Abstract
Depression is a debilitating psychiatric disorder with a huge socioeconomic burden, and its treatment relies on antidepressants including selective serotonin reuptake inhibitors (SSRIs). Recently, the melatonergic system that is closely associated with the serotonergic system has been implicated in the pathophysiology and treatment of depression. However, it remains unknown whether combined treatment with SSRI and melatonin has synergistic antidepressant effects. In this study, we applied a sub-chronic restraint stress paradigm, and evaluated the potential antidepressant effects of combined fluoxetine and melatonin in adult male mice. Sub-chronic restraint stress (6 h/day for 10 days) induced depression-like behavior as shown by deteriorated fur state, increased latency to groom in the splash test, and increased immobility time in the forced-swim test. Repeated administration of either fluoxetine or melatonin at 10 mg/kg during stress exposure failed to prevent depression-like phenotypes. However, combined treatment with fluoxetine and melatonin at the selected dose attenuated stress-induced behavioral abnormalities. Moreover, we found that the antidepressant effects of combined treatment were associated with the normalization of brain-derived neurotrophic factor (BDNF)–tropomyosin receptor kinase B (TrkB) signaling in the hippocampus, but not in the prefrontal cortex. Our findings suggest that combined fluoxetine and melatonin treatment exerts synergistic antidepressant effects possibly by restoring hippocampal BDNF–TrkB signaling.
Keywords: Melatonin, Fluoxetine, Depression, BDNF, TrkB
Introduction
Depression is a psychiatric disorder closely associated with stress exposure [1], and causes an enormous economic burden [2]. The typical symptoms of depression include anhedonia, long-lasting depressed mood, recurrent thoughts, and attempted suicide [3]. Although the currently available antidepressants are successful in alleviating the symptoms of depression [3, 4], our knowledge of the neurobiological underpinnings of depression is still limited.
5-Hydroxytryptamine (5-HT, serotonin) is a monoamine neurotransmitter crucial for mood regulation [5]. A large body of evidence has shown the critical role of 5-HT in the pathophysiology and treatment of depression [6]. Notably, selective serotonin reuptake inhibitors (SSRIs) are the most commonly-used antidepressants [7]. SSRIs such as fluoxetine exert antidepressant effects by inhibiting 5-HT turnover and increasing synaptic 5-HT levels [8]. In stress models of depression, fluoxetine has been shown to prevent depression-like behavior [9].
Melatonin is a circadian rhythm-related hormone synthesized from 5-HT and secreted primarily from the pineal gland during the dark period [10]. Disturbed circadian rhythms have been found in depressive patients, who frequently report insomnia and early-morning awakening. These findings imply a close link between the melatonergic system and depressive symptoms [11]. Recently, a disrupted level of melatonin has been implicated as a risk factor for depression [12], and its receptors MT1 and MT2 are potential therapeutic targets for depression [11]. Indeed, melatonin has been shown to exert antidepressant effects in preclinical studies [12].
Combined treatment with medications having different pharmacological properties may enhance the therapeutic effects of antidepressants [13]. In human studies, although melatonin alone does not alleviate depressive symptoms, it has antidepressant effects in combination with other antidepressants [12, 14]. Because both fluoxetine and melatonin target the 5-HT system and show antidepressant effects, it is possible that their combined administration may have synergistic antidepressant effects.
Brain-derived neurotrophic factor (BDNF) and its receptor tropomyosin receptor kinase B (TrkB) signaling is crucial for the development, plasticity, and remodeling of neuronal circuits [15]. Depression is closely associated with abnormal BDNF–TrkB signaling [16, 17] together with increased levels of circulating glucocorticoids [18, 19] that can be reversed by antidepressants. Disrupted BDNF–TrkB signaling in the hippocampus and the prefrontal cortex has been implicated in the pathophysiology of depression [15, 20–22]. In addition, glucocorticoids are released upon stress challenge and regulate metabolic, immune, and neuronal responses [19]. A dysregulated glucocorticoid level is a risk factor for depression [23]. Therefore, it is possible that fluoxetine and melatonin modulate BDNF–TrkB signaling and glucocorticoid levels to exert synergistic antidepressant effects.
To test these possibilities, we examined the effects of fluoxetine or melatonin alone and in combination, on sub-chronic stress-induced depression-like behavior, cognitive phenotypes, anxiety-related behavior, and molecular alterations in mice.
Materials and Methods
Animals
Male C57BL/6N mice (8 weeks old) were purchased from Shanghai SLAC Laboratory. The mice were maintained in a room at 21 ± 1 °C with 50%–60% relative humidity under a 12-h light/dark cycle (lights on at 08:00) with free access to food and water. All experimental procedures were approved by the Animal Care and Use Committee of the Institute of Neuroscience, Zhejiang University.
Experimental Design
The experimental design is illustrated in Fig. 1. At 1 week before experiments, the mice were individually housed. A battery of behavioral tests was performed after drug treatment and sub-chronic restraint stress. At 30 min after the forced swim test, mice were anesthetized by isoflurane inhalation and rapidly decapitated for molecular experiments.
Fig. 1.
Schematic of the experimental design.
Sub-chronic Restraint Stress and Drug Treatment
Mice were randomly assigned to the following groups (n = 7 per group): control + vehicle (CTL), sub-chronic restraint stress + vehicle (SRS), sub-chronic restraint stress + fluoxetine (FLX), sub-chronic restraint stress + melatonin (MEL), and sub-chronic restraint stress + fluoxetine + melatonin (FLX + MEL). All mice received a daily intraperitoneal injection of vehicle (normal saline containing 5% ethanol) or drug between 09:00 and 10:00. Melatonin (10 mg/kg; M5250, Sigma-Aldrich, Louis, MO) was dissolved in 5% ethanol and diluted with saline. Fluoxetine (10 mg/kg; F132, Sigma-Aldrich) was dissolved in saline containing 5% ethanol. The doses of melatonin (10 mg/kg) and fluoxetine (10 mg/kg) were selected based on previous reports [24, 25]. After injection, the mouse was placed in a restraint tube (a 50 mL centrifugal tube with punched holes) for 6 h, while the control mouse remained undisturbed in its home cage. Drug treatment and sub-chronic restraint stress lasted for 10 consecutive days. Body weight and fur state were monitored daily. Fur state was classified according to a 4-point scale, where 1 stands for a perfect and clean fur, while 4 represents a disheveled and scruffy fur. Ratings of 2 and 3 demonstrate intermediate fur states [26].
Open Field Test
Locomotor activity and anxiety-like behavior were evaluated by the open field test [27]. The apparatus (50 × 50 × 50 cm3) was made of gray polyvinyl chloride. The illumination of the central zone (25 × 25 cm2) was 30 lux. The total distance traveled, time in the central zone, number of entries to the central zone, and number of rearings were recorded by ANY-maze 4.98 (Stoelting, Wood Dale, IL) for 5 min.
Y-Maze Test
The short-term spatial working memory was tested by recording spontaneous alternation behavior in the Y-maze [28]. The apparatus was made of gray polyvinyl chloride with three symmetrical arms (30 × 10 × 15 cm3, 30 lux). The arms were marked by a triangle, bar, or plus sign as intra-maze spatial cues. Mice were placed in the center of the Y-maze and allowed to explore all arms freely for 5 min. Three consecutive choices of all three arms were counted as an alternation. The percentage of spontaneous alternation was determined by dividing the total number of alternations by the total number of choices minus 2.
Splash Test
The splash test assesses anhedonia-like behavior [29]. A 10% sucrose solution was evenly sprayed onto the dorsal coat, and the mice were individually placed in an acrylic apparatus (11 × 21 × 9 cm3). The latency to groom and the total amount of grooming were manually recorded for 5 min as indices of self-care and motivational behaviors.
Forced Swim Test
Depression-like behavior was assessed by the forced-swim test as described previously [30]. Mice were individually placed in a glass cylinder (height, 25 cm; diameter, 12 cm) containing 15 cm-deep water (21 ± 1 °C) for 6 min. The duration of immobility and struggle during the last 5 min was recorded.
Corticosterone Assay
Blood samples were collected and centrifuged at 3,000 rpm for 15 min at 4 °C. Plasma corticosterone concentrations were measured using a corticosterone ELISA kit (Cayman Chemical, Ann Arbor, MI) as described previously [31].
Western Blot
Western blot was performed as described previously [32]. Prefrontal cortex and hippocampal samples were dissected, snap-frozen, and stored at −80 °C until use. Samples were homogenized in ice-cold lysis buffer and centrifuged at 10,000 rpm for 20 min at 4 °C. Protein concentrations were measured using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL). Protein samples (20 μg) were electrophoresed in SDS-PAGE gel (15% acrylamide) and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA). The membranes were blocked with Tris-buffered saline-Tween (TBST) [150 mmol/L NaCl, 10 mmol/L Tris-HCl (pH 7.5), and 0.1% Tween] containing 5% non-fat milk for 2 h and were incubated with primary antibodies overnight at 4 °C. The primary antibodies used were as follows: rabbit anti-BDNF (1:2000; ab108319, Abcam, Cambridge), rabbit anti-TrkB (1:1000; 4603, Cell Signaling, Danvers, MA), rabbit anti-phosphorylated-TrkB (p-TrkB; 1:1000; ab109684, Abcam) and mouse anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:10000, E021010-01; EarthOx, Millbrae, CA). Next, the membranes were incubated with peroxidase-conjugated goat anti-rabbit (1:2000; ZB-2301; Zhongshan Golden Bridge Biotechnology, Beijing) or goat anti-mouse (1:2000; ZB-2305; Zhongshan Golden Bridge Biotechnology) secondary antibody for 3 h at room temperature. Bands were visualized using an enhanced chemiluminescence system and quantified by densitometry (Quantity One 4.2, Bio-Rad, Hercules, CA). All results were normalized to the value of the control group.
Statistical Analysis
Statistical analysis was performed using SPSS 20.0 (SPSS, Chicago, IL). Body weight and fur score were analyzed by one-way repeated measures analysis of variance (ANOVA) followed by the LSD post hoc test when appropriate. The remaining data were analyzed by one-way ANOVA followed by the LSD post hoc test when applicable. The level of statistical significance was set at P < 0.05. Data are expressed as mean ± SEM.
Results
Effects of Sub-chronic Stress and Drug Treatment on Body Weight, Fur State, and Plasma Corticosterone Levels
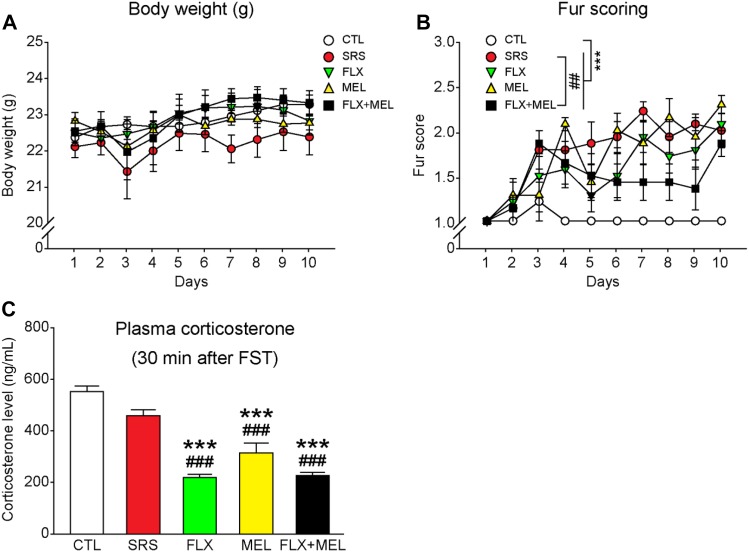
The body weight and fur state were monitored daily during sub-chronic restraint stress and drug treatment. No difference in body weight was found among groups (F 4, 30 = 0.962, P = 0.443; Fig. 2A), whereas a main effect of condition on fur score was detected (F 4, 30 = 14.403, P < 0.001). Compared with the CTL group, the SRS group displayed a significantly worse fur state, which was not improved by fluoxetine or melatonin treatment (all P < 0.001 versus CTL; Fig. 2B). However, combined fluoxetine and melatonin treatment attenuated the stress effect on fur state (P < 0.01 versus SRS), suggesting a synergistic effect of the combination.
Fig. 2.
Effects of sub-chronic restraint stress and drug treatment on body weight, fur state, and plasma corticosterone levels after an acute stress challenge. A All groups of mice had comparable body weight. B Progression of fur quality during the stress and drug treatment procedures. Combined fluoxetine and melatonin treatment improved the fur state in stressed mice. C Either fluoxetine or melatonin administration alone or combined treatment reduced the plasma corticosterone levels at 30 min after the forced swim test. ***P < 0.001 versus CTL. ## P < 0.01, ### P < 0.001 versus SRS. n = 7 mice per group.
At 30 min after the forced-swim test, blood samples were collected and plasma corticosterone was measured to evaluate the effects of stress and drug treatment on neuroendocrine activation. CTL and SRS mice had similar corticosterone levels (Fig. 2C). Fluoxetine or melatonin administration alone or in combination reduced plasma corticosterone levels (P < 0.001 versus CTL), indicating that drug treatment reduced the activation of the hypothalamic-pituitary-adrenal axis.
Sub-chronic Stress and Drug Treatment Had No Effect on Locomotion, Anxiety-Related Behavior, and Spatial Working Memory
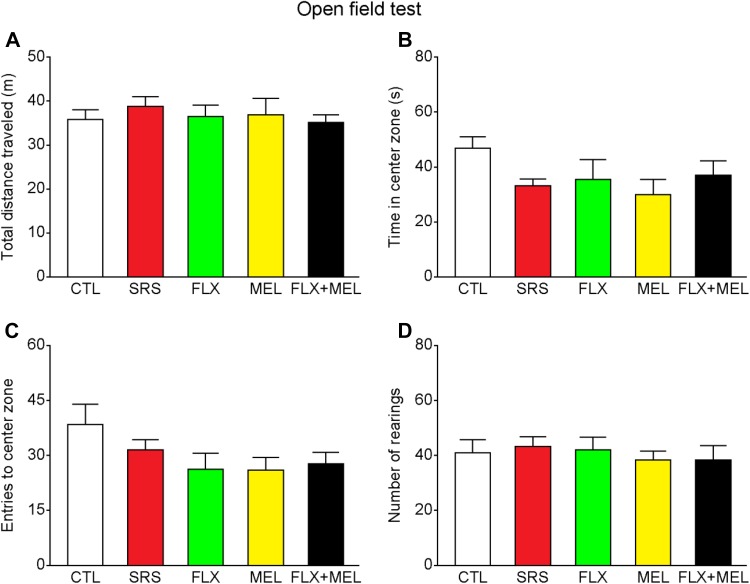
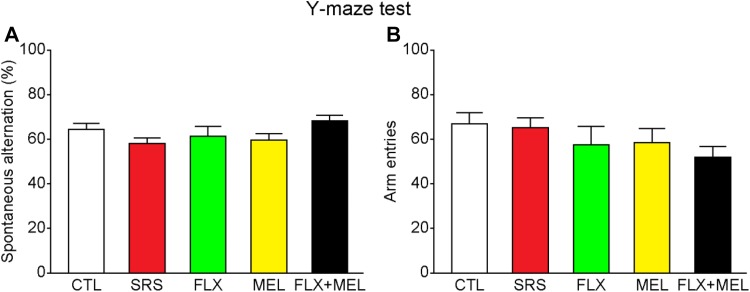
After the 10-day restraint stress, we assessed the anxiety-related behavior and spatial working memory using the open field and Y-maze tests, respectively. In the open field test, there were no differences among all groups in the total distance traveled (F 4, 30 = 0.284, P = 0.886), time in the central zone (F 4, 30 = 1.514, P = 0.223), entries to the central zone (F 4, 30 = 1.723, P = 0.171), and number of rearings (F 4, 30 = 1.514, P = 0.223; Fig. 3), indicating that sub-chronic restraint stress and drug treatment had no effect on locomotion, exploration, and anxiety-related behavior. In the Y-maze spontaneous alternation task, all mice had comparable spatial working memory performance as shown by similar spontaneous alternation rates (F 4, 30 = 1.712, P = 0.173; Fig. 4). In addition, the total number of arm entries remained unchanged in the stressed or drug treatment groups (F 4, 30 = 1.522, P = 0.221), further indicating unaltered anxiety levels.
Fig. 3.
Sub-chronic restraint stress and drug treatment did not affect locomotor activity and anxiety-related behavior in the open field test. A–D All groups of mice were similar in the total distance traveled, time in the center zone, entries to the center zone, and number of rearings. n = 7 mice per group.
Fig. 4.
Sub-chronic restraint stress and drug treatment did not affect spatial working memory in the Y-maze spontaneous alternation task. A–B All groups had comparable spontaneous alternation rates and number of arm entries. n = 7 mice per group.
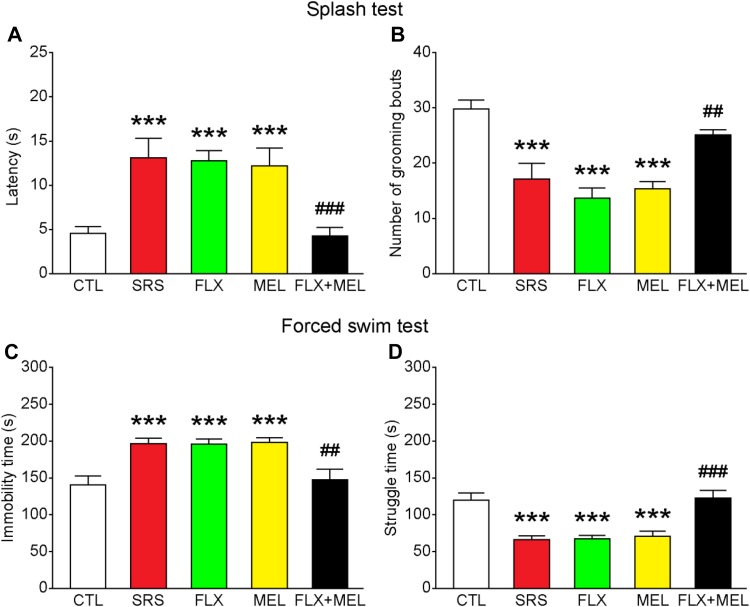
Combined Fluoxetine and Melatonin Treatment Reduced Depression-Like Behavior
Next, we assessed the effects of stress as well as melatonin and/or fluoxetine treatment on depression-like behavior. In the splash test, a significant main effect of condition on the latency to groom and number of grooming bouts was revealed (latency to groom: F 4, 30 = 8.729, P < 0.001; number of grooming bouts: F 4, 30 = 14.313, P < 0.001; Fig. 5A, B). Compared with the CTL group, mice in the SRS group had an increased latency to groom and a decreased number of grooming bouts (both P < 0.001 versus CTL; Fig. 5A, B). Administration of fluoxetine or melatonin alone did not reverse the sub-chronic stress effects (all P < 0.001 versus CTL), whereas combined fluoxetine and melatonin treatment normalized the performance in the splash test (latency to groom, P < 0.001; number of grooming bouts, P < 0.01 versus SRS). In the forced-swim test, a similar effect on immobility and struggle time was found (immobility time, F 4, 30 = 8.391, P < 0.001; struggle time, F 4, 30 = 14.504, P < 0.001; Fig. 5C, D). Sub-chronic stress significantly increased the immobility time and reduced the struggle time, and treatment with fluoxetine or melatonin alone did not attenuate these effects (all P < 0.001 versus CTL). In comparison, combined fluoxetine and melatonin treatment normalized the immobility and struggle time (immobility time, P < 0.01; struggle time, P < 0.001 versus SRS). These results support the hypothesis that combined fluoxetine and melatonin treatment at a relatively low dose exerts a synergistic antidepressant effect.
Fig. 5.
Combined fluoxetine and melatonin treatment reduced depression-like behavior. A–D Combined fluoxetine and melatonin treatment decreased the latency to groom and increased the number of grooming bouts in the splash test, and decreased immobility time but increased struggle time in the forced-swim test. ***P < 0.001 vs CTL; ## P < 0.01, ### P < 0.001 vs SRS; n = 7 mice per group.
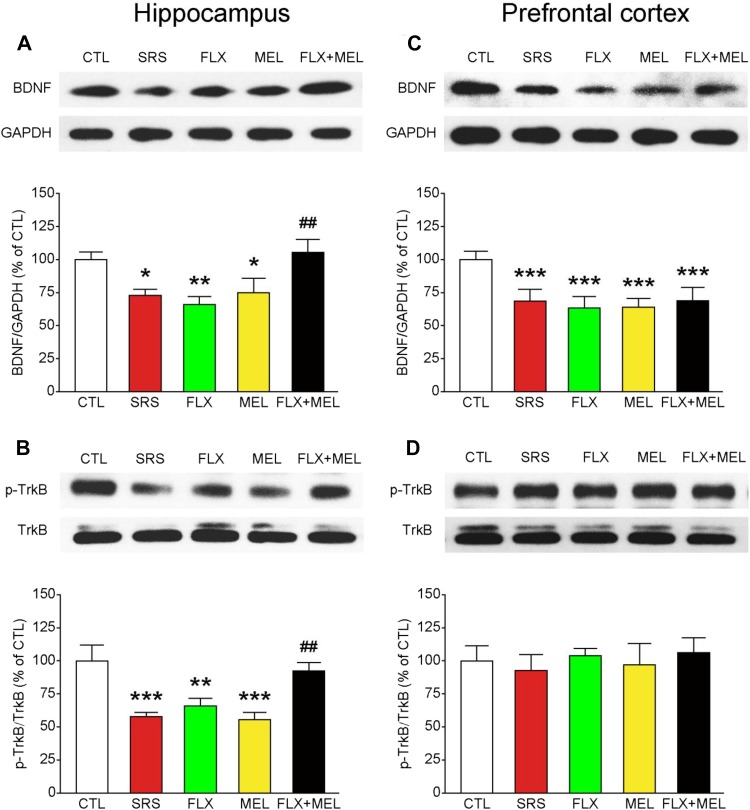
Combined Fluoxetine and Melatonin Treatment Normalized BDNF–TrkB Signaling in the Hippocampus, but Not in the Prefrontal Cortex
It has been shown that both fluoxetine and melatonin modulate BDNF–TrkB signaling [33, 34], which has been implicated in the pathogenesis of depression. Therefore, we assessed whether the synergistic antidepressant effect of combined fluoxetine and melatonin treatment was associated with the normalization of BDNF–TrkB signaling in the hippocampus and prefrontal cortex. One-way ANOVA revealed a main effect of condition on hippocampal protein levels of BDNF (F 4, 30 = 4.986, P < 0.01) and p-TrkB (F 4, 30 = 8.410, P < 0.001; Fig. 6A, B), the activated form of TrkB. Compared with the CTL group, the SRS group had reduced BDNF levels (P < 0.05) and TrkB phosphorylation (P < 0.001) in the hippocampus. Although a low dose of fluoxetine or melatonin failed to attenuate the stress effects (all P < 0.05 for CTL versus FLX or MEL), combined treatment normalized the hippocampal BDNF levels (P < 0.01 versus SRS) and TrkB signaling (P < 0.05 versus SRS). In contrast, we found that combined treatment had no effect on the protein level of BDNF in the prefrontal cortex (all P < 0.001 for CTL versus FLX, MEL, or FLX + MEL). Sub-chronic restraint stress and drug treatment did not influence prefrontal TrkB signaling (F 4, 30 = 0.885, P = 0.485). In addition, the protein levels of TrkB and GAPDH in both brain regions were comparable among groups (data not shown). These results together indicate that fluoxetine and melatonin exert their synergistic effects by modulating BDNF–TrkB signaling in the hippocampus, but not in the prefrontal cortex.
Fig. 6.
Combined fluoxetine and melatonin treatment normalized BDNF–TrkB signaling in the hippocampus (A–B), but not in the prefrontal cortex (C–D). *P < 0.05, **P < 0.01, ***P < 0.001 vs CTL; # P < 0.05, ## P < 0.01 vs SRS group; n = 7 mice per group.
Discussion
In this study, we found that combined treatment with fluoxetine and melatonin, which were administered at doses with no detectable antidepressant effect, attenuated sub-chronic restraint stress-induced depression-like behavior. Furthermore, the synergistic effect of combined treatment may be modulated by the normalization of hippocampal BDNF–TrkB signaling.
Restraint stress is a well-established animal model of depression [35], with the duration of stress exposure varying among different groups. Using the 6 h × 10 days restraint procedure, we induced depression-like behavior as shown by deteriorated fur state, increased latency to groom, and decreased number of grooming bouts in the splash test, and increased immobility time and decreased struggle time in the forced swim test in vehicle-treated mice. These results showed the validity of our stress paradigm. However, unlike many studies that report a reduction of body weight gain during or after chronic stress [36], we did not find a significant reduction of body weight gain during the sub-chronic stress period, similar to the results of Macedo et al. [37]. In addition, anxiety-related behavior and working memory remained undisturbed in sub-chronically stressed mice. It is likely that prolonged stress exposure is needed to reduce body weight gain, increase anxiety-related behavior, and impair working memory, which may also depend on the gender, age, and strain of the animals [38–40].
Fluoxetine is a commonly prescribed SSRI and is generally safe and well-tolerated in clinical use [8]. The effects of fluoxetine are dose- and time-dependent [41, 42]. Fluoxetine at a higher dose for a shorter duration [43] or at a lower dose for a longer duration [44] has more robust antidepressant effects. However, side-effects are present when higher doses are used [42]. In the current study, we found that fluoxetine at a relatively low dose (10 mg/kg) for a short duration (10 days) had no antidepressant effect, in line with previous studies [41]. Meanwhile, we also found that treatment with melatonin (10 mg/kg) alone had no effect on stress-induced depression-like behavior, consistent with human studies [45]. Although melatonin may restore the circadian rhythm in depression patients, it is not always effective in treating depression [14]. However, several animal studies have reported that exogenous melatonin has antidepressant effects [12, 14], which is inconsistent with our results. It is likely that the dose and/or the duration of fluoxetine and melatonin we used were not high or long enough to exert antidepressant effects. Nonetheless, we found that the combination of both drugs at doses that had no antidepressant effect when injected alone attenuated the deteriorated fur state, decreased the latency to groom, and increased the number of grooming episodes in the splash test, and decreased the immobility time and increased the struggle time in the forced-swim test, indicating that the two drugs interact to abolish the negative effects of sub-chronic restraint stress. In future studies, the underlying mechanisms of such synergistic antidepressant effects merit more detailed investigation.
It has been shown that both fluoxetine [46] and melatonin [47] modulate the level of circulating glucocorticoids. We found that plasma corticosterone levels at 30 min after acute severe stress (forced swim test) were inhibited by administering fluoxetine or melatonin alone. In addition, combined treatment also reduced the corticosterone levels after stress challenge. The mismatch between depression-like behavior and plasma corticosterone levels indicates that the antidepressant effects of drug treatment are independent of glucocorticoid levels.
The hippocampus and prefrontal cortex are key regions affected in depression [15]. BDNF–TrkB signaling in the hippocampus and prefrontal cortex is essential for stress adaptation, and their levels are regulated by antidepressants [16]. Accumulating evidence has implicated that stress or depression reduces BDNF–TrkB signaling in the hippocampus and prefrontal cortex, accompanied by reduced BDNF levels in the blood [16, 20]. Conversely, antidepressant treatments including SSRIs increase BDNF–TrkB signaling [16]. Furthermore, infusion of BDNF into the hippocampus has an antidepressant-like effect in animal models of depression, whereas mice lacking BDNF show blunted responses to antidepressants [20]. In line with previous findings, our data showed that hippocampal BDNF–TrkB signaling was suppressed after sub-chronic restraint stress [48]. Moreover, we found that the suppression of hippocampal BDNF–TrkB signaling was not prevented by administering fluoxetine or melatonin alone, but rather by combined fluoxetine and melatonin treatment. However, sub-chronic restraint stress and drug treatment did not alter the p-TrkB/TrkB ratio in the prefrontal cortex, and combined treatment failed to normalize prefrontal BDNF levels, findings inconsistent with previous studies [25]. This discrepancy may be ascribed to different stress paradigms. Taken together, these data suggest that the synergistic antidepressant effects of combined treatment may be mediated, at least in part, by normalizing BDNF–TrkB signaling in the stressed hippocampus. Further studies are needed to determine the molecular and cellular mechanisms underlying the modulation of hippocampal BDNF–TrkB signaling by combined fluoxetine and melatonin treatment.
The present study has a few limitations. First, it remains unclear whether the synergistic antidepressant effects of fluoxetine and melatonin are limited to the experimental paradigm we used. More evidence concerning such combined drug application on other animal models of depression and behavioral phenotypes should be provided in future studies. Second, to dissect the role of hippocampal BDNF–TrkB signaling in the therapeutic effects of combined fluoxetine and melatonin treatment, pharmacological and molecular approaches that can manipulate TrkB activation (e.g., the TrkB antagonist ANA-12) should be used.
In summary, our findings suggest that fluoxetine combined with melatonin at a relatively low dose alleviates depression-related behavioral abnormalities possibly by restoring hippocampal BDNF–TrkB signaling. These data may be helpful for optimizing the therapeutic strategy and dissecting the molecular mechanisms of depression.
Acknowledgements
We thank the Core Facilities of Zhejiang University Institute of Neuroscience for technical assistance. This work was supported by the National Natural Science Foundation of China (81471369) and Innovative Experiments on Physiology of Zhejiang University School of Medicine. The authors have no conflicting financial interests.
Footnotes
Kun Li and Si Shen have contributed equally to this work.
References
- 1.Seo JS, Zhong P, Liu A, Yan Z, Greengard P. Elevation of p11 in lateral habenula mediates depression-like behavior. Mol Psychiatry 2017. doi:10.1016/j.pbb.2017.02.004. [DOI] [PMC free article] [PubMed]
- 2.Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- 3.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/S0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 4.Cai S, Huang S, Hao W. New hypothesis and treatment targets of depression: an integrated view of key findings. Neurosci Bull. 2015;31:61–74. doi: 10.1007/s12264-014-1486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jans LA, Riedel WJ, Markus CR, Blokland A. Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol Psychiatry. 2007;12:522–543. doi: 10.1038/sj.mp.4001920. [DOI] [PubMed] [Google Scholar]
- 6.Blier P, El Mansari M. Serotonin and beyond: therapeutics for major depression. Philos Trans R Soc Lond B Biol Sci. 2013;368:20120536. doi: 10.1098/rstb.2012.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Licinio J, Wong ML. Depression, antidepressants and suicidality: a critical appraisal. Nat Rev Drug Discov. 2005;4:165–171. doi: 10.1038/nrd1634. [DOI] [PubMed] [Google Scholar]
- 8.Wong DT, Perry KW, Bymaster FP. Case history: the discovery of fluoxetine hydrochloride (Prozac) Nat Rev Drug Discov. 2005;4:764–774. doi: 10.1038/nrd1821. [DOI] [PubMed] [Google Scholar]
- 9.Kong H, Sha LL, Fan Y, Xiao M, Ding JH, Wu J, et al. Requirement of AQP4 for antidepressive efficiency of fluoxetine: implication in adult hippocampal neurogenesis. Neuropsychopharmacology. 2009;34:1263–1276. doi: 10.1038/npp.2008.185. [DOI] [PubMed] [Google Scholar]
- 10.Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. 2010;62:343–380. doi: 10.1124/pr.110.002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen MV, Danielsen AK, Hageman I, Rosenberg J, Gogenur I. The therapeutic or prophylactic effect of exogenous melatonin against depression and depressive symptoms: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2014;24:1719–1728. doi: 10.1016/j.euroneuro.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Comai S, Gobbi G. Unveiling the role of melatonin MT2 receptors in sleep, anxiety and other neuropsychiatric diseases: a novel target in psychopharmacology. J Psychiatry Neurosci. 2014;39:6–21. doi: 10.1503/jpn.130009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croxtall JD, Scott LJ. Olanzapine/Fluiloxietine A Review of its Use in Patients With Treatment-Resistant Major Depressive Disorder. CNS Drugs. 2010;24:245–262. doi: 10.2165/11203830-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Hickie IB, Rogers NL. Novel melatonin-based therapies: potential advances in the treatment of major depression. Lancet. 2011;378:621–631. doi: 10.1016/S0140-6736(11)60095-0. [DOI] [PubMed] [Google Scholar]
- 15.Yu H, Chen ZY. The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharmacol Sin. 2011;32:3–11. doi: 10.1038/aps.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22:238–249. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou D, Zhang Z, Liu L, Li C, Li M, Yu H, et al. The antidepressant-like effects of biperiden may involve BDNF/TrkB signaling-mediated BICC1 expression in the hippocampus and prefrontal cortex of mice. Pharmacol Biochem Behav. 2017;157:47–57. doi: 10.1016/j.pbb.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Nemeroff CB, Owens MJ. Treatment of mood disorders. Nat Neurosci. 2002;5(Suppl):1068–1070. doi: 10.1038/nn943. [DOI] [PubMed] [Google Scholar]
- 19.Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 21.Guo QH, Tong QH, Lu N, Cao H, Yang L, Zhang YQ. Proteomic analysis of the hippocampus in mouse models of trigeminal neuralgia and inescapable shock-induced depression. Neurosci Bull. 2017 doi: 10.1007/s12264-017-0131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu W, Mao Y, Wei D, Yang J, Du X, Xie P, et al. Structural asymmetry of dorsolateral prefrontal cortex correlates with depressive symptoms: evidence from healthy individuals and patients with major depressive disorder. Neurosci Bull. 2016;32:217–226. doi: 10.1007/s12264-016-0025-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gelman PL, Flores-Ramos M, Lopez-Martinez M, Fuentes CC, Grajeda JP. Hypothalamic-pituitary-adrenal axis function during perinatal depression. Neurosci Bull. 2015;31:338–350. doi: 10.1007/s12264-014-1508-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding K, Xu J, Wang H, Zhang L, Wu Y, Li T. Melatonin protects the brain from apoptosis by enhancement of autophagy after traumatic brain injury in mice. Neurochem Int. 2015;91:46–54. doi: 10.1016/j.neuint.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Ma M, Ren Q, Yang C, Zhang JC, Yao W, Dong C, et al. Adjunctive treatment of brexpiprazole with fluoxetine shows a rapid antidepressant effect in social defeat stress model: Role of BDNF–TrkB signaling. Sci Rep. 2016;6:39209. doi: 10.1038/srep39209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mineur YS, Prasol DJ, Belzung C, Crusio WE. Agonistic behavior and unpredictable chronic mild stress in mice. Behavior Genetics. 2003;33:513–519. doi: 10.1023/A:1025770616068. [DOI] [PubMed] [Google Scholar]
- 27.Wang XD, Labermaier C, Holsboer F, Wurst W, Deussing JM, Muller MB, et al. Early-life stress-induced anxiety-related behavior in adult mice partially requires forebrain corticotropin-releasing hormone receptor 1. Eur J Neurosci. 2012;36:2360–2367. doi: 10.1111/j.1460-9568.2012.08148.x. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt MV, Trumbach D, Weber P, Wagner K, Scharf SH, Liebl C, et al. Individual stress vulnerability is predicted by short-term memory and AMPA receptor subunit ratio in the hippocampus. J Neurosci. 2010;30:16949–16958. doi: 10.1523/JNEUROSCI.4668-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moretti M, Neis VB, Matheus FC, Cunha MP, Rosa PB, Ribeiro CM, et al. Effects of agmatine on depressive-like behavior induced by intracerebroventricular administration of 1-methyl-4-phenylpyridinium (MPP(+)) Neurotox Res. 2015;28:222–231. doi: 10.1007/s12640-015-9540-1. [DOI] [PubMed] [Google Scholar]
- 30.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioral despair in rats - new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 31.Regev L, Neufeld-Cohen A, Tsoory M, Kuperman Y, Getselter D, Gil S, et al. Prolonged and site-specific over-expression of corticotropin-releasing factor reveals differential roles for extended amygdala nuclei in emotional regulation. Mol Psychiatry. 2011;16:714–728. doi: 10.1038/mp.2010.64. [DOI] [PubMed] [Google Scholar]
- 32.Wang XD, Chen Y, Wolf M, Wagner KV, Liebl C, Scharf SH, et al. Forebrain CRHR1 deficiency attenuates chronic stress-induced cognitive deficits and dendritic remodeling. Neurobiol Dis. 2011;42:300–310. doi: 10.1016/j.nbd.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto K, Shimizu E, Iyo M. Critical role of brain-derived neurotrophic factor in mood disorders. Brain Res Brain Res Rev. 2004;45:104–114. doi: 10.1016/j.brainresrev.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Zhou WJ, Xu N, Kong L, Sun SC, Xu XF, Jia MZ, et al. The antidepressant roles of Wnt2 and Wnt3 in stress-induced depression-like behaviors. Transl Psychiatry. 2016;6:e892. doi: 10.1038/tp.2016.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griebel G, Simiand J, Steinberg R, Jung M, Gully D, Roger P, et al. 4-(2-Chloro-4-methoxy-5-methylphenyl)-N-[(1S)-2-cyclopropyl-1-(3-fluoro-4-methylp henyl)ethyl]5-methyl-N-(2-propynyl)-1, 3-thiazol-2-amine hydrochloride (SSR125543A), a potent and selective corticotrophin-releasing factor(1) receptor antagonist. II. Characterization in rodent models of stress-related disorders. J Pharmacol Exp Ther. 2002;301:333–345. doi: 10.1124/jpet.301.1.333. [DOI] [PubMed] [Google Scholar]
- 37.Macedo IC, Rozisky JR, Oliveira C, Oliveira CM, Laste G, Nonose Y, et al. Chronic stress associated with hypercaloric diet changes the hippocampal BDNF levels in male Wistar rats. Neuropeptides. 2015;51:75–81. doi: 10.1016/j.npep.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 38.Valles A, Marti O, Garcia A, Armario A. Single exposure to stressors causes long-lasting, stress-dependent reduction of food intake in rats. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1138–R1144. doi: 10.1152/ajpregu.2000.279.3.R1138. [DOI] [PubMed] [Google Scholar]
- 39.Di Liberto V, Frinchi M, Verdi V, Vitale A, Plescia F, Cannizzaro C, et al. Anxiolytic effects of muscarinic acetylcholine receptors agonist oxotremorine in chronically stressed rats and related changes in BDNF and FGF2 levels in the hippocampus and prefrontal cortex. Psychopharmacology (Berl) 2017;234:559–573. doi: 10.1007/s00213-016-4498-0. [DOI] [PubMed] [Google Scholar]
- 40.McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- 42.Wernicke JF. Safety and side effect profile of fluoxetine. Expert Opin Drug Saf. 2004;3:495–504. doi: 10.1517/14740338.3.5.495. [DOI] [PubMed] [Google Scholar]
- 43.Souza LC, Filho CB, Fabbro LD, de Gomes MG, Goes AT, Jesse CR. Depressive-like behaviour induced by an intracerebroventricular injection of streptozotocin in mice: the protective effect of fluoxetine, antitumour necrosis factor-alpha and thalidomide therapies. Behav Pharmacol. 2013;24:79–86. doi: 10.1097/FBP.0b013e32835efc2f. [DOI] [PubMed] [Google Scholar]
- 44.Samuels BA, Anacker C, Hu A, Levinstein MR, Pickenhagen A, Tsetsenis T, et al. 5-HT1A receptors on mature dentate gyrus granule cells are critical for the antidepressant response. Nat Neurosci. 2015;18:1606–1616. doi: 10.1038/nn.4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carman JS, Post RM, Buswell R, Goodwin FK. Negative effects of melatonin on depression. Am J Psychiatry. 1976;133:1181–1186. doi: 10.1176/ajp.133.10.1181. [DOI] [PubMed] [Google Scholar]
- 46.Tao W, Dong Y, Su Q, Wang H, Chen Y, Xue W, et al. Liquiritigenin reverses depression-like behavior in unpredictable chronic mild stress-induced mice by regulating PI3K/Akt/mTOR mediated BDNF/TrkB pathway. Behav Brain Res. 2016;308:177–186. doi: 10.1016/j.bbr.2016.04.039. [DOI] [PubMed] [Google Scholar]
- 47.Hoijman E, Rocha Viegas L, Keller Sarmiento MI, Rosenstein RE, Pecci A. Involvement of Bax protein in the prevention of glucocorticoid-induced thymocytes apoptosis by melatonin. Endocrinology. 2004;145:418–425. doi: 10.1210/en.2003-0764. [DOI] [PubMed] [Google Scholar]
- 48.Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]