Abstract
Cancer is one of the biggest problems in public health worldwide. Plants have been shown important role in anticancer research. Viscum album L. (Santalaceae), commonly known as mistletoe, is a semi-parasitic plant that grows on different host trees. In complementary medicine, extracts from European mistletoe (Viscum album L.) have been used in the treatment of cancer. The study was conducted to identify chemical composition and antitumor potential of Viscum album tinctures. Chemical analysis performed by high resolution chromatography equipped with high resolution mass spectrometer identified caffeic acid, chlorogenic acid, sakuranetin, isosakuranetin, syringenin 4-O-glucoside, syringenin 4-O-apiosyl-glucoside, alangilignoside C and ligalbumoside A compounds. Some of these compounds are probably responsible for the reduction of tumoral cellular growth in a dose-dependent manner. It was observed that melanoma murine cells (B16F10) were more sensitive to V. album tinctures than human leukaemic cells (K562), besides non-tumoral cells (MA-104) had a much lower cytotoxicity to them. Apoptotic-like cells were observed under light microscopy and were confirmed by a typical DNA fragmentation pattern. Additionally, flow cytometry results using Annexin-V/FITC permitted to quantify increased expression of early and late apoptotic markers on tumoral cells, confirming augmented Sub G0 population, which was probably associated with a consistent decrease in G1, and an increase in S or G2/M populations. Results indicate the chemical composition of V. album tinctures influences the mechanisms of in vitro tumoral cell death, suggesting a potential use in cancer pharmacotherapy research.
Abbreviations: % v/v, % volume/volume; TA, tincture A; TB, tincture B; TLC, Thin Layer Chromatography; NP/PEG, Diphenylboriloxyethilamine/polyetileneglicol; HPLC, high performance liquid chromatography; PDA, photodiode array detector; UFLC, ultra fast liquid chromatography; UHPLC, ultra high performance liquid chromatography; HRMS, high resolution mass; DMEM, Dulbecco’s Modified Eagle Medium
Keywords: Viscum album, Mistletoe, Lignans, Phenolic compounds, Antitumoral
1. Introduction
Cancer is a group of diseases characterized by the abnormal growth and proliferation of cells that may or may not invade or spread to other parts of the body. According to data from World Health Organization, cancer is the second cause of death worldwide and caused more than 8.8 million deaths in 2015 (WHO, 2017).
The high rates of cancer incidence have encouraged the interest of researchers in the developing of new therapeutic modalities against it, and in the finding of new drugs with better activity and lower adverse effects. In this scenario, natural products have been considered useful to the anticancer activity. It is known that approximately 55% of the anticancer drugs approved between 1940 and 2014 had their origin from natural sources (Newman and Cragg, 2016).
Several studies suggest that metabolites derived from plants may have pro-apoptotic properties. Viscum album L. (Santalaceae), commonly known as mistletoe, is a semi-parasitic plant that grows on different host trees. This species has been commonly used for complementary cancer therapy, mainly in Central Europe (Tröger et al., 2013), and it is possible to find a multitude of studies in which the immunomodulatory (Jurin et al., 1993, Gardin, 2009; Weissenstein et al., 2014), cytotoxic and pro-apoptotic (Bussing and Schietzel, 1999, Urech et al., 2005, Facina et al., 2014) properties have been described. Aqueous preparations of V. album exert several immuno-stimulatory mechanisms, possibly by interacting with the cellular and humoral compartments of the immune system, increasing the antitumor immune response (Yoon et al., 2001, Stein et al., 2002, Heinzerling et al., 2006, Gardin, 2009).
The most studied active compounds in aqueous preparations of V. album are lectins and viscotoxins. These compounds induce macrophage cytotoxicity, stimulate phagocytosis of immune cells, increase cytokine secretion and enhance in vitro cytotoxic effects on various cell lines (Timoshenko et al., 1995, Estko et al., 2015).
Other compounds, such as phenolic acids, phenylpropanoids, flavonoids, triterpenes, phytosterols, oligo and polysaccharides, were also identified in the European mistletoe (Nazaruk and Orlikowski, 2016, Delebinski et al., 2015, Strüh et al., 2013; Cebović et al., 2008) and this variety of metabolites is probably involved with the antitumoral effects of V. album extracts.
Although V. album antitumor activity is mainly associated with the aqueous preparations, the use of different solvents as well as modifications in the extraction methodology influences in vitro and in vivo antitumor activity. It must be taken into account that the chemical composition of V. album extracts is directly related to the solvent used in the extraction process.
In this context, the cytotoxicity of V. album hydroalcoholic tincture associated or not with chemotherapeutic agents was detected in the Ehrlich ascites carcinoma (Stan et al., 2013), as well as in HeLa cancer cells proliferation (Sárpataki et al., 2015), indicating that ethanol soluble compounds are also related to the antitumoral V. album activity. Moreover, Cebović et al. (2008) showed the efficacy of non-polar supercritical CO2 extract in the cytotoxicity of V. album towards Ehrlich carcinoma cells, confirming the importance of the optimization extraction methodology.
The purpose of the present study was to analyze the chemical profile of two V. album tinctures, as well as their in vitro effects in tumoral (murine melanoma cells, B16F10; human chronic myelogenic leukemia cell line, K562) and non-tumoral cells (monkey kidney cells, MA-104). The involvement of the identified chemical compounds with antitumoral V. album activity is also discussed in this paper.
2. Material and methods
2.1. Viscum album L. Tinctures
Tinctures of Viscum album L. used in this study were donated by two pharmaceutical laboratories, Homeopatia Almeida Prado (São Paulo, Brazil) and Boiron Laboratories (Lyon, France), for research purposes. Both tinctures were obtained by maceration extraction with ethanol (45% v/v) following Viscum album homeopathic monographs in pharmacopoeias (ANSM, 2010, ANVISA, 2011) and were labeled Tinctures TA and TB.
2.2. Identification of substances by thin layer chromatography
Thin layer chromatography (TLC) analyses were achieved by silica gel 60 F254 (250 µm thickness, SiliCycle, Quebec, Canada) using water/methanol/glacial acetic acid/methylene chloride (2:3:8:15) as mobile phases. The detections were done by spraying NP/PEG reagent (1% diphenylboriloxyethylamine in methanol p/v, followed by 5% polyethylene glycol 4000 in ethanol p/v). The plates were observed under ultraviolet light at 254 and 365 nm before and after spraying the reagent solution. Spots of non-diluted tinctures and standards were identified by Rf-values and color compared to the standard compounds caffeic and chlorogenic acids (ANSM, 2010) (MP Biomedicals, California, USA).
2.3. HPLC-PDA-MS conditions
Analyses were conducted using an HPLC Dionex Ultimate 3000, equipped with a photodiode array (PAD) detector (Thermo Fisher Scientific, USA) connected with LCQ Fleet Ion Trap Mass Spectrometer (Thermo Fisher Scientific, USA). The sample was prepared according to V. album monograph from French Pharmacopoeia (ANSM, 2010): in a 20.0 mL volumetric flask, 8.0 g of each tincture was diluted to 20.0 mL of a mixture of 10 volumes of acetonitrile and 90 volumes of trifluoroacetic acid (0.05 per cent v/v).
Separations were performed on a reverse-phase column (C-18, 250 mm × 4, 6 mm × 5.0 μm; Kromasil, Akzo Nobel). Water-formic acid 0.1% v/v (A) and acetonitrile (B) were used as mobile phases, as follows: (i) 0–20 min, 10% B, (ii) 20–25 min, 10–15% B, (iii) 25–45 min, 15% B, (iv) 45–50 min, 15–100% B, (v) 50–55 min 100% B, (vi) 55–57 min 100–10%, and (vii) 57–70 min 10% B. The flow rate was 1.0 mL/min and the injection volume was 20 µL. Absorption UV–VIS spectra were recorded on PDA-detector (with a total spectral range between 100 nm and 400 nm), set at detection wavelength 220 nm, simultaneously. Mass spectra were recorded in positive ion mode.
2.4. UFLC-PDA-MS/MS conditions
UFLC‑PDA‑MS/MS analyses were carried out on an ultra fast liquid chromatography (UFLC Shimadzu, model Nexera) equipped with a mass spectrometer (TOF Bruker, model Compact). The sample was prepared according to V. album monograph from French Pharmacopoeia (ANSM, 2010) as described in Section 2.3.
Separations were performed on a reverse-phase column (C-18, 100 mm × 3 mm × 2.6 μm; Kinetex, Phenomenex). A binary gradient system with water-formic acid 0.1% v/v (A) and acetonitrile (B) was used as follows: (i) 0–7.65 min, 5% B, (ii) 7.65–8.50 min, 5–15% B, (iii) 8.50–15.30 min, 15% B, (iv) 15.30–17 min, 15–100% B, (v) 17–18.70 100% B, (vi) 18.70–20.40 min 100–5% and (vii) 20.40–22 min 5% B. The flow rate was 0.5 mL/min and the injection volume was 2 µL. The detection wavelength was 220 nm. All analyses were done by electrospray ionization (ESI) in positive mode.
2.5. UHPLC-HRMS/MS conditions
Samples were prepared as described in Section 2.3 (ANSM, 2010) and analyzed on Dionex Ultimate 3000 system hyphenated to a QExactive Plus mass spectrometer (Thermo Fisher Scientific, USA) equipped with an electrospray ionization (ESI) probe. Separations were performed on a reverse-phase (C-18, 50 mm, 2, 1 mm × 1.7 μm; Syncronis, Thermo Fisher Scientific). Water-formic acid 0.1% v/v (A) and acetonitrile (B) were used as mobile phases as follows: (i) 0–2 min, 10% B, (ii) 2–3,5 min, 10–15% B, (iii) 3.5–6.4 min, 15% B, (iv) 6.4–7.0 min, 15–100% B, (v) 7.0–8.0 min 100% B, (vi) 8.0–9.2 min 100–10% and (vii) 9.2–10 min 10% B. The flow rate was 1.0 mL/min and the injection volume was 5 µL. Mass spectra were recorded in negative ion mode. The HRMS and HRMS/MS data were acquired in negative mode over a m/z range of 80–1000. The MS profile was performed in full scan mode and displayed in TIC (Total Ion Current) chromatogram. ESI conditions were as follows: capillary temperature 380 °C; spray voltage 3.9 kV; ESI voltage 2.9 kV. Nitrogen was used as sheath gas (60 Au) and auxiliary gas (20 Au). The raw data were acquired and processed with Xcalibur 2.0.7 software from Thermo Scientific.
2.6. Cell lines and culture
B16F10 cells (murine melanoma) was obtained from Laboratório de Oncobiologia Molecular (LabOMol), Federal University of Rio de Janeiro, the K562 (human chronic myelogenic leukemia cell line) and MA-104 (monkey kidney, non-tumoral) were obtained from Rio de Janeiro Cell Bank (Duque de Caxias, Rio de Janeiro, Brazil). Cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) in 25 cm2 disposable plastic bottles (TPP Techno Plastic Products AG, Switzerland), at 37 °C. They were supplemented with 10% fetal bovine serum, penicillin (100 UI/mL) and streptomycin (100 µg/mL). These supplements were obtained from Invitrogen (California, USA),
2.7. Cell viability assay
Cell viability was measured by MTT colorimetric assay as described before (Mosmann, 1983, Meira et al., 2005). Briefly, for each experiment, 1 × 104 cells were seeded per well in 96-well plates. After 24 h, cells were treated with TA and TB in concentrations varying from 1 to 5% v/v. After incubation for 24 h, the cells were centrifuged for 8 min (500g) and the supernatant was discarded. In each well, 180 µL of DMEM and 20 µL of a 5 mg/mL solution of Thiazolyl Blue Tetrazolium Bromide (MTT - Sigma, USA) were added, and cells were incubated for further 3 h at 37 °C. After incubation, cells were centrifuged, the supernatant was discarded and 200 µL of Dimethyl Sulfoxide (DMSO) were added per well to dissolve the newly formed formazan salt. The absorbance was measured at 490 nm using an ELISA plate reader (TP Reader, Thermoplate). Mean values were calculated in 3 independent experiments using 5 wells per condition.
2.8. May-Grunwald-Giemsa assay
B16F10 cells (5 × 105 cells/ well) were cultivated in 6-well plate and incubated with TA, TB and their respective controls, at the concentrations of 3% and 5% v/v for 24 h. Subsequently, cells were washed and stained with May-Grunwald-Giemsa, as described before (Meira et al., 2005, Barbosa et al., 2017). Finally, morphological alterations were examined with a light microscopy (Axioplan 2, Carl Zeiss, Göttingen, Germany), and all images were obtained using a digital camera attached to the microscope.
2.9. DNA fragmentation analysis
DNA fragmentation was performed as follows: B16F10 cells were seeded to approximately 80% of confluence (3.5 × 105 cells) in 25 cm2 disposable plastic bottles and incubated for 24 h, at 37 °C. Cells were treated with TA, TB and their respective hydro-alcoholic controls, in the concentrations of 3% and 5% v/v, and then incubated for more 6 h or 24 h at 37 °C. After that, cells were trypsinized, counted and diluted to obtain 1 × 106 cells. Following, they were washed twice with PBS, and DNA extraction was performed according to the instructions from Wizard Genomic DNA Purification Kit (Promega, São Paulo, Brazil). For DNA ladder detection, the DNA samples extracted and the 1Kb DNA Ladder Marker (M1181, Sinapse, São Paulo, Brazil) were submitted to electrophoresis in 0.8% agarose gel. After 30 min, the gel was labeled with ethidium bromide, visualized in UV and photographed (Red®, Protein Simple, California, USA).
2.10. Apoptosis measurement
Apoptosis induction was measured as follows: B16F10 cells were seeded on 6-well plates at a concentration of 1 × 105 cell/well. After 24 h, cells were treated with TB, TA and their respective controls in the concentrations of 3% and 5% v/v. After incubation for 6 h, the supernatants were collected, cells were trypsinized and added to the supernatants. Then, the suspensions were centrifuged and washed with cold PBS. The pellets were resuspended in 100 μL of FITC Annexin V Apoptosis Detection Kit I buffer (BD Biosciences, PharmingenTM), homogenized and transferred to flow cytometry tubes. Cells were then stained with annexin V-FITC and propidium iodide (PI) according to the manufacturer instructions for 15 min at room temperature, in the dark. After this time, cells were analyzed in FACSVerse (Beckton and Dickinson, USA). The percentages of apoptotic cells were evaluated using BD FACSuite software.
2.11. Cell cycle analysis
Cell cycle was evaluated by flow cytometry as follows: 24 h after cell incubation with 3% or 5% v/v TA or TB and their respective controls, cells were trypsinized, centrifuged and washed with PBS. The tubes were then centrifuged for 10 min (450g), the supernatant was discarded and the pellet was resuspended in 500 μL of propidium iodide solution (40 µg/mL) in the presence of 100 µg/ml RNase in PBS. The solution was homogenized and transferred to flow cytometry tubes. After incubation for 30 min at room temperature in the dark, the cell cycle was analyzed with a FACSVerse flow cytometer (Beckton and Dickinson, USA). The percentages of cells in the cell cycle phases G0/G1, S, G2/M and sub G0 were determined using BD FACSuite software.
2.12. Statistical analysis
All experiments were performed at least three different times, and results were analyzed by ANOVA (analysis of variance) with Dunnett post hoc test, using GraphPad 5 Software (California, USA). P values<.05 were considered statistically significant.
3. Results
3.1. Chemical profile of Viscum album tinctures
TLC plate of TA and TB showed blue zones, which are typical of phenolic acids at 365 nm UV light, after it was sprayed with NP/PEG reagent (Wagner and Bladt, 2001). TLC plate of TA sample showed one spot with Rf value (0.66) and similar color (fluorescent blue) to the chlorogenic acid reference compound. TB chromatoplate exhibited two fluorescent blue spots, one with Rf value equal to caffeic acid (Rf of 0.91) and another similar to the chlorogenic acid standard (Rf of 0.66).
UFLC-PDA-MS/MS analysis of TA tincture revealed phenolic acids and flavonoids with typical UV absorption spectrum and mass fragmentation pattern as major metabolites. In addition, these results support the previous phenolic acid and flavonoids identification by TLC plate, which showed fluorescent blue spots, typical of phenolic acid, and yellow spots, characteristic of flavonoids. UFLC chromatogram of TA, detected at 220 nm, showed three main peaks (1, 2 and 3), whose MS and MS/MS spectra were carefully analyzed and compared with literature data. Compound 1 presented a typical UV absorption spectrum for phenolic acids (Mabry et al., 1970) and protonated molecular ion at m/z 355.1036 [M+H]+, with a mass fragmentation pattern similar to chlorogenic acid (Popova, 1991). Compounds 2 and 3 showed an UV spectra with absorption spectrum corresponding to a flavonoid (Mabry et al., 1970) and protonated molecular ions at m/z 287.0925 [M+H]+ (C16H14O5) and 287.0924 [M+H]+ (C16H14O5) (Urech and Baumgartner, 2015, Nazaruk and Orlikowski, 2016). According to the MS/MS spectra of 2 and 3, m/z 285.0767, 242.0580, 167.0344, 147.0446, 119.0497 and 91.0548 [M+H]+ ions had a typical ion fragmentation for sakuranetin and naringenin 5-methyl ether, when compared with previously reported data (Hammami et al., 2004, Portet et al., 2008).
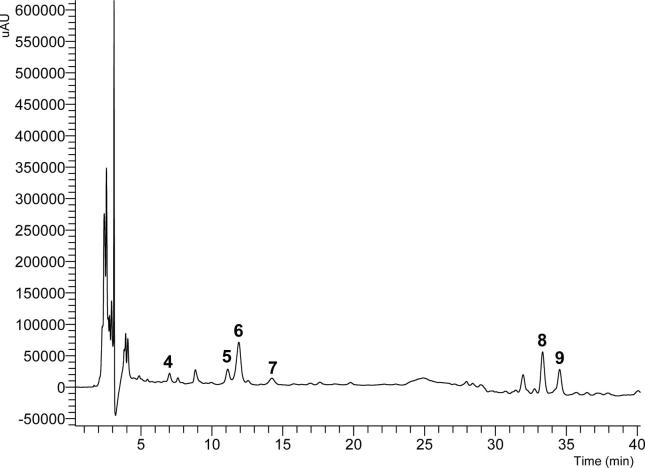
TB tincture was analyzed initially by HPLC-PDA-MS, and the chromatogram at 220 nm exhibited six main peaks (4, 5, 6, 7, 8 and 9), as shown in Fig. 1. The UV spectra of those peaks showed absorption similar to phenolic acids and lignans (Mabry et al., 1970; Benković et al., 2014). Mass analysis of each peak exhibited the following protonated molecular ions at m/z: 354.91 [M+H]+, 395.18 [M+Na]+, 527.24 [M+Na]+, 203.95 [M+Na]+, 599.87 [M+NH4]+ and 599.88 [M+NH4]+, respectively. In addition, mass spectral fragmentation pattern and UV spectrum of compounds 4–9 were compared to literature data, especially to those phenolic acids and lignans described as chemical markers of V. album (Urech and Baumgartner, 2015). Afterwards, the identification of compounds 4–9 was followed by accurate mass measurements through UHPLC-HRMS/MS analysis that presented deprotonated molecular ion peaks for compounds 4, 5, 6, 7, 8 and 9 at m/z: 353.08844 [M−H]− (C16H18O9), 371.13513 [M−H]− (C17H24O9), 503.17807 [M−H]− (C22H32O13), 179.03498 [M−H]− (C9H8O4), 581.22498[M−H]− (C28H38O13) and 581.22527 [M−H]− (C28H38O13), respectively.
Fig. 1.
UV Chromatogram at 220 nm of TB sample.
MS/MS spectra of compound 4, m/z 191.05521, 179.03458 and 135.04408 [M−H]− ions, had a typical ion fragmentation for chlorogenic acid, according to the literature (Zhang et al., 2016). For some of the constituents, such as syringenin 4-O-glucoside (5) and syringenin 4-O-apiosyl-glucoside (6) fragment ions, it was observed that m/z 209, 194 and 175 is a syringenin fragmentation characteristic (Sun et al., 2016). Compound 7 showed MS/MS spectra with ions at m/z 161 and 135 [M−H]−, similar to ion fragments of caffeic acid (Zhang et al., 2016).
In accordance with the accurate mass spectra of 8 and 9, m/z 581.22498 [M−H]− and 581.22527 [M−H]− were typical for alangilignoside C and ligalbumoside A, as already described for V. album (Nhiem et al., 2012; Benković et al., 2014).
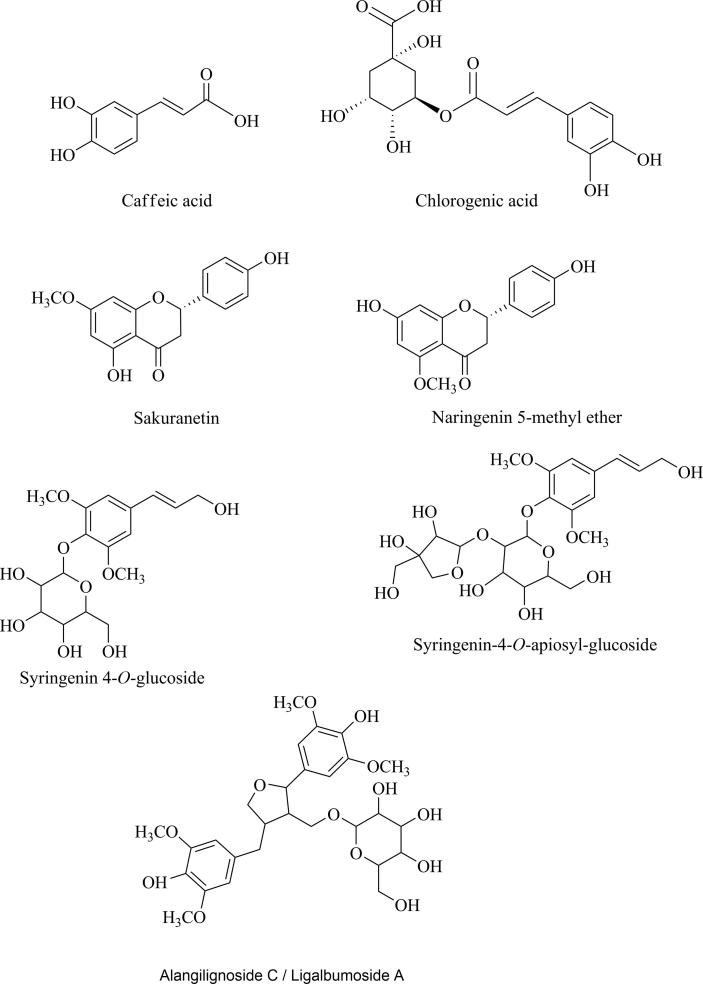
These results provided reliable information for confirming molecular weight and structure of these constituents. Therefore, compounds were identified as chlorogenic acid (4) (Ferracane et al., 2010, Mocan et al., 2016), syringenin 4-O-glucoside (Panossian et al., 1998) (eleutheroside B) (5), syringenin 4-O-apiosyl-glucoside (Panossian at al., 1998) (polygalatenoside E) (6), caffeic acid (Spagnol et al., 2015) (7), alangilignoside C (8) and ligalbumoside A (Nhiem et al., 2012; Benković et al., 2014) (9). Compounds identified in V. album tinctures are shown in the Fig. 2.
Fig. 2.
Possible structures present in TA and TB samples.
3.2. Cell viability is reduced by Viscum album L.
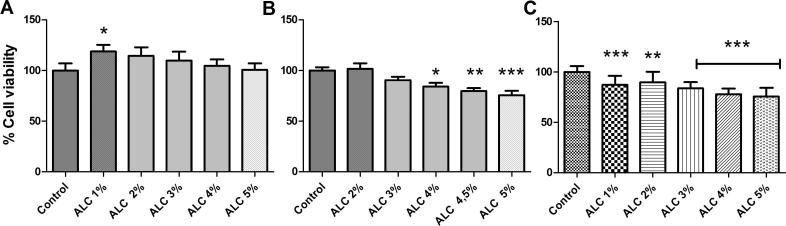
As shown in Fig. 3, control hydroalcoholic solutions decreased the viability of B16F10 and K562 cells in a dose-response way, having B16F10 presented a higher sensitivity. In contrast, MA-104 cell line was resistant to all hydroalcoholic concentrations used. Since the tumor cell lines were sensitive to the hydroalcoholic solutions, the same concentrations of these solutions were used as controls in the following experiments.
Fig. 3.
MTT assay after 24 h of treatment with 45% v/v hydro-alcoholic solution. The final concentrations varied between 1 and 5% v/v. (A) MA-104, (B) K562, (C) B16F10. Data are shown as media ± SD from at least 3 experiments. *p < .05, **p < .001 and ***p < .0001, obtained with one way ANOVA with Dunnett post-test.
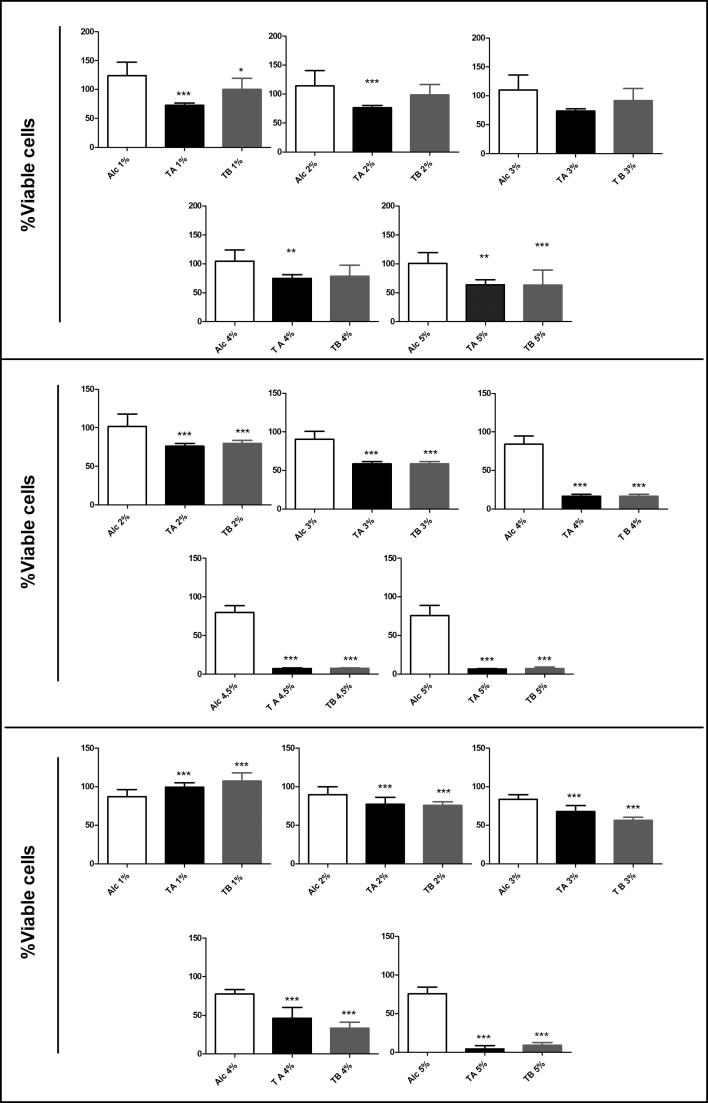
Both TA and TB were cytotoxic to the three cell lines, but this cytotoxicity was cell- and dose-dependent (Fig. 4). The MA-104 cell line was more sensitive to TA, which significantly diminished its viability in 50% (p < .001) in concentrations as low as 1% v/v, while TB reduced viable cells in 24% when compared to control (Fig. 4, panel A). However, for the concentration of 5% v/v, TA and TB were equally effective, reducing MA-104 cell viability in approximately 36%. For K562 cells (Fig. 4, panel B) and B16F10 cells, both TA and TB were almost equally efficient in reducing the cellular viability at any concentration tested. At 5% v/v, TA and TB reduced K562 and B16F10 viability in approximately 70% (Fig. 4, panels B and C). Since B16F10 cell line has a well-known high aggressive and drug-resistant phenotype and presented similar results to K562 with both tinctures treatments, the following experiments were performed only with B16F10.
Fig. 4.
MTT assay in MA-104 (panel A), K562 (panel B) and B16F10 (panel C) cells after 24 h of treatment with TA and TB solutions. The final concentrations varied between 1 and 5% v/v. Data are shown as media ± SD from at least 3 experiments. *p < .05, **p < .001 and ***p < .0001, obtained with one way ANOVA with Dunnett post-test.
3.3. Morphologic B16F10 alterations induced by TA and TB
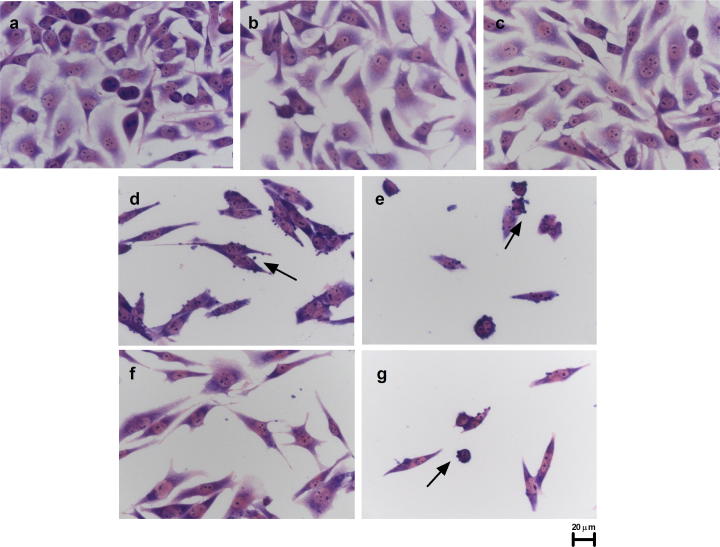
Fig. 5 shows the morphology of B16F10 stained with Giemsa after 24 h of treatment, with TA and TB at concentrations of 3% and 5% v/v. Blebs, which were absent in the hydro-alcoholic control cells, can be seen in the plasma membrane (Fig. 5 d, e, g - black arrows), suggesting apoptosis. Moreover, cells treated with 3% v/v also showed fusiform pattern cells.
Fig. 5.
Representative photography of B16F10 Giemsa staining after 24 h of treatment. Cells were treated as stated in Section 2.8. (a) Control, non-treated cells. (b) Cells treated with 3% v/v hydro-alcoholic solution. (c) Cells treated with 5% v/v hydro-alcoholic solution. (d) Cells treated with TA 3% v/v. (e) Cells treated with TA 5% v/v. (f) Cells treated with TB 3% v/v. (g) Cells treated with TB 5% v/v. The black arrows indicate possible blebs in the plasma membrane.
3.4. Viscum album tinctures induced DNA fragmentation in B16F10 cells
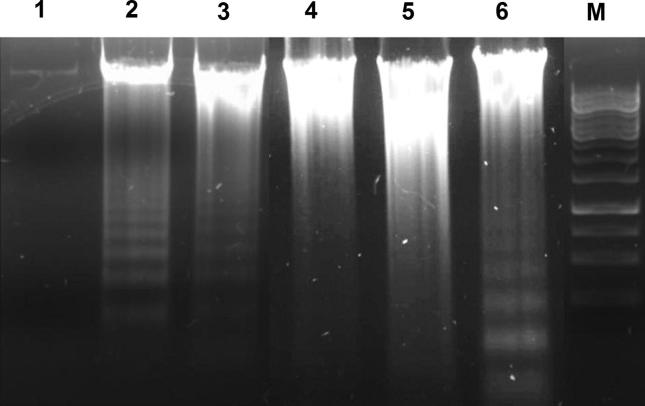
Fig. 6 shows B16F10 genomic DNA fragmentation induced by TA and TB. A DNA degradation pattern can be observed in lanes 2, 3 and 6, corresponding respectively to TB and TA at 5% v/v (6h), and TB at 3% v/v (24 h). Finally, the absence of DNA fragmentation was detected when hydro-alcoholic solvents (3% and 5% v/v) were incubated with B16F10 for 24 h (lane 1) and for 6 h (lane 4).
Fig. 6.
Genomic DNA fragmentation of B16F10 cells by Viscum album tinctures. Lane M: DNA ladder marker; Lane 1: hydro-alcoholic solution 5% v/v 6 h; Lane 2: TB 5% v/v 6 h; Lane 3: TA 5% v/v 6 h; Lane 4: hydro-alcoholic solution 3% v/v 24 h; lane 5: TA 3% v/v 24 h; lane 6: TB 3% v/v 24 h. The figure is representative of 3 (three) independent experiments.
3.5. Apoptosis evaluation by Annexin-V/FITC/PI
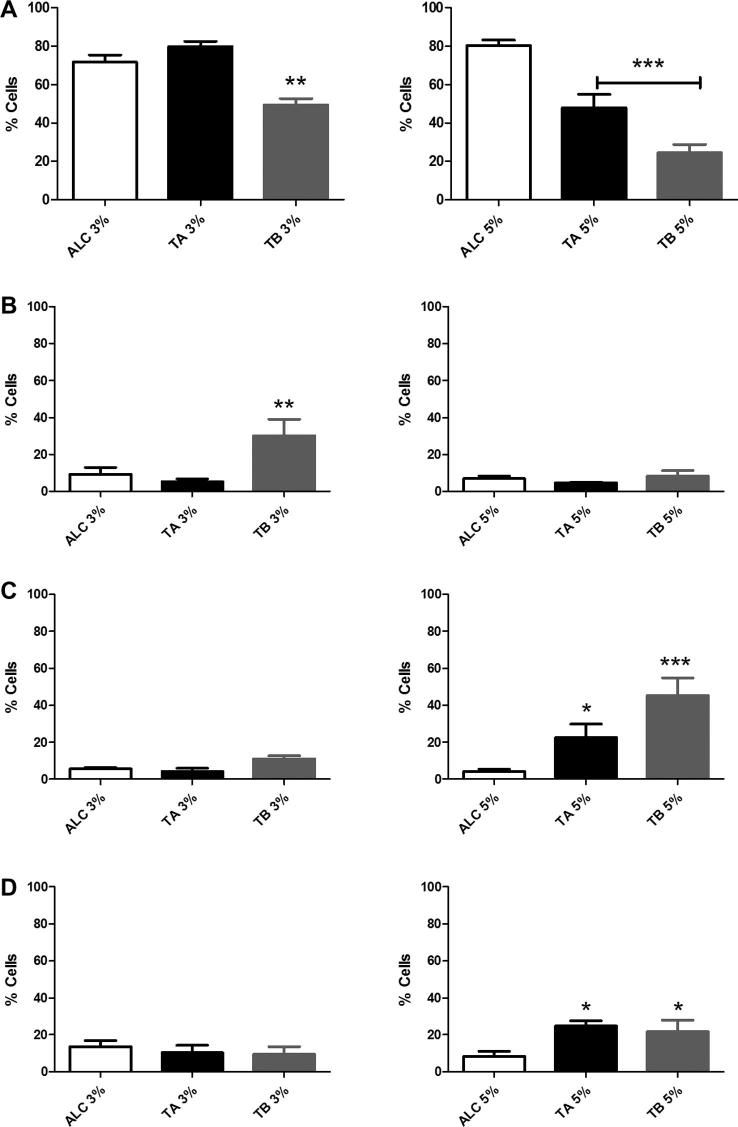
Since the results above suggested that V. album tinctures induce apoptosis in B16F10, Annexin V-FITC/PI V-PI methodology was also employed to confirm this data. As it can be seen in Fig. 8-panel A, the number of viable cells was reduced by 22.5%, in relation to its respective control, after treatment with 3% v/v of TB. This reduction was accompanied by a 21% increase in the number of early apoptotic cells (PI-/Annexin+) (Fig. 8, panel B, left). On the other hand, no statistically significant differences (p < .05), in relation to control, were detected after TA incubation.
Fig. 8.
Apoptosis induced by treatment with TA and TB (3% and 5% v/v) for 6 h. Panel A – cells not labeled with Annexin V-FITC or PI (viable cells). Panel B – cells labeled with Annexin-V/FITC (early apoptosis). Panel C – cells labeled with both Annexin-V/FITC and PI (late apoptosis). Panel D – cells labeled only with PI (necrotic cells). Data are Mean ± SD of at least three independent experiments. *p < .05; **p < .001 and ***p < .0001, as obtained by one way ANOVA with Dunnet post-test.
Additionally, using higher tincture concentrations (5% v/v), statistically significant decreases in B16F10 viability were detected by 32% (TA) and 55% (TB), with p < .0001 (Fig. 8, panel A, right). Nevertheless, Fig. 8 (panel C, right) showed an increase of 18% (TA, p < .05) and 41% (TB, p < .0001) in late apoptotic cells (PI+/Annexin+). Finally, TA and TB led to an increase in PI labeled cells with percentages of 16.5% and 13.5% (p < 0.05), respectively, suggesting necrosis or other non-apoptotic cell death pathways (Fig. 8, panel D, right).
3.6. Viscum album tinctures modified B16F10 cell cycle
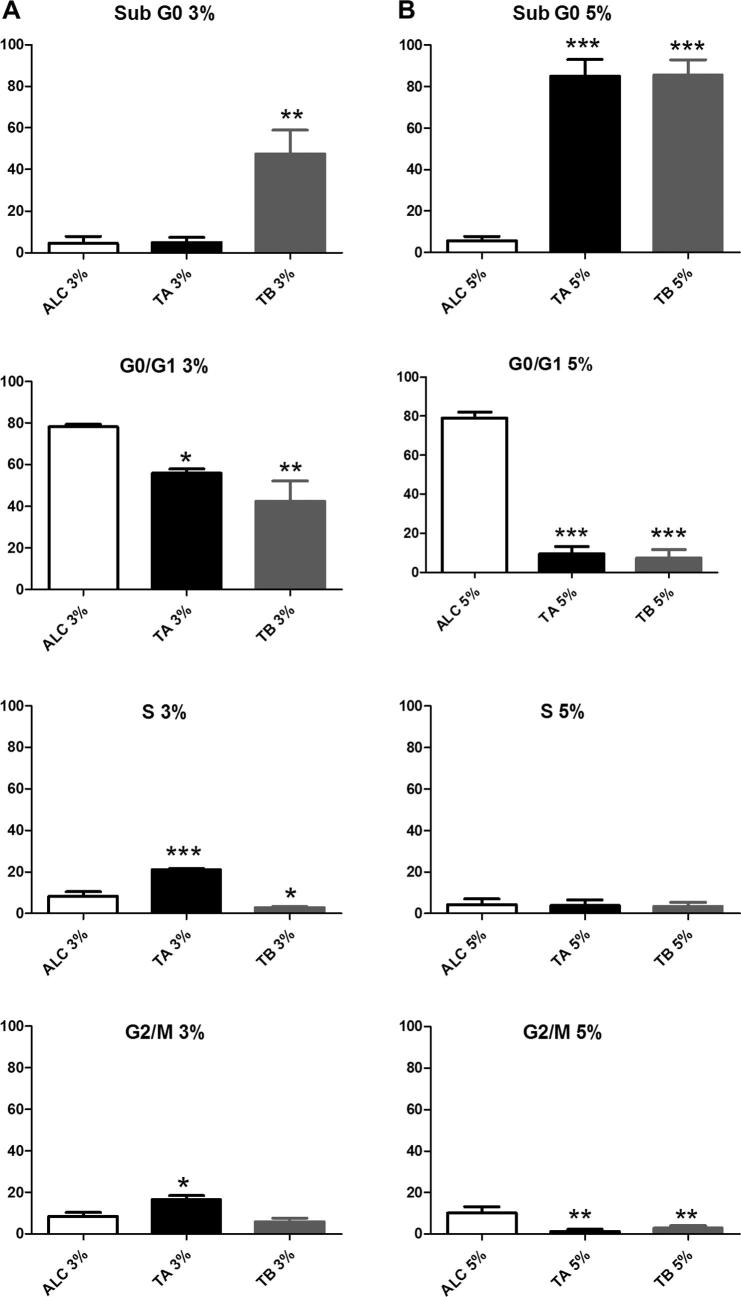
B16F10 cell cycles were evaluated after 24 h of treatment with TA and TB at 3% and 5% v/v. It can be seen in Fig. 7 that SubG0 had an increase of 43% after 24 h of incubation with TB (3% v/v), and 80% after 24 h of incubation with both tinctures at 5% v/v. The percentage of cells in G0/G1 was reduced by 22% (TA) and 36% (TB) at 3% v/v, while TA treatment (3% v/v) induced an increase in the number of cells of 13% and 8% in S and G2/M phases, respectively.
Fig. 7.
Effect of Viscum album tinctures on B16F10 cell cycle. Cells were treated with TA or TB at concentrations of 3% v/v (Panel A) or 5% v/v (panel B) for 24 h. Cells were incubated with PI and the DNA content was evaluated by flow cytometry, as described in Section 2.11. Data represent the mean ± SD of at least three independent experiments. *p < .05; **p < .001 and ***p < .0001, as measured be one-way ANOVA with Dunnet post-test.
4. Discussion
Cancer is one of the main causes of death worldwide. Natural products and their secondary metabolites have a considerable importance as anticancer agents considering their major toxicity to cancer cells when compared with normal cells (Jiang et al., 2017).
Several studies have reported cytotoxic activity of V. album aqueous extracts in different cell lines, such as MOLT-4 and Yoshida (Urech et al., 1995), cells of tongue squamous cell carcinoma (Klingbeil et al., 2013), lymphoma human cells (Seifert et al., 2008), human T cell lines CEM and monocyte cell lines HL-60 (Singh et al., 2016). The most important compounds involved with the antitumoral activity of V. album aqueous extracts are mistletoe lectins (I-III) (Valentiner et al., 2002), viscotoxins and polysaccharides (Urech et al., 1995, Tröger et al., 2013). However, its antitumoral potential is controversial because there are studies confirming that non-aqueous extracts contain compounds, such as epi-oleanolic acid (Jung et al., 2004), viscothionin (Kim et al., 2014), and alkaloids (Khwaja et al., 1980), which are also directly involved with antioxidative (Kim et al., 2016) and, consequently, anti-tumoral properties. Recently, Sárpataki et al. (2015) showed that V. album alcoholic extract reduces Hela cells proliferation with no significant effects on normal fibroblasts, confirming the involvement of other bioactive molecules.
In the present study, we observed that hydroalcoholic V. album tinctures (TA and TB) were cytotoxic to tumoral cell lines (K562 and B16F10). Since mistletoe lectins are not stable in hydroalcoholic solvents, the anticancer activity of these extracts should be also attributed to the presence of different compounds from the ones in aqueous V. album preparations. Our results showed the presence of phenolic compounds in both TA and TB tinctures, confirming previous analyses done by other authors (Pfüller, 2000, Luczkiewicz et al., 2001).
According to the literature, caffeic and chlorogenic acids presented inhibitory activities against tumoral cells, evidencing the effects in the adhesion, proliferation, migration, and invasion of tumoral cells (Yagasaki et al., 2000, Weng and Yen, 2012). Caffeic acid phenethyl ester showed a decrease of melanoma cell migration due to a nuclear factor kappa B inhibition (NF-κB) (Jones and Katiyar, 2013). Chlorogenic acid did not have cytotoxic effects on cellular viability when Hep3B cells were treated with different concentrations of this compound (Jin et al., 2005). However, the same study demonstrated that chlorogenic acid inhibited the activity of the metalloproteinase-9 (MMP-9), related with tumor progression.
Flavanone sakuranetin present in TA is well-known in V. album (Urech and Baumgartner, 2015). However, the flavanone naringenin 5-methyl ether was described for the first time in this work. Drira and Sakamoto (2016) demonstrated by MTT assay the cytotoxic effects of sakuranetin (0–75 μmol/L) in B16BL6 melanoma cells after 72 h of treatment.
Phenylpropanoids and lignan are other classes of secondary metabolites with important anticancer activity. Etoposide and teniposide, two important anticancer drugs, were derived from lignan podophyllotoxin presented in genus Podophyllum (Brandão et al., 2010). In the present work, two phenylpropanoids and two lignans were identified in TB sample, syringenin 4-O-glucoside (eleutheroside B), syringenin 4-O-apiosyl- glucoside (polygalatenoside E), alangilignoside C and ligalbumoside A, in accordance with previous works described with V. album species (Panossian et al., 1998, Popova, 1991, Nhiem et al., 2012, Urech and Baumgartner, 2015).
Eleutheroside B and polygalatenoside E presented cytotoxic activity against HL-60 and Hell-299 cell lines (Thao et al., 2015). Additionally, Zhu et al. (2012) demonstrated eleutheroside B cytotoxic activity against MCG-803 cell line with IC50 50.9 µM. Based on the literature data, the presence of phenylpropanoids and lignans in TB and flavanones in TA could be also related to their cytotoxic and apoptotic activities.
Giemsa staining revealed the occurrence of morphological B16F10 changes, induced by TA and TB treatments, mainly characterized by blebs and cell shrinkage, which are suggestive of apoptotic cell death (Zainal Ariffin et al., 2009). In fact, biochemical features of apoptosis were detected after gel electrophoresis analysis, confirming DNA ladder fragmentation into segments of 180–200 multiple base pairs, which is a typical apoptosis pattern (Veiga et al., 2005). Additionally, Annexin V-FITC/PI assay confirmed the cell death in a dose-dependent manner since the lower TB concentration (3% v/v) induced a higher percentage of early apoptotic cells. Moreover, using 5% (v/v) of TB and TA, the flow cytometry analyses showed an increase in late apoptosis or other cell death mechanisms.
Finally, the proportion of hypodiploid B16F10 cells in the total cell population was significantly higher after incubation with both tinctures at 5% (v/v), with around 80% of cells in Sub G0 (Agrawal et al., 2011) (Fig. 7), confirming the increase of late apoptotic cells detected by Annexin V-FITC/PI-FITC/PI assay (Fig. 8). The observed increase in apoptotic cells, confirmed by the augmented Sub G0 population was associated with a consistent decrease in G1, without alterations in S or G2/M populations (Fig. 7, panel B). These evidences suggest that the death-inducing mechanisms promoted by V. album tinctures 5% v/v did not impact cell cycle progression. On the other hand, cells treated with TA 3% v/v exhibited enriched populations in S and G2/M phases in association with a decline in G0/G1 after 24 h of incubation. Similar effects using plant extracts on cell cycle progression of cancer cells were previously observed. Karimian et al. (2017) reported an arrest of breast cancer cell line LA7 in S phase when treated with Kelussia odoratissima methanol extracts. Additionally, the population in G1 phase was decreased and the expression of p21 and p27 were augmented (Karimian et al., 2017). The arrest in S phase was also observed in Caco-2 and HEPG2 cells treated with Olea europaea L. ethanol extracts. This effect was accompanied by DNA fragmentation and subsequent cell death induction (Maalej et al., 2017).
Previously, Han et al. (2015) showed a G0/G1 arrest in both B16BL6 and B16F10 melanoma cells treated with Korean mistletoe lectin (V. album var. coloratum agglutinin) and its extract in vitro. They also observed an increase in both early and late apoptosis, and that this was probably caused by an increase in the activation of caspases-1, 3, 4, 5, 6, 7, 8, and 9, in a dose-dependently way, accompanied by a significant decrease in the expression of procaspase-3 and 8 (Han et al., 2015).
Corroborating these and our results, Korean mistletoe lectin (V. album var. coloratum agglutinin-VCA) increased the survival of mice inoculated with B16-BL6 melanoma cells and inhibited lung metastasis by VCA (Park et al., 2001).
They also showed that treatment of cells with VCA resulted in growth suppression, nuclear morphological changes, DNA fragmentation, and an increased fraction of cells in sub-G1 consistent with apoptosis, confirming our results. When analyzing anti-angiogenesis of VCA, they observed that vessel growth induced by fat emulsion was decreased. These results suggest that VCA has different mechanisms for inhibiting tumor growth and metastasis by increasing apoptosis and also by inhibiting angiogenesis (Park et al., 2001).
In the late decade of 1990, Antony et al. (1997) had yet observed the effects of V. album on the inhibition of lung metastatic colony formation induced by B16F10 melanoma cells in mice, and Kuttan et al. (1997) showed the anticarcinogenic and antimetastatic effects of Iscador on methylcholanthrene-induced sarcoma formation in mice, although the mechanisms in both studies were not identified at that time.
Moreover, Zarković et al. (1998) showed that low concentrations of V. album commercial extracts (Isorel) inhibited B16F10 and HeLa tumor cell lines more strongly than purified lectin-1. For the authors, the therapeutic effect of Isorel is a result of the association of low and high molecular weight components present in the extracts.
Although the above commented researches observed the effects of lectins in the antitumoral effects of V. album, in the present work, we evidenced the effects of V. album tinctures in a metastatic melanoma murine (B16F10) model for the first time, confirming the wide variety of chemical compounds in the ethanolic V. album and their promising antitumoral potential.
5. Conclusion
V. album tinctures presented anticancer activity against B16F10 and K562 tumor cell lines. Our results showed a selective tumoral cytotoxicity with apoptosis induction and cell cycle effect. Furthermore, the identified compounds chlorogenic acid, caffeic acid, sakuranetin, naringenin 5-methyl ether, syringenin 4-O-glucoside (eleutheroside B), syringenin 4-O-apiosyl-glucoside (polygalatenoside E), alangilignoside C and ligalbumoside A are the possible contributors to the antiproliferative and apoptotic effects of V. album tinctures, suggesting an interesting potential for the pharmacotherapy of cancer. Based on these results, the present authors suggest further toxicologic investigations with V. album tinctures as well as preclinical assays in order to evaluate the clinical potential of these preparations.
Conflict of interest
The authors confirm that there are no conflicts of interest associated with this publication.
Acknowledgments
The authors thank CAPES for financial support, and the pharmaceuticals laboratories Almeida Prado (Brazil) and Boiron (France) for V.album tinctures donations.
Footnotes
Peer review under responsibility of King Saud University.
References
- Agrawal S.K., Agrawal M., Sharma P.R., Gupta B.D., Arora S., Saxena A.K. Induction of apoptosis in human promyelocytic leukemia HL60 cells by an extract from Erythrina suberosa stem bark. Nutr. Cancer. 2011;63:802–813. doi: 10.1080/01635581.2011.573900. [DOI] [PubMed] [Google Scholar]
- ANSM. 2010. French Pharmacopoeia, Preparations-Homeopathiques, Mistletoe from the Apple Tree. Eleventh ed., France.
- Antony S., Kuttan R., Kuttan G. Effect of Viscum album in the inhibition of lung metastasis in mice induced by B16F10 melanoma cells. J. Exp. Clin. Cancer Res. 1997;16:159–162. [PubMed] [Google Scholar]
- ANVISA, 2011. Brazilian Homeopathic Pharmacopoeia. third ed., Brazil.
- Barbosa G.M., dos Santos E.G., Capella F.N.C., Homsani F., de Pointis Marçal C., Do Santos Valle R., de Araújo Abi-Chacra E., Braga-Silv L.A., de Oliveira Sales M.H., da Silva Neto I.D., Veiga V.F., dosSantos A.L.S., Holandino C. Direct electric current modifies important cellular aspects and ultrastructure features of Candida albicans yeasts: Influence of doses and polarities. Bioelectromagnetics. 2017;38:95–108. doi: 10.1002/bem.22015. [DOI] [PubMed] [Google Scholar]
- Benković E.T., Grohar T., Žigon D., Švajger U., Janeš D., Kreft S., Štrukelj B. Chemical composition of the silver fir (Abies alba) bark extract Abigenol® and its antioxidant activity. Ind. Crops Prod. 2014;52:23–28. [Google Scholar]
- Brandão H.N., David J.P., Couto R.D., Nascimento J.A.P., David J.M. Chemistry and pharmacology of antineoplasic chemoterapeutical derivatives from plants. Quim. Nova. 2010;33:1359–1369. [Google Scholar]
- Bussing A., Schietzel M. Apoptosis-inducing properties of Viscum album L. extracts from different host trees, correlate with their content of toxic mistletoe lectins. Anticancer Res. 1999;19:23–28. [PubMed] [Google Scholar]
- Cebović T., Spasić S., Popović M. Cytotoxic effects of the Viscum album L. Extract on Ehrlich tumour cells in vivo. Phytother. Res. 2008;22:1097–1103. doi: 10.1002/ptr.2464. [DOI] [PubMed] [Google Scholar]
- Delebinski C.I., Twardziok M., Kleinsimon S., Hoff F., Mulsow K., Rolff J., Jäger S., Eggert A., Seifert G. A natural combination extract of Viscum album L. containing both triterpene acids and lectins is highly effective against AML in vivo. PLoS One. 2015;10:e0133892. doi: 10.1371/journal.pone.0133892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drira R., Sakamoto K. Sakuranetin Induces Melanogenesis in B16BL6 Melanoma Cells through Inhibition of ERK and PI3K/AKT Signaling pathways. Phyther. Res. 2016;30:997–1002. doi: 10.1002/ptr.5606. [DOI] [PubMed] [Google Scholar]
- Estko M., Baumgartner S., Urech K., Kunz M., Regueiro U., Heusser P., Weissenstein U. Tumour cell derived effects on monocyte/macrophage polarization and function and modulatory potential of Viscum album lipophilic extract in vitro. BMC Complement. Alternat. Med. 2015;15:130. doi: 10.1186/s12906-015-0650-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facina A.S., Facina G., Silva I.D.C.G., Gonçalves G.A., Almeida F.A., Noronha S.M.R., Nakamura M.U. Viscum album modulates apoptotic related genes in melanoma tumor of mice. Am. J. Mol. Biol. Biol. 2014;4:49–58. [Google Scholar]
- Ferracane R., Graziani G., Gallo M., Fogliano V., Ritieni A. Metabolic profile of the bioactive compounds of burdock (Arctium lappa) seeds, roots and leaves. J. Pharm. Biomed. Anal. 2010;51:399–404. doi: 10.1016/j.jpba.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Gardin N.E. Immunological response to mistletoe (Viscum album L.) in cancer patients: a four-case series. Phytother Res. 2009;23:407–411. doi: 10.1002/ptr.2643. [DOI] [PubMed] [Google Scholar]
- Hammami S., Ben Jannet H., Bergaoui A., Ciavatta L., Cimino G., Mighri Z. Isolation and structure elucidation of a flavanone, a flavanone glycoside and vomifoliol from Echiochilon Fruticosum growing in Tunisia. Molecules. 2004;9:602–608. doi: 10.3390/90700602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S.Y., Hong C.E., Kim H.G., Lyu S.Y. Anti-cancer effects of enteric-coated polymers containing mistletoe lectin in murine melanoma cells in vitro and in vivo. Mol. Cell Biochem. 2015;408(1–2):73–87. doi: 10.1007/s11010-015-2484-1. [DOI] [PubMed] [Google Scholar]
- Heinzerling L., von Baehr V., Liebenthal C., von Baehr R., Volk H.D. Immunologic effector mechanisms of a standardized mistletoe extract on the function of human monocytes and lymphocytes in vitro, ex vivo, and in vivo. J. Clin. Immunol. 2006;26:347–359. doi: 10.1007/s10875-006-9023-5. [DOI] [PubMed] [Google Scholar]
- Jiang Y., Zhang L., Rupasinghe H.P.V. Antiproliferative effects of extracts from Salvia officinalis L. and Saliva miltiorrhiza Bunge on hepatocellular carcinoma cells. Biomed. Pharmacother. 2017;85:57–67. doi: 10.1016/j.biopha.2016.11.113. [DOI] [PubMed] [Google Scholar]
- Jin U.H., Lee J.Y., Kang S.K., Kim J.K., Park W.H., Kim J.G., Moon S.K., Kim C.H. A phenolic compound, 5-caffeoylquinic acid (chlorogenic acid), is a new type and strong matrix metalloproteinase-9 inhibitor: isolation and identification from methanol extract of Euonymus alatus. Life Sci. 2005;77:2760–2769. doi: 10.1016/j.lfs.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Jones V., Katiyar S.K. Emerging phytochemicals for prevention of melanoma invasion. Cancer Lett. 2013;335:251–258. doi: 10.1016/j.canlet.2013.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M., Yoo Y., Lee K., Kim J., Song K. Isolation of epi-oleanolic acid from Korean mistletoe and its apoptosis-lnducing activity in tumor cells. Arch. Pharm. Res. 2004;27:840–844. doi: 10.1007/BF02980176. [DOI] [PubMed] [Google Scholar]
- Jurin M., Zarković N., Hrzenjak M., Ilić Z. Antitumorous and immunomodulatory effects of the Viscum album L. Oncology. 1993;50:393–398. doi: 10.1159/000227217. [DOI] [PubMed] [Google Scholar]
- Karimian H., Arya A., Fadaeinasab M., Razavi M., Khan A.K., Ali H.M., Abdulla M.A., Noordin M.I. Kelussia odoratissima Mozaff. activates intrinsic pathway of apoptosis in breast cancer cells associated with S phase cell cycle arrest via involvement of p21/p27 in vitro and in vivo. Drug Des. Devel. Ther. 2017;11:337–350. doi: 10.2147/DDDT.S121518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwaja T.A., Varven J.C., Pentecos S., Pande H. Isolation of biologically active alkaloids from Korean mistletoe Viscum album coloratum. Experientia. 1980;36:599–600. doi: 10.1007/BF01965825. [DOI] [PubMed] [Google Scholar]
- Kim S., Lee D., Kim J.K., Kim J.H., Park J.H., Lee J.W., Kwon J. Viscothionin isolated from Korean mistletoe improves nonalcoholic fatty liver disease via the activation of adenosine monophosphate-activated protein kinase. J. Agric. Food Chem. 2014;62:11876–11883. doi: 10.1021/jf503535s. [DOI] [PubMed] [Google Scholar]
- Kim S.Y., Yang E.J., Son Y.K., Yeo J.H., Song K.S. Enhanced anti-oxidative effect of fermented Korean mistletoe is originated from an increase in the contents of caffeic acid and lyoniresinol. Food Funct. 2016;7:2270–2277. doi: 10.1039/c6fo00138f. [DOI] [PubMed] [Google Scholar]
- Klingbeil M.F.G., Xavier F.C.A., Sardinha L.R., Severino P., Mathor M.B., Rodrigues R.V., Pinto D.S. Cytotoxic effects of mistletoe (Viscum album L.) in head and neck squamous cell carcinoma cell lines. Oncol. Rep. 2013;30:2316–2322. doi: 10.3892/or.2013.2732. [DOI] [PubMed] [Google Scholar]
- Luczkiewicz M., Cisowski W., Kaiser P., Ochocka R., Piotrowski A. Comparative analysis of phenolic acids in mistletoe plants from various hosts. Acta Pol. Pharm. Drug. Res. 2001;58:373–379. [PubMed] [Google Scholar]
- Kuttan G., Menon L.G., Antony S., Kuttan R. Anticarcinogenic and antimetastatic activity of Iscador. Anticancer Drugs. 1997;8(Suppl 1):S15–S16. doi: 10.1097/00001813-199704001-00004. [DOI] [PubMed] [Google Scholar]
- Maalej A., Bouallagui Z., Hadrich F., Isoda H., Sayadi S. Assessment of Olea europaea L. fruit extracts: Phytochemical characterization and anticancer pathway investigation. Biomed. Pharmacother. 2017;90:179–186. doi: 10.1016/j.biopha.2017.03.034. [DOI] [PubMed] [Google Scholar]
- Mabry T., Markham K.R., Thomas M.B. first ed. Springer-Verlag; New York: 1970. The Systematic Identification of Flavonoids. [Google Scholar]
- Meira D.D., Marinho-Carvalho M.M., Teixeira C.A., Veiga V.F., Da Poian A.T., Holandino C., De Freitas M.S., Sola-Penna M. Clotrimazole decreases human breast cancer cells viability through alterations in cytoskeleton-associated glycolytic enzymes. Mol. Genet. Metab. 2005;84:354–362. doi: 10.1016/j.ymgme.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Mocan A., Schafberg M., Crisan G., Rohn S. Determination of lignans and phenolic components of Schisandra chinensis (Turcz.) Baill. using HPLC-ESI-ToF-MS and HPLC-online TEAC: Contribution of individual components to overall antioxidant activity and comparison with traditional antioxidant assays. J. Funct. Foods. 2016;24:579–594. [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nazaruk J., Orlikowski P. Phytochemical profile and therapeutic potential of Viscum album L. Nat. Prod. Res. 2016;30:373–385. doi: 10.1080/14786419.2015.1022776. [DOI] [PubMed] [Google Scholar]
- Newman D.J., Cragg G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Nhiem N.X., Lee H.Y., Kim N.Y., Park S.J., Kim E.S., Han J.E., Yang H., Kim S.H. Stereochemical assignment of five new lignan glycosides from Viscum album by NMR study combined with CD spectroscopy. Magn. Reson. Chem. 2012;50:772–777. doi: 10.1002/mrc.3875. [DOI] [PubMed] [Google Scholar]
- Panossian A., Kocharian A., Matinian K., Amroyan E., Gabrielian E., Mayr C., Wagner H. Pharmacological activity of phenylpropanoids of the mistletoe, Viscum album L., host: Pyrus caucasica Fed. Phytomedicine. 1998;5:11–17. doi: 10.1016/S0944-7113(98)80053-6. [DOI] [PubMed] [Google Scholar]
- Park W.B., Lyu S.Y., Kim J.H., Choi S.H., Chung H.K., Ahn S.H., Hong S.Y., Yoon T.J., Choi M.J. Inhibition of tumor growth and metastasis by Korean mistletoe lectin is associated with apoptosis and antiangiogenesis. Cancer Biother. Radiopharm. 2001;16(5):439–447. doi: 10.1089/108497801753354348. [DOI] [PubMed] [Google Scholar]
- Pfüller U. Chemical constituents of european mistletoe (Viscum album L.) In: Büssing A., editor. Mistletoe: The genus Viscum. Harwood Academic Publishers; Amsterdam: 2000. pp. 101–122. [Google Scholar]
- Popova O.I. Phenolic compounds of Viscum album. Chem. Nat. Comp. 1991;1:123-123. [Google Scholar]
- Portet, B., Fabre, N., Rozenberg, R., Habib-Jiwan, J.L., Moulis, C., Quetin-Leclercq, J., 2008. Analysis of minor flavonoids in Piper hostmannianum var. berbicense using liquid chromatography coupled with atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. A., vol. 1210, pp. 45–54. [DOI] [PubMed]
- Sárpataki O., Páll E., Sevastre-Berghian A.C., Stan R.L., Hanganu D., Benedec D., Hangan A.C., Sevastre B., Marcus I. Antiproliferative effect of Viscum album alcoholic extract in vitro. Bull. UASVM Vet. Med. 2015;72:170–173. [Google Scholar]
- Seifert G., Jesse P., Laengler A., Reindl T., Lüth M., Lobtz S., Henze G., Prokop A., Lode H.N. Molecular mechanisms of mistletoe plant extract-induced apoptosis in acute lymphoblastic leukemia in vivo and in vitro. Cancer Lett. 2008;264:218–228. doi: 10.1016/j.canlet.2008.01.036. [DOI] [PubMed] [Google Scholar]
- Singh B.N., Saha C., Galun D., Upreti D.K., Bayry J., Kaveri S.V. European Viscum album: a potent phytotherapeutic agent with multifarious phytochemicals, pharmacological properties and clinical evidence. R. Soc. Chem. 2016;6:23837–23857. [Google Scholar]
- Spagnol C.M., Oliveira T.S., Isaac V.L.B., Corrêa M.A., Salgado H.R.N. Validation of caffeic acid in emulsion by UV-spectrophotometric method. Phys. Chem. 2015;5:16–22. [Google Scholar]
- Stan R.L., Hangan A.C., Dican L., Sevastre B., Hanganu D., Catoi C., Sárpataki O., Ionescu C.M. Comparative study concerning mistletoe viscotoxins antitumor activity. Acta. Biol. Hung. 2013;64:279–288. doi: 10.1556/ABiol.64.2013.3.2. [DOI] [PubMed] [Google Scholar]
- Stein G.M., Bussing A., Schietzel M. Stimulation of the maturation of dendritic cells in vitro by a fermented mistletoe extract. Anticancer Res. 2002;22:4215–4219. [PubMed] [Google Scholar]
- Strüh C.M., Jäger S., Kersten A., Schempp C.M., Scheffler A., Martin S.F. Triterpenoids amplify anti-tumoral effects of mistletoe extracts on Murine B16.F10 Melanoma In Vivo. PLoS One. 2013;8:1–11. doi: 10.1371/journal.pone.0062168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Liu J., Zhang A., Zhang Y., Meng X., Han Y., Zhang Y., Wang X. Characterization of the multiple components of Acanthopanax Senticosus stem by ultra high performance liquid chromatography with quadrupole time-of-flight tandem mass spectrometry. J. Sep. Sci. 2016;39:496–502. doi: 10.1002/jssc.201500915. [DOI] [PubMed] [Google Scholar]
- Thao N.P., Luyen B.T.T., Diep C.N., Tai B.H., Kim E.J., Kang H.K., Lee S.H., Jang H.D., Cuong N.T., Van Thanh N., Cuong N.X., Nam N.H., Van Minh C., Kim Y.H. In vitro evaluation of the antioxidant and cytotoxic activities of constituents of the mangrove Lumnitzera racemosa Willd. Arch. Pharm. Res. 2015;38:446–455. doi: 10.1007/s12272-014-0429-y. [DOI] [PubMed] [Google Scholar]
- Timoshenko A.V., Cherenkevich S.N., Gabius H.J. Viscum album agglutinin-I-induced aggregation of blood cells and the lectin effects on neutrophil function. Biomed. Pharmacother. 1995;49:153. doi: 10.1016/0753-3322(96)82609-6. [DOI] [PubMed] [Google Scholar]
- Tröger W., Galun D., Reif M., Schumann A., Stanković N., Milicévić M. Viscum album L. Extract therapy in patients with locally advanced or metastatic pancreatic cancer: a randomised clinical trial on overall survival. Eur. J. Cancer. 2013;49:3788–3797. doi: 10.1016/j.ejca.2013.06.043. [DOI] [PubMed] [Google Scholar]
- Urech K., Schaller G., Ziska P., Giannattasio M. Comparative study on the cytotoxic effect of viscotoxin and mistletoe lectin on tumor cells in culture. Phyther. Res. 1995;9:49–55. [Google Scholar]
- Urech K., Scher J.M., Hostanska K., Becker H. Apoptosis inducing activity of viscin, a lipophilic extract from Viscum album L. J. Pharm. Pharmacol. 2005;57:101–109. doi: 10.1211/0022357055083. [DOI] [PubMed] [Google Scholar]
- Urech K., Baumgartner S. Chemical Constituents of Viscum album L.: implications for the pharmaceutical preparation of mistletoe, mistletoe: from mythology to evidence-based medicine. Transl. Res. Biomed. 2015;4:11–23. [Google Scholar]
- Valentiner U., Pfueller U., Baum C., Schumacher U. The cytotoxic effect of mistletoe lectins I, II and III on sensitive and multidrug resistant human colon cancer cell lines in vitro. Toxicology. 2002;171:187–199. doi: 10.1016/s0300-483x(01)00581-9. [DOI] [PubMed] [Google Scholar]
- Veiga V.F., Nimrichter L., Teixeira C.A., Morales M.M., Alviano C.S., Rodrigues M.L., Holandino C. Exposure of human leukemic cells to direct electric current. Cell. Biochem. Biophys. 2005;42:61–74. doi: 10.1385/CBB:42:1:061. [DOI] [PubMed] [Google Scholar]
- Wagner H., Bladt S. Drug containing lignans. In: Wagner H., Bladt S., editors. Plant Drug Analysis: A Thin Layer Cromatography Atlas, New York. 2001. pp. 263–273. [Google Scholar]
- Weissenstein U., Kunz M., Urech K., Baumgartner S. Interaction of standardizedmistletoe (Viscum album) extracts with chemotherapeutic drugs regarding cytostatic and cytotoxic effects in vitro. BMC Complement. Altern. Med. 2014;8:14–16. doi: 10.1186/1472-6882-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng C.J., Yen G.C. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: phenolic acids, monophenol, polyphenol, and their derivatives. Cancer. Treat. Rev. 2012;38:76–87. doi: 10.1016/j.ctrv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization) Cancer. http://www.who.int/cancer/en/2017 Accessed 17.05.17.
- Yagasaki K., Miura Y., Okauchi R., Furuse T. Inhibitory effects of chlorogenic acid and its related compounds on the invasion of hepatoma cells in culture. Cytotechnology. 2000;33:229–235. doi: 10.1023/A:1008141918852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon T.J., Yoo Y.C., Kang T.B., Her E., Kim S.H., Kim K., Azuma I., Kim J.B. Cellular and humoral adjuvant activity of lectins isolated from Korean mistletoe (Viscum album colaratum) Int. Immunopharmacol. 2001;1:881–889. doi: 10.1016/s1567-5769(01)00024-8. [DOI] [PubMed] [Google Scholar]
- Zainal Ariffin S.H., Wan Omar W.H.H., Zainal Ariffin Z., Safian M.F., Senafi S., Megat Abdul Wahab R. Intrinsic anticarcinogenic effects of Piper sarmentosum ethanolic extract on a human hepatoma cell line. Cancer Cell. Int. 2009;9 doi: 10.1186/1475-2867-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarković N., Kalisnik T., Loncarić I., Borović S., Mang S., Kissel D., Konitzer M., Jurin M., Grainza S. Comparison of the effects of Viscum album lectin ML-1 and fresh plant extract (Isorel) on the cell growth in vitro and tumorigenicity of melanoma B16F10. Cancer Biother. Radiopharm. 1998;13(2):121–131. doi: 10.1089/cbr.1998.13.121. [DOI] [PubMed] [Google Scholar]
- Zhang J.Y., Wang Z.J., Li Y., Liu Y., Cai W., Li C., Lu J.Q., Qiao Y.J. A strategy for comprehensive identification of sequential constituents using ultra-high-performance liquid chromatography coupled with linear ion trap-Orbitrap mass spectrometer, application study on chlorogenic acids in Flos Lonicerae Japonicae. Talanta. 2016;147:16–27. doi: 10.1016/j.talanta.2015.09.039. [DOI] [PubMed] [Google Scholar]
- Zhu J.X., Ren J., Qin J.J., Cheng X.R., Zeng Q., Zhang F., Yan S.K., Jin H.Z., Zhang W.D. Phenylpropanoids and lignanoids from Euonymus acanthocarpus. Arch. Pharm. Res. 2012;35:1739–1747. doi: 10.1007/s12272-012-1005-y. [DOI] [PubMed] [Google Scholar]