Abstract
Purpose/Objectives
To evaluate the role of external beam radiation therapy (EBRT) for treatment of malignant paraganglioma (PGL) and pheochromocytoma (PCC).
Methods and materials
A retrospective review was performed of all patients with malignant PGL/PCC treated with EBRT at our institution between 1973 and 2015. Local control (LC) per treated lesion and overall survival were estimated using the Kaplan-Meier method. Toxicities were scored using the Common Toxicity Criteria for Adverse Events (AE), version 4.
Results
The cohort included 41 patients with 107 sites treated. Median (range) age at EBRT was 33 (11-80) years. Treatment intention was curative in 20 patients (30 lesions) and palliative in 21 patients (77 lesions). The primary tumor was PGL (63%) and PCC (37%). Previous local therapies were surgical resection (90%) and percutaneous ablation (19%). Indications for EBRT were local control (66%), pain (22%), or spinal cord compression (12%). Treatment site included bone (69%), soft tissue (30%), and liver (1%). Median (range) EBRT dose was 40 (6.5-70) Gy. Median biologic effective dose using α/β = 10 (BED10) was 53 (9-132). Median follow-up was 3.8 years (0.04-41.5), and mean follow-up was 9.7 years. Overall survival at 5 years was 65%: 79% for curative- and 50% for palliative-intention patients (P = .028). LC at 5 years was 81% for all lesions; 91% for lesions receiving BED10 ≥53, and 62% for lesions receiving BED10 <53 (P = .001). All 11 lesions treated with stereotactic body RT or radiosurgery had LC at a median of 3.0 (0.2-5.4) years. For the symptomatic lesions, symptoms improved in 94%. There were no acute grade ≥3 treatment-related AEs, including no hypertensive crises. Two patients developed a late grade ≥3 AE.
Conclusions
EBRT is a useful treatment modality for malignant PGL and PCC. Higher RT dose was associated with improved LC.
Summary.
We retrospectively examined the outcomes of 41 patients with malignant paraganglioma or pheochromocytoma who received radiation therapy at our institution. Radiographic local control was 81% at 5 years; 94% of patients with symptomatic lesions had improvement. There were no acute Common Toxicity Criteria Assessment of Adverse Events, version 4, toxicities of grade 3 or higher; 2 (5%) grade 3 late complications occurred. No patients received extra alpha- or beta-adrenergic blockade before radiation therapy, and none experienced hypertensive crisis in response to therapy.
Alt-text: Unlabelled box
Introduction
Paraganglioma (PGL) and pheochromocytoma (PCC) are rare neuroendocrine tumors arising from chromaffin cells in the adrenal medulla (PCC) or in extra-adrenal sites (PGL). Most PGL and PCC are benign and remain localized; however, a small fraction of patients develop distant metastases, which defines malignant PGL/PCC.1 Many patients with malignant PGL/PCC experience disease progression at a relatively slow rate.
A variety of management strategies have been used for metastatic PGL/PCC, although cure is rare. For asymptomatic patients with nonimminently threatening disease, observation may be a reasonable option. For symptomatic patients, local or systemic therapies may be used to control tumor burden and improve quality of life. According to the 2017 National Comprehensive Cancer Network guidelines, management options for malignant PGL/PCC include cytoreductive surgery (when feasible), systemic chemotherapy (often cyclophosphamide, vincristine, and dacarbazine or temozolomide), or I-131 metaiodobenzylguanidine (MIBG).2 Percutaneous ablation has also been used as a minimally invasive local treatment option.3
Small retrospective series have suggested that external beam radiation therapy (EBRT) may be a useful local treatment modality in some patients with advanced/unresectable malignant PGL/PCC4, 5, 6, 7, 8, 9; however, because of the rarity of malignant PGL/PCC, the role of EBRT is incompletely defined. The purpose of the present study was to review outcomes of patients with PGL/PCC treated at our institution with EBRT. We hypothesized that EBRT would be associated with a high rate of local tumor control and symptom reduction and a low rate of treatment-related toxicity.
Methods and materials
Our institutional review board approved the conduct of this retrospective study.
Patient selection
Patients with PGL/PCC were identified from an institutional endocrinology database and an institutional radiation oncology database. Medical records were reviewed for all patients who received RT for PGL/PCC at our institution between January 1973 and September 2015. Patients with malignant PGL and PCC treated with EBRT were included. Patients with malignant PGL of the head and neck region with regional lymph node metastases were included (n = 4). Exclusion criteria were histology other than PGL/PCC, nonmetastatic (benign) disease, or inadequate follow-up or documentation.
Patients were divided into “curative” and “palliative” intention cohorts. The curative intention cohort consisted of patients being treated at all known sites of macroscopic disease. All others were considered palliative.
Patient evaluation and treatment
Because this was a retrospective study, all patients had been treated per attending physician discretion. Evaluation and treatment approaches for malignant PGL/PCC at our institution have evolved over time but generally are consistent with those included in contemporary National Comprehensive Cancer Network guidelines. Clinical evaluation included 24-hour urine and serum catecholamines and metanephrines. Imaging included computed tomography, magnetic resonance imaging, bone scan, 123I-MIBG scintigraphy, octreotide scintigraphy, and/or 18F-fluorodeoxyglucose positron emission tomography. Resection was performed of primary and metastatic tumors when anticipated morbidity was low.10 Alpha-adrenergic blockade was typically performed preoperatively before attempted resection. Percutaneous ablation techniques using ethanol, radiofrequency ablation, or cryoablation have recently been used for small, localized tumors amenable to such treatment.3 For patients with multifocal or progressive metastatic disease not amenable to local therapies, systemic therapies including cytotoxic chemotherapy, 131I-metaiodobenzylguanidine, or bone-directed therapies have been used.
EBRT has been used for patients with localized disease not amenable to other local therapies (surgical resection or percutaneous ablation) or for patients with widespread disease with symptoms from local tumor burden. From 1973 through approximately 1985, most patients were treated with 2-dimensional conformal megavoltage photon or electron RT techniques (including intraoperative electron RT). More recently, 3-dimensional conformal, intensity modulated RT, and stereotactic body RT (SBRT) megavoltage photon techniques have been used. Stereotactic radiosurgery (SRS) was used for brain metastases.
Following EBRT, patients were typically reevaluated for response and adverse events 1 to 3 months after RT with clinical examination and/or imaging, as appropriate. Subsequent follow-up and additional therapies undertaken were as deemed appropriate by involved expert providers.
The biologic effective dose (BED) was calculated for the delivered EBRT regimen, using the equation BED = nd [1 + d/α/β] with n = number of fraction, d = dose per fraction, and α/β = 10 or 3. Accordingly, this is denoted as BED10 and BED3.
Outcomes assessment
Acute (within 90 days from completion of RT) and chronic (>90 days after completion of RT) adverse events (AE) related to RT were assessed using National Cancer Institute Common Toxicity Criteria Assessment of Adverse Events, version 4.03. Local control (LC) was defined as lack of radiographic anatomical progression of the treated lesion. All other sites of progression were considered distant progression. LC included lack of local progression diagnosed before, concurrent with, or after diagnosis of distant progression.
Statistical analysis
Overall survival (OS) and LC were estimated from the first day of radiation therapy using the Kaplan-Meier method. Distant progression-free survival (dPFS) was also calculated from first day of radiation therapy for patients in the curative cohort using the Kaplan-Meier method. Univariable analyses were performed to assess for association between patient (age, sex, PGL vs PCC, tumor site, tumor size) or treatment variables (RT dose) and outcomes using Cox proportional hazards regression models. We assessed for association between RT dose and LC as a nominal variable above/below the group median and as a continuous variable, using both BED10 and BED3. Multivariable analyses were not performed because of the small sample size. Statistical significance was defined as a P value < .05. Statistical analyses were performed with JMP software (SAS Institute Inc, Cary, NC).
Results
Patient and treatment characteristics
Patient and treatment characteristics are detailed in Table 1, Table 2. Forty-one patients treated at 107 total lesions/sites were included in this series. Treatment intention was curative in 20 patients (30 lesions treated) and palliative in 21 patients (77 lesions treated). Previous local therapies were surgical resection (90%) and/or percutaneous ablation (19%), either radiofrequency or ethanol. Median BED10 was 53 (range, 9-132). Median BED3 was 75.7 (range, 13.5-300). For lesions treated with SBRT/SRS, the median dose was 24 Gy (range, 18-60) and the median number of fractions was 1 (range, 1-5).
Table 1.
Patient characteristics
| Characteristic | No. patients (%) |
|---|---|
| Age at diagnosis, median (y), range | 33 (11-80) |
| Primary tumor | |
| Paraganglioma | 26 (63) |
| Pheochromocytoma | 15 (37) |
| Sex | |
| Female | 24 (58) |
| Male | 17 (42) |
| Disease extent at initial presentation | |
| Localized | 23 (56) |
| Metastatic | 18 (44) |
| Germline mutation | |
| None | 28 (68) |
| SDHB | 12 (29) |
| SDHD | 1 (2) |
| Prior local therapiesa | |
| Surgical resection | 37 (90) |
| Radiofrequency ablation | 6 (15) |
| Cryoablation | 1 (2) |
| Ethanol ablation | 1 (2) |
| Prior medical therapiesa | |
| CVD | 8 (20) |
| Octreotide | 5 (12) |
| Zoledronic acid | 4 (10) |
| Denosumab | 4 (10) |
| 131I-MIBG | 3 (7) |
| Pazopanib | 3 (7) |
| Other | 5 (12) |
| Primary indication for EBRT | |
| Local control | 27 (66) |
| Pain | 9 (22) |
| Spinal cord compression | 5 (12) |
CVD, cyclophosphamide, vincristine, dacarbazine; EBRT, external beam radiation therapy; 131I-MIBG, 131I-metaiodobenzylguanidine.
Some patients received multiple prior treatments.
Table 2.
Treatment characteristics
| Characteristic | No. lesions (%) |
|---|---|
| Number of lesions treated | 107 |
| Lesion location | |
| Bone | 74 (69) |
| Soft tissue | 32 (30) |
| Liver | 1 (1) |
| RT technique | |
| 2D/3D RT | 77 (72) |
| IMRT | 18 (17) |
| SBRT | 10 (9) |
| Intraoperative RTa | 3 (3) |
| Stereotactic radiosurgery | 1 (1) |
| RT dose, median (Gy), range | 40 (6.5-70) |
2D, 2-dimensional; 3D, 3-dimensional; IMRT, intensity modulated radiation therapy; RT, radiation therapy; SBRT, stereotactic body radiation therapy.
2 lesions were treated with both external beam and intraoperative RT.
Outcomes
For all patients, the median follow-up was 3.8 years (range, 0.04-42), and the mean follow-up was 9.7 years. For patients alive at last follow-up, the median follow-up was 6.0 years. Median survival after RT was 11.4 years. OS at 5 years was 65% for all patients (Figure 1); 79% for curative and 50% for palliative patients (P = .028). OS was not significantly different for patients with PGL versus PCC (P = .11, 5 year OS 57% vs 89%). For the curative intention patients, median dPFS was 2.9 years (range, 0.2-20) and the dPFS at 5 years was 30%.
Figure 1.
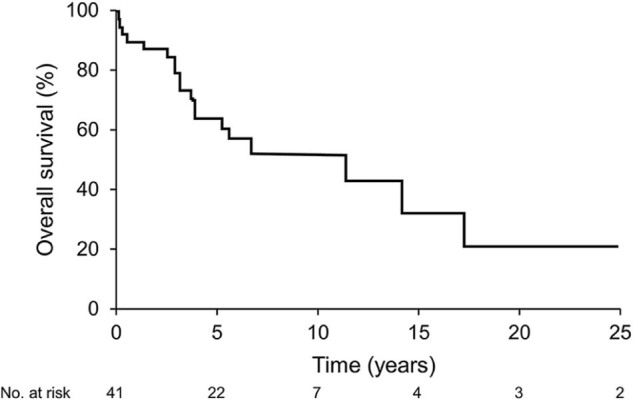
Overall survival.
LC at 5 years was 81% for all lesions. Local recurrence events occurred at a median of 2.9 years (range, 0.3-4.6) after RT. For patients experiencing local recurrence, the median BED10 was 42.5 (range, 20-60) and the median BED3 was 60.1 (range, 43.3-90). Higher RT dose using BED10 or BED3 was associated with a lower risk of tumor recurrence (BED10: risk ratio = 0.94; 95% confidence interval, 0.89-0.98; P = .004; BED3: risk ratio = 0.96, 95% confidence interval, 0.92-0.99, P = .015). LC at 5 years was 91% for lesions receiving BED10 ≥53 and 62% for lesions receiving BED10 <53 (Figure 2, P = .001). All 11 lesions treated with SBRT/SRS had LC at a median of 3.0 (range, 0.2-5.4) years. Primary tumor site (PGL vs PCC), tumor size, and location were not associated with LC.
Figure 2.
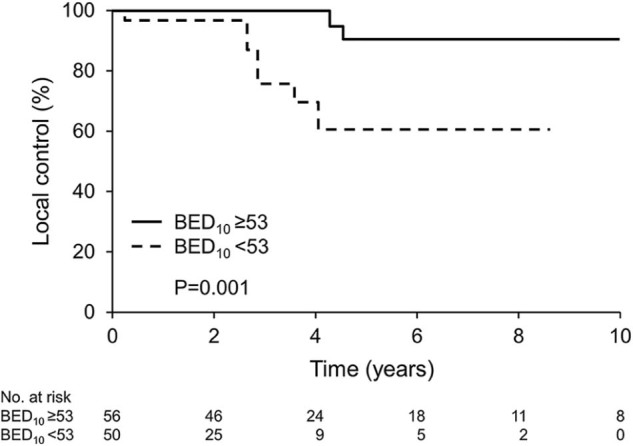
Local tumor control. BED10, biologic effective dose using α/β = 10.
For the symptomatic lesions, symptoms improved in 94%. Seven patients in the curative intention cohort had elevated blood/urine catecholamines/metanephrines before RT to all known sites of disease. Of these 7 patients, 5 patients had biochemistry repeated at first follow-up after RT; all 5 had a decline in the value of catecholamine/metanephrine levels.
AEs associated with radiation therapy
There were no acute grade ≥3 treatment-related AEs. No patient received escalated preradiation alpha-adrenergic blockade, and none experienced hypertensive crisis. Two patients developed a late grade ≥3 AE thought to be related to RT. One patient developed grade 3 premature menopause 2 months after RT. One patient who received SBRT (24 Gy in 1 fraction) for a large, painful pelvic metastasis developed grade 3 neuropathy of the sciatic nerve 20 months after RT.
Discussion
From this series of patients with malignant PGL/PCC treated with EBRT, several observations can be made. EBRT was efficacious in controlling disease locally, with most patients experiencing radiographic local tumor control and/or improvement of tumor-related symptoms. EBRT was well tolerated, with few severe treatment-related AEs, including no cases of hypertensive crises. Additionally, we observed a higher rate of local control in patients receiving higher (vs lower) biologic effective radiation doses.
Our series adds to the limited literature reporting on efficacy of EBRT for malignant PGL/PCC (Table 3). The largest published report to date from Vogel et al at the National Institutes of Health reported similarly high rates of treated-lesion radiographic control (87%) and symptomatic control (81%) in 24 patients (47 lesions) with PGL/PCC treated with EBRT.6 Additional smaller series also report efficacy of EBRT for malignant PGL/PCC.4, 5, 7, 8, 9 Similarly, a recent meta-analysis demonstrated high rates of local tumor control with EBRT for localized (nonmetastatic) PGL.11 Despite favorable disease response/control of the treated lesion, a majority of patients in our series and others experienced distant progression outside of the irradiated field. This is anticipated, considering the systemic nature of malignant PGL/PCC.
Table 3.
Select published series of external beam radiation therapy for malignant paraganglioma/pheochromocytoma
| Study | Year | No. patients | No. lesions treated | Survival | Radiographic local control | Symptom control/improvement |
|---|---|---|---|---|---|---|
| Current study | 2017 | 41 | 107 | 65% at 5 y | 81% at 5 y | 94% |
| Vogel et al6 | 2014 | 24 | 47 | 88% alive at median 52 mo | 87% | 81% |
| Fishbein et al4 | 2012 | 17 | NR | 60% alive at median 23 mo | 76% at 1 y | 94% |
NR, not reported.
Our observation of higher LC in patients receiving higher EBRT dose supports the concept of a dose-response relationship in treatment of malignant PGL/PCC with EBRT, consistent with data reported by Vogel et al.6 This suggests that, for patients with malignant PGL/PCC and limited disease burden and/or anticipated long-term survival, higher RT doses should be considered, with the goal of achieving durable local disease control and/or symptom palliation. In our series, all 11 lesions treated with SBRT/SRS experienced local control with a median follow-up of 3 years after treatment. Similarly, Vogel et al reported that all 4 lesions treated with SBRT/SRS in their series were locally controlled.6 Further data are needed to evaluate the safety and efficacy of SBRT/SRS for small, localized malignant PGL/PCC lesions, although this seems to be an attractive local treatment option because of the high rate of efficacy and for patient convenience (treatment is delivered in only 1 to 5 fractions).
Notably, no patient in our series experienced acute hypertensive crisis after EBRT. This is congruent with most other published series of EBRT for PGL/PCC,4, 6 although acute hypertensive crisis has been reported after EBRT.12 This is in contrast to other local therapies, such as surgical resection and percutaneous ablation, in which physical manipulation/destruction of the tumor may cause more immediate release catecholamines and metanephrines and resultant vasomotor instability. Resection and ablation typically produce more rapid tumor cytoreduction and palliation compared with EBRT; thus, they remain the preferred treatment options when feasible and are safe in patients with limited disease, especially for patients with secretory tumors.
There are several limitations of our study. First, because it is retrospective in nature, there are potential biases related to the receipt of EBRT and the dose delivered. Also, AEs can be difficult to accurately assess retrospectively. Furthermore, the cohort spans more than 4 decades; accordingly, the patients received heterogeneous treatment planning and delivery. Moreover, metanephrine/catecholamine assessment was not undertaken consistently before and after EBRT for many patients, limiting assessment of biochemical response to only a subset of patients. Given the rarity of malignant PGL/PCC, however, it is unlikely that prospective studies of EBRT will ever be performed; therefore, data from retrospective series are important, in spite of these inherent limitations.
In conclusion, EBRT is a useful local treatment modality for select patients with advanced/unresectable malignant PGL/PCC. For patients with limited/localized disease, cytoreductive surgery or percutaneous ablation are standard options; however, EBRT should be considered for patients with localized disease not amenable to other local therapies (surgical resection or percutaneous ablation) or for patients with widespread disease with symptoms from local tumor burden.
Footnotes
Conflicts of interest: None.
References
- 1.Goldstein R.E., O'Neill J.A., Jr, Holcomb G.W., 3rd Clinical experience over 48 years with pheochromocytoma. Ann Surg. 1999;229:755–766. doi: 10.1097/00000658-199906000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neuroendocrine tumors. 2017. http://www.NCCN.org NCCN Guidelines. 1.2017 ed.
- 3.McBride J.F., Atwell T.D., Charboneau W.J. Minimally invasive treatment of metastatic pheochromocytoma and paraganglioma: Efficacy and safety of radiofrequency ablation and cryoablation therapy. J Vasc Interv Radiol. 2011;22:1263–1270. doi: 10.1016/j.jvir.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 4.Fishbein L., Bonner L., Torigian D.A. External beam radiation therapy (EBRT) for patients with malignant pheochromocytoma and non-head and -neck paraganglioma: Combination with 131i-mibg. Horm Metab Res. 2012;44:405–410. doi: 10.1055/s-0032-1308992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pham T.H., Moir C., Thompson G.B. Pheochromocytoma and paraganglioma in children: A review of medical and surgical management at a tertiary care center. Pediatrics. 2006;118:1109–1117. doi: 10.1542/peds.2005-2299. [DOI] [PubMed] [Google Scholar]
- 6.Vogel J., Atanacio A.S., Prodanov T. External beam radiation therapy in treatment of malignant pheochromocytoma and paraganglioma. Front Oncol. 2014;4:166. doi: 10.3389/fonc.2014.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshida S., Nakagomi K., Goto S. Malignant pheochromocytoma of the urinary bladder: Effectiveness of radiotherapy in conjunction with chemotherapy. Int J Urol. 2004;11:175–177. doi: 10.1111/j.1442-2042.2003.00758.x. [DOI] [PubMed] [Google Scholar]
- 8.Gilbo P., Tariq A., Morris C.G. External-beam radiation therapy for malignant paraganglioma of the head and neck. Am J Otolaryngol. 2015;36:692–696. doi: 10.1016/j.amjoto.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Lee J.H., Barich F., Karnell L.H. National cancer data base report on malignant paragangliomas of the head and neck. Cancer. 2002;94:730–737. doi: 10.1002/cncr.10252. [DOI] [PubMed] [Google Scholar]
- 10.Strajina V., Dy B.M., Farley D.R. Surgical treatment of malignant pheochromocytoma and paraganglioma: Retrospective case series. Ann Surg Oncol. 2017;24:1546–1550. doi: 10.1245/s10434-016-5739-5. [DOI] [PubMed] [Google Scholar]
- 11.van Hulsteijn L.T., Corssmit E.P., Coremans I.E. Regression and local control rates after radiotherapy for jugulotympanic paragangliomas: Systematic review and meta-analysis. Radiother Oncol. 2013;106:161–168. doi: 10.1016/j.radonc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Teno S., Tanabe A., Nomura K. Acutely exacerbated hypertension and increased inflammatory signs due to radiation treatment for metastatic pheochromocytoma. Endocr J. 1996;43:511–516. doi: 10.1507/endocrj.43.511. [DOI] [PubMed] [Google Scholar]


