Abstract
Polymers from natural resources are attracting much attention in various fields including drug delivery as green alternatives to fossil fuel based polymers. In this quest, novel block copolymers based on renewable poly(δ-decalactone) (PDL) were evaluated for their drug delivery capabilities and compared with a fossil fuel based polymer i.e. methoxy-poly(ethylene glycol)-b-poly(ε-caprolactone) (mPEG-b-PCL). Using curcumin as a hydrophobic drug model, micelles of PDL block copolymers with different orientation i.e. AB (mPEG-b-PDL), ABA (PDL-b-PEG-b-PDL), ABC (mPEG-b-PDL-b-poly(pentadecalactone) and (mPEG-b-PCL) were prepared by nanoprecipitation method. The size, drug loading and curcumin stability studies results indicated that mPEG-b-PDL micelles was comparable to its counterpart mPEG-b-PCL micelles towards improved delivery of curcumin. Therefore, mixed micelles using these two copolymers were also evaluated to see any change in size, loading and drug release. Drug release studies proposed that sustained release can be obtained using poly(pentadecalactone) as crystalline core whereas rapid release can be achieved using amorphous PDL core. Further, mPEG-b-PDL micelles were found to be non-haemolytic, up to the concentration of 40 mg/mL. In vivo toxicity studies on rats advised low-toxic behaviour of these micelles up to 400 mg/kg dose, as evident by histopathological and biochemical analysis. In summary, it is anticipated that mPEG-b-PDL block copolymer micelles could serve as a renewable alternative for mPEG-b-PCL copolymers in drug delivery applications.
Keywords: Biodegradable polymers, Controlled release, Micelles, Polymeric drug carrier, Toxicity, Bioavailability
1. Introduction
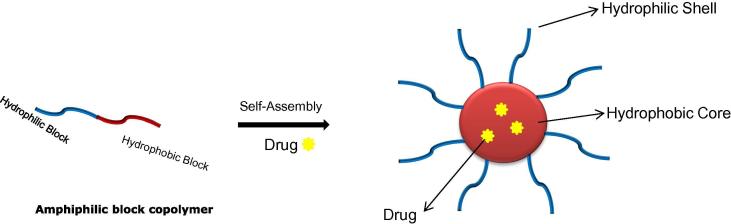
In drug delivery applications, amphiphilic block copolymers are used extensively owing their inherent self-assembly behaviour into diverse nanostructures, such as micelles (Gaucher et al., 2005, Lu and Park, 2013). The term “micelles” defines the aggregation of amphiphilic molecule in core-shell structure, above their critical micelle concentrations (CMC) when dispersed in solvent usually water (Azum et al., 2017b, Kumar and Rub, 2016) (Fig. 1). The CMC is defined as the concentration of amphiphilic molecules in solvent, above which they start forming micelles (Azum et al., 2017a). Amphiphilic block copolymers with poly(ethylene glycol) (PEG) as hydrophilic block such as PEG-b-poly(lactic acid) (PEG-b-PLA), PEG-b-poly(caprolactone) (PEG-b-PCL), PEG-b-poly(aspartic acid) (PEG-b-PA) etc., have been extensively studied as drug delivery carriers. The hydrophobic block in such copolymers can be chosen based on the required application; however, those derived from renewable resources have gained utmost interest, because of their environment friendly nature, abundant availability and in most cases biocompatibility, biodegradability and non-toxicity (Zhang et al., 2017). Additionally, polymers from renewable resources fitting in the concept of “acting responsibly to meet the needs of the present without compromising the ability of future generations to meet their own needs” (Vilela et al., 2014). Therefore, several renewable feedstocks from either plant or animal sources have been discovered, to synthesize polymers with tunable properties.
Fig. 1.
Pictorial presentation of self-assembly of an amphiphilic block copolymer into micelles when dispersed in water.
Micellar formulations have already shown their presence in the market. Genexol PM® (micelles of PEG-b-PLA) have been approved in South Korea for the treatment of breast cancer (Weissig et al., 2014) whereas NC 6004 (micelles of PEG-b-PAA) and NK 911, NK10 (micelles of PEG-b-PA) are in clinical trials (Weissig and Guzman-Villanueva, 2015). Genexol PM, though, have been fabricated using renewable polymer (PLA), but is expensive and has certain limitations. A few reports suggested that the drug loading achieved using PLA block polymer or their copolymers are usually low owing to its lower hydrophobicity. Therefore, more hydrophobic derivatives of lactide have been prepared (Trimaille et al., 2004, Trimaille et al., 2006, Yin and Baker, 1999); however, the synthesis procedure appears to be tedious and expensive. Moreover, in PLA-derived drug delivery materials, the production of excessive acid from PLA degradation can cause deleterious effects on loaded acid-sensitive drugs, thus limits its application (Kang and Schwendeman, 2002, Meyer et al., 2012). Therefore, the quest for new polymers from renewable resources continues, which could serve as a alternative for such existing polymers.
Recently, we have reported the synthesis of amphiphilic block copolymers derived from an economical and renewable monomer i.e. δ-decalactone (Bansal et al., 2015). Studies performed on the poly(δ-decalactone) (PDL) derived copolymers suggested that they have remarkable potential to act as a drug delivery carrier. Therefore, in the present study, we have compared the drug delivery capability of micelles of PDL with non-renewable PCL block copolymer using curcumin as a model drug. Micelles of block copolymers were fabricated using a revised nano-precipitation method (Schubert et al., 2011) as this offers advantages over the other methods. PCL block copolymer have been investigated earlier for the improved delivery of curcumin and therefore, chosen here for a comparative study. It has been demonstrated that mixed micelles prepared from two or more different block copolymers were capable to enhance the formulation stability and drug loading efficiency compared to the micelles prepared from single block copolymer (Attia et al., 2011). Therefore, mixed micelle formulation using mPEG-b-PDL and mPEG-b-PCL copolymer has been also fabricated to study the effect on curcumin loading content and release pattern. Another important parameter, which needs to be addressed for polymers in drug delivery, is toxicity (Ghanghoria et al., 2018). Hence, an ex vivo haemolysis study was conducted to measure haemocompatibility of mPEG-b-PDL polymers. Further, micelles were also tested on rats for in vivo sub-chronic toxicity.
2. Materials and Methods
2.1. Materials
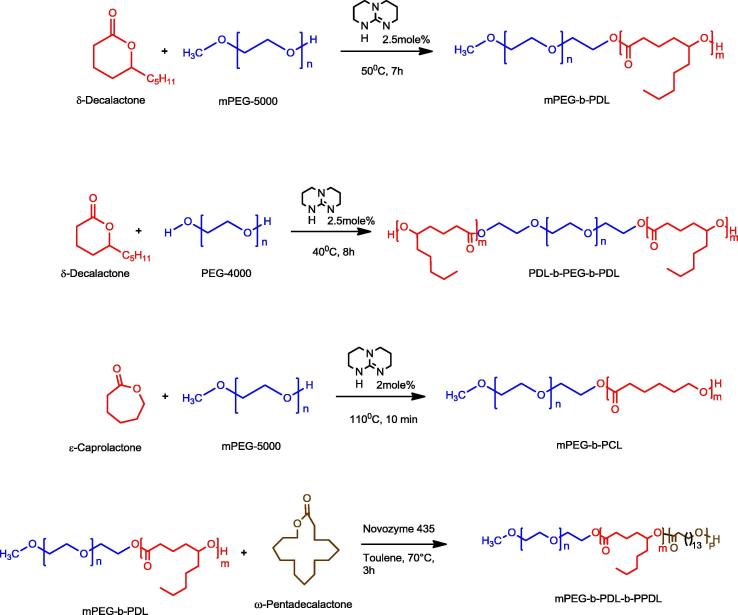
Curcumin (≥99.5%), triton X-100 (BioXtra), haematoxylin and eosin solution have been purchased from Sigma-Aldrich and used as received. Previously synthesised block copolymers of PDL and PCL have been used in all studies (Bansal et al., 2015) (Scheme 1). All the solvents used were purchased from Fischer Scientific UK.
Scheme 1.
Synthesis scheme of block copolymers used in this study (Bansal et al., 2015).
2.2. Methods
2.2.1. Micelles preparation from PDL and PCL block copolymers
Curcumin loaded micelles of block copolymers were prepared by a single-step nano-precipitation method with minor modifications (Gou et al., 2011). Briefly, curcumin (2 mg) was dissolved along with the polymer (50 mg) in acetone (5 mL) and added drop wise into Milli-Q water (10 mL) under stirring (1000 rpm). The solution was then stirred for 3 h at room temperature and left overnight (open vial) to ensure the complete removal of acetone. Curcumin is light sensitive and hence the whole process was performed in the dark . Empty micelles were prepared using same procedure without curcumin. Mixed micelles of mPEG-b-PDL and mPEG-b-PCL were fabricated by physical mixing (Bae et al., 2007) of both copolymer (25 mg each) in acetone (5 mL) and the method described above was employed to obtain curcumin-loaded mixed micelles.
Curcumin loaded micelles were purified by passing through PD10 Desalting Column (Sephadex G-25 Medium, GE Healthcare Life Sciences). In this procedure, materials of <5 K molecular weight were retained giving the sample (eluent) free from any unencapsulated drug. Separately, the crude micellar solution was also purified by filtration using a membrane syringe filter (pore size: 220 nm) (Millex-LG, Millipore Co., USA) to determine the efficiency of individual purification method. A part of the micellar solution was freeze dried for the determination of drug content.
2.2.2. Characterisation of micelles for size, zeta potential and surface morphology
For the size and polydispersity index measurements, micelle samples (50 µg/mL) in Milli-Q water were analysed on a Malvern NanoZS instrument. Surface zeta potential was measured from same instrument in HEPES 10 mM buffer (pH-7.4). TEM images were taken to confirm the size and to determine the surface morphology. Samples were imaged on TEM grids without staining. All the measurements were performed on three different batches and the mean values were reported.
2.2.3. Curcumin content, stability and in vitro release behaviour from micelles
Drug Content (DC) and Encapsulation Efficiency (EE) of curcumin in micelles were determined by dissolving the known amount of freeze dried samples of micelles in acetone followed by quantification of the drug concentration using fluorescence spectroscopy (Varian) after appropriate dilutions. For analysis, samples were excited at a fixed wavelength (λex = 420 nm) and spectra were recorded in a range of 450–600 nm (Fig. 2) (Leung and Kee, 2009). The excitation and emission slit widths were selected at 5 nm and selected emission intensity was 524 nm. Amount of curcumin present in sample was than calculated using curcumin standard calibration curve prepared in acetone. All studies were performed in triplicate and DC and EE was calculated using the formula below:
Fig. 2.
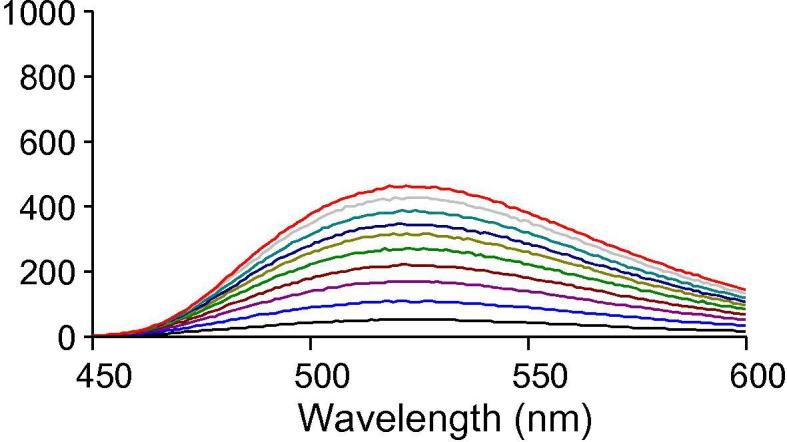
Fluorescence emission spectra of curcumin at different concentration in acetone (λex −420 nm). The fluorescence emission of curcumin solution (in acetone) was measured at wavelength of 524 nm.
The ability of mPEG-b-PDL micelles to protect the curcumin from degradation at physiological pH was tested using a reported method (Ma et al., 2008). Freeze dried micelles of mPEG-b-PDL and mPEG-b-PCL containing curcumin equivalent to 100 µg, were redispersed in 2 mL of PBS (pH 7.4) and incubated at 37 °C. For the preparation of control samples, free curcumin (100 µg) was dissolved in 2 mL of phosphate buffer saline (PBS) using methanol as co-solvent and incubated at 37 °C in a closed container. At predetermined time intervals, 100 µL of the sample was withdrawn and diluted with acetone up to 1 mL. The amount of remaining curcumin was then determined using a fluorescence spectrophotometer.
The release profile of curcumin from micelles was determined by a dialysis m ethod (Ma et al., 2008). Briefly, a calculated quantity of curcumin-loaded freeze dried micelles equivalent to 350 µg of curcumin was dissolved in PBS (2 mL) (pH-7.4). The micellar solution in PBS was then placed in dialysis tubing (Float-A-Lyzer) having the molecular weight cut off (mwco) of 3.5–5 kDa. The samples were dialysed against 500 mL of PBS (pH 7.4) at 37 °C. The release media was replaced with fresh PBS in every 24 h. The volume of solution in the dialysis tubing was measured at appropriate time intervals (~6 h), and restored to the original with PBS, if necessary. Samples (100 µL) were withdrawn directly from the dialysis tubing at predetermined time intervals and the volume of solution in the dialysis tubing was restored with fresh solvent. Samples were analysed after diluting with acetone using a fluorescence spectrophotometer to calculate the amount of curcumin remaining in the micelles.
2.2.4. Toxicity studies of novel poly(decalactone) micelles
Blank micelles solution (mPEG-b-PDL) of different concentration were used in all toxicity studies.
2.2.4.1. Ex vivo haemolytic study
The haemolytic study was performed using reported procedure with slight modifications (Evans et al., 2013). Briefly, human blood (5 mL) was drawn from an anonymous donor directly into Na2-EDTA-coated tube to prevent coagulation. Collected blood was than centrifuge at 500g for 5 min to separate plasma and red blood cells (RBCs), and plasma (yellowish, upper layer) was discarded. Separated RBCs was than washed twice with 150 mM NaCl solution followed by one wash with phosphate buffer saline (PBS, pH – 7.4). RBCs were than diluted up to 5 times with PBS (pH – 7.4) to make stock suspension.
Micelles (50 mg/mL) prepared in PBS were further diluted to make 25, 12.5, 1.25 and 0.625 mg/mL concentration with PBS. For haemolysis assay, 800 μl of each dilution of micelles (i.e. 50, 25, 12.5, 1.25 and 0.625 mg/mL) was make up to 1 mL by adding 200 μl RBCs stock suspension. Therefore, the stocks of 50, 25, 12.5, 1.25 and 0.625 mg/mL, resulting in final test concentrations of 40, 20, 10, 1 and 0.5 mg/mL of micelles, respectively. Positive control tubes was prepared by adding 800 μl of 1.25% solution of triton X-100 in 200 μl RBCs whereas for negative control tubes, 800 μl of PBS was added. Tubes were prepared in two batches (n = 3) and incubated at 37 °C for 1 h and 24 h separately with occasional shaking. After incubation, tubes were centrifuge for 5 min at 500g to pellet undamaged RBCs. The supernatant from each tube was than analysed on UV–Vis spectroscopy to measure the absorbance of released haemoglobin (λmax – 542 nm). The percentage haemolysis was calculated using formula below:
2.2.4.2. In vivo toxicity study
Six-to-seven weeks old Albino rats were divided in four groups (n = 6) in which one is control group (group-A) while rest were treated groups (group-B, C, D). The study protocol was duly approved by Institutional Animal Ethical Committee (IAEC) and experiments were performed according to the guidelines of “Committee for the Purpose of Control and Supervision of Experiments on Animals” (CPCSEA), India (approval number – GLAIPR/CPCSEA/IAEC/2016/PhD/R2). Animals were housed at 23 ± 2 °C with a 12 h day/night cycle, food and water was supplied everyday ad libitum.
Three different concentrations of blank micelles , i.e. 100, 200 and 400 mg/kg were administered intra-peritoneally (IP) to group “B”, “C” and “D” respectively while Milli-Q water was injected to group “A” animals for seven days. Body weight of animals was measured for comparison purpose before injecting micellar solution/Milli-Q water on each day to determine preliminary toxicity. A loss of body weight by ≥2 g in three or more rats in a group would be considered as the origination of toxicity (Burt et al., 1999).
-
(a)
Heamatological-Biochemical Analysis
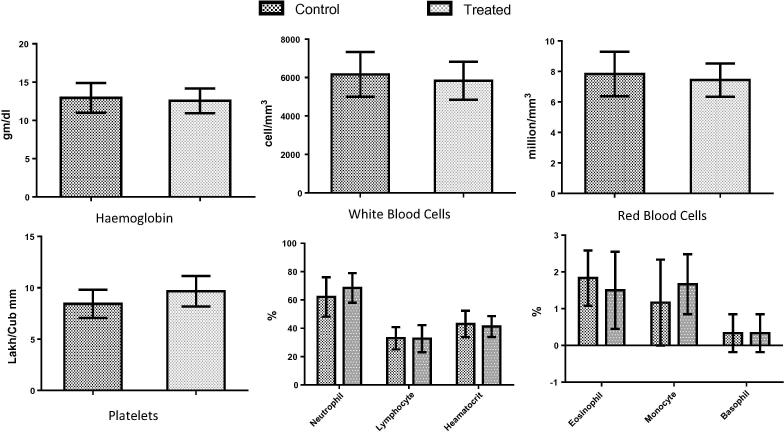
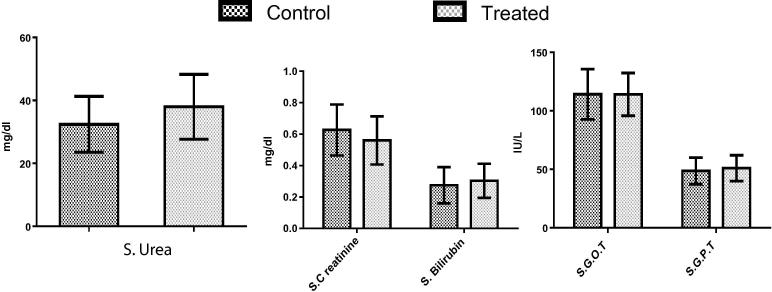
On 8th day, blood samples from each group were collected and divided in two parts for pathological analysis. Pathological test has been carried out to determine the serum level of aspartate transaminase (AST or SGOT), alanine transaminase (ALT or SGPT), urea, bilirubin and creatinine. Further, evaluation of heamatological parameters consisting haemoglobin, white blood cells, neutrophils, lymphocytes, eosinophils, haematocrit, monocytes, basophils, red blood cell count, platelets have been done.
-
(b)
Histo-Pathological Analysis
In this analysis, liver, heart, spleen, lungs and kidneys of the animals were removed surgically, washed and transferred in 10% neutral buffered formalin and left for 48 h at room temperature. Tissue sections were then prepared after dehydration and embedding the samples in paraffin. The sections were then stain with haematoxylin and eosin (H&E) and processed for histo-pathological examination under optical microscope fitted with camera. The brief procedure of dehydration and embedding is as follow:
Dehydration of tissue pieces: Tissues pieces were removed from 10% formalin solution and place in absolute alcohol for dehydration. Three changes were given in absolute alcohol each for 15 min.
Preparation of tissues for embedding: After three changes in absolute alcohol, pieces were transferred in xylene. Three changes of xylene were given each for 20 min.
Paraffin infiltration and embedding: The paraffin wax was melted by heating up to 62 ± 2 °C and kept in molten state in pan. The tissues were then transferred directly in molten wax in the first infiltration pan for 45 min at 62 ± 2 °C in an oven. After the first embedding, tissue pieces were removed and placed in second infiltration pan having molten wax and kept at controlled temperature for 45 min.
Block preparation: Molten wax was poured in the mould up to 4/5th of total height. The tissues were then removed from the infiltration, placed gently to the mould, and topped up with wax. It was allowed to stand at room temperature till solidified. The wax block was than separated, cut, and trimmed to remove excess wax.
Microtomy: The block was then cut into ribbon like sections with the help of microtome. The ribbon sections were transferred to a slide on which a fixative (Egg albumin solution) had been applied.
Staining of slide: The section on slide was de-waxed with xylol. Staining was done using haematoxylin and eosin (H&E). The sections were mounted with Canada balsam on the slides carefully with cover slip.
2.2.5. Statistical analysis
Data were represented as mean ± standard deviation (SD). Statistical analysis was conducted by one-way ANOVA with Tukey’s multiple comparisons test using P < .05 as a statistical significance threshold.
3. Results
3.1. Preparation and characterisation of curcumin loaded micelles
Micelles of poly(decalactone) and poly(caprolactone) block copolymers were prepared by nano-precipitation method, in which self-assembly of copolymers generates core-shell structures in aqueous solvent. Curcumin loading experiments was performed in Milli-Q water due to the poor stability of curcumin in alkaline conditions (Naksuriya et al., 2014). Curcumin was expected to encapsulate in the polymeric micelle cores via hydrophobic interactions during the process of self-assembly.
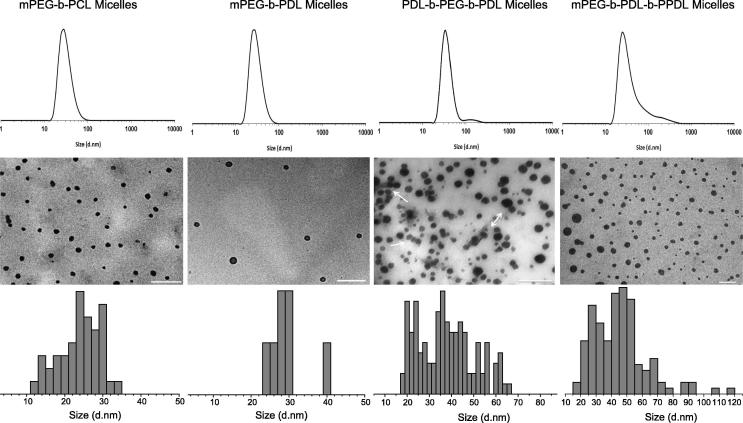
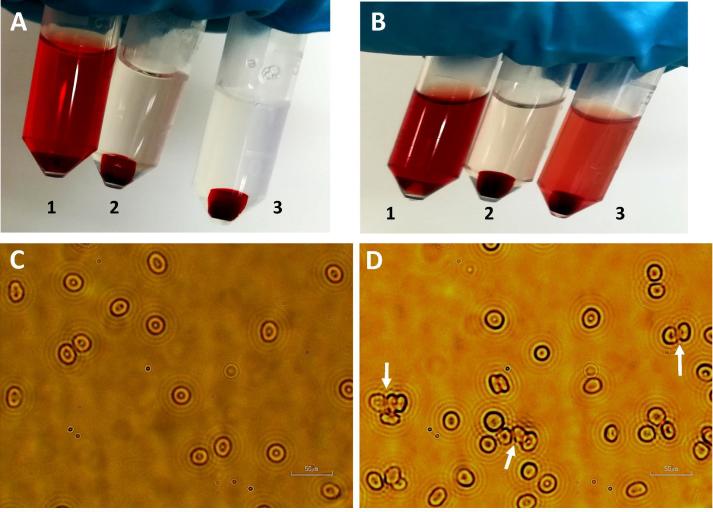
Any non-encapsulated curcumin was separated from the micelles by passing through a PD10 desalting (Sephadex) column or by filtration using syringe filter (0.22 µ). However, loss of curcumin loaded micelles was observed with Sephadex column purification method and therefore, subsequent purifications of micelles was performed by filtration only, which also provide sterility to the formulations. Post purification, cloudy appearance of PDL-b-PEG-b-PDL micelles solution advised the presence of clusters in this formulation (Fig. 3). The estimated size of micelles by DLS and TEM are presented in Table 1, Fig. 4.
Fig. 3.
Appearance of blank and curcumin loaded purified polymeric micelles solution.
Table 1.
Characterization data of polymeric micelles prepared from block copolymers of poly(decalactone) and poly(caprolactone) (CMC – critical micelles concentration, d/nm – diameter in nanometer, SD- Standard deviation, PdI – polydispersity index, mv – millivolt). *CMC, size, zeta potential and molecular weight data were reproduced from previously reported results (Bansal et al., 2015).
| Sample | CMC* (µg/mL) | Z-average size (d/nm) (±SD) (Blank)* | PdI (Blank)* | Zeta potential (mV) (±SD)* | Z-average size (d/nm) (±SD) ( loaded) | PdI (loaded) | Zeta potential (mv) (±SD) ( loaded) |
|---|---|---|---|---|---|---|---|
| PDL3K-b-PEG4K-b-PDL3K | 1.50 | 163 ± 7 | 0.26 ± 0.03 | −6.8 ± 2.6 | 123 ± 7 | 0.28 ± 0.03 | −7.3 ± 0.9 |
| mPEG5K-b-PDL6K | 1.33 | 34 ± 4 | 0.12 ± 0.02 | −3.1 ± 0.8 | 40 ± 3 | 0.14 ± 0.02 | −2.8 ± 1.2 |
| mPEG5K-b-PDL6K-b-PPDL1.5K | 1.19 | 85 ± 5 | 0.28 ± 0.01 | −2.5 ± 0.8 | 101 ± 9 | 0.28 ± 0.02 | −3.2 ± 1.8 |
| mPEG5K-b-PCL5.5K | 3.34 | 36 ± 3 | 0.14 ± 0.02 | −1.2 ± 1.2 | 40 ± 2 | 0.12 ± 0.02 | 0.1 ± 1.3 |
Fig. 4.
Size distribution curve by volume determined by DLS, TEM images and size distribution histogram from TEM images (analysed using ImageJ software) of curcumin-loaded micelles. Arrows in TEM images represents the presence of clusters in PDL-b-PEG-b-PDL micelles sample. TEM images were acquired without staining the samples. Scale bar – 200 nm.
The Z-average size observed for mPEG-b-PCL and mPEG-b-PDL micelles was almost identical while mPEG-b-PDL-b-PPDL and PDL-b-PEG-b-PDL copolymers produces micelles of larger size (∼100 nm). However, analysis of volume distribution curve generated by DLS suggested that majority of block copolymer micelles were fall in the size range of 20–40 nm. Later, TEM images of samples suggested that the micelles are of roughly spherical in shape and majority of micelles fall in the size range of 20–40 nm (Fig. 4). Presence of clusters has been observed in PDL-b-PEG-b-PDL copolymer micelles as evident by size distribution curve acquired from DLS and by TEM image. Micelles obtained from mPEG-b-PDL-b-PPDL copolymers are slightly larger compared to the micelles generated from rest of the block copolymers. A slight surge in the Z-average sizes of the micelles were observed after curcumin loading when compared to the empty micelles except with PDL-b-PEG-b-PDL (Table 1). The increase in the size of the micelles after curcumin loading has also been reported previously (Gou et al., 2011, Ma et al., 2008, Shao et al., 2011).
The sizes observed for mixed micelles by DLS were almost identical to the sizes observed for mPEG-b-PDL and mPEG-b-PCL micelles (not shown). The surface charge observed for curcumin loaded micelles showed non-significant difference compared to blank micelles (Table 1).
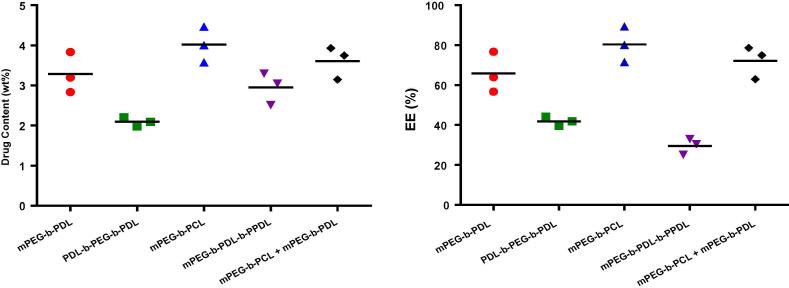
The curcumin content and encapsulation efficiency observed in amphiphilic block copolymers micelles are shown in Fig. 5. The micelles fabricated from mPEG-b-PCL copolymer demonstrated the drug content of 4.0 ± 0.4 wt%, with an encapsulation efficiency of 80.3 ± 8.9%. However, content found for mPEG-b-PDL micelles was 3.3 ± 0.5 wt%. Curcumin content in PDL-b-PEG-b-PDL micelles was found to be least (2.1 ± 0.1 wt%) when compared with other studied polymeric micelles. The micelles prepared from mPEG-b-PDL-b-PPDL copolymer showed a drug content of 2.6 ± 0.1 wt%. Curcumin content observed in mixed micelles of mPEG-b-PDL and mPEG-b-PCL (1:1) was 3.6 ± 0.4 wt%, which lies between the curcumin content observed for individual copolymers.
Fig. 5.
Curcumin content (DC wt%) and encapsulation efficiency (EE%) in different block copolymer micelles. Dots represent separate individual values and bar represents the mean value (n = 3).
The loading percentages were further compared using one-way ANOVA with Tukey’s correction for multiple comparisons. Using P < .05 as a statistical significance threshold, only PDL-b-PEG-b-PDL micelles loading content showed significant differences when compared to the other formulations. The rest formulations did not significantly differ in their drug content. Encapsulation efficiency followed the similar pattern as loading content except for mPEG-b-PDL-b-PPDL micelles because of the loss of significant amount of loaded polymeric micelles during purification by filtration.
3.2. Curcumin stability study
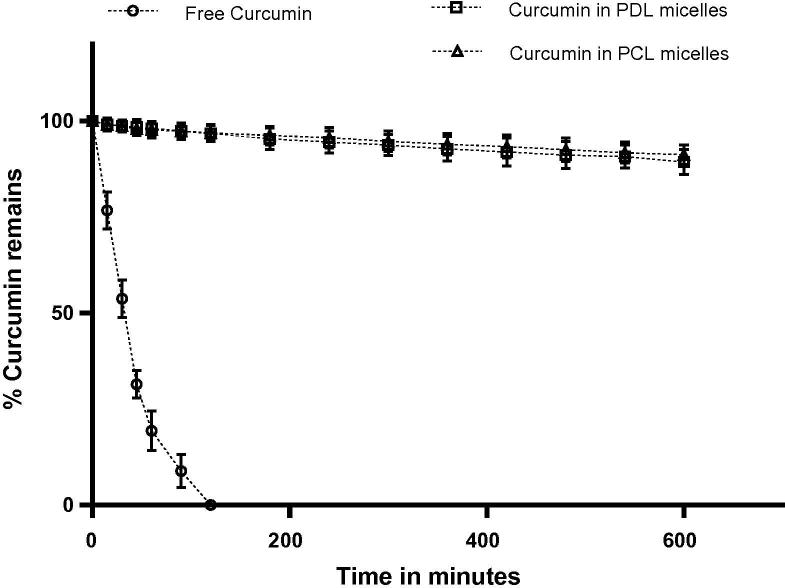
It has been reported that curcumin undergoes hydrolytic degradation at alkaline pH (Wang et al., 1997). Hence, evaluation of the degradation rate of encapsulated curcumin is an important evaluation parameter to assess the ability of micelles as protectant. mPEG-b-PDL copolymer micelles demonstrated better results compared to other PDL block copolymers in terms of size and loading, and therefore was selected for the curcumin stability study. The stabilities of free curcumin and curcumin loaded in micelles were tested for 10 h in phosphate buffer saline (PBS, pH – 7.4). After 2 h, free curcumin was degraded/ hydrolysed completely whereas only 3% of degradation was observed in encapsulated curcumin (Fig. 6). After 10 h, curcumin in PDL micelles showed 11% of degradation while PCL micelles were able to protect 91% of drug from degradation (9% of degradation). Thus, the ability of mPEG-b-PDL micelles to protect curcumin from degradation in PBS was found to be identical with their counterpart mPEG-b-PCL micelles.
Fig. 6.
Plot representing the percentage of curcumin remains in the different environment with respect to time incubated in PBS (pH-7.4) at 37 °C.
3.3. In vitro release behaviour of curcumin from block copolymers micelles
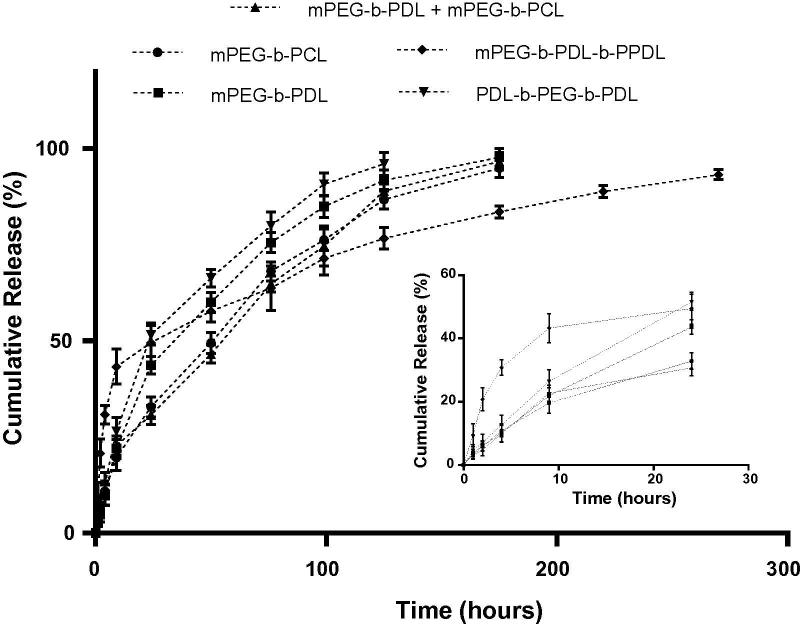
A controlled release pattern from a polymeric system is desirable to justify its potential as a drug delivery carrier (Uhrich et al., 1999). Curcumin showed rapid degradation in the chosen release medium i.e. PBS and therefore samples were collected directly from the dialysis tube to avoid any error due to its degradation (Ma et al., 2008). The release patterns of curcumin from the different amphiphilic block copolymers micelles are presented in Fig. 7.
Fig. 7.
In vitro release pattern of curcumin from different block copolymer micelles and zoomed graph showing the release pattern observed in first 24 h (inset) using PBS as release media at 37 °C.
Initial burst release of curcumin was observed in all samples where 43% release was observed with mPEG-b-PDL-b-PPDL micelles and 19% release was noticed with mPEG-b-PCL micelles in first 9 h. After 175 h, more than 95% of curcumin was released from mPEG-b-PDL, mPEG-b-PCL and mPEG-b-PDL + mPEG-b-PCL mixed micelles however, mPEG-b-PDL-b-PPDL micelles showed 83% release. Micelles of PDL-b-PEG-b-PDL copolymer displayed rapid release (96% after 125 h) whereas, slowest release pattern was observed with mPEG-b-PDL-b-PPDL micelles (76% after 125 h). In conclusion, faster release was observed with micelles containing PDL core compared to PCL or PPDL core. The rapid release from the mPEG-b-PDL could be beneficial over those drug delivery carriers where incomplete release of drug(s) was observed (Shaikh et al., 2009).
3.4. Toxicity studies of block copolymers micelles of poly(decalactone)
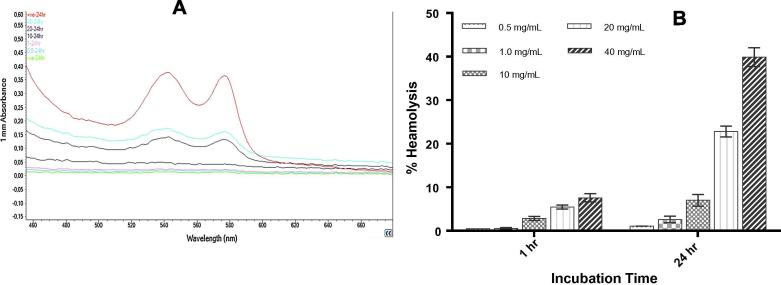
Since the performance of mPEG-b-PDL copolymer micelles is almost identical to mPEG-b-PCL micelles, this copolymer micelles have been further investigated for it’s toxicity. Heamolytic study was performed to evaluate the extent of RBCs lysis potentially caused by mPEG-b-PDL copolymer micelles upon injection (Amin and Dannenfelser, 2006). The mPEG-b-PDL micelles were tested for their heamolysis capacity up to the concentration of 40 mg/mL in static condition. Visual inspection of incubated tubes after centrifugation provided a preliminary idea of RBCs lysis. As shown in Fig. 8, absence of red color observed in supernatant (except from positive control where all cells are deliberately lysed using surfactant) after 1 h of incubation (Fig. 8A-3) proposed minimum or negligible heamolysis whereas significant amount of lysis was observed after 24 h of incubation in the sample containing 40 mg/mL micelles (Fig. 8B-3). The RBCs image also confirmed the presence of few ruptured cells in this sample (Fig. 8-D).
Fig. 8.
Appearance of tubes incubated for (A) 1 h, (B) 24 h after centrifugation and the images of red blood cells treated with 40 mg/mL micelles for (C) 1 h, (D) 24 h at 37 °C. Number 1, 2 and 3 represents positive control sample, negative control sample and sample treated with 40 mg/mL micelles respectively and white arrows represents ruptured cells present in sample.
Quantitative estimation by UV–Vis suggested 0.49 ± 0.01, 0.57 ± 0.15, 2.85 ± 0.46, 5.46 ± 0.47, 7.58 ± 0.94 percentage heamolysis after 1 h whereas 1.08 ± 0.03, 2.61 ± 0.72, 7.02 ± 1.33, 22.81 ± 1.25, 39.90 ± 2.14 percentage heamolysis was observed after 24 h for 0.5, 1.0, 10, 20 and 40 mg/mL concentration micelles respectively. The results suggested that micelles up to 40 mg/mL as drug delivery vehicle are less-heamolytic after 1 h incubation whereas, concentration and time dependent heamolysis was observed after 24 h incubation (Fig. 9).
Fig. 9.
(A) The UV–Vis graph of heamoglobin present in the supernatant layer of samples incubated for 24 h and (B) the percentage heamolysis observed with different concentration of micelles incubated for 1 h and 24 h at 37 °C.
Further, in vivo toxicity of mPEG-b-PDL micelles have been assessed by comparing the haematological and biochemical parameters of untreated (control) and treated rats. Additionally, histopathological changes, if any, in major organs were also assessed to determine the harmful effect caused by micelles.
Animals have treated with mPEG-b-PDL micellar solution for six days up to the concentration of 400 mg/kg. Multiple IP doses of micelles up to 400 mg/kg concentration in rats did not produce any mortality during the study duration. Further, no significant reduction in weight (i.e. ≥2 g) has been observed in rats post injections.
Haematology study results reveals no statistically significant changes in blood parameters in treated group animals compared to control group animals. The results obtained from the blood samples collected from group “D” animals (400 mg/kg dose) are presented in Fig. 10, Fig. 11. In addition, values of biochemical parameters of liver and kidneys functions suggested that micellar solution did not elicit any toxic effect up to the tested concentration in rats (Fig. 11).
Fig. 10.
Comparison of various heamatological parameters obtained from control and treated rats (dose – 400 mg/kg). The data has been analysed by graphpad prism and no statistically significant difference has been found between the estimated values (P > .05, n = 6).
Fig. 11.
Comparison of various biochemical parameters obtained from control and treated rats (dose – 400 mg/kg). The data has been analysed by graphpad prism and no statistically significant difference has been found between the estimated values (P > .05, n = 6).
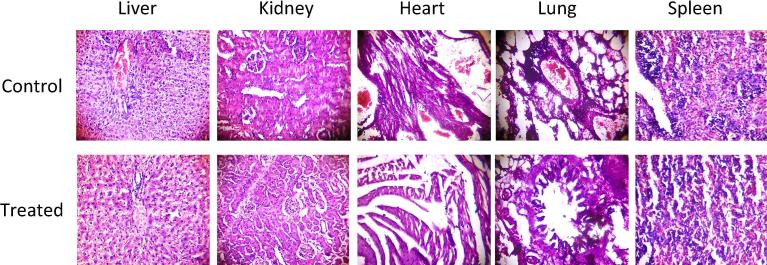
Furthermore, to examine the toxic effect of micelles on specific organs, histopathological changes in heart, lung, liver, kidney and spleen were observed by light microscope. The tissue images of treated organs did not showed any abnormalities/inflammation compared to the control organs tissue images (Fig. 12).
Fig. 12.
Histological evaluation of major organs of the rat treated with mPEG-b-PDL micelles (400 mg/kg, IP). No abnormalities/inflammation were observed in the tissue images compared to control group. Images were taken at 10X magnification with haematoxylin and eosin staining.
Additionally, comprehensive investigation of pictures of treated liver tissues revealed absence of activated Kupffer cells, sinusoidal dilatation and cytoplasmic vacuolation, which advised absence of any toxic reaction caused by micelles. Similarly, non-existence of granular cast, cellular cast and protein cast in kidney tissue images suggesting non-toxic nature of micelles up to the tested concentration (Siti et al., 2014). Likewise, no apparent histopathologic changes such as cells necrosis, inflammation were observed in images of heart, lung and spleen of treated group compared to control group (El-Refaiy and Eissa, 2013, Al-Forkan et al., 2016).
4. Discussion
In the presented study, novel block copolymers of PDL has been investigated for the improved delivery of curcumin and the results were compared with PCL block copolymer. Poly(decalactone) block copolymers with different orientation like AB (mPEG-b-PDL), ABA (PDL-b-PEG-b-PDL) and ABC (mPEG-b-PDL-b-PPDL) type have been evaluated at one platform to determine the effect of polymer structure on particle size, drug loading and release. The micelles were prepared by nanoprecipitation method due the disadvantages associated with other commonly used techniques. For instance, with the solvent evaporation (film method) method, incomplete reconstitution of the polymeric film was observed in aqueous solvent resulting in polymer loss and consequently decreases the micelles efficiency (Aliabadi and Lavasanifar, 2006). Emulsion method for micelle preparation was not preferred due to the requirement of toxic solvents such as dichloromethane and chloroform (Aliabadi and Lavasanifar, 2006, Naksuriya et al., 2014). In the dialysis method, use of organic solvent in dialysis bag is required. However, solvents other than DMSO is not recommended for use with the regenerated cellulose membrane and none of the solvents (such as DMF, DMSO) is recommended with the cellulose membrane (Kim et al., 1998, Zhang et al., 2007) as this could damage the membrane causing undesired loss of micelles in the release medium. Additionally, good solubility of copolymer and drug is desirable in the above mentioned solvent. Further, large-scale preparation of micelles using dialysis method seems not feasible.
The un-encapsulated drug was removed in subsequent purification steps to avoid false results. It was found that the purification by filtration was better than PD10 column method in this study. Incomplete recovery of drug-loaded micelles was observed with column purification and thus avoided to achieve the high yield. The micelles produced by nano-precipitation using mPEG-b-PDL and mPEG-b-PCL copolymers are of comparable size. This could be due to the similar hydrophobic to hydrophilic ratio and same orientation (AB type, di-block) of both copolymer. However, as expected, triblock copolymers produced larger size micelles. ABA type block copolymer showed cluster formation whereas ABC copolymer produced larger size micelles due to its poor solubility in acetone (Bansal et al., 2015). Therefore, mPEG-b-PDL-b-PPDL copolymer is not a good candidate to fabricate micelles using nanoprecipitation method with acetone as a solvent. The insignificant change in zeta potential of micelles after curcumin loading suggested that the curcumin was located in the micellar core, shielded by the PEG corona.
Curcumin loading in PDL block copolymers was found to be less compared with PCL block copolymer. It has been reported that the ability of a hydrophobic core to encapsulate a drug is mainly depends upon its compatibility with the drug molecule (Letchford et al., 2008, Lu and Park, 2013). Therefore, it was assumed that curcumin could be less compatible with PDL polymer and thus micelles with PDL core forming block demonstrated low loading compared to micelles with PCL core. However, low loading with PDL micelles may not be observe with all drugs since, recent studies suggested that amorphous core could produce formulations with high drug payload (Gou et al., 2015, Kakde et al., 2016).
Curcumin content observed with mPEG-b-PCL micelles in this study was significantly less when compared with previously reported results. The variation in the results might have been due to the different parameters used for loading in the individual studies. Encapsulation procedure, drug to polymer ratio, molecular weight of polymer, solvent used etc. could significantly influence the loading content (Feng et al., 2012, Gong et al., 2013, Gou et al., 2015, Ma et al., 2008, Mohanty et al., 2010) and hence these parameters can be tailored to achieve the maximum drug loading. The objective in current study was to compare the loading results obtained from novel and well established block copolymers using identical parameters. Thus, the optimisation of the formulation parameters was not performed in this work but could be considered in future studies.
Curcumin loading observed with the mixed micelles suggested that mixing of the novel renewable copolymer (mPEG-b-PDL) with a copolymer synthesised from non-renewable feedstock (such as mPEG-b-PCL) could provide similar drug loading when compared to the results obtained from single block copolymer. Thus, the mixed micelle approach might be useful to reduce the amount of non-renewable copolymer(s) in final formulation.
In curcumin stability study, the degradation rate of curcumin encapsulated in mPEG-b-PDL micelles was found to be slower compared to the free drug. This result suggests that the curcumin was located inside the highly hydrophobic PDL core. Due to this reason, the direct contact of curcumin with PBS was hampered and thus; the degradation rate was reduced. The mPEG-b-PDL micelle’s ability to protect the degradation of curcumin was also found to be similar to the mPEG-b-PCL micelles.
In the release experiment, burst release was observed from all micelle formulations, which could be attributed to the presence of drug on the exterior part (surface) of the hydrophobic block. The initial burst release of curcumin from polymeric micelles was also reported earlier in which 30–40% of drug was released within 10 h (Bisht et al., 2007, Shao et al., 2011, Zhao et al., 2012). On the basis of the release results, it can be proposed that the mechanism responsible for the release of curcumin from the studied micelles is mainly diffusion. It has been reported that polymers with low Tm, Tg value and low crystallinity release their loaded content rapidly (Karavelidis et al., 2010, Papadimitriou and Bikiaris, 2009) and therefore, the drug permeated faster from the amorphous PDL core compared to semicrystalline (PCL) or crystalline (PPDL) core polymeric micelles (Teng et al., 1998). A similar release pattern has been reported with PLGA nano carriers where increase in the composition of D, L-PLA (amorphous polymer) increased the release rate of loaded drug (Jain, 2000, Nasongkla et al., 2006).
Delayed release observed from mPEG-b-PDL-b-PPDL micelles was most likely due to the restricted diffusion of drug from the more crystalline poly(pentadecalactone) core (Liu et al., 2011, Liu et al., 2009). Similar results were reported earlier with poly(propylene succinate-co-caprolactone) copolymer nanoparticles where mixing of crystalline polymer with amorphous polymer decreased the release rate compared to the release observed from the amorphous polymer alone (Papadimitriou and Bikiaris, 2009). The release rate observed for mPEG-b-PCL micelles in this study was more sustained than previously reported release pattern for mPEG-b-PCL micelles of similar molecular weight (Ma et al., 2008). This difference in release pattern can be attributed to the concentration of curcumin, sample withdrawal method and release media used for the study. It was reported that when higher drug concentration was used for the release study, a more sustained release pattern was observed (Layre et al., 2006, Soo et al., 2002). Additionally, the release pattern could be different in the different release media, based on the partition coefficient of the drug at different pH.
In the previous report, we have demonstrated the comparable toxicity profile of mPEG-b-PDL micelles with mPEG-b-PCL micelles in vitro (Bansal et al., 2015). Therefore, in the current study, we further evaluated mPEG-b-PDL micelles to assess their toxicity ex vivo and in vivo. Heamolysis assay results suggested that the tested concentration of mPEG-b-PDL micelles are non-heamolytic after 1 h incubation (heamolysis ≤10%). However, after 24 h incubation, concentration above 10 mg/mL was appeared to be heamolytic (Amin and Dannenfelser, 2006). Although, in dynamic condition i.e. inside the human body, the concentration of mPEG-b-PDL micelles interacting with RBCs would probably not reach above 10 mg/mL due to the dilution with approximately 5 l of blood. Therefore, it can be proposed that the drug delivery via injectable route using this novel carrier system could be harmless. The preliminary in vivo toxicity studies was performed via IP route based on previous published literature and the limitations of the IV route in repeated-dose administration (Binkhathlan et al., 2017, Bulcão et al., 2013, Kim et al., 2003). The results suggested that IP dose of mPEG-b-PDL micelles up to the concentration of 400 mg/kg was well tolerated by tested animals without producing any noticeable adverse effects. These initial toxicity results on mPEG-b-PDL micelles have paved the way for future investigations in generation of broader toxicity profile for human use.
5. Conclusion
The block copolymer micelles of renewable poly(decalactone) with different orientation was evaluated for their drug delivery capability and results were compared with its non-renewable counterpart mPEG-b-PCL micelles. Based on the sizes observed for different copolymers, it can be assumed that polymer orientation (di or tri block) does make difference on micelles assembly. The drug loading results suggested that curcumin could be less compatible with PDL block compared to PCL block and thus showed a lesser amount of loading in triblock PDL micelles. Release experiment demonstrated that the drug released more quickly from amorphous PDL block compared to semicrystalline (PCL) or crystalline block (PPDL). However, based on the results, it could be proposed that the performance of novel mPEG-b-PDL micelles as a carrier was almost equivalent to the mPEG-b-PCL micelles. Further, ex vivo and in vivo toxicity results suggested the low toxicity profile of novel mPEG-b-PDL micelles. Thus, this study results suggested that mPEG-b-PDL copolymer could act as a renewable alternative for mPEG-b-PCL copolymer in drug delivery applications.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aliabadi H.M., Lavasanifar A. Polymeric micelles for drug delivery. Expert Opin. Drug Del. 2006;3(1):139–162. doi: 10.1517/17425247.3.1.139. [DOI] [PubMed] [Google Scholar]
- Al-Forkan, M., Islam, S., Akter, R., Shameen Alam,S., Khaleda, L., 2016. A sub-chronic exposure study of arsenic on hematological parameters, liver enzyme activities, histological studies and accumulation pattern of arsenic in organs of Wistar Albino rats. J. Cytol. Histol., 1–7.
- Amin K., Dannenfelser R.-M. In vitro hemolysis: guidance for the pharmaceutical scientist. J. Pharm. Sci. 2006;95:1173–1176. doi: 10.1002/jps.20627. [DOI] [PubMed] [Google Scholar]
- Attia A.B.E., Ong Z.Y., Hedrick J.L., Lee P.P., Ee P.L.R., Hammond P.T., Yang Y.-Y. Mixed micelles self-assembled from block copolymers for drug delivery. Curr. Opin. Colloid Interface Sci. 2011;16:182–194. [Google Scholar]
- Azum N., Rub M.A., Asiri A.M. Interaction of triblock-copolymer with cationic gemini and conventional surfactants: a physicochemical study. J. Dispersion Sci. Technol. 2017;38:1785–1791. [Google Scholar]
- Azum N., Rub M.A., Asiri A.M., Bawazeer W.A. Micellar and interfacial properties of amphiphilic drug–non-ionic surfactants mixed systems: Surface tension, fluorescence and UV–vis studies. Colloids Surf., A. 2017;522:183–192. [Google Scholar]
- Bae Y., Diezi T.A., Zhao A., Kwon G.S. Mixed polymeric micelles for combination cancer chemotherapy through the concurrent delivery of multiple chemotherapeutic agents. J. Control. Release. 2007;122:324–330. doi: 10.1016/j.jconrel.2007.05.038. [DOI] [PubMed] [Google Scholar]
- Bansal K.K., Kakde D., Purdie L., Irvine D.J., Howdle S.M., Mantovani G., Alexander C. New biomaterials from renewable resources – amphiphilic block copolymers from [small delta]-decalactone. Polym. Chem. 2015;6:7196–7210. [Google Scholar]
- Binkhathlan Z., Qamar W., Ali R., Kfoury H., Alghonaim M. Toxicity evaluation of methoxy poly(ethylene oxide)-block-poly(ε-caprolactone) polymeric micelles following multiple oral and intraperitoneal administration to rats. Saudi Pharm. J. 2017;25:944–953. doi: 10.1016/j.jsps.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisht, S., Feldmann, G., Soni, S., Ravi, R., Karikar, C., Maitra, A., Maitra, A., 2007. Polymeric nanoparticle- encapsulated curcumin (“nanocurcumin”): a novel strategy for human cancer therapy. J. Nanobiotechnol. 5, 3-Article No.: 3. [DOI] [PMC free article] [PubMed]
- Bulcão R.P., Freitas F.A., Venturini C.G., Dallegrave E., Durgante J., Göethel G., Cerski C.T.S., Zielinsky P., Pohlmann A.R., Guterres S.S., Garcia S.C. Acute and subchronic toxicity evaluation of poly(ɛ-caprolactone) lipid-core nanocapsules in rats. Toxicol. Sci. 2013;132:162–176. doi: 10.1093/toxsci/kfs334. [DOI] [PubMed] [Google Scholar]
- Burt H.M., Zhang X., Toleikis P., Embree L., Hunter W.L. Development of copolymers of poly(d, l-lactide) and methoxypolyethylene glycol as micellar carriers of paclitaxel. Colloids Surf., B. 1999;16:161–171. [Google Scholar]
- Schubert S., Delaney J.T., Jr, Schubert U.S. Nanoprecipitation and nanoformulation of polymers: from history to powerful possibilities beyond poly(lactic acid) Soft Matter. 2011;7(5):1581–1588. [Google Scholar]
- El-Refaiy A.I., Eissa F.I. Histopathology and cytotoxicity as biomarkers in treated rats with cadmium and some therapeutic agents. Saudi J. Biol. Sci. 2013;20:265–280. doi: 10.1016/j.sjbs.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, B.C., Nelson, C.E., Yu, S.S., Beavers, K.R., Kim, A.J., Li, H., Nelson, H.M., Giorgio, T.D., Duvall, C.L., 2013. Ex vivo red blood cell hemolysis assay for the evaluation of pH-responsive endosomolytic agents for cytosolic delivery of biomacromolecular drugs. J. Vis. Exp., e50166. [DOI] [PMC free article] [PubMed]
- Feng R., Song Z., Zhai G. Preparation and in vivo pharmacokinetics of curcumin-loaded PCL-PEG-PCL triblock copolymeric nanoparticles. Int. J. Nanomed. 2012;7:4089–4098. doi: 10.2147/IJN.S33607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaucher G., Dufresne M.H., Sant V.P., Kang N., Maysinger D., Leroux J.C. Block copolymer micelles: preparation, characterization and application in drug delivery. J. Control. Release. 2005;109:169–188. doi: 10.1016/j.jconrel.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Ghanghoria R., Kesharwani P., Tekade R.K., Jain N.K. Targeting luteinizing hormone-releasing hormone: a potential therapeutics to treat gynecological and other cancers. J. Control. Release. 2018;269:277–301. doi: 10.1016/j.jconrel.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Gong C., Deng S., Wu Q., Xiang M., Wei X., Li L., Gao X., Wang B., Sun L., Chen Y., Li Y., Liu L., Qian Z., Wei Y. Improving antiangiogenesis and anti-tumor activity of curcumin by biodegradable polymeric micelles. Biomaterials. 2013;34:1413–1432. doi: 10.1016/j.biomaterials.2012.10.068. [DOI] [PubMed] [Google Scholar]
- Gou J., Feng S., Xu H., Fang G., Chao Y., Zhang Y., Xu H., Tang X. Decreased core crystallinity facilitated drug loading in polymeric micelles without affecting their biological performances. Biomacromolecules. 2015;16:2920–2929. doi: 10.1021/acs.biomac.5b00826. [DOI] [PubMed] [Google Scholar]
- Gou M., Men K., Shi H., Xiang M., Zhang J., Song J., Long J., Wan Y., Luo F., Zhao X., Qian Z. Curcumin-loaded biodegradable polymeric micelles for colon cancer therapy in vitro and in vivo. Nanoscale. 2011;3:1558–1567. doi: 10.1039/c0nr00758g. [DOI] [PubMed] [Google Scholar]
- Jain R.A. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials. 2000;21:2475–2490. doi: 10.1016/s0142-9612(00)00115-0. [DOI] [PubMed] [Google Scholar]
- Kakde D., Taresco V., Bansal K.K., Magennis E.P., Howdle S.M., Mantovani G., Irvine D.J., Alexander C. Amphiphilic block copolymers from a renewable ε-decalactone monomer: prediction and characterization of micellar core effects on drug encapsulation and release. J. Mater. Chem. B. 2016;4:7119–7129. doi: 10.1039/c6tb01839d. [DOI] [PubMed] [Google Scholar]
- Kang J.C., Schwendeman S.P. Comparison of the effects of Mg(OH)(2) and sucrose on the stability of bovine serum albumin encapsulated in injectable poly(D, L-lactide-co-glycolide) implants. Biomaterials. 2002;23:239–245. doi: 10.1016/s0142-9612(01)00101-6. [DOI] [PubMed] [Google Scholar]
- Karavelidis V., Giliopoulos D., Karavas E., Bikiaris D. Nanoencapsulation of a water soluble drug in biocompatible polyesters. Effect of polyesters melting point and glass transition temperature on drug release behavior. Eur. J. Pharm. Sci. 2010;41:636–643. doi: 10.1016/j.ejps.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Kim S.Y., Lee Y.M., Baik D.J., Kang J.S. Toxic characteristics of methoxy poly(ethylene glycol)/poly(ε-caprolactone) nanospheres; in vitro and in vivo studies in the normal mice. Biomaterials. 2003;24:55–63. doi: 10.1016/s0142-9612(02)00248-x. [DOI] [PubMed] [Google Scholar]
- Kim S.Y., Shin I.L.G., Lee Y.M., Cho C.S., Sung Y.K. Methoxy poly(ethylene glycol) and epsilon-caprolactone amphiphilic block copolymeric micelle containing indomethacin. II. Micelle formation and drug release behaviours. J. Controlled Release. 1998;51(1):13–22. doi: 10.1016/s0168-3659(97)00124-7. [DOI] [PubMed] [Google Scholar]
- Kumar D., Rub M.A. Aggregation behavior of amphiphilic drug promazine hydrochloride and sodium dodecylbenzenesulfonate mixtures under the influence of NaCl/urea at various concentration and temperatures. J. Phys. Org. Chem. 2016;29:394–405. [Google Scholar]
- Layre A., Couvreur P., Chacun H., Richard J., Passirani C., Requier D., Benoit J.P., Gref R. Novel composite core-shell nanoparticles as busulfan carriers. J. Control. Release. 2006;111:271–280. doi: 10.1016/j.jconrel.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Letchford K., Liggins R., Burt H. Pharmaceutics, preformulation and drug delivery. J. Pharm. Sci. 2008;97:1179–1190. doi: 10.1002/jps.21037. [DOI] [PubMed] [Google Scholar]
- Leung M.H.M., Kee T.W. Effective stabilization of curcumin by association to plasma proteins: human serum albumin and fibrinogen. Langmuir. 2009;25:5773–5777. doi: 10.1021/la804215v. [DOI] [PubMed] [Google Scholar]
- Liu J., Jiang Z., Zhang S., Liu C., Gross R.A., Kyriakides T.R., Saltzman W.M. Biodegradation, biocompatibility, and drug delivery in poly (omega-pentadecalactone-co-p-dioxanone) copolyesters. Biomaterials. 2011;32:6646–6654. doi: 10.1016/j.biomaterials.2011.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Jiang Z., Zhang S., Saltzman W.M. Poly(omega-pentadecalactone-co-butylene-co-succinate) nanoparticles as biodegradable carriers for camptothecin delivery. Biomaterials. 2009;30:5707–5719. doi: 10.1016/j.biomaterials.2009.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Park K. Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs. Int. J. Pharm. 2013;453:198–214. doi: 10.1016/j.ijpharm.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z., Haddadi A., Molavi O., Lavasanifar A., Lai R., Samuel J. Micelles of poly(ethylene oxide)-b-poly(epsilon-caprolactone) as vehicles for the solubilization, stabilization, and controlled delivery of curcumin. J. Biomed. Mater. Res. Part A. 2008;86A:300–310. doi: 10.1002/jbm.a.31584. [DOI] [PubMed] [Google Scholar]
- Meyer F., Wardale J., Best S., Cameron R., Rushton N., Brooks R. Effects of lactic acid and glycolic acid on human osteoblasts: a way to understand PLGA involvement in PLGA/calcium phosphate composite failure. J. Orthop. Res. 2012;30:864–871. doi: 10.1002/jor.22019. [DOI] [PubMed] [Google Scholar]
- Mohanty C., Acharya S., Mohanty A.K., Dilnawaz F., Sahoo S.K. Curcumin-encapsulated MePEG/PCL diblock copolymeric micelles: a novel controlled delivery vehicle for cancer therapy. Nanomedicine. 2010;5:433–449. doi: 10.2217/nnm.10.9. [DOI] [PubMed] [Google Scholar]
- Naksuriya O., Okonogi S., Schiffelers R.M., Hennink W.E. Curcumin nanoformulations: a review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials. 2014;35:3365–3383. doi: 10.1016/j.biomaterials.2013.12.090. [DOI] [PubMed] [Google Scholar]
- Nasongkla N., Bey E., Ren J., Ai H., Khemtong C., Guthi J.S., Chin S.-F., Sherry A.D., Boothman D.A., Gao J. Multifunctional polymeric micelles as cancer-targeted, MRI-ultrasensitive drug delivery systems. Nano Lett. 2006;6:2427–2430. doi: 10.1021/nl061412u. [DOI] [PubMed] [Google Scholar]
- Papadimitriou S., Bikiaris D. Novel self-assembled core-shell nanoparticles based on crystalline amorphous moieties of aliphatic copolyesters for efficient controlled drug release. J. Control. Release. 2009;138:177–184. doi: 10.1016/j.jconrel.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Shaikh J., Ankola D.D., Beniwal V., Singh D., Kumar M.N.V.R. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharm. Sci. 2009;37:223–230. doi: 10.1016/j.ejps.2009.02.019. [DOI] [PubMed] [Google Scholar]
- Shao J., Zheng D., Jiang Z., Xu H., Hu Y., Li X., Lu X. Curcumin delivery by methoxy polyethylene glycol-poly(caprolactone) nanoparticles inhibits the growth of C6 glioma cells. Acta Biochim. Biophys. Sin. 2011;43:267–274. doi: 10.1093/abbs/gmr011. [DOI] [PubMed] [Google Scholar]
- Siti, S.A., Norhaizan, M.E., Hazilawati, H., 2014. Histopathologic changes in liver and kidney tissues from male Sprague Dawley rats treated with Rhaphidophora Decursiva (Roxb.) Schott Extract. J. Cytol. Histol., 1–6.
- Soo P.L., Luo L.B., Maysinger D., Eisenberg A. Incorporation and release of hydrophobic probes in biocompatible polycaprolactone-block-poly(ethylene oxide) micelles: Implications for drug delivery. Langmuir. 2002;18:9996–10004. [Google Scholar]
- Teng Y., Morrison M.E., Munk P., Webber S.E., Prochazka K. Release kinetics studies of aromatic molecules into water from block polymer micelles. Macromolecules. 1998;31:3578–3587. [Google Scholar]
- Trimaille T., Moller M., Gurny R. Synthesis and ring-opening polymerization of new monoalkyl-substituted lactides. J. Polym. Sci. Part A-Polym. Chem. 2004;42:4379–4391. [Google Scholar]
- Trimaille T., Mondon K., Gurny R., Moeller M. Novel polymeric micelles for hydrophobic drug delivery based on biodegradable poly(hexyl-substituted lactides) Int. J. Pharm. 2006;319:147–154. doi: 10.1016/j.ijpharm.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Uhrich K.E., Cannizzaro S.M., Langer R.S., Shakesheff K.M. Polymeric systems for controlled drug release. Chem. Rev. 1999;99:3181–3198. doi: 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- Vilela C., Sousa A.F., Fonseca A.C., Serra A.C., Coelho J.F.J., Freire C.S.R., Silvestre A.J.D. The quest for sustainable polyesters – insights into the future. Polym. Chem. 2014;5:3119–3141. [Google Scholar]
- Wang Y.J., Pan M.H., Cheng A.L., Lin L.I., Ho Y.S., Hsieh C.Y., Lin J.K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997;15:1867–1876. doi: 10.1016/s0731-7085(96)02024-9. [DOI] [PubMed] [Google Scholar]
- Weissig V., Guzman-Villanueva D. Nanopharmaceuticals (part 2): products in the pipeline. Int. J. Nanomed. 2015;10:1245–1257. doi: 10.2147/IJN.S65526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissig V., Pettinger T.K., Murdock N. Nanopharmaceuticals (part 1): products on the market. Int. J. Nanomed. 2014;9:4357–4373. doi: 10.2147/IJN.S46900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin M., Baker G.L. Preparation and characterization of substituted polylactides. Macromolecules. 1999;32:7711–7718. [Google Scholar]
- Zhang S., Jin L., Arshad M., Ullah A. Renewable biomaterials as nanocarriers for drug and gene delivery. In: Sharma H.S., Muresanu D.F., Sharma A., editors. Drug and Gene Delivery to the Central Nervous System for Neuroprotection: Nanotechnological Advances. Springer International Publishing; Cham: 2017. pp. 1–32. [Google Scholar]
- Zhang J.X., Yan M.Q., Li X.H. Local delivery of indomethacin to arthritis-bearing rats through polymeric micelles based on amphiphilic polyphosphazenes. Pharmaceut. Res. 2007;24(10):1944–1953. doi: 10.1007/s11095-007-9322-4. [DOI] [PubMed] [Google Scholar]
- Zhao L., Du J., Duan Y., Zang Y.N., Zhang H., Yang C., Cao F., Zhai G. Curcumin loaded mixed micelles composed of Pluronic P123 and F68: preparation, optimization and in vitro characterization. Colloids Surf. B-Biointerfaces. 2012;97:101–108. doi: 10.1016/j.colsurfb.2012.04.017. [DOI] [PubMed] [Google Scholar]