Abstract
Infection with pathogenic microbes often results in a significant inflammatory response. A cascade of proinflammatory cytokines including tumor necrosis factor α (TNF-α) and IL-1 initiates this response. Although there is a clear role for IL-1 during infection, little is known to distinguish the role of IL-1α from that of IL-1β during this process. With the use of Yersinia enterocolitica as a model enteric pathogen, we have identified a specific role for IL-1α in inducing pathologic inflammation during bacterial infection. Depletion of IL-1α in mice infected with wild-type Y. enterocolitica results in significantly decreased intestinal inflammation. Furthermore, a bacterial mutant that does not induce IL-1α expression but induces normal levels of IL-1β, TNF-α, and IFN-γ, causes greatly reduced intestinal inflammation and is attenuated by LD50 analysis in the C57BL/6 mouse model. These results demonstrate a distinct and unrecognized role for IL-1α in inducing intestinal inflammation that cannot be compensated for by the endogenous levels of IL-1β, TNF-α, or IFN-γ that are produced in response to Y. enterocolitica. Additionally, these results suggest that IL-1α-induced inflammation is a major contributor to the pathology of yersiniosis.
Yersinia enterocolitica (Ye) is a Gram-negative enteric pathogen that infects both humans and mice (1). Ye infection leads to an acute inflammatory disease, typically manifesting as a self-limiting infection of the gastrointestinal tract and the mesenteric lymph nodes, resulting in gastroenteritis and lymphadenitis. The bacteria are usually ingested with contaminated food or water and travel to the terminal ileum, where they attach to and invade through the M cells that overlay the Peyer's patches (PPs) (2). The bacteria then survive and replicate extracellularly in the PPs before dissemination to deeper tissues.
To survive in the tissues of the host animal, Ye, as well as several other Gram-negative enteric pathogens (Shigella and Salmonella), have developed complex strategies for the modulation and evasion of the host immune response. Many of the proteins responsible for the modulation of the host response are delivered to the host cell via a type III secretion system (3). For example, the ability of Ye to modulate the respiratory burst in vitro, as well as to resist phagocytosis by professional phagocytes, is the result of several Ye proteins that are delivered by the type III system. Despite this ability to modulate certain aspects of the immune response, a key feature of the pathology of a Ye infection is intestinal inflammation (4). Although it is likely that this inflammatory response is part of an attempt by the host to eliminate the pathogen, it may also contribute to the virulence of the organism. Thus, this inflammatory response may be a common virulence mechanism used by enteric pathogens, as intestinal inflammation is a common feature of the infectious process of these bacteria (5).
Recently it has become evident that in addition to the plasmid-encoded virulence genes, there are numerous virulence genes encoded on the Ye chromosome (6, 7). The global virulence regulator, RovA, is required for the expression of a variety of chromosomally encoded virulence genes including inv, which encodes the primary invasion factor invasin (8, 9). However, RovA does not appear to regulate the virulence plasmid-encoded effector genes that have been implicated in the modulation of the immune response to Ye infection (10).
As mentioned above, a common feature of the innate immune response to microbial pathogens is the localized production of proinflammatory cytokines, leading to the influx and activation of neutrophils and macrophages at the site of infection, resulting in inflammation. One of the most potent and pleiotropic proinflammatory cytokines is IL-1. There are two distinct forms of IL-1, designated IL-1α and IL-1β, which bind to the IL-1 receptor, eliciting responses ranging from the costimulation of T cells to anorexia, fever, and the induction of acute-phase responses (11). Because the two forms of IL-1 bind to the same receptor, it generally has been assumed that the two forms of the cytokine elicit similar responses (12). However, in this report we present evidence that IL-1α plays a distinct role in the induction of intestinal inflammation in the PPs in response to infection with the bacterial pathogen Ye. Furthermore, IL-1α expression appears to depend on the presence of a functional copy of the rovA gene. Interestingly, the defect in inflammation cannot be compensated for by IL-1β, tumor necrosis factor α (TNF-α), or IFN-γ, nor is the expression of these proinflammatory cytokines sufficient for the intestinal inflammatory response to a Ye infection.
Materials and Methods
Mice.
Six- to eight-week-old female C57BL/6, BALB/c, and 129SV/j mice were purchased from Charles River Breeding Laboratories and maintained in the barrier facility at Washington University School of Medicine. Mice were given free access to food and water throughout all experiments. Animals were killed by carbon dioxide asphyxiation. The Washington University committee on animal studies approved all animal experiments.
Reverse transcription–PCR was done as follows. PPs were excised and placed in RNAlater solution (Ambion, Austin, TX) until they were used. PP tissue was mechanically disrupted in Trizol solution (GIBCO/BRL) according to the manufacturer's instructions and then treated with DNase. Total RNA was treated with 20 units of RNase-free DNase (Roche Molecular Biochemicals) for 2 h at 37°C. Twenty-five micrograms of total RNA was reverse-transcribed with the use of Moloney murine leukemia virus reverse transcriptase and a random hexanucleotide. The product from the reverse-transcription reaction was then used in a PCR with TAQ DNA polymerase (Qiagen, Chatsworth, CA) and primers specific for the indicated cytokine: IL-1α, 5′-GATGCAAGCTATGGCTCACTTCATG; IL-1α 3′-GCAGCTGATGTGAAGTAGTTC; IL-1β, 5′-CTGTTCCTGAACTCAACTGTG; IL-1β, 3′- GGGTATTGCTTGGGATCCACA; IFN-γ, 5′-AGCGGCTGACTGAACTCAGATTGTAG; IFN-γ, 3′-GTCACAGTTTTCAGCTGTATAGGG; TNF-α, 5′- GGCAGGTCTACTTTGGAGTCATTGC; TNF-α, 3′-ACATTCGAGGCTCCAGTGAATTCGG; β-actin, 5′-TGGAATCCTGTGGCATCCATGAAAC, and β-actin, 3′-TAAAACGCAGCTCAGTAACAGTCCG. PCR products were separated by electrophoresis on a 1.5% agarose gel and then stained with ethidium bromide to visualize the DNA.
IL-1 Immunohistochemistry.
C57BL/6 mice were infected with 1 × 107 colony-forming units (cfu) for wild type and 1 × 109 cfu for the rovA mutant. Ye strains used in this study were derivatives of the serogroup 08 strain 8081. For more details about the strains see Revell and Miller (10) and the references therein. The rovA mutant used in this study (YVM 641) was produced by replacing the chromosomal copy of the rovA gene in our wild-type strain with an erythromycin cassette. The mice were then killed after 3 or 7 days, and the small intestines were removed, embedded in OCT compound, and flash-frozen. Sections were cut on a cryostat and fixed in methanol before staining. Tissues were rehydrated in PBS. Peroxidase activity was blocked by incubation with 0.3% hydrogen peroxide and then blocked with BSA and milk. Sections were incubated overnight with the indicated antibody at a concentration of 1 μg/ml. The antibodies used were hamster anti-IL-1α and anti-IL-1β mAb (mAb 161.1 and B122, respectively) (13, 14). Sections were washed with PBS and then incubated for 1 h with a biotinylated goat anti-hamster antibody (Pierce) in blocking buffer with 10% normal goat serum. Sections were washed in PBS and then incubated with 1 μg/ml streptavidin-horseradish peroxidase for 1 h. Sections were washed in PBS, and then positive staining was detected with the use of Tyramide Signal Amplification 3′ cyanine (DuPont/NEN) and fluorescence microscopy.
Peritoneal Macrophages.
C57BL/6 mice were injected i.p. with 3 ml of a sterile lipopolysaccharide-free 2% starch solution. After 4 days the mice were killed, and the peritoneal cavity was washed with DMEM containing 10% FCS. Peritoneal exudate cells were collected and plated in 6-well dishes at 107 cells per well. After 4 h the cells were extensively washed with PBS to remove the nonadherent cells and then maintained in RPMI medium 1640/10% FCS until they were used. Eighteen hours before they were used the peritoneal macrophages were activated with 100 units of recombinant IFN-γ (R & D Systems), and then the indicated bacteria were cocultured with the macrophages for 4 h before RNA extraction for reverse transcription–PCR. For assays involving the inducible rovA plasmid, cells were washed and then resuspended in RPMI-1640–glucose, supplemented with 0.2% arabinose and 10% FCS. The wild-type copy of the rovA gene was placed under the control of the PBAD promoter carried on the pBAD33 plasmid.
Immunodepletion of IL-1α.
C57BL/6 mice were injected i.p. with 200 μg of mAb 161.1 or irrelevant hamster IgG at day −1 and day +1.5 relative to the inoculation of the wild-type bacteria. On day 3 after infection the mice were killed, and the small intestines were removed. The lumen of the intestine was flushed with PBS and then fixed in 10% neutral buffered formaldehyde before being embedded in paraffin and stained with hematoxylin and eosin. Statistical significance (unpaired two-tailed P value) was calculated with the Mann–Whitney test.
Results and Discussion
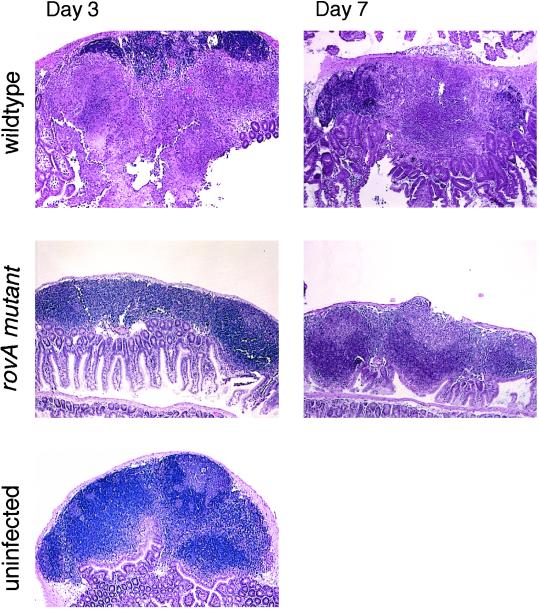
When mice were infected with high doses of the rovA mutant bacteria (1 × 109 cfu), gross examination of the intestines at necroscopy revealed very little intestinal inflammation as compared with mice infected with wild-type Ye (not shown). Histological examination of PPs from mice infected with rovA or wild-type Ye revealed significant differences in pathology (Fig. 1). During a wild-type Ye infection pathological changes were apparent by day 3; there was infiltration of both neutrophils and macrophages, initiation of granuloma formation, and changes in the architecture of the lymphoid follicle. By day 7 necrosis was usually evident in the mice infected with wild-type Ye, consistent with previous results (15, 16). In contrast, mice infected with the rovA mutant were indistinguishable from the baseline controls showing no detectable inflammation. The rovA mutant never showed necrotic changes at the time points investigated, even at day 7, when necrosis was common during the wild-type Ye infection. These results suggest that there is a fundamental difference in the inflammatory response to the rovA mutant.
Figure 1.
Histological examination of PPs after infection with Ye. C57BL/6 mice were orally infected with either wild-type Ye (1 × 107 cfu) or the rovA mutant (5 × 109 cfu) for the indicated amount of time. To visualize inflammation intestinal sections were stained with hematoxylin and eosin. As a control mice were mock-infected with PBS. (Original magnification: ×40.)
To further investigate the molecular basis of the differences in inflammatory response to the rovA mutant, mice were infected orally with either wild-type Ye or the rovA mutant. Then on days 1, 3, and 7, RNA was extracted from the PPs and subjected to reverse transcription–PCR, with primers specific for the proinflammatory cytokines IL-1α, IL-1β, TNF-α, and IFN-γ and, as a control, β-actin. The mice infected with the rovA mutant failed to induce IL-1α at any time point investigated (Fig. 2A). Expression of IL-1β, TNF-α, IFN-γ, and β-actin was comparable between mice infected with either the rovA mutant or wild-type Ye, ruling out a general defect in an innate immune response to bacterial infection. Interestingly, the differences in IL-1α expression were independent of invasin expression, as an inv mutant was indistinguishable from the wild-type Ye when tested as described above (not shown). Likewise, similar results were observed in BALB/c, 129SV/j, and C57BL/6 mice, suggesting that these results are not mouse strain-specific (not shown).
Figure 2.
Mice orally infected with the rovA mutant do not express IL-1α in PPs. (A) Reverse transcription–PCR of total RNA from the PPs of C57BL/6 mice orally infected with either wild-type Ye (1 × 107 cfu) or the rovA mutant (1 × 109 cfu) for 1, 3, or 7 days. Amplified DNA was separated on a 1.5% agarose gel and visualized with ethidium bromide staining. (B) Immunohistochemical staining of PPs from C57BL/6 mice orally infected with wild-type Ye or the rovA mutant as described above. PPs were stained with antibodies specific to IL-1α or IL-1β followed by detection with the use of tyramide signal amplification. PPs were visualized by fluorescence microscopy. (Original magnification: ×200.) The number of viable Ye in the PPs was determined by plating PP tissue extracts on Petri plates specific for Ye. Results are presented as cfu/g for the surviving mice in each group. (C) RovA will complement the defect in IL-1α expression. Peritoneal macrophages were obtained from C57BL/6 mice, activated with IFN-γ for 18 h and then cocultured with (lane 1) wild-type Ye, (lane 2) rovA mutant, or (lane 3) the rovA mutant carrying an inducible copy of the rovA gene on a plasmid for 4 h. RNA was extracted and treated as described for A.
To determine whether the differences in IL-1α mRNA levels correlated with differences in protein expression in vivo, PPs were examined immunohistochemically. Mice were infected orally with either the rovA mutant or wild-type Ye. On days 3 and 7, the mice were killed, and the small intestines were removed and prepared for immunohistochemistry. Tissue sections were then stained with antibodies specific for either IL-1α or IL-1β (mAb 161.1 and mAb B122, respectively) (13, 14). Mice infected with wild-type Ye showed positive staining with antibodies specific for IL-1α and IL-1β, whereas the PPs from mice infected with the rovA mutant only showed reactivity with the IL-1β antibody (Fig. 2B). Samples from uninfected mice (baseline control) did not show staining with either antibody (data not shown).
To determine the number of bacteria in the PPs of the mice, PPs were removed, and the number of cfu was determined. The rovA-infected mice were colonized with about two logs fewer bacteria than the wild-type infected mice, consistent with the difference in LD50 observed (Fig. 2B) (10). However, the differences in IL-1α expression appear to be independent of bacterial load in the doses tested (see below). These results show a correlation between mRNA and protein expression for IL-1α and IL-1β. Importantly, they also show that while there are dramatic differences in the levels of IL-1α in the PPs of the rovA and wild-type infected mice, the levels of IL-1β are comparable. Thus, the observed differences in inflammation during the infection with the rovA mutant correlate with differences in IL-1α levels.
The role of the rovA gene in the induction of IL-1α was further investigated in vitro. Because plasmid vectors are extremely unstable in vivo it was not possible to complement the rovA mutant in the mouse infection experiments. However, an in vitro assay was developed to test for complementation. When IFN-γ-activated peritoneal macrophages were cocultured with equal numbers of either wild-type Ye or the rovA mutant, results similar to those observed in vivo were obtained (Fig. 2C). Macrophages cocultured with wild-type Ye expressed IL-1α, whereas macrophages cocultured with the rovA mutant did not. Furthermore, the loss of IL-1α expression could be complemented; when the macrophages were cocultured with the rovA mutant carrying an inducible copy of the rovA gene on a plasmid, expression of IL-1α was restored. IL-1α expression levels obtained with the rovA complemented bacteria were comparable to those observed when the macrophages were cultured with wild-type Ye. The expression of TNF-α and IL-1β was not altered when activated macrophages were cultured with the rovA mutant versus the wild type (not shown), consistent with the in vivo data. These data suggest that differences in IL-1α expression levels are due to the loss of RovA and presumably the loss of one or more of the RovA regulated genes, which is an interesting result because, like the rovA mutant, the inv mutant shows decreased bacterial numbers in the PPs at early time points (17). Nevertheless, the inv mutant is capable of inducing wild-type levels of IL-1α (not shown), suggesting that a RovA-regulated gene other than invasin is responsible for the induction of IL-1α. Furthermore, these data suggest that the lower bacterial numbers encountered in the PPs during an inv mutant or a rovA mutant infection are sufficient to induce the expression of IL-1α.
To determine whether the failure to induce IL-1α could be responsible for any of the observed pathological and virulence phenotypes associated with the rovA mutation, the biological role of IL-1α in the inflammatory pathology of yersiniosis was tested in vivo. Mice were depleted of IL-1α with the use of an IL-1α-specific neutralizing mAb (mAb 161.1) and then infected with wild-type Ye. After 3 days the intestines were removed from the mice and prepared for histology. Mice depleted of IL-1α and infected with the wild-type Ye were indistinguishable from the rovA-infected mice and the baseline control mice when examined by hematoxylin and eosin staining of the PPs. These mice had no discernible intestinal inflammation when compared with the isotype control mice or the PBS-treated mice (P = 0.0014 and 0.08, respectively), suggesting that IL-1α is a required mediator of intestinal inflammation during a Ye infection (Fig. 3A). In the mAb161.1-treated mice and the rovA-infected mice, one mouse in 10 showed slight inflammation that correlated with 7% and 4%, respectively, of the total PPs examined for these mice (Fig. 3B). These mice had more polymorphonuclear leukocytes present in the PPs than the baseline controls. Nevertheless, these PPs did not show the extent of infiltration seen with a wild-type infection, nor were there any changes to follicular architecture or necrosis. The presence of polymorphonuclear leukocytes was not totally unexpected, because of the role of the PPs in intestinal immune surveillance. In contrast, mice infected with wild-type Ye that had been treated with an irrelevant isotype control antibody or mock-treated with PBS had significant intestinal inflammation (Fig. 3A). The wild-type infected PPs showed infiltration of both neutrophils and macrophages, changes to follicular architecture, as well as necrotic changes in several of the PPs (Fig. 3A). The pathological changes observed are consistent with changes observed in previous studies (15, 16). The majority of these mice had intestinal inflammation: the isotype control-treated group with 57% of the examined PPs showed inflammatory changes, and the PBS-treated group with 36% of the examined PPs showed inflammatory changes (Fig. 3B).
Figure 3.
IL-1α induces intestinal inflammation in response to Ye infection. (A) C57BL/6 mice were treated with an IL-1α neutralizing antibody 161.1, an irrelevant hamster isotype control antibody, or PBS. These mice were then infected orally with wild-type Ye (1 × 107 cfu). Mice were infected for 3 days and were then prepared for histology as described in Fig. 1. For comparison, PBS-treated mice were infected with the rovA mutant (1 × 109 cfu) and then processed in the same way as the mice treated with antibodies. (B) Quantification of the inflammatory response. PPs treated as described in A were examined in a blind fashion by three separate investigators. Ten mice were examined in two independent experiments of five mice each and scored for inflammation. If a mouse had an inflamed PP a shaded box represents it. The ratio of inflammed to noninflammed patches is presented. n = the total number of PPs examined for each experimental group.
The possibility that any changes in pathology due to IL-1α depletion were a result of an inability of the bacteria to colonize the PPs of IL-1α-depleted mice was ruled out as follows. Mice were treated with neutralizing antibody or control antibody as described above and then were infected with wild-type Ye for 3 days. The mice were killed, PPs were aseptically removed, and a single cell suspension was made and plated on Petri plates selective for Ye. Depletion of IL-1α did not have a significant effect on the numbers of bacteria recovered from the PPs of these mice. The mAb 161.1-treated group had an average bacterial load of 5 × 107 cfu/g of tissue, and the isotype control-treated mice had 3.5 × 107 cfu/g of tissue, suggesting that any differences observed in pathology at this time point were not due to differences in the numbers of colonizing bacteria. All together, these data indicate that IL-1α is a major mediator of intestinal inflammation during yersiniosis.
Intestinal inflammation is a common aspect of the pathology of several enteric bacterial pathogens, including Ye. Inflammation is typically mediated by proinflammatory cytokines, including IL-1 and TNF-α. Although there is a significant amount of data supporting the role of TNF-α in inducing an inflammatory response to bacterial pathogens, very little is known about the role of IL-1 in the host response to these pathogens (18). One important exception is the work done on Shigella flexneri, which demonstrates the importance of IL-1β in inducing inflammatory pathology in response to Shigella infection (19). It has also been known for several years that both IL-1α and IL-1β are expressed in the PPs of mice infected with Ye, but the relevance of the expression of these cytokines during infection was not examined (20).
Although the inflammatory response to enteric pathogens is clearly an attempt by the host to contain the bacterial infection, it may also help the bacteria gain access to deeper tissues. Neutrophils and macrophages are the predominant inflammatory cells involved in the early response to Ye infection. Neutrophils exert their antimicrobial effects by releasing granules that contain reactive oxygen and nitrogen compounds as well as proteases. All of these molecules cause coincidental tissue damage at the site of infection. Ye may exploit this damage to gain access to deeper tissues. For example, the lamina propria of PPs from mice infected with wild-type Ye is breeched, allowing access to both the mucosa and the lumen of the intestine (Figs. 1 and 3). Furthermore, it is not uncommon for the dome of the PP to be breeched at later points in infection, giving the bacteria access to the peritoneum and systemic infection (not shown). The defect the rovA mutant exhibits in inducing an inflammatory response may explain why the rovA mutant is defective in the colonization of the deeper tissues (10) when it should have an apparent survival advantage due to the decreased inflammatory response.
The exact mechanism for the observed differences in proinflammatory cytokine expression in response to Ye is not yet clearly understood. One possible model is that wild-type Ye has a general mechanism for the down-regulation of proinflammatory signals and that the bacteria produce a specific factor that induces IL-1α signaling leading to inflammation. It is clear that in vitro Ye is capable of modulating host-cell signaling cascades involved in inflammatory signaling. The YopJ/P protein is capable of blocking signaling through NF-κB, p38, c-Jun NH2-terminal kinase, and extracellular signal-regulated kinase in vitro, which leads to decreased levels of proinflammatory cytokine expression (21, 22). This model would partially explain the subtle virulence phenotype for the YopP protein in vivo (6). Presumably wild-type Ye induces IL-1α in a rovA-dependent manner, and, as mentioned above, IL-1α-induced inflammation is required for full virulence.
Until now very little data were available that distinguished specific roles for IL-1α and IL-1β in causing inflammatory disease. In this report we provide evidence that IL-1α is an essential mediator of Ye-induced intestinal inflammation. Results obtained with both a bacterial mutant and mice immunodepleted for IL-1α suggest a distinct role for IL-1α during a bacterial infection that has not been recognized. Significantly, IL-1β, TNF-α, and IFN-γ are not sufficient to induce the inflammatory changes observed during Ye infection. These data provide insight into the role of IL-1α in the inflammatory pathology of a Ye infection and may help in the understanding of the pathology of gastrointestinal disease caused by other enteric pathogens.
Acknowledgments
We thank Andrew Darwin and William Goldman for critical reading of the manuscript. Histological sections were prepared by the Washington University Digestive Diseases Research Core Facility, funded by Grant P30-DK52574 from the National Institutes of Health. We are thankful to Jacquie McDonough for expert assistance with immunohistochemistry. This study was supported by National Institutes of Health Grant AI27342 (to V.L.M.). D.D.C. is an Investigator of the Howard Hughes Medical Institute. P.H.D. was supported by a Keck postdoctoral fellowship and Infectious Diseases Training Grant AI07172–21. P.A.R. was supported by a Lucille P. Markey predoctoral fellowship and by Cellular and Molecular Biology Training Grant 5T32 GM07067.
Abbreviations
- Ye
Yersinia enterocolitica
- PP
Peyer's patch
- TNF-α
tumor necrosis factor α
- cfu
colony-forming units
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Carter P B. Infect Immun. 1975;11:164–170. doi: 10.1128/iai.11.1.164-170.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isberg R R, Leong J M. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 3.Cornelis G R, Wolf-Watz H. Mol Microbiol. 1997;23:861–867. doi: 10.1046/j.1365-2958.1997.2731623.x. [DOI] [PubMed] [Google Scholar]
- 4.West A. In: Pathology of Infectious Diseases. Conner D, Chandler F, Manz H, Baird K, Schwartz D, Lack E, Utz J, editors. Stamford, CT: Appleton and Lange; 1997. pp. 917–925. [Google Scholar]
- 5.Perdomo J, Cavaillon M, Huerre H, Ohayon P, Gounon P, Sansonetti P J. J Exp Med. 1994;180:1307–1319. doi: 10.1084/jem.180.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Darwin A J, Miller V L. Mol Microbiol. 1999;32:51–62. doi: 10.1046/j.1365-2958.1999.01324.x. [DOI] [PubMed] [Google Scholar]
- 7.Young G M, Miller V L. Mol Microbiol. 1997;25:319–328. doi: 10.1046/j.1365-2958.1997.4661829.x. [DOI] [PubMed] [Google Scholar]
- 8.Isberg R R, Falkow S. Nature (London) 1985;317:262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- 9.Miller V L, Falkow S. Infect Immun. 1988;56:1242–1248. doi: 10.1128/iai.56.5.1242-1248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Revell P A, Miller V L. Mol Microbiol. 2000;35:677–85. doi: 10.1046/j.1365-2958.2000.01740.x. [DOI] [PubMed] [Google Scholar]
- 11.Patarca R, Fletcher M A. Crit Rev Oncog. 1997;8:143–188. doi: 10.1615/critrevoncog.v8.i2-3.20. [DOI] [PubMed] [Google Scholar]
- 12.Dinarello C. Cytokine Growth Factor Rev. 1998;8:253–265. doi: 10.1016/s1359-6101(97)00023-3. [DOI] [PubMed] [Google Scholar]
- 13.Fuhlbrigge R, Sheehan K, Scrieber R, Chaplin D, Unanue E. J Immunol. 1988;141:2643–2650. [PubMed] [Google Scholar]
- 14.Shornick L, De Togni P, Mariathasan S, Goellner J, Strauss-Schoenberger J, Karr R, Ferguson T, Chaplin D. J Exp Med. 1996;183:1427–1436. doi: 10.1084/jem.183.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pepe J C, Wachtel M R, Wagar E, Miller V L. Infect Immun. 1995;63:4837–4848. doi: 10.1128/iai.63.12.4837-4848.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Autenrieth I B, Hantschmann P, Heymer B, Heesemann J. Immunobiology. 1993;187:1–16. doi: 10.1016/S0171-2985(11)80241-X. [DOI] [PubMed] [Google Scholar]
- 17.Pepe J C, Miller V L. Proc Natl Acad Sci USA. 1993;90:6473–6477. doi: 10.1073/pnas.90.14.6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Autenrieth I B, Heesemann J. Med Microbiol Immunol. 1992;181:333–338. doi: 10.1007/BF00191545. [DOI] [PubMed] [Google Scholar]
- 19.Sansonetti P, Arondel J, Cavaillon J, Huerre M. J Clin Invest. 1995;96:884–892. doi: 10.1172/JCI118135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beuscher H U, Rausch U-P, Otterness I G, Röllinghoff M. J Exp Med. 1992;175:1793–1797. doi: 10.1084/jem.175.6.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orth K, Palmer L E, Bao Z Q, Stewart S, Rudolph A E, Bliska J B, Dixon J E. Science. 1999;285:1920–1923. doi: 10.1126/science.285.5435.1920. [DOI] [PubMed] [Google Scholar]
- 22.Palmer L E, Pancetti A R, Greenberg S, Bliska J B. Infect Immun. 1999;67:708–716. doi: 10.1128/iai.67.2.708-716.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]