Parkinson’s disease (PD) is recognized as the second most common neurodegenerative disorder after Alzheimer disease. Although a fascinating 200-year journey of research has revealed the multifaceted nature of PD [1, 2], its fundamental features are the loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) and depletion of dopamine (DA) in the striatum.
Iron accumulates in normal brains with aging. Such deposition has been reported to be exacerbated in acquired neurodegenerative disorders and in genetic neurological disorders such as neurodegeneration with brain iron accumulation and Friedreich ataxia [3]. Especially in PD, potential mechanisms have been offered to explain why iron metabolism is disturbed, as well as how elevated iron leads to dopaminergic neuronal degeneration [4]. However, it has been noted that this degeneration occurs in the SNpc, while other iron-rich areas remain unaffected. Meanwhile, dopaminergic neurons survive in the adjacent ventral tegmental area, where the iron levels are lower than in the SNpc. This emphasizes the close relationship between iron and DA, two key chemical components, and thus a toxic couple in the degeneration of dopaminergic neurons [5].
The revealed aspects of iron-DA coupling are largely derived from their pro-oxidant properties. As the central neurotransmitter involved in PD, DA is synthesized by tyrosine hydroxylase in dopaminergic neurons and then stored in synaptic vesicles ready for neurotransmission. The released DA is recycled into the cytoplasm by the DA transporter. DA easily forms toxic metabolites and these processes occur predominantly in the cytoplasm. Physiologically, H2O2 is produced by a DA enzymatic process via monoamine oxidase that converts DA to 3,4-dihydroxyphenylacetic acid and then homovanillic acid. Meanwhile, oxidative circumstances facilitate DA auto-oxidation and then the non-enzymatic catalytic production of o-quinones and quinones. Apart from the processes of biosynthesis and biodegradation, the free cytoplasmic DA is tightly restricted to a minimum by the modulation of trafficking from the presynaptic terminals via the DA transporter and synaptic vesicles via vestibular monoamine transporter 2. Accumulation of a high level of free cytoplasmic DA thus triggers DA-derived cytotoxicity. Although DA is depleted in the pathological progression of PD, it has to be noted that the cytotoxicity of free DA plays a part in the vulnerability of dopaminergic neurons.
Excess iron induces oxidative stress via the formation of hydroxyl radicals, leading to the peroxidation of DNA, proteins, and lipids. Recently, ferroptosis has been introduced and highlighted for triggering non-apoptotic cell death dependent on iron and reactive oxygen species. The induction of ferroptosis further leads to ferritinophagy (degradation of ferritin), which releases iron and in turn increases the labile cytoplasmic iron pool. This emphasizes the pro-oxidant nature of iron [6]. The elevated cytoplasmic iron elicits a hazardously pro-oxidant environment, although there are still arguments about whether this elevated iron is the primary insult or secondarily released from the degenerating neurons in PD. Iron becomes toxic in dopaminergic neurons by (1) reacting with H2O2 produced in the enzymatic processes of DA metabolism, and (2) accelerating the non-enzymatic catalytic processes and producing neurotoxic intermediates or the end-products O·−2 and ·OH. Hare et al. have proposed an alternative mechanism that the oxidation of DA by iron forms 6-hydroxydopamine (6-OHDA). As a commonly-used neurotoxin in PD models, 6-OHDA liberates iron from ferritin. The H2O2 produced in 6-OHDA metabolism in turn participates in the Fenton reaction with iron. The mutual relationships suggest that 6-OHDA formed in DA metabolism contributes to the iron and DA interactions. These biochemical changes and deleterious events eventually overwhelm compensatory antioxidant mechanisms and make the dopaminergic neurons in neurochemically distinct region extremely vulnerable, considering that these neurons contain high concentrations of both iron and DA.
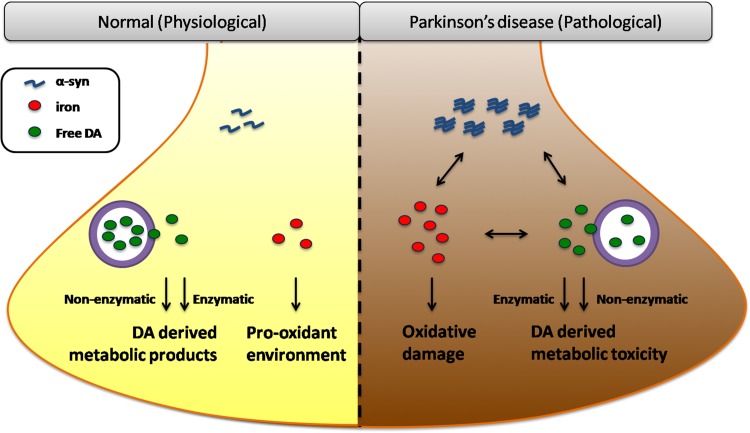
In terms of iron-DA coupling, another crucial player, α-synuclein (α-syn), has to be taken into account. Aggregated α-syn and deposited iron are present in Lewy bodies – the neuropathological hallmark of PD. Evidence of the close relationships between these two molecules is emerging. The structural links first come from the fact that iron binding to α-syn directly increases its aggregation and induces the formation of larger oligomers even at low micromolar concentrations. These conformational changes dramatically facilitate the tendency of α-syn to aggregate and thus impart the synergistic neurotoxicity of iron-α-syn coupling. An alternative structural link has been proposed following the report of an iron-responsive element in the α-syn 5′-untranslated region, indicating that α-syn expression is modulated by iron at the post-transcriptional level. Iron and α-syn interactions are also related to iron-induced oxidative stress and post-translational modification. In turn, overexpressed α-syn, acting as an intrinsic ferrireductase, increases the ferrous iron levels and induces susceptibility to the toxicity of the oligomers [7]. As for α-syn and DA interactions, α-syn physiologically regulates vesicle packing and DA release. However, in the disease state of PD, elevated or mutated α-syn impairs vesicle docking and recycling, preventing vesicular incorporation; these defects in handling cytoplasmic DA eventually result in the retention of a high concentration of free DA. On the other hand, DA promotes α-syn aggregation into oligomers and the subsequent cytotoxicity, and post-translational modifications of α-syn further regulate DA and iron transport [8]. Therefore, a cyclically accelerated process has been suggested in dopaminergic neurons displaying the characteristics high iron, abundant DA, and misfolded α-syn, which make them extremely susceptible to cell death (Fig. 1).
Fig. 1.
Iron, dopamine, and α-syn in dopaminergic neurons. Under physiological conditions, iron is essential for some biological processes, while contributing to a pro-oxidant environment in cells. DA is in balance between the free cytoplasmic form and the stored vesicular form and participates in both enzymatic and non-enzymatic metabolic processes. Alpha-syn functions in membrane-associated processes and regulates vesicular trafficking in its native form. In the disease state of PD, elevated iron elicits oxidative damage, accelerates DA-derived metabolic toxicity, and aggravates α-syn dysfunction by promoting its expression and aggregation. Alpha-syn-induced defects in vesicular incorporation retain free DA while overexpressed α-syn increases ferrireductase activity and ferrous iron levels. DA promotes α-syn aggregation and the metabolic products react with iron. These manifest a toxic vicious cycle among iron, DA, and α-syn in PD
Last but not least, the neuronal aspects of iron, dopamine, and α-syn interactions have been extensively discussed. Neurodegenerative processes trigger universal and conserved glial reactions in PD. Glia are extensively involved in brain iron metabolism as well as participating in the cell-to-cell transmission of α-syn. Astrocytes are able to take up DA and regulate its release, indicating their functions in managing DA levels [9]. The involvement of activated glia in iron metabolism, DA metabolism, and α-syn pathology implies that they play significant roles in the pathogenesis of PD. Selective degeneration occurs in neurons rich in iron, free DA, and aggregated α-syn. It may be that packaging these interactive molecules into a “safe” place, such as vesicles/compartments (considering the neuroprotective effects of vestibular monoamine transporter 2 and ferritin/mitochondrial ferritin), might be a promising strategy for preventing or rescuing the dopaminergic neurons at risk in PD.
Acknowledgements
This Research Highlight article was supported by grants from the National Natural Science Foundation of China (81430024, 31771124, 31571054, and 31371081), Excellent Innovative Team of Shandong Province and Taishan Scholars Construction Project. We thank Dr. Junichi Matsumoto for his assistance of graph preparation.
References
- 1.Przedborski S. The two-century journey of Parkinson disease research. Nat Rev Neurosci. 2017;18:251–259. doi: 10.1038/nrn.2017.25. [DOI] [PubMed] [Google Scholar]
- 2.Li S, Le W. Milestones of Parkinson’s Disease Research: 200 Years of History and Beyond. Neurosci Bull. 2017;33:598–602. doi: 10.1007/s12264-017-0178-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014;13:1045–1060. doi: 10.1016/S1474-4422(14)70117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang H, Wang J, Rogers J, Xie J. Brain Iron Metabolism Dysfunction in Parkinson’s Disease. Mol Neurobiol. 2017;54:3078–3101. doi: 10.1007/s12035-016-9879-1. [DOI] [PubMed] [Google Scholar]
- 5.Hare DJ, Double KL. Iron and dopamine: a toxic couple. Brain. 2016;139:1026–1035. doi: 10.1093/brain/aww022. [DOI] [PubMed] [Google Scholar]
- 6.Latunde-Dada GO. Ferroptosis: Role of lipid peroxidation, iron and ferritinophagy. Biochim Biophys Acta. 2017;1861:1893–1900. doi: 10.1016/j.bbagen.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Angelova DM, Jones HBL, Brown DR. Levels of alpha-and beta-synuclein regulate cellular susceptibility to toxicity from alpha-synuclein oligomers. FASEB J 2017. 10.1096/fj.201700675R [DOI] [PubMed]
- 8.Duce JA, Wong BX, Durham H, Devedjian JC, Smith DP, Devos D. Post translational changes to alpha-synuclein control iron and dopamine trafficking; a concept for neuron vulnerability in Parkinson’s disease. Mol Neurodegener. 2017;12:45. doi: 10.1186/s13024-017-0186-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cacace F, Mineo D, Viscomi MT, Latagliata EC, Mancini M, Sasso V, et al. Intermittent theta-burst stimulation rescues dopamine-dependent corticostriatal synaptic plasticity and motor behavior in experimental parkinsonism: Possible role of glial activity. Mov Disord. 2017;32:1035–1046. doi: 10.1002/mds.26982. [DOI] [PubMed] [Google Scholar]