Abstract
Medical and environmental microbiology have two distinct, although very short, histories stemming, the first from the pioneering works of Sommelweiss, Pasteur, Lister and Koch, the second mainly from the studies of Bejerink and Winogradsky. These two branches of microbiology evolved and specialized separately producing distinct communities and evolving rather different approaches and techniques. The evidence accumulated in recent decades indicate that indeed most of the medically relevant microorganisms have a short circulation within the nosocomial environment and a larger one involving the external, i.e. non-nosocomial, and the hospital environments. This evidence suggests that the differences between approaches should yield to a convergent approach aimed at solving the increasing problem represented by infectious diseases for the increasingly less resistant human communities. Microbial biofilm is one of the major systems used by these microbes to resist the harsh conditions of the natural and anthropic environment, and the even worse ones related to medical settings. This paper presents a brief outline of the converging interest of both environmental and medical microbiology toward a better understanding of microbial biofilm and of the various innovative techniques that can be employed to characterize, in a timely and quantitative manner, these complex structures. Among these, micro-Raman along with micro-Brillouin offer high hopes of describing biofilms both at the subcellular and supercellular level, with the possibility of characterizing the various landscapes of the different biofilms. The possibility of adding a taxonomic identification of the cells comprising the biofilm is a complex aspect presenting several technical issues that will require further studies in the years to come.
Keywords: Biofilm, Candida, Environmental microbiology, Medical microbiology, Micro-Brillouin, Micro-Raman, Yeasts
Introduction
Several opportunistic fungi are often found in food, environmental substrates and medical settings [1]. Of these, Aspergillus is probably one of the best known [2], although the problem involves several yeast species that can produce both exogenous and endogenous nosocomial infections [3]. The latter are carried out by strains already present in the hospital and propagating from one patient to another, possibly surviving on surfaces, devices and even operators’ hands [4, 5]. Although the isolates from hospitals appear to be more virulent than others [6], hospital conditions and therapies can produce relatively rapid selections producing a significant microevolution toward increased resistance to drugs [7] and to detergents [8]. One of the key factors in the circulation of yeasts from the environment to the patient, and potentially vice versa, is the biofilm. This structure is well known to impart increased resistance to the cells with a variety of mechanisms [9, 10].
In this paper, we propose the concept that biofilm is one of the most important factors to ensure the survival and also the dissemination of the fungal cells throughout different habitats, including those of medical and clinical environments. At the same time, we propose a novel way of characterizing biofilm through its metabolomics and mechanical characteristics, aiming at understanding their role in their stability on surfaces and the conditions allowing the delivering of large amounts of cells for the colonization of other surfaces. This article does not contain any studies with human participants or animals performed by any of the authors.
Opportunistic Yeasts are Present in Different Environments and Food
The idea that pathogenic and opportunistic species are typical of medical and clinical settings is well confirmed for species such as Candida albicans which can cause both endogenous and exogenous infections [3]. In contrast, other species can be isolated from both food and environmental samples. Probably the best known cases are the presence of Cryptococcus neoformans in pigeon droppings [11]. C. gattii has been found in soil, tree surfaces, air, and water in throughout the year [12]. Meyerozyma guilliermondii (formerly Candida guilliermondii), a species widely present in food [13] and on fruit [14], is also found in clinical settings. It has also been shown using different markers that fruit isolates are rather different from those deriving from environmental and clinical samples, outlining a kind of ongoing speciation [15]. M. guilliermondii has been found in cheese in association with C. parapsilosis, which is also frequently found on environmental samples together with its sibling species, C. orthopsilosis and C. metapsilosis [16]. In a recent unpublished survey, we found large amounts of C. parapsilosis on fruits in the Mediterranean area (Cardinali, personal communication).
Together, this evidence suggests that not only pathogenic bacteria [17] and molds [18] are isolated from food and environmental samples but also a large number of yeast species. Considering the expanding realm of pathogenic fungi, these observations suggest that even more environmental and food-borne yeasts will be likely enumerated among opportunistic species in the years to come.
Biofilm is Critical in Ensuring the Success of the Species
Invasive fungal infections cause more than 1.6 milliom deaths worldwide every year with a burden similar to tuberculosis, yet are the most understudied group of pathogens in microbiology [19]. The ability to produce biofilm is one of the risk factors for mortality in patients [20], and one of the most effective mechanisms for the resistance to drugs [20]. The mechanisms of the resistance of fungal planktonic and sessile cells are quite complex and numerous [21]. Among them, the outstanding efficacy of efflux pumps is being actively studied [22], whereas less is known about the mechanism, role and positioning within the biofilm of the “persister” cells. The situation is further complicated by the large variability due to the presence of five prominent species, in addition to C. albicans [23]. Moreover, the many recent cases caused by C. auris has led to the considering of this yeast as a worldwide threat and has enlarged the set of highly dangerous opportunistic yeasts of the genus Candida [24].
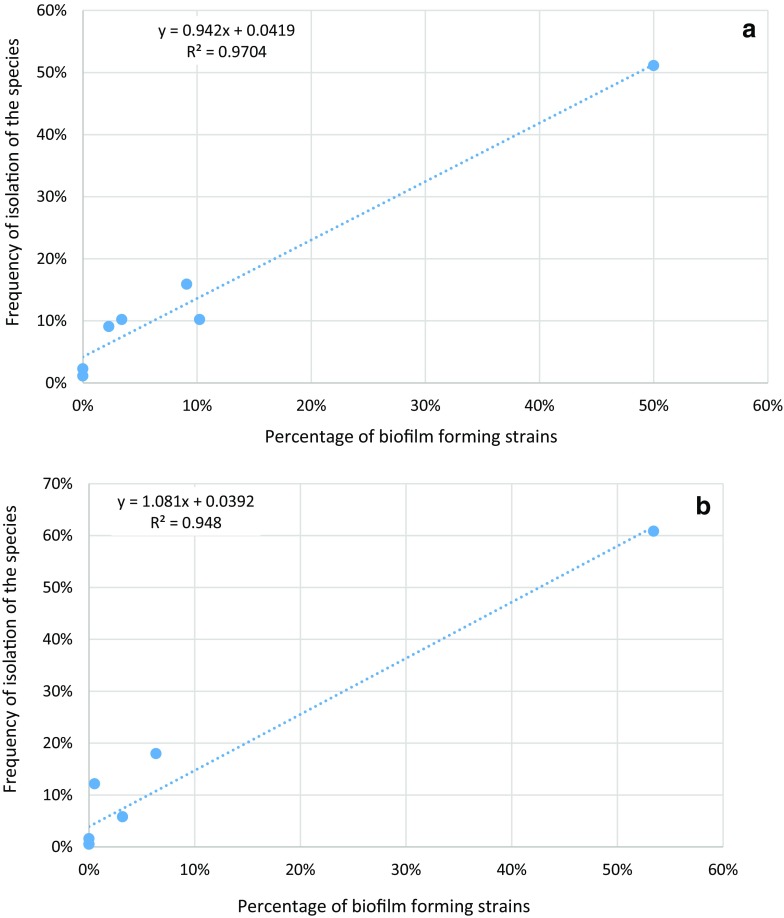
We have recently demonstrated that biofilm production is the key factor in the success of opportunistic yeasts in the hospital environment [25]. In fact, a linear relationship has been found between the percentage of biofilm-forming strains within each species and the frequency of isolation of that very species in the hospital (Fig. 1). Even more interestingly, the regression functions obtained with the isolates at the Pisa and Udine Hospitals in Italy are rather different (Fig. 1a vs. b) and both have a strong support (R2 = 0.97 and 0.95 for Pisa and Udine, respectively). This experimental evidence suggests that the permanence and spreading of yeast biofilms may be due to intrinsic characteristics, either of the specific hospital or of the biofilm structure of different strains and species. The second hypothesis calls for the setting up of an ad hoc system to evaluate the mechanical properties that could be instrumental in the ability of biofilms to remain attached to surfaces even in the presence of mechanical and chemical stresses such as those caused by the process of surface cleaning.
Fig. 1.
Regression between the percentage of biofilm forming strains and the frequency of isolation: a data from Pisa Hospital; b data from Udine Hospital
A New Characterization of Microbial Biofilm, Through Its Mechanical Parameters
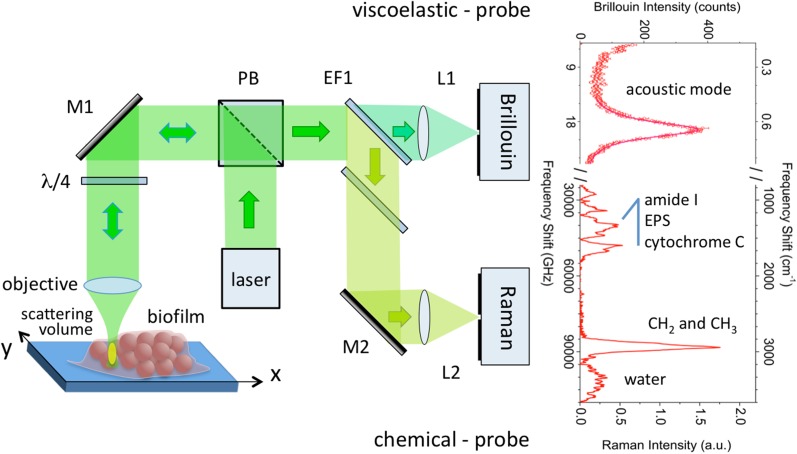
In order to meet the need for an accurate system to determine the mechanical properties of yeast biofilm, a set-up was recently realized to perform Brillouin–Raman micro-spectroscopy measurements [26] for the mechanical mapping with chemical specificity of biological samples [27–30] (Fig. 2). The Brillouin spectrum, with a peak at a frequency shift of about 18 GHz, shows the mechanical (viscoelastic) properties, while the relative intensities of the Raman peaks show the chemical composition inside the scattering volume. The square of the Brillouin frequency shift is proportional to the longitudinal elastic modulus, while the linewidth of the Brillouin peaks is related to the viscosity of the sample inside the scattering volume [28, 30]. In addition, when the thickness of the sample is lower than that of the scattering volume, the intensity of the Brillouin peak is a measure of the thickness of the sample (the thicker the sample, the more scattered the light).
Fig. 2.
Schematic of the set-up for Brillouin-Raman micro spectroscopy measurements [26], with (right side) typical Brillouin and Raman spectra from one single point of the microfilm. Light from a laser source is reflected by a polarizing beam splitter (PB) into a ×50 microscope objective lens and focused though a ~ 1 μm × 1 μm area of the sample. The light back-scattered by the (few micrometers thick) scattering volume is split by an edge filter (EF1), the high-frequency-shift component is sent to the Raman monochromator and the low-frequency shift is sent to the Fabry–Perot interferometer. The sample is mounted on top of a xyz translation stage for 1- and 2-D mapping of the mechanical and chemical properties of the sample. 3-D mapping is also possible, in the case of transparent samples. More details are reported in Ref. [26]
Linear Mapping of a C. albicans Biofilm
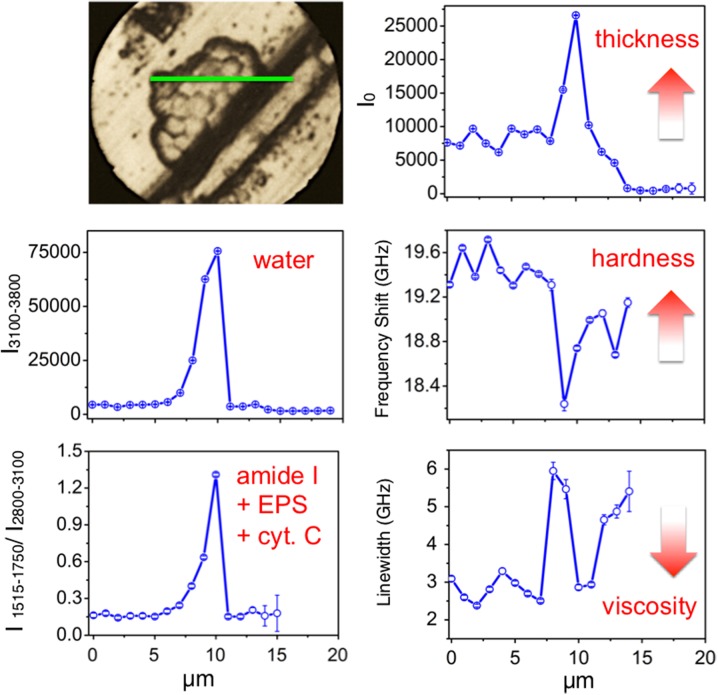
The morphology of the C. albicans biofilm grown over an aluminum substrate is visible in the photomicrograph of Fig. 3. Brillouin and Raman spectra have been recorded along a 20-μm linear region (green line in Fig. 2), with 1-μm steps. The intensity, frequency shift and width of the Brillouin lines are reported in the righthand panels of Fig. 2, showing, in the central region of the scan, a ~ 6% reduction of Brillouin frequency together with an increase by a factor of 2 in the linewidth. These features are correlated with an increase by a factor of 3 of the intensity, I0, suggesting that a three-layer structure of the biofilm exists in the central region. The joint Raman investigation (lefthand panels in Fig. 3) shows an order of magnitude increase of water content in the same region. On this basis, the combined reduction of stiffness and static viscosity can be attributed to the plasticizing effect associated with a local increase of residual water, as previously observed in dehydrated tissues [28]. These results (the larger the thickness, the more plastic the behavior, and the more intense the Raman signals) can be consistently interpreted in terms of a region in which a chemico-physical condition is preserved that is favorable to the survival of Candida cells. This hypothesis is reinforced by the corresponding increase of Raman signals in the 1510- to 1750-cm−1 region, which can be, at least partially, attributed to the resonant scattering from cytochrome c, a marker of cell vitality [31, 32]. This result is in line with the microbiological evidence that the biofilm acts as a structure which increases the resistance of the cells to challenging agents.
Fig. 3.
Photomicrograph and results of the analysis of Brillouin and Raman spectra of C. albicans on an aluminum substrate [26, 34]. The green line denotes a 20-μm-long region where Brillouin and Raman spectra were collected, using a 1-μm step size. Righthand panels Brillouin intensity I0, frequency shift and linewidth, proportional to thickness, hardness and viscosity, respectively. Lefthand panels Raman integrated intensity of the OH region (3100–3800 cm−1), and of amide I + EPS constituents + cytochrome c (1515–1750 cm−1) normalized to the intensity of the CH2 and CH3 stretching region (2800–3100 cm−1)
2D Mapping of C. parapsilosis Biofilm
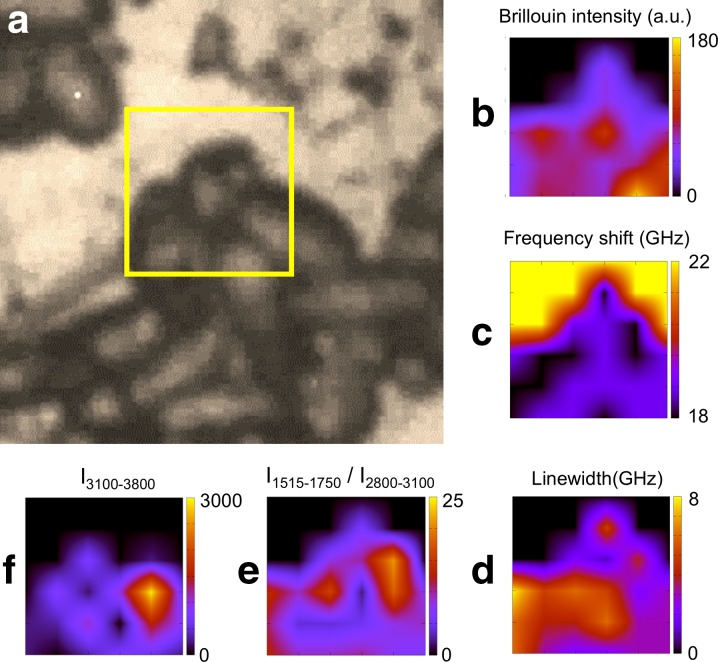
A C. parapsilosis biofilm grown on stainless steel was analyzed by our combined spectroscopic technique. Brillouin and Raman spectra have been recorded along a 6 μm × 6 μm map, with 1-μm steps in both directions (yellow square frame in Fig. 4a). The intensity of Brillouin lines (Fig. 4b) shows a maximum, twice the average, in the bottom right corner, indicating a two-layer morphology in that region. In this case, the maximum of residual water (Fig. 4f) is not located inside the buried cell but inside a cell participating in the single layered border of the biofilm. The 1515- to 1750-cm−1 Raman intensity (Fig. 4e), comprising the cytochrome c signal, is maximum in the same region, suggesting, as in the case of C. albicans, the presence of a cell which has survived to drying. In addition, the maximum of the 1515- to 1750-cm−1 Raman signal extends in a larger region, revealing the presence of the EPS component. In fact, superimposed on the micrograph, Fig. 4e is more intense in the interstitial region between yeast cells. The minimum of the Brillouin frequency shift and the maximum of the linewidth correlate well with both the more hydrated region and the region reaching more to the EPS, both of them corresponding to a softer viscoelastic behavior.
Fig. 4.
Photomicrograph and results of the analysis of Brillouin and Raman spectra of C. parapsilosis grown on stainless steel. a Yellow box a 6 μm × 6 μm region where Brillouin and Raman spectra were collected, using a 1-μm step-size in both directions. b Brillouin intensity, I0, c frequency shift and d linewidth. Raman integrated intensity: e of amide I + EPS constituents + cytochrome c (1515–1750 cm−1) and f of the OH region (3100–3800 cm−1)
Conclusion
Oportunistic yeasts are a serious threat to public health; their presence and circulation involves various environments and food beyond the medical settings. One of the key factors governing the success of these organisms is their ability to produce biofilms, complex structures with peculiar mechanical features. A micro-Raman coupled with a micro-Brillouin device, developed in our laboratories, was able to characterize some of these mechanical characteristics and will be used in future to define whether they have strain- or species-specific variability. These results corroborate previous findings of our working groups [26, 33], and similar results, although limited to just the Brillouin technique, have been obtained with the bacterium Pseudomonas aeruginosa [34]. Taken together, these findings indicate that the present technique has a huge potential in unravelling the mechanical properties of the complex structure of both bacterial and fungal biofilms.
Acknowledgements
Funding
This article was partly supported by a fellowship Gilead grant on the biofilm of C. parapsilosis and partly by a regional grant for seed safety, deployed by the Bavicchi Company. Luca Roscini was partially supported by a grant from Fondazione Cassa di Risparmio di Perugia. No funding or sponsorship was received for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Prior Presentation
Part of this paper was presented at the conference “Critically ill patients: from translational research to clinical management” (Kalamata–Greece June 17th, 2017) in the cooperation with the RAMAN4Clinics COST project.
Disclosures
In the past 2 years, Dr. Carlo Tascini has been paid for lectures on behalf of Pfizer, Novartis, Merck Astra, Angelini, Gilead and Astellas. In the past year, Prof. Gianluigi Cardinali won a Gilead fellowship grant for research on yeast biofilm. Drs. Luca Roscini, Alice Vassiliou, Laura Corte, Debora Casagrande Pierantoni, Vincent Robert, Sara Mattana, Martina Alunni Cardinali, Stylianos E. Orfanos and Daniele Fioretto have nothing to disclose.
Compliance with Ethics Guidelines
This article does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to 10.6084/m9.figshare.5878114.
References
- 1.Fridkin SK, Jarvis WR. Epidemiology of nosocomial fungal infections. Clin Microbiol Rev. 1996;9:499–511. doi: 10.1128/cmr.9.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.VandenBergh MFQ, Verweij PE, Voss A. Epidemiology of nosocomial fungal infections: invasive aspergillosis and the environment. Diagn Microbiol Infect Dis. 1999;34:221–227. doi: 10.1016/S0732-8893(99)00026-7. [DOI] [PubMed] [Google Scholar]
- 3.Gallè F, Catania M, Liguori G. Nosocomial Candida infections: epidemiology of candidaemia. J Prev Med Hyg. 2015;47:119–126. [PubMed] [Google Scholar]
- 4.Weinstein RA, Hota B. Contamination, disinfection, and cross-colonization: are hospital surfaces reservoirs for nosocomial infection? Clin Infect Dis. 2004;39:1182–1189. doi: 10.1086/424667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Traore O, Springthorpe V, Sattar S. A quantitative study of the survival of two species of Candida on porous and non-porous environmental surfaces and hands. J Appl Microbiol. 2002;92:549–555. doi: 10.1046/j.1365-2672.2002.01560.x. [DOI] [PubMed] [Google Scholar]
- 6.Sabino R, Sampaio P, Carneiro C, Rosado L, Pais C. Isolates from hospital environments are the most virulent of the Candida parapsilosis complex. BMC Microbiol. 2011;11:180. doi: 10.1186/1471-2180-11-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blasi E, Brozzetti A, Francisci D, Neglia R, Cardinali G, Bistoni F, et al. Evidence for microevolution in a clinical case of recurrent Cryptococcus neoformans meningoencephalitis. Eur J Clin Microbiol Infect Dis. 2001;20:535–543. doi: 10.1007/s100960100549. [DOI] [PubMed] [Google Scholar]
- 8.Kalkanci A, Elli M, Adil Fouad A, Yesilyurt E, Jabban Khalil I. Assessment of susceptibility of mould isolates towards biocides. Journal de Mycologie Médicale J Med Mycol. 2015;25:280–286. doi: 10.1016/j.mycmed.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Sanguinetti M, Posteraro B, Lass-Flörl C. Antifungal drug resistance among Candida species: mechanisms and clinical impact. Mycoses. 2015;58:2–13. doi: 10.1111/myc.12330. [DOI] [PubMed] [Google Scholar]
- 10.Sherry L, Rajendran R, Lappin DF, Borghi E, Perdoni F, Falleni M, et al. Biofilms formed by Candida albicans bloodstream isolates display phenotypic and transcriptional heterogeneity that are associated with resistance and pathogenicity. BMC Microbiol. 2014;14:182. doi: 10.1186/1471-2180-14-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costa AK, Sidrim JJ, Cordeiro RA, Brilhante RS, Monteiro AJ, Rocha MF. Urban pigeons (Columba livia) as a potential source of pathogenic yeasts: a focus on antifungal susceptibility of Cryptococcus strains in Northeast Brazil. Mycopathologia. 2010;169:207–213. doi: 10.1007/s11046-009-9245-1. [DOI] [PubMed] [Google Scholar]
- 12.Kidd SE, Chow Y, Mak S, Bach PJ, Chen H, Hingston AO, et al. Characterization of environmental sources of the human and animal pathogen Cryptococcus gattii in British Columbia, Canada, and the Pacific Northwest of the United States. Appl Environ Microbiol. 2007;7:1433–1443. doi: 10.1128/AEM.01330-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wrent P, Rivas E-M, de Prado EG, Peinado JM, de Silóniz M-I. Assessment of the factors contributing to the growth or spoilage of Meyerozyma guilliermondii in organic yogurt: comparison of methods for strain differentiation. Microorganisms. 2015;3:428–440. doi: 10.3390/microorganisms3030428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Cagno R, Cardinali G, Minervini G, Antonielli L, Rizzello CG, Ricciuti P, et al. Taxonomic structure of the yeasts and lactic acid bacteria microbiota of pineapple (Ananas comosus L. Merr.) and use of autochthonous starters for minimally processing. Food Microbiol. 2010;27:381–389. doi: 10.1016/j.fm.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 15.Corte L, di Cagno R, Groenewald M, Roscini L, Colabella C, Gobbetti M, et al. Phenotypic and molecular diversity of Meyerozyma guilliermondii strains isolated from food and other environmental niches, hints for an incipient speciation. Food Microbiol. 2015;48:206–215. doi: 10.1016/j.fm.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Silva AP, Miranda IM, Lisboa C, Pina-Vaz C, Rodrigues AG. Prevalence, distribution, and antifungal susceptibility profiles of Candida parapsilosis, C. orthopsilosis, and C. metapsilosis in a tertiary care hospital. J Clin Microbiol. 2009;47:2392–2397. doi: 10.1128/JCM.02379-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vantarakis A, Affifi M, Kokkinos P, Tsibouxi M, Papapetropoulou M. Occurrence of microorganisms of public health and spoilage significance in fruit juices sold in retail markets in Greece. Anaerobe. 2011;17:288–291. doi: 10.1016/j.anaerobe.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Brandi G, Sisti M, Paparini A, Gianfranceschi G, Schiavano GF, De Santi M, et al. Swimming pools and fungi: an environmental epidemiology survey in Italian indoor swimming facilities. Int J Environ Health Res. 2007;17:197–206. doi: 10.1080/09603120701254862. [DOI] [PubMed] [Google Scholar]
- 19.Nature Microbiology Editor. Stop neglecting fungi. Nat Microbiol. 2017;2:17120. [DOI] [PubMed]
- 20.Rajendran R, Sherry L, Nile CJ, Sherriff A, Johnson E, Hanson M, et al. Biofilm formation is a risk factor for mortality in patients with Candida albicans bloodstream infection—Scotland, 2012–2013. Clin Microbiol Infect. 2016;22:87–93. doi: 10.1016/j.cmi.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akins RA. An update on antifungal targets and mechanisms of resistance in Candida albicans. Med Mycol. 2005;43:285–318. doi: 10.1080/13693780500138971. [DOI] [PubMed] [Google Scholar]
- 22.Mukherjee PK, Chandra J, Kuhn DM, Ghannoum MA. Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols. Infect Immun. 2003;71:4333–4340. doi: 10.1128/IAI.71.8.4333-4340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silva S, Negri M, Henriques M, Oliveira R, Williams DW, Azeredo J. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol Rev. 2012;36(2):288–305. doi: 10.1111/j.1574-6976.2011.00278.x. [DOI] [PubMed] [Google Scholar]
- 24.Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis. 2016;64(2):134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corte L, Roscini L, Colabella C, Tascini C, Leonildi A, Sozio E, et al. Exploring ecological modelling to investigate factors governing the colonization success in nosocomial environment of Candida albicans and other pathogenic yeasts. Sci Rep. 2016;6:26860. doi: 10.1038/srep26860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scarponi F, Mattana S, Corezzi S, Caponi S, Comez L, Sassi P, et al. High-performance versatile setup for simultaneous Brillouin–Raman microspectroscopy. Phys Rev X. 2017;7(3):031015. [Google Scholar]
- 27.Traverso AJ, Thompson JV, Steelman ZA, Meng Z, Scully MO, Yakovlev VV. Dual Raman–Brillouin microscope for chemical and mechanical characterization and imaging. Anal Chem. 2015;87(15):7519–7523. doi: 10.1021/acs.analchem.5b02104. [DOI] [PubMed] [Google Scholar]
- 28.Palombo F, Madami M, Stone N, Fioretto D. Mechanical mapping with chemical specificity by confocal Brillouin and Raman microscopy. Analyst. 2014;139:729–733. doi: 10.1039/C3AN02168H. [DOI] [PubMed] [Google Scholar]
- 29.Palombo F, Madami M, Fioretto D, Nallala J, Barr H, David A, et al. Chemico-mechanical imaging of Barrett’s oesophagus. J Biophotonics. 2016;9:694–700. doi: 10.1002/jbio.201600038. [DOI] [PubMed] [Google Scholar]
- 30.Mattana S, Caponi S, Tamagnini F, Fioretto D, Palombo F. Viscoelasticity of amyloid plaques in transgenic mouse brain studied by Brillouin microspectroscopy and correlative Raman analysis. J Innov Opt Health Sci. 2017;10:1742001. doi: 10.1142/S1793545817420019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okada M, Smith NI, Palonpon AF, Endo H, Kawata S, Sodeoka M, et al. Label-free Raman observation of cytochrome c dynamics during apoptosis. Proc Natl Acad Sci USA. 2012;109:28–32. doi: 10.1073/pnas.1107524108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caponi S, Mattana S, Ricci M, Sagini K, Urbanelli L, Sassi P, et al. Raman micro-spectroscopy study of living SH-SY5Y cells adhering on different substrates. Biophys Chem. 2016;208:48–53. doi: 10.1016/j.bpc.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Mattana S, Cardinali MA, Caponi S, Pierantoni DC, Corte L, Roscini L, et al. High-contrast Brillouin and Raman micro-spectroscopy for simultaneous mechanical and chemical investigation of microbial biofilms. Biophys Chem. 2017;229:123–129. doi: 10.1016/j.bpc.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Karampatzakis A, Song CZ, Allsopp LP, Filloux A, Rice SA, Cohen Y, et al. Probing the internal micromechanical properties of Pseudomonas aeruginosa biofilms by Brillouin imaging. NPJ Biofilms Microbiomes. 2017;3:20. doi: 10.1038/s41522-017-0028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.