Graphical abstract
Keywords: Antimicrobial peptides, Marine antimicrobial peptides, Antibacterial, Antifungal, Antiviral, Antiparasitic
Abstract
Antimicrobial peptides are group of proteins showing broad-spectrum antimicrobial activity that have been known to be powerful agents against a variety of pathogens. This class of compounds contributed to solving the microbial resistance dilemma that limited the use of many potent antimicrobial agents. The marine environment is known to be one of the richest sources for antimicrobial peptides, yet this environment is not fully explored. Hence, the scientific research attention should be directed toward the marine ecosystem as enormous amount of useful discoveries could be brought to the forefront. In the current article, the marine antimicrobial peptides reported from mid 2012 to 2017 have been reviewed.
1. Introduction
Antimicrobial peptides (AMPs) are molecules known to be essential components of the innate immune response that can also participate in certain organisms as immune modulators (Zanetti, 2004). The living organisms use the AMPs as an important vital tool for survival. They combat the invading pathogens to the host via a broad spectrum of antimicrobial activity that can vary according to the nature of the pathogens (Lehrer et al., 1983, Ganz et al., 1985, Zasloff, 1987). The wide spectrum is in part attributed to the diversity in the structures of AMPs that vary from alpha helix to beta strands (Hancock, 2001). AMPs play defensive role for the producing organisms. When it comes to their selective toxicity, the AMPs explore the differences in the lipid composition of the membrane between eukaryotic and prokaryotic organism that serves as their target of action sparing the host from harmful effects. Nevertheless, the selectivity of these molecules also rise from the effect of the uniform net charge and hydrophobicity which are characteristic trades for AMPs. The majority of these compounds have been described to be carrying a uniform positive charge ranging from +2 to +9, with only few reported to be anionic in nature (Zasloff, 2002).
The cationic nature accounts for the AMPs mechanism of action, as it enables their binding to the negatively charged lipopolysaccharides invading pathogen membrane via electrostatic attractive forces. During this interaction, the AMPs acquire an amphipathic confirmation that optimizes the binding to the pathogen’s membrane. This kind of AMPs are classified to be membrane permeabilizing peptides due to their capability of forming pores in the membrane after their electrostatic interaction, thus resulting in expulsion of cellular content and subsequent cell death (Sengupta et al., 2008). In addition to the previously mentioned mechanism, AMPs can act by interfering with some vital cellular processes inhibiting them after their translocation across the cellular membrane. These peptides are classified as non membrane-permeabilizing peptides (Kondejewski et al., 1999, Brogden, 2005).
AMPs nowadays are an interesting field of research for potential drug candidates owing to their broad spectrum of activity and more importantly to the fact that AMPs may overcome the antimicrobial resistance. Since AMPs target the lipid components in the membrane of the invading pathogens, this interference with such a vital structure in microbes creates a barrier against resistance development. This fact has directed the effort of scientific research to exploit the vast world of AMPs where we can find more than 2000 AMPs reported in antimicrobial peptide databases [http://aps.unmc.edu/AP/main.php/].
Despite the promising activity of the AMPs, they suffer from the following drawbacks. (i) limited stability at certain pHs; (ii) disrupt the cellular membrane of eukaryotic organisms causing hemolytic side effects; (iii) elevated production cost and technical issues are limitations of their manufacturing (Mygind et al., 2005); (iv) lack of data on their toxicity, pharmacodynamics, and pharmacokinetics properties (Evans et al., 1999, Michalopoulos et al., 2005); (v) reduced activity in the presence of some cations such as calcium, iron and certain serum conditions (Smith et al., 1996, Thackray and Moir, 2003).
We have previously reviewed the chemical and biological properties of the marine AMPs reported from 2009 to June 2012 (El-Gamal et al., 2013). Herein, we reviewed the very recent research results in the field of marine AMPs, which were characterized and identified in the time period from July 2012 till 2017. We have classified marine AMPs according to their biological effects into antibacterial, antimycobacterial, antifungal, antiviral, and antiprotozoal.
2. Antibacterial marine peptide
Almost there is no marine organism that does not produce natural antibacterial compounds as an essential line of defense to survive, hence marine antibacterial peptides is a rich class of antimicrobials with new discoveries.
Aurelin is an AMP derived from the mesoglea of a scyphoid jellyfish called Aurelia aurita. It is composed of 40 amino acid residues and described to be cationic in nature with a molecular weight of 4296.4 Da. It has demonstrated modest antibacterial activity against both Gram-positive and Gram-negative bacteria with MIC of 10 μM against the Bacillus megaterium, strain B-392, and 40 μM against Micrococcus luteus, strain Ac-2229 which were determined to be the most sensitive species to Aurelin. Fig. 1 shows that Aurlein is the first AMP to acquire Shk fold that enables Aurelin to have a diversity in its mechanism of action which can be attributed to two different mechanisms; (i) acting as a peptide toxin bocking K+ channels, (ii) acting as a membrane active antimicrobial peptide (Shenkarev et al., 2012).
Fig. 1.
Spatial structure and backbone dynamics of aurelin in aqueous solution (Shenkarev et al., 2012).
Mytimacin-AF is an AMP derived from marine mollusks, particularly from the mucus of Achatina fulica snail. It is characterized to be rich in cysteine as it is composed of 80 amino acid residues 10 of which are cysteines, and its molecular weight is 9711.41 Da. Mytimacin-AF showed activity against both Gram-positive and Gram-negative bacteria, however it was most potent against Staphylococcus aureus with minimum inhibitory concentration (MIC) value of 1.9 μg/ml. In addition, the most promising feature about mytimacin-AF is its activity against the human Klebseilla pneumonia, one of the most common hospital-acquired bacterial infections, what makes mytimacin-AF a very promising antibacterial agent (Zhong et al., 2013).
Myticusin-1 (TDHQMAQSACIGVSQDNAYASAIPRDCHGGKTCEGICADATATMDRYSDTGGPLSIARCVNAFHFYKRRGEENVSYKPFVVSWKYGVAGCFYTHCGPNFCCCIS) is another cysteine-rich peptide derived from mussels. It was characterized and identified from the hemolymph of Mytilus coruscus. Its involvement in the host immune response has been proven, hence it plays a role in bacterial infection eradication. The cysteine amino acid comprises 10 out of 104 amino acid residues which makes myticusin −1 a long polypeptide chain with a molecular weight of 11,279.63 Da. This molecule has demonstrated greater potency against Gram-positive compared to Gram-negative bacteria, as it has recorded an MIC < 5 µM against a variety of tested Gram-positive strains including S. aureus compared to an MIC >10 µM against a variety of Gram-negative bacteria including E. coli (Liao et al., 2013).
EC-hepcidin3 (APAKCTPYCYPTHDGVFCGVRCDFQ), is a novel isoform that belongs to the hepcidin class of AMPs. This class is also known to be rich in cysteine, but this isoform is rather a four cysteinehepcidine unlike the typical eight cysteinehepcidin. It was cloned from the marine fish, the orange spotted grouper Epinephelus coioides. The purified Ec–hepcidin 3 has a theoretical molecular weight of 2798.2 Da. The kinetic studies proved that this molecule has a rapid and strong antibacterial activity against Staphylococcus aureus (MIC 1.5–3 μM, MBC 1.5–3 μM) and Pseudomonas stutzeri (MIC < 1.5 μM and minimum bactericidal concentration (MBC) < 1.5 μM) (Qu et al., 2013).
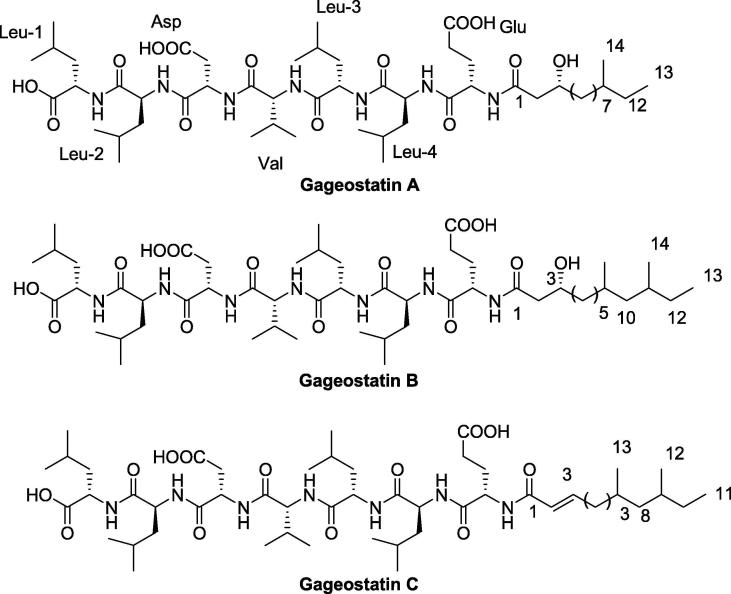
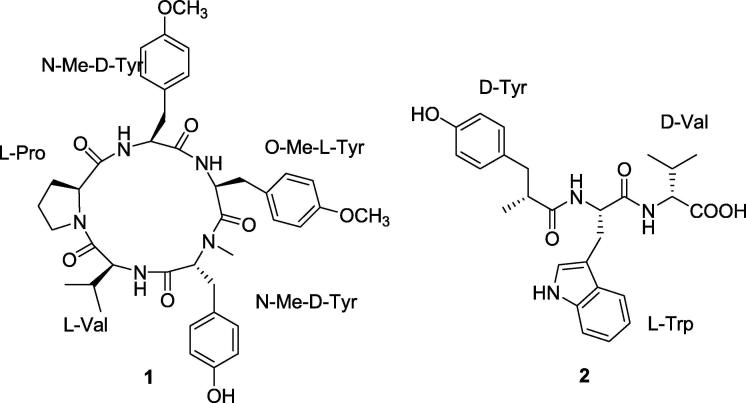
Marine’s bacteria are also important source for AMP, Gageostatins A-C (Fig. 2). They are examples of three new non-ribosomal lipopeptide molecules that were derived from the broth of the bacterium Bacillus subtilis. These three molecules are consisting of heptapeptides and new fatty acid which is 3 beta hydroxy fatty acid and are anionic in nature with a uniform net charge of −3. Those molecules were tested as anticancer agents against a panel of six cancer cell lines; MDA-MB-231 breast cancer cell line, HCT-15 colon cancer cell line, PC-3 prostate cancer cell line, NCI-H23 lung cancer cell line, NUGC-3 stomach cancer cell line, and ACHN renal cancer cell line, and their GI50 values ranged from 4.6 to 19.6 µg/ml. They were also tested for antifungal and bacterial activities against R. solani, C. acutatum, and B. cinera fungi, in addition to S. aureus, B. subtilis, S. typhi, and P. aeruginosa with MIC ranging from 4 to 64 µg/ml. It was reported that a combination between Gageostatins A and B showed greater antibacterial activity than individuals. The three compounds differ in molecular weight and structure, but they all possess the same amino acid sequence (ELLVDLL) (Tareq et al., 2014a).
Fig. 2.
Structures of Gageosatins A–C.
Acosta et al. (2013) were capable of identifying three AMPs from piscidin that were isolated from the Teloest fish, tilapia gills called (Oreochromis niloticus). These peptides had the names and molecular weights of oreochromicins (Oreoch-1, 2524.40 Da; Oreoch-2, 2981.60 Da; and Oreoch-3, 3654.19 Da), the three AMPs are composed of a signal peptide which is highly cationic in nature with uniform net charges of 5, 7, and 10, and constituted of 23, 25, and 32 amino acid residues, respectively. The three peptides displayed activity against a wide range of bacteria and fungi, specifically Oreoch-1 (FIHHIIGGLFSVGKHIHGLIHGH) that showed activity against Gram-positive bacteria like S. aureus (MIC = 5 µM) and B. subtilis (MIC = 3 µM), and Gram-negative bacteria like P. aeruginosa (MIC = 35 µM) and E. coli (MIC = 6.7 µM), as well as antifungal activity against C. albicans (MIC = 20 µM) (Mygind et al., 2005). While Oreoch-2 (FIHHIIGGLFSAGKAIHRLIRRRRR) showed similar potency to Oreoch-1 against S. aureus (MIC = 5 µM), B. subtilis (MIC = 1.7 µM), P. aeruginosa (MIC = 6.7 µM), E. coli (MIC = 5 µM), E. tarda (MIC = 20 µM), and C. albicans (MIC = 26.7 µM). Lastly, the third AMPs Oreoch-3 (IWDAIFHGAKHFLHRLVNPGGKDAVKDVQQKQ) showed greater antifungal activity compared to antibacterial, where it was active mainly against C. albicans (MIC = 40 µM) but very weak against B. subtilis (MIC = 106 µM), E. tarda (MIC = 160 µM), and Vibrio sp. (MIC = 106 µM). The ability of oreoch compounds to induce an immune response and serve as adjuvant candidates for vaccine development was investigated by their co-administration to fish and mice infected with inactivated bacteria and virus that served as the subunit antigen. The results of this in vivo study indicated that the three oreoch compounds were capable of inducing a hormonal and cellular responses in the infected species as well. The study described the powerful impact of the combination of Oreoch-2 along with Oreoch-3 as this combination resulted in a significant increase in the produced antibody amounts. Hence, according to the study results, oreoch compounds are to be considered as strong and successful candidates for molecular adjuvants in vaccination (Acosta et al., 2014).
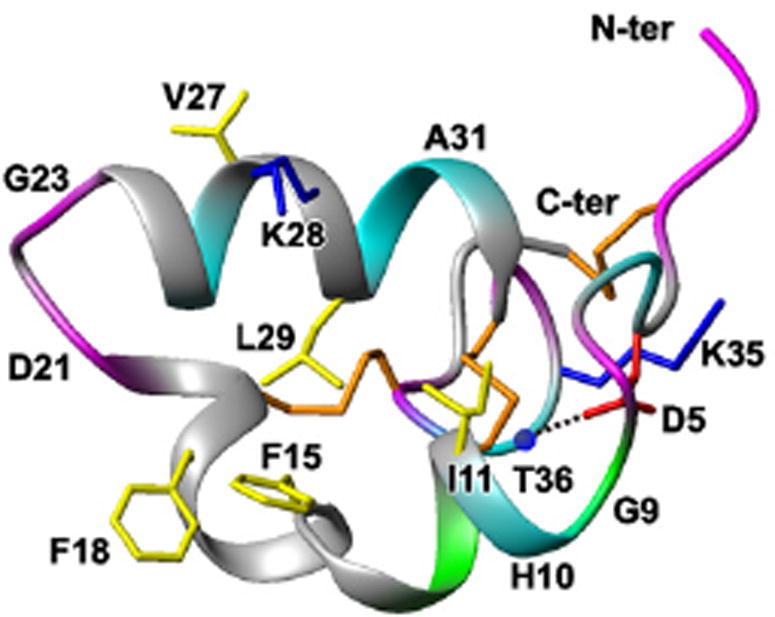
β-defensins are a large family of broad-spectrum AMPs. Cod β-defensin [defb] [WSCPTLSGVCRKVCLPTEMFFGPLGCGKEFQCCVSHFF] is a novel antimicrobial peptide that belongs to the Defenisins family, this family of compounds has a diversity of functions and protective roles. Defb was identified from the Atlantic cod Gadusmorhua. The structure is enriched with six cysteine residues that make three disulfide bridges, which are accommodated in the compound's tertiary structure composed of one α helix and three ß sheets (Fig. 3). The compound has a molecular weight of 4490 Da, and a uniform net charge of +1. Defb shows activity against Gram-positive M. lutes ATCC 4698 (MIC = 25–50 µM) and P. citreus NCIMB 1493 (MIC = 0.4–0.8 µM). However, the in vivo study suggested potential activity for this compound against Gram-negative bacteria as this gene expression was highly upregulated in the head kidney of the challenged atlantic cod with Vibrio anguillarum. In addition, the Defb showed phagocytic activity on the prepared head kidney leucocytes obtained from five Atlantic cods (Ruangsri et al., 2013).
Fig. 3.
3D structure of cod defb (Acosta et al., 2014).
The Clam antimicrobial peptide V. philippinarum defensin (VpDef) (GFGCPEDEYECHNHCKNSVGCRGGYCDAGTLRQRCTCYGCNQKGRSIQE) possesses combined helix and beta structure, and four potential disulfide bonds. The recombinant VpDef (rVpDef) was expressed in E. coli Origami (DE3) as a C-terminal His6-tagged fusion protein. VpDef transcripts were significantly induced in the hemocytes post Vibrio anguillarum infection. rVpDef showed high activity against Gram-positive bacteria Micrococcus luteus (MIC = 6.25–12.5 µM) and less activity against Gram-negative bacteria like Proteus mirabilis (MIC = 50–100 µM) (Zhang et al., 2015).
CATH-BRALE is a salmonoid cathelicidin that is expressed from an ancient fish called Bracgymysttazlenok. This AMP belongs to the Cathelicidins family which are considered to be an essential component of the innate immune system that is capable of playing antibacterial and immunomodulatory role. This compound is composed of 199 amino acids, rich in arginine and glycine giving the compound an intense positive charge [uniform net charge +13]. CATH-BRALE is described to be composed of antiparallel β-sheets protruded by α-helices. This compound exhibited greater potency against Gram-negative bacteria where it has shown to effectively inhibit Gram negative fish bacterial pathogens, Aeromonas salmonicida and Aeromonas hydrophila with a low MIC value against both pathogens (9.38 μM). It has also displayed some antifungal activity against C. albicans ATCC 2002 (MIC = 2.3 µM) (Li et al., 2013).
As-CATH4 (amino acid sequence: RRGLFKKLRRKIKKGFKKIFKRLPPVGVGVSIPLAGRR) and As-CATH5 (amino acid sequence: TRRKFWKKVLNGALKIAPFLLG) are two novel compounds that belong to the cathelicidins family of AMPs. These compounds impact on the immune system was tested by their administration to the Chinese mitten crab. The injection of both compounds in the crab induced an increase in the activity of two enzymes, acid phosphatase and alkaline phosphatase. Hence, this in vivo study demonstrates the role of these compounds as immunostimulants. Additionally, this immunostimulatory effect had a positive impact on improving the anti-infective capacity of the drugs, as the bacterial load in the two groups of infected crabs was significantly reduced upon As-CATH4 and As-CATH5 administration. The bactericidal activity was further supported by the in vitro results where both compounds were effective against the tested ten different bacterial strains. Among these ten strains, eight strains were known to be ampicillin-resistant pathogens, and yet the compounds proved efficacy in low concentrations against them. This highlights the potential unique application of As-CATH4 and As-CATH 5 against drug resistant pathogens. The killing kinetics assay revealed the feature of the rapid killing effect where it took less than 20 min to have the colony-forming unit of the treated bacteria with As-CATH5 to be reduced and remain zero. When the bactericidal mechanism was investigated for these compounds, it showed their ability of targeting the cell membrane and increasing the permeability and cell disruption. These results provide evidence of the potent anti-infective ability of both As-CATH4 and As-CATH5 in crab’s aquatic organisms (Guoa et al., 2017).
A cathelicidin from the sea snake Hydrophis cyanocinctus was identified as Hc-CATH having the amino acid sequence KFFKRLLKSVRRAVKKFRKKPRLIGLSTLL. It demonstrated potent and broad-spectrum antimicrobial activity through disruption of the cell membrane and lysis of the bacterial cell. Hc-CATH displayed powerful activity against human pathogenic microorganisms, with MICs ranging from 0.16 µM (toward Shigella dysenteriae) to 20.67 µM (toward Klebsiella 8). Keeping into consideration that the α helix conformation as well as the positive charge of the peptide are essential for the antimicrobial activity. It is unlikely that the bacteria would develop resistance to this peptide because of its rapid bactericidal effect. In addition to that, Hc-CATH is reported to possess anti-inflammatory effects, extreme stability in aqueous solutions and very low cytotoxicity toward tested mammalian cells. It was also shown that human serum proteins can degrade and bind to Hc-CATH. On the other hand, unlike many antimicrobial peptides, Hc-CATH’s antimicrobial activity is highly salt resistant implying great potency for application (Wei et al., 2015).
Li et al. (2014) were capable of identifying, characterizing, and purifying a new AMP from the hemolymph of Mytilud coruscus a mollusks species called mytichitin-CB (TVKCGMNGKMPCKHGAFYTDTCDKNVFYRCVWGRPVKKHCGRGLVWNPRGFCDYA) with a molecular weight of 6621.55 Da. This molecule has some special features that include the chitin-binding domain for the drug, where preformed studies have shown C-terminal chitin-binding domain of human ChT allowed the drug from binding to chitin. It is constituted of 55 amino acid 6 of which are cysteines forming 3 disulfide linkages. This drug is specific against Gram-positive bacteria, and some fungi where it showed activity against B. subtilis, S. aureus, S. luteus; and B. megaterium (MIC < 5 µM), fungi C. albicans and M. albicans (MIC 6.2–25 µM).
SM HEP1p and SM HEP2p are two hepcidin AMPs that are rich in cysteines which make 4 disulfide bond needed for the molecule's tertiary structure. They are isolated from turbot called Scophthalmus maximus. SMHEP1 which is composed of 26 amino acids has a uniform net charge of 4 and its sequence is (QSHISLCRWCCNCCKANKGCGFCCKF) with a molecular weight of 2940 Da while SMHEP2 which is made up of 22 amino acid residues has a uniform net charge of 3 and the following sequence (GMKCKFCCNCCNLNGCGVCCRF) and its molecular weight is 2410 Da. When it comes to their antimicrobial activity, it was noted that against Gram-positive bacteria SEM HEP1p showed greater potency, where it was active against both S. aureus (MIC = 2 µM) and M. luteus (MIC = 1 µM). Unlike SEM HEP2p that showed much greater activity against Gram-negative bacteria, where it was potent against the following Gram-negative E. tarda (MIC = 1 µM) and V. anguillarum (MIC = 2 µM), and both compounds displayed antiviral activity. As for their mechanism of action, it is most likely that they act as bactericidal by inducing cell membrane destruction and causing severe permeability disruption. Despite the described activity for both compounds, the preformed in vivo and in vitro studies confirm that SmHep2p was superior to SmHep1p as antimicrobial agents. The preformed in vitro studies showed that both compounds were successful in inhibiting bacterial growth in fish cells. However, the in vivo study done on the turbot given the two compounds showed an increase in its bacterial and viral resistance (Zhang et al., 2014).
SA-hepcidin1 (QSHLSMCRYCCNCCRNNKGCGFCCKF) and SA-hepcidin2 (NPAGCRFCCGCCPNMIGCGVCCRF) are cationic hepcidins from the spotted scat fish (Scatophagus argus). The antibacterial activity of both SA-hepcidin1 and SA-hepcidin2 were similar against Gram-positive bacteria Staphylococcus aureus (MIC = 50 µM) and Gram-negative bacteria Vibrio anguillarum (MIC = 50 µM) and Vibrio alginolyticus (MIC = 50 µM). Moreover, SA-hepcidin1 showed strong antibacterial activity against Gram-negative bacteria Vibrio fluvialis (MIC = 25 µM) and Escherichia coli (MIC = 50 µM), whereas SAhepcidin2 did not. In addition to the antibacterial effect, SA-hepcidin2 showed antiviral activity against enveloped and non-enveloped viruses, being most effective against MsReV. One possible mechanism of action as antiviral is causing virion collapse, thereby preventing viral attachment to host cells (Gui et al., 2016).
YFGPA (VKVGINGFGRIGRLVTRAAFHGKKVEVVAIND) AMP is considered as a GAPDH related compound. GAPDH is the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase that has multiple essential intracellular functions, in addition to the newly discovered antimicrobial properties. YFGPA was obtained from the yellowfin tuna, Thunnus albacares. Its molecular weight is 3400 Da. The antimicrobial spectrum was proven to cover Gram-positive bacteria such as Bacillus subtilis, Micrococcus luteus, and Streptococcus iniae with minimal effective concentrations (MECs) of 1.2–17.0 µg/mL, as well it showed activity against Gram-negative bacteria like Aeromonas hydrophila, Escherichia coli D31, and Vibrio parahaemolyticus (MECs = 3.1–12.0 µg/mL). The preformed killing kinetic study against B. subtilis, and E. coli D31 showed that YFGPA has a bacteriostatic activity rather than bactericidal activity, as it acts through non-lytic pathway. The low cytotoxicity of YFGPA makes it a potential alternative therapeutic agent for humans or a substitute to conventional antibiotics for managing fish diseases (Seo et al., 2014a).
Piscidins are known to have good antimicrobial activity and are important for innate host defense. Rock bream piscidin (Rbpisc) (GEGFLGMLLHGVGHAIHGLIHGK) has a tertiary structure which includes amphipathic helix-loop-helix structure. This unique structure of the peptide enables it to interact electrostatically with the negatively-charged bacterial membrane inserting the hydrophobic region into the lipid bilayer and puncturing the bacterial membrane, which is a crucial step to the antimicrobial activity. In vitro, it showed very strong antibacterial activity against S. iniae, V. harveyi and V. ordalii with MICs lower than 0.9 µM, and against V. ordalii (7.8–15.6 µM). Rbpisc also showed weak antimicrobial activity against E. coli, Vibrio alginolyticus and V. campbellii, as indicated by the MIC and IC50 values (1.9–3.9 and 125–250 µM, respectively). It is known that in order to apply any antimicrobial peptide as an antibiotic, it should have low toxicity or hemolytic activity, unfortunately the analysis done by Bae et al. (2016) revealed that the peptide exhibited significant hemolytic effect against fish erythrocytes at concentrations higher than 500 μM which is the concentration that effectively inhibited E. tarda and V. vulnificus. However, Rbpisc did not exhibit cytotoxicity at the effective concentration against S. iniae, V. alginlyticus, V. harveyi, and V. ordalii.
Peng et al. discovered five piscidin-like AMPs, two of which are having potent antimicrobial activity which are named TP3 (FIHHIIGGLFSVGKHIHSLIHGH) and TP4 (FIHHIIGGLFSAGKAIHRLIRRRRR). These compounds were derived from Nile tilapia, Oreochromis niloticus. They share similar amphipathic α-helical structure and cationic nature that attribute in part to their activity. The selective toxicity and hemolytic activity of these two compounds were examined by comparing the toxicity against human mammalian normal cells and Hela cells of cervical tumors. The preformed in vitro study conveyed that TP3 was selective to tumor cells over normal cells. In contrast, TP4 showed no selectivity between both the normal and tumor cells. In addition, the hemolytic studies showed that TP4 is capable of inducing hemolysis to human erythrocytes while TP3 exerted less capability of inducing it. Both compounds showed antibacterial activity against both Gram-positive and Gram-negative bacteria where TP3 which is O. niloticus dicentracin-like peptide had a minimal inhibitory concentration range of 0.6–20 µg/mL against Gram-positive S. agalactiae819, E. faecalis BCRC 10066, and S. agalactiae BCRC 10787 and Gram-negative V. vulnificus 204, V. alginolyticus. TP4 which is O. niloticus moronecidin-like peptide had a minimal inhibitory concentration range of 0.03–2 μg/ml against a variety of Gram-positive including Acteria S. agalactiae 819, E. faecalis BCRC 10066, and S. agalactiae BCRC 10787 and Gram-negative including V. vulnificus 204, A. hydrophila BCRC 13018, V. alginolyticus, and P. aeruginosa ATCC 19660. Also TP4 showed promising activity against H. pylori, and triple-negative breast cancer (Peng et al., 2012).
In addition, the activities of TP3 and TP4 were examined in vivo, where the antimicrobial potencies of TP3 and TP4 were investigated by the eradication of acute bacterial infection from hybrid tilapia fish. Upon infection of the Tilepia fish with V. vulnificus, the co-administration of TP3 and TP4 at a dose of 20 µg/fish for each agent, resulted in a survival rate of 95.3% and 88.9%, respectively, seven days following the treatment. Moreover, this study demonstrated that the pretreatment of the fish with TP3 and TP4 prior to infection would result in a survival rates of 28.9% and 37.8%, respectively, while injecting the fish with TP3 and TP4 30 min after infection gave 33.3% and 48.9% survival rates, respectively (Pan et al., 2017).
SpHyastatin protein from the Crab Scylla paramamosain displays a broad antimicrobial spectrum. Potent activities against Gram-positive bacteria (Micrococcus luteus, Staphylococcus aureus, Corynebacterium glutamicum, Micrococcusluteus) and Gram-negative bacteria (Pseudomonas stutzeri, Pseudomonas fluorescens, Aeromonashydrophila) with MIC values of 0.63–2.5 µM, as well as values of MBC lower than 10 µM were demonstrated. In an in vivo study, Scylla paramamosain crab was infected with V. parahaemolyticus to compare whether suppression of SpHyastatin would affect the survival rates following infection with V. parahaemolyticus. The results revealed that at 60 h, 13% of the control group survived while none of the SpHyastatin-suppressed crabs survived. It was proposed that this compound was capable of eradicating the bacterial infection via an immune reaction. Furthermore, this in vivo study revealed a unique property about SpHyastatin, it has the highest transcription level among six other tested AMPs on the infected crab, indicating the high potential of pathogen resistance to this compound (Shan et al., 2016).
Sphistin is a cationic, amphiphilic α-helical antimicrobial peptide with a uniform net charge of +9. The 38 amino acid sequence of Sphistin peptide (MAGGKAGKDSGKAKAKAVSRSARAGLQFPVGRIHRHLK) was derived from the N terminus of the crab (Scylla paramamosain) histone H2A. It exerts its antimicrobial function through electrostatic attraction because of its cationic characteristics. Sphistin was effective against the growth of Gram-positive bacteria (S. aureus, C. glutamicum, Bacillus subtilis, Micrococcus lysodeikticus, and Micrococcus luteus) and Gram-negative bacteria (Shigella flexneri, Pseudomonas stutzeri, and Pseudomonas fluorescens) with MIC values lower than 1.5 µM as well MBC lower than 12 µM. It was demonstrated that sphistin exerted its antimicrobial activity via adsorption, followed by permeabilization and finally damaging the bacterial cell membranes. Fortunately, sphistin did not display toxicity towards either mammal or crab normal cells (Chen et al., 2015).
Pt5e is a peptide derived from fish egg yolk protein phosvitin. Its recombinant analogue (rPt5e) was expressed in E. coli Transetta (DE3) strain. It was purified by inverse transition cycling (ITC) technique, and the yield was 2.39 mg from 100 ml culture. It has shown the ability to kill clinical multidrug resistant bacteria; E. coli 577 (MIC = 2.1 µM), E. coli 4457 (MIC = 2.1 µM), Klebsiella pneumoniae 2182 (MIC = 2.1 µM), E. coli 140237 (MIC = 2.1 µM), and Acinetobacter baumannii7225 (MIC = 1.4 µM) in a dose-dependent manner. To confirm the interaction between Pt5e and the bacteria, the membrane proteins were extracted from the multidrug resistant (MDR) bacteria and treated with Pt5e, then they were subjected to Western blotting analysis. The extracted membrane proteins of the MDR bacteria that were treated with Pt5e reacted with anti-His tag mouse monoclonal antibody, on the other hand, the membrane proteins of the same bacteria treated with phosphate buffered saline (PBS) did not react, indicating that the extracted proteins of the MDR bacteria treated with Pt5e contained recombinant Pt5e. These findings suggested that Pt5e interacted with the membranes of the MDR bacteria causing membrane permeabilization, depolarization, and increased the intracellular reactive oxygen species, leading to bacterial cell death (Li et al., 2016).
From the fish orange-spotted grouper (Epinephelu scoioides), ecPis-4 (FFRHIKSFWKGAKAIFRGARQG) was identified as having strong antibacterial activities, in addition to the two others; ecPis-2 and ecPis-3. The three piscidins were synthesized in order to determine their biological activity; they demonstrated antibacterial activities against fish pathogen V. parahaemolyticus, and human pathogen E. coli and S. aureus. MBCs were lower than 5.0 µmol/l for EcPis-2 and ecpis-4. However ecPis-3 MBCs were 5.0 µmol/l against V. parahaemolyticus, 10 µmol/l against E. coli, and 20 µmol/l against S. aureus (Zhuang et al., 2017).
MoroPC-NH2 (FFGHLFRGIINVGKHIHGLLSG-NH2) is synthetic, amidated moronecidin-like peptides from the Antarctic Fish Parachaenichthyscharcoti. It showed strong activity against Gram-negative Shigellasonnei, Psychrobacter sp., and E. coli DH5α (MICs < 12.5 μM). In addition, Gram-positive bacteria; Enterococcus faecalis, Streptococcus pyogenes, Staphylococcus aureus, and Listeriamonocytogenes are sensitive to the peptide below 25 μM. On the other hand, moroNC-NH2 (FFWHHIGHALDAAKRVHGMLSG-NH2) is another synthetic, amidated moronecidin-like peptides obtained from the Antarctic Fish Notothenia coriiceps; having lower antibacterial activity and a narrower spectrum. This might be a result from its lower hydrophobicity. Salts can decrease the activity of antimicrobial peptides since they disrupt the electrostatic interactions needed for forming pores in the microbial membrane. The activity of moro-NH2 was affected by salt concentration as evidenced by the increase in the MIC in the presence of 500 µM NaCl or 5 µM CaCl2. Besides, it was concluded that it is difficult to consider moroNC-NH2 for clinical use since it has a narrow spectrum of antibacterial activity and high salt sensitivity. Upon examining the effect of the temperature on the antimicrobial peptides (moro-NH2, moroNC-NH2, and moroPC-NH2), none of the temperatures tested (15 °C, 20 °C, 30 °C, and 37 °C) affected the activities of the peptides (Shin et al., 2017).
PaLEAP-2 (MTPLWRVMGNKPFGAYCQDHVECSTGICKGGHCITSQPIKS) is an antimicrobial peptide from the teleost fish Plecoglossus altivelis, after being chemically synthesized; it exhibited selective antimicrobial activity in vitro against various bacteria. Its potencies towards E. tarda and V. anguillarum are the highest (6.25 µg/ml). While the MIC value against Escherichia coli DH5α is 50 µg/ml. Also, P. putida, V. alginolyticus, and V. vulnificus were sensitive to PaLEAP-2 (MIC = 100 µg/ml). The mode of action of LEAP-2 was suggested as hydrolyzing the DNA as a means to kill the bacteria. In addition, in vivo studies revealed that at high concentrations of PaLEAP-2 the mortality caused by V. anguillarum infection was significantly decreased; this is promising since the main causative agent for Vibriosis, which is a prevalent disease in ayu culture in China, is V. anguillarum (Li et al., 2015).
EeCentrocin 1 (GWWRRTVDKVRNAGRKVAGFASKACGALGH), EeCentrocin 2 (WGHKLRSSWNKVKHAVKKGAGYASGACRVLGH) and EeStrongylocin 2 (WNPFKKIAHRHCYPKNECITTNGKKTCKDYSCCQIVLFGKKTRSACTVVAQ) are peptides isolated from the edible sea urchin Echinus esculentus. EeCentrocin 1 is potent against the Gram-positive bacteria; C. glutamicum (MIC = 0.78 μM) and S. aureus (MIC = 0.78 μM), and against the Gram-negative bacteria, E. coli (MIC = 0.1 μM) and P. aeruginosa (MIC = 0.78 μM), whereas the MIC of EeStrongylocin 2 was found to range from 0.78 to 3.13 μM (Solstad et al., 2016).
From the large yellow croaker (Larimichthys crocea) which is a marine fish in China, Lc-NK-lysin was extracted with the amino acid sequence: MNSSSVLFVCILGACSVWTVHGRNLKVNDDDQEGAELDISVEARKLPGLCWVCKWSLNKVKKLLGRNTTAESVKEKLMRVCNEIGLLKSLCKKFVKGHLGELIEELTTSDDVRTICVNLKACKPKELSELDFESDEDAHTEMNDLLFE. Lc-NK-lysin mature peptide was divided into Lc-NK-lysin-1 and Lc-NK-lysin-2, and both of these two peptides showed antibacterial activities. The Lc-NK-lysin-1 peptide exerted activity that could inhibit and kill S. aureus (MIC = 12–24 µM) and V. harveyi (MIC = 12–24 µM). In addition, it could inhibit but could not kill B. subtilis (MIC = 24–48 µM) and E. coli (MIC = 12–24 µM). In contrast, even the highest concentration of Lc-NK-lysin-1 peptide could not affect V. parahaemolyticus, A. hydrophila, and P. damselae. For the Lc-NK-lysin-2 peptide, it exerted activities against S. aureus (MIC = 12–24 µM) and E. coli (MIC = 24–48 µM) (Zhou et al., 2016).
WB Piscidin 5 (LIGSLFRGAKAIFRGARQGWRSHKAVSRYRARYVRRPVIYYHRVYP) is a host defense peptide found in the white bass Morone chrysops. It was chemically synthesized and its antibacterial activity was tested. WB Piscidin 5 exhibited activity against E. faecalis (MIC = 4.52 µM), S. aureus (MIC = 4.52 µM), Shigella flexneri (MIC = 1.13–2.26 µM), and E. coli (MIC = 1.13 µM) (Salger et al., 2016).
Lysozyme from the seahorse Hippocampus abdominalis can catalyze the hydrolysis of the bacterial cell wall, making it a good antibacterial agent in the innate immune system. ShLysG is a polypeptide obtained from H. abdominalis containing 184 amino acids (MGYGDIMKVDTSGASMKTAGQDRLTYAGVAASNTMAQTDLGRMNNYKAIIQRVGGKKDVDPAIIAGIISRESRAGNVLVNGWGDNGNAWGLMQVDKRYHTPQGGWNSEEHLSQGTDIISFIKQVQGKFPSWTAEQQLKGGIAAYNIGLGGVQTYERMDVGTTGDDYSSDVVARAQWYKSQGGF). It has a molecular mass of 20,000 Da. The conserved catalytic bacterial soluble lytic transglycosylase domain in ShLysG contains three catalytic residues and seven N-acetyl-d-glucosamine binding sites. This domain is crucial for the hydrolysis of the β-1,4-glycosidic bonds between the N-acetylmuramic acid and N-acetylglucosamine of peptidoglycan in the bacterial cell wall. X-ray crystal structure reported that Glu73 and two aspartic acid residues (Asp90 and Asp101) are also directly associated with the catalytic reaction. rShLysG presented antimicrobial activity towards the five bacterial strains; V. salmonicida, V. parahemolyticus, L. monocytogenes, S. iniae, and V. anguillarum (Ko et al., 2016).
A set of Histone H2A fragments were isolated from the Russian sturgeon (Acipenser gueldenstaedtii). They were called acipensins1, 2, 6 (Ac1, Ac2, Ac6). The peptides Ac1 and Ac2 displayed potent antimicrobial activity towards Gram-negative and Gram-positive bacteria, while Ac6 was only effective against Gram-negative bacteria. Ac1 (SGRGKTGGKARAKAKTRSSRAGLQFPVGRVHRLLRKGNYAQRVGAGAPVY) presented MIC = 0.7 µM against E. coli ML35p, MIC = 1.1 µM against Listeria monocytogenes EGD, and MIC = 0.9 µM against methicillin-resistant Staphylococcus aureus (MRSA) ATCC 33591. Meanwhile, Ac2 (SGRGKTGGKARAKAKTRSSRAGLQFPVGRVHRLLR) presented MIC of 0.3 µM against E. coli, MIC = 1 µM against L. monocytogenes, and MIC = 0.6 µM against MRSA. Finally, Ac6 (ILELAGNAARDNKKTRIIPRHLQL) had activity only against E. coli with MIC = 2.5 µM. All three peptides were devoid of hemolytic activity against human erythrocytes at concentrations of 1–40 μM and at concentrations 1–20 µM they did not exert toxic effects on the cells (Shamova et al., 2014).
SJGAP is another AMP (VKVGINGFGRIGRLVTRAAFHGKKVEIVAIND) obtained from skin extract of skipjack tuna (Katsuwonus pelamis). It exhibited potent antimicrobial activity against Gram-positive bacteria, such as B. subtilis, M. luteus, S. aureus, and S. iniae (MEC = 1.2–17.0 µg/mL), and Gram-negative bacteria, such as A. hydrophila, E. coli D31, and V. parahaemolyticus (MEC = 3.1–12.0 µg/mL). SJGAP consists of one α helix and two parallel β-strands, and it forms an amphipathic structure. Also, it acts through bacteriostatic process rather than bactericidal one since it displays a small degree of leakage ability (Seo et al., 2014b).
LEAP-2 is an important molecule of miiuy croaker’s innate immune system as it provides resistance against bacterial infections. It was found that LEAP-2 (MTPLWRIMNSKPFGAYCQNNYECSTGLCRAGHCSTSHRATSETVNY) has a molecular mass of 5000 Da and its instability index is 34.74, which means that the polypeptide is a stable protein. It was effective in controlling A. hydrophila showing bacteriostatic diameter of 10 mm (Liu et al., 2014).
Hemocyanins are multifunctional proteins that can be found in invertebrates including molluscs. Zhuang et al. (2015) identified haliotisin, a hemocyanin-derived antimicrobial peptide from the mollusk Haliotis tuberculate. Haliotisin peptide 3-4-5 (DTFDYKKFGYRYDSLELEGRS ISRIDELIQQRQEKDRTFAGFLLKGFGTSAS) exhibited antibacterial activities. Peptide 3 displayed MIC = 0.3–1 µM against B. subtilis, while peptide 4 displayed MIC = 1.6–2.6 µM against E. carotovor, in addition to peptide 5 which showed 1.2–1.5 µM against E. carotovar.
The novel 55 amino acid peptide cgMolluscidin (AATAKKGAKKADAPAKPKKATKPKSPKKAAKKAGAKKGVKRAGKKGAKKTTKAKK) with a molecular weight of 5500 Da, extracted from the pacific oyster Crassostrea giga, exerted potent antimicrobial activity against both Gram-positive bacteria including B. subtilis, M. luteus, and S. aureus (MEC = 1.3–31.3 µg/mL), and Gram-negative bacteria including E. coli, S. enterica, and V. parahaemolyticus (MEC = 0.4–2.3 µg/mL) (Seo et al., 2013a, Seo et al., 2013b).
PdBD-2 (YDTGIQGWTCGSRGLCRKHCYAQEHTVGYHGCPRRYRCCALRF) was identified from the Chinese loach fish, Paramisgurnus dabryanus and it significantly inhibited the growth of the Gram-negative bacteria A. hydrophila and the Gram-positive B. subtilis (Chen et al., 2013a).
From the pacific oyster Crassostrea gigas, the 74 amino acid cgUbiquitin (MQIFVKTLTGKTITLEVEPSDTIENVKAKIQDKEGIPPDQQRLIFAGKQLEDGRTLSDYNIQKESTLHLVLRLR) was identified, having a molecular weight of 8471 Da. It is composed of three α-helices and four β-strands separated by 7 loop regions. It is active against both Gram-positive and negative bacteria including S. iniae (MEC = 7.8 µg/mL) and V. parahemolyticus (MEC = 9.8 µg/mL) without causing hemolysis to human red blood cells up to 100 µg/mL. Moreover, according to the kinetics of killing and membrane permeabilization studies, it was illustrated that cgUbiquitin was not membrane permeable and acted through a bacteriostatic process (Seo et al., 2013a, Seo et al., 2013b).
Chionodracine (FFGHLYRGITSVVKHVHGLLSG), isolated from the icefish species Chionodracohamatus, exhibited significant effect against Antarctic psychrophilic bacteria strains Psychrobacter sp. TAD1 (MIC = 10 µM) and TA144 (MIC = 15 µM at 15 °C), the Gram-positive B. cereus (MIC = 5 µM at 25 °C), and the Gram-negative E. coli (MIC = 5 µM at 25 °C). However, when the activity of the peptide was tested at 37 °C against E. coli and B. cereus the MIC levels increased to 20 µM and 10 µM, respectively. Buonocore et al. suggested that this could be an indication that chionodracine is adapted to low temperatures or that there was a conformational change in the bacterial membrane due to the lower temperature, leading to higher susceptibility to the peptide. A test was done in vitro on human erythrocytes and no significant lysis occurred with the use of Chionodracine until the concentration reached 50 µM (Buonocore et al., 2012).
The NZ17074 (N1) is a marine peptides derived from the marine invertebrate lugworm Arenicola marina. It is characterized by disulfide bonds. It was proven to show a potent antibacterial activity against Gram-negative bacteria, antifungal and cytotoxicity. However, to reduce its cytotoxicity and to improve its physicochemical properties, varieties of analogues were prepared including, N2, N3, N4, N5, N6 and N7 by structural modification of the natural peptide N1 through changing the number and locations of the disulfide bonds. The NMR spectral analysis identified four different classes of peptides designed based on structural determinants: (I) Rocket analogues with two disulfide bonds (N2: Gly1, 12 → Ala; N3: Trp4,Asn5 → Ala; N4: Trp0). (II) Kite analogue with a Cys7-Cys16 disulfide bond (N6: Cys3,20 → Ala). (III) Bullet analogue with a Cys3-Cys20 disulfide bond (N7: Cys7,16 → Ala). (IV) Other disulfide bond-free linear analogues (N5: ΔCys3,7,16,20; N8: Cys3,7,16,20 → Ala) (Harwing et al., 1996, Yang et al., 2017). The four classes displayed in vitro activity against a wide range of microorganisms including Gram-negative and Gram-positive bacteria, and fungi. The Rocket N2 analogue, with two disulfide bonds, showed the strongest antimicrobial activity against Gram-negative bacteria with MIC values ranging from 0.25 to 1 µg/ml against E. coli, Salmonella, and Pseudomonas strains and lower cytotoxicity compared to other analogues (Wang et al., 2017). N2 and N6 analogues were less cytotoxic against RAW 264.7 macrophages, compared with N1. Both N2 and N6 analogues induced cell cycle arrest in I- and R-phases, respectively, in E. coli and S. enteritidis. In E. coli and in S. enteritidis, both peptides inhibited DNA/RNA/cell wall synthesis by 18.7–43.8% and 5.7–61.8%, respectively. They also modified the morphology of E. coli cells to become collapsed and filamentous after treatment with the two peptides. The survival rate of peritonitis- and endotoxemia-induced mice was prolonged, the serum levels of interleukin-6 (IL-6), IL-1β, and tumor necrosis factor α (TNF-α) decreased following doses ranging from 2.5 to 7.5 mg/kg body weight. These peptides prevented lipopolysaccharide (LPS)-induced lung injury in mice (Harwing et al., 1996, Yang et al., 2017).
3. Antimycobacterial marine peptides
The development of resistance to both first and second line anti-TB medications has decreased the effective management of TB. Thereby, it is essential to increase the efforts and focus on the investigation and development of new anti-TB drugs. Accordingly, research was directed to the naturally occurring compounds such as peptides.
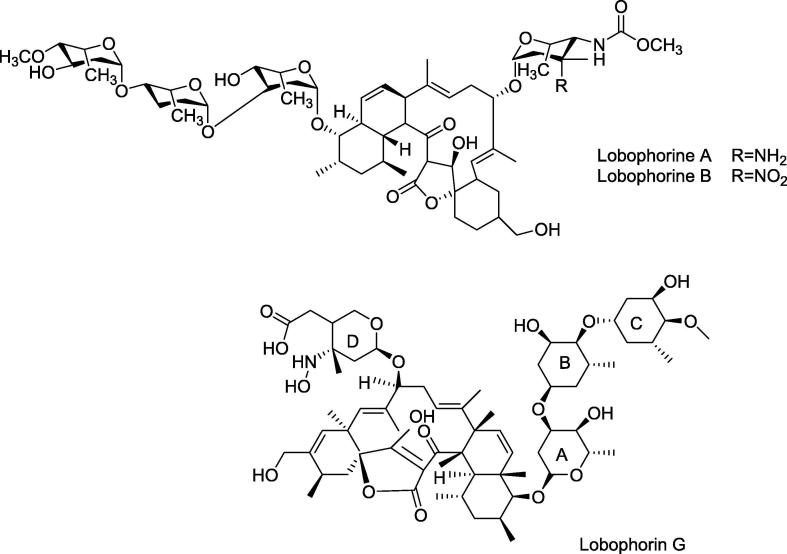
Lobophorin G, Lobophorin A, and Lobophorin B (Fig. 4) are isolated from an Actinomycete strain. The molecular weights of Lobophorin G, A, and B are 1225.75 Da, 1161.70 Da, and 1191.68 Da, respectively. As for the biological activity, the three compounds have showed a moderate activity against M. tuberculosis yet a strong activity against Mycobacterium bovis BCG. Lobophorin G and lobophorin A have produced the same MIC values against M. bovis BCG with (MIC = 1.56 µg/ml) and the same MIC value against M. tuberculosis which is 32 µg/ml. However, lobophorin B has a lower MIC value that is almost half of that produced by lobophorin G and lobophorin A, which is 0.78 µg/mL against M. bovis BCG and 16 µg/ml against M. tuberculosis, indicating a higher potency of lobophorin B against the two microorganisms when compared to lobophorin G and lobophorin A (Chen et al., 2013b).
Fig. 4.
Structures of Lobophorins G, A, and B, antimycobacterial peptides.
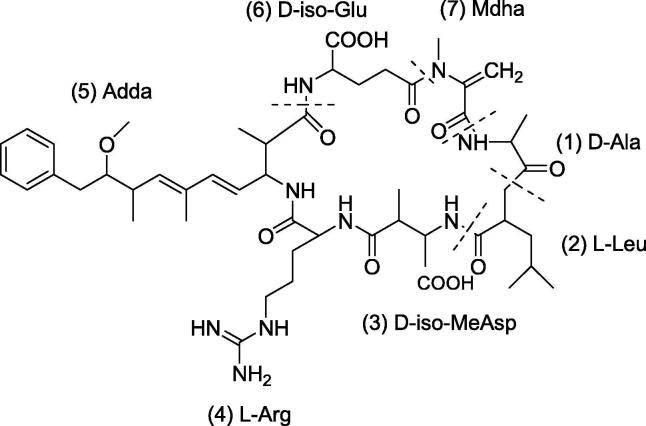
Microcystin-LR (D-Leu1) MC-LR obtained from Microcystis aeruginosa, are cyclic heptapeptides that are made up of seven amino acids, two of which are protein amino acids which are essential for the discrimination of the structural variants of microcystins, while the remaining five are non-protein amino acids which are common between the variants. An example of a compound that belongs to the microcystins is microcystin-LR (D-Leu1) MC-LR. It contains a leucine and an arginine residue in its structure, with a leucine residue that is present at position 2 (Fig. 5). The amino acid residues present at that position may vary in other microcystins. The mechanism of action involves the inhibition of the serine/threonine phosphatases 1 and 2A which are responsible for protein phosphorylation, this will result in cytoskeletal damage, liver necrosis, and hemorrhage in the liver that is related to the cytotoxic as well as tumor promoting activities of microcystins. As for the antimycobacterial activity, microcystin-LR (D-Leu1) MC-LR has produced an MIC of 13.2 µM against both the susceptible and rifampin-resistant M. tuberculosis strains and 26.5 µM against isoniazid-resistant M. tuberculosis strains, indicating that it is a promising candidate for developing new antimycobacterial drug (Ramos et al., 2015).
Fig. 5.
General structure of Microcystin-LR (D-Leu1) MC-LR with leucine (L) in the amino acid position 2 and arginine (R) in the amino acid position 4 and a leucine in the amino acid position 1.
4. Antifungal marine peptides
A crude extract of Red Sea sponge Theonella swinhoei located in Hurghada, Egypt, in the Red Sea coast, was subjected to multiple chromatographic separations and a final HPLC purification, where theonellamide G a bicyclic glycopeptide was determined (Fig. 6). Theonellamide G was reported to have strong antifungal activity and cytotoxic activity. The antifungal activity was measured against two strains of Candida albicans, the wild type and amphotericin B-resistant, with MIC values of 4.49 and 2.0 μM, respectively. Furthermore, theonellamide G cytotoxic activity was measured against the human colon adenocarcinoma cell line (HCT-16) with IC₅₀ of 6.0 μM (Youssef et al., 2014).
Fig. 6.
Chemical structure of Theonellamide G, antifungal peptide.
From a deep-sea sediment in Calyptogenacommunity located in Sagami Bay of Japan an Aneurinibacillus sp. YR247 was isolated and tested for antimicrobial activity, resulting in an inhibitory activity on Aspergillus brasiliensis with an inhibitory zone of 20.3 mm yet no inhibitory activity on Candida albicans. Upon the antifungal activity findings, the crude extract of YR247 was purified using silica gel chromatography, and the purified compound had an average molecular weight of 1167.9 Da which was identified via E.I-MS/MS. Furthermore, the effects of temperature and pH on antifungal activity were studied which resulted that the compound is stable at 4–70 °C and pH 2.0–12.0, respectively (Kurata et al., 2017).
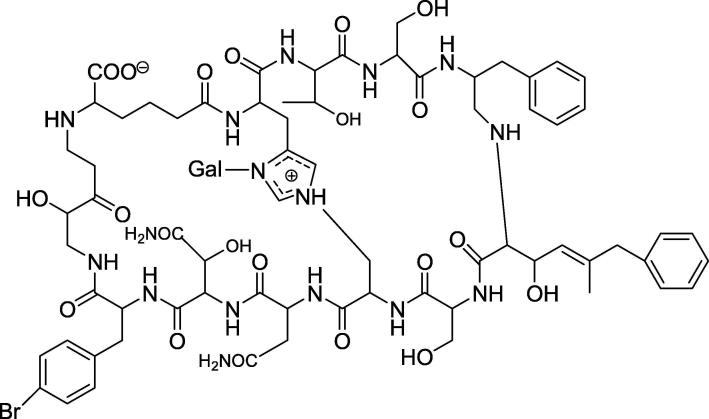
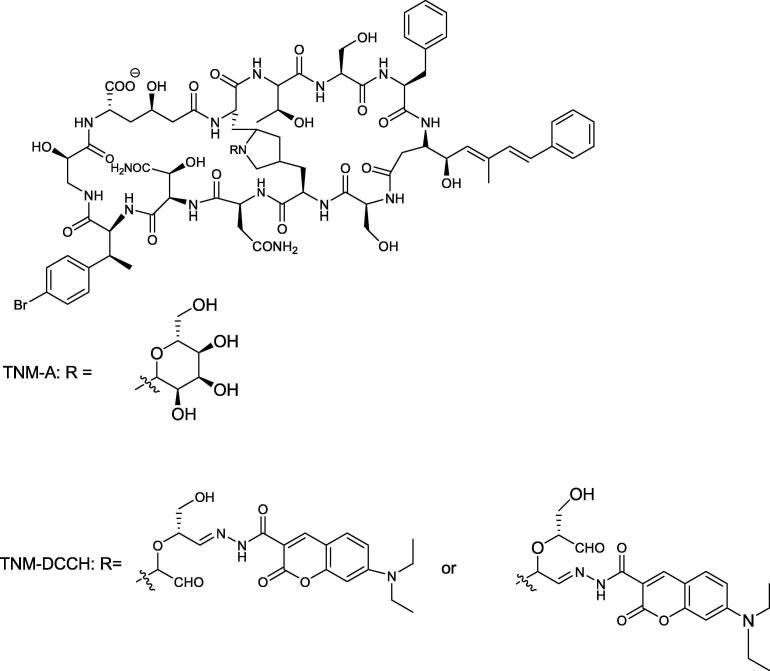
Based on previous studies, which report findings of bicyclic dodecapeptidestheonellamide (TNMs) A-E that butanol extract of marine sponge Theonella species showed antifungal and cytotoxic activity (Matsunaga and Fusetani, 1995). The structure of theonellamide-A is illustrated in Fig. 7. Theonellamides bind specifically to 3-β-hydroxysterols, resulting in 1,3-β-d-glucan overproduction and membrane damage in yeasts. In this study, they specifically examined (TNM-A) membrane action, that was assessed by 31P nuclear magnetic resonance (NMR) and confocal microscopy, where they were able to conclude in combination with earlier studies, that TNM-A binding to the membrane is facilitated by the direct interaction of TNM-A with sterol found on the membrane surface, consequently the accumulated TNM-A resulted in disruption of the membrane integrity and distortion in the membrane morphology (Espiritu et al., 2013, Espiritu et al., 2016).
Fig. 7.
Structure of Theonellamide A.
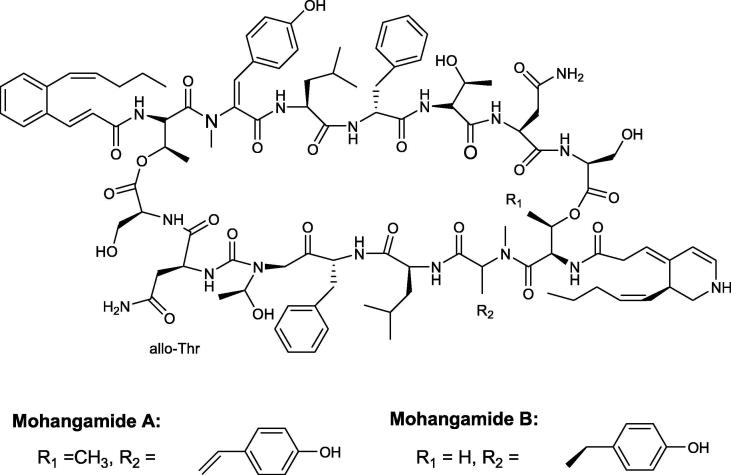
Mohangamides A and B were isolated from marine Streptomyces sp. found in seashore mud located in Busan, Korea. Upon multiple chromatographic and spectroscopic studies mohangamides structures were identified as novel structures, because it contained a unique dihydropyridine acyl chain and a dilactone-tethered pseudodimeric peptides (Fig. 8). Mohangamide A activity was tested against C. albicans where it exhibited a strong inhibitory activity against isocitratelyase with an IC50 of 4.4 μM, while Mohangamide B with IC50 = 20.5 μM (Bae et al., 2015).
Fig. 8.
Structures of Mohangamides A and B.
From earlier studies, the researchers isolated Cm-p1 from a coastal snail C. muricatus, which possessed antifungal activity. Whilst in Lopez-Abarrategui et al. (2015) study, which described the preparation of new Cm-p1 derivatives by adding 2 more residues to the C-terminus of Cm-p1 aiming at increasing the antifungal activity. This addition gave rise to three new derivatives, and out of the three Cm-p5 (SRSELIVHQRLF) seemed the most promising. Cm-p5 had a MIC value of 10 µg/ml; EC50 of 1.146 µg/ml against C. albicans, hence increased fungistatic activity compared to the parent peptide. Cm-p5 characterized by circular dichroism and nuclear magnetic resonance revealed an α-helical structure in lipophilic conditions and random coil folding in aqueous conditions. In silico studies showed Cm-p5 binding to phosphatidylserine bilayer, and isothermal titration calorimetry showed Cm-p5 having different affinities, where it possessed the highest affinity to phospholipids of fungal membranes, intermediate affinity to mammalian membrane phospholipid, lowest affinity to ergosterol, and no affinity to chitin, respectively. Furthermore, in mice with candidiasis, Cm-p5 was administered i.p., but failed to control the fungal kidney burden.
The heptacyclopeptide Stylissamide G (Fig. 9) was isolated from the Bahamian marine sponge Stylissa caribica located in the Caribbean Sea. In the study performed by Dahiya et al., they were able to synthesize stylissamide G. Quantitative analysis were used to confirm the structure of the synthesized stylissamide G, which was evaluated against different fungal strains, and it exhibited antifungal activity at a concentration of 6 μg/ml, against pathogenic C. albicans and dermatophytes Trichophyton mentagrophytes with an inhibitory zone of 22 mm and Microsporum audouinii with an inhibitory zone of 23 mm. In addition, the antifungal activity might be linked to the inhibition of glucan/cell wall chitin/sphingolipids synthesis. Stylissamide G can be delivered intravenously, subcutaneously, transdermally, or intranasaly to avoid possible degradation and limited absorption in the gastrointestinal tract (GIT) and hepatic first-pass metabolism. Although oral delivery is challenging, structural modification such as cyclization provides resistance to proteolytic degradation and has higher than expected absorption after oral administration in comparison to the linear counterparts (Dahiya et al., 2016).
Fig. 9.
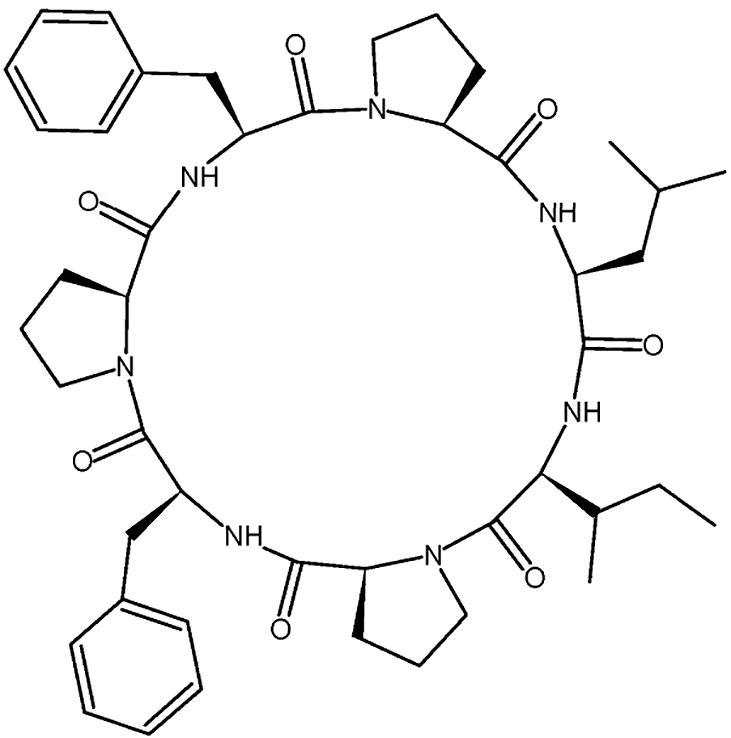
Structure of Stylissamide G.
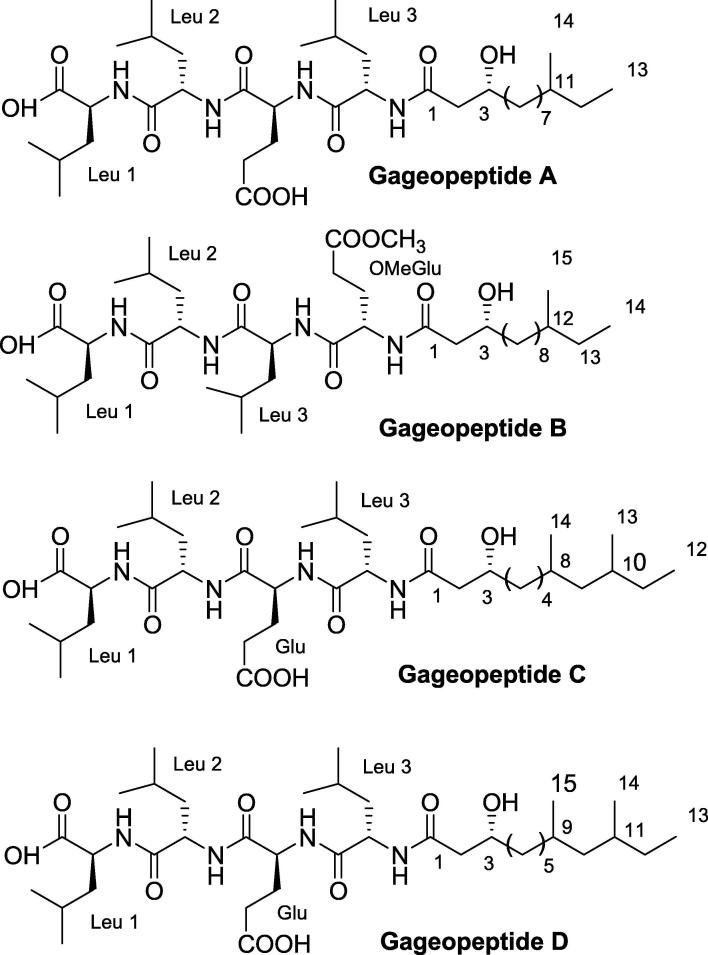
Gageopeptides A − D (Fig. 10) are four lipopeptides that were obtained from marine-derived bacterium B. subtilis. They showed antifungal activity with MIC values of 0.02–0.06 μM against Rhizoctonia solani, Botrytis cinerea, and Colletotrichum acutatum that are considered pathogenic fungi. In addition, Gageopeptides A–D showed no cytotoxicity when tested on cancer cell line (Tareq et al., 2014b).
Fig. 10.
Structures of Gageopeptides A-D.
5. Antiviral marine peptide
Marine antiviral peptides are unique natural antimicrobials, due to the presence of bioactive metabolites. These bioactive metabolites come from different sources like sponges, fungi, and fish. The bioactive peptides are either cyclic or linear peptides containing atypical amino acids. They inhibit viral infection through different mechanisms: (i) inhibition of virus attachment and entry; (ii) inhibition of virus penetration; (iii) inhibition of nucleic acid synthesis; (iv) inhibition of protein synthesis; (v) inhibition of virus release (Sadorn et al., 2016). Among the marine antiviral agents, the following agents were reported; Asperterrestide A, EcDefensin, and Aspergillipeptide.
Asperterrestide A (Fig. 11) is a cyclic tetrapeptide, containing 3-OH-N-CH3-Ph. residue, was isolated from the fungus Aspergillus terreus SCSG AF0162. It showed an inhibitory effect against the influenza strain A/WSN/33 H1N1 (an M2-resistant strain) and strain A/Hong Kong/8/68 H3N2 (an M2-sensitive strain) with IC50 values of 15 and 8.1 µM, respectively (He et al., 2013, Kang et al., 2015, Moghadamtousi et al., 2015, Sadorn et al., 2016, Abdelmohsen et al., 2017).
Fig. 11.
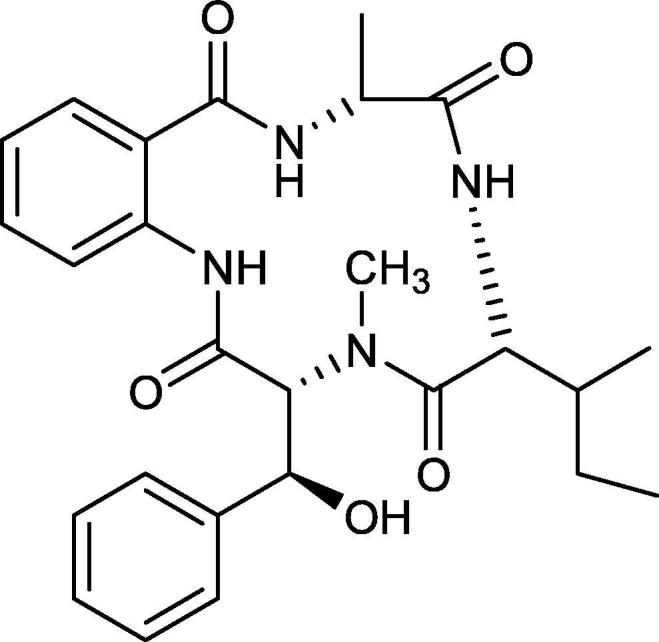
Structure of Asperterrestide A.
EcDefensin possesses dual antiviral activity, inhibiting the infection and replication of SGIV (an enveloped DNA virus of Singapore grouper iridovirus) and VNNV non-enveloped RNA virus of viral nervous necrosis virus. It comes from marine organisms called Epinephelus coioides. In vivo, EcDefensin was remarkably up-regulated in both spleen and liver tissues in presence of the pathogens, including LPS, SGIV and polyI:C (a synthetic dsRNA). When Ec-defenisn was used in concentrations less than 400 mg/ml, it did not show cytotoxic effects on GS or EAGB cells. Regarding the antiviral mechanism of this peptide, it exerts direct effect on virions in addition to some activity on target cell and both the innate and adaptive immunity. Fortunately, the majority of microbial pathogens have not developed resistance to defensins as antimicrobial compounds (Cheung et al., 2014, Ma et al., 2017). Aspergillipeptides (1–2) (Fig. 12), are new cyclic pentapeptide (compound 1) and linear peptide (compound 2). They were isolated from fungus Aspergillus sp. SCSIO 41501. Compounds 1 and 2 showed antiviral activity against Herpes simplex virus type 1 (HSV-1) with IC50 values of 9.5 and 19.8 µM, respectively. In addition compound 1 also had antiviral activity against acyclovir-resistant clinical isolates of HSV-1 (Cheung et al., 2015).
Fig. 12.
Structures of Aspergillipeptides.
6. Anitprotozoal marine peptides
Pc-pis is a cationic AMP isolated from Pseudosciaena crocea. Quantitative PCR analysis revealed that the overexpression of Pc-pis in the fish vital organs could be regulated during cryptocaryon irritans infection and post C. irritans falling off, highlighting the role of Pc-pis in immune defense against C. irritans and secondary bacterial infections. The synthetic Pc-pis peptide was a new member of the piscidin family were it exhibited a broad antimicrobial and antiparasitic activities. Antiparasitic assays were done to trophont of C. irritans that was rubbed off from the skin, pterygiophore, and gill of heavily infected S. latus to see the change in morphology and survival at different Pc-pis concentrations. Results revealed that at low concentration of synthetic Pc-pis it was strongly active against C. irritanstrophonts. When trophonts were exposed to high concentrations of Pc-pis, such as 24 or 48 μM, within minutes of exposure cytoplasmic infiltration from the trophonts which was dose-dependent was seen, at concentration of 48 μM the trophonts plasma membrane at several cell positions was completely and immediately lysed, where at 24 μM Pc-pis lysing of parasites was slower and cytoplasmic infiltration was in one or two cell positions. Lastly at the lowest concentration (6 μM), no cell rupture was seen but it was able to inhibit most trophonts swimming and unusual morphological changes with minimum cytoplasmic infiltration were observed (Niu et al., 2013).
EcPis-2, EcPis-3 and EcPis-4 are three putative piscidin paralogues taken from orange-spotted grouper (Epinephelus coioides). They exhibited different activities against the theronts of C. irritans. When the theronts were exposed to these piscidin, they became spherical, their motility decreased, and their membrane was ruptured. EcPis-3S exhibited the most potent activity against the infective stage of C. irritanstheronts, where by using 5 µmol/l ecPis-3S at 5 min post-treatment over 90% of the theronts were rapidly disintegrated and lysed. About 30 to 50% of theronts when 10 µmol/L were used of ecPis-2S, -2L, ecPis-3L, and ecPis-4S, -4L at 2 h post-treatment, were disintegrated and lysed and the rest theronts were less motile. In vivo, the peptide FFFHIVKGLFHAGRMIHGLV was identified with 99% confidence by HPLC and ESI-LCMS analysis, demonstrating high possibility that the short N-terminal fragment of ecPis-2, ecPis-2S, exists as a mature peptide of ecPis-2 in orange-spotted grouper (Zhuang et al., 2017).
Compounds 2,3,4 S Furthermore, two piscidins isolated from the fish Moronechrysops were identified to have activity against the parasite Tetrahymena pyriformis. White bass piscidin 6 (LFGSVKAWFKGAKKGFQDYRYQKDMAKMNKRYGPNWQQRGGQEPPADAQANDQPP) showed PCmin<0.25 µM and PC100 of 0.99. While (WB) Piscidin 5 (LIGSLFRGAKAIFRGARQGWRSHKAVSRYRARYVRRPVIYYHRVYP) displayed PCmin of 0.56 µM and PC100 of 1.1 µM (Salger et al., 2016).
7. Conclusion
Marine microorganisms are considered a rich source of antimicrobial agents. They can be used for different types of infections like bacterial, viral, fungal and parasitic infections. The structure of marine AMPs can be utilized to develop novel compounds with greater activity and stability, and to be used orally.
Because of multidrug resistance in bacterial, fungal, viral and parasitic infections, there is demand for developing new classes of antimicrobial agents to overcome this problem. So, AMPs are considered a good solution for this problem.
Most of the reported marine AMPs have been tested in vitro only, but some of them showed promising in vivo results. For example, Oreoch-2 and Oreoch-3 which significantly enhanced the antibody production when used in combination. As-CATH4 demonstrated in vivo immunostimulatory effect that can enhance the anti-infective potency of drugs used in combination with it. TP3/TP4 combination elongated the fish survival time after infection with V. vulnificus. PaLEAP-2 was reported to have in vivo activity against V. anguillarum. Cod β-defensin was active in vivo against Gram-negative bacteria. Moreover, arenicin derivatives N2 and N6 showed improved antibacterial activity and physicochemical properties, and prevented bacterial peritonitis and endotoxemia in mice.
Further researches are needed to explore the marine environment, and structural modifications of the natural peptides are required to get semi-synthetic or synthetic peptides possessing improved spectrum of activity, potency, and pharmacokinetic profile which will be a great addition to the battle against microbial infections.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Mohammad H. Semreen, Email: msemreen@sharjah.ac.ae.
Mohammed I. El-Gamal, Email: drmelgamal2002@gmail.com.
References
- Abdelmohsen U.R., Balasubramanian S., Oelschlaeger T.A., Grkovic T., Pham N.B., Quinn R.J., Hentschel U. Potential of marine natural products against drug-resistant fungal, viral, and parasitic infections. Lancet Infect Dis. 2017;17(2):30–41. doi: 10.1016/S1473-3099(16)30323-1. [DOI] [PubMed] [Google Scholar]
- Acosta J., Carpio Y., Valdés I., Velázquez J., Zamora Y., Morales R., Morales A., Rodríguez E., Estrada M.P. Co-administration of tilapia alpha-helical antimicrobial peptides with subunit antigens boost immunogenicity in mice and tilapia (Oreochromis niloticus) Vaccine. 2014;32(2):23–229. doi: 10.1016/j.vaccine.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Acosta J., Montero V., Carpio Y., Velazquez J., Garay H.E., Reyes O., Cabrales A., Masforrol Y., Morales A., Estrada M.P. Cloning and functional characterization of three novel antimicrobial peptides from tilapia (Oreochromis niloticus) Aquaculture. 2013;372–375:9–18. [Google Scholar]
- Bae J.S., Jung J.M., An C.M., Kim J.W., Hwang S.D., Kwon M.G., Park M.A., Kim M.C., Park C.I. Piscidin: antimicrobial peptide of rock bream, Oplegnathus fasciatus. Fish Shellfish Immunol. 2016;51:136–142. doi: 10.1016/j.fsi.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Bae M., Kim H., Moon K., Nam S.J., Shin J., Oh K.B., Oh D.C. Mohangamides A and B, new dilactone-tethered pseudo-dimeric peptides inhibiting Candida albicans isocitrate lyase. Org Lett. 2015;17(3):712–715. doi: 10.1021/ol5037248. [DOI] [PubMed] [Google Scholar]
- Brogden K.A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria. Nat. Rev. Microbiol. 2005;3(3):238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- Buonocore F., Randelli E., Casani D., Picchietti S., Belardinelli M.C., Pascale D.D., Santi C.D., Scapigliati G. A piscidin-like antimicrobial peptide from the icefish Chionodraco hamatus (Perciformes: Channichthyidae): molecular characterization, localization and bactericidal activity. Fish Shellfish Immunol. 2012;33(5):1183–1191. doi: 10.1016/j.fsi.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Chen B., Fan D.Q., Zhu K.X., Shan Z.G., Chen F.Y., Hou L., Cai L., Wang K.J. Mechanism study on a new antimicrobial peptide Sphistin derived from the N-terminus of crab histone H2A identified in haemolymphs of Scylla paramamosain. Fish Shellfish Immunol. 2015;47:833–846. doi: 10.1016/j.fsi.2015.10.010. [DOI] [PubMed] [Google Scholar]
- Chen Y., Zhao H., Zhang X., Luo H., Xue X., Li Z., Yao B. Identification, expression and bioactivity of Paramisgurnus dabryanus β-defensin that might be involved in immune defense against bacterial infection. Fish Shellfish Immunol. 2013;35(2):399–406. doi: 10.1016/j.fsi.2013.04.049. [DOI] [PubMed] [Google Scholar]
- Chen C., Wang J., Guo H., Hou W., Yang N., Ren B., Liu M., Dai H., Liu X., Song F., Zhang L. Three antimycobacterial metabolites identified from a marine-derived Streptomyces sp. MS100061. Appl. Microbiol. Biotechnol. 2013;97(9):3885–3892. doi: 10.1007/s00253-012-4681-0. [DOI] [PubMed] [Google Scholar]
- Cheung R.C., Wong J.H., Pan W.L., Chan Y.S., Yin C., Dan X.L., Wang H.X., Fang E.F., Lam S.K., Ngai P.K., Xia L.X., Liu F., Ye X.Y., Zhang G.Q., Liu Q.H., Sha O., Lin P., Ki C., Bekhit A.A., Bekhit A.E., Wan D.C., Ye X.J., Xia J., Ng T.B. Antifungal and antiviral products of marine organisms. Appl. Microbiol. Biotechnol. 2014;98(8):3475–3494. doi: 10.1007/s00253-014-5575-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung R.F., Ng T.B., Wong J.H. Marine peptides: bioactivities and applications. Mar. Drugs. 2015;13(7):4006–4043. doi: 10.3390/md13074006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahiya R., Singh R., Sharma A., Chennupati S., Maharaj S. First total synthesis and biological screening of a proline-rich cyclopeptide from a caribbean marine sponge. Mar. Drugs. 2016;14(12):228. doi: 10.3390/md14120228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gamal M.I., Abdel-Maksoud M.S., Oh C.H. Recent advances in the research and development of marine antimicrobial peptides. Curr. Top. Med. Chem. 2013;13(16):2026–2033. doi: 10.2174/15680266113139990127. [DOI] [PubMed] [Google Scholar]
- Espiritu R.A., Cornelio K., Kinoshita M., Matsumori N., Murata M., Nishimura S., Kakeya H., Yoshida M., Matsunaga S. Marine sponge cyclic peptide theonellamide A disrupts lipid bilayer integrity without forming distinct membrane pores. Biochim. Biophys. Acta. 2016;1858(6):1373–1379. doi: 10.1016/j.bbamem.2016.03.019. [DOI] [PubMed] [Google Scholar]
- Espiritu R.A., Matsumori N., Murata M., Nishimura S., Kakeya H., Matsunaga S., Yoshida M. Interaction between the marine sponge cyclic peptide theonellamide A and sterols in lipid bilayers as viewed by surface plasmon resonance and solid-state 2H nuclear magnetic resonance. Biochemistry. 2013;52(4):2410–2418. doi: 10.1021/bi4000854. [DOI] [PubMed] [Google Scholar]
- Evans M.E., Feola D.J., Rapp R.P. Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant gram-negative bacteria. Ann. Pharmacother. 1999;33(9):60–967. doi: 10.1345/aph.18426. [DOI] [PubMed] [Google Scholar]
- Ganz T., Selsted M.E., Szklarek D., Harwig S.S., Daher K., Bainton D.F., Lehrer R.I. Defensins. Natural peptide antibiotics of human neutrophils. J. Clin. Investig. 1985;76(4):1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui L., Zhang P., Zhang Q., Zhang J. Two hepcidins from spotted scat (Scatophagus argus) possess antibacterial and antiviral functions in vitro. Fish Shellfish Immunol. 2016;50:191–199. doi: 10.1016/j.fsi.2016.01.038. [DOI] [PubMed] [Google Scholar]
- Guoa Z., Qiaob X., Chenga R., Shib N., Wange A., Fengd T., Chena Y., Zhanga F., Yub H., Wang Y. As-CATH4 and 5, two vertebrate-derived natural host defense peptides, enhance the immuno-resistance efficiency against bacterial infections in Chinese mitten crab, Eriocheir sinensis. Fish Shellfish Immunol. 2017;71:202–209. doi: 10.1016/j.fsi.2017.10.015. [DOI] [PubMed] [Google Scholar]
- Hancock R.E. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect. Dis. 2001;1(3):156–164. doi: 10.1016/S1473-3099(01)00092-5. [DOI] [PubMed] [Google Scholar]
- Harwing S.S., Waring A., Yang H.J., Cho Y., Tan L., Lehrer R.I. Intramolecular disulfide bonds enhance the antimicrobial and lytic activities of protegrins at physiological sodium chloride concentrations. Eur. J. Biochem. 1996;240:352–357. doi: 10.1111/j.1432-1033.1996.0352h.x. [DOI] [PubMed] [Google Scholar]
- He F., Bao J., Zhang X.Y., Tu Z.C., Shi Y.M., Qi S.H. Asperterrestide A, a cytotoxic cyclic tetrapeptide from the marine-derived fungus Aspergillus terreus SCSGAF0162. J. Nat Prod. 2013;76(6):1182–1186. doi: 10.1021/np300897v. [DOI] [PubMed] [Google Scholar]
- Kang H.K., Seo C.H., Park Y. Marine peptides and their anti-infective activities. Mar Drugs. 2015;13(1):618–654. doi: 10.3390/md13010618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J., Wan Q., Bathige S., Lee J. Molecular characterization, transcriptional profiling, and antibacterial potential of G-type lysozyme from seahorse (Hippocampus abdominalis) Fish Shellfish Immunol. 2016;58:622–630. doi: 10.1016/j.fsi.2016.10.014. [DOI] [PubMed] [Google Scholar]
- Kondejewski L.H., Jelokhani-Niaraki M., Farmer S.W., Lix B., Kay C.M., Sykes B.D., Hancock R., Hodges R.S. Dissociation of antimicrobial and hemolytic activities in cyclic peptide diastereomers by systematic alterations in amphipathicity. J. Biol. Chem. 1999;274(19):13181–13192. doi: 10.1074/jbc.274.19.13181. [DOI] [PubMed] [Google Scholar]
- Kurata A., Yamaura Y., Tanaka T., Kato C., Nakasone K., Kishimoto N. Antifungal peptidic compound from the deep-sea bacterium Aneurinibacillus sp. YR247. World J. Microbiol. Biotechnol. 2017;33(4):73. doi: 10.1007/s11274-017-2239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R.I., Selsted M.E., Szklarek D., Fleischmann J. Antibacterial activity of microbicidal cationic proteins 1 and 2, natural peptide antibiotics of rabbit lung macrophages. Infect. Immun. 1983;42(1):10–14. doi: 10.1128/iai.42.1.10-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.X., Lu X.J., Li C.H., Chen J. Molecular characterization of the liver-expressed antimicrobial peptide 2 (LEAP-2) in a teleost fish, Plecoglossus altivelis: antimicrobial activity and molecular mechanism. Mol Immunol. 2015;65(2):406–415. doi: 10.1016/j.molimm.2015.02.022. [DOI] [PubMed] [Google Scholar]
- Li Q.C., Huang W., Zhou S.Q., Wang X.C., Liu H.H., Fan M.H., Wang R.X., Gao P., Liao Z. Characterization of a novel antimicrobial peptide with chitin-binding domain from Mytilus coruscus. Fish Shellfish Immunol. 2014;41(2):362–370. doi: 10.1016/j.fsi.2014.09.019. [DOI] [PubMed] [Google Scholar]
- Li Z., Wang P., Jiang C., Cui P., Zhang P. Antibacterial activity and modes of action of phosvitin-derived peptide Pt5e against clinical multi-drug resistance bacteria. Fish Shellfish Immunol. 2016;58:370–379. doi: 10.1016/j.fsi.2016.09.044. [DOI] [PubMed] [Google Scholar]
- Li Z., Zhang S., Gao J., Guang H., Tian Y., Zhao Z., Wang Y., Yu H. Structural and functional characterization of CATH_BRALE, the defense molecule in the ancient salmonoid, Brachymystax lenok. Fish Shellfish Immunol. 2013;34:1–7. doi: 10.1016/j.fsi.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Liao Z., Wang X.C., Liu H.H., Fan M.H., Sun J.J., Shen W. Molecular characterization of a novel antimicrobial peptide from Mytilus coruscus. Fish Shellfish Immunol. 2013;34(2):610–616. doi: 10.1016/j.fsi.2012.11.030. [DOI] [PubMed] [Google Scholar]
- Liu T., Gao Y., Wang R., Xu T. Characterization, evolution and functional analysis of the liver-expressed antimicrobial peptide 2 (LEAP-2) gene in miiuy croaker. Fish Shellfish Immunol. 2014;41(2):191–199. doi: 10.1016/j.fsi.2014.08.010. [DOI] [PubMed] [Google Scholar]
- Lopez-Abarrategui C., McBeth C., Mandal S.M., Sun Z.J., Heffron G., Alba-Menendez A., Migliolo L., Reyes-Acosta O., Garcia-Villarino M., Nolasco D.O., Falcao R., Cherobim M.D., Dias S.C., Brandt W., Wessjohann L., Starnbach M., Franco O.L., Otero-Gonzalez A.J. Cm-p5: an antifungal hydrophilic peptide derived from the coastal mollusk Cenchritis muricatus (Gastropoda: Littorinidae) FASEB J. 2015;29(8):3315–3325. doi: 10.1096/fj.14-269860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Nong X.H., Ren Z., Wang J., Liang X., Wang L., Qi S.H. Antiviral peptides from marine gorgonian-derived fungus Aspergillus sp. SCSIO 41501. Tetrahedron Lett. 2017;58(12):1151–1155. [Google Scholar]
- Matsunaga S., Fusetani N. Theonellamides A-E, cytotoxic bicyclic peptides, from a marine sponge Theonella sp. J. Org. Chem. 1995;60:1177–1181. [Google Scholar]
- Michalopoulos A.S., Tsiodras S., Rellos K., Mentzelopoulos S., Falagas M.E. Colistin treatment in patients with ICU-acquired infections caused by multiresistant Gram-negative bacteria: the renaissance of an old antibiotic. Clin. Microbiol. Infect. 2005;11(2):115–121. doi: 10.1111/j.1469-0691.2004.01043.x. [DOI] [PubMed] [Google Scholar]
- Moghadamtousi S.Z., Nikzad S., Kadir H.A., Abubakar S., Zandi K. Potential antiviral agents from marine fungi: an overview. Mar. Drugs. 2015;13(7):4520–4538. doi: 10.3390/md13074520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mygind P.H., Fischer R.L., Schnorr K.M., Hansen M.T., Sönksen C.P., Ludvigsen S., Raventós D., Buskov S., Christensen B., Maria L.D., Taboureau O., Yaver D., Jorgensen S.G., Sorensen M.V., Christensen B.E., Kjærulff S., Moller N.F., Lehrer R.I., Zasloff M., Kristensen H.H. Plectasin is a peptide antibiotic with therapeutic potential from a saprophytic fungus. Nature. 2005;437(7061):975–980. doi: 10.1038/nature04051. [DOI] [PubMed] [Google Scholar]
- Niu S.F., Jin Y., Xu X., Qiao Y., Wu Y., Mao Y., Su Y.Q., Wang J. Characterization of a novel piscidin-like antimicrobial peptide from Pseudosciaena crocea and its immune response to Cryptocaryon irritans. Fish Shellfish Immunol. 2013;35(2):513–524. doi: 10.1016/j.fsi.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Pan C.Y., Tsai T.Y., Su B.C., Hui C.F., Chen J.Y. Study of the antimicrobial activity of Tilapia Piscidin 3 (TP3) and TP4 and their effects on immune functions in hybrid Tilapia (Oreochromis spp.) PLoS ONE. 2017;12(1):e0169678. doi: 10.1371/journal.pone.0169678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng K.C., Lee S.H., Hour A.L., Pan C.Y., Lee L.H., Chen J.Y. Five different piscidins from Nile tilapia, Oreochromis niloticus: analysis of their expressions and biological functions. PLoS ONE. 2012;7(12):e50263. doi: 10.1371/journal.pone.0050263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu H., Chen B., Peng H., Wang K.J. Molecular cloning, recombinant expression, and antimicrobial activity of EC-hepcidin3, a new four-cysteine hepcidin isoform from Epinephelus coioides. Biosci. Biotechnol. Biochem. 2013;77(1):103–110. doi: 10.1271/bbb.120600. [DOI] [PubMed] [Google Scholar]
- Ramos D.F., Matthiensen A., Colvara W., Votto A.P.S.D., Trindade G.S., Silva P.E.A.D., Yunes J.S. Antimycobacterial and cytotoxicity activity of microcystins. J. Venom Anim. Toxins. Incl. Trop. Dis. 2015;21:9. doi: 10.1186/s40409-015-0009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruangsri J., Kitani Y., Kiron V., Lokesh J., Brinchmann M.F., Karlsen B.O., Fernandes J.O. A novel beta-defensin antimicrobial peptide in Atlantic cod with stimulatory effect on phagocytic activity. PLoS ONE. 2013;8(4):e62302. doi: 10.1371/journal.pone.0062302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadorn K., Saepua S., Boonyuen N., Laksanacharoen P., Rachtawee P., Prabpai S., Kongsaeree P., Pittayakhajonwut P. Allahabadolactones A and B from the endophytic fungus, Aspergillus allahabadii BCC45335. Tetrahedron Lett. 2016;72:489–495. [Google Scholar]
- Salger S.A., Cassady K.R., Reading B.J., Noga E.J. A diverse family of host-defense peptides (Piscidins) exhibit specialized anti-bacterial and anti-protozoal activities in fishes. PLoS ONE. 2016;11(8):e0159423. doi: 10.1371/journal.pone.0159423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta D., Leontiadou H., Mark A.E., Marrink S.J. Toroidal pores formed by antimicrobial peptides show significant disorder. Biochim. Biophys. Acta. 2008;1778(10):2308–2317. doi: 10.1016/j.bbamem.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Seo J.K., Lee M.J., Go H.J., Kim G.D., Jeong H.D., Nam B.H., Park N.G. Purification and antimicrobial function of ubiquitin isolated from the gill of Pacific oyster, Crassostrea gigas. Mol. Immunol. 2013;53(1–2):88–98. doi: 10.1016/j.molimm.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Seo J.K., Lee M., Go H.J., Park T.H., Park N.G. Purification and characterization of YFGAP, a GAPDH-related novel antimicrobial peptide, from the skin of yellowfin tuna, Thunnus albacares. Fish Shellfish Immunol. 2014;33(4):743–752. doi: 10.1016/j.fsi.2012.06.023. [DOI] [PubMed] [Google Scholar]
- Seo J.K., Lee M.J., Go H.J., Kim Y.J., Park N.G. Antimicrobial function of the GAPDH-related antimicrobial peptide in the skin of skipjack tuna, Katsuwonus pelamis. Fish Shellfish Immunol. 2014;36(2):571–581. doi: 10.1016/j.fsi.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Seo J.K., Lee M.J., Nam B.H., Park N.G. cgMolluscidin, a novel dibasic residue repeat rich antimicrobial peptide, purified from the gill of the Pacific oyster, Crassostrea gigas. Fish Shellfish Immunol. 2013;35(2):480–488. doi: 10.1016/j.fsi.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Shamova O.V., Orlov D.S., Balandin S.V., Shramova E.I., Tsvetkova E.V., Panteleev P.V., Leonova Y.F., Tagaev A.A., Kokryakov V.N., Ovchinnikova T.V. Acipensins—novel antimicrobial peptides from Leukocytes of the Russian Sturgeon Acipenser gueldenstaedtii. Acta Nat. 2014;6:99–109. [PMC free article] [PubMed] [Google Scholar]
- Shan Z., Zhu K., Peng H., Chen B., Liu J., Chen F., Ma X., Wang S., Qiao K., Wang K. The new antimicrobial peptide SpHyastatin from the mud crab Scylla paramamosain with multiple antimicrobial mechanisms and high effect on bacterial infection. Front Microbiol. 2016;7:1140. doi: 10.3389/fmicb.2016.01140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenkarev Z.O., Panteleev P.V., Balandin S.V., Gizatullina A.K., Altukhov D.A., Finkina E.I., Kokryakov V.N., Arseniev A.S., Ovchinnikova T.V. Recombinant expression and solution structure of antimicrobial peptide aurelin from jellyfish Aurelia aurita. Biochem. Biophys. Res. Commun. 2012;429(1–2):63–69. doi: 10.1016/j.bbrc.2012.10.092. [DOI] [PubMed] [Google Scholar]
- Shin S.C., Ahn I.H., Ahn D.H., Lee Y.M., Lee J., Lee J.H., Kim H.W., Park H. Characterization of two antimicrobial peptides from antarctic fishes (Notothenia coriiceps and Parachaenichthys charcoti) PLoS ONE. 2017;12(1):e0170821. doi: 10.1371/journal.pone.0170821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.J., Travis S.M., Greenberg E.P., Welsh M.J. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85(2):229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- Solstad R.G., Li C., Isaksson J., Johansen J., Svenson J., Stensvåg K., Haug T. Novel antimicrobial peptides EeCentrocins 1, 2 and EeStrongylocin 2 from the Edible Sea Urchin Echinus esculentus have 6-Br-Trp post-translational modifications. PLoS ONE. 2016;11(3):e0151820. doi: 10.1371/journal.pone.0151820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tareq F.S., Lee M.A., Lee H.S., Lee J.S., Lee Y.J., Shin H.J. Gageostatins A-C, antimicrobial linear lipopeptides from a marine Bacillus subtilis. Mar. Drugs. 2014;12(2):871–885. doi: 10.3390/md12020871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tareq F.S., Lee M.A., Lee H.S., Lee Y.J., Lee J.S., Hasan C.M., Islam M.T., Shin H.J. Non-cytotoxic antifungal agents: isolation and structures of gageopeptides A-D from a Bacillus strain 109GGC020. J. Agric. Food Chem. 2014;62(24):5565–5572. doi: 10.1021/jf502436r. [DOI] [PubMed] [Google Scholar]
- Thackray P.D., Moir A. SigM, an extracytoplasmic function sigma factor of Bacillus subtilis, is activated in response to cell wall antibiotics, ethanol, heat, acid, and superoxide stress. Int. J. Bacteriol. 2003;185(12):3491–3498. doi: 10.1128/JB.185.12.3491-3498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Teng D., Mao R., Yang N., Hao Y., Wang J. Combined systems approaches reveal a multistage mode of action of a marine antimicrobial peptide against pathogenic Escherichia coli and its protective effect against bacterial peritonitis and endotoxemia. Antimicrob. Agents Chemother. 2017;61:e01056–e1116. doi: 10.1128/AAC.01056-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L., Gao J., Zhang S., Wu S., Xie Z., Ling G., Kuang Y.Q., Yang Y., Yu H., Wang Y. Identification and characterization of the first Cathelicidin from sea snakes with potent antimicrobial and anti-inflammatory activity and special mechanism. J. Biol. Chem. 2015;290:16633–16652. doi: 10.1074/jbc.M115.642645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N., Liu X., Teng D., Li Z., Wang X., Mao R., Wang X., Hao Y., Wang J. Antibacterial and detoxifying activity of NZ17074 analogues with multi-layers of selective antimicrobial actions against Escherichia coli and Salmonella Enteritidis. Sci. Rep. 2017;7:3392. doi: 10.1038/s41598-017-03664-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef D., Shaala L., Mohamed G., Badr J., Bamanie F., Ibrahim S. Theonellamide G, a potent antifungal and cytotoxic bicyclic glycopeptide from the Red Sea marine sponge Theonella swinhoei. Mar Drugs. 2014;12(4):1911–1923. doi: 10.3390/md12041911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J. Leukoc. Biol. 2004;75(1):39–48. doi: 10.1189/jlb.0403147. [DOI] [PubMed] [Google Scholar]
- Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. USA. 1987;84(15):5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415(6870):389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- Zhang J., Yu L.P., Li M.F., Sun L. Turbot (Scophthalmus maximus) hepcidin-1 and hepcidin-2 possess antimicrobial activity and promote resistance against bacterial and viral infection. Fish Shellfish Immunol. 2014;38:127–134. doi: 10.1016/j.fsi.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Zhang L., Yang D., Wang Q., Yuan Z., Wu H., Pei D., Cong M., Li F., Ji C., Zhao J. A defensin from clam Venerupis philippinarum: molecular characterization, localization, antibacterial activity, and mechanism of action. Dev. Comp. Immunol. 2015;51(1):29–38. doi: 10.1016/j.dci.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Zhong J., Wang W., Yang X., Yan X., Liu R. Peptides. 2013;39:1–5. doi: 10.1016/j.peptides.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Zhou Q.J., Wang J., Liu M., Qiao Y., Hong W.S., Su Y.Q., Han K.H., Ke Q.Z., Zheng W.Q. Identification, expression and antibacterial activities of an antimicrobial peptide NK-lysin from a marine fish Larimichthys crocea. Fish Shellfish Immunol. 2016;55:195–202. doi: 10.1016/j.fsi.2016.05.035. [DOI] [PubMed] [Google Scholar]
- Zhuang J., Coates C.J., Zhu H., Zhu P., Wu Z., Xie L. Identification of candidate antimicrobial peptides derived from abalone hemocyanin. Dev. Comp. Immunol. 2015;49(1):96–102. doi: 10.1016/j.dci.2014.11.008. [DOI] [PubMed] [Google Scholar]
- Zhuang Z.R., Yang X.D., Huang X.Z., Gu H.X., Wei H.Y., He Y.J., Deng L. Three new piscidins from orange-spotted grouper (Epinephelus coioides). Phylogeny, expression and functional characterization. Fish Shellfish Immunol. 2017;66:240–253. doi: 10.1016/j.fsi.2017.04.011. [DOI] [PubMed] [Google Scholar]