Abstract
Background
Ethnic variability in the pharmacokinetics of organic anion transporting polypeptide (OATP) 1B1 substrates has been observed, but its basis is unclear. A previous study hypothesizes that, without applying an intrinsic ethnic variability in transporter activity, allele frequencies of transporters cannot explain observed ethnic variability in pharmacokinetics. However, this hypothesis contradicts the data collected from compounds that are OATP1B1 substrates but not breast cancer resistance protein (BCRP) substrates.
Objective
The objective of this study is to evaluate a hypothesis that is physiologically reasonable and more consistent with clinical observations.
Methods
We evaluated if allele frequencies of two transporters (OATP1B1 and BCRP) are key contributors to ethnic variability. In this hypothesis, the same genotype leads to the same activity independent of ethnicity, in contrast to the previous hypothesis of intrinsic ethnic variability in OATP1B1 activity. As a validation, we perform mechanistic pharmacokinetic modeling for SLCO1B1 (encoding OATP1B1) and ABCG2 (encoding BCRP) genotyped pharmacokinetic data from 18 clinical studies with healthy Caucasian and/or Asian subjects.
Results
Simulations based on the current hypothesis reasonably describe SLCO1B1 and ABCG2 genotyped pharmacokinetic time course data for five transporter substrates (atorvastatin, pitavastatin, pravastatin, repaglinide, and rosuvastatin) in Caucasian and Asian populations.
Conclusion
This hypothesis covers the observations that can (e.g., ethnic differences in rosuvastatin pharmacokinetics) or cannot (e.g., lack of differences for pitavastatin pharmacokinetics) be explained by the previous hypothesis. It helps to characterize sources of ethnic variability and provides a foundation for predicting ethnic variability in transporter substrate pharmacokinetics.
Electronic supplementary material
The online version of this article (doi:10.1007/s40262-017-0568-7) contains supplementary material, which is available to authorized users.
Key Points
| Ethnic variability in the pharmacokinetics of organic anion-transporting polypeptide (OATP) 1B1 substrates has been observed for some drugs, but the basis of the differences has been unclear. A previous hypothesis proposing intrinsic ethnic variability in activity was inconsistent with data collected from compounds that are OATP1B1 substrates but not breast cancer resistance protein (BCRP) substrates (e.g., pitavastatin and repaglinide). |
| We evaluated an alternative hypothesis for ethnic variability in the pharmacokinetics of transporter substrates based upon allelic frequencies of both OATP1B1 and BCRP. Simulations based on this hypothesis can reasonably describe OATP1B1 or BCRP genotyped pharmacokinetic time course data in Caucasian, Chinese, Japanese, and Korean populations. |
| The hypothesis and mathematical model developed in this study provide insights into the sources of ethnic variability and provide a foundation for prospectively predicting ethnic variability in the pharmacokinetics of transporter substrates. |
Introduction
Many efforts have been undertaken in the past decade to understand the roles played by transporters in drug pharmacokinetics, pharmacodynamics, and toxicity. Their importance in drug absorption, disposition, and elimination has been recognized by academia, industry, and regulatory agencies. Among drug transporters, organic anion transporting polypeptide (OATP) 1B1, a hepatic uptake transporter encoded by gene SLCO1B1, has probably received the most attention. Previous studies have shown that SLCO1B1 genetic variations may lead to changes in OATP1B1 activity in both in-vitro and clinical studies [1]. In addition, pharmacokinetic profiles of OATP1B1 substrates differ among ethnic groups (particularly Asian and Caucasian populations) [2]. These differences contribute to the inter-individual variability in the clinical pharmacokinetics of drugs, raising questions of how these observations may guide future clinical practice to achieve pharmacological effects while avoiding toxicological effects in each patient. For example, should the dose be adjusted for ethnic differences, genetic variations, or both? To answer these questions, it is important first to understand the mechanism governing the observations.
A previous report states that with the same genotype of SLCO1B1, OATP1B1 activity in an Asian population is about half of that in a Caucasian population, suggesting that along with allele frequencies of transporter genes, an intrinsic ethnic variability in activity could be an independent source of pharmacokinetic variation [2]. This conclusion is supported by the fact that the allele frequencies of SLCO1B1 per se cannot describe the higher plasma exposure of rosuvastatin, an OATP1B1 substrate, in the Japanese population than that in the Caucasian population [2]. However, it is hard to interpret physiologically the source of the proposed intrinsic ethnic variability; given that a recent study shows similar or slightly elevated hepatic expression levels of OATPs in Asians relative to Caucasians [3]. In addition, when a unified intrinsic ratio between Japanese and Caucasian populations is applied to multiple compounds, improved agreement between observed and predicted clearances is observed for some compounds but not for others [2], suggesting the intrinsic ethnic variability may be compound dependent. For certain OATP1B1 substrates (e.g., repaglinide and pitavastatin), Asian and Caucasian populations have similar pharmacokinetic profiles [4, 5]. Applying both the intrinsic ethnic variability of OATP1B1 and the allele frequency-caused variability will over-predict the clearance difference between Asian and Caucasian groups for these compounds. As a result, this hypothesis has been challenged [3]; thus, we evaluate alternative proposals by a quantitative approach.
For an improved understanding of the ethnic variability of the pharmacokinetics of transporter substrates, we analyze OATP1B1-genotyped clinical pharmacokinetic data for atorvastatin, repaglinide, pitavastatin, pravastatin, and rosuvastatin in Caucasians and three Asian groups (i.e., Chinese, Japanese, and Korean) with a physiologically based pharmacokinetic (PBPK) model. We also include in the analysis the allele frequency of breast cancer resistance protein (BCRP, i.e., ATP Binding Cassette Protein G2) encoded by the ABCG2 gene. The BCRP polymorphism and its allele frequency previously have been proposed as sources for the observed ethnic variability [3]. Two extensively studied transporter mutations SLCO1B1 c.521T>C and ABCG2 c.421C>A are included in the current study. We show that a PBPK model can reasonably simulate the clinical pharmacokinetic time course data for SLCO1B1 c.521TT, c.521CC, ABCG2 c.421CC, and c.421AA carriers in four ethnic groups, without assuming an intrinsic ethnic variability.
Methods
Constructing Physiologically Based Pharmacokinetic Models to Analyze Genotyped Pharmacokinetic Data
Time course pharmacokinetic data for OATP1B1 transporter substrates available from healthy subjects in more than one ethnic group are included in this analysis. A previously published PBPK model structure incorporating hepatic active uptake (CLact), passive diffusion (CLpass), and biliary excretion (CLbile) or metabolism (CLmet) [6] was used to analyze all the pharmacokinetic data in this study. Briefly, all tissues were connected with circulating blood. The compound distribution was perfusion limited in all tissues except for the liver, which was modeled as permeability limited with five sequential segments. Every segment included one pair of extracellular and intracellular sub-compartments with CLact, CLpass, and CLbile or CLmet incorporated. Absorption was described empirically using two sequential compartments with first-order absorption rates (k a) and F a F g (i.e., the product of the fraction of a dose absorbed and the fraction of a drug passing though the gut wall without metabolism) in each compartment. The parameter values are assumed the same for the two compartments. To simulate the enterohepatic recirculation, there are five sequential compartments, with the same first-order transit rate (k bile), between the liver intracellular compartments and the first absorption compartment. MATLAB was used for the modeling (Mathworks, Natick, MA, USA). The schematic for the model structure is provided in Fig. S1 of the Electronic Supplementary Material (ESM).
Assuming that OATP1B1 is the major transporter responsible for CLact, in the model, SLCO1B1 c.521TT carriers in different ethnic groups have the same CLact, as do the c.521CC carriers. The impact of BCRP genetic variation on the pharmacokinetics is reflected through the empirical term, F a F g. To simplify the analysis, only wild-type (SLCO1B1 c.521TT or ABCG2 c.421CC) and homogenous mutation (SLCO1B1 c.521CC or ABCG2 c.421AA) data are included. To our knowledge, no pharmacokinetic time course data have been published with both SLCO1B1 and ABCG2 genotyped. As such, in modeling SLCO1B1-genotyped data, we have to assume that the allele frequencies of ABCG2 genotypes in these studies are the same as previously published values (Table 1). A similar approach is taken when modeling ABCG2 genotyped data, and making assumptions about SLCO1B1 frequencies [7, 8].
Table 1.
Frequencies of BCRP c.421CC, c.421CA, and c.421AA, and OATP1B1 c.521TT, c.521TC, and c.521CC in Caucasian, Chinese, Japanese, and Korean populations
| Ethnicity | BCRP | Sample size | References | ||
|---|---|---|---|---|---|
| c.421CC | c.421CA | c.421AA | |||
| Caucasian | 0.82 | 0.17 | 0.01 | 982 | [7, 33, 34] |
| Chinese | 0.43 | 0.45 | 0.12 | 115 | [33, 35] |
| Japanese | 0.50 | 0.41 | 0.09 | 244 | [36, 37] |
| Korean | 0.52 | 0.40 | 0.08 | 250 | [31] |
| Ethnicity | OATP1B1 | Sample size | References | ||
|---|---|---|---|---|---|
| c.521TT | c.521TC | 521CC | |||
| Caucasian | 0.64 | 0.32 | 0.04 | 468 | [38] |
| Chinese | 0.85 | 0.14 | <0.01 | 128 | [13, 38–41] |
| Japanese | 0.69 | 0.30 | 0.01 | 120 | [42] |
| Korean | 0.78 | 0.21 | 0.01 | 200 | [43] |
BCRP breast cancer resistance protein, OATP organic anion transporting polypeptide
The Model Parameterization
The values for physiological parameters (e.g., tissue weights and blood flows, Table S1 of the ESM) have been previously published [9]. The compound-specific values (e.g., plasma and liver intracellular free fraction, blood-to-plasma ratio, pKa, and LogD7.4) are provided in Table S2 of the ESM. For non-liver tissues, the compound partition coefficients between tissue and blood are calculated using a previously published approach [10]. The renal clearances of pravastatin and rosuvastatin are fixed at 26.5 and 12.3 L h−1 [11, 12], and are assumed to be the same across different populations according to a previous study [2].
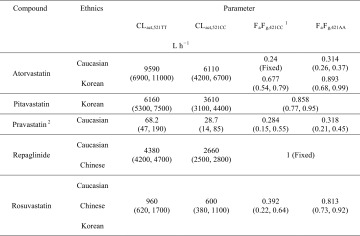
The hepatic CLact values are shared by the carriers with the same SLCO1B1 c.521T>C genotype, independent of ethnicity. Except for atorvastatin, F a F g values for each compound are shared by the different ethnic groups with the same ABCG2 c.421C>A genotype. BCRP activity may also affect k a. However, except for rosuvastatin, all the groups share k a values for each compound owing to limited data in the absorption phase. All groups assumed the same CLpass values for each compound. Compound-specific CLmet, CLbile, and k bile have different values for different ethnic groups, but are assumed the same within each ethnic group independent of the SLCO1B1 or ABCG2 genotype. The impact of the ABCG2 genotype on CLbile is considered be minimal [7, 13].
Hepatic clearance processes, k a, and F a F g, are simultaneously estimated by fitting all genotyped data for a given compound. Because all the genotyped pharmacokinetic data included here are generated with oral dosing, it becomes challenging to precisely estimate both clearance and F a F g. As such, F a F g is estimated first for every compound with its ungenotyped Caucasian pharmacokinetic data following intravenous and oral dosing. To be specific, for rosuvastatin, pitavastatin, and pravastatin for which pharmacokinetic profiles are affected by biliary excretion and enterohepatic recirculation of the parent drug, ungenotyped F a F g is determined using the PBPK model (Fig. S2 of the ESM); while for repaglinide for which metabolism is the major elimination pathway, F a F g can be estimated with empirical equations, i.e., bioavailability/(1 − blood clearance/hepatic blood flow). No atorvastatin (i.e., another compound with a metabolism-driven disposition) pharmacokinetic time course data following intravenous dosing are published; thus, F a F g is fixed at a previously reported value of 0.24 [14]. The value of F a F g in ungenotyped studies is assumed to be the same as the value of F a F g in c.421CC groups because of the very low frequency of c.421AA in the Caucasian population (about 1%, Table 1), and very similar pharmacokinetic profiles between c.421CC and c.421CA carriers [7, 8]. Then, F a F g values for the c.421CC groups are fixed when estimating other parameters including F a F g values for the c.421AA groups.
SLCO1B1 c.521T>C genotyped rosuvastatin data in Caucasian, Chinese, and Korean populations, pitavastatin data in the Korean population, pravastatin data in the Caucasian population, atorvastatin data in Caucasian and Korean populations, and repaglinide data in Caucasian and Chinese populations are used in fitting. ABCG2 c.421C>A genotyped rosuvastatin data in Caucasian and Chinese populations, pravastatin data in a Caucasian population, and atorvastatin data in a Caucasian population are also used in fitting. Assuming linear kinetics, all data were scaled to a 10-mg dosing amount. Clinical data were digitized from published mean pharmacokinetic time courses, with references provided in Table S4 of the ESM.
Given the complexity of the model structure and the number of estimated parameters, a numerical global optimization method (i.e., differential evolution) initially optimized the parameter values. For a better understanding of the uncertainty associated with parameter estimation, the globally optimized values were fed into a Markov chain Monte Carlo (MCMC) method [15] as the starting point to approximate mean estimates and their 95% confidence intervals. Table 2 and Table S3 of the ESM show the parameter values and confidence intervals. Both differential evolution and MCMC methods have been previously implemented in MATLAB with codes published online (http://www1.icsi.berkeley.edu/~storn/code.html#matl; http://helios.fmi.fi/~lainema/mcmc/#sec-4).
Table 2.
Estimated values of CLact and F a F g in Caucasian and Asian populations (presented as the mean and 95% confidence intervals approximated with the Markov chain Monte Carlo method)
BCRP breast cancer resistance protein, CL act hepatic active uptake, CL pass passive diffusion, F a F g the product of the fraction of a dose absorbed and the fraction of a drug passing though the gut wall without metabolism
a F a F g,421CC is determined using ungenotyped Caucasian data based on the facts that the BCRP c.421AA frequency is very low in the Caucasian population, and that c.421CC and c.421CA carriers have similar pharmacokinetic profiles. Other parameters are determined by fitting genotyped data with F a F g,421CC fixed at its mean values
bIn estimating parameter values for pravastatin, CLpass is fixed at an assumed value to avoid huge uncertainty because of a correlation between CLact and CLpass. Details are given in the Electronic Supplementary Material
Simulating SLCO1B1 or ABCG2 Genotyped Pharmacokinetics of Transporter Substrates in Asian and Caucasian Populations
Time course pharmacokinetic profiles are simulated in c.521TT-c.421CC, c.521TT-c.421AA, c.521CC-c.421CC, and c.521CC-c.421AA groups in Caucasian, Chinese, Japanese, and Korean populations. To simplify the modeling, heterozygous mutations are not included in the current analysis. In fitting SLCO1B1 genotyped but ABCG2 ungenotyped data, c.521TT pharmacokinetics is simulated as an average of c.521TT-c.421CC and c.521TT-c.421AA weighted by the frequencies of c.421CC and c.421AA in four populations (Table 1). c.421CC and c.421CA carriers have similar pharmacokinetic profiles [7, 8], hence c.421CC frequencies in simulations are the sums of reported c.421CC and c.421CA frequencies. ABCG2 c.421CC pharmacokinetics are simulated as an average of c.521TT-c.421CC and c.521CC-c.421CC weighted by the frequencies of c.521TT and c.521CC in different populations (Table 1). c.521CC frequencies in the simulations include reported c.521CC and c.521TC frequencies, assuming the pharmacokinetics of c.521CC are close to those of c.521TC [16]. SLCO1B1 c.521CC and ABCG2 c.421AA pharmacokinetic profiles are simulated similarly.
To validate further the modeling, for pravastatin and pitavastatin, we also prospectively predict ungenotyped pharmacokinetics. Time courses for four genotyped groups (i.e., c.521TT-c.421CC, c.521TT-c.421AA, c.521CC-c.421CC, and c.521CC-c.421AA) are generated. As mentioned above, our hypothesis is that differences in pharmacokinetics among different ethnic groups is the result of different activity associated with each genotype and different allele frequencies in each ethnic group. Based on this hypothesis, to simulate ungenotyped pharmacokinetics in an ethnic population, we take the average pharmacokinetics of four genotyped ‘ethnicity-independent’ groups, but weighted by the allele frequencies of SLCO1B1c.521T>C and ABCG2 c.421C>A in that specific ethnic population. Only if both pharmacokinetics and allele frequencies in the four groups were right, could the model reproduce the ungenotyped data, with the underlying assumption that OATP1B1 and BCRP are the major determinants of pharmacokinetics for the tested compounds.
Results
Analyzing Genotyped Rosuvastatin Pharmacokinetic Data in Caucasian, Chinese, and Korean Groups
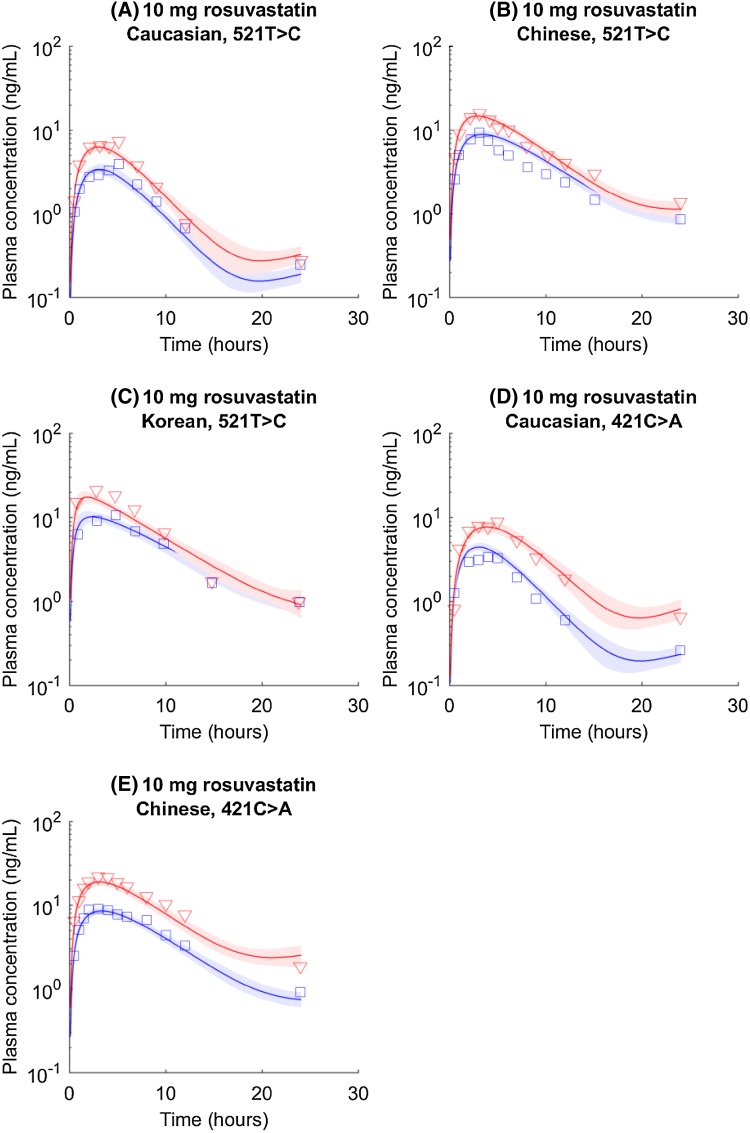
Assuming the same SLCO1B1 genotypes have the same hepatic uptake activity, and the same ABCG2 genotypes have the same F a F g, independent of ethnicity, the model can reasonably describe genotyped rosuvastatin pharmacokinetic time course data from Caucasian, Chinese, and Korean groups (Fig. 1). c.421CC carriers have lower F a F g values than c.421AA carriers (Table 2). Because the allele frequency of ABCG2 c.421C>A is much higher in Asian populations than that in Caucasian populations with the same SLCO1B1 genotype, the average Asian exposure is expected to be higher than the average Caucasian exposure. c.421CC groups seem to have faster k a rates than c.421AA groups, although the difference between c.421CC and c.421AA is not statistically significant in Asian groups (Table S3). In addition, with the same ABCG2 c.421C>A genotypes, the Caucasian population has a slower k a rate than Asian populations. Overall, the Caucasian population also has a faster CLbile than the Asian groups. A brief discussion about these differences is provided in Sect. 4. The empirical bile transit rates (k bile, representing bile flow from the liver to the gut) are similar across three populations.
Fig. 1.
SLCO1B1 c.521T>C and ABCG2 c.421C>A genotyped rosuvastatin pharmacokinetic time courses in Caucasian, Chinese, and Korean populations. Blue circles and lines represent data and simulations for the wild type (i.e., SLCO1B1 c.521TT or ABCG2 c.421CC). Red triangles and lines represent data and simulations for homogenous mutations (i.e., SLCO1B1 c.521CC or ABCG2 c.421AA). Shaded areas indicate 95% confidence intervals approximated with parameter values generated in Markov chain Monte Carlo (confidence intervals presented here are to visualize the numerical uncertainty associated with mean simulations, but not inter-individual or inter-study variability. The same implication applies to the following figures)
Analyzing Genotyped Atorvastatin Pharmacokinetic Data in Caucasian and Korean Groups
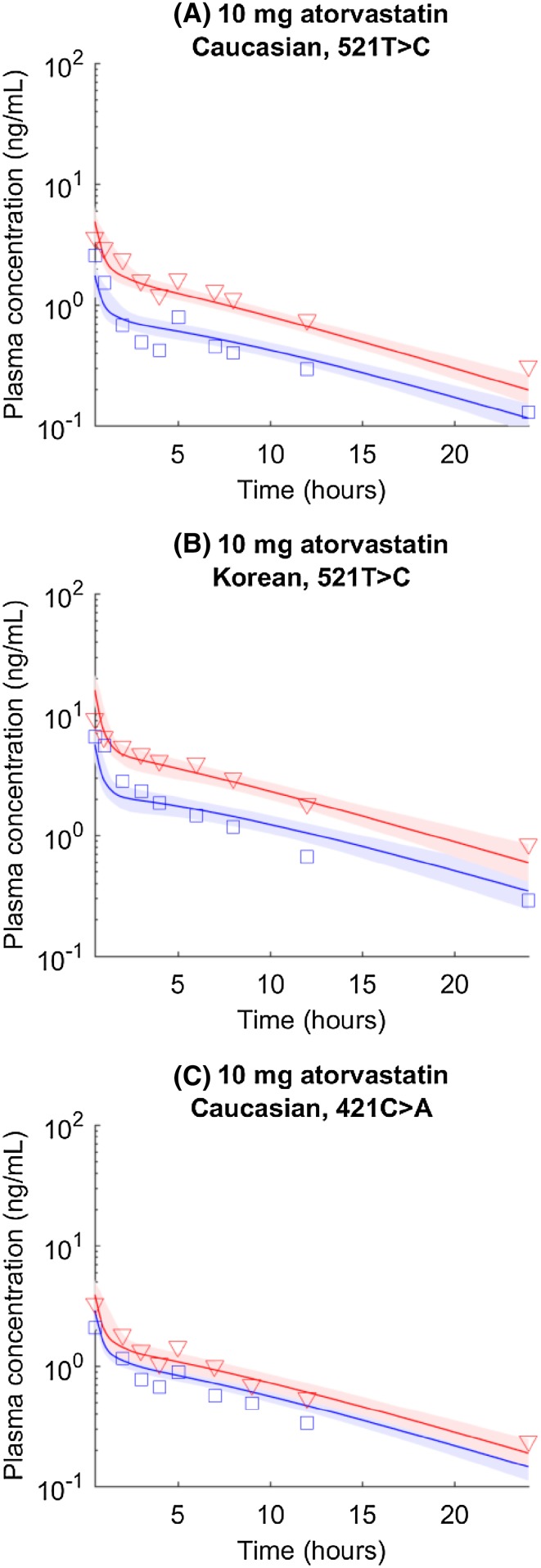
Genotyped atorvastatin pharmacokinetic data (i.e., SLCO1B1 genotyped atorvastatin data in Caucasian and Korean populations, and ABCG2 genotyped atorvastatin data in the Caucasian population) are analyzed similarly to rosuvastatin data. Overall, the results (Fig. 2; Table 2) are similar to those for rosuvastatin. Caucasian and Korean populations require different F a F g values to describe atorvastatin data, which may be owing to the genetic variations of intestinal metabolic enzymes. Because of the lack of genotyped atorvastatin data in the absorption phase, we reduced the number of fitted parameters with assumptions that different populations share the same k a values. However, the estimated k a is still associated with large uncertainty.
Fig. 2.
SLCO1B1 c.521T>C and ABCG2 c.421C>A genotyped atorvastatin pharmacokinetic time courses in Caucasian, Japanese, and Korean populations. Blue circles and lines represent data and simulations for the wild type (i.e., SLCO1B1 c.521TT or ABCG2 c.421CC). Red triangles and lines represent data and simulations for homogenous mutations (i.e., SLCO1B1 c.521CC or ABCG2 c.421AA). Shaded areas indicate 95% confidence intervals approximated with parameter values generated in Markov chain Monte Carlo
Analyzing Genotyped Repaglinide Pharmacokinetic Data in Caucasian and Chinese Groups
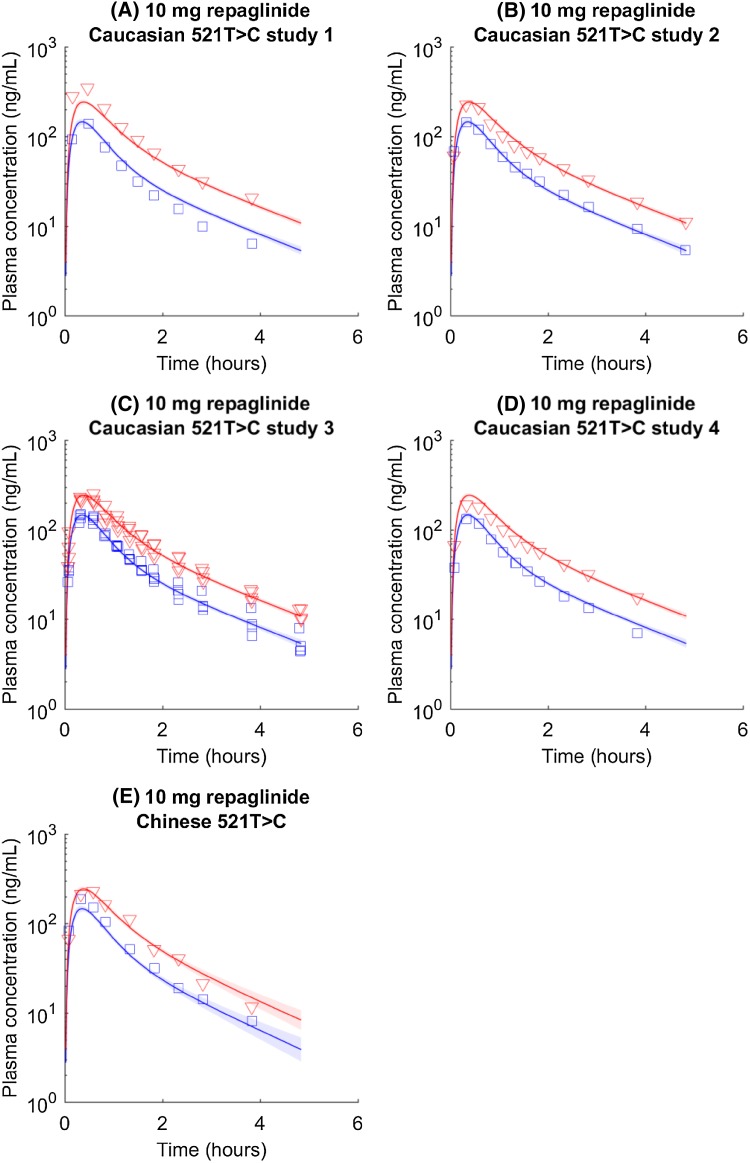
The F a F g value for repaglinide is determined to be 1. As such, its pharmacokinetics is unlikely to be affected by the BCRP activity or the polymorphism of the ABCG2 gene. Not being confounded by BCRP activity, the compound is chosen to further validate our hypothesis: Asian and Caucasian participants with the same SLCO1B1 genotype have similar uptake activity. The results are as expected, that both observations and simulations have similar pharmacokinetic profiles between Caucasian and Chinese groups (Fig. 3; Table 2).
Fig. 3.
SLCO1B1 c.521T>C genotyped repaglinide pharmacokinetic time courses in Caucasian and Chinese populations. Blue circles and lines represent data and simulations for the wild type (i.e., SLCO1B1 c.521TT). Red triangles and lines represent data and simulations for homogenous mutations (i.e., SLCO1B1 c.521CC). Caucasian data are from four independent clinical studies. Shaded areas indicate 95% confidence intervals approximated with parameter values generated in Markov chain Monte Carlo
Analyzing Genotyped Pitavastatin Pharmacokinetic Data in the Korean Group and Predicting Ungenotyped Pharmacokinetics in the Caucasian Group
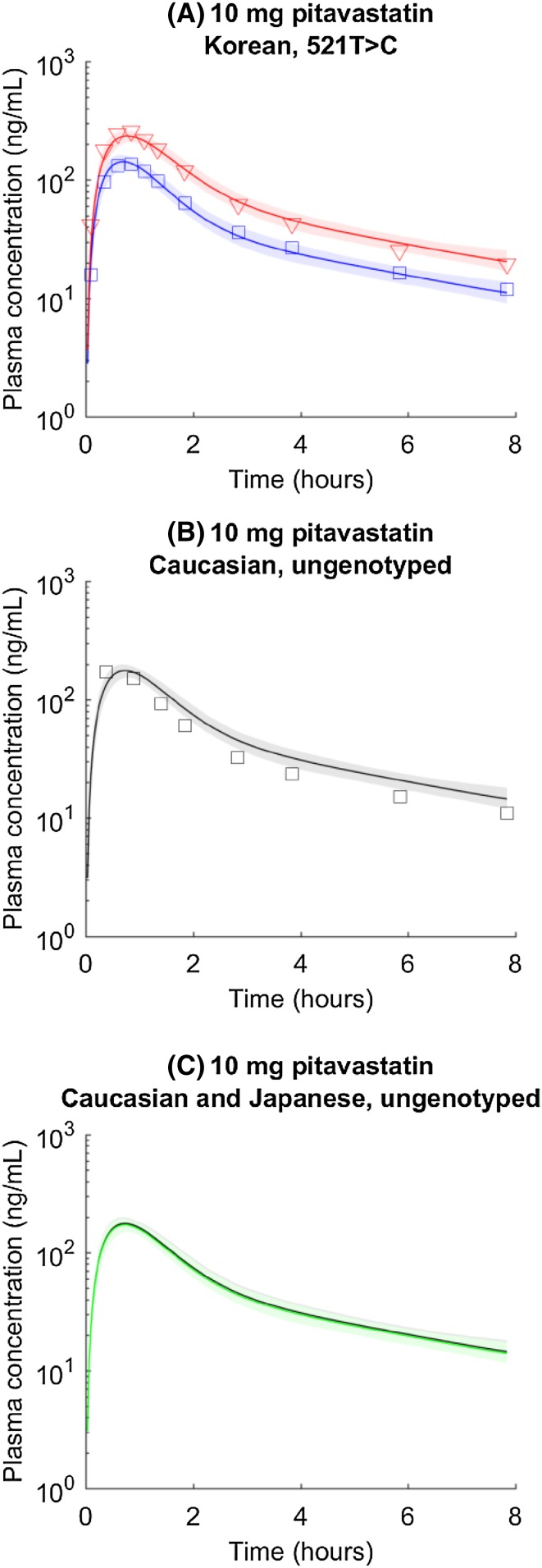
Clinical observations suggest that ABCG2 c.421C>A is not associated with pitavastatin pharmacokinetic variability [17, 18], consistent with in vitro observation that pitavastatin may not be a BCRP substrate [19]. The model is trained only with Asian SLCO1B1 c.521T>C data (Fig. 4a; Table 2), as there is no genotyped Caucasian data available. To test our hypothesis, we simulate pharmacokinetics in c.521TT and c.521CC groups, and then prospectively predict ungenotyped plasma concentrations in the Caucasian population as the average concentration of c.521TT and c.521CC groups weighted by the Caucasian c.521T>C allele frequency, with the underlying assumption that all parameter values (e.g., CLact,521TT, CLact,521CC, CLpass, k a, and other parameters listed in Table 2; Table S3) are the same between Caucasian and Korean populations. The prediction matches observed pharmacokinetics in Caucasian individuals (Fig. 4b). The ungenotyped Japanese pharmacokinetics is generated similarly. The model successfully predicts that there is a minimal difference in ungenotyped pharmacokinetics between Japanese and Caucasian populations (Fig. 4c), as shown in a previous study [5]. The difference between c.521TT and c.521CC activity and the differences in allele frequencies among populations are not enough to introduce an obvious difference in simulated pharmacokinetics.
Fig. 4.
a SLCO1B1 c.521T>C genotyped pitavastatin pharmacokinetic time courses in the Korean population. Blue circles and lines represent data and simulations for the wild type (i.e., SLCO1B1 c.521TT). Red triangles and lines represent data and simulations for homogenous mutations (i.e., SLCO1B1 c.521CC). Shaded areas indicate 95% confidence intervals approximated with parameter values generated in Markov chain Monte Carlo. b Prospectively predicted ungenotyped pharmacokinetic time courses of pitavastatin in Caucasian populations. Circles, line, and shaded area represent observation, mean prediction, and confidence interval. c Predicted ungenotyped pharmacokinetic time courses of pitavastatin in Caucasian (black) and Japanese (green) populations
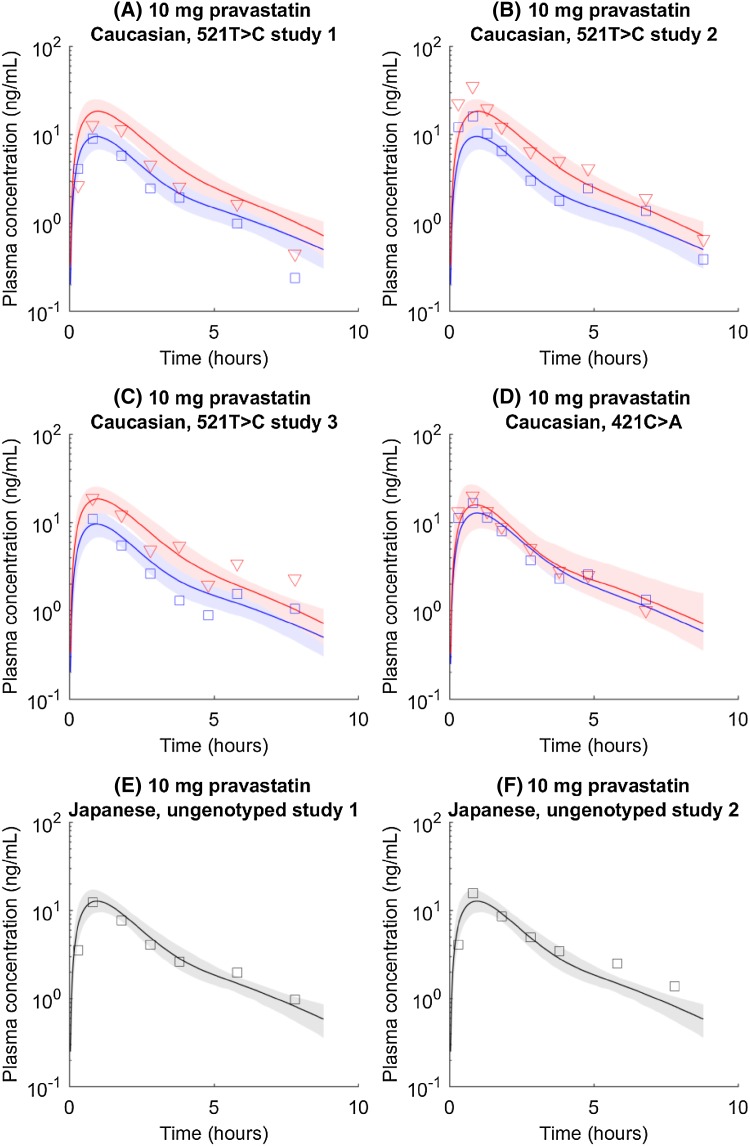
Analyzing Genotyped Pravastatin Pharmacokinetic Data in Caucasian Groups and Predicting Ungenotyped Pharmacokinetics in Japanese Groups
Pravastatin was chosen to perform another prospective prediction. Parameter values are estimated by fitting genotyped Caucasian data (Fig. 5a–d). With estimated parameter values (Table 2; Table S3), we simulate pharmacokinetics in four groups: (1) c.521TT c.421CC, (2) c.521TT c.421AA, (3) c.521CC c.421CC, and (4) c.521CC c.421AA. The ungenotyped pharmacokinetics in the Japanese population is prospectively predicted as the average concentration of the four groups, with the same assumption made for pitavastatin. The prediction matches observed data in Japanese from two independent studies (Fig. 5e, f).
Fig. 5.
a–d SLCO1B1 c.521T>C and ABCG2 c.421C>A genotyped pravastatin pharmacokinetic time courses in the Caucasian population. Blue circles and lines represent data and simulations for wild type (i.e., SLCO1B1 c.521TT or ABCG2 c.421CC). Red triangles and lines represent data and simulations for homogenous mutations (i.e., SLCO1B1 c.521CC or ABCG2 c.421AA). Shaded areas indicate 95% confidence intervals approximated with parameter values generated in Markov chain Monte Carlo. e, f Prospectively predicted ungenotyped pharmacokinetic time courses of pitavastatin in Japanese populations. Circles, line, and shaded area represent observation, mean prediction, and confidence interval
Discussion
In this study, by accounting for the allele frequencies of both OATP1B1 and BCRP, we could reproduce the observed pharmacokinetic time course data of five transporter substrates in a Caucasian population and three Asian populations. As such, the observed ethnic variability can be explained with a more mechanistic approach, which not only provides insight into the source of the ethnic variability, but also a foundation for predicting such pharmacokinetic variability of other transporter substrates and in other populations.
In the pioneering work published by Tomita et al., the authors proposed a hypothesis that both intrinsic ethnic variability of OATP1B1 activity and allele frequencies of OATP1B1 contribute to the observed ethnic variability [2]. With this hypothesis, the ethnic variability in the pharmacokinetics of rosuvastatin in Caucasian and Japanese populations can be better described [2, 20]. However, the hypothesis will over-predict the ethnic variability of the pharmacokinetics of compounds minimally affected by BCRP activity, for example, pitavastatin [5] and repaglinide [4], which a recent study noted [3]. The inconsistency between BCRP substrates and non-substrates leads to the conclusion that the intrinsic variability may be an artifact owing to an allele frequency of BCRP. The Tomita et al. hypothesis rejects such an explanation because estimated F a F g values of an individual compound (i.e., rosuvastatin) are close between Caucasian and Japanese populations [2]. However, rosuvastatin is a compound whose exposure is determined by multiple processes (absorption, hepatic uptake, and biliary excretion). Even if F a F g values are similar between Caucasian and Asian populations groups, it does not necessarily prove that the difference in exposure is the result of different OATP1B1 intrinsic activity (i.e., uptake). Essentially, in the analysis with PBPK, we find that variability in biliary excretion can have a great influence on ethnic variability in rosuvastatin exposure, although with the data available, we do not know the source of the variability in biliary excretion.
In this study, only SLCO1B1 c.521C>T and ABCG2 c.421C>A allele frequencies are taken into consideration because of limited data, although in reality, other mutations of SLCO1B1 and ABCG2, as well as mutations of other proteins may be involved in the pharmacokinetic variability. For example, with a quantitative proteomic approach, Peng et al. reported somewhat elevated hepatic expression levels of OATP1B1 in Asian individuals relative to Caucasian individuals [3]. Because SLCO1B1 c.388A>G leads to increased expression levels [3], the result can be explained by the composition of SLCO1B1 c.388A>G in each population: AA:AG:GG is 0.38:0.45:0.17 in Caucasian samples, and 0.05:0.61:0.33 in Asian samples. Genetic variations in multidrug resistance-associated protein 2 are associated with pravastatin exposure variability in the Caucasian population [21]. However, these data are either only available for a single compound, in a single population, or not presented as time course pharmacokinetics [22], and hence excluded from this analysis.
Owing to limited genotyped time course data for transporter substrates, we did not extend the analysis to other ethnic groups, but the approach may explain observations in these groups. Ho et al. reported that African Americans have lower pravastatin exposure than European Americans [23]. It is very likely because of a high SLCO1B1 c.388A>G frequency combined with a low c.521T>C frequency in African Americans [3, 23]. We excluded heterozygous mutants to simplify the analysis, with assumptions that activity of the ABCG2 c.421CA carrier is similar to wild-type (c.421CC) activity, and that activity of the SLCO1B1 c.521TC carrier is similar to a homozygous mutation (c.521TT). However, the activity of a heterozygous mutant, particularly for SLCO1B1 c.521T>C, requires additional study because it is not consistent across clinical studies (i.e., c.521TC activity can be close to c.521CC, between c.521CC and c.521TT, or below c.521CC [16, 24]).
It is interesting to see that the estimated ratio of CLact between SLCO1B1 c.521CC and c.521TT groups is around 0.6 for all compounds except for pravastatin (~0.4). It is unclear if this phenomenon is a coincidence, given the fact that this ratio depends on both the fractional OATP1B1 contribution to CLact, and the intrinsic activity difference between the two genotypes. It is also worth noting that the c.521T>C mutation does not necessarily reduce OATP1B1 activity, which has been shown in in-vitro and in-vivo studies of fluvastatin [25–27]. Unlike the other compounds studied here, atorvastatin requires different F a F g values for different ethnic groups to describe the data. Atorvastatin undergoes extensive gut metabolism [14]; hence, genetic variations of metabolic enzymes [28, 29] may also be involved in its ethnic variability.
The impact of the BCRP polymorphism on renal clearance and CLbile is ignored in this analysis based on previous studies [7, 13], where area under the plasma concentration–time curves and maximum plasma concentration of atorvastatin and rosuvastatin but not their elimination half-lives are affected by the ABCG2 genotype, strongly suggesting that the BCRP function mainly affects the absorption rather than biliary excretion. As such, when data are available (i.e., rosuvastatin analysis), individual CLbile values are assigned to different ethnic groups but not different ABCG2 genotypes. The estimated CLbile is higher in the Caucasian group than that in the Asian groups. However, Caucasian individuals have a slower rate than Asian individuals with the same ABCG2 c.421C>A genotype. The differences may be the result of factors other than ABCG2 mutations (e.g., genetic variation of transporters and their allele frequencies involved in biliary excretion).
For rosuvastatin, c.421CC groups seem to have faster k a rates than c.421AA groups (Table S3), which although is only a reflection of the trend in the data, may contradict a general expectation that greater efflux activity in the c.421CC group should lead to slower absorption. Although both F a F g and k a are empirical parameters that lump multiple kinetic processes together, F a F g is a parameter reflecting the overall absorption, while k a is used to empirically describe longitudinal data (i.e., time course). It is unclear how BCRP activity may affect k a values. It also worth noting that the difference between c.421CC and c.421AA groups is insignificant in the Asian groups. In the Caucasian population, the difference is a result of fitting mean data from a single clinical study; we cannot rule out the possibility that this is an artifact in data averaging, particularly for pharmacokinetic data in the absorption phase. As to the other compounds we tested, such comparisons on absorption rates between c.421CC and c.421AA groups are either not achievable owing to limited data in absorption phase (i.e., atorvastatin and pravastatin), or not worthwhile because compounds are not BCRP substrates (i.e., repaglinide and pitavastatin). More data are required to fully understand this phenomenon.
We note that polymorphisms of multiple transporters are rarely sequenced in the same study. For a few studies where more than one transporter is sequenced simultaneously [13, 17, 30], the results are presented as non-compartmental parameters with analysis on one transporter separated from analysis on the other transporter, which is not helpful for further mechanistic analysis that may be performed in the future. For instance, in this study, we have to combine data from multiple independent studies, and assume that the allele frequency estimated from one study can be applied to another study. This assumption is supported by previous reports that allele frequencies estimated from different studies are similar [31, 32].
For future clinical studies, it may be helpful to simultaneously sequence genes of several key transporters to understand the combined effect from genetic variations of multiple transporters, and to publish time course data to facilitate a mechanistic analysis. By validating a more mechanistic hypothesis, the current study may serve as a starting point for prospectively predicting pharmacokinetics and guiding dose adjustment for different ethnic groups in the future. Here, we return to the question asked in Sect. 1: should we adjust dose based on ethnicity, genotype, or both? For a given drug dosed to a given patient, if the dominant transporters or metabolic enzymes in absorption and elimination are identified and well characterized, then mechanistic adjustment based on the genotype is a superior approach. It is worth noting that for a given drug, understanding of certain pharmacokinetic processes (e.g., hepatic uptake) may be better than understanding of other processes (e.g., biliary excretion). As such, if both processes dramatically influence drug exposure, it may be beneficial to combine mechanistic adjustment based on the genotype for well understood processes with empirical adjustment based on ethnicity for less understood processes.
Conclusion
For compounds with transporter-mediated activity as dominant pathways for absorption and disposition, we propose that allele frequencies of genes of multiple transporters could contribute significantly to the ethnic variability. Simulations based on this hypothesis can reasonably describe SLCO1B1 or ABCG2 genotyped pharmacokinetic time course data in Caucasian, Chinese, Japanese, and Korean populations. By eliminating the compound-dependent intrinsic ethnic variability, the work accomplished here can facilitate efforts to predict prospectively ethnic variability in the pharmacokinetics of transporter substrates.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Compliance with Ethical Standards
Funding
No funding was received for the conduct of this study or preparation of this article.
Conflict of interest
Rui Li and Hugh A. Barton are full time employees of Pfizer Inc. Hugh A. Barton holds stock options or shares in the company.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s40262-017-0568-7) contains supplementary material, which is available to authorized users.
References
- 1.Niemi M, Pasanen MK, Neuvonen PJ. Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011;63(1):157–181. doi: 10.1124/pr.110.002857. [DOI] [PubMed] [Google Scholar]
- 2.Tomita Y, Maeda K, Sugiyama Y. Ethnic variability in the plasma exposures of OATP1B1 substrates such as HMG-CoA reductase inhibitors: a kinetic consideration of its mechanism. Clin Pharmacol Ther. 2013;94(1):37–51. doi: 10.1038/clpt.2012.221. [DOI] [PubMed] [Google Scholar]
- 3.Peng KW, et al. Ethnic variability in the expression of hepatic drug transporters: absolute quantification by an optimized targeted quantitative proteomic approach. Drug Metab Dispos. 2015;43(7):1045–1055. doi: 10.1124/dmd.115.063362. [DOI] [PubMed] [Google Scholar]
- 4.Thomsen MS, et al. Pharmacokinetics of repaglinide in healthy caucasian and Japanese subjects. J Clin Pharmacol. 2003;43(1):23–28. doi: 10.1177/0091270002239702. [DOI] [PubMed] [Google Scholar]
- 5.Warrington S, Nagakawa S, Hounslow N. Comparison of the pharmacokinetics of pitavastatin by formulation and ethnic group: an open-label, single-dose, two-way crossover pharmacokinetic study in healthy Caucasian and Japanese men. Clin Drug Investig. 2011;31(10):735–743. doi: 10.2165/11592480-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Li R, et al. A “middle-out” approach to human pharmacokinetic predictions for OATP substrates using physiologically-based pharmacokinetic modeling. J Pharmacokinet Pharmacodyn. 2014;41(3):197–209. doi: 10.1007/s10928-014-9357-1. [DOI] [PubMed] [Google Scholar]
- 7.Keskitalo JE, et al. ABCG2 polymorphism markedly affects the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther. 2009;86(2):197–203. doi: 10.1038/clpt.2009.79. [DOI] [PubMed] [Google Scholar]
- 8.Wan Z, et al. Marked alteration of rosuvastatin pharmacokinetics in healthy Chinese with ABCG2 34G>A and 421C>A homozygote or compound heterozygote. J Pharmacol Exp Ther. 2015;354(3):310–315. doi: 10.1124/jpet.115.225045. [DOI] [PubMed] [Google Scholar]
- 9.Shah DK, Betts AM. Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J Pharmacokinet Pharmacodyn. 2012;39(1):67–86. doi: 10.1007/s10928-011-9232-2. [DOI] [PubMed] [Google Scholar]
- 10.Rodgers T, Rowland M. Physiologically based pharmacokinetic modelling 2: predicting the tissue distribution of acids, very weak bases, neutrals and zwitterions. J Pharm Sci. 2006;95(6):1238–1257. doi: 10.1002/jps.20502. [DOI] [PubMed] [Google Scholar]
- 11.Martin PD, et al. Absolute oral bioavailability of rosuvastatin in healthy white adult male volunteers. Clin Ther. 2003;25(10):2553–2563. doi: 10.1016/S0149-2918(03)80316-8. [DOI] [PubMed] [Google Scholar]
- 12.Singhvi SM, et al. Disposition of pravastatin sodium, a tissue-selective HMG-CoA reductase inhibitor, in healthy subjects. Br J Clin Pharmacol. 1990;29(2):239–243. doi: 10.1111/j.1365-2125.1990.tb03626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birmingham BK, et al. Rosuvastatin pharmacokinetics and pharmacogenetics in Caucasian and Asian subjects residing in the United States. Eur J Clin Pharmacol. 2015;71(3):329–340. doi: 10.1007/s00228-014-1800-0. [DOI] [PubMed] [Google Scholar]
- 14.Lennernas H. Clinical pharmacokinetics of atorvastatin. Clin Pharmacokinet. 2003;42(13):1141–1160. doi: 10.2165/00003088-200342130-00005. [DOI] [PubMed] [Google Scholar]
- 15.Haario H, et al. DRAM: efficient adaptive MCMC. Stat Comput. 2006;16(4):339–354. doi: 10.1007/s11222-006-9438-0. [DOI] [Google Scholar]
- 16.Pasanen MK, et al. Different effects of SLCO1B1 polymorphism on the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther. 2007;82(6):726–733. doi: 10.1038/sj.clpt.6100220. [DOI] [PubMed] [Google Scholar]
- 17.Oh ES, et al. Impact of ABCC2, ABCG2 and SLCO1B1 polymorphisms on the pharmacokinetics of pitavastatin in humans. Drug Metab Pharmacokinet. 2013;28(3):196–202. doi: 10.2133/dmpk.DMPK-12-RG-068. [DOI] [PubMed] [Google Scholar]
- 18.Ieiri I, et al. SLCO1B1 (OATP1B1, an uptake transporter) and ABCG2 (BCRP, an efflux transporter) variant alleles and pharmacokinetics of pitavastatin in healthy volunteers. Clin Pharmacol Ther. 2007;82(5):541–547. doi: 10.1038/sj.clpt.6100190. [DOI] [PubMed] [Google Scholar]
- 19.Prueksaritanont T, et al. Pitavastatin is a more sensitive and selective organic anion-transporting polypeptide 1B clinical probe than rosuvastatin. Br J Clin Pharmacol. 2014;78(3):587–598. doi: 10.1111/bcp.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li R, Barton HA, Maurer TS. Toward prospective prediction of pharmacokinetics in OATP1B1 genetic variant populations. CPT Pharmacomet Syst Pharmacol. 2014;3:e151. doi: 10.1038/psp.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niemi M, et al. Association of genetic polymorphism in ABCC2 with hepatic multidrug resistance-associated protein 2 expression and pravastatin pharmacokinetics. Pharmacogenet Genom. 2006;16(11):801–808. doi: 10.1097/01.fpc.0000230422.50962.91. [DOI] [PubMed] [Google Scholar]
- 22.Ieiri I, Higuchi S, Sugiyama Y. Genetic polymorphisms of uptake (OATP1B1, 1B3) and efflux (MRP2, BCRP) transporters: implications for inter-individual differences in the pharmacokinetics and pharmacodynamics of statins and other clinically relevant drugs. Expert Opin Drug Metab Toxicol. 2009;5(7):703–729. doi: 10.1517/17425250902976854. [DOI] [PubMed] [Google Scholar]
- 23.Ho RH, et al. Effect of drug transporter genotypes on pravastatin disposition in European- and African-American participants. Pharmacogenet Genomics. 2007;17(8):647–656. doi: 10.1097/FPC.0b013e3280ef698f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niemi M, et al. High plasma pravastatin concentrations are associated with single nucleotide polymorphisms and haplotypes of organic anion transporting polypeptide-C (OATP-C, SLCO1B1) Pharmacogenetics. 2004;14(7):429–440. doi: 10.1097/01.fpc.0000114750.08559.32. [DOI] [PubMed] [Google Scholar]
- 25.Choi MK, et al. Differential effect of genetic variants of Na(+)-taurocholate co-transporting polypeptide (NTCP) and organic anion-transporting polypeptide 1B1 (OATP1B1) on the uptake of HMG-CoA reductase inhibitors. Xenobiotica. 2011;41(1):24–34. doi: 10.3109/00498254.2010.523736. [DOI] [PubMed] [Google Scholar]
- 26.Deng JW, et al. The effect of SLCO1B1*15 on the disposition of pravastatin and pitavastatin is substrate dependent: the contribution of transporting activity changes by SLCO1B1*15. Pharmacogenet Genom. 2008;18(5):424–433. doi: 10.1097/FPC.0b013e3282fb02a3. [DOI] [PubMed] [Google Scholar]
- 27.Niemi M, Pasanen MK, Neuvonen PJ. SLCO1B1 polymorphism and sex affect the pharmacokinetics of pravastatin but not fluvastatin. Clin Pharmacol Ther. 2006;80(4):356–366. doi: 10.1016/j.clpt.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Riedmaier S, et al. Paraoxonase (PON1 and PON3) polymorphisms: impact on liver expression and atorvastatin-lactone hydrolysis. Front Pharmacol. 2011;2:41. doi: 10.3389/fphar.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riedmaier S, et al. UDP-glucuronosyltransferase (UGT) polymorphisms affect atorvastatin lactonization in vitro and in vivo. Clin Pharmacol Ther. 2010;87(1):65–73. doi: 10.1038/clpt.2009.181. [DOI] [PubMed] [Google Scholar]
- 30.Birmingham BK, et al. Impact of ABCG2 and SLCO1B1 polymorphisms on pharmacokinetics of rosuvastatin, atorvastatin and simvastatin acid in Caucasian and Asian subjects: a class effect? Eur J Clin Pharmacol. 2015;71(3):341–355. doi: 10.1007/s00228-014-1801-z. [DOI] [PubMed] [Google Scholar]
- 31.Kim KA, Joo HJ, Park JY. ABCG2 polymorphisms, 34G>A and 421C>A in a Korean population: analysis and a comprehensive comparison with other populations. J Clin Pharm Ther. 2010;35(6):705–712. doi: 10.1111/j.1365-2710.2009.01127.x. [DOI] [PubMed] [Google Scholar]
- 32.Nakanishi T, Tamai I. Genetic polymorphisms of OATP transporters and their impact on intestinal absorption and hepatic disposition of drugs. Drug Metab Pharmacokinet. 2012;27(1):106–121. doi: 10.2133/dmpk.DMPK-11-RV-099. [DOI] [PubMed] [Google Scholar]
- 33.de Jong FA, et al. ABCG2 pharmacogenetics: ethnic differences in allele frequency and assessment of influence on irinotecan disposition. Clin Cancer Res. 2004;10(17):5889–5894. doi: 10.1158/1078-0432.CCR-04-0144. [DOI] [PubMed] [Google Scholar]
- 34.Mizuarai S, Aozasa N, Kotani H. Single nucleotide polymorphisms result in impaired membrane localization and reduced atpase activity in multidrug transporter ABCG2. Int J Cancer. 2004;109(2):238–246. doi: 10.1002/ijc.11669. [DOI] [PubMed] [Google Scholar]
- 35.Zamber CP, et al. Natural allelic variants of breast cancer resistance protein (BCRP) and their relationship to BCRP expression in human intestine. Pharmacogenetics. 2003;13(1):19–28. doi: 10.1097/00008571-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Imai Y, et al. C421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141K protein and low-level drug resistance. Mol Cancer Ther. 2002;1(8):611–616. [PubMed] [Google Scholar]
- 37.Kobayashi D, et al. Functional assessment of ABCG2 (BCRP) gene polymorphisms to protein expression in human placenta. Drug Metab Dispos. 2005;33(1):94–101. doi: 10.1124/dmd.104.001628. [DOI] [PubMed] [Google Scholar]
- 38.Pasanen MK, et al. Frequencies of single nucleotide polymorphisms and haplotypes of organic anion transporting polypeptide 1B1 SLCO1B1 gene in a Finnish population. Eur J Clin Pharmacol. 2006;62(6):409–415. doi: 10.1007/s00228-006-0123-1. [DOI] [PubMed] [Google Scholar]
- 39.Sui SM, et al. Effect of OATP1B1 521T>C heterogenesis on pharmacokinetic characteristics of rosuvastatin in Chinese volunteers. Acta Pharm Sin. 2011;46(6):695–700. [PubMed] [Google Scholar]
- 40.Lee E, et al. Rosuvastatin pharmacokinetics and pharmacogenetics in white and Asian subjects residing in the same environment. Clin Pharmacol Ther. 2005;78(4):330–341. doi: 10.1016/j.clpt.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 41.Zhu J, et al. Effect of pitavastatin in different SLCO1B1 backgrounds on repaglinide pharmacokinetics and pharmacodynamics in healthy Chinese males. Pak J Pharm Sci. 2013;26(3):577–584. [PubMed] [Google Scholar]
- 42.Nishizato Y, et al. Polymorphisms of OATP-C (SLC21A6) and OAT3 (SLC22A8) genes: consequences for pravastatin pharmacokinetics. Clin Pharmacol Ther. 2003;73(6):554–565. doi: 10.1016/S0009-9236(03)00060-2. [DOI] [PubMed] [Google Scholar]
- 43.Choi JH, et al. Influence of OATP1B1 genotype on the pharmacokinetics of rosuvastatin in Koreans. Clin Pharmacol Ther. 2008;83(2):251–257. doi: 10.1038/sj.clpt.6100267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.