Abstract
Introduction:
Ovarian cancer has the highest mortality rate of all gynecological tumors and represent fifth leading cause of cancer death in women, due to the absence of early symptoms of the disease and the lack of screening tests. A new HE4 ovarian cancer biomarker was found in various studies, with different populations having quite different sensitivity and specificity values as well as reference values. The tumor marker HE4 is a useful marker for ovarian cancer detection with only minimal expression in normal ovarian tissue. HE4 is particularly promising as a marker for early detection, showing its potential in differentiating women with ovarian cancer than those with benign (nonmalignant) ovarian conditions.
Material and methods:
Samples were taken in serum gel tubes, centrifuged and stored until analysis. A total of 300 respondents was included, of whom 188 were premenopausal women and 112 postmenopausal women. Respondents were divided into two groups by age: women aged 50-75 years and Control group of women aged 20-50 years. The HE4 assay was performed by the Immunological Test (ECLIA) for the quantitative determination of HE4 in human serum or plasma. Solid phase test, two-step reaction using immune 2HP and 3HD8 monoclonal antibodies („sandwich principle“).
Results:
Tumor markers HE4 analysis by groups indicate that in the postmenopausal group there was 92 (82.14%) of subjects with normal tumor markers HE4 and 170 (90.43%) in the premenopausal group. The elevated values of HE4 tumor markers in the postmenopausal group were in 20 (17.86%) cases and in the premenopausal group 18 (9.57%). Statistical analysis (nonparametric method) established the upper limit of the reference value of HE4 tumor markers in premenopausal and postmenopausal women.
Conclusion:
The established reference value for the women population in Canton Sarajevo for premenopausal women is <78.6 pmol/l, and for postmenopausal women <122.5 pmol/l. We found that the values of HE4 tumor markers differ significantly in premenopausal and postmenopausal women (p=0.0391). Postmenopausal women have a higher incidence of high-value tumor markers HE4. By comparison of the reference interval of the tumor marker HE4 for the population of women from Canton Sarajevo, determined by our research, with a reference interval for the German and Asian populations, we found a significant difference between the upper limit of the reference value in postmenopausal women (p<0.05), while in premenopausal women the difference was not statistically significant (p=0.4314).
Keywords: HE4, tumor marker, ovarian cancer, reference values
1. INTRODUCTION
Ovarian cancer has the highest mortality rate of all gynecological tumors and represent fifth leading cause of cancer-related death in women, due to the absence of early symptoms of the disease and the lack of screening tests. This tumor is mostly diagnosed at an advanced stage when ovarian cancer has spread beyond the ovaries. Annually are diagnosed 200,000 cases worldwide with a higher incidence in US and Northern Europe and lower incidence in Africa and Asia. Frequency varies from country to country (lowest in Japan and highest in Scandinavia) (1).
Although uterus neck and uterus carcinomas occur more often than ovarian cancer, a far greater percentage of women die from ovarian cancer. The reason for this is later diagnosis due to lack of symptoms at early stages of disease and faster spread of malignant cells in the body (2).
With early detection of the disease 5-years survival could be 95%. In most literature on ovarian carcinoma, it is stated that, as a rule, there are no early symptoms of illness and it is often called „silent killer“ (3).
The authors then demonstrated that in 95% of women who had some symptoms of the disease, and most often gastrointestinal problems, ovarian cancer was diagnosed only after many years (4). Late detection of malignant ovarian and endometrial cancers usually has a poor prognosis. That is why a marker that will enable early diagnosis is needed. Due to the difficult diagnosis and the insufficient sensitivity and specificity of the CA125 tumor marker, many potential markers for ovarian and endometrial cancers have been studied, among which only HE4 has shown great potential for clinical use (5).
Determination of tumor markers is a mandatory part of the diagnostic process, treatment course and monitoring of patients with various types of ovarian cancer. Reference values for serum tumor markers are not sufficiently well defined and can only be used for general orientation. The main principle in determination of reference values according to IFCC recommendations requires that working conditions should not differ from daily routine (6).
The determination of reference values for blood components and other body fluids is carried out according to the following research plan:
Crating the form, determine criteria for selection of reference persons and establish a list of diagnoses according to the International Classification of Diseases (ICD) for previous diseases acceptable in the selection of reference persons.
Determination of the sample size from the reference population and the relationship of subjects by age and gender within the sample.
Review and selection of reference persons.
Standardize the preparation of reference persons for taking blood and other human material.
Taking blood and other body fluids and standardizing the preparation of human material for analysis.
Statistical processing and making reference persons.
The tumor marker HE4 is a useful marker for ovarian cancer detection with only minimal expression in normal ovarian tissue. HE4 is particularly promising as a marker for early detection, showing its potential in differentiating women with ovarian cancer from those with benign (nonmalignant) ovarian states. Immunochemical analyzes have shown that 32% of ovarian tumors that do not have elevated CA125 values have elevated HE4 values (7).
The International Federation for Clinical Chemistry and Laboratory Medicine and the Institute for Clinical and Laboratory Standards have described methods for determining reference intervals for biomarkers. The upper 95% reference limit of the 95th reference interval is used with 90% accuracy interval. The upper limit for premenopausal women is 118.9 pM (90% CI: 97.7–167.4) and for postmenopausal women the upper limit is 167.8 pM (90% CI: 140.8–212.7). The upper limit of normal value is 146.3 pM (90% CI 138.0 -191.5) equivalent to 150 pM thresholds reported in the FDA annex for HE4 EIA test. When testing the cut-off of 60 years for all women (before and after menopause), women under the age of 60 had an upper limit of 116.9 pM (90% CI: 102.6-138.0) and women over the age of 60 had the upper limit of normal values of 212.7 (90% CI: 152.7-234.3) (8).
2. MATERIAL AND METHODS
The study was conducted as prospective in the period from June 1, 2014 to June 0, 2015 in the Biochemical and Hematological laboratory “Medical Laboratory” in Ilidza (Sarajevo). During this period, blood was taken by the subjects for analysis, which, after complete gynecological examinations, were referred to laboratory tests. Sampling was performed according to NACB (National Academy of Clinical Biochemistry) and EGTM (European Group for Tumor Markers). Samples were taken in serum gel tubes, centrifuged and stored until analysis. A total of 300 subjects were interviewed, of whom 188 were premenopausal women and 112 postmenopausal women.
Subjects were divided into two groups by age:
Group I–Subjects aged 50-75 years;
Group II–The control group of respondents 20-50 years old.
All samples were divided according to the time taken into the A–samples taken from 9 to 11 hours and the B samples taken from 16 to 18 hours. In the period from 9 to 11 hours, 300 samples were taken, and 16 samples were taken from 16 to 18 hours.
HE4 determination procedure: Immunological Test (ECLIA) for Quantitative Determination of HE4 in Human Serum or Plasma. Technically, this is a solid phase test, a two-step reaction using immune 2HP and 3HD8 monoclonal antibodies. The test principle is the so-called, sandwich principle.
3. RESULTS
We determined the precision and accuracy of the method for determination of the tumor marker HE4 on the Elecsys 2010 analyzer. With the univariate statistical analysis of the control serum HE4 tumor marker determined is high confidence in the determination of this test (p>0.05). It was found that the method for determination at the analyzer Elecsys 2010 showed a satisfactory repeatability of results. The normal distribution test in case of control values indicated acceptable values. Values of HE4 tumor marker serum samples ranged within ± 1-2 SD. For the determination of the accuracy of the HE4 tumor marker method we investigated the deviation of the PreciCotrol HE4 1 and 2 control serum values from the expected value.
The method of determining the tumor marker HE4 meets the accuracy criteria because the deviation for the PreciControl TM1 serum sample is 0.031% and from PreciControl TM2 is 2.61%. Analysis of the HE4 tumor marker after comparing 20 samples taken from 9 to 11 hours and 20 of the same samples taken from 16 to 18 hours we found that the correlation coefficient r = 0.9825 and the significant level is 0.0009, indicating that the sampling time had no effect on the values of the HE4 tumor marker. We found the values of HE4 tumor markers in samples in which are the values of the parameters: creatinine, GFR, ABS and CRPhs outside the reference interval. By analyzing the values of the tumor marker HE4 in the samples with elevated values of the examined laboratory parameters, we found that the highest effect on elevated values of HE4 tumor markers from all examined parameters had elevated CRPhs, GFR and creatinine values.
To determine the reference interval of the HE4 tumor marker in the Sarajevo Canton respondents, we included only subjects with normal gynecological findings and subjects with normal laboratory parameters: creatinine, GFR, potassium, ABS and CRPhs. We examined the age of the respondents and divided them into two groups according to the reproductive age into the premenopausal and postmenopausal women. By analyzing age distribution of respondents, we found that there is a total of 112 (37.33%) postmenopausal women samples and 188 (62.67%) samples from premenopausal women. By the analysis of tumor markers HE4 per group, we found that in the postmenopausal group there were 92 (82.14%) subjects with normal tumor markers HE4 and 170 (90.43%) in the premenopausal group. In the postmenopausal group elevated values of HE4 was determined in 20 (17.86%) and in the premenopausal group in 18 (9.57%) cases. Compared to the reproductive age value of the HE4 tumor marker, values were statistically significantly different (p=0.0391). In the postmenopausal group there is a higher incidence of high values of the tumor marker HE4 (Table 1) as showed by the presentation of the HE4 tumor marker values per age groups (Figure 1).
Table 1. Values of HE4 tumor markers by age groups.
| N | t | df | Sig. (2-tailed) | Mean value difference | 95% confidence interval | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| (20-29) years | 28 | 51.140 | 27 | .000 | 261.786 | 25.128 | 27.229 |
| HE4 | 29 | 16.406 | 28 | .000 | 417.140.091.590 | 365.057.261.178 | 469.222.922.003 |
| (30-39) years | 78 | 109.082 | 77 | .000 | 341.923 | 33.568 | 34.816 |
| HE4 | 79 | 29.369 | 78 | .000 | 48.033.888.532.950 | 4.477.780.079.781 | 51.289.976.268.083 |
| (40-49) years | 81 | 136.072 | 80 | .000 | 442.593 | 43.612 | 44.907 |
| HE4 | 82 | 32.361 | 81 | .000 | 5.158.861.507.624.920 | 484.167.708.707 | 54.760.459.281.709 |
| (50-65) years | 99 | 137.058 | 98 | .000 | 562.828 | 55.468 | 57.098 |
| HE4 | 95 | 27.074 | 94 | .000 | 54.663.145.800.223 | 50.654.292.481.858 | 58.671.999.118.588 |
| >65 years | 13 | 72.984 | 12 | .000 | 703.077 | 68.209 | 72.407 |
| HE4 | 13 | 9.916 | 12 | .000 | 57.709.270.582.005 | 45.028.909.277.122 | 70.389.631.886.887 |
Figure 1. Concentration of HE4 tumor marker by age groups.
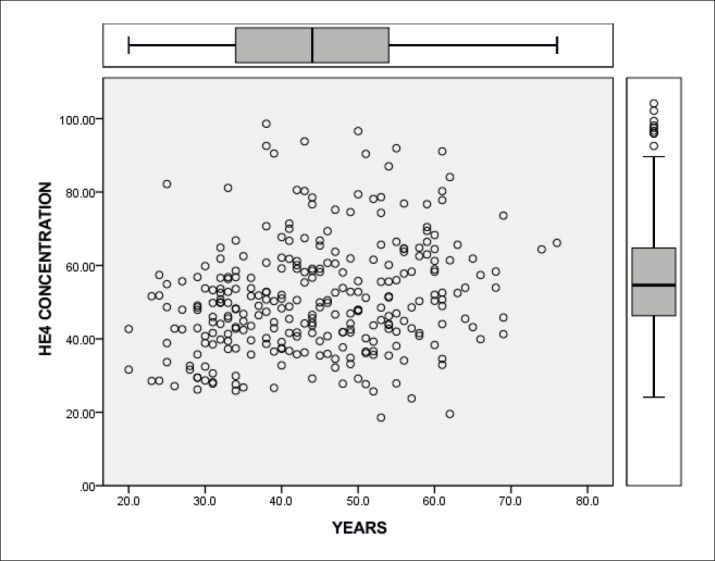
Statistical analysis (nonparametric method) determined the upper limit of the reference value of the tumor marker HE4 in premenopausal and postmenopausal women (Table 2).
Table 2. Values of HE4 tumor markers per group.
| Variable | Group I | Group II | |||
| No. of samples | 94 | 168 | |||
| Minimum value | 19.5 | 25.87 | |||
| Maximum value | 161.1 | 134.1 | |||
| Mean | 60.3771 | 49.6692 | |||
| Percent-iles | 95% Confidence Interval | 95% Confidence Interval | |||
| 2.5 | 25.40 | 27.00 | 25.9310 to 28.5332 | ||
| 5 | 29.76 | 21.9347 to 35.1913 | 28.49 | 26.7337 to 29.8073 | |
| 10 | 35.37 | 28.6827 to 398592 | 31.60 | 28.5910 to 34.9833 | |
| 25 | 43.81 | 39.9110 to 47.6880 | 39.01 | 36.4749 to 41.6072 | |
| 75 | 65.63 | 62.2429 to 76.8530 | 57.12 | 53.6171 to 60.5431 | |
| 90 | 87.34 | 76.9194 to 129.4841 | 67.68 | 62.4160 to 76.4969 | |
| 95 | 122.50 | 87.9929 to 200.8108 | 78.69 | 70.1123 to 94.5114 | |
We found that the upper limit of reference values for the female population of Canton Sarajevo at postmenopausal women group is <122 pmol/l, and in premenopausal women <78.68 pmol/l. The upper limits of the reference values for the female population of Canton Sarajevo were compared with the upper limits for the German population determined by the research of Roche diagnostics and for the Asian population as determined by Mokhtar N and associates (Table 3).
Table 3. Comparison of the tumor marker HE4 reference values.
| Age | Canton Sarajevo | German population | Asia population |
|---|---|---|---|
| Postmenopause | < 122.50 pmol/l HE4 | < 104.00 pmol/l HE4 | < 140.00 pmol/l HE4 |
| Premenopause | < 78.68 pmol/l HE4 | < 76.20 pmol/l HE4 | < 70.00 pmol/l HE4 |
By comparison of the upper limit of the reference values for the population of the Canton Sarajevo in comparison to the German and Asian populations, we notice that there is a significant difference between the upper limit of the reference value in postmenopausal women (p<0.05), while in premenopausal women the difference is not statistically significant (p = 4314).
4. DISCUSSION
Ovarian cancer has a high mortality rate and represents fifth leading cause of cancer-related death in women. In ovarian cancer diagnosis, therefore, all efforts are directed towards finding a new, better biomarker that would be more specific to this disease. In the present practice, the most commonly used ovarian cancer biomarker is CA125. More recently, from all of the potential biomarkers tested, the HE4 had the best results for clinical use (9).
Research in this field can accelerate the process of introducing the tumor marker HE4 for early detection and monitoring of ovarian cancer therapy. A new HE4 ovarian cancer biomarker in different studies, with different populations have quite different sensitivity and specificity values as well as reference values. The value obtained within the reference values does not exclude malignancy, while the tumor marker concentration above the reference value does not necessarily represent the presence of the cancer. Changes in values over time are probably more clinically useful than value obtained at one-time point (10).
Researchers found that HE4 and Mesothelin are more effective in recognizing serous subtype ovarian carcinoma than endometrial and mucinous subtypes. Given that serous ovarian cancer cells are the most common cellular form and are most difficult to diagnose if they are only restricted to ovaries, markers such as HE4 and Mesothelium may bring major improvement in the detection of ovarian cancer (11).
The precision of the tumor marker HE4 determination method was tested by 30 serum control samples and their target values. It was estimated using the univariate statistical analysis and showed satisfactory results (p> 0.05). It has been established that the Elecsys 2010 assay method demonstrates a satisfactory degree of repeatability of the results, which is consistent with the clinical study in Mannheim. For the determination of the accuracy of the tumor marker method we investigated the deviation of the values of the PreciCotrol HE4 1 and 2 control serum from the expected value. The method for determining the tumor marker HE4 meets the accuracy criteria because the deviation of the PreciControl TM1 control serum samples is 0.031% and PreciControl TM2 is 2.61%. The HE4 tumor markers can also influence hormonal changes such as menstrual cycle, menopause, medication or contraception (12).
Roche Diagnostics has conducted a study at one clinic in Germany with Elecsys HE4 test on a series of 358 healthy women. Likewise, the distribution of HE4 test values was established in two clinic centers in Spain and Germany with Elecsys HE4 test on 896 samples. In this study, 98% of healthy women had a HE4 test value at or below 140 pmol/l.
It is recommended that each laboratory establishes its reference value for the population of interest (13).
By analysis of the HE4 tumor marker according to age groups we found that in the postmenopausal group there was 92 (82.14%) samples with normal values of HE4 tumor marker and 170 (90.43%) in premenopausal women. In the postmenopausal group elevated HE4 was found in 20 (17.86%) and in the premenopausal group in 18 (9.57%) samples. The study for the determination of the reference interval included 262 subjects with normal gynecological findings without kidney, lung, cardiovascular system as well as infectious diseases. The statistical value of the tumor marker HE4 showed the reference values of this tumor marker for premenopausal women <78.68 pmol/l and for postmenopausal women 122.5 pmol/l. It was found that relative risk for increased values of HE4 tumor marker exists in postmenopausal women compared to premenopausal women. Our analysis showed that age was a significant predictor of high values of HE4 tumor marker, which is consistent with study of Huthinen and colleagues. This study found that in women who were considered healthy, by the method of random sampling was found that 38 (12.66%) had the positive values of the tumor marker HE4.
Mokhtar et al. conducted research and reached the upper 95% (90% CI) reference interval for all women at 64.6 pmol/l, 58.4 pmol/l for premenopausal and 69.0 pmol/l for postmenopausal women. It has been observed that the concentration of HE4 increases with age, especially in women over the age of 50 years. They found that their suggested reference limit was lower compared to the level given by Abbott Architect HE4 insert (58.4 vs. 70 pmol/l for premenopausal and 69.0 vs. 140 pmol/l in postmenopausal group). The study also showed a significant difference in the concentration of HE4 between ethnic groups (Malesia and Indian). The HE4 level in group of Indian women was higher than in Malays (p <0.05), while there were no significant differences between Malaysian and Chinese ethnic groups (14).
In Hungary, a study has been conducted and the following conclusions have been reached. When a woman is in menopause age and if the HE4 level is <147 pmol/l, and what is very similar to the internationally accepted value of <140 pmol/l, there is a 9% chance of cancer. If HE4 is higher than 147 pmol/l and eGFR is not below 48 ml/min/1.73m2, 95% of cases are probably malignant. In cases of those patients where the HE4 level is abnormal and eGFR is below 48 ml/min/1.73m2, CA125 concentration should be considered. If it is below 66 kIU/l, there is a relatively moderate chance of cancer (21%), while in the case of an increased CA125 level, the risk of malignancy is much higher (71%). The AUC ROC curve value was 0.951 (confidence interval 0.920-0.982). In women in postmenopause with HE4 over 210 pmol/l and with eGFR N 28 ml/min/1.73m2, there is a very high risk for ovarian cancer (92%). If HE4 is normal, the chance of cancer is practically equal to zero. Within the HE4 range of 112-210 pmol/l, CA125 should be considered: if it is greater than 69 kIU/l, patients have an increased likelihood for cancer (67%), women in premenopause, nor CA125 nor eGFR have shown significant effect on HE4, therefore a reference value for HE4 <91 pmol/l is taken (15).
Some studies and determination of reference intervals of HE4 were conducted in Croatia. The results of this studies are mostly consistent with current literature data. Although the AUC for HE4 is higher than for CA125 has been shown to be statistically not significant. The results showed that the CA 125 limit value should be shifted to 35 kIU/l at 44 kIU/l because it is optimal and the limit value of HE4 at 140 pmol/L to 120 pmol/l (16).
By comparison of the upper limit of the reference values for the population of Canton Sarajevo in relation to the German and Asian populations we notice that there is a significant difference between the upper limit of the reference value in postmenopausal women (p<0.05), while in premenopausal women the difference is not statistically significant (p=4314). By comparing the values of the tumor marker HE4 according to the reference values for the German population of Roche diagnostics compared to the reference values determined by our research, we have found that the number of positive values according to the reference values determined by our research was significantly lower (17.67%) versus 38 (12.67%) of positive values according to the reference values for the German population. The number of negative values is significantly higher 283 (94.33%) versus 262 (87.33%). Such results are in line with the fact that in postmenopausal women in our population there are often elevated values of the HE4 tumor marker, that cannot be evidence of ovarian cancer, lungs, kidney any other disease. One of the advantages of HE4 is that it seems to be able to correctly identify benign lesions in relation to CA125. However, due to extreme heterogeneity and genetic abnormalities in ovarian cancer, there is a need to use multiple markers, of which HE4 and CA125 may be components. By combining these two markers, detection of those CA125 non-expression cases is enabled. HE4 shows high sensitivity to ovarian cancer detection and can be used with CA125 as a predictor of malignancy. Additional benefits of HE4 are to help respond to therapy in patients with invasive ovarian cancer and as a marker to detect relapses following primary tumor treatment (17).
5. CONCLUSIONS
The established reference value for the female population in Canton Sarajevo for premenopausal women is <78.6 pmol/l, and for postmenopausal women <122.5 pmol/l.
We found that the values of HE4 tumor markers differ significantly in premenopausal and postmenopausal women (p=0.0391). Postmenopausal women have a higher incidence of high values of tumor marker HE4.
By comparing the reference interval of the tumor marker HE4 for the population of women of Sarajevo Canton, determined by our research with a reference interval for the German and Asian populations, we found that these values for postmenopausal women significantly differ, while in premenopausal women the difference is not statistically significant.
Conflict of interest:
none declared.
Contribution by the author:
Lejla Hasanbegović carried out research and participated in the writing of the paper. Nedeljka Šljivo participated in the writing of the paper and she’s in charge of correspondence.
REFERENCES
- 1.Allard JW. HE4 and CA125 combined for the improved managment of ovarian cancer. Oncologie. 2009;3:20–1. [Google Scholar]
- 2.Schapira MM, Matchar DB, Young MJ. Die Wirksamkheit von Eierstock-Krebs Screening: eine Entscheidung Analyse-Modell. Annals of Internal Medicine. 1993;118(11):838–43. doi: 10.7326/0003-4819-118-11-199306010-00002. [DOI] [PubMed] [Google Scholar]
- 3.Huthinen K. Serum HE4 concentracion differentiates malignant ovarian tumors from ovarian endometriotic cysts. 2009;100:1315–20. doi: 10.1038/sj.bjc.6605011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crombach G, Scharl A, et al. Detection of squamous cell carcinoma antigen in normal squamous epithelial and in squamous cell carcinomas of the uterine cervix. Cancer. 1989;63(Supll 1):1337. doi: 10.1002/1097-0142(19890401)63:7<1337::aid-cncr2820630719>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 5.Hellstrom, et al. The He4 protein is a biomarker for ovarian carcinoma. Cancer Research. 2003;63:3695–3700. [PubMed] [Google Scholar]
- 6.Ćorić J, i saradnici . Kontrola kvaliteta rada u laboratorijskoj medicini. 5. Vol. 71. Sarajevo: Univerzitetsko izdanje; 2015. p. 75. [Google Scholar]
- 7.Journal of Womens Health. 2011;20(2):2273–5. [Google Scholar]
- 8.Selby C. Interference in immunoassay. Ann Clin Biohem. 1999;36:704–21. doi: 10.1177/000456329903600603. [DOI] [PubMed] [Google Scholar]
- 9.Moore GR, Miller MC, Eklund EE, Lu HK, Bast CR, Jr, Lambert-Messerlian G. Serum Levels of the Ovarian Cancer Biomarker HE4 are decreased in Pregnancy and Increase with Age. [2012 Apr];Am J Obstet. 206(4):349. doi: 10.1016/j.ajog.2011.12.028. Rerterived: https://www.ncbi.nlm.nih.gov/pubmed/22301440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molina R, Filella X, Jo J, Augusti C, Ballesta AM. CA125 in biological fluids. Int J Biol Markers. 1998 Oct-Dec;13(4):224–30. doi: 10.1177/172460089801300410. [DOI] [PubMed] [Google Scholar]
- 11.Jelic S, ESMO Guidelines Working Group Hepatocellular carcinoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009 May;20(Suppl 4):41–5. doi: 10.1093/annonc/mdp124. [DOI] [PubMed] [Google Scholar]
- 12.Moore R, et al. HE4 and Epithelial Ovarian Cancer. Gynecol Oncol. 108:402–8. [Google Scholar]
- 13.Chudecka-Glaz A, Cymbaluk-Ploska A, Strojna A, Menkiszak J. HE4 Serum Levels in Patients with BRCA 1 Gene Mutation Undergoing Prophylactic Surgery as well as in Other Benign and Malignant Gynecological Diseases. Journals Disease Markers. 2017;13:9792756. doi: 10.1155/2017/9792756. Retreived: http://www.cobas.com/content/dam/cobas_com/pdf/product/Elecsys%20HE4%20Human%20Epididymal%20Protein%204/HE4%20fact%20she. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mokhtar N, Thevarajah M, Ma N MI. Human Epididymis protein 4 reference intervals in a multiethnic asian women population. Asian Pac J Cancer Prev. 2012;13(12):6391–5. doi: 10.7314/apjcp.2012.13.12.6391. [DOI] [PubMed] [Google Scholar]
- 15.János Kappelmayer ⁎, Péter Antal-Szalmás, Béla Nagy., Jr Human epididymis protein 4 (HE4) in laboratory medicine and an algorithm in renal disorders. Clinica Chimica Acta. 2015;38:35–42. doi: 10.1016/j.cca.2014.07.040. [DOI] [PubMed] [Google Scholar]
- 16.Troha M. Diplomski rad, Sveučilište u Zagrebu, Farmaceutsko-biokemijski fakultet; 2016. Dijagnostička vrijednost tumorskog biljega HE4 u diferencijalnoj dijagnostici ginekoloških oboljenja; p. 41. [Google Scholar]
- 17.Bignotti E, Ragnoli M, Zanotti L, Calza S, Falchetti M, Lonardi S, Bergamelli S, Bandiera E, Tassi RA, Romani C, Todeschini P, Odicino FE, Facchetti F, Pecorelli S, Ravaggi A. Diagnostic and Prognostic impact of serum HE4 detection in Endometrial carcinoma Patients. Br J Cancer. 2011 Apr 26;104(9):1418–25. doi: 10.1038/bjc.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]


