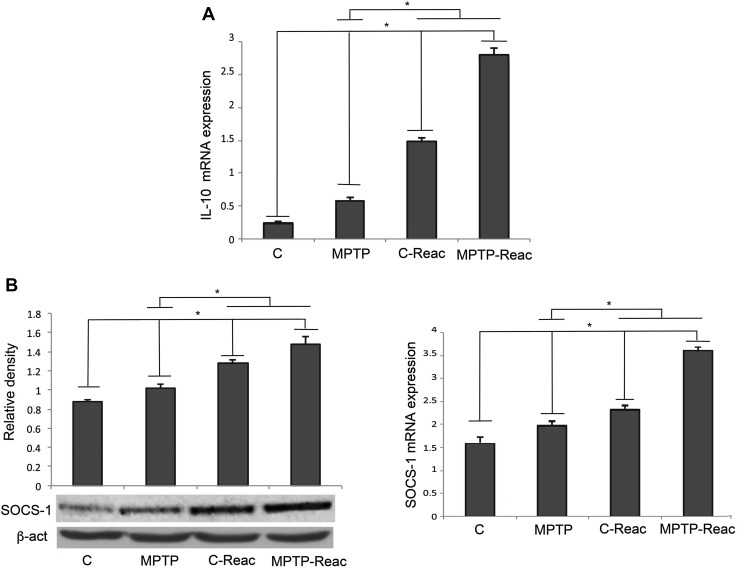
IL-10 and SOCS-1 Analysis
In order to test a possible regulation of the anti-inflammatory responses in the brain of mice exposed to REAC TO-RGN-N, we analyzed the expression of the well-known anti-inflammatory cytokine IL-10 in the SNpc, in terms of mRNA expression by qRT-PCR analysis. MPTP treatment significantly increased the IL-10 mRNA level in comparison to control mice. However, a significant up-regulation of IL-10 mRNA was detected in REAC TO-RGN-N-treated mice in comparison to MPTP-treated animals. More interestingly, combined REAC TO-RGN-N + MPTP induced an additional increase of IL-10 mRNA expression in comparison to both MPTP alone and REAC TO-RGN-N alone (Fig. 6A).
Fig. 6.
IL-10 and SOCS-1 expression in the SNpc. A qRT-PCR analysis of IL-10 mRNA levels in untreated (C), MPTP, C-Reac, and MPTP-Reac groups. B Left: Western blots showing SOCS-1 expression in untreated (C), MPTP, C-Reac, and MPTP-Reac groups (relative optical density after normalization against β-actin). Right: expression of SOCS-1mRNA transcripts detected by qRT-PCR in untreated (C), MPTP, C-Reac, and MPTP-Reac groups (fold-change relative to GAPDH). All values are expressed as means ± SD (n = 5/group, 5 replicates; *P < 0.05).
Suppressor of cytokine signaling (SOCS) proteins are among the possible modulators mediating feedback inhibition of cytokine-induced responses, acting as a negative regulator of cytokine signaling and able to suppress pro-inflammatory cytokine activity. Therefore, we tested the possible effect of REAC TO-RGN-N treatment on the expression of SOCS-1, one of the negative regulators of cytokine expression, in the brain of tested animals. Western blot analysis revealed a significant increase of SOCS-1 protein level in MPTP-treated animals in comparison to control. In mice exposed to REAC TO-RGN-N, SOCS-1 expression was up-regulated in comparison to MPTP-treated mice. Interestingly, also in mice exposed to REAC TO-RGN-N treatment with MPTP intoxication, the SOCS-1 level was significantly higher than in both untreated and MPTP-alone mice (Fig. 6B, left). Accordingly, qRT-PCR analysis revealed that SOCS-1 mRNA was over-expressed in MPTP-treated mice exposed to REAC TO-RGN-N (Fig. 6B, right).
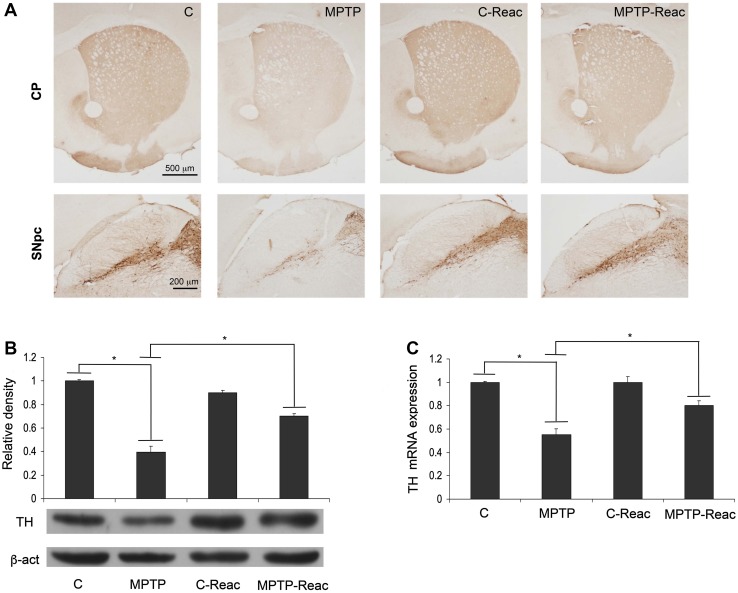
Neuronal Damage
TH expression analysis was used to assess neurodegeneration (Fig. 7). TH immunoreactivity decreased in both the caudate-putamen (CP) and SNpc following MPTP administration (Fig. 7, panel A). Besides, animals treated with REAC TO-RGN-N alone exhibited a TH immunoreactivity comparable to that in controls. Remarkably, TH immunostaining in MPTP-treated mice exposed to REAC TO-RGN-N was more intense than in animals that received neurotoxic treatment only. Western blot analysis of TH protein expression in SNpc lysates (Fig. 7B) showed that in the brain of animals treated with MPTP alone, TH expression was significantly lower than in controls. Interestingly, MPTP + REAC TO-RGN-N treatment induced a significant increase of TH level in comparison to mice exposed to MPTP alone. No differences between REAC TO-RGN-N treatment and controls were found (Fig. 7B).
Fig. 7.
TH analysis. A TH immunoreactivity in the CP (upper, scale bar = 500 μm) and SNpc (lower, scale bar = 200 μm) in control (C), MPTP, C-Reac, and MPTP-Reac groups. B Western blots showing TH expression levels in untreated (C), MPTP, C-Reac, and MPTP plus Reac groups (relative optical density after normalization against β-actin). C Real-time PCR analysis of TH mRNA expression levels in SNpc of untreated (C), MPTP, C-Reac, and MPTP-Reac groups (fold-change relative to GAPDH). All values are expressed as mean ± SD (n = 5/group, 5 replicates; *P < 0.05).
Similarly, TH transcript expression significantly increased in animals exposed to MPTP + REAC TO-RGN-N in comparison to animals given MPTP alone (Fig. 7C).
Discussion
In this work we showed that REAC TO-RGN-N treatment is able to reduce pro-inflammatory responses in the brain of MPTP mice. In addition, REAC TO-RGN-N treatment was able to up-regulate the anti-inflammatory cytokine, IL-10, as well as SOCS-1 expression. These results strongly suggest the possibility that REAC TO-RGN-N treatment can have neuroprotective effects in MPTP-induced brain damage, reducing the amount of inflammatory mediators that are potentially neurotoxic and responsible for exacerbation of the neurodegenerative picture.
MPTP is a product of the chemical synthesis of a meperidine analog with potent heroin-like effects and induces parkinsonian features in humans, rodents, and non-human primates, causing rapid and selective dopaminergic (DA) neurotoxicity through oxidative mitochondrial damage, which results in neuronal death [37]. MPTP readily crosses the blood-brain barrier, and systemic administration produces lesions of the nigrostriatal DA pathway, leading to motor disorders including tremors, bradykinesia, abnormal postural reflexes, rigidity, and akinesia [38]. For these reasons, it is considered an excellent option in animal models for studies of pathogenesis, for testing neuro-protective therapies, and for assessing biomarkers to detect the disease pre-symptomatically [39].
MPTP also leads to a sustained inflammatory response, probably linked to astroglial and microglial activation [40]. This is notably demonstrated when microglia are added to neuronal cultures, leading to a greatly increased level of MPTP-induced DA toxicity [41]. Astroglia and microglia activation typical of neurodegenerative diseases leads to the chronic release of pro-inflammatory cytokines, such as IL-1β, TNF-α, and NO, which exacerbate the loss of DA neurons [42–44].
In this respect, iNOS up-regulation, IL-1β, and IL-6 have already been demonstrated in the brain of MPTP-treated mice [45, 46]. Also, MPTP administration induces the expression of other cytokines involved in the process of inflammation including IL-10 and TNF-α [47] and their receptors [11]. In this work, we found that the levels of pro-inflammatory cytokines were significantly reduced by REAC TO-RGN-N in MPTP-induced parkinsonism. Apart from the reduction in cytokine expression, we also clearly demonstrated that REAC TO-RGN-N treatment in PD animals was able to significantly reduce the expression of other pro-inflammatory mediators, including iNOS and COX-2.
iNOS expression increases significantly in pathological conditions where robust gliosis is present. In fact, high levels of iNOS expression have been clearly demonstrated in many pharmacologically-induced parkinsonism models [48]. Once expressed, iNOS produces high levels of NO continuously from microglia or astrocytes [49, 50]. Other studies have also suggested that inhibition of iNOS is neuroprotective in the MPTP-induced PD model [51, 52].
COX-2 is a pro-inflammatory enzyme that mediates the prostaglandin expression playing an important role in the etiology of PD [53]. Actually, it has been demonstrated that COX-2 is up-regulated in substantia nigra DA neurons in both PD patients and animal models, and that non-steroidal anti-inflammatory drug pre-treatment is able to protect against MPTP- or 6-hydroxy dopamine-induced nigro-striatal DA neuron degeneration [54]. Intriguingly, we found reduced levels of these pro-inflammatory molecules, both in terms of mRNA and protein, in REAC TO-RGN-N-treated PD mice, thus suggesting the beneficial effects of REAC TO-RGN-N treatment in attenuating the pro-inflammatory responses.
IL-10 is a classic immunoregulatory and anti-inflammatory cytokine produced by activated microglia and is a potent inhibitor of inflammation. In fact, it has been shown to share a variety of immunomodulatory functions, including inhibiting the production of pro-inflammatory cytokines by microglia [55, 56]. It is notable that anti-inflammatory cytokines, including IL-10, have been correlated with neuroprotection, recovery, and repair in various neurodegenerative diseases as well as in animal models of PD [57, 58]. We found that REAC TO-RGN-N was able to up-regulate IL-10 expression in mice exposed to MPTP, suggesting a possible modulatory effect of the inflammatory responses by activated microglia.
In the present study, we also suggest a possible mechanism involved in the protective action of REAC TO-RGN-N, by evaluating SOCS-1 expression in all tested animals. In this regard, we demonstrated SOCS up-regulation, both in terms of mRNA transcripts and protein, in REAC TO-RGN-N treated mice. This result may also be correlated with the reduced expression of pro-inflammatory cytokines and their receptors, with the increased expression of the anti-inflammatory cytokine IL-10, as well as with the reduction of neuronal damage, in MPTP + REAC TO-RGN-N-treated mice, while it was not detectable in mice exposed to MPTP alone.
In order to prevent uncontrolled inflammation in brain tissues, SOCS proteins play a key role, restricting cellular signaling pathways by enhancing the degradation of activated receptors and removing the stimuli for continued activation [59, 60]. Qin et al. reported that SOCS-1 expression induced by pro-inflammatory cytokines in astrocytes negatively regulates immune cell migration by reducing the expression of key chemokines [61]. Another study showed a decrease of SOCS-1 mRNA expression in peripheral blood leukocytes from multiple sclerosis patients [62]. Finally, in a transgenic model of AD, reduced levels of SOCS-1 have been correlated with enhanced neuroinflammation and neuropathology [63].
The impact of REAC treatment has also been explored in other inflammation-based neurodegenerative diseases. In particular, it has recently been reported that REAC RGN-N treatment modifies pathological neuroinflammation and mitigates part of the changes in complex motor behavior observed in Tg2576 mice, an mouse model of AD [28].
Overall, we showed an association between increased SOCS-1 expression and reduced pro-inflammatory responses in mice exposed to REAC TO-RGN-N.
Consistent with these results, we found that microglia activation, evaluated in terms of CD11β expression, in tissue sections after REAC TO-RGN-N treatment of MPTP-exposed animals, was significantly lower than in animals with MPTP only, thus suggesting a possible role for REAC TO-RGN-N treatment in modulating microglial activation. In the central nervous system, microglia play their physiological role by protecting neurons from invading microorganisms through a receptor of the innate immune system, TLR-4 [64–66]. Through the activated TLR4-dependent pathway, microglia produce NO and other pro-inflammatory mediators that, if produced at high levels, may cause neuronal degeneration [67]. Up-regulation of TLRs, including TLR-4, in the brain has been demonstrated in neurodegenerative conditions [12, 68], indicating that innate immunity is a novel therapeutic target in these disorders. Also, MPTP toxicity is less severe in TLR4-deficient mice than in WT mice [10].
Our results showed that in PD mice exposed to REAC TO-RGN-N there was a significant down-regulation of TLR-4 expression, suggesting involvement of the TLR-4-mediated signaling pathways in the inflammatory responses active in neurodegenerative diseases.
In summary, REAC TO-RGN-N exposure protects DA neurons against MPTP-induced neurotoxicity in mice by modulating the activation of astroglia and microglia. These effects were accompanied on the one hand by attenuated expression of pro-inflammatory factors and on the other hand by up-regulation of anti-inflammatory responses, as demonstrated by increased IL-10 mRNA and SOCS-1 up-regulation. Further studies are needed to evaluate the potential application of REAC TO-RGN-N as therapeutic support in the treatment of neuroinflammatory diseases.
Acknowledgements
We are grateful to Mr. Diego Mangiullo for technical assistance in animal cage assembly. This work was partially supported by a Fondazione Umberto Veronesi 2011 grant to RR, by a grant from the University of Bari (Fondi di Ateneo 2014), and by a grant from the University of Salento (Fondi di Ateneo 2014).
Compliance with Ethical Standards
Conflict of interest
Salvatore Rinaldi and Vania Fontani are the inventors of REAC technology. They are also founders of the company that produces REAC technology.
Footnotes
Maria Antonietta Panaro, Alessandra Aloisi, and Giuseppe Nicolardi have contributed equally to this work.
References
- 1.González-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci. 1999;22:219–240. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- 2.Nagele RG, Wegiel J, Venkataraman V, Imaki H, Wang K-C, Wegiel J. Contribution of glial cells to the development of amyloid plaques in Alzheimer’s disease. Neurobiol Aging. 2004;25:663–674. doi: 10.1016/j.neurobiolaging.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Chen Z, Zhong C. Oxidative stress in Alzheimer’s disease. Neurosci Bull. 2014;30:271–281. doi: 10.1007/s12264-013-1423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su F, Bai F, Zhang Z. Inflammatory cytokines and Alzheimer’s disease: a review from the perspective of genetic polymorphisms. Neurosci Bull. 2016;32:469–480. doi: 10.1007/s12264-016-0055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eder C. Regulation of microglial behavior by ion channel activity. J Neurosci Res. 2005;81:314–321. doi: 10.1002/jnr.20476. [DOI] [PubMed] [Google Scholar]
- 6.Jurgens HA, Johnson RW. Dysregulated neuronal-microglial cross-talk during aging, stress and inflammation. Exp Neurol. 2012;233:40–48. doi: 10.1016/j.expneurol.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kingwell K. Neurodegenerative disease: Microglia in early disease stages. Nat Rev Neurol. 2012;8:475. doi: 10.1038/nrneurol.2012.172. [DOI] [PubMed] [Google Scholar]
- 8.Yuan H, Zheng JC, Liu P, Zhang SF, Xu JY, Bai LM. Pathogenesis of Parkinson’s disease: oxidative stress, environmental impact factors and inflammatory processes. Neurosci Bull. 2007;23:125–130. doi: 10.1007/s12264-007-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan H, Zhang ZW, Liang LW, Shen Q, Wang XD, Ren SM, et al. Treatment strategies for Parkinson’s disease. Neurosci Bull. 2010;26:66. doi: 10.1007/s12264-010-0302-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noelker C, Morel L, Lescot T, Osterloh A, Alvarez-Fischer D, Breloer M, Henze C, Depboylu C, Skrzydelski D, Michel PP, Dodel RC, Lu L, Hirsch EC, et al. Toll like receptor 4 mediates cell death in a mouse MPTP model of Parkinson disease. Sci Rep. 2013;3:1393. doi: 10.1038/srep01393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lofrumento DD, Saponaro C, Cianciulli A, De Nuccio F, Mitolo V, Nicolardi G, et al. MPTP-induced neuroinflammation increases the expression of pro-inflammatory cytokines and their receptors in mouse brain. Neuroimmunomodulation. 2011;18:79–88. doi: 10.1159/000320027. [DOI] [PubMed] [Google Scholar]
- 12.Panaro MA, Lofrumento DD, Saponaro C, De Nuccio F, Cianciulli A, Mitolo V, et al. Expression of TLR4 and CD14 in the Central Nervous System (CNS) in a MPTP mouse model of Parkinson’s-like disease. Immunopharmacol Immunotoxicol. 2008;30:729–740. doi: 10.1080/08923970802278557. [DOI] [PubMed] [Google Scholar]
- 13.Collodel G, Fioravanti A, Pascarelli NA, Lamboglia A, Fontani V, Maioli M, et al. Effects of regenerative radioelectric asymmetric conveyer treatment on human normal and osteoarthritic chondrocytes exposed to IL-1B: A biochemical and morphological study. Clin Interv Aging. 2013;8:309–316. doi: 10.2147/CIA.S42229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maioli M, Rinaldi S, Santaniello S, Castagna A, Pigliaru G, Gualini S, et al. Radiofrequency energy loop primes cardiac, neuronal, and skeletal muscle differentiation in mouse embryonic stem cells: A new tool for improving tissue regeneration. Cell Transplant. 2012;21:1225–1233. doi: 10.3727/096368911X600966. [DOI] [PubMed] [Google Scholar]
- 15.Rinaldi S, Maioli M, Santaniello S, Castagna A, Pigliaru G, Gualini S, et al. Regenerative treatment using a radioelectric asymmetric conveyor as a novel tool in antiaging medicine: an in vitro beta-galactosidase study. Clin Interv Aging. 2012;7:191–194. doi: 10.2147/CIA.S33312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maioli M, Rinaldi S, Santaniello S, Castagna A, Pigliaru G, Delitala A, et al. Radioelectric asymmetric conveyed fields and human adipose-derived stem cells obtained with a nonenzymatic method and device: a novel approach to multipotency. Cell Transplant. 2014;23:1489–1500. doi: 10.3727/096368913X672037. [DOI] [PubMed] [Google Scholar]
- 17.Mura M, Castagna A, Fontani V, Rinaldi S. Preliminary pilot fMRI study of neuropostural optimization with a noninvasive asymmetric radioelectric brain stimulation protocol in functional dysmetria. Neuropsychiatr Dis Treat. 2012;8:149–154. doi: 10.2147/NDT.S29971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rinaldi S, Fontani V, Castagna A. Brain activity modification produced by a single radioelectric asymmetric brain stimulation pulse: a new tool for neuropsychiatric treatments. Preliminary fMRI study. Neuropsychiatr Dis Treat. 2011;7:649–654. doi: 10.2147/NDT.S26123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rinaldi S, Fontani V, Moretti E, Rosettani B, Aravagli L, Saragò G, et al. A new approach on stress-related depression & anxiety: Neuro-Psycho- Physical-Optimization with Radio Electric Asymmetric-Conveyer. Indian J Med Res. 2010;132:189–194. [PubMed] [Google Scholar]
- 20.Rinaldi S, Fontani V, Aravagli L, Margotti ML. Psychological and symptomatic stress-related disorders with radio-electric treatment: psychometric evaluation. Stress Heal. 2010;26:350–358. doi: 10.1002/smi.1298. [DOI] [Google Scholar]
- 21.Rinaldi S, Fontani V, Mannu P, Castagna A. Social anxiety disorder: radio electric asymmetric conveyor brain stimulation versus sertraline. Patient Prefer Adherence. 2011;5:581–586. doi: 10.2147/PPA.S27409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rinaldi S, Fontani V, Aravagli L, Mannu P, Castagna A, Lotti M. Noninvasive radioelectric asymmetric brain stimulation in the treatment of stress-related pain and physical problems: psychometric evaluation in a randomized, single-blind placebo-controlled, naturalistic study. Int J Gen Med. 2011;4:681–686. doi: 10.2147/IJGM.S24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rinaldi S, Mannu P, Fontani V, Castagna A. Long-term treatment of bipolar disorder with a radioelectric asymmetric conveyor. Neuropsychiatr Dis Treat. 2011;7:373–379. doi: 10.2147/NDT.S22007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Olivieri EB, Vecchiato C, Ignaccolo N, Mannu P, Castagna A, Aravagli L, et al. Radioelectric brain stimulation in the treatment of generalized anxiety disorder with comorbid major depression in a psychiatric hospital: a pilot study. Neuropsychiatr Dis Treat. 2011;7:449–455. doi: 10.2147/NDT.S23420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rinaldi S, Fontani V, Aravagli, Lotti M, Castagna A, Piero Mannu. Neuropsychophysical optimization by REAC technology in the treatment of: sense of stress and confusion. Psychometric evaluation in a randomized, single blind, sham-controlled naturalistic study. Patient Prefer Adherence 2012, 6: 195–199. [DOI] [PMC free article] [PubMed]
- 26.Rinaldi S, Mannu P, Fontani V, Castagna A. Radio electric asymmetric brain stimulation in the treatment of behavioral and psychiatric symptoms in Alzheimer disease. Clin Interv Aging. 2011;6:207–211. doi: 10.2147/CIA.S23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olazarán J, González B, López-Álvarez J, Castagna A, Osa-Ruiz E, Herrero-Cano V, et al. Motor effects of REAC in advanced Alzheimer’s disease: results from a pilot trial. J Alzheimers Dis. 2013;36:297–302. doi: 10.3233/JAD-130077. [DOI] [PubMed] [Google Scholar]
- 28.Luca L, Alessandro G, Sandra S, Antonio BV, Mercedes F, Matteo LM, et al. REAC technology modifies pathological neuroinflammation and motor behaviour in an Alzheimer’s disease mouse model. Sci Rep. 2016;6:35719. doi: 10.1038/srep35719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maioli M, Rinaldi S, Santaniello S, Castagna A, Pigliaru G, Delitala A, et al. Anti-senescence efficacy of radio-electric asymmetric conveyer technology. Age (Omaha) 2014;36:9–20. doi: 10.1007/s11357-013-9537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maioli M, Rinaldi S, Santaniello S, Castagna A, Pigliaru G, Gualini S, et al. Radio electric conveyed fields directly reprogram human dermal skin fibroblasts toward cardiac, neuronal, and skeletal muscle-like lineages. Cell Transplant. 2013;22:1227–1235. doi: 10.3727/096368912X657297. [DOI] [PubMed] [Google Scholar]
- 31.Maioli M, Rinaldi S, Migheli R, Pigliaru G, Rocchitta G, Santaniello S, et al. Neurological morphofunctional differentiation induced by REAC technology in PC12: A neuro protective model for Parkinson’s disease. Sci Rep. 2015;5:10439. doi: 10.1038/srep10439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rinaldi S, Maioli M, Pigliaru G, Castagna A, Santaniello S, Basoli V, et al. Stem cell senescence. Effects of REAC technology on telomerase-independent and telomerase-dependent pathways. Sci Rep. 2014;4:6373. doi: 10.1038/srep06373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rinaldi S, Mura M, Castagna A, Fontani V. Long-lasting changes in brain activation induced by a single REAC technology pulse in Wi-Fi bands: Randomized double-blind fMRI qualitative study. Sci Rep. 2014;4:5668. doi: 10.1038/srep05668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zippo AG, Rinaldi S, Pellegata G, Caramenti GC, Valente M, Fontani V, et al. Electrophysiological effects of non-invasive Radio Electric Asymmetric Conveyor (REAC) on thalamocortical neural activities and perturbed experimental conditions. Sci Rep. 2015;5:18200. doi: 10.1038/srep18200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- 36.Panaro MA, Carofiglio V, Calvello C, Aloisi A, Rinaldi R, Nicolardi G, et al. Modulation of pro-inflammatory response in a mouse model of Parkinson’s disease by non-invasive physical approach: preliminary evaluation. Proc 2015 IEEE 15th Mediterr Microw Symp 2015: 1–4.
- 37.Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA, Karluk D. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann Neurol. 1999;46:598–605. doi: 10.1002/1531-8249(199910)46:4<598::AID-ANA7>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 38.Miller KM, Okun MS, Marsiske M, Fennell EB, Bowers D. Startle reflex hyporeactivity in Parkinson’s disease: An emotion-specific or arousal-modulated deficit? Neuropsychologia. 2009;47:1917–1927. doi: 10.1016/j.neuropsychologia.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/S0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 40.McGeer PL, McGeer EG. Inflammation and neurodegeneration in Parkinson’s disease. Parkinsonism Relat Disord. 2004;10:S3–7. doi: 10.1016/j.parkreldis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 41.Gao HM. Synergistic dopaminergic neurotoxicity of MPTP and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson’s disease. FASEB J. 2003;17:1957–1959. doi: 10.1096/fj.03-0203fje. [DOI] [PubMed] [Google Scholar]
- 42.Nagatsu T, Sawada M. Molecular mechanism of the relation of monoamine oxidase B and its inhibitors to Parkinson’s disease: possible implications of glial cells. J Neural Transm Suppl. 2006;71:53–65. doi: 10.1007/978-3-211-33328-0_7. [DOI] [PubMed] [Google Scholar]
- 43.Panaro MA, Cianciulli A. Current opinions and perspectives on the role of immune system in the pathogenesis of Parkinson’s disease. Curr Pharm Des. 2012;18:200–208. doi: 10.2174/138161212799040574. [DOI] [PubMed] [Google Scholar]
- 44.Doherty GH. Nitric oxide in neurodegeneration: potential benefits of non-steroidal anti-inflammatories. Neurosci Bull. 2011;27:366–382. doi: 10.1007/s12264-011-1530-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurkowska-Jastrzębska I, Wrońska A, Kohutnicka M, Członkowski A, Członkowska A. The inflammatory reaction following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine intoxication in mouse. Exp Neurol. 1999;156:50–61. doi: 10.1006/exnr.1998.6993. [DOI] [PubMed] [Google Scholar]
- 46.Nagatsu T, Mogi M, Ichinose H, Togari A. Cytokines in Parkinson’s disease. J Neural Transm Suppl. 2000;54:143–151. [PubMed] [Google Scholar]
- 47.Mandel S, Weinreb O, Youdim MB. Using cDNA microarray to assess Parkinson’s disease models and the effects of neuroprotective drugs. Trends Pharmacol Sci. 2003;24:184–191. doi: 10.1016/S0165-6147(03)00067-1. [DOI] [PubMed] [Google Scholar]
- 48.Knott C, Stern G, Wilkin GP. Inflammatory regulators in Parkinson’s disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Mol Cell Neurosci. 2000;16:724–739. doi: 10.1006/mcne.2000.0914. [DOI] [PubMed] [Google Scholar]
- 49.Bal-Price A, Brown GC. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J Neurosci. 2001;21:6480–6491. doi: 10.1523/JNEUROSCI.21-17-06480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bal-Price A, Matthias A, Brown GC. Stimulation of the NADPH oxidase in activated rat microglia removes nitric oxide but induces peroxynitrite production. J Neurochem. 2002;80:73–80. doi: 10.1046/j.0022-3042.2001.00675.x. [DOI] [PubMed] [Google Scholar]
- 51.Broom L, Marinova-Mutafchieva L, Sadeghian M, Davis JB, Medhurst AD, Dexter DT. Neuroprotection by the selective iNOS inhibitor GW274150 in a model of Parkinson disease. Free Radic Biol Med. 2011;50:633–640. doi: 10.1016/j.freeradbiomed.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 52.Gahm C, Holmin S, Wiklund PN, Brundin L, Mathiesen T. Neuroprotection by selective inhibition of inducible nitric oxide synthase after experimental brain contusion. J Neurotrauma. 2006;23:1343–1354. doi: 10.1089/neu.2006.23.1343. [DOI] [PubMed] [Google Scholar]
- 53.Hunter RL, Dragicevic N, Seifert K, Choi DY, Liu M, Kim HC, et al. Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J Neurochem. 2007;100:1375–1386. doi: 10.1111/j.1471-4159.2006.04327.x. [DOI] [PubMed] [Google Scholar]
- 54.Esposito E, Di Matteo V, Benigno A, Pierucci M, Crescimanno G, Di Giovanni G. Non-steroidal anti-inflammatory drugs in Parkinson’s disease. Exp Neurol. 2007;205:295–312. doi: 10.1016/j.expneurol.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 55.Molina-Holgado F, Grencis R, Rothwell NJ. Actions of exogenous and endogenous IL-10 on glial responses to bacterial LPS/cytokines. Glia. 2001;33:97–106. doi: 10.1002/1098-1136(200102)33:2<97::AID-GLIA1009>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 56.Cianciulli A, Dragone T, Calvello R, Porro C, Trotta T, Lofrumento DD, et al. IL-10 plays a pivotal role in anti-inflammatory effects of resveratrol in activated microglia cells. Int Immunopharmacol. 2015;24:369–376. doi: 10.1016/j.intimp.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 57.Stoll G, Jander S, Schroeter M. Cytokines in CNS disorders: neurotoxicity versus neuroprotection. J Neural Transm Suppl. 2000;59:81–89. doi: 10.1007/978-3-7091-6781-6_11. [DOI] [PubMed] [Google Scholar]
- 58.Calvello R, Cianciulli A, Nicolardi G, De Nuccio F, Giannotti L, Salvatore R. Vitamin D treatment attenuates neuroinflammation and dopaminergic neurodegeneration in an animal model of Parkinson’s disease, Shifting M1 to M2 microglia responses. J Neuroimmune Pharmacol. 2017;12:327–339. doi: 10.1007/s11481-016-9720-7. [DOI] [PubMed] [Google Scholar]
- 59.Dragone T, Cianciulli A, Calvello R, Porro C, Trotta T, Panaro MA. Resveratrol counteracts lipopolysaccharide-mediated microglial inflammation by modulating a SOCS-1 dependent signaling pathway. Toxicol In Vitro. 2014;28:1126–1135. doi: 10.1016/j.tiv.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 60.Lofrumento DD, Nicolardi G, Cianciulli A, De Nuccio F, La Pesa V, Carofiglio V, et al. Neuroprotective effects of resveratrol in an MPTP mouse model of Parkinson’s-like disease: Possible role of SOCS-1 in reducing pro-inflammatory responses. Innate Immun. 2014;20:249–260. doi: 10.1177/1753425913488429. [DOI] [PubMed] [Google Scholar]
- 61.Qin H, Niyongere SA, Lee SJ, Baker BJ, Benveniste EN. Expression and functional significance of SOCS-1 and SOCS-3 in astrocytes. J Immuno. 2008;181:3167–3176. doi: 10.4049/jimmunol.181.5.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sedeño-Monge V, Arcega-Revilla R, Rojas-Morales E, Santos-López G, Perez-García JC, Sosa-Jurado F, et al. Quantitative analysis of the suppressors of cytokine signaling 1 and 3 in peripheral blood leukocytes of patients with multiple sclerosis. J Neuroimmunol. 2014;273:117–119. doi: 10.1016/j.jneuroim.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 63.Guedes JR, Custodia CM, Silva RJ, de Almeida LP, Pedroso de Lima MC, Cardoso AL. Early miR-155 upregulation contributes to neuroinflammation in Alzheimer’s disease triple transgenic mouse model. Hum Mol, Gene. 2014;23:6286–6301. doi: 10.1093/hmg/ddu348. [DOI] [PubMed] [Google Scholar]
- 64.Mansell A, Smith R, Doyle SL, Gray P, Fenner JE, Crack PJ, et al. Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat Immunol. 2006;7:148–155. doi: 10.1038/ni1299. [DOI] [PubMed] [Google Scholar]
- 65.Jung DY, Lee H, Jung BY, Ock J, Lee MS, Lee WH, et al. TLR4, but not TLR2, signals autoregulatory apoptosis of cultured microglia: a critical role of IFN-beta as a decision maker. J Immunol. 2005;174:6467–6476. doi: 10.4049/jimmunol.174.10.6467. [DOI] [PubMed] [Google Scholar]
- 66.Liu HY, Chen CY, Hsueh YP. Innate immune responses regulate morphogenesis and degeneration: roles of Toll-like receptors and Sarm1 in neurons. Neurosci Bull. 2014;30:645–654. doi: 10.1007/s12264-014-1445-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Trotta T, Porro C, Calvello R, Panaro MA. Biological role of Toll-like receptor-4 in the brain. J Neuroimmunol. 2014;268:1–12. doi: 10.1016/j.jneuroim.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 68.Letiembre M, Liu Y, Walter S, Hao W, Pfander T, Wrede A, et al. Screening of innate immune receptors in neurodegenerative diseases: A similar pattern. Neurobiol Aging. 2009;30:759–768. doi: 10.1016/j.neurobiolaging.2007.08.018. [DOI] [PubMed] [Google Scholar]