Abstract
Purpose
The purpose of this study was to report toxicity and long-term survival outcomes of 2 prospective trials evaluating mitomycin C (MMC) with 5-fluorouracil–based adjuvant chemoradiation in resected periampullary adenocarcinoma.
Methods and materials
From 1996 to 2002, 119 patients received an adjuvant 4-drug chemotherapy regimen of 5-fluorouracil, leucovorin, MMC, and dipyridamole with chemoradiation on 2 consecutive trials (trials A and B). Trial A patients received upfront chemoradiation (50 Gy split-course, 2.5 Gy/fraction) followed by 4 cycles of the 4-drug chemotherapy with bolus 5-fluorouracil. Trial B patients received 1 cycle of the 4-drug chemotherapy with continuous infusion 5-fluorouracil followed by continuous chemoradiation (45-54 Gy, 1.8 Gy/fraction) and 2 additional cycles of chemotherapy. Cox proportional hazards models were performed to identify prognostic factors for overall survival (OS).
Results
Of the 62 trial A patients, 61% had pancreatic and 39% nonpancreatic periampullary carcinomas. Trial B (n = 57) consisted of 68% pancreatic and 32% nonpancreatic periampullary carcinomas. Resection margin and lymph node status were similar for both trials. Median follow-up was longer for trial A than trial B (197.5 vs 107.0 months), with median OS of 32.2 and 24.2 months, respectively. Rates of 3-, 5-, and 10-year OS were 48%, 31%, and 26% in trial A and 32%, 23%, and 9% in trial B. On multivariate analysis, lymph node–positive resection was the strongest prognostic factor for OS. A pancreatic primary and positive margin status were also associated with inferior survival (P < .05). Rates of grade ≥3 treatment-related toxicity in trials A and B were 2% and 7%, respectively.
Conclusions
This is the first study to report long-term outcomes of MMC with 5-fluorouracil–based adjuvant chemoradiation in periampullary cancers. Because MMC may be considered in DNA repair-deficient carcinomas, randomized trials are needed to determine the true benefit of adjuvant MMC.
Summary.
This is the first prospective study with long-term follow-up that evaluates mitomycin C with standard split-course or continuous 5-fluorouracil–based adjuvant chemoradiation in resected pancreatic or periampullary cancer. Incorporation of mitomycin C appears safe and effective in the adjuvant setting. In an era in which the resected periampullary tumor can be sequenced and analyzed for mutations in DNA repair pathways, it may be worthwhile to integrate mitomycin C into a standard regimen for the patients.
Alt-text: Unlabelled box
Introduction
Periampullary adenocarcinomas originate in one of 4 anatomical locations: the pancreatic head or uncinate process (pancreatic ductal adenocarcinoma [PDAC]), distal common bile duct, ampulla of Vater, or duodenum. Their incidence has been increasing and the associated prognosis is generally poor. Projected 5-year postresection survival rates range from 7% to 29% for patients with PDAC and 23% to 69% for patients with non-PDAC periampullary adenocarcinoma.1, 2, 3, 4, 5, 6, 7, 8
No standard adjuvant therapy regimen has been established for these patients, and management is typically extrapolated from PDAC paradigms as treatment strategies continue to evolve.4, 9 Historic studies used split-course radiation therapy,10, 11, 12 whereas modern studies incorporate continuous radiation therapy.13, 14, 15 Previously, Isacoff et al reported improved outcomes with 5-fluorouracil (5-FU), leucovorin (LV), mitomycin C (MMC), and dipyridamole (DPM) in patients with locally advanced PDAC.16, 17 Studies have suggested that MMC may be most effective in PDAC patients with certain mutations, in particular those who have a family history of PDAC and/or harbor mutations in a gene coding for DNA repair proteins (such as BRCA2 or PALB2).18, 19, 20, 21, 22, 23 With the current rise in next-generation sequencing and precision medicine, the impact of DNA-intercalating agents such as MMC on outcomes in patients with such dismal prognoses as periampullary cancer is brought into question.
In an effort to further investigate the efficacy of MMC integration with 5-FU–based chemoradiation therapy (CRT) for these malignancies, we enrolled patients with resected periampullary adenocarcinoma on a pair of prospective trials. In 1996 and 1999, respectively, we initiated 2 consecutive clinical trials incorporating adjuvant 5-FU, leucovorin, MMC, and DPM with CRT for patients with resected periampullary adenocarcinoma. Patients in trial A received upfront CRT with bolus 5-FU and split-course CRT as outlined previously,1 with timing similar to the Gastrointestinal Tumor Study Group trial, followed by 4 cycles of the same chemotherapy.11 The second trial (trial B) incorporated 1 cycle of the 4-drug chemotherapy with continuous infusion 5-FU followed by a continuous course of CRT and 2 additional cycles of the same chemotherapy. Herein, we present the long-term clinical and therapeutic outcomes of the first 2 prospective clinical trials with long-term follow-up to evaluate the integration of MMC with standard 5-FU–based adjuvant CRT in resected PDAC or non-PDAC periampullary cancer.
Methods and materials
Patient eligibility
Both trials A and B were approved by our institutional review board. The study populations consisted of patients with PDAC or non-PDAC periampullary adenocarcinoma who underwent curative resection. Patients with negative (R0), microscopic (R1), or macroscopic residual disease (R2) at the time of resection were eligible. Restaging after surgery included complete history and physical examination, computed tomography scan of the chest/abdomen/pelvis, and laboratory studies. The final cohort included 62 patients in trial A and 57 patients in trial B.
Eligibility criteria included (1) age ≥18 years; (2) Karnofsky performance status ≥70%; (3) adequate bone marrow function; (4) adequate hepatic function; and (5) adequate renal function. Patients were excluded for: (1) prior malignancy within 5 years; (2) prior abdominal irradiation; (3) distant metastatic disease; and (4) poorly controlled medical condition(s) that could be exacerbated by the treatment.
Surgery
All patients underwent pancreaticoduodenectomy. Resection margins were positive (R1) if the carcinoma was close (within 1 mm) or present at the final soft-tissue margin. Gross residual disease (R2) was based on the surgical report and/or residual disease seen on first postoperative imaging. Postoperatively, patients were evaluated by radiation and medical oncologists to discuss treatment options and determine eligibility.24
Adjuvant therapy
Therapy schemas are outlined in Table S1 (available as supplementary material online only at www.advancesradonc.org). Radiation for trials A and B consisted of 15-MV photons. All patients were simulated on the Picker AcQ Sim-CT simulator (Picker, Inc., Cleveland, OH), and treatment planning was completed using computerized dosimetry. Isodose curves were generated on axial slices at the isocenter and at least 2 additional levels. Critical normal organs at risk and tumor target volumes were electronically contoured by 1 radiation oncologist. The treatment volume was designed to include the preoperative tumor volume, primary lymph node drainage stations, and the para-aortic lymph nodes with a 1.5- to 2-cm margin. The encompassed vertebral body levels were T11-L4 inclusive. For both trials, the dose range was 50.4 to 54 Gy.
Trial A (9625): Adjuvant chemoradiation followed by maintenance chemotherapy
Patients were treated on trial A from April 1996 to July 1999.1 Chemotherapy details can be found in Table S1. Radiation was delivered as a split course of 50 Gy with a 2-week break after the initial delivery of 25 Gy. Irradiation was delivered using 3- or 4-field technique, custom alloy blocking, 2.5 Gy per fraction, 1 fraction per day, and 5 fractions per week. The daily spinal cord dose did not exceed 1.9 Gy per fraction. Portions of the kidney, specifically the right kidney, received a full dose, although the treatment plan ensured that 50% of the functioning renal parenchyma received no more than 35% of the daily dose or a total of 17.5 Gy. Radiation and chemotherapy began concurrently on day 1, 41 to 86 days after surgery. Two cycles of chemotherapy were administered during radiation, followed by 4 additional cycles of identical chemotherapy.
Trial B (9940): Chemotherapy prior to and following adjuvant chemoradiation
Patients were treated on trial B from August 1999 to April 2002. Chemotherapy was administered similarly to trial A, with the exception that patients received continuous infusion 5-FU and an 8 mg/m2/day dose of MMC (2 mg lower). Radiation was delivered to patients in trial B according to the technical aspects outlined for trial A; however, daily fractions of 1.8 Gy were delivered continuously (with no planned break) for 25 to 30 fractions. Furthermore, 1 cycle of chemotherapy was administered before CRT and 2 additional cycles were delivered after CRT.
Toxicity analysis and dose modifications
All patients were evaluated for toxicity weekly during therapy. Toxicity was graded using the National Cancer Institute Common Toxicity Criteria version 1.0 (1994). Treatment was held for absolute neutrophil count <1000/mm3 and platelet count <75,000/mm3. For any nonhematologic toxicity of grade ≥3 attributable to radiation, further treatments were held until toxicity resolved to grade ≤1 toxicity. Radiation dose escalation was not allowed. If treatment delayed radiation for >12 weeks, the patient was removed from the study.
Modifications of 5-FU, LV, DPM, and MMC were based on nadir absolute neutrophil count and platelet counts or worst-grade nonhematologic toxicity attributable to these chemotherapies in the immediately preceding cycle. The 5-FU/LV/DPM was delayed until toxicity had resolved to grade 0 or 1. The dose of DPM was decreased by 25% for DPM-related toxicities such as headache, whereas 5-FU and LV doses were not modified.
Hematologic toxicities were measured at different time points on the 2 protocols. For the split-course treatment in trial A, toxicity was assessed twice, once during CRT and once after CRT. Toxicities were assessed once after CRT in trial B.
Family history
Paper and electronic charts as well as the National Familial Pancreatic Tumor Registry25 were reviewed to examine possible correlations of family history of cancer with overall survival (OS). A family history of PDAC was defined as having at least 1 first-degree relative with a diagnosis of PDAC. A family history of breast or ovarian cancer was defined as having at least 1 first- or second-degree relative with either of these carcinomas.
Statistical analysis
The primary statistical endpoints are toxicity and OS of patients treated on trials A and B. Toxicity is reported descriptively. Follow-up information was obtained from medical records, with restaging occurring every 3 months for year 1, every 4 months for year 2, and every 6 months thereafter. Outcomes between the 2 trials were not directly compared because the study designs were single-arm and treatment was not randomized. Event time distributions were estimated using the Kaplan-Meier method26 and comparisons within each trial were made using the log-rank statistic27 or the proportional hazards regression model.28 All factors in Table 1, in addition to family history of PDAC, gastrointestinal cancers, and breast or ovarian cancer, were tested for an association with OS. Median follow-up was calculated with the reverse Kaplan-Meier method. Binomial probabilities were compared with χ2 tests and reported with exact binomial 95% confidence intervals (CIs). The Cochran-Mantel-Haenszel test was used to evaluate the association of PDAC with positive margins stratifying for protocol. The Breslow-Day test for homogeneity of odds ratios was used to confirm assumptions of stratified analyses. 2-sided significance testing was used throughout the analysis, and a P value of .05 was considered statistically significant.
Table 1.
Patient characteristics: demographics and surgical outcomes
| Trial A (9625) (n = 62) |
Trial B (9940) (n = 57) |
|
|---|---|---|
| Study dates (y) | April 29, 1996-July 16, 1999 | August 6, 1999-April 10, 2002 |
| Median age (IQR), y | 60 (39-79) | 50 (29-77) |
| Sex, n (%) | ||
| Male | 35 (56) | 29 (51) |
| Female | 27 (44) | 28 (49) |
| Race, n (%) | ||
| Caucasian | 60 (97) | 53 (93) |
| African American | 1 (2) | 2 (4) |
| Other | 1 (2) | 2 (4) |
| Primary tumor site, n (%) | ||
| Pancreas | 38 (61) | 39 (68) |
| Ampullary | 7 (11) | 8 (14) |
| DCBD | 24 (39) | 18 (32) |
| Duodenum | 2 (3) | 3 (5) |
| T stage | ||
| T1 | 2 (3) | 0 (0) |
| T2 | 6 (10) | 6 (11) |
| T3 | 41 (66) | 49 (86) |
| T4 | 13 (21) | 2 (4) |
| Tumor size, n (%) | ||
| ≥ 3 cm | 36 (58) | 30 (53) |
| < 3 cm | 26 (42) | 27 (47) |
| Histologic grade, n (%) | ||
| Well-differentiated | 0 (0) | 2 (4) |
| Well to moderately | 1 (2) | 3 (5) |
| Moderately differentiated | 31 (50) | 24 (42) |
| Moderately to poorly | 11 (18) | 14 (25) |
| Poorly differentiated | 19 (31) | 14 (25) |
| Negative margins, n (%) | 45 (73) | 46 (81) |
| Positive margins, n (%) | 17 (27) | 11 (19) |
| Microscopic | 14 (23) | 9 (16) |
| Macroscopic | 3 (5) | 2 (4) |
| Radical lymph node dissection, n (%) | 19 (31) | 7 (12) |
| Positive lymph nodes, n (%) | 53 (85) | 49 (86) |
| Median number of positive lymph nodes (IQR), n | 3 (0-24) | 2 (0-16) |
| Lymphovascular invasion, n (%) | 26 (42) | 22 (39) |
| Perineural invasion, n (%) | 48 (77) | 45 (80) |
| Median time to start RT (IQR), days | 67 (35-96) | 64 (41-87) |
DCBD, distal common bile duct; IQR, interquartile range; RT, radiation therapy.
Results
Demographic, tumor, and treatment characteristics for both trials are given in Table 1. The patients on these protocols had very similar demographics and disease characteristics. The median (interquartile range [IQR]) age of patients on trials A and B was 60 (IQR, 56, 67) and 59 (IQR, 54, 67), respectively. Both trials had approximately 60% of patients with PDAC, included more than 92% Caucasian patients, and were approximately evenly split on gender. Tumor characteristics were mostly similar (Table 1). One notable difference was radical lymph node dissection, which was more common on trial A (31%; 95% CI, 19.56-43.65) compared with 12% (95% CI, 5.08-23.68) on trial B, likely because of a separate overlapping surgical clinical trial.29, 30, 31
Univariate analysis
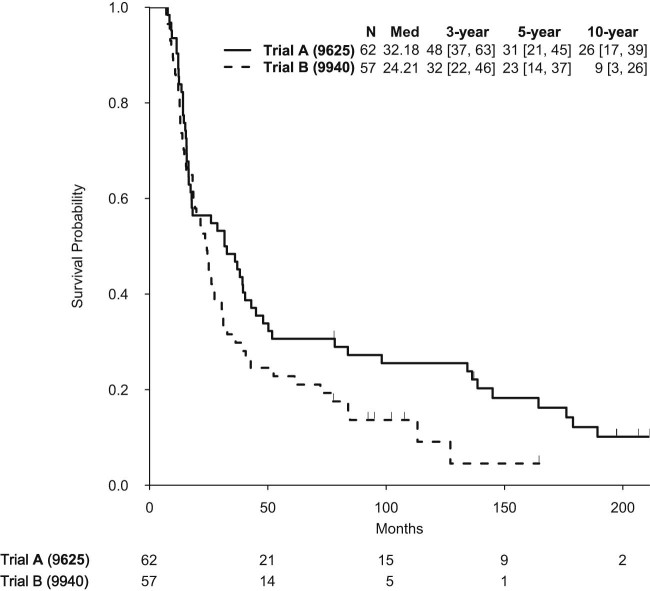
Median follow-up was 197.5 months for trial A and 107.7 months for trial B. Median OS in trial A was 32.2 months and 24.2 months in trial B (Fig 1). Rates of 3-, 5-, and 10-year OS were 48%, 31%, and 26% in trial A and 32%, 23%, and 9% in trial B. Univariate OS analyses for both trials are shown in Table 2.
Figure 1.
Kaplan-Meier overall survival curve of all patients separated by trial A versus trial B.
Table 2.
Significant univariate associations with OS for trials A and B
| N | Median | 3-y OS | 5-y OS | 10-y OS | HR | 95% CI | P value | |
|---|---|---|---|---|---|---|---|---|
| Trial A (9625) | ||||||||
| Diagnosis | ||||||||
| Non-PDAC | 24 | 49.9 | 71 (55-92) | 46 (30-71) | 32 (18-58) | 1.00 | - | .016 |
| PDAC | 38 | 16.9 | 34 (22-53) | 21 (11-39) | 21 (11-39) | 2.02 | 1.14-3.58 | |
| Margins | ||||||||
| Negative | 45 | 39.5 | 60 (47-76) | 36 (24-53) | 33 (22-50) | 1.00 | - | .026 |
| Positive | 17 | 15.7 | 18 (6-49) | 18 (6-49) | 6 (1-39) | 1.98 | 1.09-3.6 | |
| Node status | ||||||||
| Negative | 9 | 164.4 | 89 (71-00) | 78 (55-100) | 65 (39-100) | 1.00 | - | .017 |
| Positive | 53 | 18.2 | 42 (30-57) | 23 (14-37) | 19 (11-33) | 2.84 | 1.21-6.69 | |
| Family of breast or ovarian cancer | ||||||||
| No | 52 | 34.4 | 50 (38-66) | 37 (26-52) | 30 (20-46) | 1.00 | - | .039 |
| Yes | 10 | 22.6 | 40 (19-85) | 0 (NA-NA) | 0 (NA-NA) | 2.13 | 1.04-4.36 | |
| T stage | ||||||||
| 1-2 | 8 | 164.4 | 75 (50-100) | 75 (50-100) | 75 (50-100) | 1.00 | - | .05 |
| 3-4 | 54 | 27.3 | 44 (33-60) | 24 (15-39) | 18 (10-32) | 2.37 | 1-5.61 | |
| Trial B (9940) | ||||||||
| Diagnosis | 18 | 38.5 | 56 (37-84) | 39 (22-69) | 28 (13-59) | 1.00 | - | .026 |
| Non-PDAC PDAC |
39 | 21.7 | 21 (11-38) | 15 (7-32) | 0 (NA-NA) | 2.07 | 1.09-3.94 | |
| Size, cm | ||||||||
| < 3 | 20 | 25.3 | 45 (28-73) | 35 (19-64) | 25 (12-53) | 1.00 | - | .054 |
| ≥ 3 | 37 | 21.7 | 24 (14-43) | 16 (8-34) | 0 (NA-NA) | 1.84 | 0.99-3.4 | |
| Perineural invasion | ||||||||
| No | 11 | 61.3 | 73 (51-100) | 55 (32-94) | 23 (5-87) | 1.00 | - | .011 |
| Yes | 45 | 21.6 | 22 (13-38) | 16 (8-31) | 6 (2-20) | 2.92 | 1.28-6.64 | |
| Lymph node dissection | ||||||||
| Standard | 50 | 22.54 | 26 (16-42) | 18 (10-33) | 4 (1-20) | 1.00 | - | .015 |
| Radical | 7 | NR | 71 (45-100) | 57 (30-100) | 57 (30-100) | 0.23 | 0.07-0.75 | |
| Tumor differentiation | ||||||||
| Well to moderately poorly | 43 | 26.1 | 37 (25-55) | 28 (17-45) | 10 (4-30) | 1.00 | - | .045 |
| Poorly | 14 | 15.6 | 14 (4-52) | 7 (1-47) | 7 (1-47) | 1.93 | 1.01-3.66 | |
CI, confidence interval; HR, hazard ratio; OS, overall survival; NA, not available; NR, not reported; PDAC, pancreatic ductal adenocarcinoma.
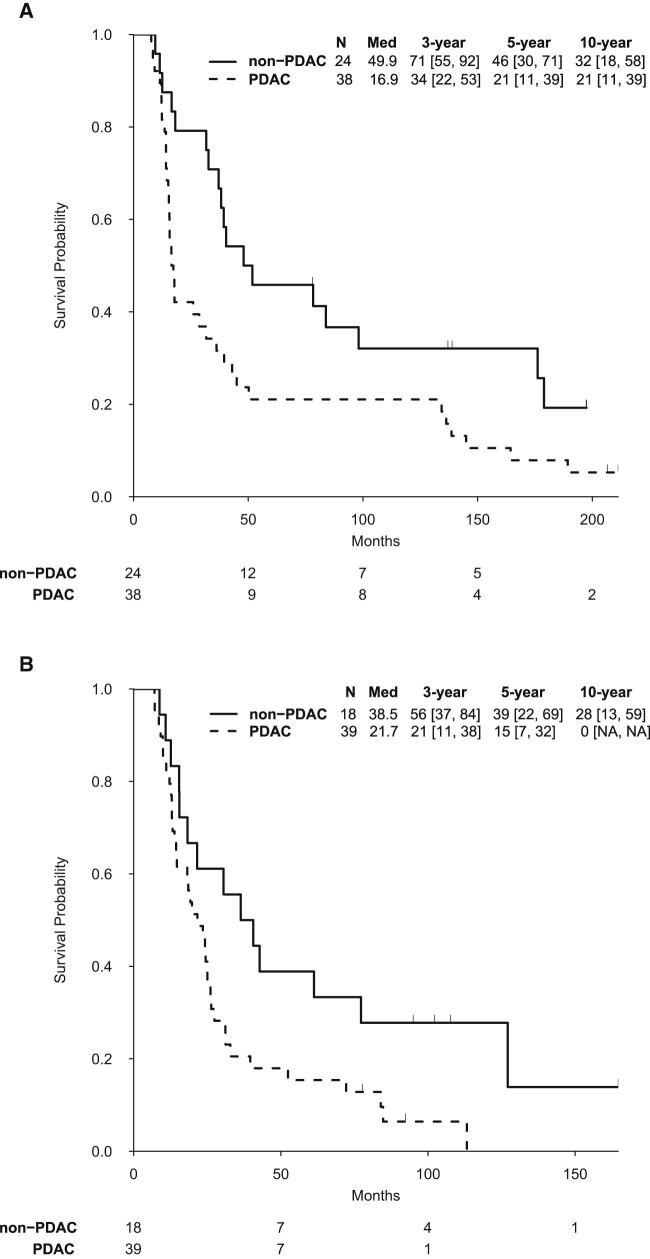
On trial A, a PDAC diagnosis, margin-positive resection, lymph node–positive resection, and T stage 3-4 were associated with decreased OS (Table 2). Although PDAC patients in trial A had a significantly inferior OS than non-PDAC patients (median, 16.9 vs 49.9 months; P = .016), long-term OS rates in PDAC were impressive (34% 3-year, 21% 5-year, and 21% 10-year; Fig 2A). Patients on trial A with a family history of PDAC had a trend toward improved OS (median, 164.4 vs 28.7 months, P = .058), whereas those with a family history of breast or ovarian cancer had decreased OS (P = .039; Fig S1).
Figure 2.
Kaplan-Meier overall survival curve of (A) trial A and (B) trial B patients separated by a PDAC versus non-PDAC diagnosis. Med, median; PDAC, pancreatic ductal adenocarcinoma.
A PDAC diagnosis was also associated with decreased OS in trial B (Table 2). Additional risk factors for worse OS included female sex, tumor size ≥3 cm, perineural invasion, and poor differentiation (Table 2). Of note, the 3-, 5-, and 10-year OS rates for patients with PDAC on trial B were 21%, 15%, and 0%, and 56%, 39%, and 28% for non-PDAC patients (Fig 2B). A family history of cancer, PDAC or breast/ovarian, was not associated with OS in this trial. The few patients with radical lymph node dissections (n = 7) had significantly improved OS (P = .015).
Multivariate analysis
In multivariate analysis, lymph node-positive resection was the strongest factor associated with OS (Table 3). Positive margins were more likely in patients with PDAC (31.2% vs 9.5%, Cochran-Mantel-Haenszel P = .01). This correlation makes it difficult to determine whether the PDAC diagnosis or margin status is a greater contributing factor to inferior OS; however, each factor alone is also significantly associated with decreased OS after adjusting for lymph node status (all P < .05).
Table 3.
Significant multivariate associations with OS for trials A and B
| HR (95% CI) | P value | |
|---|---|---|
| Trial A (9625) | ||
| PDAC vs non-PDAC diagnosis | 1.65 (0.91-2.98) | .10 |
| Positive vs negative margins | 1.69 (0.91-3.12) | .10 |
| Positive vs negative node status | 2.48 (1.04-5.90) | .04 |
| Trial B (9940) | ||
| PDAC vs non-PDAC diagnosis | 1.90 (0.97-3.71) | .06 |
| Female vs male sex | 1.89 (1.04-3.44) | .04 |
| PNI vs no PNI | 2.80 (1.18-6.63) | .02 |
| Poorly vs well to moderately-poorly differentiated tumors | 2.41 (1.22-4.78) | .01 |
PNI, perineural invasion. All other abbreviations as in Table 2.
Adjusting for other significant factors on multivariate analysis, a diagnosis of PDAC was also marginally associated with decreased OS in trial B (Table 3). Margin status was not a significant factor for patients on trial B on univariate or multivariate analyses. Factors significantly associated with worse OS included female sex, perineural invasion, and poor differentiation. A radical lymph node dissection was not significantly associated with OS on multivariate analysis when adjusted for other factors.
Long-term survivors
For strictly descriptive purposes, we also report characteristics of long-term survivors defined as ≥5 years of OS from surgery. Trial A had 19 patients who survived longer than 5 years, in comparison with 11 patients in trial B, for a total of 30 of 119 (25%) long-term survivors. Long-term survivors were more likely to be male (63%) and had a median age of 60 years (IQR, 56, 65). The majority of long-term survivors had PDAC (43%), followed by distal common bile duct (30%), ampullary (17%), and duodenal (10%) carcinomas. The majority had a margin-negative resection (87%) and moderately differentiated tumors (47%), with a median tumor size of 3.0 cm (IQR, 2.0, 4.0).
Toxicity
Nonhematologic and hematologic grade ≥3 toxicities on both trials are itemized in Table 4. Overall, rates of grade ≥3 nonhematologic toxicity in trials A and B were 14.4% and 23.4%, respectively. For all types of nonhematologic toxicity, the proportion on either protocol was ≤5%. The acute and late grade ≥3 toxicity rates in trial A were 6.4% and 8.0%, respectively. In trial B, there were more late versus acute grade ≥3 toxicities (19.8% vs 3.6%, respectively). All 9 adverse events on trial A were standalone events, whereas on trial B, 1 patient had 3 adverse events, another patient had 2 adverse events, and the remaining 8 patients had 1 adverse event. Two patients on trial A had a grade 5 toxicity, with 1 patient dying of gangrenous bowel 6 months after local disease recurrence and another experiencing a gut infarct 3 years after CRT. No grade 5 toxicities were observed in trial B. Upon further evaluation, only 1 (1.6%) adverse event was deemed attributed to CRT in trial A, whereas 4 (7.2%) adverse events were considered attributable to CRT in trial B.
Table 4.
Hematologic and nonhematologic grade ≥3 toxicity
| Trial A (9625, n = 62) |
Trial B (9940, n = 57) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Total grade ≥3, % | Grade 3, % | Grade 4, % | Grade 5, % | Total grade ≥3, % | Grade 3, % | Grade 4, % | Grade 5, % | |
| Nonhematologic | ||||||||
| Gastrointestinal bleed | 4.8 | 4.8 | 0 | 0 | 3.6 | 1.8 | 1.8 | 0 |
| Ischemia | 3.2 | 0 | 0 | 3.2 | 0 | 0 | 0 | 0 |
| Ulcer | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bowel obstruction | 1.6 | 1.6 | 0 | 0 | 1.8 | 1.8 | 0 | 0 |
| Gastric obstruction | 0 | 0 | 0 | 0 | 3.6 | 3.6 | 0 | 0 |
| Pancreatitis | 1.6 | 1.6 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cholangitis | 1.6 | 1.6 | 0 | 0 | 1.8 | 1.8 | 0 | 0 |
| Enteritis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Colitis | 0 | 0 | 0 | 0 | 3.6 | 1.8 | 1.8 | 0 |
| Esophagitis | 0 | 0 | 0 | 0 | 1.8 | 1.8 | 0 | 0 |
| Pain | 0 | 0 | 0 | 0 | 5.4 | 5.4 | 0 | 0 |
| Sepsis | 1.6 | 1.6 | 0 | 0 | 1.8 | 1.8 | 0 | 0 |
| Total | 14.4 | 11.2 | 0 | 3.2 | 23.4 | 19.8 | 3.6 | 0 |
| Hematologica | ||||||||
| Neutropenia | 39 | 23 | 16 | 0 | 39 | 23 | 16 | 0 |
| 38 | 23 | 15 | 0 | |||||
| Thrombocytopenia | 6 | 19% | 3 | 0 | 22 | 19 | 3 | 0 |
| 32 | 16% | 16 | 0 | |||||
| Anemia | 2 | 2 | 0 | 0 | 10 | 10 | 0 | 0 |
| 10 | 10 | 0 | 0 | |||||
Hematologic toxicity was measured at 2 separate time points in trial A.
Discussion
Through next-generation sequencing, it is now possible to determine whether a patient's cancer has mutations in DNA repair pathways.32 Studies suggest integration of MMC into the treatment paradigm of patients with these mutations may result in improved outcomes.20, 33 This is the first prospective study with long-term follow-up to evaluate this approach. In summary, these 2 clinical trials suggest that MMC may be safely incorporated into either split-course or continuous 5-FU–based (continuous infusion or bolus) adjuvant CRT regimens in patients with resected PDAC or non-PDAC periampullary adenocarcinoma. Furthermore, these regimens (trials A and B) resulted in as high as an 18-month OS benefit in comparison with other studies.2, 6, 7, 34, 35, 36 Also consistent with the literature, patients with non-PDAC periampullary tumors were found to have a more favorable prognosis and extended OS after surgical resection than patients with PDAC.1, 2, 35
The rationale behind the novel adjuvant regimens used in this study was based on innovative agents, including MMC and DPM, under investigation during the development of the study.16 Although the proportion of long-term survivors (>5 years) was larger in trial A, it is unclear if this difference is attributable to patient selection, the duration or type of chemotherapy, or the split-course radiation treatment (2.5 vs 1.8 Gy/fraction), which had about 5% to 8% more biological equivalent dose for α/β ratios of 10 or 6, respectively, and about 15% more potent for an α/β ratio of 3 (not counting repopulation). In addition, the split-course regimen in trial A was associated with similar rates of toxicity when compared with the continuous CRT regimen used in trial B, suggesting that MMC can be safely integrated into adjuvant CRT for patients with periampullary cancers, even when combined with bolus 5-FU (in trial A), which has been shown to be associated with unfavorable toxicity.37
Consistent with previous studies, positive resection margins and lymph nodes appear to be independently associated with worse OS.3, 7, 38, 39 Because neoadjuvant therapy has been associated with a higher likelihood of margin- and node-negative resection in patients with borderline resectable and resectable PDAC,40, 41 delivery of chemotherapy and/or CRT before surgery may confer improved local control and long-term survival in patients with resectable PDAC and/or non-PDAC periampullary adenocarcinoma.42, 43 Earlier detection of these tumors and a better understanding of the genomic profile before surgery may be coupled with neoadjuvant therapy to select patients who may benefit from surgery. Positron emission tomography imaging, in particular, may allow for detection of nodal disease before resection. In fact, 1 study suggests that lymph nodes with maximum standard uptake value ≥2.8 were an independent factor for prognosis after resection.44, 45
To our knowledge, this is the largest report prospectively evaluating the role of MMC in the adjuvant management of periampullary cancers.1, 46 MMC has been suggested to convert unresectable patients to surgical candidates,42 enhance the therapeutic effects of adjuvant CRT,47 and improve local control48 in periampullary adenocarcinoma. Although it has not been extensively studied in PDAC, MMC has been used in other cancers such as anal cancer,49, 50, 51 and recent studies suggest its promising role in PDAC patients with a family history of PDAC and/or carcinomas with an inactivating mutation in a Fanconi anemia pathway gene (such as BRCA1, BRCA2, or PALB2).18, 19, 20, 21, 22, 23 Study results from trial A demonstrated that having a family history of PDAC specifically resulted in a borderline significant improvement in OS (164.4 vs 28.7 months, P = .058). In contrast, however, having a family history of breast or ovarian cancer was associated with inferior OS (22.6 vs 34.4 months, P = .039). These conclusions are limited by the fact that only a small proportion of patients fit these criteria; therefore, larger trials are needed to further determine whether MMC is beneficial in 1 subgroup or another.
In addition to the relatively small sample size, there are numerous limitations to this study. To achieve an analyzable sample size for these rare cancers, all periampullary cancers were included; however, this increases the difficulty of interpreting the results because of the differences in prognoses. To acknowledge this, we separated survival outcomes into PDAC and non-PDAC periampullary cancers. Of note, the American Joint Committee on Cancer staging system at the time in which patients were enrolled on these clinical trials differs from that in current use today; although it appears that patients with advanced disease were enrolled on the clinical trials, this is not the case and, as such, T stage was not included in the multivariate model. Furthermore, the study design of these historic prospective trials is outdated with the use of bolus 5-FU and split-course CRT. Nonetheless, the purpose of this study is to share the long-term results of these studies and to suggest that MMC can indeed be combined with standard adjuvant CRT in periampullary cancers and may be beneficial in patients with mutations in the DNA repair pathway.
These prospective studies indicate that an MMC may be safely incorporated into a 5-FU–based CRT regimen in patients with PDAC and non-PDAC periampullary cancers, with promising results of long-term survival. A family history of PDAC correlated with a trend toward improved OS in trial A. It is unclear if these improvements in OS are due to MMC and will therefore need to be prospectively evaluated in a larger, randomized series with integrated cancer sequencing for DNA repair defects.32
Footnotes
Sources of support: National Institutes of Health SPORE grant CA62924.
Conflict of interest: Dr. Hruban receives royalty payments from Myriad Genetics for the PALB2 invention.
Supplementary material for this article (http://dx.doi.org/10.1016/j.adro.2017.07.008) can be found at www.advancesradonc.org.
Supplementary data
The following is the supplementary data to this article:
Kaplan-Meier OS curve of patients enrolled onto Trial A with (A) a family history of pancreatic ductal adenocarcinoma (PDAC) or (B) breast or ovarian cancer.
Treatment schema for Trials A and B.
References
- 1.Chakravarthy A., Abrams R.A., Yeo C.J. Intensified adjuvant combined modality therapy for resected periampullary adenocarcinoma: Acceptable toxicity and suggestion of improved 1-year disease-free survival. Int J Radiat Oncol Biol Phys. 2000;48:1089–1096. doi: 10.1016/s0360-3016(00)00755-0. [DOI] [PubMed] [Google Scholar]
- 2.Morak M.J., van der Gaast A., Incrocci L. Adjuvant intra-arterial chemotherapy and radiotherapy versus surgery alone in resectable pancreatic and periampullary cancer: A prospective randomized controlled trial. Ann Surg. 2008;248:1031–1041. doi: 10.1097/SLA.0b013e318190c53e. [DOI] [PubMed] [Google Scholar]
- 3.Gutierrez J.C., Franceschi D., Koniaris L.G. How many lymph nodes properly stage a periampullary malignancy? J Gastrointest Surg. 2008;12:77–85. doi: 10.1007/s11605-007-0251-7. [DOI] [PubMed] [Google Scholar]
- 4.Neoptolemos J.P., Moore M.J., Cox T.F. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: The ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308:147–156. doi: 10.1001/jama.2012.7352. [DOI] [PubMed] [Google Scholar]
- 5.Aiura K., Takahashi S., Matsui J., Ueda M., Kitagawa Y. Beneficial effects of 5-fluorouracil and heparin-based portal infusion chemotherapy combined with mitomycin C and cisplatin after curative resection of pancreatic cancer. Pancreatology. 2010;10:250–258. doi: 10.1159/000244265. [DOI] [PubMed] [Google Scholar]
- 6.Jiang Z.Q., Varadhachary G., Wang X. A retrospective study of ampullary adenocarcinomas: Overall survival and responsiveness to fluoropyrimidine-based chemotherapy. Ann Oncol. 2013;24:2349–2353. doi: 10.1093/annonc/mdt191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He J., Ahuja N., Makary M.A. 2564 resected periampullary adenocarcinomas at a single institution: Trends over three decades. HPB (Oxford) 2014;16:83–90. doi: 10.1111/hpb.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Cancer Society Cancer facts & figures 2017. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017.pdf Available at:
- 9.Wolfgang C.L., Herman J.M., Laheru D.A. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63:318–348. doi: 10.3322/caac.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moertel C.G., Frytak S., Hahn R.G. Therapy of locally unresectable pancreatic carcinoma: A randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil: The gastrointestinal tumor study group. Cancer. 1981;48:1705–1710. doi: 10.1002/1097-0142(19811015)48:8<1705::aid-cncr2820480803>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Kalser M.H., Ellenberg S.S. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 12.Neoptolemos J.P., Dunn J.A., Stocken D.D. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: A randomised controlled trial. Lancet. 2001;358:1576–1585. doi: 10.1016/s0140-6736(01)06651-x. [DOI] [PubMed] [Google Scholar]
- 13.Regine W.F., Winter K.A., Abrams R.A. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: A randomized controlled trial. JAMA. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 14.Tempero M.A., Malafa M.P., Behrman S.W. Pancreatic adenocarcinoma, version 2.2014: Featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2014;12:1083–1093. doi: 10.6004/jnccn.2014.0106. [DOI] [PubMed] [Google Scholar]
- 15.Ling T.C., Slater J.M., Mifflin R. Evaluation of normal tissue exposure in patients receiving radiotherapy for pancreatic cancer based on RTOG 0848. J Gastrointest Oncol. 2015;6:108–114. doi: 10.3978/j.issn.2078-6891.2014.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Todd K.E., Gloor B., Lane J.S., Isacoff W.H., Reber H.A. Resection of locally advanced pancreatic cancer after downstaging with continuous-infusion 5-fluorouracil, mitomycin-C, leucovorin, and dipyridamole. J Gastrointest Surg. 1998;2:159–166. doi: 10.1016/s1091-255x(98)80008-5. [DOI] [PubMed] [Google Scholar]
- 17.Isacoff W.H., Bendetti J.K., Barstis J.J., Jazieh A.R., Macdonald J.S., Philip P.A. Phase II trial of infusional fluorouracil, leucovorin, mitomycin, and dipyridamole in locally advanced unresectable pancreatic adenocarcinoma: SWOG S9700. J Clin Oncol. 2007;25:1665–1669. doi: 10.1200/JCO.2006.06.7637. [DOI] [PubMed] [Google Scholar]
- 18.van der Heijden M.S., Brody J.R., Dezentje D.A. In vivo therapeutic responses contingent on Fanconi anemia/BRCA2 status of the tumor. Clin Cancer Res. 2005;11:7508–7515. doi: 10.1158/1078-0432.CCR-05-1048. [DOI] [PubMed] [Google Scholar]
- 19.Wang L., Brune K.A., Visvanathan K. Elevated cancer mortality in the relatives of patients with pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:2829–2834. doi: 10.1158/1055-9965.EPI-09-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villarroel M.C., Rajeshkumar N.V., Garrido-Laguna I. Personalizing cancer treatment in the age of global genomic analyses: PALB2 gene mutations and the response to DNA damaging agents in pancreatic cancer. Mol Cancer Ther. 2011;10:3–8. doi: 10.1158/1535-7163.MCT-10-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui Y., Brosnan J.A., Blackford A.L. Genetically defined subsets of human pancreatic cancer show unique in vitro chemosensitivity. Clin Cancer Res. 2012;18:6519–6530. doi: 10.1158/1078-0432.CCR-12-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Navarrete A., Armitage E.G., Musteanu M. Metabolomic evaluation of mitomycin C and rapamycin in a personalized treatment of pancreatic cancer. Pharmacol Res Perspect. 2014;2:e00067. doi: 10.1002/prp2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vyas O., Leung K., Ledbetter L. Clinical outcomes in pancreatic adenocarcinoma associated with BRCA-2 mutation. Anticancer Drugs. 2015;26:224–226. doi: 10.1097/CAD.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 24.Herman J.M., Swartz M.J., Hsu C.C. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: Results of a large, prospectively collected database at the johns hopkins hospital. J Clin Oncol. 2008;26:3503–3510. doi: 10.1200/JCO.2007.15.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johns Hopkins National Familial Pancreatic Tumor Registry http://pathology.jhu.edu/pancreas/nfptr/index.php Available at:
- 26.Kaplan E.L., Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 27.Mantel N., Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 28.Cox D. Regression models and life tables. JR Stat Soc. 1972;B34:187–220. [Google Scholar]
- 29.Riall T.S., Cameron J.L., Lillemoe K.D. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma—part 3: Update on 5-year survival. J Gastrointest Surg. 2005;9:1191–1206. doi: 10.1016/j.gassur.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 30.Yeo C.J., Cameron J.L., Sohn T.A. Pancreaticoduodenectomy with or without extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma: Comparison of morbidity and mortality and short-term outcome. Ann Surg. 1999;229:613–624. doi: 10.1097/00000658-199905000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeo C.J., Cameron J.L., Lillemoe K.D. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: Randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236:355–368. doi: 10.1097/00000658-200209000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Humphris J.L., Patch A.M., Nones K. Hypermutation in pancreatic cancer. Gastroenterology. 2017;152:68–74. doi: 10.1053/j.gastro.2016.09.060. e2. [DOI] [PubMed] [Google Scholar]
- 33.Chalasani P., Kurtin S., Dragovich T. Response to a third-line mitomycin C (MMC)-based chemotherapy in a patient with metastatic pancreatic adenocarcinoma carrying germline BRCA2 mutation. JOP. 2008;9:305–308. [PubMed] [Google Scholar]
- 34.Abrams R.A., Grochow L.B., Chakravarthy A. Intensified adjuvant therapy for pancreatic and periampullary adenocarcinoma: Survival results and observations regarding patterns of failure, radiotherapy dose and CA19-9 levels. Int J Radiat Oncol Biol Phys. 1999;44:1039–1046. doi: 10.1016/s0360-3016(99)00107-8. [DOI] [PubMed] [Google Scholar]
- 35.Lee J.H., Whittington R., Williams N.N. Outcome of pancreaticoduodenectomy and impact of adjuvant therapy for ampullary carcinomas. Int J Radiat Oncol Biol Phys. 2000;47:945–953. doi: 10.1016/s0360-3016(00)00537-x. [DOI] [PubMed] [Google Scholar]
- 36.Turan N., Benekli M., Unal O.U. Impact of adjuvant treatment modalities on survival outcomes in curatively resected pancreatic and periampullary adenocarcinoma. Chin J Cancer Res. 2015;27:408–416. doi: 10.3978/j.issn.1000-9604.2015.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahaseth H., Brutcher E., Kauh J. Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma. Pancreas. 2013;42:1311–1315. doi: 10.1097/MPA.0b013e31829e2006. [DOI] [PubMed] [Google Scholar]
- 38.Schnelldorfer T., Ware A.L., Sarr M.G. Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: Is cure possible? Ann Surg. 2008;247:456–462. doi: 10.1097/SLA.0b013e3181613142. [DOI] [PubMed] [Google Scholar]
- 39.Smith R.A., Bosonnet L., Ghaneh P. Preoperative CA19-9 levels and lymph node ratio are independent predictors of survival in patients with resected pancreatic ductal adenocarcinoma. Dig Surg. 2008;25:226–232. doi: 10.1159/000140961. [DOI] [PubMed] [Google Scholar]
- 40.Katz M.H., Marsh R., Herman J.M. Borderline resectable pancreatic cancer: Need for standardization and methods for optimal clinical trial design. Ann Surg Oncol. 2013;20:2787–2795. doi: 10.1245/s10434-013-2886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sen N., Falk S., Abrams R.A. Role of chemoradiotherapy in the adjuvant and neoadjuvant settings for resectable pancreatic cancer. Clin Oncol (R Coll Radiol) 2014;26:551–559. doi: 10.1016/j.clon.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Weese J.L., Nussbaum M.L., Paul A.R. Increased resectability of locally advanced pancreatic and periampullary carcinoma with neoadjuvant chemoradiotherapy. Int J Pancreatol. 1990;7:177–185. doi: 10.1007/BF02924235. [DOI] [PubMed] [Google Scholar]
- 43.Reilley M.J., Shroff R., Varadhachary G.R. Adjuvant/perioperative therapy in pancreatic and periampullary cancer. Indian J Surg. 2015;77:403–408. doi: 10.1007/s12262-015-1361-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalady M.F., Clary B.M., Clark L.A. Clinical utility of positron emission tomography in the diagnosis and management of periampullary neoplasms. Ann Surg Oncol. 2002;9:799–806. doi: 10.1007/BF02574503. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi S., Nagano H., Hoshino H. Diagnostic value of FDG-PET for lymph node metastasis and outcome of surgery for biliary cancer. J Surg Oncol. 2011;103:223–229. doi: 10.1002/jso.21811. [DOI] [PubMed] [Google Scholar]
- 46.Splinter T.A., Obertop H., Kok T.C., Jeekel J. Adjuvant chemotherapy after resection of adenocarcinoma of the periampullary region and the head of the pancreas. A non-randomized pilot study. J Cancer Res Clin Oncol. 1989;115:200–202. doi: 10.1007/BF00397924. [DOI] [PubMed] [Google Scholar]
- 47.Coia L., Hoffman J., Scher R. Preoperative chemoradiation for adenocarcinoma of the pancreas and duodenum. Int J Radiat Oncol Biol Phys. 1994;30:161–167. doi: 10.1016/0360-3016(94)90531-2. [DOI] [PubMed] [Google Scholar]
- 48.Tomikawa M., Kubota T., Matsuzaki S.W. Mitomycin C and cisplatin increase survival in a human pancreatic cancer metastatic model. Anticancer Res. 1997;17:3623–3625. [PubMed] [Google Scholar]
- 49.Kachnic L.A., Winter K., Myerson R.J. RTOG 0529: A phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2013;86:27–33. doi: 10.1016/j.ijrobp.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vinayan A., Glynne-Jones R. Anal cancer—what is the optimum chemoradiotherapy? Best Pract Res Clin Gastroenterol. 2016;30:641–653. doi: 10.1016/j.bpg.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 51.Peixoto R.D., Wan D.D., Schellenberg D., Lim H.J. A comparison between 5-fluorouracil/mitomycin and capecitabine/mitomycin in combination with radiation for anal cancer. J Gastrointest Oncol. 2016;7:665–672. doi: 10.21037/jgo.2016.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Kaplan-Meier OS curve of patients enrolled onto Trial A with (A) a family history of pancreatic ductal adenocarcinoma (PDAC) or (B) breast or ovarian cancer.
Treatment schema for Trials A and B.