Abstract
Pharmacovigilance is vital to public health. Adopting a robust spontaneous reporting system for adverse drug events can counteract most hazards that arise from utilizing medicinal products. Prior to the establishment of the Saudi Food and Drug Authority (SFDA), the number of pharmacovigilance-related activities in Saudi Arabia was limited. In 2009, the SFDA established the National Pharmacovigilance and Drug Safety Center (Saudi Vigilance). The pharmacovigilance system has remarkably improved during the past few years. Several initiatives have been taken to improve the program’s performance. These initiatives include initiation of pharmacovigilance guidelines, enhancement of communication and reporting tools, training sessions for concerned staff and healthcare providers, and compliance from stakeholders. This review article provides an overview of what the Saudi Vigilance program is, focusing on the scope, mission and vision, hierarchy, operational themes, and overall work processes. Additionally, we will shed light on the challenges we encountered during the early phase and on our future plans.
Keywords: Pharmacovigilance, Saudi Vigilance Program, Saudi Food and Drug Authority
1. Introduction
The Saudi Food and Drug Authority (SFDA) was established as an independent body in 2003 to regulate food, drugs, and medical devices as well as set necessary regulations and specifications for both imported and locally manufactured products. Prior to the SFDA’s establishment, most regulations related to these products were created by different ministries in Saudi Arabia, such as the Ministry of Health (MOH), Ministry of Commerce, Ministry of Municipality, and Ministry of Agriculture. Basically, the SFDA consists of three major sectors: the drug, food, and medical devices sectors. All three sectors are equipped with highly competitive technical associates and robust information technology systems.
One of the drug sector’s main tasks is to establish a suitable regulatory framework to monitor the risk-benefit balance of all registered products throughout their life cycles in the Saudi market. Therefore, the SFDA has established a pharmacovigilance system that operates under the National Pharmacovigilance and Drug Safety Center (NPC). The process of establishing the Saudi pharmacovigilance system, the challenges of implementing it, lessons learned, and more will be described in this paper.
2. Pharmacovigilance activities prior to the establishment of the SFDA
The earliest program for adverse drug event (ADE) reporting was established in 1975 as a hospital-based program at King Faisal Specialist Hospital and Research Center in Riyadh. In 1998, the MOH in Saudi Arabia established a post-marketing program, which focused mainly on early detection of unexpected and serious adverse drug reactions (ADRs), detecting increases in frequency of known ADEs, identifying quality defect issues of registered products, and disseminating necessary safety information. A training program was carried out in collaboration with the United States Food and Drug Administration (USFDA) in the main regions of Saudi Arabia. The program was launched in the main hospitals and private community pharmacies, and ADE reporting forms were dispatched to those institutions. In addition, a national database for aggregating received ADEs was initiated in 2002, and in 2003, an advisory committee was assigned to oversee, study, and classify ADE reports and other drug safety issues. Unfortunately, the program suffered from a lack of staff and technical support (Bawazir, 2006).
3. Legal basis for pharmacovigilance activities
The legal framework for pharmacovigilance of pharmaceutical products for human use in the community is given in the law of Pharmaceutical Institutions and Pharmaceutical Products number M/31 (Saudi Council of Ministers, 2005) as well as Council of Ministers directive number 168, dated September 2, 2002. These laws described the respective obligations of the marketing authorization holders (MAHs) and the national regulatory agency in Saudi Arabia to set up systems for pharmacovigilance to collect, collate, and evaluate ADEs and take the appropriate regulatory corrective actions to mitigate the risks certain medicines pose. The regulations required that the MAHs and national regulatory agency share all available information related to drug safety and effectiveness to ensure favorable risk-benefit balance of marketed pharmaceutical products.
To facilitate compliance with these obligations, the SFDA developed pharmacovigilance guidelines to describe the roles and responsibilities of all relevant stakeholders. These guidelines were developed by a committee that included representatives of a variety of Saudi institutions, such as the MOH, universities, and tertiary hospitals, to ensure involvement of different stakeholders. To cope with global harmonization efforts, the regulations implemented were based on the guidelines of the International Conference on Harmonization (ICH) and the European Medicine Agency (EMA) (European Commission, 2008). When the guidelines were being developed, interested entities including MAHs were given the opportunity to provide feedback concerning the beta version of the guidelines. That stage was deemed a fine-tuning period prior to the guidelines’ final implementation and adoption in 2009.
The SFDA officials believed that involving of all stakeholders in guideline development was important to initiating a solid, well-built pharmacovigilance system, even though the pharmacovigilance concept was relatively new to many MAHs with limited capabilities at that time. Because of capacity limitations, some companies expressed concern about implementing certain requirements. For example, obeying technical requirements for individual case safety reports (ICSRs) submission, such as generating XML-E2B files, was especially challenging for small and local companies. Therefore, during the transitional period it was acceptable to submit ICSRs using the Council for International Organizations of Medical Sciences (CIOMS) (Faich et al., 1990) format.
To facilitate the process of establishing a pharmacovigilance system, the guidelines emphasized the role of a qualified person responsible for pharmacovigilance (QPPV). Each MAH was asked to nominate a QPPV residing within the Kingdom of Saudi Arabia who would be responsible for the establishment and maintenance of the pharmacovigilance system. The legislations stated that a QPPV should be appropriately qualified and sufficiently trained in pharmacovigilance to fulfill the responsibilities outlined in the national pharmacovigilance guidelines. A database containing QPPV names, 24/7 contact details, and backup procedures was created to facilitate prompt follow-ups with the MAHs for any safety concerns.
Guideline development was challenging. There were few experts in pharmacovigilance. Furthermore, regulations supporting pharmacovigilance-related activities in the country were limited. The only way to overcome these shortcomings was training. Thus, the SFDA offered pharmacovigilance training for members of pharmacovigilance guidelines committee. In addition, available international pharmacovigilance guidelines were reviewed to select the most appropriate model for local implementation.
Selecting appropriate references for the new guidelines was another challenge. Adopting advanced regulations in a country where basic pharmacovigilance activities were lacking would not have made sense. Therefore, the committee members made massive effort to select the best model that pharmaceutical companies could implement and follow locally.
The committee chose to select the European Union’s (EU) Volume 9 Pharmacovigilance Guidelines as an authorized reference for pharmacovigilance-related activities for many reasons. First, the European Medicines Agency (EMA) represents the world’s largest union for drug regulatory authorities, and its regulations are widely respected because of its market size. Second, the Volume 9 guidelines were primarily based on international agreements regarding pharmacovigilance practices within the framework of the International Council for Harmonization (ICH). Third, it was deemed a solid platform for linking regulatory authorities with the pharmaceutical industry to discuss scientific requirements for drug registration and therefore improve harmonization in the interpretation and application of technical guidelines.
One of the difficulties experienced with the EMA’s pharmacovigilance guidelines was that the guidelines contained several sections describing internal EMA procedures (e.g., centralized and decentralized procedures) that were irrelevant to non-EMA members. Therefore, during the process of creating Saudi pharmacovigilance guidelines, the committee focused only on requirements related to pharmacovigilance activities and disregarded sections related to EMA procedures.
4. Building the center’s infrastructure
Establishing a new national pharmacovigilance center takes time, vision, dedication, expertise, and continuous follow-up. It was decided that to achieve its goals of ensuring post-marketing safety of authorized products, the center should be part of the SFDA, Saudi Arabia’s national regulatory authority. This decision provided the center more power to achieve its goals and objectives and ensure operational continuity. Many efforts were carried out, especially to build up the center’s infrastructure and increase the engagement of all relevant stakeholders.
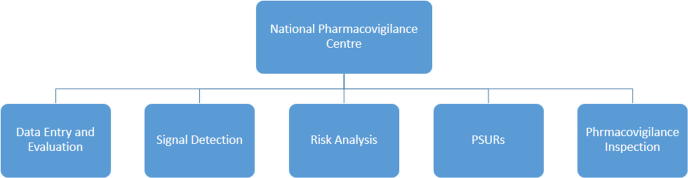
Building the center’s infrastructure included developing the organizational chart (Fig. 1), recruiting technical staff, training associates, designing reporting forms, setting up electronic platforms (databases), and initiating an advisory committee. The organizational chart was benchmarked to resemble international pharmacovigilance centers, and it was created to reflect all essential pharmacovigilance activities. The SFDA recruited 12 full-time employees with qualifications ranging from undergraduate degrees to postgraduate degrees in pharmaceutical sciences.
Fig. 1.
Organizational chart of the National Pharmacovigilance and Drug Safety Center (NPC). PSURs: periodic safety update reports.
5. Strategic plans
Since the SFDA’s establishment, two strategic plans have been implemented. Establishing and activating a pharmacovigilance center was clearly one of the SFDA’s top strategic initiatives. The first strategic plan (2006–2011) focused mainly on how to create a national pharmacovigilance center and transfer pharmacovigilance responsibilities from the MOH to the SFDA. Most of this plan’s objectives and action items pertained to manpower, training, and development; leveraging an operational model that mimics global best practices; detailed center design; and information technology (IT) support. The second strategic plan (2012–2016) focused on developing all available tools to increase stakeholders’ engagement in such pharmacovigilance activities as improving communication, establishing an inspection process, and detailing the penalties MAHs face if they violate the pharmacovigilance requirements.
6. Pharmacovigilance best practice model
To develop a clear understanding of optimal operational models it could adopt from international best practices, some of the SFDA’s staff visited several international regulatory authorities that have well-established pharmacovigilance systems. These institutions included the United Kingdom’s Medicines and Healthcare Products Regulatory Agency (MHRA), the Uppsala Monitoring Center (UMC) in Sweden, and the Netherlands’ Pharmacovigilance Centre (Lareb). The visits covered most of the daily business related to pharmacovigilance, including workflows, databases, and standard operating procedures (SOPs). These visits were useful in providing a clear understanding of the resources needed to establish the center and how to build a solid model that draws on international best practices in pharmacovigilance.
7. Program name and logo
To promote any public health program, the selection of a program name and logo is crucial. The name should be unique, reflective of the mission, and expressive. We chose Saudi Vigilance as the name for the program the National Pharmacovigilance Center runs. We designed the logo to give the center a unique identity and to visually represent the center’s vision and mission (Fig. 2). We selected a logo that displays the program’s name in both Arabic and English. An eye was vertically inserted to give the logo the sense of a “monitoring” function. The logo’s colors were chosen to match those of the SFDA logo (Fig. 3).
Fig. 2.

Logo of the National Pharmacovigilance & Drug Safety Center.
Fig. 3.

Logo of the Saudi Food and Drug Authority.
8. Training and professional development
People working in pharmacovigilance units needs continuous education and training. During the establishment phase and after the center opened, the SFDA has continued to offer extensive training programs for the center’s staff both inside or outside of Saudi Arabia. The training plan includes courses in causality assessment, signal detection, risk management, pharmacoepidemiology, critical appraisal, biostatistics, administrative skills, project management, and scientific writing. Elementary-level training courses have focused on basics of pharmacovigilance, such as the International Pharmacovigilance Training course, an intensive, three-week program provided by the UMC. This course covers essential pharmacovigilance topics to improve the performance of associates working at national pharmacovigilance centers. It also offers a platform for participants from different international authorities to have informative discussions and share their experiences in conducting and managing pharmacovigilance activities. A training manual has also been developed to provide essential information for newly recruited staff. The manual contains a clear training plan with mandatory beginner courses in pharmacovigilance regulations, data collection, validation, and coding.
9. Organizational chart
The NPC has five sections through which it will succeed at its mission. These five sections are Data Entry, Signal Detection, Risk Analysis, Periodic Safety Update Report Evaluation, and Pharmacovigilance Inspection. Data Entry is responsible for receiving, evaluation, entering, and archiving all ADEs from pharmaceutical companies and spontaneous reports from both healthcare providers or the public.
As underreporting is a global challenge to any spontaneous reporting system, data entry teams experience the same issue. To overcome this problem, many strategic solutions have been proposed. These strategic solutions included but not are limited to conducting workshops in hospitals and healthcare facilities and developing a regional committee in each region of Saudi Arabia to facilitate communication between the NPC and healthcare professionals (HCPs) working in that region. In addition, social media has been used to reach out to a larger group of patients to make them aware of the importance of reporting as well as of what could happen if they fail to report ADEs to the NPC. The other challenge was database selection and evaluation. Most commercially available databases are designed for and tailored to the pharmaceutical industry. One solution to this challenge is to develop an in-house database according to the center’s needs. This approach is being discussed with the SFDA’s information technology department.
The second section within the NPC is Signal Detection. This section reviews all safety signals that originate from different sources (e.g., local databases, literature, international authorities, and media). The signal detection team periodically reviews the local ADE databases to investigate issues raised by specific drugs or specific pharmacological groups. After a safety signal has been refined and detected, the section prepares a signal validation report with appropriate recommendations against the concerned medication(s), which the advisory committee will subsequently discuss to take the appropriate regulatory action.
The third section, Risk Analysis, manages, evaluates, and approves risk management plans (RMPs), including risk minimization measures (RMMs) and healthcare professional communications. As an example of approved RMMs, a pregnancy prevention program for isotretinoin products has been implemented to mitigate the teratogenic risks of isotretinoin products on women who are pregnant or plan to be. The measures include English- and Arabic-language patient consent forms and medication guides for both the public and HCPs that explain the risks associated with isotretinoin treatment and suggest precautions to minimize these risks. Continuous education of the public and HCPs about these measures has also been carried out by newsletters, the SFDA website, and official memos. One of the main challenges Risk Analysis faces is how to measure the implemented RMMs’ effectiveness. The NPC started to require certain effectiveness indicators from MAHs to ensure that implemented interventions were efficient enough to minimize potential harm. Another challenge is that some of the proposed RMMs apply only in countries with appropriate healthcare and information technology infrastructure. To overcome this issue, we require that QPPVs in Saudi Arabia review the proposed RMPs and examine their applicability in Saudi Arabia.
The fourth section within the NPC is Periodic Safety Update Report (PSUR) Evaluation. Each registered medication in Saudi Arabia should have a PSUR that MAH must periodically submit. The product’s safety profile and international birth date determine the period. After receiving the PSUR, the section’s staff will conduct a thorough safety evaluation. If there are any potential safety concerns, the PSUR team will communicate with other NPC sections or the MAH to recommend safety measures based on the identified risk(s).
The fifth section is Pharmacovigilance Inspection. This section conducts pharmacovigilance inspection activities according to SFDA requirements as per the published pharmacovigilance guidelines. Inspection coordination, MAH priority lists, findings reports, and follow-up are among this section’s routine tasks.
10. Official launch of the NPC
The NPC was officially launched in March 2009. The launch was announced via an international symposium held in Riyadh, the capital of Saudi Arabia. During the two-day symposium, experts in pharmacovigilance from different parts of the world were invited to deliver presentations on drug safety, post-marketing surveillance, collection and assessment of ADEs, and their pharmacovigilance experience in Arabic-speaking countries. Several awareness materials displaying the center’s logo of the center were distributed, including key rings, pens, coasters, booklets, flyers, and bookmarks.
The SFDA became a full member of the Uppsala Monitoring Center (UMC) of the World Health Organization (WHO) in 2009, shortly after the NPC was established to participate in the global effort to monitor drug safety (Uppsala Monitoring Center, 2017).
11. Electronic systems
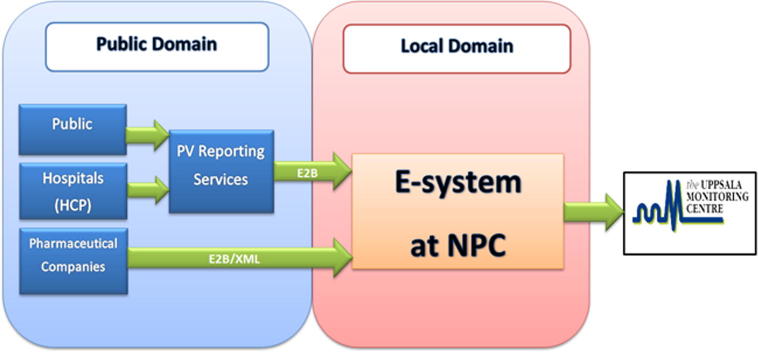
One of the cornerstones of developing an effective national spontaneous drug safety program is to establish an electronic system for ADE reports collection. In determining the best system for managing ADE reports, we had to consider several factors. One was the electronic interaction between different stakeholders, such as the SFDA, hospitals, the pharmaceutical industry, and the UMC (Fig. 4). The ICH’s standards of E2B guidance had to be implemented for both the working system and the database (International Conference on Harmonisation, 2001). Data ownership, confidentiality, security, and availability in the local electronic database also required investigation during the selection process. These factors were discussed in detail with different electronic solution vendors in accordance with best international practices.
Fig. 4.
Illustration of different stakeholders and their electronic interaction. PV: pharmacovigilance; HCPs: healthcare professionals; NPC: National Pharmacovigilance and Drug Safety Center.
Prior to the system’s installation, a detailed performance qualifications plan was conducted. This plan safeguarded the system’s production environment. System compatibility with available electronic drug and disease dictionaries such as the Medical Dictionary for Regulatory Activities (MedDRA), a dictionary developed and maintained by the ICH and updated twice per year, was required during the selection process. This process was carried out to avoid any possible complications during system start-up. Another key requirement was the system’s ability to detect duplicate cases. This is an important feature for case evaluations in the system as it prevents false frequencies and odd trends among reported ADE cases. The vendor customized new fields—for instance, “action taken by the reporter”—per a request from the NPC staff.
To choose an appropriate case management system, a number of factors should be considered. Factors considered during the establishment of the NPC included, among others, case data–entry methods in the system, support of electronic dictionaries, ease of use, export and import functionality based on E2B guidance, training, and technical support required.
To guarantee that the spontaneous reporting system operates efficiently, staff should be able to enter ADE data from different stakeholders manually, automatically, or semi-automatically. These entry methods are also useful when working with cases received from MAHs and healthcare professionals. The system must be sensitive to drug as well as events dictionaries, e.g., MedDRA coding language. Therefore, a pivotal aspect of any case management system is its ability to perform dictionary updates smoothly and with minimal technical involvement. The web interface should be easy to use and accessible via the organization’s intranet. This will help staff members become skillful users of the system and keep it secure at all times.
An important aspect of any case management system is the availability of cases reporting on international guidance (E2B). This function is important to facilitate communication with different stakeholders, as when receiving individual case safety reports (ICSRs) from MAHs or sending ADE cases to the WHO. Another important aspect for drug regulators in case management systems is the ability to add newly registered drugs to the system when they hit the market. The addition of new drug lists should not significantly affect the system.
Case duplication screening is also essential. The system should be able to detect duplicate cases automatically. In addition, the system should be able to track these cases with unique case identifiers and log all processes done on each case entered. This will help the head of the center to track all cases and staff members’ workloads during all stages of case entry, coding, and evaluation.
When considering the procurement of a case management system, the provider must fulfill the above factors. Web demonstration of the system must be organized for department staff, who should be able to first examine the system’s functionalities in a testing environment. Such sessions with all staff usually raise issues that may arise with the system in the future.
12. Spontaneous reporting
A spontaneous report is an unsolicited communication that healthcare professionals or consumers send to a company, regulatory authority, or other organization. It describes one or more adverse drug reactions in a patient who was given one or more medicinal products; it does not derive from a study or any organized data-collection scheme (Ahmad, Goetsch, & Marks, 2007). Spontaneous reporting of ADEs is the most important source of information in pharmacovigilance. Therefore, it is crucial to establish a solid reporting system with a user-friendly interface.
The NPC developed three methods for reporting ADEs: (a) online submission; (b) paper-based reporting forms; and (c) traditional methods, such as verbal, fax, and telephone reporting. All forms were compatible with ICH guidelines and included four essential elements: an identifiable patient, at least one suspected product, at least one suspected ADE, and an identifiable reporter (International Conference on Harmonisation, 2003). An agreement with the Saudi Post was signed to facilitate paper submission to the NPC at no cost (i.e., prepaid postage) to reporters.
There were two versions of the online form. The first version was designed as a Web-based, drop-list form with Naranjo Probability Scale for causality assessment purposes (Théophile et al., 2013). This version had several limitations, including slow website navigation, unfavorable design, and a drop-list design that led to system instability and loss of pre-filled data. In addition, attachment upload of some documents proved difficult, and feedback recommended against using the Naranjo scale in the forms.
Based on this feedback, the online reporting form was revised. The new form was developed as a wizard to save the data before moving forward. The Naranjo scale was removed, navigation speed was enhanced, and the system was updated to accept attachments, such as photos of the products reported to have quality defects. In the updated version, there were three different types of reporting forms for different types of reporters. Two forms targeted HCPs to report suspected ADEs or product quality issues. The third form was purposely designed for members of the public to report either suspected ADEs or product quality defects. The latter form used nontechnical, layman’s Arabic to facilitate understanding of the terminology and to streamline the overall reporting process.
13. Pharmacovigilance advisory committee
The Pharmacovigilance Advisory Committee is an independent expert panel responsible for assessing drug-related safety issues. This group’s main responsibilities are to evaluate potential signals raised by spontaneous reports, evaluate documents submitted by the NPC pertaining to marketed products’ risk-benefit profiles, propose necessary actions to minimize potential drug-originated risks, and discuss any emerging safety concerns based on NPC requests.
Basically, the advisory committee consists of 18 members who represent their institutions as drug safety experts. They have been chosen because of their qualifications, experience, specialties, and interest in aspects of pharmacovigilance and drug safety. Members are usually physicians from different disciplines (cardiology, nephrology, internal medicine, etc.); clinical pharmacists; and academic scientists. The SFDA periodically reviews the committee’s membership. All members are required to disclose potential conflicts of interests before partaking in committee businesses.
The Pharmacovigilance Advisory Committee meets when a safety issue arises. The committee has met 37 times since its launch date and has discussed more than 100 safety issues and 19 MAH responses.
14. NPC coordinators
Saudi Arabia is a country with a large area, and to facilitate communication between the NPC and health institutions, the SFDA decided to assign a nationwide network of pharmacovigilance coordinators. Members of this network are HCPs—primarily pharmacists working in tertiary hospitals. The NPC coordinators split into five regional committees corresponding to the country’s five provinces (central, western, eastern, southern, and northern).
The major role of pharmacovigilance coordinators is to liaise between the NPC and their institutions. They also play a significant role in raising awareness about the NPC’s existence and stressing the importance of submitting ADE reports from their individual health institutions. Each committee meets at least four times annually with NPC officials to discuss updates, any difficulties or challenges related to their jobs as coordinators, and address these when possible. In addition, they provide valuable input on how to improve the NPC’s reporting tools.
In 2013, the SFDA held an annual meeting for all coordinators. The purpose of this meeting was to share the experiences of all coordinators, address challenges coordinators faced, and discuss what the NPC or coordinators could do to overcome those challenges. Furthermore, the NPC staff delivered several presentations about signal detection, risk-minimization strategies, pharmaceutical products’ quality defects, pharmacoepidemiological studies, medication errors, and vaccine safety. The SFDA president commended the coordinators for their efforts and appreciated their continuous support of the pharmacovigilance program. This meeting was a unique experience because it entailed thorough discussion of reporting barriers, and several suggestions were flagged and later implemented. In addition, the coordinators felt that they were engaged within the context of the NPC’s mission, vision, and initiatives. Furthermore, they felt that their voluntary work with the NPC was recognized.
15. Awareness campaigns
For the sake of raising awareness of the importance of reporting ADEs to the NPC, many promotional campaigns have been initiated in a great number of healthcare settings, such as hospitals, academic institutions, and public places (e.g., shopping malls). More than 80 workshops have been conducted in different parts of Saudi Arabia. The presentations delivered in these workshops have included information detailing the nation’s pharmacovigilance system and the importance of engaging various stakeholders in ADE reporting. These workshops usually demonstrate all methods of reporting ADEs and include sessions to discuss the challenges each institution faces. To encourage concerned healthcare professionals to attend NPC workshops, the SFDA has worked with the Saudi Commission for Health Specialty (SCHS) to grant each attendee three continuous medical education hours (CMEs).
Over the years, the NPC has also collaborated with several large community pharmacy chains in Saudi Arabia to raise awareness and understanding of the importance of running an effective pharmacovigilance program in a community pharmacy. These community pharmacies have distributed packaged NPC promotional materials to the public, including detailed information on how to fill out reports and general information about drug safety and quality.
16. Current situation and future plans
Today, the NPC efficiently conducts several pharmacovigilance activities, such as ADE evaluation, vaccine safety evaluation, signal detection, assessment of periodic safety update reports, risk assessment and analysis, oversight of proper RMP application, pharmacovigilance communication, medication error evaluation, and pharmacovigilance inspection. Fig. 5, Fig. 6, Fig. 7 show some statistics related to the center’s activities, including cases uploaded to the database.
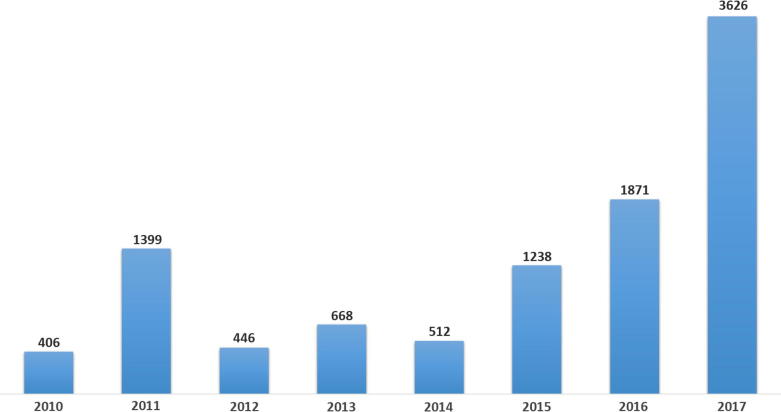
Fig. 5.
Local adverse drug event cases (from Saudi Arabia) reported to the center (January 2010–September 2017).
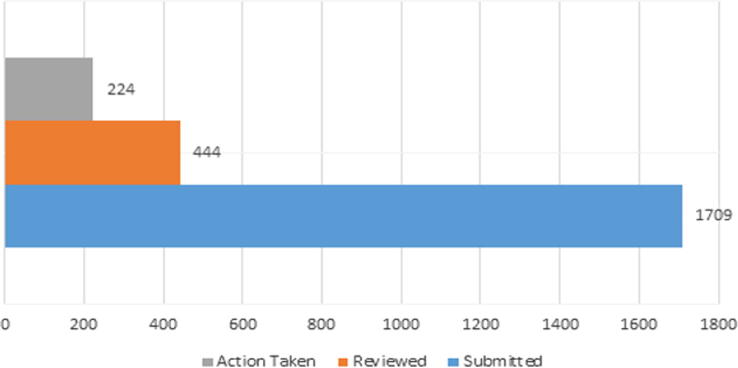
Fig. 6.
Submitted and reviewed periodic safety update reports (PSURs).
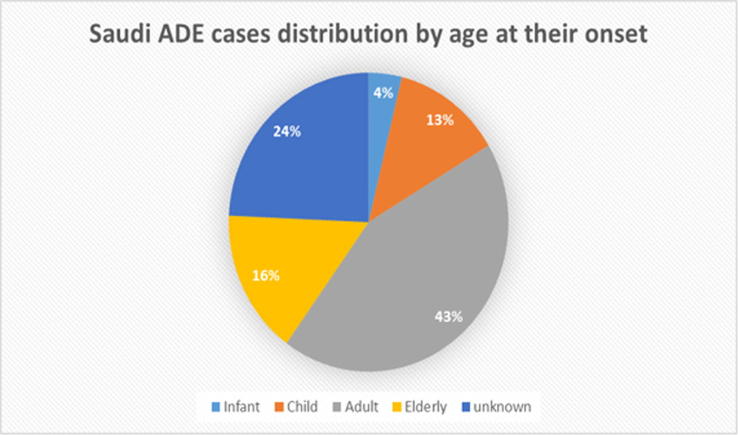
Fig. 7.
Local Saudi adverse drug events (ADEs) stratified by age at onset of adverse event.
As Fig. 5 illustrates, the trend of local ADEs has increased significantly in the last few years. Such increase may be due to several implemented measures. Since 2014, the center improved most of communication tools with both the public and healthcare providers including public campaigns in shopping malls and the media and participation in scientific conferences. In addition, several meetings with managements of large hospitals in the country were held to bolster collaboration and reporting. The publication of the new pharmacovigilance guideline in late 2015 and increased number of inspections to pharmaceutical companies may also contributed to the enhanced reporting.
Most of the center’s staff have attended pharmacovigilance and pharmacoepidemiology courses at well-known institutions around the world. In addition, to support the center’s mission with qualified drug safety professionals, almost half of employees have been awarded educational scholarships to pursue graduate degrees in pharmacovigilance-related disciplines. The SFDA and NPC leadership are making efforts to further enhance the staff’s technical competency and skills to bolster pharmacovigilance activities in the country.
17. Challenges
One of the most notable challenges in pharmacovigilance is underreporting. This phenomenon can be seen in many countries (Biagi et al., 2013). The center is trying hard to engage both HCPs and the public in its activities to improve the reporting rate. Another challenge that was encountered during the early phase of the center’s establishment was limited cooperation from some national hospitals. This obstacle was overcome by continuous appreciation and promotion of the importance of these hospitals’ contributions and workshops that emphasize the vital importance of ADE reporting. Also, we developed a tool that allows hospitals’ representatives to access the online reporting system to search all electronically submitted cases from their hospitals; this tool serves as a database for follow-up purposes. Staff turnover and lack of highly qualified personnel are major problems that the center continues to encounter.
18. Conclusion
Establishing an effective national pharmacovigilance center requires political commitments from health authorities; continuous, dedicated work; and financial and human resources support. Collaboration with all relevant stakeholders (i.e., MAHs, hospitals, and other international regulatory agencies) is crucial to supporting pharmacovigilance activities. Because pharmacovigilance is a rapidly growing field, there is a constant need to update pharmacovigilance guidelines, invest in staff training and development, monitor international pharmacovigilance initiatives, upgrade computer systems, and implement all available tools to ensure a positive risk-benefit balance in pharmaceutical products.
Source of funds
This research received no grant support from funding agencies in the public, commercial, or non-profit sectors.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmad S.R., Goetsch R.A., Marks N.S. Pharmacoepidemiology. fourth ed. John Wiley & Sons Ltd.; Chichester, UK: 2007. Spontaneous reporting in the United States. [Google Scholar]
- Bawazir S. Attitude of community pharmacists in Saudi Arabia towards adverse drug reaction reporting. Saudi Pharm. J. 2006;14(1):75–83. [Google Scholar]
- Biagi C., Montanaro N., Buccellato E., Roberto G., Vaccheri A., Motola D. Underreporting in pharmacovigilance: an intervention for Italian GPs (Emilia–Romagna region) Eur. J. Clin. Pharmacol. 2013;69(2):237–244. doi: 10.1007/s00228-012-1321-7. [DOI] [PubMed] [Google Scholar]
- European Commission Volume 9A of the Rules Governing Medicinal Products in the European Union: Guidelines on Pharmacovigilance for Medicinal Products for Human Use. 2008. http://ec.europa.eu/health/documents/eudralex/vol-9/index_en.htm Retrieved from.
- Faich G.A., Castle W., Bankowski Z. International adverse drug reaction reporting: the Cioms project. Drug Inform. J. 1990;24(2):419–425. [PubMed] [Google Scholar]
- International Conference on Harmonisation Maintenance of the ICH Guideline on Clinical Safety Data Management: Data Elements for Transmission of Individual Case Safety Reports. 2001. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E2B/Step4/E2B_R2__Guideline.pdf Retrieved from.
- International Conference on Harmonisation Post-Approval Safety Data Management: Definitions and Standards for Expedited Reporting. 2003. http://academy.gmp-compliance.org/guidemgr/files/E2D_GUIDELINE.PDF Retrieved from.
- The Saudi Council of Ministers Law of Pharmaceutical Products and Pharmaceutical Institutions. 2005. http://www.sfda.gov.sa/ar/drug/drug_reg/DocLib/InstitutionsandPharmaceuticalProductsGuidelines.pdf Retrieved from.
- Théophile H., André M., Miremont-Salamé G., Arimon Y., Bégaud B. Comparison of three methods (an updated logistic probabilistic method, the Naranjo and Liverpool algorithms) for the evaluation of routine pharmacovigilance case reports using consensual expert judgement as reference. Drug Safety. 2013;36(10):1033–1044. doi: 10.1007/s40264-013-0083-1. [DOI] [PubMed] [Google Scholar]
- Uppsala Monitoring Center Uppsala Monitoring Center, Members of the WHO Programme [Online] 2017. https://www.who-umc.org/global-pharmacovigilance/members/ Retrieved from.