Abstract
Tropical peatlands, which play a crucial role in the maintenance of different ecosystem services, are increasingly drained for agriculture, forestry, peat extraction and human settlement purposes. The present study investigated the differences between natural and drained sites of a tropical peatland in the community structure of soil bacteria and archaea and their potential to perform nitrogen transformation processes. The results indicate significant dissimilarities in the structure of soil bacterial and archaeal communities as well as nirK, nirS, nosZ, nifH and archaeal amoA gene-possessing microbial communities. The reduced denitrification and N2-fixing potential was detected in the drained tropical peatland soil. In undisturbed peatland soil, the N2O emission was primarily related to nirS-type denitrifiers and dissimilatory nitrate reduction to ammonium, while the conversion of N2O to N2 was controlled by microbes possessing nosZ clade I genes. The denitrifying microbial community of the drained site differed significantly from the natural site community. The main reducers of N2O were microbes harbouring nosZ clade II genes in the drained site. Additionally, the importance of DNRA process as one of the controlling mechanisms of N2O fluxes in the natural peatlands of the tropics revealed from the results of the study.
Introduction
Nitrogen (N) is a naturally occurring element that is vital for all organisms, as N is component of proteins and nucleic acids. Therefore, the N cycle is one of the most important nutrient cycles in an ecosystem1. This cycle is driven by abiotic, decomposition, assimilative and dissimilative processes. The latter includes different microorganism-mediated pathways such as N fixation2, nitrification3,4, denitrification5,6, dissimilatory nitrate reduction to ammonium (DNRA)7 and anaerobic ammonium oxidation (ANAMMOX)8, as well as the newly described complete oxidation of ammonium to nitrate (comammox)9,10. The ongoing discovery of new processes and organisms involved in these complex systems widens our understanding of N cycling.
Tropical peatlands are particularly important in terms of nutrient cycling. Several recent studies suggest that far more peat exists in the tropics than was previously estimated11,12. The tropical peat area (ca 1.7 million km2), volume (ca. 7,268 km3) and carbon pool (350 Gt) may be more than three times larger from previous estimates and nearly a half of tropical peatlands can be found in South America12. Tropical peatlands play a crucial role in the maintenance of different ecosystem services, including sources for groundwater or surface water, biodiversity conservation, reduction of excess nutrient flows from surface waters and the sequestration and storage of atmospheric carbon13. Large areas of tropical peatlands (e.g., two-thirds of the Southeast Asian tropical peatlands) are drained for agriculture, forestry, peat extraction and human settlement purposes14. The drainage ditch network across the peatland regulates the soil water content to achieve the best conditions for different land use practices, which affect the distribution and supply of nutrients, such as carbon and N, and microbial community abundance and composition in peat15. Since the management of N is economically, ecologically and environmentally critical16,17, there is an emerging interest in understanding the ecology of microbes involved in N transformation processes in tropical ecosystems18,19.
The integration of soil conditions, soil microbial diversity and functional traits has proven to be an informative approach for exploring ecosystem responses to a changing environment20–22. Furthermore, understanding the link between changing environmental factors and microbial community dynamics provides insights into the N cycle in soil. Several studies have shown that the effect of environmental parameters on N cycling is dependent on the ecosystem type23–25. Although some research concerning the abundance and activity of microorganisms has been performed in tropical peatlands in Southeast Asia (reviewed by Hatano et al.18; Nurulita et al.26) and Central America27, studies focusing on microbial processes in South American tropical peatlands are lacking. Furthermore, modelling studies on greenhouse gas emissions, which are essential for estimations of these ecosystems’ impacts on climate change, are also limited due to the paucity of knowledge regarding microbial processes governing emissions from this region28.
We hypothesise that the drainage of a tropical peatland will significantly affect the soil microbial community structure and drive N transformation towards the prevalence of aerobic processes. The aim of this study was to assess the differences in the community structure of soil bacteria and archaea and their potential to perform different N transformation processes between natural and drained sites of a tropical peatland in French Guiana.
Results
Soil physicochemical conditions and N gas emissions from soil
The physicochemical conditions in the top 10-cm layer of soil were significantly different between the natural and drained site (Fig. 1a and Supplementary Table 1). The natural and drained sites differed in their N2O and N2 emission from soil (Fig. 2). The average N2O flux from the natural site was significantly lower (F = 6.98, p < 0.05) than that for the drained site. The N2 emission potential was highly variable in the 0- to 10-cm soil layers of both study sites, but it was significantly greater at the natural site (F = 6.76, p < 0.05).
Figure 1.
Characteristics of physicochemical and gene parameters in the natural and drained peatland sites. Principal components analysis (PCA) ordination plots with 95% confidence ellipses demonstrating the grouping of soil samples according to their physicochemical parameters (a) and target gene abundances (obtained by qPCR) (b). Abbreviations: SWC – soil water content, 16S bacteria – bacterial 16S rRNA gene, 16S archaea – archaeal 16S rRNA gene.
Figure 2.
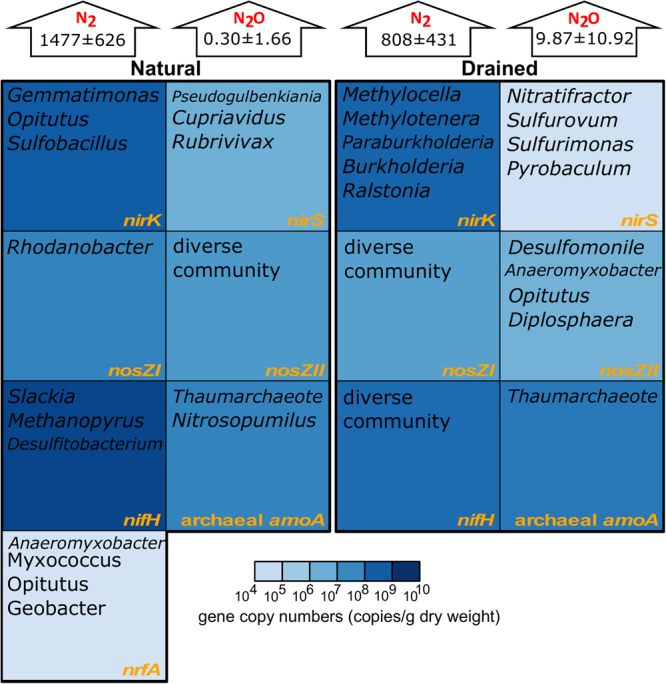
Comparison of the N-transforming microbial groups’ abundance and diversity between the top soil of natural and drained peatland sites. The most abundant microbial groups revealed by edge principal components analysis (edge PCA) (nirK, nirS, nosZ, nifH and archaeal amoA genes; Supplementary Fig. 1–5) and appropriately averaged phylogenetic tree (nrfA gene; Supplementary Fig. 6) are shown. Means and standard deviations (n = 9) of the measured nitrogen gases (N2 and N2O) emissions (μg m−2 h−1) are shown.
Soil microbial community abundance and phylogenetic composition
Quantitative PCR (qPCR) results showed that the proportion of bacteria and archaea in prokaryotic communities differed between the natural and drained soils (F = 83.51, p < 0.001, Supplementary Table 2). The archaeal abundance exceeded the bacterial abundance by more than one order of magnitude in the drained site, whereas at the natural site, the two were almost equally represented in the community. The total bacterial abundance was higher (F = 38.89, p < 0.001) and the archaeal abundance lower (F = 17.08, p < 0.01) at the natural site than at the drained site (Fig. 1b).
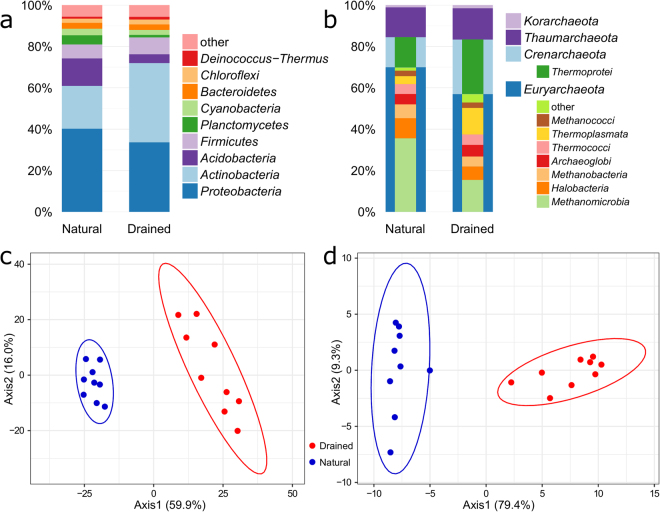
The structure of the bacterial and archaeal communities also differed between the natural and drained sites revealed from the analyses of metagenomes (Fig. 3). Overall, 32 different bacterial phyla were identified. The average proportions of nine dominant bacterial phyla were different by 0.1 to 17.5% between the sites (Fig. 3a) and statistical analysis confirmed significant difference (F = 32.46–760.22, p < 0.001 in all cases) between almost all of these phyla (except for Proteobacteria). The natural and drained sites also significantly differed in their bacterial genera composition (F = 53.86, p < 0.001; Fig. 3c, Supplementary Fig. 7), although Mycobacterium, Conexibacter, Burkholderia, Rhodoplanes, Pseudomonas and Paenibacillus were the dominant genera at both sites.
Figure 3.
Microbial community structures of the natural and drained peatland sites from metagenome. The dominant bacterial phyla (a) and archaeal phyla (column edges) and classes (column interior) (b) in soil samples. PCA ordination plots with 95% confidence ellipses demonstrating the grouping of soil samples according to the proportions of bacterial (c) and archaeal (d) genera.
The dominant archaeal phyla were Euryarchaeota, Crenarchaeota (class Thermoprotei), Thaumarchaeota and Korarchaeota at both study sites (Fig. 3b), although three of them were significantly different between sites (F = 11.75–216.26, p < 0.01 for Korarchaeota and p < 0.001 for Euryarchaeota and Crenarchaeota). At the natural site, more than half of the Euryarchaeota belonged to the class Methanomicrobia, whereas Methanomicrobia and Thermoplasmata were the most abundant euryarchaeal classes at the drained site. PCA of the archaeal genera showed that two distinct clusters were formed for both study sites (F = 134.70, p < 0.001; Fig. 3d). “Ca. Nitrosotenuis”, Nitrosopumilus, Thermococcus, Methanobacterium, “Ca. Nitrosopelagicus”, Pyrococcus, Methanobrevibacter, Methanocaldococcus, Geoglobus, Methanothermobacter, Ferroglobus and Halobacterium were among the most abundant genera at both study sites. A number of archaeal genera were differentially abundant between the natural and drained sites (Supplementary Fig. 8).
Combining the proportions obtained from metagenomes with 16S rRNA gene abundances (qPCR data), the obtained abundances of all dominant bacterial phyla were higher at the natural site and archaeal phyla at the drained site (Supplementary Fig. 9).
Abundance and diversity of N-transforming microbial groups in the study soils
nirS, nirK, nosZI, nosZII, nifH and archaeal amoA genes were detected in all studied soil samples, whereas nrfA genes were detected only in soil samples from the natural site (Figs 1b and 2, Supplementary Table 2). Bacterial amoA and ANAMMOX-specific 16S rRNA genes were not detected from either of the sites. These results were also confirmed by metagenomic analysis, in which neither hzsA (ANAMMOX) nor bacterial amoA/pmoA genes were detected in the study site samples, and nrfA genes were not detected in samples from the drained site.
A clear separation between the natural and drained sites was revealed by PCA according to the abundance of functional genes (Fig. 1b). The abundances of nirS, nosZI, nosZII and nifH were significantly higher in the natural site soil (F = 57.09–521.85, p < 0.001 for nirS, nosZI and nifH; F = 23.68, p < 0.01 for nosZII), and the archaeal amoA abundance was higher in the drained soil (F = 7.34, p < 0.05).
The proportions of nirS, nirK, nosZI, nosZII and nifH in prokaryotic communities were significantly higher in the natural site soil than in the drained site soil (F = 70.35–460.54, p < 0.001 in all cases), whereas the proportions of archaeal amoA appeared to be fairly similar at the natural and drained sites (Supplementary Table 2).
The levels of nirS/nirK and nosZ/nir were significantly higher (F = 72.56–104.05, p < 0.001 in both cases) at the natural sites than at the drained sites (Supplementary Table 2). The ratio of nosZI/nosZII ranged from 1.2 to 35.9 across the study sites, and this parameter was not significantly different between the two sites.
Metagenomic analysis revealed the presence of a comammox bacterium (“Ca. Nitrospira inopinata”) whose sequence abundance was 0.14–0.19% and 0.09–0.13% among all classified bacteria at the natural and drained sites, respectively. However, quantification of this group failed when using the available comammox Nitrospira specific primers due to unspecific amplification.
The proportion of studied nitrogen cycling genes reads in the metagenome dataset for natural and drained peatland sites were shown in Fig. 4. Based on the balanced weighted phylogenetic diversity (BWPD) index, we found that nirK (t = 5.55, p < 0.001), nirS (t = –3.25, p < 0.01), nosZ (t = 2.99, p < 0.01), nifH (t = 6.73, p < 0.001) and archaeal amoA (t = –6.37, p < 0.001) gene phylogenetic diversities were significantly different at the natural and drained sites (Supplementary Table 3). nosZ alpha diversity and potential of N2 emission were significantly positively correlated (R = 0.78, p < 0.05) at the natural site but nirS alpha diversity and potential of N2 emission were significantly negatively related (R = –0.82, p < 0.01) at the drained site.
Figure 4.
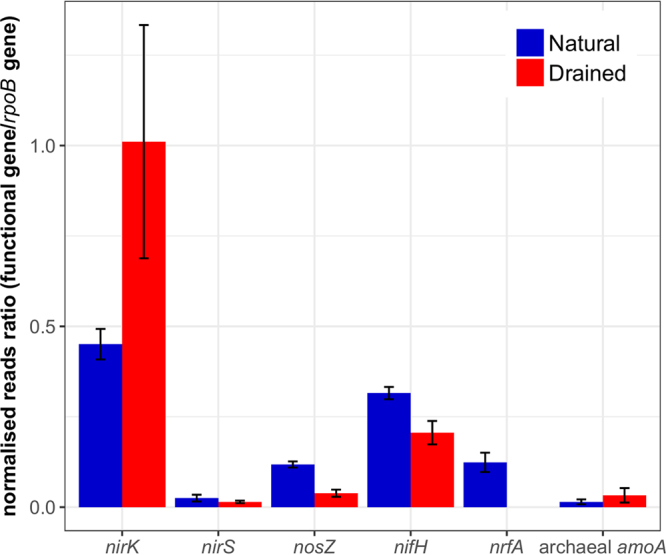
Average values (n = 9) and standard deviations of proportions of studied nitrogen cycling gene reads in the metagenome dataset for natural and drained peatland sites. Functional gene read abundances are shown as proportions of total rpoB (RNA polymerase) gene read abundance. Read counts were normalised according to gene length.
Edge PCA results indicate to variable extent grouping of soil samples according to sampling location for nirK, nirS, nosZ (clade I and II), nifH and archaeal amoA gene phylogenetic diversity (Figs 2 and 5). PERMANOVA (permutational multivariate analysis of variance) confirmed the significance of the differences in N-cycling microbial community structure between the natural and drained sites (F = 17.80–203.0, p < 0.001 in all cases). In all cases, the first principal component from the edge PCAs provided main separation of the samples.
Figure 5.
Ordination of soil samples for different N-transforming microbial groups in the natural and drained peatland sites. Edge PCA ordination plots with 95% confidence ellipses demonstrating the grouping of soil samples according to the diversity of nirK (Supplementary Fig. 1), nirS (Supplementary Fig. 2), nosZ (clade I and II) (Supplementary Fig. 3), nifH (Supplementary Fig. 4) and archaeal amoA (Supplementary Fig. 5) gene-possessing microbes.
For nirK-harbouring microorganisms, the edge PCA first principal component was related to the higher relative abundance of the genera Gemmatimonas, Opitutus and Sulfobacillus at the natural site, while the genera Methylocella, Methylotenera, Paraburkholderia, Burkholderia and Ralstonia were more abundant at the drained site (Supplementary Fig. 1). The diversity of nirS-harbouring microorganisms varied along the first PCA axis for the drained site. The difference in nirS-harbouring microbes between the natural and drained soils was primarily due to the higher contribution of the genera Pseudogulbenkiania, Cupriavidus and Rubrivivax at the natural site and of Nitratifractor, Sulfurovum, Sulfurimonas and Pyrobaculum at the drained site (Supplementary Fig. 2). A superposition of the nosZI-harbouring microbial community composition on the phylogenetic tree showed that the drained site has a diverse and heterogeneous community, while only the nosZI gene-possessing genus Rhodanobacter was abundant at the natural site (Supplementary Fig. 3). In contrast, the natural site possessed a diverse community of nosZII-harbouring microbes, while the drained site was colonised with only four genera: Desulfomonile, Anaeromyxobacter, Opitutus and Diplosphaera. The edge PCA of nifH-harbouring microorganisms indicated that the natural site had a higher abundance of Slackia, Methanopyrus and Desulfitobacterium, while the drained soil had a more diverse set of genera (Supplementary Fig. 4). The separation of amoA-harbouring archaea between the natural and drained sites occurred primarily along the edge PCA first principal component and was related to the higher abundance of Nitrosopumilus at the natural site; other differences were attributed to an uncultured thaumarchaeote (Supplementary Fig. 5). nrfA gene-possessing microbes were detected only at the natural site (mainly from the genera Anaeromyxobacter, Myxococcus, Opitutus and Geobacter) (Supplementary Fig. 6).
Similar patterns for community differences across the sites were observed for nirK, nirS, nosZ, nifH and archaeal amoA functional genes, as indicated by the Procrustes analysis, with highly significant goodness-of-fit measures (M2 ranging from 0.31 to 0.55; p < 0.001 in all cases, except between nirS and archaeal amoA genes (p < 0.01)) (Supplementary Table 4). Looking at the results of the beta diversity partitioning for N-transforming microbial groups (Supplementary Table 3), it appeared that in case of nirK gene-possessing microbes the replacement was dominating process, which accounted for 89.5% of the total beta diversity. In case of other studied N-cycle genes the replacement component contribution was still high but the richness difference accounted for one third or more of the total beta diversity.
Relationships between target genes, physicochemical parameters and gas emissions
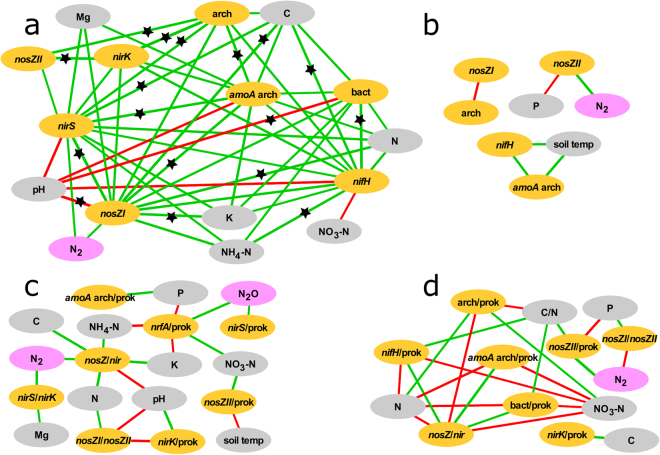
The data analysis detected several statistically significant relationships between target gene parameters and environmental factors, but the patterns of these relationships were different for the drained and natural sites (Fig. 6, Supplementary Tables 5 and 6). At the natural site, the archaeal 16S rRNA gene abundance was strongly related to most of the detected functional gene abundances, while only two significant correlations were found between gene abundances in the drained soil. The relationships between bacterial 16S rRNA gene abundance and nosZI and archaeal amoA and the particularly strong relationship with nifH abundance were revealed by a correlation analysis of the natural site. For the drained site, strong correlations between the bacterial and archaeal 16S rRNA proportions and the nifH and archaeal amoA proportions were detected. The nifH abundance was found to be related to most of the studied denitrification pathway genes at the natural site, while at the drained site, the proportion of nifH was related to nosZI and archaeal amoA proportion in the prokaryotic community. We did not detect any relationships between nrfA and other targeted genes for either of the study sites.
Figure 6.
Spearman correlation networks (p < 0.05) for target gene abundances (obtained by qPCR) (a – natural site, b – drained site) and target gene proportions and ratios (obtained by qPCR) (c – natural site, d – drained site). The pair wise Spearman correlation was calculated for each target gene parameter and environmental factor pair (exact R and p values are shown in Supplementary Tables 5 and 6). The Benjamini-Hochberg corrected (p < 0.05) Spearman correlations are specified with black stars. Green is used to represent positive relationships, and red indicates negative relationships; yellow stands for gene abundances, grey for soil physicochemical variables and pink for gaseous parameters. Abbreviations: soil temp – soil temperature, bact – bacterial 16S rRNA gene abundance, arch – archaeal 16S rRNA gene abundance, prok – prokaryotic community (total 16S rRNA gene proportion), N2 – potential N2 emission, N2O – N2O emission rate.
The soil C, N and C/N content was related to most of the gene parameters, but the effect differed between the two study sites. In addition, the pH had a strong effect on many gene parameters in the natural site soil, but this was not the case for the drained site. The nrfA proportion in the natural site community showed correlations with the soil chemical composition, while no correlations were found between this gene and environmental parameters in the drained soils.
Abundances and ratios of different denitrification genes were significantly related to the potential of N2 emission from the topsoil layer. The proportion of nirS and nrfA in the natural site microbial community correlated with N2O emission from the soil.
Distance-based regression analysis showed that soil chemical variables (especially NO3-N) explained significant amount of the variation in the functional gene-based microbial community structure (Table 1).
Table 1.
Results of a distance-based regression analysis showing soil physicochemical variables explaining the variation in target gene community structure (p < 0.05).
| Target gene | Physicochemical parameter | Variation explained (%) |
|---|---|---|
| nirK | Ca, NH4-N, NO3-N, K | 68.0 |
| nrfA | pH | 44.5 |
| nifH | NO3-N, Mg | 32.6 |
| nosZ | Ca, NO3-N, C, K | 26.4 |
| nirS | NO3-N, Mg | 26.1 |
| archaeal amoA | Ca, NO3-N | 12.0 |
Discussion
In this study, the effect of drainage on the phylogenetic composition of a microbial community and its genetic N transformation potential was linked for the first time to the site-specific characteristics of a tropical peatland. We applied a combined methodological approach by utilizing advantages of both methods (e.g., the broad-spectrum profile provided by metagenomics combined with higher sensitivity and quantitative capacity of qPCR). This approach could compensate for the weaknesses such as sequencing depth issues and adequacy of reference databases for metagenomic analysis29 or low-throughput, and limited availability and coverage of primers and nonspecific amplification for qPCR analysis30.
Our results show that the structure of a soil microbial community is significantly different in natural and drained sites of this ecosystem. At the natural site, the archaeal proportion was almost equal to the bacterial proportion in the prokaryotic community, while at the drained site, the proportion of archaea was notably higher.
Similar to most of the natural soils22,31, Proteobacteria, Actinobacteria, Acidobacteria and Firmicutes were among the most dominant bacterial phyla at both study sites, but the proportions of the most abundant phyla were not similar for the natural and drained sites.
Our results agree with a meta-analysis study, which found similar differences in bacterial phyla proportions (except Planctomycetes, whose proportion was increased) in tropical soils before and after the conversion of natural sites into agricultural systems31. Found at the natural site, the members of Acidobacteria, which have a broad range of transporters for the uptake of different substrates, have an advantage in complex environments and in adaptation to oligotrophic conditions32, and Proteobacteria, which are known to be important in the C, S and N cycles33, were more abundant. At the drained site, the proportion of Actinobacteria and Firmicutes, which are involved in the degradation and mineralisation of plant and humic materials in soil34–36, was higher compared to the natural site.
Our results highlight the importance of archaea, especially in a drained tropical peatland. Differences in the proportions of Euryarchaeota and Crenarchaeota between the natural and drained site were significant. The proportion of organic matter mineralising archaea (euryarchaeal classes Thermoplasmata, Archaeoglobi and Thermococci, as well as the phyla Crenarchaeota and Korarchaeota37) was approximately 20% higher in the drained soil, at the expense of the methanogenic euryarchaeal class Methanomicrobia. In addition, a difference between organisms performing the same methanogenic function (smaller proportion of Methanomicrobia and bigger proportion of Thermoplasmata) was detected at the drained site. Similar trends in the proportions of Methanomicrobia and Thermoplasmata were reported in an upper layer of peat (0–30 cm) in pristine and boreal ecosystems (i.e., bog, fen, spruce swamp forest) after long-term drainage38. The results of this study demonstrate that the proportion of the phylum Crenarchaeota involved in the S cycle (e.g., Caldivirga in sulphidogenesis and Sulfolobus and Metallosphaera in sulphide and S oxidation37) was twice as high in the drained peat compared to the natural peat.
Although the soil carbon content was about 5% lower compared to the natural, it was still high (around 30%) at the drained site. On the other hand, the organic matter quality was different from the natural site (indicated by C/N ratio) and more suitable for the archaeal metabolic activity at the drained site (confirmed by the significant negative relationship between proportion of archaea in the microbial community and C/N in soil). The lower ammonia content and higher nitrate concentration refer to the higher nitrification activity that is conducted by autotrophic nitrifying archaea whose proportion was also higher in the prokaryotic community of the drained site. One factor that can be related to the differences in archaeal (including ammonium-oxidising archaea (AOA)) abundances between the studied sites can be the more pronounced effect of physical factors such as temperature in upper layer of drained soils. Almost 2 °C higher temperature was recorded at the sampling time in the drained peat. Studies have shown that bacteria and archaea expressing similar activity can have different temperature preferences. Wu et al.39 showed that AOA have higher temperature optimum than ammonium-oxidising bacteria (AOB) and proved autotrophic growth of AOA under warmer experimental conditions.
We did not detect bacterial amoA in the studied tropical peatlands with the applied tools, while archaeal amoA genes were abundant at both study sites. These results are in agreement with Lu et al.40, who found a similar trend in acidic tea orchard and forest soils (pH 3.8–5.4). Because of the highly efficient anabolic pathways of AOA, which provide an ecological advantage compared to AOB in some environments41, AOA are known to be a dominant ammonia oxidiser in a wide range of soils19,42,43. Archaeal amoA gene diversity was substantially higher in the drained peatland soil and both replacement and to lesser extent richness difference contributed to dissimilarity in archaeal amoA communities between two study sites. We found higher abundance and proportion of different amoA-harbouring Thaumarchaeota members and lower proportion of Nitrosopumilus in the drained site soil. The study results also indicate that the soil chemical composition (including pH and C and N concentration) was strongly related to the abundance of AOA at the natural site. Soil pH has been shown to be a key factor for controlling the abundance and community composition of AOA, but many other soil characteristics (e.g., salinity, temperature, water, N, organic C and O content) may also affect the AOA community structure42,43.
Although the presence of a recently discovered complete nitrification (comammox) process has been already shown in several different ecosystems9,10,44, its ecological background remains unclear. In our study, the results from a metagenomic analysis suggest that this microbial group may be present in both tropical peatland soils.
Denitrification is shown to be a major microbial pathway for nitrate reduction and losses from soil in natural environments45,46. We focused on the key functional genes involved in the modular denitrification pathway, where either the nirS or nirK gene encodes nitrite reductase and the nosZ clade I or II gene encodes nitrous oxide reductase. Not all denitrifiers have a complete denitrification pathway47, and molecular studies have shown that the gene copy abundance of nir usually significantly exceeds that of nosZ in various environments24,48. We also found by applying qPCR that the balance between nosZ and nir genes was in favour of the latter genes at both study sites. In our study, the abundance and proportion of organisms harbouring the targeted denitrification genes (nirS, nosZI and nosZII) were higher in the drained soils; however, nirK-type denitrifiers were equally represented at both study locations. The abundance of nirS-type and nosZI-type denitrifiers showed a similar pattern in response to the edaphic factors in the natural soil. Our results are consistent with those of Stone et al.49, who found that the abundances of nirS and nosZ were positively correlated with soil C, N and P concentrations in humid tropical forests in Puerto Rico. The observed correlations suggest that nirS-type and nosZI-type denitrifiers play an important role in controlling N2O and N2 gas fluxes in the natural peatland soil, while microbes harbouring the nosZII gene more likely perform N2O transformation to N2 at the drained site. It could be assumed that the discrepancy between the denitrification communities of the two sites might be caused by a selective pressure of different environments in which two different N2O reductase mechanisms are preferred48. We cannot exclude that the obtained differences in N cycling genes’ abundances between the study sites could be partly related to the selected primers that could be more specific for members of the N-cycling microbial community at one of the study sites30,50.
The metagenomic data indicate that the community structure of organisms possessing either nir genes differed between the study sites. The nirK gene diversity was higher at the natural site and the dissimilarity between nirK possessing communities two locations was dominated by replacement process. The nirK-type denitrifiers belonging to the genera Gemmatimonas, Opitutus and Sulfobacillus were dominant in the natural site soil, while the genera Methylocella, Methylotenera, Paraburkholderia, Burkholderia and Ralstonia were predominant at the drained site. The nirS-type denitrifiers belonging primarily to Pseudogulbenkiania, Cupriavidus and Rubrivivax were observed at the natural site, while at the drained site their diversity was slightly higher. Nitratifractor, Sulfurovum, Sulfurimonas and Pyrobaculum nirS-type denitrifiers were dominant at the drained site. According to Graf et al.51, the majority of the abovementioned nirS-type denitrifiers also possess the nosZ gene, while only half of the listed nirK-type denitrifiers have the ability to reduce N2O. At the natural site, in addition to the diverse community of nosZII-harbouring microbes, nosZI gene-possessing genus Rhodanobacter was found to be abundant. The genus Rhodanobacter has been shown to be an important group of denitrifiers in acidic soils52. At the drained site, a diverse community of nosZI-harbouring organisms and nosZII-type denitrifiers (Desulfomonile, Anaeromyxobacter, Opitutus and Diplosphaera) were predominant. The results indicate that the soil C and N content significantly affects the denitrifying community structure in tropical peatland soil. In addition, denitrification ability has been described for several archaea including the genera observed in the studied tropical peatland (Haloferax, Halobacterium and Ferroglobus)53.
Another N reduction pathway, DNRA, is favoured in competition with denitrifiers under nitrate-limited conditions with suitable organic C source availability54. Depending on the environmental conditions, microbes performing DNRA may release N2O as a by-product of the reduction process or may reduce N2O produced by themselves or other microorganisms55. We detected nrfA-specific sequences (mainly from the genera Anaeromyxobacter and Myxococcus) at only the natural site. The importance of DNRA as one of the controlling mechanisms of N2O fluxes in the natural peatlands of the tropics can be assumed from the positive relationship between the nrfA proportion and N2O emission values. Templer et al.56 reported that DNRA rates were much higher than N2O production rates from denitrification (approximately 35% of gross nitrification) in a humid tropical forest soil in Puerto Rico.
In addition to DNRA, which is generally considered a process that conserves N in the ecosystem even though many microorganisms conducting DNRA also produce N2O54,55, biological N fixation (symbiotic and free-living) is another process that promotes N retention in soil25. Current evidence suggests that terrestrial ecosystems receive a critical N input from free-living diazotrophs, especially when the system includes a limited number of symbiotic N2-fixing plants (roots infected by Rhizobia, Bradyrhizobia or actinomycetes)57. In this study, free-living N2-fixing microbes were responsible for the differences in N-fixing microbial communities between the natural and drained sites. The soil nitrate was primary factor affecting this community structure. The metagenomic data indicate that the N2-fixer diversity was higher in the drained soil than in the natural soil, whereas the abundance and proportion of N2-fixing organisms (obtained by qPCR) was lower. Like all soil microorganisms, N2-fixers are affected by a wide variety of abiotic and biotic factors in different ecosystems (forests, grasslands, oceans, etc.) worldwide (reviewed by Reed et al.57). The results of the present study showed that only soil temperature is correlated with the abundance of N2-fixing organisms in a drained tropical peatland, while a number of different chemical parameters regulated the abundance of N2-fixers in a wet natural site. In addition to bacteria, N2 fixation is shown to be widespread among methanogenic Euryarchaeota53, which were significantly more abundant at the natural site.
Hu et al.58 obtained an enriched ANAMMOX culture from a N-loaded peat soil and showed some potential for ANAMMOX activity in the top 10-cm layer of peat. Nevertheless, neither metagenomic nor qPCR analyses detected ANAMMOX organisms from the studied tropical peatland sites, confirming that the ANAMMOX process plays only a minor role in most terrestrial soils59.
Our results indicate the coupling of several N transformation processes in the studied tropical peatland soil and a strong effect of soil drainage on the microorganisms performing these transformations. Nelson et al.60 suggested that the abundant soil microbial groups utilising a given N pathway usually support prokaryotes that can use other N pathways, and many microorganisms are able to perform multiple functions in the N cycle. For example, some microorganisms conducting DNRA are capable of N2O reduction to N2 because they possess the nosZ gene (mainly clade II)61. This study also demonstrated that the proportions of nrfA and nosZ clade II genes were positively correlated at the natural site. Additionally, numerous other positive relationships were revealed by the juxtaposition of the different microbial community abundances or proportions at the study sites, although the causes of these relationships are not yet entirely clear.
Analysis of N-cycle genes phylogenetic diversity in the current study is based on bioinformatics pipeline with the multiple steps. The results of each intermediate data analysis step have an inherent uncertainty and potentially impact the as-yet-unmeasured statistical significance of downstream analyses. Some of the studied N-cycle genes have highly conserved motifs separated by highly variable regions, and the short sequence limit of metagenome sequences might be insufficient for detecting a real hit from distant homologs that do not share the same function. We encountered this type of the problem of possible false positives with nirS and especially in case nirK gene (Supplementary Figs 1 and 2). In order to overcome this problem, improved bioinformatic data analysis methods are needed to reliably detect and quantify target genes in short-read metagenomes.
The results of this study show that modifications in the water regime of tropical peatlands may cause substantial shifts in the microbially mediated N cycle but more extensive studies are needed to confirm the revealed tendencies.
Methods
Site description, soil and gas sampling and analyses
The studied peatland is located in the northern region of French Guiana, where the average monthly temperature is approximately 26 °C, with little variation between seasons, and the average annual rainfall is 3000–4000 mm62. In situ measurements and soil sampling were performed at two sites of this peatland in October 2013 (dry season): a natural site located close to the village of Tonate (4°59'27“N, 52°27'14“W) and a drained site near the town of Kourou (5°09′42″N, 52°39′06″W). Further detailed information regarding the sampling sites, soil and gas sampling as well as analyses is provided in the Supplementary Information.
DNA extraction
Three parallel DNA extractions were performed from each sample (0.15 g of soil) with a PowerSoil DNA Isolation kit (MO BIO Laboratories Inc., Carlsbad, CA, USA) according to the manufacturer’s instructions. Homogenisation of samples was performed using a Precellys® 24 (Bertin Technologies, Montigny-le-Bretonneux, France) at 5000 rpm for 20 s. The Infinite M200 spectrophotometer (Tecan AG, Grödig, Austria) was used to determine quality and quantity of extracted DNA (Supplementary Table 7). The parallel extracts were pooled and stored at −20 °C.
Preparation of DNA libraries, sequencing and data processing
DNA samples were purified using a Genomic DNA Clean and Concentrator kit (Zymo Research, Irvine, CA, USA) according to the protocol provided by the manufacturer. Paired-end sequencing libraries were constructed for each sample using the TruSeq DNA PCR-Free Library Preparation kit (Illumina, San Diego, CA, USA) according to the manufacturer’s instructions. DNA concentrations of individual samples were quantified with a Qubit Fluorometer (Thermo Fisher Scientific Inc., MA, USA), and finally, the sample DNA was pooled in equal proportions. The library was sequenced using the NextSeq. 500 Illumina sequencing system (Illumina, San Diego, CA, USA). See the Supplementary Information and Supplementary Table 7 for further analysis details.
Quantitative PCR
qPCR was applied to evaluate the bacterial and archaeal community abundance by quantifying the abundances of specific 16S rRNA genes and to evaluate the genetic potential of the following N transformation processes by quantifying the respective functional genes: denitrification (nirS, nirK, nosZ clade I and nosZ clade II), N fixation (nifH), DNRA (nrfA), nitrification (bacterial and archaeal amoA), comammox (amoA clade A and clade B) and ANAMMOX (ANAMMOX-specific 16S rRNA genes). See the Supplementary Information and Supplementary Tables 8 and 9 for further details.
Statistical analyses
In all tests, statistical significance was determined at a 95% confidence level. For data analyses, samples were grouped according to the study sites: natural (n = 9) and drained (n = 9) soil groups. Separate PCAs were performed on soil physicochemical and microbiological data (gene copy numbers, proportions of bacterial and archaeal genera) using the R package ade4 v. 1.7–463. To evaluate the significance of the differences between study sites with respect to physicochemical variables, gene parameters, phylogenetic data (the log-ratio (clr) transformation64 and genetic marker rpoB gene was used for the normalisation of metagenomic reads) and emission values, multivariate linear models were constructed, and models were tested using the anova function in R package mvabund v. 3.11.965. Spearman’s rank correlation coefficients were used to assess the relationships between environmental factors and target gene abundances as well as the relationships between different gene abundances. The p-values were also adjusted for the false discovery rate by the Benjamini-Hochberg method with significance at p < 0.05. Spearman correlation networks were visualised with Cytoscape v. 3.4.066.
In case of each N-cycling gene, edge PCA was performed to detect differences between soil samples containing closely related taxa67, and graphics were produced with the R package ggplot2 v. 2.1.068 and the Archaeopteryx tree viewer v. 0.992069. nrfA phylogenetic tree with edges fattened in proportion to the number of reads was made for natural site by guppy tool from pplacer suite70. BWPD indices, which account for abundance and are robust to sampling depth differences between samples, were computed at theta 0.5 for each N-cycling gene to investigate the diversity of the soil community at the natural and drained sites (alpha diversity)71. Alpha diversity indices were compared between the natural and drained sites using t-test. The Spearman correlations were computed between alpha diversities and N gas emissions. To evaluate beta diversity among sites, we calculated percentage difference indices and decomposed beta diversity into its components of replacement and richness difference for each N-cycling gene using the R package adespatial v. 0.0-972.
For PCA and edge PCA, differences in microbial community structure between the natural and drained sites were evaluated using PERMANOVA with 9999 permutations, using the R package vegan v. 2.4-173. The same package was used to assess pairwise marker gene community structure concordance, and the ordination results of different edge PCAs were compared with a Procrustes rotation using the protest function with 9999 permutations74.
Distance-based regression analysis was applied using the DISTLM program75 with the forward selection procedure and 9999 permutations to identify soil physicochemical variables explaining variations in marker gene community structure.
Beta diversity evaluation, PERMANOVA and distance-based regression analysis based on Kantorovich-Rubinstein distance matrices, which were calculated by guppy tool from pplacer suite70.
Data availability
All raw sequencing reads have been deposited in the European Nucleotide Archive under the study accession number PRJEB21930. The data that support the findings of this study can be requested from M.E.
Electronic supplementary material
Acknowledgements
This was supported by the Estonian Research Council (grant IUT2-16); and the EU through the European Regional Development Fund through Centre of Excellence EcolChange and the European Social Fund (Doctoral School of Earth Sciences and Ecology). We would like to thank the PhD students participating in the field works.
Author Contributions
K.K. and M.M. performed the field sampling. M.E., T.L., H.N. and M.M. carried out the laboratory analysis. Ü.M. contributed to the financial support. M.E., J.T. and K.O. performed the data analyses. M.E., M.T. and J.T. wrote the paper but received feedback from all co-authors.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-23032-y.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thamdrup B. New Pathways and Processes in the Global Nitrogen Cycle. Annu. Rev. Ecol. Evol. Syst. 2012;43:407–428. doi: 10.1146/annurev-ecolsys-102710-145048. [DOI] [Google Scholar]
- 2.Zehr JP, Jenkins BD, Short SM, Steward GF. Nitrogenase gene diversity and microbial community structure: a cross-system comparison. Env. Microbiol. 2003;5:539–554. doi: 10.1046/j.1462-2920.2003.00451.x. [DOI] [PubMed] [Google Scholar]
- 3.Holmes AJ, Costello A, Lidstrom ME, Murrell JC. Evidence that particulate methane monooxygenase and ammonia monooxygenase may be evolutionary related. FEMS Microbiol.Lett. 1995;132:203–208. doi: 10.1111/j.1574-6968.1995.tb07834.x. [DOI] [PubMed] [Google Scholar]
- 4.Könneke M, et al. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature. 2005;437:543–546. doi: 10.1038/nature03911. [DOI] [PubMed] [Google Scholar]
- 5.Wrage N, Velthof GL, Van Beusichem ML, Oenema O. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem. 2001;33:1723–1732. doi: 10.1016/S0038-0717(01)00096-7. [DOI] [Google Scholar]
- 6.Shoun H, Fushinobu S, Jiang L, Kim S-W, Wakagi T. Fungal denitrification and nitric oxide reductase cytochrome P450nor. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2012;367:1186–1194. doi: 10.1098/rstb.2011.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tiedje, J. M. Ecology of denitrification and dissimilatory nitrate reduction to ammonium. Environ. Microbiol. Anaerobes 179–244 (1988).
- 8.Mulder A, van de Graaf AA, Robertson LA, Kuenen JG. Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor. FEMS Microbiol. Ecol. 1995;16:177–183. doi: 10.1111/j.1574-6941.1995.tb00281.x. [DOI] [Google Scholar]
- 9.Daims H, et al. Complete nitrification by Nitrospira bacteria. Nature. 2015;528:504–509. doi: 10.1038/nature16461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Kessel MAHJ, et al. Complete nitrification by a single microorganism. Nature. 2015;528:555–559. doi: 10.1038/nature16459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dargie GC, et al. Age, extent and carbon storage of the central Congo Basin peatland complex. Nature. 2017;542:86–90. doi: 10.1038/nature21048. [DOI] [PubMed] [Google Scholar]
- 12.Gumbricht, T. et al. An expert system model for mapping tropical wetlands and peatlands reveals South America as the largest contributor. Glob. Chang. Biol. 23, 3581–3599 (2017). [DOI] [PubMed]
- 13.Page SE, Rieley JO, Wüst R. Lowland tropical peatlands of Southeast Asia. Dev. Earth Surf. Process. 2006;9:145–172. doi: 10.1016/S0928-2025(06)09007-9. [DOI] [Google Scholar]
- 14.Parish, F. et al. Assessment on Peatlands, Biodiversity and Climate Change: Main Report (2008).
- 15.Limpens J, et al. Peatlands and the carbon cycle: from local processes to global implications – a synthesis. Biogeosciences. 2008;5:1475–1491. doi: 10.5194/bg-5-1475-2008. [DOI] [Google Scholar]
- 16.Galloway JN, et al. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science. 2008;320:889–892. doi: 10.1126/science.1136674. [DOI] [PubMed] [Google Scholar]
- 17.Gruber N, Galloway J. An Earth-system perspective of the global nitrogen cycle. Nature. 2008;451:293–296. doi: 10.1038/nature06592. [DOI] [PubMed] [Google Scholar]
- 18.Hatano, R. et al. In Tropical Peatland Ecosystems (eds. Osaki, M. & Tsuji, N.) 339–351 10.1007/978-4-431-55681-7 (Springer, 2016).
- 19.Pajares S, Bohannan BJM. Ecology of nitrogen fixing, nitrifying, and denitrifying microorganisms in tropical forest soils. Front. Microbiol. 2016;7:1–20. doi: 10.3389/fmicb.2016.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh BK, Bardgett RD, Smith P, Reay DS. Microorganisms and climate change: terrestrial feedbacks and mitigation options. Nat Rev Microbiol. 2010;8:779–790. doi: 10.1038/nrmicro2439. [DOI] [PubMed] [Google Scholar]
- 21.Graham EB, et al. Microbes as engines of ecosystem function: When does community structure enhance predictions of ecosystem processes? Front. Microbiol. 2016;7:1–10. doi: 10.3389/fmicb.2016.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Truu M, et al. Elevated Air Humidity Changes Soil Bacterial Community Structure in the Silver Birch Stand. Front. Microbiol. 2017;8:1–15. doi: 10.3389/fmicb.2017.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ligi T, et al. Characterization of bacterial communities in soil and sediment of a created riverine wetland complex using high-throughput 16S rRNA amplicon sequencing. Ecol. Eng. 2014;72:56–66. doi: 10.1016/j.ecoleng.2013.09.007. [DOI] [Google Scholar]
- 24.Ligi T, et al. The genetic potential of N2 emission via denitrification and ANAMMOX from the soils and sediments of a created riverine treatment wetland complex. Ecol. Eng. 2014;80:181–190. doi: 10.1016/j.ecoleng.2014.09.072. [DOI] [Google Scholar]
- 25.van Groenigen JW, et al. The soil N cycle: new insights and key challenges. Soil. 2015;1:235–256. doi: 10.5194/soil-1-235-2015. [DOI] [Google Scholar]
- 26.Nurulita Y, Adetutu EM, Gunawan H, Zul D, Ball AS. Restoration of tropical peat soils: The application of soil microbiology for monitoring the success of the restoration process. Agric. Ecosyst. Environ. 2016;216:293–303. doi: 10.1016/j.agee.2015.09.031. [DOI] [Google Scholar]
- 27.Sjögersten S, Cheesman AW, Lopez O, Turner BL. Biogeochemical processes along a nutrient gradient in a tropical ombrotrophic peatland. Biogeochemistry. 2011;104:147–163. doi: 10.1007/s10533-010-9493-7. [DOI] [Google Scholar]
- 28.Farmer J, Matthews R, Smith JU, Smith P, Singh BK. Assessing existing peatland models for their applicability for modelling greenhouse gas emissions from tropical peat soils. Curr. Opin. Environ. Sustain. 2011;3:339–349. doi: 10.1016/j.cosust.2011.08.010. [DOI] [Google Scholar]
- 29.Yang Y, Li B, Ju F, Zhang T. Exploring Variation of Antibiotic Resistance Genes in Activated Sludge over a Four-Year Period through a Metagenomic Approach. Environ. Sci. Technol. 2013;47:10197–10205. doi: 10.1021/es4017365. [DOI] [PubMed] [Google Scholar]
- 30.Bonilla-Rosso G, Wittorf L, Jones CM, Hallin S. Design and evaluation of primers targeting genes encoding NO- forming nitrite reductases: implications for ecological inference of denitrifying communities. Sci. Rep. 2016;6:1–8. doi: 10.1038/srep39208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trivedi P, Delgado-Baquerizo M, Anderson IC, Singh BK. Response of Soil Properties and Microbial Communities to Agriculture: Implications for Primary Productivity and Soil Health Indicators. Front. Plant Sci. 2016;7:990. doi: 10.3389/fpls.2016.00990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kielak AM, Barreto CC, Kowalchuk GA, van Veen JA, Kuramae EE. The ecology of Acidobacteria: Moving beyond genes and genomes. Front. Microbiol. 2016;7:1–16. doi: 10.3389/fmicb.2016.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kersters, K. et al. in The Prokaryotes (eds. Dwarkin, M., Falkow, S., Rosenberg, E., Schleifer, K.-H. & Stackebrandt, E.) 3–37, 10.1007/0-387-30741-9 (Springer New York, 2006).
- 34.Galperin MY. Genome Diversity of Spore-Forming. Firmicutes. Microbiol. Spectr. 2013;1:1–15. doi: 10.1128/microbiolspectrum.TBS-0015-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mandic-Mulec, I., Stefanic, P. & Elsas, J. D. V. Ecology of Bacillaceae. Microbiol. Spectr. 1–24 10.1128/microbiolspec.TBS-0017-2013 (2015). [DOI] [PubMed]
- 36.Lewin GR, et al. Evolution and Ecology of Actinobacteria and Their Bioenergy Applications. Annu. Rev. Microbiol. 2016;70:235–254. doi: 10.1146/annurev-micro-102215-095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Offre P, Spang A, Schleper C. Archaea in biogeochemical cycles. Annu. Rev. Microbiol. 2013;67:437–457. doi: 10.1146/annurev-micro-092412-155614. [DOI] [PubMed] [Google Scholar]
- 38.Urbanová Z, Bárta J. Effects of long-term drainage on microbial community composition vary between peatland types. Soil Biol. Biochem. 2016;92:16–26. doi: 10.1016/j.soilbio.2015.09.017. [DOI] [Google Scholar]
- 39.Wu Y, et al. Autotrophic Growth of Bacterial and Archaeal Ammonia Oxidizers in Freshwater Sediment Microcosms Incubated at Different Temperatures. Appl. Environ. Microbiol. 2013;79:3076–3084. doi: 10.1128/AEM.00061-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu L, et al. Nitrification of archaeal ammonia oxidizers in acid soils is supported by hydrolysis of urea. ISME J. 2012;6:1978–1984. doi: 10.1038/ismej.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Könneke M, et al. Ammonia-oxidizing archaea use the most energy-efficient aerobic pathway for CO2 fixation. Proc. Natl. Acad. Sci. 2014;111:8239–8244. doi: 10.1073/pnas.1402028111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hatzenpichler R. Diversity, physiology, and niche differentiation of ammonia-oxidizing archaea. Appl. Environ. Microbiol. 2012;78:7501–7510. doi: 10.1128/AEM.01960-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oton EV, Quince C, Nicol GW, Prosser JI, Gubry-Rangin C. Phylogenetic congruence and ecological coherence in terrestrial Thaumarchaeota. ISME J. 2015;10:85–96. doi: 10.1038/ismej.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pjevac, P. et al. AmoA-targeted polymerase chain reaction primers for the specific detection and quantification of comammox Nitrospira in the environment. bioRxiv 96891 10.1101/096891 (2017). [DOI] [PMC free article] [PubMed]
- 45.Fang Y, et al. Microbial denitrification dominates nitrate losses from forest ecosystems. Proc. Natl. Acad. Sci. USA. 2015;112:1470–1474. doi: 10.1073/pnas.1416776112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morse JL, et al. Soil denitrification fluxes from three northeastern North American forests across a range of nitrogen deposition. Oecologia. 2015;177:17–27. doi: 10.1007/s00442-014-3117-1. [DOI] [PubMed] [Google Scholar]
- 47.Zumft WG. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 1997;61:533–616. doi: 10.1128/mmbr.61.4.533-616.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones CM, Graf DRH, Bru D, Philippot L, Hallin S. The unaccounted yet abundant nitrous oxide-reducing microbial community: a potential nitrous oxide sink. ISME J. 2013;7:417–426. doi: 10.1038/ismej.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stone MM, Kan J, Plante AF. Parent material and vegetation influence bacterial community structure and nitrogen functional genes along deep tropical soil profiles at the Luquillo Critical Zone Observatory. Soil Biol. Biochem. 2015;80:273–282. doi: 10.1016/j.soilbio.2014.10.019. [DOI] [Google Scholar]
- 50.Gaby JC, Buckley DH. A Comprehensive Evaluation of PCR Primers to Amplify the nifH Gene of Nitrogenase. PLoS One. 2012;7:e42149. doi: 10.1371/journal.pone.0042149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Graf DRH, Jones CM, Hallin S. Intergenomic comparisons highlight modularity of the denitrification pathway and underpin the importance of community structure for N2O emissions. PLoS One. 2014;9:e114118. doi: 10.1371/journal.pone.0114118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van den Heuvel RN, van der Biezen E, Jetten MSM, Hefting MM, Kartal B. Denitrification at pH 4 by a soil-derived Rhodanobacter-dominated community. Environ. Microbiol. 2010;12:3264–3271. doi: 10.1111/j.1462-2920.2010.02301.x. [DOI] [PubMed] [Google Scholar]
- 53.Cabello P, Roldán MD, Moreno-Vivián C. Nitrate reduction and the nitrogen cycle in archaea. Microbiology. 2004;150:3527–3546. doi: 10.1099/mic.0.27303-0. [DOI] [PubMed] [Google Scholar]
- 54.Rütting T, Boeckx P, Müller C, Klemedtsson L. Assessment of the importance of dissimilatory nitrate reduction to ammonium for the terrestrial nitrogen cycle. Biogeosciences. 2011;8:1779–1791. doi: 10.5194/bg-8-1779-2011. [DOI] [Google Scholar]
- 55.Mania D, Heylen K, van Spanning RJM, Frostegård Å. The nitrate-ammonifying and nosZ-carrying bacterium Bacillus vireti is a potent source and sink for nitric and nitrous oxide under high nitrate conditions. Environ. Microbiol. 2014;16:3196–3210. doi: 10.1111/1462-2920.12478. [DOI] [PubMed] [Google Scholar]
- 56.Templer PH, Silver WL, Pett-Ridge J, DeAngelis KM, Firestone MK. Plant and microbial controls on nitrogen retention and loss in a humid tropical forest. Ecology. 2008;89:3030–3040. doi: 10.1890/07-1631.1. [DOI] [PubMed] [Google Scholar]
- 57.Reed SC, Townsend AR, Cleveland CC. Functional Ecology of Free-Living Nitrogen Fixation: A Contemporary Perspective. Annu. Rev. Ecol. Evol. Syst. 2011;42:489–512. doi: 10.1146/annurev-ecolsys-102710-145034. [DOI] [Google Scholar]
- 58.Hu BL, et al. New anaerobic, ammonium-oxidizing community enriched from peat soil. Appl. Environ. Microbiol. 2011;77:966–971. doi: 10.1128/AEM.02402-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Butterbach-Bahl, K. et al. Nitrogen processes in terrestrial ecosystems. Eur. Nitrogen Assess. 99–125, 10.1017/CBO9780511976988.009 (2011).
- 60.Nelson MB, Martiny AC, Martiny JBH. Global biogeography of microbial nitrogen-cycling traits in soil. Proc. Natl. Acad. Sci. 2016;113:8033–8040. doi: 10.1073/pnas.1601070113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sanford RA, et al. Unexpected nondenitrifier nitrous oxide reductase gene diversity and abundance in soils. Proc. Natl. Acad. Sci. 2012;109:19709–19714. doi: 10.1073/pnas.1211238109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cubizolle H, Mayindza Mouandza M, Muller F. Mires and histosols in French Guiana (South America): new data relating to location and area. Mires Peat. 2013;12:1–10. [Google Scholar]
- 63.Dray S, Dufour AB. The ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007;22:1–20. doi: 10.18637/jss.v022.i04. [DOI] [Google Scholar]
- 64.Gloor GB, Macklaim JM, Pawlowsky-Glahn V, Egozcue JJ. Microbiome Datasets Are Compositional: And This Is Not Optional. Front. Microbiol. 2017;8:1–6. doi: 10.3389/fmicb.2017.02224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y, Naumann U, Wright ST, Warton DI. Mvabund- an R package for model-based analysis of multivariate abundance data. Methods Ecol. Evol. 2012;3:471–474. doi: 10.1111/j.2041-210X.2012.00190.x. [DOI] [Google Scholar]
- 66.Shannon P, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsen FA, IV, Evans SN. Edge Principal Components and Squash Clustering: Using the Special Structure of Phylogenetic Placement Data for Sample Comparison. PLoS One. 2013;8:e56859. doi: 10.1371/journal.pone.0056859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wickham, H. ggplot2: Elegant Graphics for Data Analysis (Springer, 2016).
- 69.Han MV, Zmasek C. M. phyloXML: XML for evolutionary biology and comparative genomics. BMC Bioinformatics. 2009;10:356. doi: 10.1186/1471-2105-10-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Matsen FA, Kodner RB, Armbrust E. V. pplacer: linear time maximum-likelihood and Bayesian phylogenetic placement of sequences onto a fixed reference tree. BMC Bioinformatics. 2010;11:538. doi: 10.1186/1471-2105-11-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mccoy CO, Matsen FA., IV Abundance-weighted phylogenetic diversity measures distinguish microbial community states and are robust to sampling depth. PeerJ. 2013;1:e157. doi: 10.7717/peerj.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Legendre P. Interpreting the replacement and richness difference components of beta diversity. Glob. Ecol. Biogeogr. 2014;23:1324–1334. doi: 10.1111/geb.12207. [DOI] [Google Scholar]
- 73.Oksanen, J. et al. vegan: Community Ecology Package. (2016).
- 74.Peres-Neto PR, Jackson DA. How well do multivariate data sets match? The advantages of a procrustean superimposition approach over the Mantel test. Oecologia. 2001;129:169–178. doi: 10.1007/s004420100720. [DOI] [PubMed] [Google Scholar]
- 75.Mcardle BH, Anderson MJ. Fitting Multivariate Models to Community Data: A Comment on Distance-Based Redundancy Analysis. Ecology. 2001;82:290–297. doi: 10.1890/0012-9658(2001)082[0290:FMMTCD]2.0.CO;2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All raw sequencing reads have been deposited in the European Nucleotide Archive under the study accession number PRJEB21930. The data that support the findings of this study can be requested from M.E.