Summary.
We report, with complete follow-up, a case of medically-inoperable Upper Urinary Tract Urothelial Carcinoma (UUTUC) treated with stereotactic body radiation therapy (SBRT) consisting of 50 Gy in 4 fractions. The patient's symptomatic hematuria resolved approximately 10 days after treatment. Furthermore, the patient had no clinical signs of acute or late adverse events with complete response (RECIST criteria v1.1) and no evidence of locoregional recurrence or distant metastasis at final follow-up 31 months after completing SBRT.
Alt-text: Unlabelled box
Background
Upper urinary tract urothelial carcinoma (UUTUC) is rare. Current estimates claim that urothelial (transitional) cell malignancies within the renal pelvis account for less than 10% of all renal tumors.1 Although related, ureteric lesions are far less prevalent than their renal pelvis counterpart, with renal pelvis-to-ureter ratios exceeding 3:1.1 The Memorial Sloan Kettering Cancer Center reported a large longitudinal series consisting of primary urothelial carcinoma (UC) cases, of which 88% were bladder, 7% urethra, 3% renal pelvis, and 2% ureter.2 Other studies have corroborated the low prevalence of UC in the upper urinary tract.1, 3, 4, 5, 6 Consequent to the rarity of UUTUC, the literature is largely limited to retrospective analyses and case reports.
For UUTUC, prognosis is strongly linked to age at diagnosis, T-category, histology grade, and lymphatic involvement.3, 4 The standard treatment for a UUTUC includes radical nephroureterectomy (RNU) with removal of the bladder cuff.7, 8 There are alternative kidney-sparing techniques, but patient selection is crucial when opting for less invasive approaches because the risk of disease recurrence is high.6 With limited data on UUTUC and no prospective randomized trials, the exact role of nonoperative therapies, including chemotherapy, immunotherapy, and radiation therapy, is currently unclear.6 Unfortunately, there is no standard treatment for patients with UUTUC who are not candidates for surgery as a result of poor functional status, overwhelming disease burden, or refusal of surgical intervention. Novel, noninvasive treatments are needed for this clinical scenario and stereotactic body radiation therapy (SBRT) may be an option, as presented in this report.
Case presentation
A 95-year old male patient presented with a 1-year history of intermittent gross hematuria. His medical history was notable for coronary artery disease status, postcoronary artery bypass grafting and stent placement, atrial fibrillation, and systolic congestive heart failure with an ejection fraction of <20%. The patient was on aspirin, clopidogrel, and warfarin for cardiovascular disease. Upon presentation, the patient was found to be anemic and was admitted for further evaluation. He received 2 units of packed red blood cells. After being appropriately stabilized, a cystoscopy was performed but showed no evidence of malignancy in the bladder or urethra. Laboratory evaluation was notable for hemoglobin of 13.8 g/dL and creatinine of 1.2 mg/dL. Voided urinary cytology was positive for urothelial carcinoma. A computed tomography (CT) scan of the chest, abdomen, and pelvis revealed a soft tissue mass in the upper left intrarenal collecting system that measured 4.5 cm × 2.2 cm × 1.9 cm and was consistent with a urothelial neoplasm. There was no evidence of regional lymphadenopathy or distant metastatic disease. The patient was staged as urothelial carcinoma, clinical T2, N0, M0, and GX in accordance with the criteria by the American Joint Committee on Cancer, 7th edition.
The patient was evaluated by both urology and cardiology. Given his age and cardiovascular comorbidities, he was determined to be at high risk for complications with general anesthesia and RNU. Consequently, he was deemed medically inoperable. The patient was then evaluated by interventional radiology and deemed an unsuitable candidate for percutaneous ablation due to the location of the tumor in the collecting system and his inability to receive anesthesia. As a result, he was referred to radiation oncology for consideration of palliative radiation therapy.
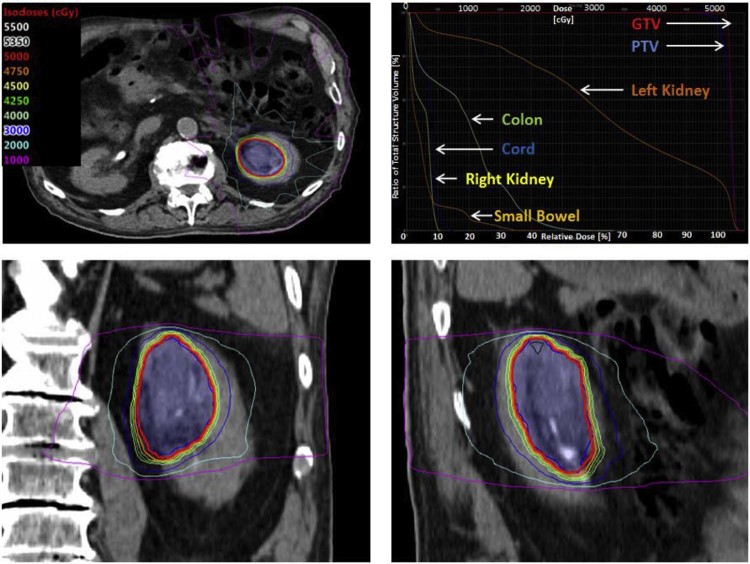
The patient was treated with SBRT to the tumor with a prescription dose of 50 Gy in 4 fractions delivered over 4 consecutive days (Fig 1). For simulation, he was immobilized in a full-body BodyFix system (Elekta, Stockholm, Sweden). A 4-dimensional CT scan with and without intravenous contrast was obtained for treatment planning. An internal gross tumor volume was delineated, representing the gross tumor volume on all respiratory phases. The planning target volume was created by isometrically expanding the internal gross tumor volume by 0.5 cm. The dose was prescribed to the planning target volume with target objectives of V100%[%] ≥95% (95.1% achieved), D90%[%] ≥100% (101.3% achieved), and V120%[cc] < 0.03 cc (0 cc achieved).
Figure 1.
Stereotactic body radiation therapy plan and dose volume histogram of clinical T2, N0, M0 urothelial carcinoma of the left renal pelvis.
Treatment planning was performed using the Eclipse Treatment Planning System (Varian, Palo Alto, CA) using a 10-field static intensity modulated radiation therapy technique. The following beam arrangements were used: A30R, A10R, A15L, A40L, A65L, left lateral, P60L, P40L, P20L, and a posterior beam, wherein A vs. P designates anterior versus posterior and L vs. R designates left versus right of midline. The number between the letters designates the gantry angle with respect to the isocenter. Treatment delivery was performed using a TrueBeam 6-MV photon system (Varian). Dose constraints were followed per the report by the American Association of Physicists in Medicine Task Group 101 on stereotactic body radiation therapy.9 Dose to organs at risk was constrained per the 3-fraction recommendations outlined in Table III of the American Association of Physicists in Medicine Task Group 101 report. The 3-fraction constraints were chosen as conservative objectives for the proposed 4-fraction treatment. Key constraint objectives with actual achieved values included cord V21.9 Gy [cc] < .03 cc (0 cc achieved), skin V30 Gy [cc] < 10 cc (0 cc achieved), stomach V16.5 Gy [cc] < 10 cc (0 cc achieved), small bowel V17.7 Gy [cc] < 5 cc (1.71 cc achieved), and V25.2 Gy [cc] < .03 cc (0 cc achieved), colon V 28.2 Gy [cc] < 1 cc (0.65 cc achieved), and V24 Gy [cc] < 20 cc (13.16 cc achieved), right kidney V10.5 Gy [%] <66% (0% achieved), and liver CV19.2 Gy [cc] > 700 cc (1299.4 cc achieved). No attempt was made to limit the dose to the involved left kidney (V10.5% was 87.9%). Image guidance was performed with daily cone beam CT using a robotic couch with 3 degrees of freedom.
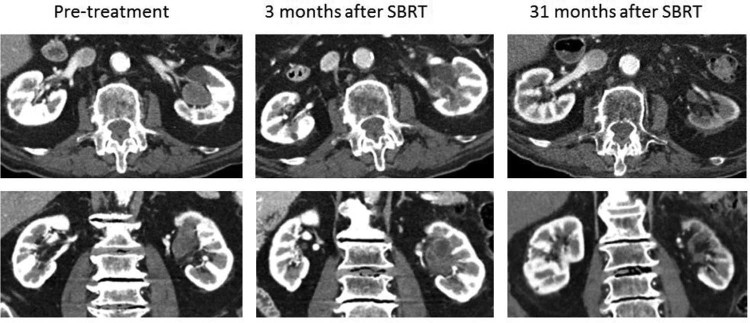
Prospectively collected, physician-reported adverse events per the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) Version 4.03 were recorded in an ARIA Oncology Information System (Varian) before SBRT delivery and at all follow-up visits. On completion of SBRT, the patient experienced no acute adverse effects (CTCAE v4.03 grade 0 gastrointestinal and genitourinary domains). Approximately 10 days after completing SBRT, the patient's previously noted grade 1 hematuria resolved and never recurred. The patient was reevaluated 3 months after completion of therapy, at which time he had no adverse effects (CTCAE v4.03 grade 0 gastrointestinal and genitourinary domains). His hemoglobin was stable at 13.5 g/dL, and creatinine was 1.1 mg/dL. A CT urogram revealed a significant reduction in size of the treated tumor, which was necrotic and appeared consistent with a partial response using RECIST criteria v1.1 (Fig 2).10 There was new thickening and enhancement of the wall of the left renal pelvis and upper ureter, consistent with posttreatment inflammation. He was followed with clinical examination; CT scans of the chest, abdomen, and pelvis; and laboratory tests every 3 to 4 months.
Figure 2.
Left renal pelvis tumor 1 week before, 3 months after, and 31 months after SBRT.
At the patient's last radiation oncology reevaluation 31 months after completing SBRT, he had no clinically apparent late adverse effects related to SBRT (CTCAE v4.03 grade 0 gastrointestinal and genitourinary domains). His creatinine levels remained within the normal limits at 1.3 mg/dL. A CT urogram showed left renal atrophy with no residual mass or filling defect in the left kidney, consistent with a complete response using RECIST criteria v1.1 (Fig 2). Complete imaging showed no regional or distant metastatic disease. The patient experienced decompensated systolic heart failure 33 months after SBRT, enrolled in hospice care, and passed away.
Discussion
RNU with systematic bladder cuff excision for primary resection of UUTUC has been the gold standard treatment dating back to 1957 when it was first described by Dr. Francis Twinem.7 It remains the preferred treatment, especially in cases of high-risk disease.3, 6, 7, 11 Catton et al showed durable locoregional control after complete RNU with a recurrence rate of 15% when compared with a 46% recurrence rate after incomplete RNU.3 Recent findings demonstrating positive outcomes for low-risk disease have incited researchers to investigate kidney-sparing approaches to treatment, including percutaneous, endoscopic, and segmental approaches.6, 11, 12 Preliminary data on these newer treatments are encouraging and indicate a strong role for less invasive approaches in cases of low-risk disease.6, 13
The overall prognosis for UUTUC is quite poor, with reports of 5-year disease-specific survival <50% for patients with pT2/pT3 and <10% for pT4 tumors, regardless of interventions.6 Specific adverse risk factors are well defined in the medical literature and include age >60 years at diagnosis, T3/T4 staging, lymph node involvement, ureteral localization, high-grade tumors, and the inability to attain negative surgical margins.3, 4, 11, 12, 14, 15 Recurrence rates are 35% versus 70% for early and locally advanced disease, respectively.3, 16
As early as 1941, Cook et al argued for postoperative radiation in cases of extraureteral extension or locally advanced disease.17 Several retrospective series suggest potential benefits with postoperative radiation in select patient populations at high risk for locoregional recurrence, namely those with high-risk features including high T-category staging, lymph node involvement, high-grade tumors, and positive resection margins.3, 4, 12, 16, 18 These data have provided evidence that radiation therapy may be an effective adjunctive treatment for selected patients with UUTUC.
SBRT, also known as stereotactic ablative radiation therapy, is an ablative radiation technique that delivers high doses of radiation typically in 5 or fewer fractions. The American Society for Radiation Oncology has defined SBRT as a radiation therapy course not to exceed 5 fractions when using the technical Current Procedural Terminology code.19 Radiotherapeutic innovations have allowed for more conformal delivery of radiation while minimizing radiation to dose-limiting adjacent healthy tissue. SBRT is commonly used for medically inoperable patients with early stage lung cancer.20, 21 It is also frequently used for liver and osseous metastases (eg, spine).
Additionally, recent data suggest that SBRT is a safe and effective treatment option for localized cancers of the pancreas, prostate, and kidney.22 In a recent systematic review of 110 patients with localized renal cell carcinoma of the kidney who were treated with SBRT, local control was 94% and severe adverse events (grade 3 or higher) occurred in only 4% of patients.23 Phase 1 to 2 studies are ongoing to better define the safety, efficacy, and optimal dose/fractionation regimen of SBRT for localized renal cell carcinoma of the kidney.24, 25
Maehata et al reported on their experience with aggressive hypofractionated radiation therapy, using between 50 and 60 Gy in 10 fractions in 3 elderly patients with medically inoperable UUTUC.26 In all 3 cases, this hypofractionated regimen was effective at controlling the treated tumor. One patient, who had a primary lesion in the distal ureter, experienced an out-of-field bladder recurrence 22 months after treatment. In a similar manner, we present a case of medically inoperable UUTUC treated with 4-fraction SBRT. Our case supports the experience of Maehata et al in that our patient experienced durable local control and disease-specific survival with an ablative radiation therapy dose. When assuming an α/β ratio of 10, a fractionation schedule of 50 Gy in 4 fractions has a biologically effective dose (BED) of 112.5 Gy10 and an equivalent dose in 2 Gy per fraction (EQD2) of 93.75 Gy, which is greater than that delivered with 60 Gy in 10 fractions (BED 96 Gy10 and EQD2 80 Gy) or 50 Gy in 10 fractions (BED 75 Gy10 and EQD2 62.5 Gy). To our knowledge, this is the first case report of a patient with inoperable UUTUC treated with SBRT as defined by the American Society for Radiation Oncology definition (eg, 5 fractions or fewer).19
Conclusions
We report a favorable outcome in a patient with medically inoperable, clinically localized UUTUC who was treated with SBRT. Further investigation of SBRT in this patient population is warranted.
Footnotes
Sources of support: None.
Conflicts of interest: The authors declare that they have no competing interests.
References
- 1.Hall M.C., Womack J.S., Roehrborn C.G., Carmody T., Sagalowsky A.I. Advanced transitional cell carcinoma of the upper urinary tract: Patterns of failure, survival and impact of postoperative adjuvant radiotherapy. J Urol. 1998;160:703–706. doi: 10.1016/S0022-5347(01)62763-0. [DOI] [PubMed] [Google Scholar]
- 2.Batata M.A., Whitmore W.F., Hilaris B.S., Tokita N., Grabstald H. Primary carcinoma of the ureter: A prognostic study. Cancer. 1975;35:1626–1632. doi: 10.1002/1097-0142(197506)35:6<1626::aid-cncr2820350623>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 3.Catton C.N., Warde P., Gospodarowicz M.K. Transitional cell carcinoma of the renal pelvis and ureter: Outcome and patterns of relapse in patients treated with postoperative radiation. Urol Oncol. 1996;2:171–176. doi: 10.1016/s1078-1439(96)00095-6. [DOI] [PubMed] [Google Scholar]
- 4.Chen B., Zeng Z.C., Wang G.M. Radiotherapy may improve overall survival of patients with T3/T4 transitional cell carcinoma of the renal pelvis or ureter and delay bladder tumour relapse. BMC Cancer. 2011;11:297. doi: 10.1186/1471-2407-11-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genega E.M., Porter C.R. Urothelial neoplasms of the kidney and ureter. An epidemiologic, pathologic, and clinical review. Am J Clin Pathol. 2002;117:S36–S48. doi: 10.1309/CCTJ-MKK1-XU0T-X3DY. [DOI] [PubMed] [Google Scholar]
- 6.Roupret M., Babjuk M., Comperat E. European Association of Urology Guidelines on upper urinary tract urothelial cell carcinoma: 2015 Update. Eur Urol. 2015;68:868–879. doi: 10.1016/j.eururo.2015.06.044. [DOI] [PubMed] [Google Scholar]
- 7.Twinem F.P. Primary tumors of the ureter. J Am Med Assoc. 1957;163:808–813. doi: 10.1001/jama.1957.02970450010004. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology for Bladder Cancer (Version 2.2017) 2017. https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site Available at.
- 9.Benedict S.H., Yenice K.M., Followill D. Stereotactic body radiation therapy: The report of AAPM Task Group 101. Med Phys. 2010;37:4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 10.Eisenhauer E.A., Therasse P., Bogaerts J. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Brookland R.K., Richter M.P. The postoperative irradiation of transitional cell carcinoma of the renal pelvis and ureter. J Urol. 1985;133:952–955. doi: 10.1016/s0022-5347(17)49328-1. [DOI] [PubMed] [Google Scholar]
- 12.Cozad S.C., Smalley S.R., Austenfeld M., Noble M., Jennings S., Reymond R. Adjuvant radiotherapy in high stage transitional cell carcinoma of the renal pelvis and ureter. Int J Radiat Oncol Biol Phys. 1992;24:743–745. doi: 10.1016/0360-3016(92)90723-u. [DOI] [PubMed] [Google Scholar]
- 13.Fang D., Seisen T., Yang K. A systematic review and meta-analysis of oncological and renal function outcomes obtained after segmental ureterectomy versus radical nephroureterectomy for upper tract urothelial carcinoma. Eur J Surg Oncol. 2016;42:1625–1635. doi: 10.1016/j.ejso.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozsahin M., Zouhair A., Villa S. Prognostic factors in urothelial renal pelvis and ureter tumours: A multicentre Rare Cancer Network study. Eur J Cancer. 1999;35:738–743. doi: 10.1016/s0959-8049(99)00012-x. [DOI] [PubMed] [Google Scholar]
- 15.Jang N.Y., Kim I.A., Byun S.S., Lee S.E., Kim J.S. Patterns of failure and prognostic factors for locoregional recurrence after radical surgery in upper urinary tract transitional cell carcinoma: Implications for adjuvant radiotherapy. Urol Int. 2013;90:202–206. doi: 10.1159/000343729. [DOI] [PubMed] [Google Scholar]
- 16.Czito B., Zietman A., Kaufman D., Skowronski U., Shipley W. Adjuvant radiotherapy with and without concurrent chemotherapy for locally advanced transitional cell carcinoma of the renal pelvis and ureter. J Urol. 2004;172:1271–1275. doi: 10.1097/01.ju.0000137910.38441.8a. [DOI] [PubMed] [Google Scholar]
- 17.Cook E.N., Counseller V.S. Primary epithelioma of the ureter. JAMA. 1941;116:122–127. [Google Scholar]
- 18.Fan K.H., Chen Y.C., Leung W.M., Chuang C.K., Pang S.T., Hong J.H. Adjuvant and salvage radiotherapy for urothelial cell carcinoma of the upper urinary tract: Experience in a single institution. Chang Gung Med J. 2012;35:247–254. doi: 10.4103/2319-4170.106147. [DOI] [PubMed] [Google Scholar]
- 19.ASTRO Model policies for stereotactic body radiation therapy (SBRT) 2014. https://www.astro.org/uploadedFiles/_MAIN_SITE/Daily_Practice/Reimbursement/Model_Policies/Content_Pieces/ASTROSBRTModelPolicy.pdf Am Med Assoc; Available at.
- 20.Fakiris A.J., McGarry R.C., Yiannoutsos C.T. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: Four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75:677–682. doi: 10.1016/j.ijrobp.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 21.Timmerman R., Paulus R., Galvin J. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lo S.S., Fakiris A.J., Chang E.L. Stereotactic body radiation therapy: A novel treatment modality. Nat Rev Clin Oncol. 2010;7:44–54. doi: 10.1038/nrclinonc.2009.188. [DOI] [PubMed] [Google Scholar]
- 23.Siva S., Pham D., Gill S., Corcoran N.M., Foroudi F. A systematic review of stereotactic radiotherapy ablation for primary renal cell carcinoma. BJU Int. 2012;110:E737–E743. doi: 10.1111/j.1464-410X.2012.11550.x. [DOI] [PubMed] [Google Scholar]
- 24.Pham D., Thompson A., Kron T. Stereotactic ablative body radiation therapy for primary kidney cancer: A 3-dimensional conformal technique associated with low rates of early toxicity. Int J Radiat Oncol Biol Phys. 2014;90:1061–1068. doi: 10.1016/j.ijrobp.2014.07.043. [DOI] [PubMed] [Google Scholar]
- 25.Ponsky L., Lo S.S., Zhang Y. Phase I dose-escalation study of stereotactic body radiotherapy (SBRT) for poor surgical candidates with localized renal cell carcinoma. Radiother Oncol. 2015;117:183–187. doi: 10.1016/j.radonc.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 26.Maehata Y., Kuriyama K., Aoki S., Araya M., Marino K., Onishi H. Stereotactic body radiotherapy for localized ureter ransitional cell carcinoma: Three case reports. Case Rep Urol. 2015;2015:519897. doi: 10.1155/2015/519897. [DOI] [PMC free article] [PubMed] [Google Scholar]