Abstract
In the present study, we describe various pharmacological effects and computational analysis of nepetolide, a tricyclic clerodane-type diterpene, isolated from Nepeta suavis. Nepetolide concentration-dependently (1.0–1000 µg/mL) exhibited 1,1-diphenyl,2-picrylhydrazyl free radical scavenging activity with maximum effect of 87.01 ± 1.85%, indicating its antioxidant potential, as shown by standard drug, ascorbic acid. It was moderately active against bacterial strain of Staphylococcus aureus. In brine shrimp’s lethality model, nepetolide potently showed cytotoxic effect, with LC50 value of 8.7 µg/mL. When evaluated for antitumor activity in potato disc tumor assay, nepetolide exerted tumor inhibitory effect of 56.5 ± 1.5% at maximum tested concentration of 1000 µg/mL. Nepetolide at 20 mg/kg reduced carrageenan-induced inflammation (P < .001 vs. saline group) in rat paw. Nepetolide dose-dependently (100–500 mg/kg) decreased acetic acid evoked writhes, as exhibited by diclofenac sodium. In-silico investigation of nepetolide was carried out against cyclooxygenase-2, epidermal growth factor receptor and lipoxygenase-2 targets. Virtual screening through Patchdock online docking server identified primarily hydrophobic interactions between ligand nepetolide and receptors proteins. Enhanced hydrogen bonding was predicted with Autodock showing 6–8 hydrogen bonds per target. These results indicate that nepetolide exhibits antioxidant, antibacterial, cytotoxic, anticancer, anti-inflammatory and analgesic activities and should be considered as a lead compound for developing drugs for the remedy of oxidative stress-induced disorders, microbial infections, cancers, inflammations and pain.
Keywords: Nepetolide, Antioxidant, Antimicrobial, Cytotoxic, Anticancer, Anti-inflammatory, Analgesic, Computational Pharmacology
1. Introduction
Nepeta genus belonging to the family Lamiaceae and is the largest genus contains approximately 250 species, found in Central and Southern Europe, the North Africa and Southern Asia. The plants of this genus are used in folk medicine as anti-septic, snake bite, scorpion bite, anti-asthmatic, anti-tussive, anti-spasmodic, diuretic and astringent agents (Newall et al., 1996). In Iranian medicine, they are used for treatment of various nervous, respiratory and gastrointestinal diseases (Amin, 1991). The genus Nepeta is named on the ancient Italian city of Nephi (Simonovic, 1959). About 67 species of the genus Nepeta are found in Iran and 58 in Pakistan. Phytochemical screening of plant reveals that Nepeta genus are rich fatty acids, flavones, flavone-glycosides, coumarins, steroids, iridoid-glycosides, monoterpenic lactones, eudesmane sesquiterpenoids, abietane diterpenoids, triterpenoids, and carbohydrates (Khan, 2010). Phytochemical investigation of Nepeta Suavis has led to the isolation of one new diterpene compound, nepetolide (Fig. 1), as reported earlier (Hussain et al., 2008). We previously observed antispasmodic effect of nepetolide, mediated through Ca++ channel blocking mechanisms (Khan et al., 2016). The present research project is designed to investigate the nepetolide different pharmacological activities i.e. antioxidant, antibacterial, cytotoxic, antitumor, anti-inflammatory and analgesic as well as to evaluate it against cyclooxygenase-2 (Cox-2), epidermal growth factor receptor (EGFR) and 15-lipoxygenase-2 (Lox-2) targets, using molecular docking techniques.
Fig. 1.
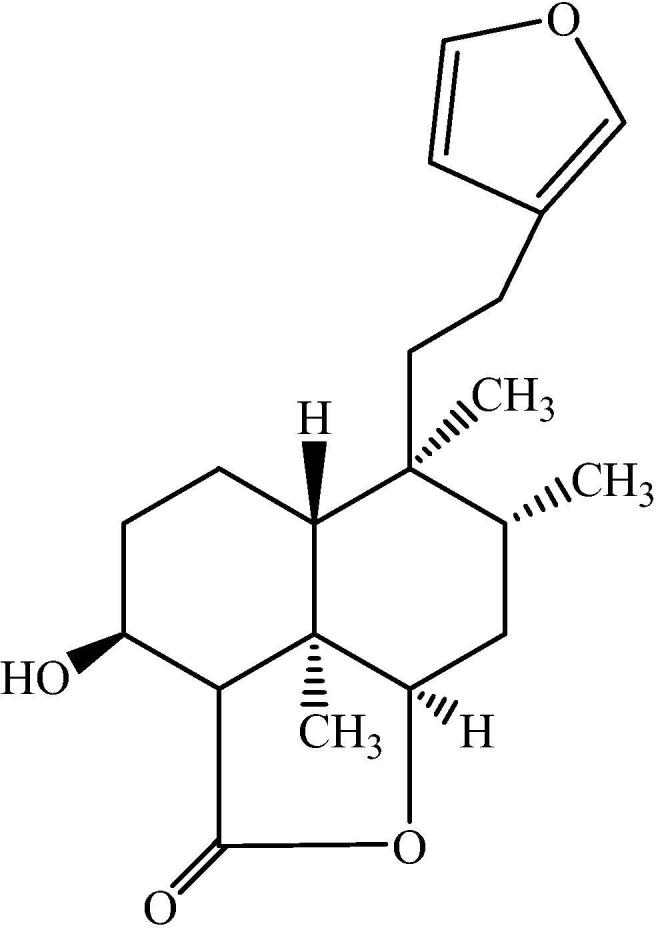
Chemical structure of nepetolide: a diterpene compound isolated from Nepeta Suavis.
2. Results
2.1. Effect on DPPH free radical-scavenging assay
The results of antioxidant activity of different concentrations of nepetolide are shown in Fig. 2. Nepetolide showed significant antioxidant activity when compared with the standard. Result are expressed as% DPPH free radical scavenging and represents the ability of nepetolide and standard to prevent free radical generation. Response of the test compound was in concentration dependent manner. Nepetolide at the test concentrations of 1, 3, 5, 10, 100, 300 and 1000 µg/mL showed 0.50 ± 1.76, 0.55 ± 2.87, 1.29 ± 3.56, 1.59 ± 2.47, 34.01 ± 2.71, 47.07 ± 2.35, 87.01 ± 1.85% free radical scavenging respectively. Ascorbic acid showed 3.27 ± 2.45, 16.65 ± 3.11, 21.36 ± 3.45, 40.95 ± 4.67, 44.48 ± 3.70, 51.76 ± 3.05 and 91.11 ± 1.65% free radical scavenging, at of 1, 3, 5, 10, 100, 300 and 1000 µg/mL respectively. Nepetolide showed maximum percentage scavenging of 87.01% ± 1.85% which is comparable to the free radical scavenging of ascorbic acid. EC50 value of ascorbic acid was 231.1 µg/mL while for nepetolide it was 330.0 µg/mL.
Fig. 2.
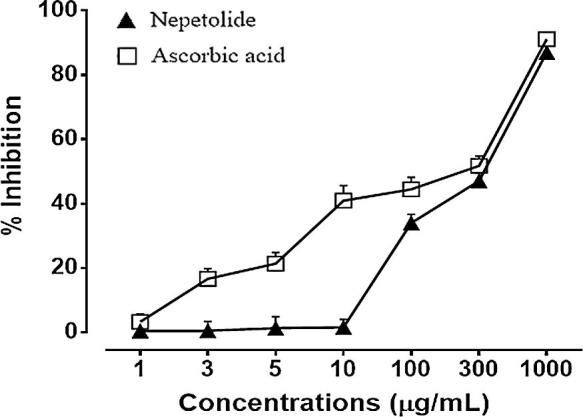
Free radical scavenging activity of nepetolide and ascorbic acid measured as percent inhibition of 1,1-diphenyl-2-picryl hydrazyl radical. n = 3.
2.2. Bactericidal effect
The antibacterial potential of the nepetolide against four different bacterial strains are shown in Table 1. Nepetolide was only active against Staphylococcus aureus having the zone of inhibitions of 8.7 ± 0.5, 9.1 ± 1.7, 9.3 ± 1.4, 10.1 ± 2.1, 11.5 ± 2.5, 11.8 ± 0.9 and 12.2 ± 0.7 at 1, 3, 5, 10, 100, 300 and 1000 µg/mL. Nepetolide was ineffective against Acinobacter, Escherichia coli and Methicillin resistant Staphylococcus aureus at all the tested concentrations and hence no zone of inhibition was observed. The standard drugs ciprofloxacin showed activity against all four bacterial strains while negative control i.e. 5% DMSO showed no activity against any bacterial strain.
Table 1.
Antibacterial effect of different concentrations of nepetolide and ciprofloxacin against Staphylococcus aureus, Acinobacter, Escherichia coli and Methicillin resistant Staphylococcus aureus, measured as diameter of inhibitory zone (mm).
| Microorganisms | Concentrations (µg/mL) |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 10 | 100 | 300 | 1000 | Ciprofloxacin (5 µg) | |
| Staphylococcus aureus | 8.7 ± 0.5 | 9.1 ± 1.7 | 9.3 ± 1.4 | 10.1 ± 2.1 | 11.5 ± 2.5 | 11.8 ± 0.9 | 12.2 ± 0.7 | 25.2 ± 2.2 |
| Acinobacter | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 20 ± 1.4 |
| Escherichia coli | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 8.3 ± 0.9 |
| Methicillin Resistant Staphylococcus Aureus | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 0 ± 0.0 | 9.5 ± 1.5 |
Values shown are mean ± SEM, n = 3.
2.3. Fungicidal effect
Nepetolide at the test concentrations of 1, 3, 5, 10, 100, 300 and 1000 µg/mL did not show any inhibitory effect against Candida albican and Aspergillus niger (data not shown).
2.4. Effect on brine shrimp lethality
The results of cytotoxic activity at different concentrations of nepetolide are shown in Table 2. Mean percentage of larvae killed by nepetolide were 10 ± 5.77, 13.3 ± 8.82, 26.7 ± 6.67, 53.3 ± 13.33, 93.3 ± 6.67, 96.7 ± 3.33 and 100 ± 3.33% at of 1, 3, 5, 10, 100, 300 and 1000 µg/mL respectively. Etoposide killed 12 ± 4.5, 46.3 ± 3.5, 48.7 ± 3.5, 79.3 ± 2.7, 90.7 ± 5.5, 98.1 ± 0.1 and 100 ± 3.33% of mean larvae at 1, 3, 5, 10, 100, 300 and 1000 µg/mL respectively. The cytotoxic potential of nepetolide was found to be significant, having LC50 8.70 µg/mL, which was comparable to that of standard drug etoposide having LC50 value of 5.64 µg/mL.
Table 2.
Cytotoxic effect of nepetolide and etoposide at different concentrations (µg/mL) in brine shrimp lethality assay.
| Test drug | 1 | 3 | 5 | 10 | 100 | 300 | 1000 | LC50 µg/mL |
|---|---|---|---|---|---|---|---|---|
| Nepetolide | 10 ± 5.77 | 13.3 ± 8.82 | 26.7 ± 6.67 | 53.3 ± 13.33 | 93.3 ± 6.67 | 96.7 ± 3.33 | 100 ± 3.33 | 8.70 |
| Etoposide | 12 ± 4.5 | 46.3 ± 3.5 | 48.7 ± 3.5 | 79.3 ± 2.7 | 90.7 ± 5.5 | 98.1 ± 0.1 | 100 ± 0.0 | 5.64 |
Values shown are mean ± SEM, n = 3.
2.5. Effect on inhibition of tumor formation
Nepetolide was screened for their antitumor activity through ‘potato disc tumor assay’. Table 3 represents the antitumor potential of the nepetolide. Antitumor activity of nepetolide was moderate. At different test doses of 1, 3, 5, 10, 100, 300 and 1000 µg/mL the percent tumor inhibition of nepetolide was 1.3 ± 1.5, 8.7 ± 1.5, 18.7 ± 1.5, 26.1 ± 1.5, 36.1 ± 1.5, 40.4 ± 1.5 and 56.5 ± 1.5% respectively, with LC50 value of 412.3 µg/mL. Standard drug vincristine at 250 µg/mL showed 91.3% tumor inhibition.
Table 3.
Percent Inhibition of tumor formation at different concentrations (µg/mL) of nepetolide.
| Concentrations | Percentage tumor inhibition |
|---|---|
| 1 | 1.3 ± 1.5% |
| 3 | 8.7 ± 1.5% |
| 5 | 18.7 ± 1.5% |
| 10 | 26.1 ± 1.5% |
| 100 | 36.1 ± 1.5% |
| 300 | 40.4 ± 1.5% |
| 1000 | 56.5 ± 1.5% |
| LC50 | 412.3 µg/mL |
Vincristine (250 µg/mL) = 91.3% tumor inhibition. Values shown are mean ± SEM, n = 3.
2.6. Effect on carrageenan-induced hind paw edema
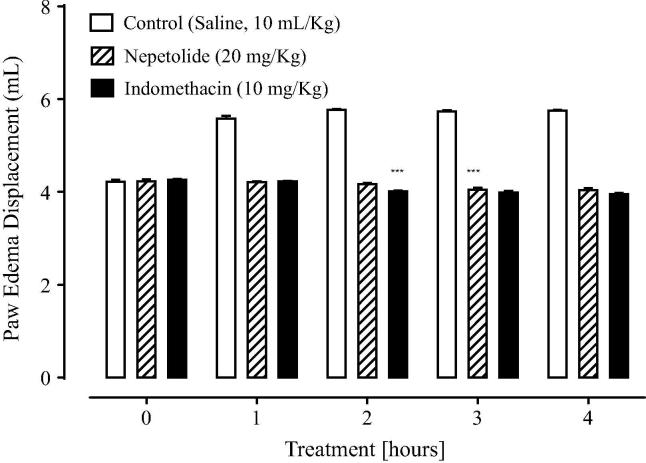
Fig. 3 shows the response of carrageenan-induced paw edema model after the administration of test drug nepetolide. In case of control group, that was treated with 0.5 mL of normal saline, there was gradual increase in the paw thickness after the administration of carrageenan. The animals of saline group at 0, 1, 2, 3 and 4 h were having mean paw edema thickness of 4.22 ± 0.04, 5.58 ± 0.06, 5.77 ± 0.02, 5.74 ± 0.02 and 5.75 ± 0.02 mm respectively. In case of treated group there was inhibitory effect on the paw edema. Nepetolide at the dose of 20 mg/kg shows significant reduction (P < .001 vs. saline group) in edema after 2 h of carrageenan administration. Mean paw edema thickness in nepetolide tested group were 4.26 ± 0.02, 4.23 ± 0.01, 4.01 ± 0.02, 3.99 ± 0.03 and 3.95 ± 0.03 mm respectively, after 0, 1, 2, 3 and 4 h of carrageenan administration. Indomethacin caused mark reduction in edema, having 4.23 ± 0.04, 4.21 ± 0.02, 4.17 ± 0.02, 4.05 ± 0.04 and 4.04 ± 0.04 mm paw edema thickness after 0, 1, 2, 3 and 4 h of carrageenan administration respectively. Indomethacin significantly decreased (P < .001 vs. saline group) the paw edema after 3 h of carrageenan administration.
Fig. 3.
Effect of nepetolide and indomethacin on carrageenan-induced hind paw edema. Values shown are mean ± SEM, n = 5. ***P < .001 vs. saline group. One-way analysis of variance followed by Tukey’s test.
2.7. Effect on acetic acid-induced writhing
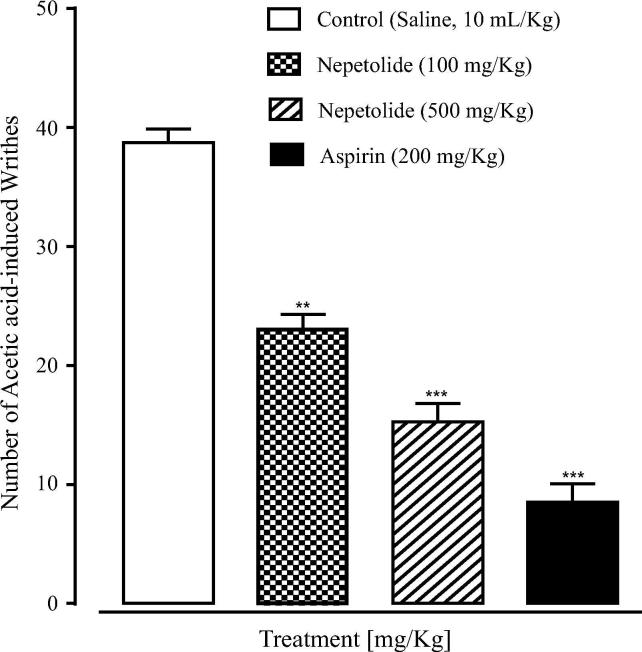
Analgesic potential of nepetolide was evaluated using acetic acid induced writhing test. Percent inhibition of writhes reflects the analgesic potential of nepetolide. The results showed that the nepetolide at doses of 100 mg/kg and 500 mg /kg causes 40.68% (P < .01 vs. saline group) and 60.89% (P < .001 vs. saline group) inhibition of writhes respectively as shown in Fig. 4. Aspirin causes 77.86% inhibition of writhes at 200 mg/kg dose. Percent inhibition of writhes shows that the nepetolide has a good analgesic activity at 500 mg/kg dose when compared with standard drug aspirin.
Fig. 4.
Bar chart showing inhibitory effect of nepetolide and aspirin on the acetic acid-induced writhes in mice. Values shown are mean ± SEM, n = 5. **P < .01, ***P < .001 vs. saline group, one-way analysis of variance with post-hoc Tukey test.
2.8. In-silico evaluation
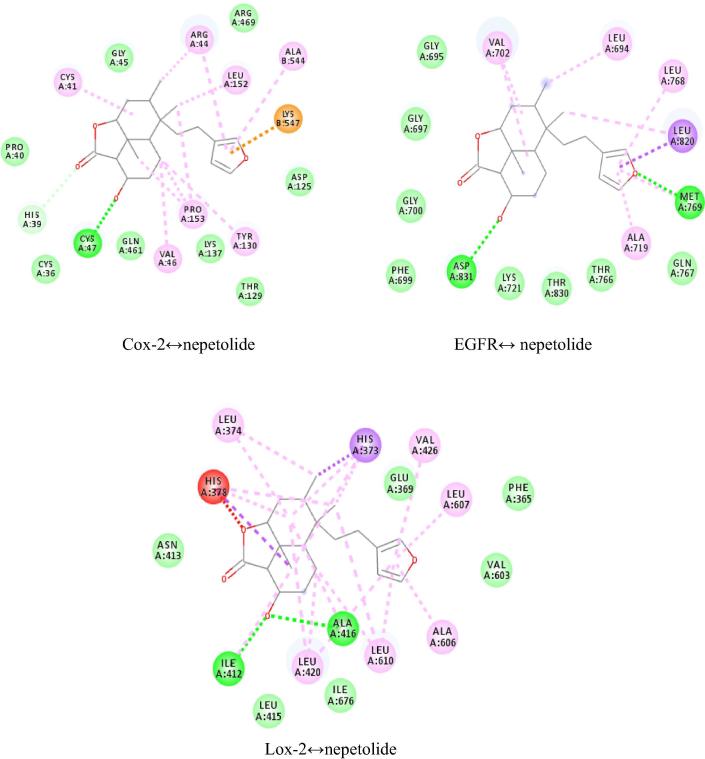
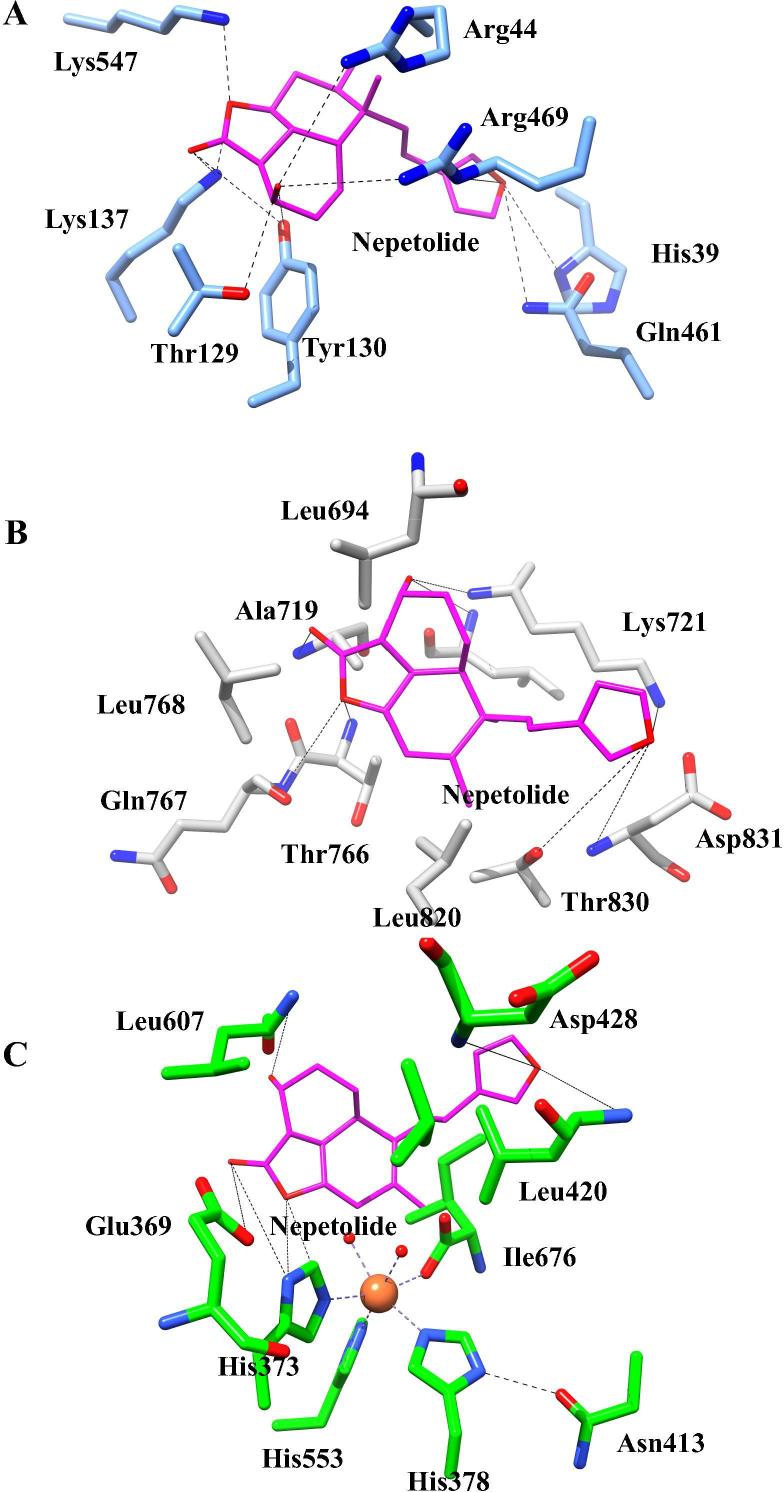
Patchdock online docking server was used for initial screening to dock the nepetolide against the target receptors i.e. Cox-2 (PDB code 5IKQ) (Orlando and Malkowski, 2016), EGFR (PDB code 5JEB) (Novontny et al., 2016) and Lox-2 (PDB code 4NRE) (Kobe et al., 2014). Atomic contact energies and hydrogen bonds formed between the ligand and the target receptors from Patchdock and Autodock are given in the Table 4. Analysis of the docking results of nepetolide in complex with Cox-2 with Patchdock revealed that the ligand is inserted into the binding cage surrounded by CYS A:47, CYS A:36, HIS A:39, PRO A:40, GLY A:45, ARG A:469, ASP A:125, THR A:129, LYS A: 137, and GLN A:461 as shown in Fig. 5. These interactions between the residues of the receptor protein and ligand account for the atomic contact energy of −11.83 kcal/mol in Patchdock. Interaction of the EGFR with nepetolide have the atomic contact energy of −10.69 kcal/mol in Patchdock. Analysis of the interaction of nepetolide with EGFR showed that the ligand is surrounded by GLY A:695, GLY A:697, GLY A:700, PHE A:699, ASP A:831, LYS A:721, THR A:830, THR A:766, GLN A:767 and MET A:769 (Fig. 5). Atomic contact energy of Lox-2 with nepetolide is −13.44 kcal/mol (Patchwork). Nepetolide is surrounded by PHE A:365, GLU A:369, ILE A:412, ASN A:413, LEU A:415, ALA A:416, VAL A:603 and ILE A:676 residues of the receptor proteins (Fig. 5). In contrast while Patchdock results predicted primarily hydrophobic interactions, enhanced hydrogen bonding was found in the same region of the protein (predicted binding pockets were the same) when nepetolide was docked with Cox-2, EGFR, and Lox-2 using Autodock as shown in Fig. 6A–C and Table 4. The hydrogen bonding range used was 1.8–3.75 Å.
Table 4.
Atomic contact energies (kcal/mol) and hydrogen bonds formed by nepetolide with cyclooxygenase-2 (Cox-2), epidermal growth factor receptor (EGFR) and 15-lipoxygenase-2 (Lox-2).
| Atomic contact energies (kcal/mol) |
|||
|---|---|---|---|
| Cox-2 | EGFR | Lox-2 | |
| Nepetolide | −11.83 (−38.75) | −10.69 (−26.54) | −13.44(−29.25_ |
| Hydrogen bonds | 1(9) | 2 (6) | 2(7) |
The results represent the values for the best solution from docking programs. The numbers in parenthesis represent Autodock results.
Fig. 5.
Binding interactions of nepetolide with cyclooxygenase-2 (Cox-2), epidermal growth factor receptor (EGFR) and 15-lioxygenase-2 (Lox-2) using Patchdock (Duhovny et al., 2002, accelrys.com, 2017).
Fig. 6.
Binding interactions of nepetolide with A) cyclooxygenase-2 (Cox-2), B) epidermal growth factor receptor (EGFR) and C) 15-lioxygenase-2 (Lox-2) using Autodock and rendered in Chimera (Morris et al., 2009, Pettersen et al., 2004).
3. Discussion
Nepeta species are used traditionally as a medicine in the treatment of various diseases (Amin, 1991). Nepetolide isolated from Nepeta suavis, was screened for pharmacological activities and evaluated as a lead compound using in silico docking studies. A antioxidant is an agent capable of inhibiting the oxidation of other molecule. It is actually a radical scavenger which protects the human body against free radicals which may be one of the reason of certain pathological conditions. These free radicals are produced as result of oxidation reactions and have the capability to start chain reactions that damages the cells. Antioxidants remove the free radicals intermediates thus terminate these chain reactions. Actually antioxidants oxidize themselves and causes the reduction of other compounds. Decrease in absorbance by increase in concentration of nepetolide revealed the increase in free radicals scavenging activity of nepetolide (Kumar et al., 2011). The antioxidant potential of the nepetolide was quite significant as compared to the ascorbic acid standard, especially at the doses of 300 and 1000 µg/mL. Results indicate nepetolide may be a good candidate to develop new drugs for oxidative stress disorders relative to human, as formation of reactive oxygen species may lead to serious health consequences (Aruoma, 2003).
Recent research approaches give the evidence of discoveries of new antimicrobial agents and most of these compounds are isolated directly from medicinal plants (Trusheva et al., 2006). The results of antimicrobial assays showed that the nepetolide has limited antimicrobial activity as it was inactive against the fungal strains while it was only moderately active against one of the bacterial strain i.e. Staphylococcus aureus. Nepetolide was found to be effective through brine shrimp lethality bioassay. To determine the cytotoxic potential of nepetolide, brine shrimp lethality bioassay was used because it is less time consuming, inexpensive and convenient bioassay tool. Cytotoxic potential is considered as a preliminary screening for the drugs having anticancer potential (Karadeniz et al., 2011). Nepetolide was found to have good cytotoxic potential, with LC50 value of 8.70 ug/mL that was nearly comparable with the standard drug etoposide having LC50 value of 5.64 µg/mL. The nepetolide with the significant cytotoxic potential may be a source of anticancer potential as there is established literature that shows the correlation between the brine shrimp toxicity and human nasopharyngeal carcinoma (Rehman et al., 2009). Prior to screening the nepetolide for antitumor activity through potato disc tumor assay, antibacterial activity of nepetolide was performed in order to confirm that whether the test compound is inhibiting the tumor formation through killing of Agrobacterium tumefaciens or through interfering with the DNA transfer mechanism of bacteria. No zone of inhibition was observed, suggesting that nepetolide is inhibiting the tumor through interfering with the some of the vital mechanism inside the cell of bacteria. Nepetolide exhibited moderate antitumor activity with 56.5 ± 1.5% tumor inhibition at the dose of 1000 µg/mL. Results indicate that nepetolide can prove to be a potential antitumor agent in a dose-dependent manner. Potato disc tumor assay is the simple method used for the determination of antitumor potential of natural compounds (Stachel and Zambryski, 1989). Tumor in potato has the resemblance with the tumors in human, giving the evidence that the agents that are active as an antitumor agent in potato, can also prove to be active in humans as well (Mclaughlin, 1991, Agrios, 1997). Carrageenan-induced hind paw edema model is the most frequently used to determine the anti-inflammatory effect of natural products. When the carrageenan is injected into the hind paw of rat, it produces biphasic response. First phase involves release of histamine, serotonin and kinins. This phase is because of trauma of injection and begins within 1st hour and lasts for 2 h (Okokon and Nwafor, 2010, Rahman et al., 2010). In the second phase there is release of prostaglandins that begins after 2nd hour. Indomethacin was used as a standard drug in this assay. Indomethacin seems to interfere with inflammatory mediation, by inhibiting prostaglandin synthesis, that is in the second phase of inflammatory response, as it significantly decreases the paw edema after 3 h of carrageenan administration (Lu et al., 2007). The possible mechanism behind the nepetolide anti-inflammatory activity may also be due to inhibition of prostaglandin synthesis as it significantly decreases the paw edema after 2 h of carrageenan administration. As non-steroidal anti-inflammatory drugs (NSAIDs) cause 3500 hospitalizations and 400 deaths from ulcer bleeding per annum in the United Kingdom in those aged 60 years and above and about 30–60% of NSAIDs users have gastrointestinal effects such as dyspepsia and some abdominal discomfort (Al Mofleh and Al Rashed, 2007). Since nepetolide is also a potent free radical scavenger, it may have sufficient potential to inhibit the inflammatory response by acting on Cox-2. Nepetolide may prove to be a potent anti-inflammatory agent (Brahmbhatt et al., 2010). Acetic acid induced writhing test is commonly used to investigate the potential of peripherally acting analgesic drugs (Hossain et al., 2011, Viana et al., 2011). Acetic acid causes the constriction of abdominal muscles. In writhing test, writhes are induced by acetic acid administration and peripheral analgesic activity of nepetolide is investigated. Intraperitoneal injection of acetic acid causes algesia by the activation of chemo-sensitive nociceptors or irritation of visceral surface that results the release of free arachidonic acid from tissue phospholipids, which also causes the release of histamine, serotonin, bradykinin and prostaglandin along with increased level of these mediator in the peritoneal fluid. This response is mediated by acid sensing ion channels, eicosanoids pathway and peritoneal mast cells (Taïwe et al., 2011). In the present study, nepetolide showed convincing analgesic activity at higher dose of 500 mg/kg. This represents the analgesic potential of nepetolide against the peripheral pain mechanism that may be attributed to the liberation of inflammatory mediators or through the blockade of eicosanoid system by inhibition of cyclooxygenase (Cox-1 and Cox-2) and may be due to receptor blockade. Docking studies of nepetolide strongly suggest nepetolide has affinity towards Cox-2. Apart from anti-inflammatory response, Cox-2 may also have the potential of causing carcinogenesis due to generation of free radical. These free radicals may cause carcinomas. So inhibition of Cox-2 enzyme may also have role in inhibition of cancer and their levels are up-regulated in various carcinomas. (Howe et al., 2001). As shown in Table 4, the docking results of Cox-2, EGFR, and Lox-2 strongly supports nepetolide interaction with these 3 targets, making a convincing case of nepetolide as a promising lead compound in drug design.
Catalytic products of lipoxygenases are associated with carcinogenic processes such as tumor cell proliferation, differentiation, apoptosis and has been recognized as drug targets for treatment of inflammation (Wisastra and Dekker, 2014). Therefore, six hydrogen bonds formed between nepetolide and Lox-2 and atomic contact energy of −26.50 kcal/mol suggests that nepetolide may prove to be a potent anti-inflammatory as well as anticancer agent.
4. Conclusions
Different pharmacological activities of nepetolide that includes antioxidant, antibacterial, cytotoxic, anti-tumor, anti-inflammatory and analgesic activity showed significant results. Thus there is need for further in-vivo investigation of nepetolide, so as to validate the activity, to optimize its dose and to confirm its safety profile further to be designed into new drugs having better efficacy and safety.
5. Experimental
5.1. Chemicals
1,1-diphenyl-2-picrylhydrazyl (DPPH), acetic acid, ascorbic acid, carrageenan 1% w/v solution, ciprofloxacin, diclofenac sodium, di-methyl sulphoxide (DMSO), etoposide, ethanol, indomethacin, lugol’s solution, iodine (I), mercuric cyanide, potassium iodide (KI), ciprofloxacin and vincristine (Sigma Chemicals Co., St. Louis, MO, USA) of analytical grade were used.
5.2. Animals
Sprague Dawley rats (100–150 gm) and Balb-C mice (25–30 gm) of either sex were employed to investigate anti-inflammatory and analgesic effects of the test drug respectively. Animals were housed at the Animal House of Riphah Institute of Pharmaceutical Sciences, maintained at 23–25 °C and were given standard diet and tap water ad libitium. Experiments performed complied with the rules of Institute of Laboratory Animal Resources, Commission on Life Sciences University, National Research Council (1996) and approval was granted by Ethical Committee of Riphah Institute of Pharmaceutical Sciences, Riphah International University (Ref. No. REC/RIPS/2015/007).
5.3. Antioxidant analysis
2,2-diphenyl-1-picrylhydrazyl (DPPH) radical-scavenging assay was used to determine the antioxidant potential of nepetolide (Duh et al., 1999). DPPH is a free and stable radical so it is used for the determination of antioxidant activity (Chen et al., 1999). Serial dilutions of nepetolide were having concentrations of 1, 3, 5, 10, 100, 300 and 1000 ug/mL in 5% DMSO. 1 mM fresh DPPH was prepared in ethanol. Ascorbic acid was used as a standard (positive control). 1 mL of freshly prepared DPPH solution was added to each of the concentration of nepetolide and control containing no nepetolide. The solutions were vortexed and then were allowed to stand for 30 min at room temperature. Absorbance of each solution was then measured at 517 nm by using UV spectrophotometer (SP-3000 PLUS Spectrophotometer, Optima, Japan). The procedure was carried out in triplicate. At high concentration of the test drug the absorbance decreases that is the evidence of increase in free radical scavenging (Kumar et al., 2011). Percentage of free radical scavenging was calculated by using following formula
where, A = Absorbance of control, B = Absorbance of test sample.
EC50 was calculated by plotting the graph between concentration and% scavenging.
5.4. Antibacterial evaluation
Disc diffusion method was used to determine the antibacterial potential of the nepetolide (Rodrigues et al., 2008). Serial dilutions of nepetolide were prepared having concentrations of 1, 3, 5, 10, 100, 300 and 1000 µg/mL. 5% DMSO was used as a vehicle. Filter paper (Whatman No. 3) was cut into the shape of circular discs of 7 mm. These discs were then separately impregnated with already prepared test compound solution, allowed to dry at room temperature and then were placed in refrigerator. Antibacterial activity of the nepetolide was evaluated against Acinobacter (ATCC-6539) and Staphylococcus aureus (ATCC-6538) that were obtained from the microbiology laboratory of Quaid-e-Azam University Islamabad and against two clinical isolates obtained from Islamabad Diagnostic Laboratory i.e. Escherichia coli and Methicillin resistant Staphylococcus aureus. First the nutrient broth was prepared and was then autoclaved. Already prepared nutrient broth bacterial culture of 1.5 × 108 colony forming units (CFU) was taken and uniformly distributed on nutrient agar medium plates with the help of sterilized cotton swab. Discs of paper containing different concentrations of nepetolide were placed on surface of inoculated nutrient agar plates and were then incubated for 24 h at 36 °C. After incubation growth and zone of inhibition was observed. Ciprofloxacin 5 ug disc was used as a standard and 5% DMSO was used as a negative control. Experiment was performed in triplicate. The lowest concentration inhibiting the growth was taken as minimum inhibitory concentration (MIC).
5.5. Antifungal evaluation
Fungal strains with ATCC number used in the assay include: Candida albican (ATCC-60387) and Aspergillus niger (ATCC-16404). These fungal strains were obtained from microbiology laboratory of Quaid-e-Azam University Islamabad. Nutrient broth media was prepared according to the reported procedure. Media was then inoculated with fungal strains and incubated at 25 °C for initial growth. Some of the media was transferred to test tubes (10 mL) in specific quantity. 1 mL solution of each of the serial dilutions of the four test compounds were then added to the test tubes containing nutrient broth medium. These test tubes were inoculated with the fungal strains and then incubated at 25 °C for 8 days. After the incubation period fungal growth was observed and analyzed (Hussain et al., 2010, Ismail et al., 2012). Lowest test compound concentration inhibiting the growth was taken as MIC.
5.6. Cytotoxic bioassay
Brine shrimp lethality test was proposed to estimate and determine the cytotoxic potential of nepetolide. Brine shrimp eggs were allowed to hatch in artificial sea water for 48 h at 22–29 °C (Ali et al., 2011). Serial dilutions of the nepetolide were prepared in the concentration of 1, 3, 5, 10, 100, 300 and 1000 ug/mL. 100 µL from each of these solution was then transferred to 90-well microplate in triplicates. 50 µL larvae solution containing 10 napulii larvae were transferred to each well with the help of pasteur pipette. Standard etoposide was used as a positive control while 5% DMSO as a negative control. Micro-well plate was then covered and incubated at 22–29 °C for 24 h. The number of live and dead shrimps was counted after 24 h in each well under 3x glass of microscope and percentage larvae killed were determined. LC50 was calculated using Graphpad prism 6.0.
5.7. Antitumor evaluation
Antitumor potential of the nepetolide was evaluated by using potato disc method with slight modification (Ferrigni et al., 1982, Mclaughlin, 1991). Fresh potatoes (red skinned) were sterilized with Clorox (20% solution) and then were cut down into were cut into discs of 1 cm × 0.5 cm. Five discs of potato were placed in each of petri dish containing 1.5% agar solution, in such a way that two third of each discs are dipped in the agar solution. A 48-hour-old bacterial culture of Agrobacterium tumefaciens (AtTp0120) was used as bacterial culture. Test compound solution of 1, 3, 5, 10, 100, 300 and 1000 µg/mL prepared. 100 µL from each of the serial dilution was mixed with 100 µL of bacterial solution. Vincristine 250 µg/mL was used as a positive control and 5% DMSO was used as a negative control. 50 uL from this mixed solution was then poured onto the surface of the discs in the petri dish and were incubated at room temperature for 21 days. After 21 days, potato discs were stained with Lugol's solution (5% I2 and 5% KI). Tumors on the potato discs were then counted under microscope. Experiment was performed in triplicates. Percentage inhibition of tumor was calculated by following formula.
In order to check, that whether compounds are inhibiting the tumor formation through killing of Agrobacterium tumefaciens, or through some other mechanism sensitivity of Agrobacterium tumefaciens against the newly synthesized compounds, was tested through disc diffusion method (Bauer et al., 1966). Serial dilutions of the test compounds i.e. 1, 3, 5, 10, 100, 300 and 1000 µg/mL, were made. 100 µL of nutrient broth bacterial culture (106 CFU/mL) was spread on muller hinton agar plates. Ciprofloxacin and imipenam were used as positive control. Filter paper discs impregnated with 10 μL of test compounds, were placed on muller hinton agar plates and were incubated for 24 h at 28–30 °C. Viability of the bacterium against the tested compounds was assessed by measuring zone of inhibition. Test was performed in triplicate.
5.8. Anti-inflammatory activity
Anti-inflammatory activity of nepetolide was determined using carrageenan-induced hind paw edema method in rats (De et al., 2010). Animals were divided into three groups having four animals in each group and were kept fastened overnight. Control group was treated with normal saline (10 mL/kg) solution by intraperitoneal route. A group was treated with nepetolide 20 mg/kg. Another group was treated with 10 mg/kg of indomethacin. After 30 min 0.1 mL of 1% carrageenan solution was injected to the hind paw of each of the following group. Paw volumes were measured with help of vernier caliper after 0, 1, 2, 3 and 4 h of carrageenan administration. Results were represented as mean ± SEM and evaluated statistically by applying one-way ANOVA followed by Tukey’s test, represented graphically.
5.9. Analgesic action
Analgesic activity of nepetolide was determined using acetic acid induced writhing test in mice (Sadaiah et al., 2011). Animals were divided into four groups each having sample size of four. Group 1 was considered as control group and was administered 5% normal saline solution. Group 2 and 3 were treated with 100 mg/kg and 500 mg/kg of nepetolide. Group 4 was treated with aspirin 200 mg/kg. After 30 min writhing was induced by intraperitoneal administration of 1% acetic acid solution. After acetic acid administration muscular contractions were counted over a period of 20 min. Percent inhibition of writhing was calculated by following formula:
5.10. Computational study
The structures of nepetolide was retrieved from the Pubchem database (www.ncbi.nlm.nih.gov/Pubchem) (Fig. 1). Protein data bank database (www.rcsb.pdb) was used to obtain the three dimensional structure of the Cox-2, EGFR and Lox-2 with pdb IDs 5IKQ (Orlando and Malkowski, 2016), 5JEB (Novontny et al., 2016) and 4NRE (Kobe et al., 2014). The initial in silico screening docking process was done by submitting the pdb coordinates of above proteins and ligand nepetolide to Patchdock online docking server (Duhovny et al., 2002, Schneidman-Duhovny et al., 2005). The atomic contact energies of the best of ten docked poses were obtained for ligand and receptor proteins. Contact analysis of the docked complexes was done using discovery studio 3.1 visualizer (accelrys.com, 2017). Autodock (Morris et al., 2009) was also utilized to verify top 10 nepetolide conformations from Patchdock and Chimera (Pettersen et al., 2004) was used to visualize the top 10 poses of Autodock output. PRODRG (Schüttelkopf and van Aalten, 2004) software suite was used to convert nepetolide conformations output from Patchwork PDB format to PDB format that could be visualized and analyzed in Pymol (pymol.org, 2017) with Autodock output. Pymol was used to superimpose the top scoring Patchwork and Autodock output nepetolide PDBs. Both Patchwork and Autodock are automated docking tool which operate by Lamarckian genetic algorithm predicting how small molecules such as potential drug candidates like nepetolide bind to a receptor of known 3D structures.
Conflict of interest
Authors reports no conflict of interest.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sector.
Acknowledgement
The authors are thankful to Samaviya Taslem and Mr. Saadat Ali Khan for his co-operation and for her kind support to carry out the analgesic activity. Authors also express their gratitude to Mr. Hassan Afzal and Mr. Fawad Ali for their co-operation and guidance during entire experimental analysis.
Footnotes
Peer review under responsibility of King Saud University.
References
- http://accelrys.com/products/collaborative-science/biovia-discovery-studio/ (Last accessed 4/1/17).
- Agrios G.N. Plant path. 1997;4:407–470. [Google Scholar]
- Al Mofleh I.A., Al Rashed R.S. Saudi J. Gastroenterol. 2007;13:107–113. doi: 10.4103/1319-3767.33460. [DOI] [PubMed] [Google Scholar]
- Ali N., Ahmed G., Shah S.W.A., Shah I., Ghias M., Khan I. BMC Comp. Alter. Med. 2011;11:99. doi: 10.1186/1472-6882-11-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin G.R. Popular Medicinal Plants of Iran. Ministry of Health Publications; Tehran: 1991. pp. 40–41. [Google Scholar]
- Aruoma O.I. Mut. Res/Fund. Molec. Mech. Mutagenesis. 2003;523:9–20. doi: 10.1016/s0027-5107(02)00317-2. [DOI] [PubMed] [Google Scholar]
- Bauer A.W., Kirby W.M.M., Sherris J.C., Turck M. Amer. J. Clinic. Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- Brahmbhatt M.R., Patel J.M., Patel V.B., Saluja A.K. J Pharmacogn. Phytotherap. 2010;2:001–003. [Google Scholar]
- Chen Y., Wang M., Rosen R.T., Ho C.T. J. Agri. Food Chem. 1999;47:2226–2228. doi: 10.1021/jf990092f. [DOI] [PubMed] [Google Scholar]
- De S., Dey Y.N., Ghosh A.K. Inter. J. Pharma. Bio. Sci. 2010;1 BS15 ref.17. [Google Scholar]
- Duh P.D., Tu Y.Y., Yen G.C. LWT-Food Sci. Tech. 1999;32:269–277. [Google Scholar]
- Duhovny, D., Nussinov, R., Wolfson, H.J., et al., 2002. Efficient Unbound Docking of Rigid Molecules. In Gusfield et al., Ed. Proceedings of the 2'nd Workshop on Algorithms in Bioinformatics(WABI) Rome, Italy, Lecture Notes in Computer Science 2452 Springer Verlag. 185–200.
- Ferrigni N.R., Putnam J.E., Anderson B., Jacobsen L.B., Nichols D.E., Moore D.S., Smith C.R., Jr J. Nat. Pro.. 1982;45:679–686. doi: 10.1021/np50024a005. [DOI] [PubMed] [Google Scholar]
- Hossain M.S., Alam M.B., Chowhdury N.S., Asadujjaman M., Zahan R., Islam M.M., Islam A. Antioxidant, analgesic and anti-inflammatory activities of the herb. Eclipta prostrata J. Pharma Toxicol. 2011;6:468–480. [Google Scholar]
- Howe L.R., Subbaramaiah K., Brown A.M., Dannenberg A.J. Endocrine Relat. Cancer. 2001;8:97–114. doi: 10.1677/erc.0.0080097. [DOI] [PubMed] [Google Scholar]
- Hussain J., Ullah F., Hussain H., Hussain T., Shah M.R. Zeitschrift für Naturforschung. B. 2008;63:591–594. [Google Scholar]
- Hussain J., Khan F.U., Gilani S.A., Abbas G., Ahmed S., Khan A.U., Choudhary M.I. Latin Amer. J. Pharm. 2010;29:573–578. [Google Scholar]
- Ismail M., Hussain J., Khan A.U., Khan A.L., Ali L., Khan F.U., Lee I.J. Evidence-based Comp. Alter. Med. 2012 [Google Scholar]
- Karadeniz B., Ulker Z., Alpsoy L. Toxicol Indus. Health. 2011 doi: 10.1177/0748233711428642. 0748233711428642. [DOI] [PubMed] [Google Scholar]
- Khan F.U. Kohat University of Science and Technology; 2010. Phytochemical Investigation on the Chemical Constituents of Nepeta Suavis and its Biological Activities. Ph.D. Thesis. [Google Scholar]
- Khan F.U., Khan A.U., Hussain J., Khan I.U., Muhammad N., Khan A., Gilani A.H. Nat Pro Commun. 2016;11:591–592. [PubMed] [Google Scholar]
- Kobe M.J., Neau D.B., Mitchell C.E., Bartlett S.G., Newcomer M.E. J. Biol Chem. 2014;289:8562–8569. doi: 10.1074/jbc.M113.543777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Kumar V., Chandrashekhar M.S. Inter. J. Pharm. Tech. Res. 2011;3:889–894. [Google Scholar]
- Lu T.C., Ko Y.Z., Huang H.W., Hung Y.C., Lin Y.C., Peng W.H. J. Ethnopharm. 2007;113:142–148. doi: 10.1016/j.jep.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Mclaughlin J.L. Methods Plant Biochem. 1991;6:1–32. [Google Scholar]
- Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. J. Computat. Chem. 2009;16:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newall C.A., Anderson L.A., Phillipson J.D. The pharmaceutical press; 1996. Herbal medicines. A Guide for Health-care Professionals. [Google Scholar]
- Novontny C.J., Pollari S., Park J.H., Lemmon M.A., Shen W., Shokat K.M. Nat. Chem. Biol. 2016;12:923–930. doi: 10.1038/nchembio.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okokon J.E., Nwafor P.A. Pak J .Pharm. Sci. 2010;23:383–390. [PubMed] [Google Scholar]
- Orlando B.J., Malkowski M.G. J. Biol. Chem. 2016;291:15069–15081. doi: 10.1074/jbc.M116.725713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E., Comput J. Chem. 2004;25(13):1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- https://www.pymol.org/ (Last accessed 4/1/17).
- Rahman M.A., Bachar S.C., Rahmatullah M. Pak J. Pharm. Sci. 2010;23:256–258. [PubMed] [Google Scholar]
- Rehman A.U., Mannan A., Inayatullah S., Akhtar M.Z., Qayyum M., Mirza B. Pharma Bio. 2009;47:628–633. [Google Scholar]
- Rodrigues J., Michelin D.C., Rinaldo D., Zocolo G.J., dos Santos L.C., Vilegas W., Salgado H.R.N. J Med Food. 2008;11:120–126. doi: 10.1089/jmf.2007.557. [DOI] [PubMed] [Google Scholar]
- Sadaiah B., Kumar K.T.S., Kavitha C.H.N., Babu S.M., Reddy V.P. Der. Pharmacia Sinica. 2011;2:190–197. [Google Scholar]
- Schneidman-Duhovny D., Inbar Y., Nussinov R., Wolfson H.J. Nucl. Acids. Res. 2005;2005(33):W363–W367. doi: 10.1093/nar/gki481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüttelkopf A.W., van Aalten D.M.F. Acta Crystallogr D. 2004;60:1355–1363. doi: 10.1107/S0907444904011679. [DOI] [PubMed] [Google Scholar]
- Simonovic D. Botanical Dictionary. Institute for Serbo-Croatian Language; Belgrad: 1959. p. 3153. [Google Scholar]
- Stachel S.E., Zambryski P.C. Nature. 1989;340:190–191. doi: 10.1038/340190a0. [DOI] [PubMed] [Google Scholar]
- Taïwe G.S., Bum E.N., Talla E., Dimo T., Weiss N., Idiki S.N., Waard M.D. Pharm Bio. 2011;49:15–25. doi: 10.3109/13880209.2010.492479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusheva B., Popova M., Bankova V., Simova S., Marcucci M.C., Miorin P.L., Tsvetkova I. Evidence-Based Compl. Alter Med. 2006;3:249–254. doi: 10.1093/ecam/nel006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana A.F., Maciel I.S., Motta E.M., Leal P.C., Pianowski L., Campos M.M., Calixto J.B. Evidence-Based Comp. Alter Med. 2011:2011. doi: 10.1093/ecam/nep144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisastra R., Dekker F.J. Cancers. 2014;6:1500–1521. doi: 10.3390/cancers6031500. [DOI] [PMC free article] [PubMed] [Google Scholar]