Abstract
Curcumin as the active compound of turmeric has antioxidative, antiinflammatory, antimicrobial and anticancer properties among others. However, its disadvantageous properties like low solubility, poor bioavailability and rapid degradation under neutral or alkaline pH conditions or when exposed to light limit its clinical application. These problems can be solved by a smart combination of using a natural enhancer like piperine and preparing nanoparticles by a proper method like electrospray.
Due to these facts it was aimed in this study to develop curcumin and piperine loaded zein-chitosan nanoparticles step by step. For that purpose various formulation parameters like the concentrations of zein, curcumin, piperine and chitosan and the preparation parameters like the applied voltage and the nozzle diameter were investigated step by step. The nanoparticles were characterised by investigating their shapes, morphologies, particle sizes with help of SEM images and the cytotoxicity on neuroblastoma cells.
It was succeeded to prepare curcumin and piperine loaded zein-chitosan nanoparticles having a mean particle size of approximately 500 nm and high encapsulation efficencies for curcumin (89%) and piperine (87%). Using a curcumin concentration of 10–25 µg/ml resulted in reduction of the viability of approximately 50% of the neuroblastoma cells. The here developed nanoparticle formulation consisting of solely natural compounds showed good cytotoxic effects and is a promising approach with appropriate properties for final consumption.
Keywords: Curcumin, Piperine, Nanoparticles, Electrospray, Cytotoxicity
1. Introduction
The preparation of curcumin and piperine loaded zein-chitosan nanoparticles, having a particle size of approximately 500 nm and appropriate properties for final consumption is the main objective of this study. Curcumin [1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione] is a diphenolic, active compound of turmeric (Curcuma longa), with antioxidative (Jayaprakasha et al., 2006), antiinflammatory (Jurenka, 2009), antimicrobial (Wang et al., 2009a) and anticancer (Villegas et al., 2008, Yoysungnoen et al., 2008) properties. Unfortunately, curcumin has some disadvantageous properties such as low solubility and poor bioavailability (Wang et al., 2009b, Yang et al., 2007), which limit its clinical application. This combination of low solubility and poor bioavailability negatively affects its biological efficacy (Shaikh et al., 2009). One important approach to improve the poor biopharmaceutical properties of curcumin is to improve its aqueous solubility using nanotechnology and nanoparticles, having a small size in the nanometer range (Torchilin, 2009, Ruenraroengsak et al., 2010, Sultana et al., 2013).
Due to these facts the starting-point to solve these problems consists of two crucial factors: nanoencapsulation of curcumin to enhance its poor solubility and to protect it against degradation, and combination of curcumin with the natural enhancers like piperin, which was proven to enhance the absorption of curcumin (Han et al., 2008), and chitosan in order to enhance its poor bioavailability.
Nanoencapsulation systems exhibit a high potential as carriers of bioactive substances due to their subcellular size, allowing relatively higher intracellular uptake (Mora-Huertas et al., 2010) and improved stability and protection of labile substances against degradation (Preetz et al., 2008).
Encapsulation includes the immobilization of a particular compound in a material that coats it or in which it is dispersed. Encapsulation, in general, can improve disadvantageous properties of a substance, like a low aqueous solubility and protect a molecule from degradation or loss of functionality due to the effects of light, oxygen, pH or moisture. All these properties are occurring with curcumin. Encapsulating curcumin by several methods has been described like incorporating into cyclodextrins (Baglole et al., 2005), liposomes (Li et al., 2007a), microemulsions (Lin et al., 2009) and micelles (Yu and Huang, 2010). In addition, the development of micro- or nanoparticles by spray-drying (Wang et al., 2009b), solvent emulsion-evaporation (Shaikh et al., 2009, Mukerjee and Vishwanatha, 2009, Prajakta et al., 2009), electrohydrodynamic atomization and so-called electrospray (Gomez-Estaca et al., 2012) were referred. A simple and one-step method had been developed to encapsulate actives (Jaworek, 2008, Zhang et al., 2012, Cao et al., 2014), which is called electrospray or electrohydrodynamic atomization. Electrospray is a well-known method for preparing polymeric nanoparticles and for encapsulating drugs with poor aqueous solubility, just like curcumin. Electrospray is based on the break-up of a liquid into fine charged droplets under the action of an electric field. When a liquid is passed through a thin metal tube such as a nozzle, needle or capillary and the liquid meniscus located at the tip of the tube is electrically stressed by applying a potential difference between the tube and the counter electrode, the electrospray is generated. Electrospray offers several advantages compared to other nanoparticle manufacturing methods, like monodispersed particles, the fact that the particle size can be easily controlled by adjusting the preparation parameters (Yun et al., 2009, Ding et al., 2005), a high encapsulation efficiency and no need for a tedious separation process to remove the particles from the solvent, like for many encapsulation methods. Furthermore, hydrophobic or hydrophilic drugs can be loaded into electrosprayed particles with high entrapment efficiency (Kim and Kim, 2011, Valo et al., 2009) and core–shell structured particles can be conveniently obtained (Zhang et al., 2011). The complex electrospraying process is affected by many variables like the electrostatic field strength, needle/nozzle diameter, the solution flow rate and concentration of the compounds. Although the size of electrosprayed particles was always within micron to submicron range many strategies have been developed to decrease the particle size. Particles with sizes ranging from 275 to 860 nm were obtained by varying the polymer concentration, feed rate and applied voltage (Chang et al., 2010). Chitosan particles with an average diameter of 124 nm were prepared by decreasing the polymer concentration and reducing the fluid flow rate (Zhang and Kawakami, 2010). It must be pointed out that decreasing the particle size by decreasing the solution concentration or flow rate can lead to a low particle production capacity. Nanoparticles showed an enhanced colon bioadhesion and increased oral bioavailability compared to microparticles (Lamprecht et al., 2001, Sigfridsson et al., 2011).
Due to previous experiences of encapsulating lycopene with zein (Kose and Bayraktar, 2016) this maize protein was selected as the encapsulating material for dispersing curcumin. Zein comprises a group of alcohol soluble but aqueous insoluble proteins (Shukla and Cheryan, 2001). There are many reports about the use of zein as a wall material for encapsulating a variety of compounds (Patel et al., 2010, Liu et al., 2005, Zhong and Jin, 2009). Biofunctionality and the use of non-toxic materials are the most fundamental conditions for the release of bioactive compounds in the pharmaceutical industry to be possible. Therefore, natural polymers like zein or polyelectrolytes such as chitosan are competitive candidates as materials for the formation of nanoparticles. After zein was certained as matrix for curcumin, a second multifunctional biopolymer for the shell of the nanoparticles was appreciated. There are several studies in which viscosity modified macromolecules such as sodium alginate (Tonnesen, 2006), chitosan (Mazzarino et al., 2012), acrylic derivates of cellulose (Jaiswal et al., 2010) alone or in combination, were tested as a curcumin bioavailability promoter, because of their ability to increase water solubility, their affinity for the biological membranes, time release and membrane permeation. The biocompatible and biodegradable polymer chitosan is currently employed to prepare nanoparticles with mucoadhesive properties. This cationic copolymer can form nanoparticles by different methods like ionotropic gelation, microemulsion, emulsification solvent diffusion, and polyelectrolytes complex formation (Nagpal et al., 2010). Chitosan exhibits some promising properties like being nontoxic, biodegradable and biocompatible (Helander et al., 2001). Chitosan is a naturally positively-charged polymer with the propensity to easily interact with negatively-charged sites on a cell surface (Gordon et al., 2008). These beneficial properties made chitosan to one of the most popular biopolymers for the development of bioactive compounds delivery systems for a wide range of applications (Luo and Wang, 2014). Due to the fact that the surface of all physiological membranes including the intestine, have a negative surface charge (Rojanasakul et al., 1992), positively charged nanoparticles containing chitosan, proven to enhance the absorption (Thanou et al., 2001), makes them very attractive.
The major component of black pepper (Piper nigrum L.) piperine is the first and most potent bioenhancer till date (Atal and Bedi, 2010), enhances the bioavailability of many drugs by increasing the absorption from the intestine, suppresses the drug metabolism via inhibiting CYP3A4 and P-glycoprotein (P-gp) (Makhov et al., 2012). The ATP-dependent efflux transporter P-gp pumps various drug molecules out of the cells (Zhu et al., 2007) and is responsible for multidrug resistance in cancer cells (Ampasavate et al., 2010). Piperine has diverse important effecst like anti-microbial and anti-parasitic (Raay et al., 1999), antiinflammatory (Vaibhav et al., 2012), antidepressant (Li et al., 2007b), antiangiogenic (Doucette et al., 2013) and anticancer activities (Pradeep and Kuttan, 2002). Although piperine suppresses tumor growth and metastasis in vitro as well as in vivo (Lai et al., 2012), its use alone as chemotherapeutic molecule for cancer therapeutics is limited because of its high concentration requirement, due to its hydrophobic nature. The co-administered piperine (10 mg) increased the plasma concentration and delayed the elimination of drugs like phenytoin (Velpandian et al., 2001) and rifampin (Zutshi et al., 1985), both P-gp substrates (Schuetz et al., 1996).
In another study it was shown that piperine abolished the P-gp function in Caco-2 and L-MDR1 cells at a concentration of 50 μM. Piperine was non-cytotoxic at concentrations up to 100 μM to the Caco-2 and L-MDR1 cells after 4 h exposure, shown with the in vitro MTT assay (Han et al., 2008). Piperine suppressed tumor growth and metastasis in vitro and in vivo in a 4T1 murine breast cancer model (Lai et al., 2012). Co-administration with piperin is one of the strategies to increase the bioavailability of curcumin. The application of 2 g of curcumin with 20 mg piperine increased the curcumin bioavailability in human and in rats (Shoba et al., 1998). However, this was not a fixed combination, like it was aimed in this study. As the way of action for piperine, it was postulated that it acts as an apolar molecule forming an apolar complex with drugs. The activity of piperine against P-gp mediated efflux was shown in several studies (Khajuria et al., 1998, Okura et al., 2010). In a clinical trial it could be shown that twice daily application of 5 mg piperine enhanced the absorption of 2 g curcumin (Han et al., 2008).
The cytotoxic effect of piperine for the mouse tumor cell lines B16-F10 melanoma cells and 4T1 mammary carcinoma cells could be shown (Pradeep and Kuttan, 2002, Lai et al., 2012). Piperine also inhibited hepatic CYP3A4-mediated metabolism of docetaxel (Makhov et al., 2012) and down-regulated the expression of transporter genes that mediate multidrug resistance (Li et al., 2011). In addition piperine inhibited human rectal cancer cells (Yaffe et al., 2013).
Due to these facts it was aimed in this study to prepare curcumin and piperine loaded zein-chitosan nanoparticles having a particle size of approximately 500 nm and appropriate properties for final consumption. For that purpose, curcumin was encapsulated with the zein in the core of the nanoparticles and piperine was incorporated in the shell with help of the biopolymer chitosan. The nanoparticles were prepared using electrospray by varying the concentrations of curcumin and piperine as the actives and the concentrations of the polymer compounds zein and chitosan, all as ratios, and the applied voltage and nozzle diameter of the electrospray. The nanoparticles were characterised by investigating their shaper, morphologies and determinig their particle sizes and with help of scanning electron microscopy (SEM) images.
2. Materials and methods
2.1. Materials
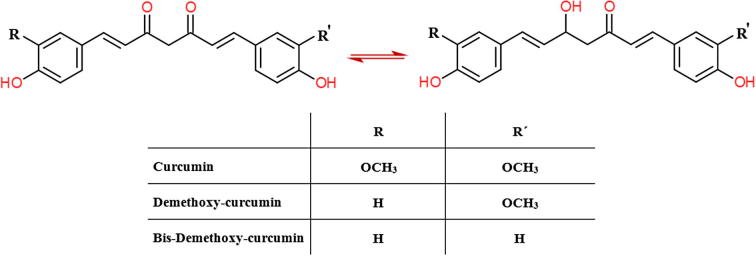
Curcumin (Fig. 1) was extracted from turmeric and piperine (Fig. 2) from black pepper, respectively (Baspinar et al., 2017), which were supplied from India. Ethanol with analytical grade was purchased from Merck (Merck, Darmstadt, Germany). Medium molecular weight chitosan and zein were purchased from Sigma-Aldrich (Sigma, St. Louis, MO, USA), just like the reference substances of curcumin and piperine for the High Performance Liquid Chromatography (HPLC) analysis.
Fig. 1.
The chemical structure of curcumin (keto and enol form), desmethoxy-curcumin and bis-desmethoxy-curcumin.
Fig. 2.
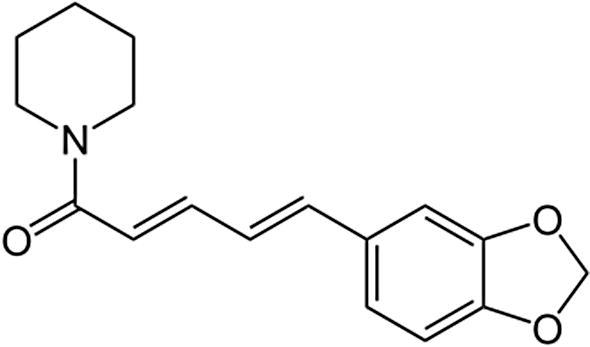
The chemical structure of piperine.
2.2. Analyses of curcumin and piperine with HPLC
For determining the encapsulation efficiency of curcumin and piperine, their concentrations were analyzed by reversed phase HPLC (Thermo Scientific Ultimate 3000) equipped with a pump and an ultraviolet-visible spectroscopy detector at 262 nm at a column temperature of 33 °C. The mobile phase consisted of a mixture of 0.1% phosphoric acid and acetonitrile (45:55%, v/v), and the flow rate was set at 0.8 ml/minute. A C18 column Acclaim® 120 (Thermo Scientific 120 Å C18 3 μm 4.6 × 150 mm) was used, and the injection volume was 20 μl. HPLC assay validation was performed five times a day for 5 consecutive days at a curcumin and piperine concentration range 0.0625–0.03125–0.01562–0.00675 mg/ml, respectively. This method was modified after (Moorthi et al., 2013).
2.3. Preparation of the micro- and nanoparticles by electrospray
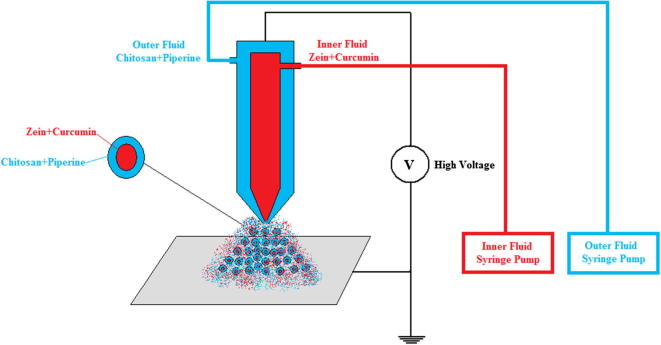
All nanoparticles were prepared by electrospray using an electrospray apparatus (Fig. 3) (IMKASAN Basic System Electrospray/coating, Manisa, Turkey). This apparatus provides an opportunity to spray two fluids separately at the same time, an inner fluid and an outer fluid. The expected nanoparticles are designed as core and shell. The materials used for the core of the nanoparticles were curcumin as the active and zein as the biopolymer and piperine and chitosan as the second biopolymer for the shell.
Fig. 3.
Schematic presentation of the electrospray apparatus.
For the development of the nanoparticles various formulation parameters like the influence of the concentrations of curcumin, zein, piperine and chitosan and the preparation parameters like the applied voltage and the nozzle diameter were investigated. For that purpose first the unloaded zein microparticles, then the unloaded zein nanoparticles without curcumin, piperine and chitosan were prepared. Next curcumin was loaded to these nanoparticles and finally the nanoparticles were prepared including piperine and chitosan as the shell.
2.3.1. Preparation of unloaded zein micro- and nanoparticles
Before preparing the curcumin and piperine loaded chitosan nanoparticles, the concentration of the biopolymer zein must be determined to obtain particles with a suitable shape and particle size. For that purpose zein was dissolved in 70% ethanol: water (v/v) solution and stirred at room temperature until it was completely dissolved. The microparticles were prepared by electrospray using a constant flow rate of 0.3 ml/h, a voltage of 15 kV and a nozzle with a diameter of 0.3 mm, but by varying the zein concentration (1, 2.5, 5, 10 and 20%) (Table 1, ZM). The curcumin-zein mixture solutions as the inner fluid were used during the electrospraying process with a blunt end steel needle syringe which was attached to the positive electrode of a direct current of power supply. For feeding the solution to the syringe needle an injection pump (New Era, Model NE-1000, Programmable Single Syringe Pump, U.S.A.) was used. At a distance of 10 cm away from the needle tip an aluminum foil covered collector plate was fixed and connected to the counter electrode of the power supply. The solution was fed to process with a plastic syringe and for controlling the flow rate of the solution a syringe pump was used.
Table 1.
Electrospray conditions for preparing the unloaded zein microparticles (ZM), unloaded zein nanoparticles (ZN), curcumin loaded zein nanoparticles (CZN) and curcumin and piperine loaded zein-chitosan nanoparticles (CPZN).
| ZM (1–5) | ZN (1–3) | CZN (1–9) | CPZN (1–9) | |
|---|---|---|---|---|
| Flow rate [ml/h] | 0.3 | 0.3 | 0.3 | 0.3 |
| Voltage [kV] | 15 | 17.5; 20; 22.5 | 20 | 20 |
| Nozzle diameter [mm] | 0.3 | 0.3 | 0.6 | 0.6 |
| Ethanol concentration [%, v/v] | 70 | 70 | 70 | 70 |
| Zein concentration [%, w/w] | 1; 2.5; 5; 10; 20 | 5 | 5 | 5 |
| Curcumin solution: zein solution | – | – | 1:10; 1:20; 1:30 | 1:10 |
| Piperine solution:chitosan solution | – | 1:10; 1:30; 1:50 |
ZM = zein microparticles; ZN = zein nanoparticles; CZN = curcumin loaded zein nanoparticles; CPZN = curcumin and piperine loaded zein nanoparticles.
Due to the fact that this step is just a preliminary test, only the morphology of the prepared microparticles was evaluated by using SEM in order to find a suitable zein concentration, the particle size was not further investigated yet. It was assumed that the prepared particles with the relative low applied voltage of 15 kV are in the micrometer range or have some particles in that range.
For obtaining monodispersed nanoparticles with electrospray process, the applied voltage is a key parameter. Due to that fact the applied voltage was also increased from 15 to 17.5, 20 and 22.5 kV (Table 2), respectively and the resulted nanoparticles were characterised according to their shape and particle size with help of SEM images.
Table 2.
Investigated parameter for the preparation of unloaded zein micro- (ZM) and nanoparticles (ZN).
| ZM1 | ZM2 | ZM3 | ZM4 | ZM5 | ZN1 | ZN2 | ZN3 | |
|---|---|---|---|---|---|---|---|---|
| Voltage [kV] | 15 | 15 | 15 | 15 | 15 | 17.5 | 20 | 22.5 |
| Zein concentration [%] | 1 | 2.5 | 5 | 10 | 20 | 5 | 5 | 5 |
2.3.2. Preparation of curcumin loaded zein nanoparticles
After optimization of the zein concentration and of the applied voltage, curcumin loaded zein nanoparticles (CZN) were prepared by adding curcumin at different weight ratios ranging from 1:10 to 1:30 to the zein solution (curcumin: zein) and a voltage of 20 kV. The curcumin concentration used in this preparation step resulted from the extraction assays (Baspinar et al., 2017). Curcumin was dissolved in 70% (v/v) ethanol and the needed relation of the zein solution to the curcumin solution (1:10, 1:20 and 1:30) for preparing suitable curcumin and piperine loaded zein-chitosan nanoparticles was investigated (Table 3). In addition the nozzle diameter was changed from 0.3 mm to 0.6 mm.
Table 3.
The investigated parameter for the preparation of the curcumin loaded zein nanoparticles (CZN).
| CZN1 | CZN2 | CZN3 | CZN4 | CZN5 | CZN6 | CZN7 | CZN8 | CZN9 | |
|---|---|---|---|---|---|---|---|---|---|
| Voltage [kV] | 17.5 | 17.5 | 17.5 | 20 | 20 | 20 | 22.5 | 22.5 | 22.5 |
| Curcumin solution: zein solution | 1:10 | 1:20 | 1:30 | 1:10 | 1:20 | 1:30 | 1:10 | 1:20 | 1:30 |
2.3.3. Preparation of curcumin and piperine loaded zein-chitosan nanoparticles
For the preparation of the curcumin and piperine loaded zein-chitosan nanoparticles the first solution of zein and curcumin as an inner fluid for the core and the second solution of piperine and chitosan as an outer fluid for the shell of the nanoparticles have to be prepared (Fig. 4). For that purpose the piperine concentration obtained from the extraction assays was used. Due to the fact that medium molecular weight chitosan has not a good aqueous solubility, a 1% acetic acid solution was used to dissolve 2% chitosan. For obtaining the best possible nanoparticle formulation different concentrations of the 2% chitosan solution (10, 20 and 30%) and different relations of the piperine:chitosan solutions (ranging from 1:10 to 1:50) were investigated (Table 4). The inner nozzle of the electrospray apparatus was fed with formulation of CZN4 and different relations of piperine-chitosan solutions were used as the outer fluid. This preparation was performed by application of a voltage of 20 kV and a flow rate of 0.3 ml/h.
Fig. 4.
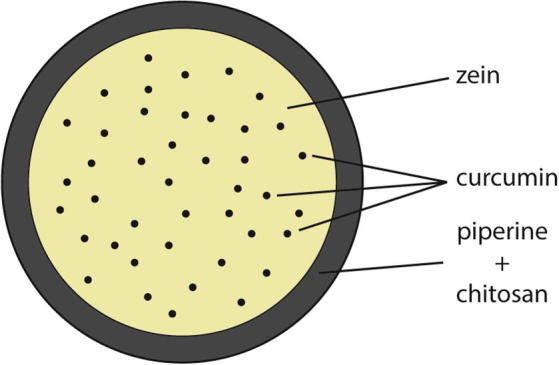
The core shell model of the curcumin and piperine loaded zein-chitosan nanoparticles.
Table 4.
The investigated parameter for the preparation of the curcumin and piperine loaded zein- chitosan nanoparticles (CPZChNs).
| Sample | % of chitosan (2%) | Piperine solution: chitosan solution |
|---|---|---|
| CPZChN1 | 10 | 1:10 |
| CPZChN2 | 1:30 | |
| CPZChN3 | 1:50 | |
| CPZChN4 | 20 | 1:10 |
| CPZChN5 | 1:30 | |
| CPZChN6 | 1:50 | |
| CPZChN7 | 30 | 1:10 |
| CPZChN8 | 1:30 | |
| CPZChN9 | 1:50 | |
2.4. Characterisation of the micro- and nanoparticles
2.4.1. Morphology, size and structure characterisation of particles
The morphology and size of curcumin and piperine loaded zein-chitosan nanoparticles were measured using SEM (Philips XL 30S FEG, Holland) at a voltage of 5 kV. Before testing, the samples were mounted on the SEM stubs with double-sided adhesive tape and coated with platinum under vacuum to make the sample conductive. The size of the particle was calculated as the average of the shortest and longest dimensions.
2.4.2. Particle size measurement with dynamic light scattering
A Zetasizer Nanoseries (Malvern Instruments, Malvern, UK) was used for measuring the mean particle size of the curcumin and piperine loaded zein-chitosan nanoparticles per photon correlation spectroscopy (PCS) with dynamic light scattering (DLS).
2.5. Cytoxicity of curcumin and piperine loaded zein-chitosan nanoparticles
The cytotoxicity of the curcumin and piperine loaded zein-chitosan nanoparticles was determined by XTT cell proliferation assay using the neuroblastoma cell line SH-SY5Y cells. The SH-SY5Y cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% heat-inactivated fetal bovine serum (FBS), penicillin (100 U/mL), streptomycin (100 μg/mL) and 2 mM l-glutamine in a 37 °C incubator under 5% CO2 atmosphere. Prior to treatment, cells were trypsinized and seeded in a final volume of 100 μl (5 × 104 cells/ml) into each well of 96-well plates, and incubated for 24 h. At the day of tratment the medium was aspirated, cells were washed with PBS and 50 μl of fresh medium was added to each well. Treatment formulations were diluted in the growth medium and added as 50 μl portions to the corresponding wells (final volume 100 μl). The viability (%) of the cells was investigated with increasing the concentration of curcumin and piperine loaded zein-chitosan nanoparticles from 5 to 100 µg/ml. Cells were incubated for 24 h in a 37 °C incubator under 5% CO2 in the presence of the formulations. After the incubation the medium was aspirated, cells were washed with PBS and 50 μl of fresh medium was added to each well. Subsequently 50 μl of XTT reagent prepared as per manufacturers instructions was added and incubated for 2 h at 37 °C. The absorbance of formed orange-colored formazan compound was measured by using an automatic microplate reader (Varioskan Flash microplate reader, Thermo Fisher Scientific, USA) at 450 nm. All experiments were performed in triplicate.
2.6. Encapsulation efficiency (EE)
The samples of the curcumin and piperine loaded zein-chitosan nanoparticles were collected on an aluminum foil placed over the collection plate and stored at 0% RH, in the dark, until they were analyzed. The amounts of curcumin and piperine loaded in the nanoparticles were measured by dissolving the nanoparticles in acetone and centrifugating this mixture. Then the upper phase was taken and the concentration of curcumin and piperine, respectively was measured with HPLC. The concentration of curcumin and piperine, respectively, in the sample was calculated from the previously prepared standard calibration curve for curcumin and piperine in 80% (w/w) ethanol. The EE of curcumin and piperine were obtained as the mass ratio between the curcumin and piperine, respectively, determined in the nanoparticles and that used in the preparation of the nanoparticles according to the following Eq. (1):
| (1) |
3. Results and discussion
3.1. Characterisation of the micro- and nanoparticles
The main variables that affect the electrospraying process include the concentration of the polymers zein and chitosan in solution, the concentration of curcumin and piperine and the preparation conditions (applied voltage and nozzle diameter). A previous study have shown that the flow rate had only a minor effect on the final properties of zein nanoparticles (Kose and Bayraktar, 2016).
3.1.1. Characterisation of unloaded zein micro- and nanoparticles
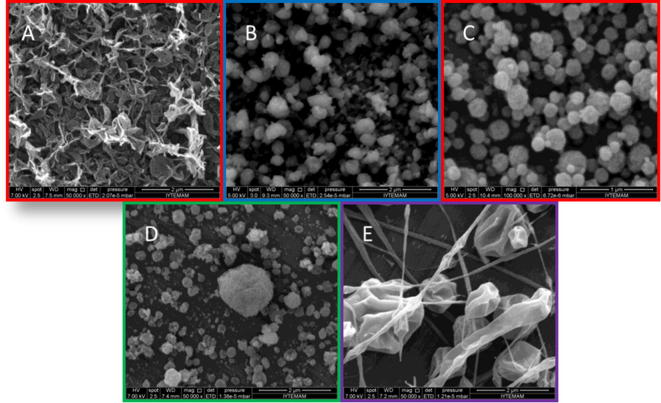
The effect of the zein concentration on the morphology of the resulting microparticle strucures was studied for concentrations ranging from 1 to 20% (w/w) (Table 2) in 70% ethanol (v/v). For that purpose the flow rate was maintained at 0.3 ml/h and the voltage applied was fixed at 15 kV, in order to study the effect of the zein concentration in the formation of the microparticle structures, shown with SEM images (Fig. 5).
Fig. 5.
SEM images of zein microparticles with varying zein concentrations of 1–20% (A:ZM1, B:ZM2, C:ZM3, D:ZM4, E:ZM5).
The morphology of the zein structures obtained with increasing concentrations of zein are shown in Fig. 5. It is apparent that 1% (Fig. 5A) of zein in the solution was too low for particle formation. At this low concentration of 1%, there were not enough intermolecular entanglements among polypeptide chains of this low molecular weight polymer to allow the chains to aggregate into spheres after solvent evaporation. It can be seen that droplets carrying a low concentration of 1% zein formed a discontinuous film. Increasing the concentration of zein to 2.5% lead to the formation of compact particles (Fig. 5B). These particles were round in shape and showed a relatively smooth surface. Thus, increasing the concentration of zein from 1% to 2.5% promotes the entanglement of polymer chains, which impedes droplet fission and leads to the formation of particles. Compact particles have been associated with small droplet size and a low concentration of solutes (Raula et al., 2004). In the present study, compact particles in the nanometer range of smaller than 500 nm could not be clearly achieved with a zein concentrations of 1% and 2.5%, respectively, which can be related to the low molecular weight of zein compared to other polymers. After increasing the concentration of zein to 5% (Fig. 5C) the particles maintained their morphology, showing a round shape and compact structure, and the size clearly decreased under 500 nm.
A further increase in the zein concentration to 10% (Fig. 5D) revealed particles with an increased particle size. For other polymers like Eudragit (Raula et al., 2004), polycaprolactone (Xie et al., 2006a) and an elastin-like polypeptide (Wu et al., 2009) an increase in particle size with higher polymer concentration has been reported, too. Such an increase could be related to the greater mass of the polymer in the droplets generated during the electrospray process. An increase of the zein concentration to 20% (Fig. 5E) resulted in the transition from particles to fibers, which is in accordance with the results reported in the literature (Gomez-Estaca et al., 2012, Li et al., 2009). There is a relationship between the concentration and the viscocity, respectively of zein. It could be shown that with increasing concentration of zein from 15% to 20%, the viscosity of the zein solution also increased. In that case a stable elongated jet can be obtained if the viscosity is high enough. Increasing the viscosity of the zein solution further resulted in a high cohesion and entanglement between the polymer chains. Hence, the liquid was prevented from breaking up into droplets, leading to a transition from electrospray to electrospinning, which greatly depends on the viscocity of the zein solution. The entangled polymer network is stretched in the electrospinning process, resulting finally in the formation of electrospun fibers.
It could be shown with help of the SEM images that the ideal zein concentration for further development of the nanoparticles was 5%. The particles obtained after electrospraying with 5% zein revealed the best shape of the spherical particles and the smallest particle size.
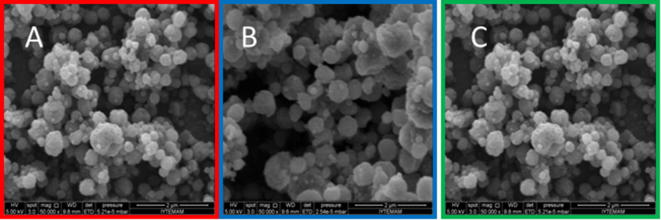
As the next step curcumin loaded zein nanoparticles were prepared with 5% zein and an increasing applied voltage of 17.5, 20 and 22.5 kV. These nanoparticles were characterised according to the shape of the particles and the particles size with help of SEM images (Fig. 6).
Fig. 6.
SEM images of zein nanoparticles with varying applied voltages of 17.5, 20 and 22.5 kV (A: ZN1, B: ZN2, C: ZN3).
For obtaining monodispersed nanoparticles with electrospray process, the applied voltage is a key parameter. Due to that fact the applied voltage was also increased from 15 to 17.5, 20 and 22.5 kV (Table 2), respectively, and the resulted nanoparticles were characterised according to their shape and particle size with help of SEM images.
Increasing the voltage from 15 to 17.5, 20 and 22.5 kV, respectively, did not change the shape of the nanoparticles compared to the nanoparticles prepared with 15 kV. There was a slight agglomeration of some particles, prepared with 17.5 and 22.5 kV (Fig. 6A and C), which is in agreement with previous studies (Hartman et al., 2000, Hong et al., 2008). It was reported that the applied voltage affected the jet break-up mechanism and depended on the stress ratio at the jet surface, expressed as the ratio of the normal electric stress to the surface tension stress (Hartman et al., 1999).
Axisymmetric instabilities, also known as varicose instabilities, lead to a break up at a low stress ratio value. As a result monodisperse droplets are produced and the number of secondary droplets is lower than the number of primary droplets. If a stress ratio threshold value is reached, the jet begins to whip and lateral instabilities contribute to the break-up of the jet, resulting in a rise in the number of secondary droplets and satellites, as in the case of the present work when the voltage was increased from 15 to 17.5, 20 and 22.5 kV, respectively.
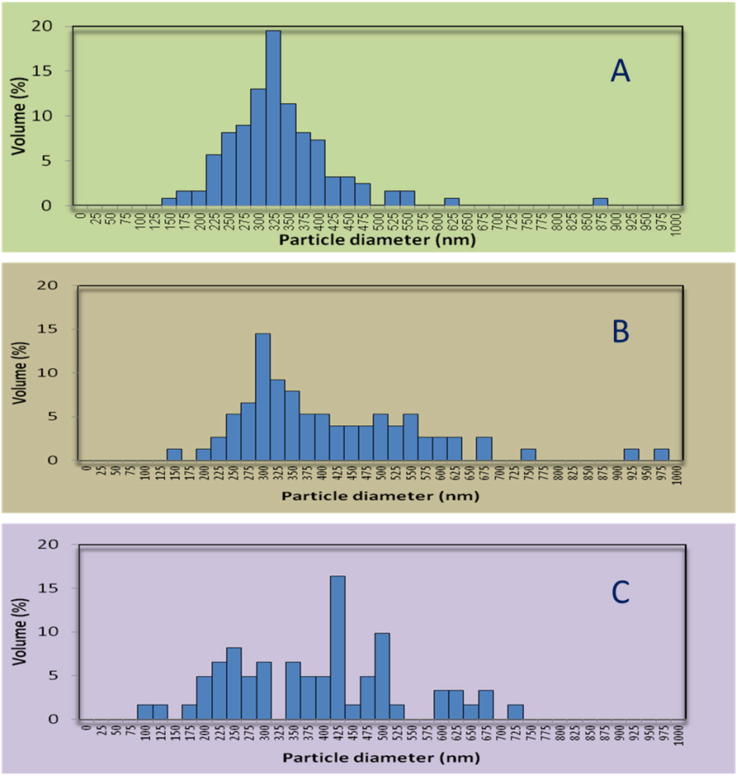
Using the SEM images and the software Photoshop the particle size of each particle seen at the SEM image was determined and expressed as particle size, with distribution (Fig. 7).
Fig. 7.
Particle size results of unloaded zein nanoparticles (A: ZN1, B: ZN2 and C: ZN3).
The mean particles size of the zein nanoparticles increased from 337 nm to 413 nm and 389 nm, with increasing applied voltage from 17.5 kV to 20 kV and 22.5 kV, respectively.
Due to the fact that there is not a clear tendency according to the particle size of zein nanoparticles prepared by electrospray, the curcumin loaded nanoparticles were also prepared with increasing applied voltage from 17.5 kV to 20 kV and 22.5 kV, respectively, and varying curcumin solution to zein solution ratios of 1:10, 1:20 and 1:30 (Table 3).
3.1.2. Characterisation of curcumin loaded zein nanoparticles
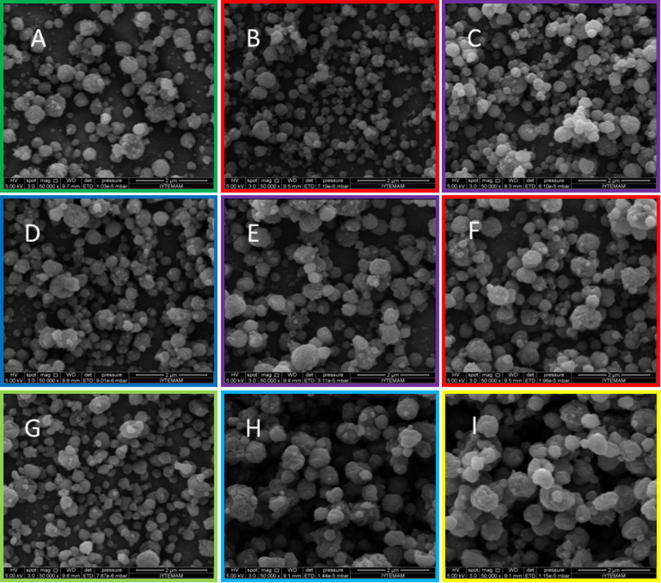
The particles size of the curcumin loaded zein nanoparticles decreased from 339 nm to 326 nm and 310 nm, respectively, with increasing curcumin solution to zein solution ratios from 1:10 to 1:20 and 1:30, respectively, and at a constant applied voltage of 17.5 kV. For the nanoparticles prepared with 20 kV an opposite effect was observed, namely an increase of the particle size from 328 nm to 390 nm and 379 nm, respectively, with increasing curcumin solution to zein solution ratios from 1:10 to 1:20 and 1:30, respectively. A high similarity to this was shown for the nanoparticles prepared with 22.5 kV, where an increase of the particle size from 322 nm to 482 nm and 527 nm, respectively, was shown. Curcumin loaded zein nanoparticles with the lowest mean particle size are CZN1 (339 nm), CZN2 (326 nm), CZN3 (310 nm), CZN4 (328 nm) and CZN7 (322 nm). Although the mean particle size of these formulations is around 300 nm, there are some particles observed of more than 600 nm. Nanoparticles with such a inhomogenous particle distribution could have some long-term stability problems. To sum up, combining the SEM images (Fig. 8) and the mean particles size results revealed that the best curcumin loaded zein nanoparticle formulation from these above mentioned is CZN4.
Fig. 8.
SEM images of curcumin loaded zein nanoparticles with varying voltages of 17.5–22.5 kV and ratios of curcumin solution to zein solution from 1:10 to 1:30 (A: CZN1, B: CZN2, C: CZN3, D: CZN4, E: CZN5, F: CZN6, G: CZN7, H: CZN8, I: CZN9).
Due to that fact the formulations of CZN4 was used as core for the preparation of the curcumin and piperine loaded zein-chitosan nanoparticles.
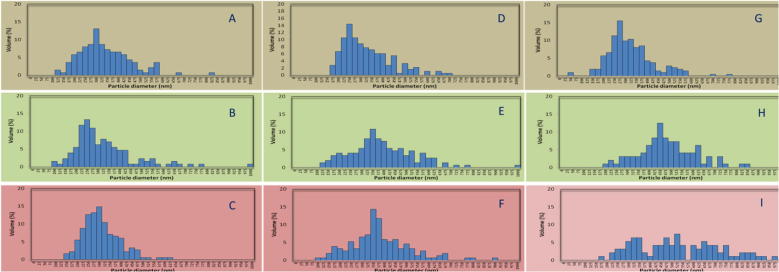
Using SEM images, the mean particle size of the curcumin loaded zein nanoparticles (CZN1, CZN2, CZN3, CZN4, CZN5, CZN6, CZN7, CZN8 and CZN9) were determined (Fig. 9, Table 5).
Fig. 9.
Particle size distribution of the curcumin loaded zein nanoparticles (A: CZN1, B: CZN2, C: CZN3, D: CZN4, E: CZN5, F: CZN6, G: CZN7, H: CZN8, I: CZN9).
Table 5.
Mean particle size results of the curcumin loaded zein nanoparticles (CZN1-CZN9).
| CZN1 | CZN2 | CZN3 | CZN4 | CZN5 | CZN6 | CZN7 | CZN8 | CZN9 | |
|---|---|---|---|---|---|---|---|---|---|
| Voltage [kV] | 17.5 | 17.5 | 17.5 | 20 | 20 | 20 | 22.5 | 22.5 | 22.5 |
| Curcumin solution: zein solution | 1:10 | 1:20 | 1:30 | 1:10 | 1:20 | 1:30 | 1:10 | 1:20 | 1:30 |
| Particle size [nm] | 339 | 326 | 310 | 328 | 390 | 379 | 322 | 482 | 527 |
At all the applied voltages of 17.5, 20 and 22.5 kV, respectively, the curcumin loaded zein nanoparticles showed a similar morphology (Fig. 9) and size distribution (Fig. 9) to those of the unloaded zein nanoparticles (Fig. 6, Fig. 7) obtained with an applied voltage of 20 kV. Hence, adding curcumin to the zein solution seems not to have affected the electrospray process and thus the formation of the zein nanoparticles, at least at the applied voltages investigated in this study.
3.1.3. Characterisation of curcumin and piperine loaded zein-chitosan nanoparticles
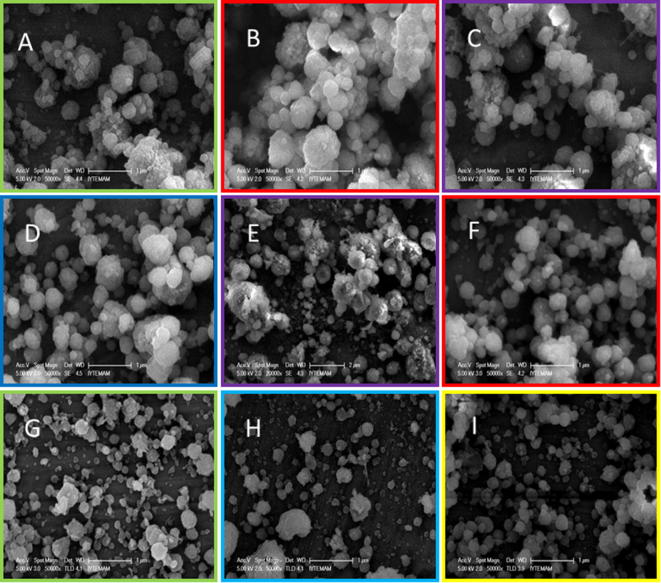
The morphology of the curcumin and piperine loaded zein-chitosan nanoparticles was investigated with help of SEM images (Fig. 10). After investigating the SEM images of the curcumin and piperine loaded zein-chitosan nanoparticles (CPZChN1, CPZChN2, CPZChN3, CPZChN4, CPZChN5, CPZChN6, CPZChN7, CPZChN8, CPZChN9) it was concluded that the formulation CPZN6 revealed particles with a round shape, a compact structure and a more homogenous distribution. The rest of the formulations had some disadvantages like a not satisfying shape of the particles, or too big particles with an inappropriate distribution.
Fig. 10.
SEM images of curcumin and piperine loaded zein-chitosan nanoparticles with varying ratios of piperine solution to chitosane solution from 1:10 to 1:50 (A: CPZChN1, B: CPZChN2, C: CPZChN3, D: CPZChN4, E: CPZChN5, F: CPZChN6, G: CPZChN7, H: CPZChN8, I: CPZChN9).
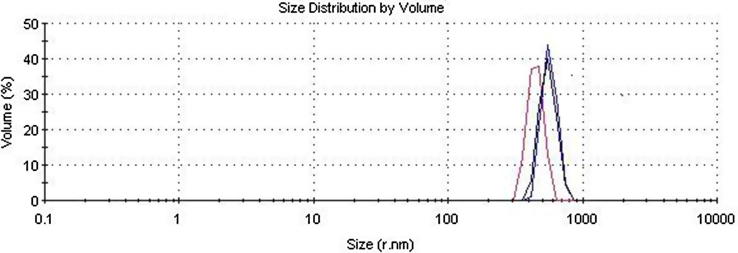
The mean particle size of this formulation was measured using DLS (Fig. 11) and resulted in a value of 550 nm, just slightly over the aimed mean particle size of 500 nm. It can be seen that this formulation has a homogenous particle size distribution.
Fig. 11.
Mean particle size measurement results of ZCPN6 performed with DLS.
Adding the chitosan-piperine solution to the curcumin loaded zein nanoparticles resulted in an increase of the particle size of 328 nm (CZN4, Fig. 8, Table 5) to 550 nm (Fig. 10), while the morphology did not change (Fig. 9). In contrast to the curcumin loaded nanoparticles, where adding curcumin to the zein solution appears not to have affected the formation of the nanoparticles, adding piperin-chitosan solution did significantly changed the particles size.
To sum up, for obtaining curcumin and piperine loaded zein-chitosan compact and spherical nanoparticles with a mean particle size of 550 nm by using electrospray, 5% zein, 20 kV applied voltage, a curcumin to zein ratio of 1:20 and a piperine to chitosan ratio of 1:50 were necessary (Table 6).
Table 6.
The concentrations of the components, their ratios and the electrospray conditions for preparing the final curcumin and piperine loaded zein-chitosan nanoparticle formulation.
| Component | Concentration | Electrospray parameter | Electrospray conditions |
|---|---|---|---|
| Zein | 5% | Flow rate | 0.3 ml/h |
| Curcumin | 0.49 mg/ml | Voltage | 20 kV |
| Curcumin:zein ratio | 1:10 | Nozzle diameter | 0.6 mm |
| Piperine | 1.21 mg/ml | ||
| Chitosan | 2% | ||
| Piperine:chitosan ratio | 1:50 |
3.2. Cytotoxicity of curcumin and piperine loaded zein-chitosan nanoparticles
The cytotoxicity results revealed that increasing the concentration of curcumin and piperine loaded zein-chitosan nanoparticles from 5 to 10, 25, 50 and 100 µg/ml, respectively, decreased the viability of the neuroblastoma cells from 115 to 99, 30, 8 and finally 3% (Fig. 12).
Fig. 12.
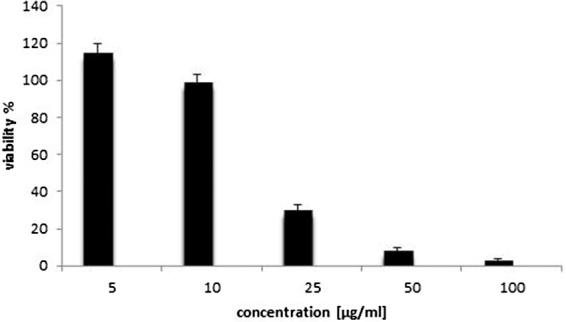
The cytotoxicity results of the curcumin and piperine loaded zein-chitosan nanoparticles using neuroblastoma cells.
This decrease of the viability by increasing the nanoparticle concentration can be explained by the fact that a concentration of 10–25 µg/ml nanoparticle are necessary to kill the majority of the neuroblastoma cells.
3.3. Encapsulation efficiency
The reason for the use of acetone for the determination of the encapsulation efficiencies for curcumin and piperine was the fact that zein and chitosan are not soluble in acetone, while curcumin and piperine are soluble. Thus, free (not encapsulated) curcumin and free piperine are located in the upper phase and can be determined with HPLC. The encapsulation efficiency of curcumin ranged from 80 to 92% and while for piperine values of 72–87% were observed (Table 7). Changing the curcumin to zein ratio from 1:10 to 1:20 and 1:30, respectively, at a fixed applied voltage did not change significantly the EE of curcumin. The highest EE were obtained at a applied voltage of 20 kV. A high encapsulation efficiency achieved by electrospraying, ranging from 80% to 96% was reported by other authors, too (Ding et al., 2005, Xie et al., 2006b, Xie et al., 2008). In a work hydrophilic bovine serum albumin was encapsulated in the hydrophobic polymers PLGA and PCL, resulting in that the EE greatly depends on the interactions between the polymer, protein, and organic solvent.
Table 7.
The encapsulation efficiency results of curcumin and piperine for the curcumin and piperine loaded zein-chitosan nanoparticles.
| Sample | EE (%) curcumin | EE (%) piperine |
|---|---|---|
| CPZChN1 | 80 | 72 |
| CPZChN2 | 83 | 75 |
| CPZChN3 | 82 | 79 |
| CPZChN4 | 89 | 78 |
| CPZChN5 | 91 | 83 |
| CPZChN6 | 92 | 87 |
| CPZChN7 | 79 | 73 |
| CPZChN8 | 80 | 77 |
| CPZChN9 | 84 | 80 |
For determining the optimum zein to curcumin ratio, the formulations CZN1, CZN2, CZN3, CZN4 and CZN7 had a satisfying particle size of approximately 300 nm. Considering the EE for curcumin of these formulation, ranging from 79 to 89%, revealed that the decision for CZN4 was right due to the fact of a curcumin EE of 89%, the highest EE of these formulations.
Changing the piperine to chitosan ratio from 1:10 to 1:30 and 1:50, respectively, lead to an increase of the EE (Table 7). The highest EE of piperine was found in the optimised formulation CPZChN6 with 87%. Once again the step by step optimised development of the curcumin and piperine loaded zein-chitosan nanoparticle was successful and justified.
The good solubility of curcumin and piperine in the solvent and the intimate contact between the components could be a feasible explanation for the good EE of curcumin and piperine in zein-chitosan nanoparticles.
4. Conclusion
For the development of the curcumin and piperine loaded zein-chitosan nanoparticles various formulation parameters like the influence of the concentrations of curcumin, zein, piperine and chitosan and the preparation parameters like the applied voltage and the nozzle diameter were investigated step by step.
First, the zein concentration (1–20%) was tested and compact spherical nanoparticles were obtained with 5% zein solution. Then, the voltage was increased from 15 to 17.5, 20 and 22.5 kV, respectively. A slight agglomeration of some particles, prepared with 17.5 kV and 22.5 kV was the reason to use 20 kV for further investigations. Next, the curcumin solution to zein solution ratio was increased from 1:10 to 1:20 and 1:30, respectively, and the particle size of the nanoparticles was investigated. Except for the formulation prepared with a curcumin solution to zein solution ratio of 1:10 particles of more than 600 nm were observed. Due to the fact that except for the formulation prepared with a piperine to chitosan ratio of 1:50 with satisfying properties, the rest of the formulations had some handicaps like a not appropriate shape of the particles, or too big particles with a disadvantageous distribution.
For obtaining compact and sperical curcumin and piperine loaded zein-chitosan nanoparticles with a mean particle size of 550 nm, 5% zein, 20 kV applied voltage, a curcumin to zein ratio of 1:20 and a piperine to chitosan ratio of 1:50 were necessary.
The fact that a concentration of 10–25 µg/ml curcumin and piperine loaded zein-chitosane nanoparticle are necessary to kill the majority of the neuroblastoma cells revealed the impact of the developed formulation.
The choice of the formulation CPZChN6 was confirmed the results that the highest encapsulation efficiency of curcumin (92%) and piperine (87%), respectively, were found in this optimised formulation.
It was succeed to prepare curcumin and piperine loaded zein-chitosan nanoparticles having a mean particle size of 550 nm and appropriate properties for final consumption (Fig. 13).
Fig. 13.
The final curcumin and piperine loaded zein-chitosan nanoparticle product dispersed in a mixture of glycerole (19%, w/w) and water and as powder filled in capsules.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ampasavate C., Sotanaphun U., Phattanawasin P., Piyapolrungroj N. Effects of Curcuma spp. on P-glycoprotein function. Phytomedicine. 2010;17:506–512. doi: 10.1016/j.phymed.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Atal N., Bedi K.L. Bioenhancers: revolutionary concept to market. J. Ayur. Integr. Med. 2010;1:96–99. doi: 10.4103/0975-9476.65073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglole K.N., Boland P.G., Wagner B.D. Fluorescence enhancement of curcumin upon inclusion into parent and modified cyclodextrins. J. Photochem. Photobiol. A – Chem. 2005;173(3):230–237. [Google Scholar]
- Baspinar Y., Üstündaş M., Bayraktar O., Sezgin C. Response surface methodology for extraction of curcumin from turmeric and piperine from black pepper. Celal Bayar Univ., J. Sci. 2017;13(3):747–754. [Google Scholar]
- Cao L., Luo J., Tu K., Wang L.-Q., Jiang H. Generation of nano-sized core–shell particles using a coaxialtri-capillary electrospray-template removal method. Coll. Surf. B: Biointerf. 2014;115:212–218. doi: 10.1016/j.colsurfb.2013.11.046. [DOI] [PubMed] [Google Scholar]
- Chang M.-W., Stride E., Edirisinghe M. A new method for the preparation ofmonoporous hollow microspheres. Langmuir. 2010;26:5115–5121. doi: 10.1021/la903592s. [DOI] [PubMed] [Google Scholar]
- Doucette D., Hilchie L., Liwski R., Hoskin W. Piperine, a dietary phytochemical, inhibits angiogenesis. J. Nutr. Biochem. 2013;24:231–239. doi: 10.1016/j.jnutbio.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L., Lee T., Wang C.H. Fabrication of monodispersed taxol-loaded parti-cles using electrohydrodynamic atomization. J. Control. Release. 2005;102:395–413. doi: 10.1016/j.jconrel.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Gomez-Estaca J., Balaguer M.P., Gavara R., Hernandez-Munoz P. Formation of zein nanoparticles by electrohydrodynamic atomization: effect of the main processing variables and suitability for encapsulating the food coloring and active ingredient curcumin. Food Hydrocolloids. 2012;28:82–91. [Google Scholar]
- Gordon S., Saupe A., McBurney W., Rades T., Hook S. Comparison of chitosan nanoparticles and chitosan hydrogels for vaccine delivery. J. Pharm. Pharmacol. 2008;60:1591–1600. doi: 10.1211/jpp/60.12.0004. [DOI] [PubMed] [Google Scholar]
- Hartman R.P.A., Brunner D.J., Camelot D.M.A., Marijnissen J.C.M., Scarlett B. Electrohydrodynamic atomization in the cone-jet mode physical modeling of the liquid cone and jet. J. Aerosol Sci. 1999;30(7):823–849. [Google Scholar]
- Hartman R.P.A., Brunner D.J., Camelot D.M.A., Marijnissen J.C.M., Scarlett B. Jet break-up in electrohydrodynamic atomization in the cone-jet mode. J. Aerosol Sci. 2000;31(1):65–95. [Google Scholar]
- Han Y., Chin Tan T.M., Lim L.-Y. In vitro and in vivo evaluation of theeffects of piperine on P-gp function and expression. Toxicol. Appl. Pharmacol. 2008;230:283–289. doi: 10.1016/j.taap.2008.02.026. [DOI] [PubMed] [Google Scholar]
- Helander I.M., Nurmiaho-Lassila E.L., Ahvenainen R., Rhoades J., Roller S. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int. J. Food Microbiol. 2001;71(2–3):235–244. doi: 10.1016/s0168-1605(01)00609-2. [DOI] [PubMed] [Google Scholar]
- Hong Y.L., Li Y.Y., Yin Y.Z., Li D.M., Zou G.T. Electrohydrodynamic atomization of quasi-monodisperse drug-loaded spherical/wrinkled microparticles. J. Aer. Sci. 2008;39(6):525–536. [Google Scholar]
- Jaiswal M., Dinda A.K., Gupta A., Koul V. Polycaprolactone diacrylatecrosslinked biodegradable semi-interpenetrating networks ofpolyacrylamide and gelatin for controlled drug delivery. Biomed. Mater. 2010;5:065014. doi: 10.1088/1748-6041/5/6/065014. [DOI] [PubMed] [Google Scholar]
- Jaworek A. Electrostatic micro- and nanoencapsulation and electroemulsification: a brief review. J. Microencapsul. 2008;25(7):443–468. doi: 10.1080/02652040802049109. [DOI] [PubMed] [Google Scholar]
- Jayaprakasha G.K., Rao L.J., Sakariah K.K. Antioxidant activities of curcumin, demethoxycurcumin and bisdemethoxycurcumin. Food Chem. 2006;98(4):720–724. [Google Scholar]
- Jurenka J.S. Anti-inflammatory properties of curcumin, a major constituent of curcuma longa: a review of preclinical and clinical research. Altern. Med. Rev. 2009;14(2):141–153. [PubMed] [Google Scholar]
- Khajuria A., Zutshi U., Bedi K.L. Permeability characteristics ofpiperine on oral absorption—an active alkaloid from peppersand a bioavailability enhancer. Indian J. Exp. Biol. 1998;36:46–50. [PubMed] [Google Scholar]
- Kim W., Kim S.S. Synthesis of biodegradable triple-layered capsules using atriaxial electrospray method. Polymer. 2011;52:3325–3336. [Google Scholar]
- Kose D., Bayraktar O. Encapsulation of lycopene using electrospraying method. Biointerf. Res. Appl. Chem. 2016;6(4):1417–2142. [Google Scholar]
- Lai L.H., Fu Q.H., Liu Y., Jiang K., Guo Q.M., Chen Q.Y., Yan B., Wang Q.Q., Shen J.G. Piperine suppresses tumor growth and metastasis in vitro and in vivo in a 4T1 murine breast cancer model. Acta Pharmacol. Sin. 2012;33:523–530. doi: 10.1038/aps.2011.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamprecht A., Schafer U., Lehr C.M. Size-dependent bioadhesion of micro- and nanoparticulate carriers to the inflamed colonic mucosa. Pharm. Res. 2001;18:788–793. doi: 10.1023/a:1011032328064. [DOI] [PubMed] [Google Scholar]
- Li L., Ahmed B., Mehta K., Kurzrock R. Liposomal curcumin with and without oxaliplatin: effects on cell growth, apoptosis, and angiogenesis in colorectal cancer. Mol. Cancer Ther. 2007;6(4):1276–1282. doi: 10.1158/1535-7163.MCT-06-0556. [DOI] [PubMed] [Google Scholar]
- Li S., Wang C., Wang M.W., Li W., Matsumoto K., Tang Y.Y. Antidepressant like effects of piperine in chronic mild stress treated mice and its possible mechanisms. Life Sci. 2007;80:1373–1381. doi: 10.1016/j.lfs.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Li Y., Lim L., Kakuda Y. Electrospun zein fibers as carriers to stabilize (-)-epigallocatechin gallate. J. Food Sci. 2009;74(3):C233–C240. doi: 10.1111/j.1750-3841.2009.01093.x. [DOI] [PubMed] [Google Scholar]
- Li S., Lei Y., Jia Y., Li N., Wink M., Ma Y. Piperine, a piperidine alkaloid from Piper nigrum re-sensitizes P-gp, MRP1 and BCRP dependent multidrug resistant cancer cells. Phytomedicine. 2011;19:83–87. doi: 10.1016/j.phymed.2011.06.031. [DOI] [PubMed] [Google Scholar]
- Lin C., Lin Y., Chen C., Yu W., Lee H. Stability and characterisation of phospholipid-based curcumin-encapsulated microemulsions. Food Chem. 2009;116(4):923–928. [Google Scholar]
- Liu X.M., Sun Q.S., Wang H.J., Zhang L., Wang J.Y. Microspheres of corn protein, zein, for an ivermectin drug delivery system. Biomaterials. 2005;26(1):109–115. doi: 10.1016/j.biomaterials.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Luo Y., Wang Q. Recent development of chitosan-based polyelectrolytecomplexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014;64:353–367. doi: 10.1016/j.ijbiomac.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Makhov P., Golovine K., Canter D., Kutikov A., Simhan J., Corlew M.M., Uzzo R.G., Kolenko V.M. Co-administration of piperine and docetaxel results in improved anti-tumor efficacy via inhibition of CYP3A4 activity. Prostate. 2012;72:661–667. doi: 10.1002/pros.21469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzarino L., Travelet C., Ortega-Murillo S., Otsuka I., Pignot-Paintrand I., Lemos Senna E., Borsali R. Elaboration ofchitosan-coated nanoparticles loaded with curcumin formucoadhesive applications. J. Colloid Interf. Sci. 2012;370:58–66. doi: 10.1016/j.jcis.2011.12.063. [DOI] [PubMed] [Google Scholar]
- Mora-Huertas C.E., Fessi H., Elaissari A. Polymer-based nanocapsules fordrug delivery. Int. J. Pharm. 2010;385:113–142. doi: 10.1016/j.ijpharm.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Moorthi C., Kumar C.S., Mohan S., Krishnan K., Kathiresan K. Application of validated RP-HPLC-PDA method for the simultaneous estimation of curcumin and piperine in Eudragit E 100 nanoparticles. J. Pharm. Res. 2013;7:224–229. [Google Scholar]
- Mukerjee A., Vishwanatha J.K. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res. 2009;29(10):3867–3875. [PubMed] [Google Scholar]
- Nagpal K., Singh S.K., Mishra D.N. Chitosan nanoparticles: a promising system in novel drug delivery. Chem. Pharm. Bull. (Tokyo) 2010;58:1423–1430. doi: 10.1248/cpb.58.1423. [DOI] [PubMed] [Google Scholar]
- Okura T., Ibe M., Umegaki K., Shinozuka K., Yamada S. Effects ofdietary ingredients on function and expression of P-glycoprotein in human intestinal epithelial cells. Biol. Pharm. Bull. 2010;33:255–259. doi: 10.1248/bpb.33.255. [DOI] [PubMed] [Google Scholar]
- Patel A., Hu Y.C., Tiwari J.K., Velikov K.P. Synthesis and characterisation of zein-curcumin colloidal particles. Soft Matter. 2010;6(24):6192–6199. [Google Scholar]
- Pradeep C.R., Kuttan G. Effect of piperine on the inhibition of lung metastasis induced B16F–10 melanoma cells in mice. Clin. Exp. Metast. 2002;19:703–708. doi: 10.1023/a:1021398601388. [DOI] [PubMed] [Google Scholar]
- Prajakta D., Ratnesh J., Chandan K., Suresh S., Grace S., Meera V., Vandana P. Curcumin loaded ph-sensitive nanoparticles for the treatment of colon cancer. J. Biomed. Nanotech. 2009;5:445–455. doi: 10.1166/jbn.2009.1038. [DOI] [PubMed] [Google Scholar]
- Preetz C., Rübe A., Reiche I., Hause G., Mäder K. Preparation andcharacterization of biocompatible oil-loaded polyelectrolyte nanocapsules. Nanomed.: Nanotech. Biol. Med. 2008;4(2):106–114. doi: 10.1016/j.nano.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Raay B., Medda S., Mukhopadhyay S., Basu M.K. Targeting of piperine intercalated in mannose-coated liposomes in experimental leishmaniasis. Indian J. Biochem. Biophys. 1999;36:248–251. [PubMed] [Google Scholar]
- Raula J., Eerikainen H., Kauppinen E.I. Influence of the solvent composition on the aerosol synthesis of pharmaceutical polymer nanoparticles. Int. J. Pharm. 2004;284:13–21. doi: 10.1016/j.ijpharm.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Rojanasakul Y., Wang L.Y., Bhat M., Glover D.D., Malanga C.J., Ma J.K.H. The transport barrier of epithelia: a comparative study on membrane permeability and charge selectivity in the rabbit. Pharm. Res. 1992;9:1029–1034. doi: 10.1023/a:1015802427428. [DOI] [PubMed] [Google Scholar]
- Ruenraroengsak P., Cook J.M., Florence A.T. Nanosystem drug targeting: facing up to complex realities. J. Control. Release. 2010;141:265–276. doi: 10.1016/j.jconrel.2009.10.032. [DOI] [PubMed] [Google Scholar]
- Schuetz E.G., Schinkel A.H., Relling M.V., Schuetz J.D. P-glycoprotein: a major determinant of rifampicin-inducible expression of cytochrome P450 in mice and humans. Proc. Natl. Acad. Sci. USA. 1996;93:4001–4005. doi: 10.1073/pnas.93.9.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh J., Ankola D.D., Beniwal V., Singh D., Ravi Kumar M.N.V. Nanoparticle encapsulation improves oral bioavailability ofcurcumin by at least 9-times when compared to curcuminadministered with piperine as absorption enhancer. Eur. J. Pharm. Sci. 2009;37:223–230. doi: 10.1016/j.ejps.2009.02.019. [DOI] [PubMed] [Google Scholar]
- Shoba G., Joy D., Joseph T., Majeed M., Rajendran R., Srinivas P.S.S.R. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998;64(4):353–356. doi: 10.1055/s-2006-957450. [DOI] [PubMed] [Google Scholar]
- Shukla R., Cheryan M. Zein: the industrial protein from corn. Ind. Crops Prod. 2001;13(3):171–192. [Google Scholar]
- Sigfridsson K., Nordmark A., Theilig S., Lindahl A. A formulation compari-son between micro- and nanosuspensions: the importance of particle size forabsorption of a model compound, following repeated oral administration torats during early development. Drug Dev. Ind. Pharm. 2011;37:185–192. doi: 10.3109/03639045.2010.504209. [DOI] [PubMed] [Google Scholar]
- Sultana S., Khan M.R., Kumar M., Kumar S., Ali M. Nanoparticles-mediated drug delivery approaches for cancer targeting: a review. J. Drug Target. 2013;21:107–125. doi: 10.3109/1061186X.2012.712130. [DOI] [PubMed] [Google Scholar]
- Thanou M., Verhoef J.C., Junginger H.E. Chitosan and its derivatives as intestinal absorption enhancers. Adv. Drug Del. Rev. 2001;50:S91–S101. doi: 10.1016/s0169-409x(01)00180-6. [DOI] [PubMed] [Google Scholar]
- Tonnesen H.H. Solubility and stability of curcumin in solutionscontaining alginate and other viscosity modifying macromolecules. Studies of curcumin and curcuminoids. Pharmazie. 2006;61(8):696–700. [PubMed] [Google Scholar]
- Torchilin V. Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. Eur. J. Pharm. Biopharm. 2009;7:431–444. doi: 10.1016/j.ejpb.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaibhav K., Shrivastava P., Javed H., Khan A., Ahmed M.E., Tabassum R., Khan M.M., Khuwaja G., Islam F., Siddiqui M.S., Safhi M.M. Piperine suppresses cerebral ischemia-reperfusion-induced inflammation through the repression of COX-2, NOS-2, and NF-kappaB in middle cerebral artery occlusion rat model. Mol. Cell. Biochem. 2012;367:73–84. doi: 10.1007/s11010-012-1321-z. [DOI] [PubMed] [Google Scholar]
- Valo H., Peltonen L., Vehvilainen S., Karjalainen M., Kostiainen R., Laaksonen T., Hirvonen J. Electrospray encapsulation of hydrophilic and hydrophobic drugsin poly(l-lactic acid) nanoparticles. Small. 2009;5:1791–1798. doi: 10.1002/smll.200801907. [DOI] [PubMed] [Google Scholar]
- Velpandian T., Jasuja R., Bhardwaj K., Jaiswal J., Gupta K. Piperine in food: interference in the pharmacokinetics of phenytoin. Eur. J. Drug Metab. Pharmacokinet. 2001;26:241–247. doi: 10.1007/BF03226378. [DOI] [PubMed] [Google Scholar]
- Villegas I., Sanchez-Fidalgo S., de la Lastra A. New mechanisms and therapeutic potential of curcumin for colorectal cancer. Mol. Nutr. Food Res. 2008;52(9):1040–1061. doi: 10.1002/mnfr.200700280. [DOI] [PubMed] [Google Scholar]
- Wang Y., Lu Z.X., Wu H., Lv F.X. Study on the antibiotic activity of microcapsule curcumin against foodborne pathogens. Int. J. Food Microbiol. 2009;136(1):71–74. doi: 10.1016/j.ijfoodmicro.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Wang Y., Lu Z.X., Lv F.X., Bie X.M. Study on microencapsulation of curcumin pigments by spray drying. Eur. Food Res. Tech. 2009;229(3):391–396. [Google Scholar]
- Wu Y.Q., MacKay J.A., McDaniel J.R., Chilkoti A., Clark R.L. Fabrication of elastin-like polypeptide nanoparticles for drug delivery by electrospraying. Biomacromolecules. 2009;10(1):19–24. doi: 10.1021/bm801033f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Ng W.J., Lee L.Y., Wang C.-H. Encapsulation of protein drugs in biodegradable microparticles by co-axial electrospray. J. Coll. Interf. Sci. 2008;317:469–476. doi: 10.1016/j.jcis.2007.09.082. [DOI] [PubMed] [Google Scholar]
- Xie J.W., Lim L.K., Phua Y.Y., Hua J.S., Wang C.H. Electrohydrodynamic atomization for biodegradable polymeric particle production. J. Coll. Interf. Sci. 2006;302(1):103–112. doi: 10.1016/j.jcis.2006.06.037. [DOI] [PubMed] [Google Scholar]
- Xie J.W., Marijnissen J.C.M., Wang C.H. Microparticles developed by electrohydrodynamic atomization for the local delivery of anticancer drug to treat c6 glioma in vitro. Biomaterials. 2006;27:3321–3332. doi: 10.1016/j.biomaterials.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Yaffe P.B., Doucette C.D., Walsh M., Hoskin D.W. Piperine impairs cell cycle progression and causes reactive oxygen species-dependent apoptosis in rectal cancer cells. Exp. Mol. Pathol. 2013;94:109–114. doi: 10.1016/j.yexmp.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Yang K., Lin L., Tseng T., Wang S., Tsai T. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC-MS/MS. J. Chromatogr. B. 2007;853:183–189. doi: 10.1016/j.jchromb.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Yoysungnoen P., Wirachwong P., Changtam C., Suksamram A., Patumraj S. Anti-cancer and anti-angiogenic effects of curcumin and tetrahydrocurcumin on implanted hepatocellular carcinoma in nude mice. World J. Gastroenterol. 2008;14(13):2003–2009. doi: 10.3748/wjg.14.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H.L., Huang Q.R. Enhanced in vitro anti-cancer activity of curcumin encapsulated in hydrophobically modified starch. Food Chem. 2010;119(2):669–674. [Google Scholar]
- Yun K.M., Suryamas A.B., Hirakawa C., Iskandar F., Okuyama K. A new physicalroute to produce monodispersed microsphere nanoparticle–polymer composites. Langmuir. 2009;25:11038–11042. doi: 10.1021/la901343j. [DOI] [PubMed] [Google Scholar]
- Zhang S., Kawakami K. One-step preparation of chitosan solid nanoparticlesby electrospray deposition. Int. J. Pharm. 2010;397:211–217. doi: 10.1016/j.ijpharm.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Zhang L., Huang J., Si T., Xu R.X. Coaxial electrospray of microparticles and nanoparticles for biomedical applications. Expert Rev. Med. Dev. 2012;9(6):595–612. doi: 10.1586/erd.12.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Kawakami K., Yamamoto M., Masaoka Y., Kataoka M., Yamashita S., Sakuma S. Coaxial electrospray formulations for improving oral absorption of apoorly water-soluble drug. Mol. Pharm. 2011;8:807–813. doi: 10.1021/mp100401d. [DOI] [PubMed] [Google Scholar]
- Zhong Q.X., Jin M.F. Nanoscalar structures of spray-dried zein microcapsules and in vitro release kinetics of the encapsulated lysozyme as affected by formulations. J. Agric. Food. Chem. 2009;57(9):3886–3894. doi: 10.1021/jf803951a. [DOI] [PubMed] [Google Scholar]
- Zhu H.J., Wang J.S., Markowitz J.S., Donovan J.L., Gibson B.B., DeVane C.L. Risperidone and paliperidone inhibit P-glycoprotein activity in vitro. Neuropsychopharmacology. 2007;32:757–764. doi: 10.1038/sj.npp.1301181. [DOI] [PubMed] [Google Scholar]
- Zutshi R.K., Singh R., Zutshi U., Johri R.K., Atal C.K. Influence of piperine on rifampicin blood levels in patients of pulmonary tuberculosis. J. Assoc. Phys. India. 1985;33:223–224. [PubMed] [Google Scholar]