Abstract
Background
Colorectal cancer (CRC) is the third most common cancer worldwide, making it is a serious threat to human health. It is imperative to develop new therapeutics to improve the CRC treatment efficiency. The aim of this study was to investigate the role of NPRL2 in improving sensitivity to CPT-11 in colon cancer cells.
Material/Methods
NPRL2 overexpression was established by transfecting the recombinant lentivirus-encoding NPRL2 gene into HCT116 colon cancer cells. Cell proliferation was identified using Cell Counting Kit-8 (CCK8) assay. Cell cycle and apoptosis were examined by flow cytometry. An immunofluorescence staining assay was conducted to examine the expression of γ-H2AX. Wound-healing and Transwell assays were utilized to show cell migration and invasion capability. The expression of apoptosis-related proteins (cleaved caspase-3, caspase-9, cleaved PARP, BAX, and Bcl-2), invasion-related proteins (MMP2, MMP9, p-PI3K, and p-AKT), and DNA damage checkpoint pathway proteins (p-ATM, p-Chk2, Cdc25C, Cdc2, and Cyclin B1) were quantified by Western blotting.
Results
A CCK8 assay revealed that the overexpression of NPRL2 improved the sensitivity of CPT-11 in HCT116 cells (P<0.05). Functionally, NPRL2 overexpression elevated the sensitivity of CPT-11 by preventing colon cancer cell proliferation, cell movement, and invasion, and promoting cell apoptosis and G2/M cell cycle arrest. Mechanistically, NPRL2 overexpression enhanced CPT-11 sensitivity by activating the DNA damage checkpoint pathway.
Conclusions
NPRL2 overexpression enhances sensitivity to CPT-11 treatment in colon cancer cells, and it may serve as a molecular therapeutic agent to treat patients with CRC.
MeSH Keywords: Apoptosis, Colonic Neoplasms, DNA Damage
Background
Colorectal cancer (CRC), the third most frequently diagnosed cancer across the globe, is a grave danger to human health [1]. Despite clinical improvements with chemotherapy for CRC, resistance exists in most patients and the survival rate of CRC patients is significantly reduced once drug resistance is determined. Therefore, it is critical to determine and create new and safe therapies to improve treatment efficiency, lower toxicities, and halt the advancement of CRC. An emerging treatment method combines DNA-damaging drugs with the overexpression of tumor-suppressor genes to improve therapeutic efficacy.
Nitrogen permease regulator like-2 (NPRL2) is a proposed tumor-suppressor gene that is found at chromosome 3p21.3 [2]. Lowering or halting expression of the NPRL2 gene has been identified in numerous types of cancer, such as lung cancer and renal cell carcinoma [2,3]. NPRL2 down-regulation has been noted in CRC tissues and NPRL2 overexpression has been shown to prevent proliferation and prompt apoptosis in different tumor cell lines [4,5]. Evaluations have indicated that NPRL2 is involved in DNA mismatch repair, cell cycle checkpoint signaling, and control of the apoptotic pathway [2,3].
Irinotecan (CPT-11) is a topoisomerase I inhibitor that induces lethal replication-mediated double-strand breaks (DSBs) [6]. Drug-induced DSBs produce a DNA damage response that features the stimulation of serine-threonine kinases in the ATM-Chk2-Cdc25C-mediated checkpoint pathways and cell cycle arrest at the G2/M cell cycle phase transitions. Based on the level of DNA lesions, the stimulation of DNA damage signaling leads to DNA repair or apoptosis [7]. Evaluations have revealed that NPRL2 elevates vulnerability to anticancer drugs and apoptosis and we previously showed that NPRL2 improves sensitivity to oxaliplatin in colon cancer cells [5,8]. CPT-11 and oxaliplatin are the most often utilized chemotherapy treatments for CRC [9].
These results prompted us to posit that NPRL2 can improve the sensitivity of colon cancer cells to CPT-11 by stimulating the DNA damage checkpoint pathway. In the present study, we investigated the effects of NPRL2 combined with CPT-11 in vitro on CRC cell proliferation, cell cycle progression, cell apoptosis, and cell migration and invasion. We found that NPRL2 enhances the anticancer effects of CPT-11 in colon cancer cells.
Material and Methods
Cell culture
The HCT116 colon cancer cell line was acquired from Boster Biological (Wuhan, China) and cultured in McCoy’s 5A medium along with 10% fetal bovine serum (Boster Biological) and 1% penicillin/streptomycin.
Lentiviral transfection for stable expression clone
LV5-V9797-1 −GFP + Puro plasmids with the NPRL2 gene and negative control (LV-NPRL2 and LV-NC) were purchased from Sangon Biotech (Shanghai, China). HCT116 cells stably expressing NPRL2 were established by transfecting the lentivirus. The empty vector (EV) clones were established with the same method. The transfection effect was detected by Western blotting.
Cell viability assay
HCT116 cells transduced (with or without) the NPRL2 gene were seeded in 96-well plates with 5000 cells per well. A CCK8 assay (Dojindo, Japan) was used to identify the cell viability following after various concentrations of CPT-11 (Selleck Chemicals, Boston, MA) (3.75, 7.5, 15, 30, and 60 μg/ml CPT-11) or throughout in the culture (24, 48, and 72 h). The OD450 was measured by a microplate reader (Multiskan MK3; Thermo Fisher Scientific Inc., Rockford, IL, USA).
Cell apoptosis detection
At 48 h following the inoculation, the cells were digested and rinsed with phosphate-buffered saline (PBS) 2 times and then resuspended in binding buffer at a final density of 2×106 cells/ml. After that, the cells were stained with PE-labeled Annexin-V and 7-AAD (4A Biotech Co., Ltd., Beijing, China) for 5 min at 4°C in the dark. Apoptosis was evaluated using a flow cytometer (Becton Dickinson, Franklin Lakes, NJ).
Cell cycle analysis
Cells transduced with NPRL2 were treated with CPT-11 for 48 h and 7×105 cells were collected. After trypsinization, the cells were rinsed with PBS and then set in 95% ethanol. After that, the cells were rinsed with 1× PBS, resuspended in PBS/1% FCS containing with PI and RNase A (5 mg/ml) (4A Biotech), and then incubated for 30 min at 37°C. The cell cycle distribution was examined by flow cytometry (Becton Dickinson, Franklin Lakes, NJ).
Western blot analysis
Cellular protein extracts were isolated by electrophoresis on a 12% or 8% SDS-polyacrylamide gel and electrophoretically moved onto a PVDF membrane (Millipore, Bedford, MA), which was then obstructed with 5% nonfat powdered milk (Sangon Biotech, Shanghai, China) for 1 h and then incubated overnight at 4°C with primary antibodies against NPRL2 (ab88691, 1μg/ml, Abcam, USA), p-ATM (ab81292, 1: 50000, Abcam, USA), γ-H2AX (ab81299, 1: 1000, Abcam, USA), CyclinB1 (ab32053, 1: 3000, Abcam, USA), p-PI3K (ab191606, 1: 1000, Abcam, USA), p-AKT (ab81283, 1: 5000, Abcam, USA), Chk2 (#2197, 1: 1000, Cell Signaling Technology, USA), Cdc2 (#77055, 1: 1000, Cell Signaling Technology, USA), Bcl-2 (12789-1-AP, 1: 1000, Proteintech Group, USA), BAX (50599-2-AP, 1: 1000, Proteintech Group, USA), Cleaved caspase-3 (25546-1-AP, 1: 1000, Proteintech Group, USA), Caspase-9 (103801-1-AP, 1: 1000, Proteintech Group, USA), MMP2 (10373-2-AP, 1: 200, Proteintech Group, USA), MMP9 (10375-2-AP, 1: 200, Proteintech Group, USA), Cdc25C (16485-1-AP, 1: 500, Proteintech Group, USA) and GADPH (10494-1-AP, 1: 2000, Proteintech Group, USA). After rinsing with PBS and Tween-20, the membrane was incubated with IRDye 800CW goat anti-rabbit IgG (H + L) secondary antibody (1/5000; LI-COR Biosciences, Lincoln, NE) for 1 h. Then, the antigen-antibody complexes were established by an infrared imaging system (Odyssey; LI-COR Biosciences, Lincoln, NE).
Immunofluorescence staining
Cultured HCT116 cells were set with 4% paraformaldehyde for 20 min. After that, the cell membrane was infiltrated with 0.4% Triton X-100 for 1 h and obstructed by goat serum for 1h, and then primary antibody against γ-H2AX (phosphor S139) (ab195189, 1: 200, Abcam, USA) staining at 4°C overnight. After rinsing with PBS, the HCT116 cells were incubated with the correlating Alexa Fluor 647-conjugated secondary antibody (ab150079, 1: 200, Abcam, USA) for 1 h. The cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Beyotime, Haimen, China) for 5 min and examined with a confocal microscope. The area of each γ-H2AX focus was quantified by using the Image J software.
Cell migration assay
A wound-healing assay was utilized to establish cell migration capability. After confluence approached about 90%, a pipette tip was used to produce a scratch on a consistent layer of cells. The scratch was rinsed with PBS 2 times to eliminate unattached cells. Images of the scratch were obtained at 0 h and 24 h for the HCT116 cells with a microscope at 4×10 magnification to quantify the width of the wound, which was measured using Image J software.
Cell invasion assay
Matrigel (BD Biosciences) was placed in the Transwell inserts and polymerized at 37°C. Then, the HCT116 cells in serum-free medium were seeded within the upper wells (1×105 cells/well), and 10% serum-containing medium was placed in the outer wells. After treatment with the CPT-11 for 24 h, the medium was removed from the upper wells. The non-invading cells were removed with a cotton swab; while the invading cells were set with 4% paraformaldehyde, stained with 0.1% crystal violet, and photographed and quantified in 3 indiscriminately chosen fields with a microscope. The number of invasive cells was counted using Image J software.
Statistical analysis
Differences among the cell lines following treatment with empty vector (with or without CPT-11) or with NPRL2 (with or without CPT-11) were analyzed using the repeated measures analysis of variance. The data are the mean ± standard deviation (SD) of 3 individual experiments. Variations between the 2 groups were examined with the two-tailed t test. P<0.05 was regarded as being statistically significant. Results were analyzed using GraphPad Prism 5 (La Jolla, CA, USA) and SPSS software (version 17.0) (Chicago, IL, USA).
Results
Lentiviral transduction of NPRL2
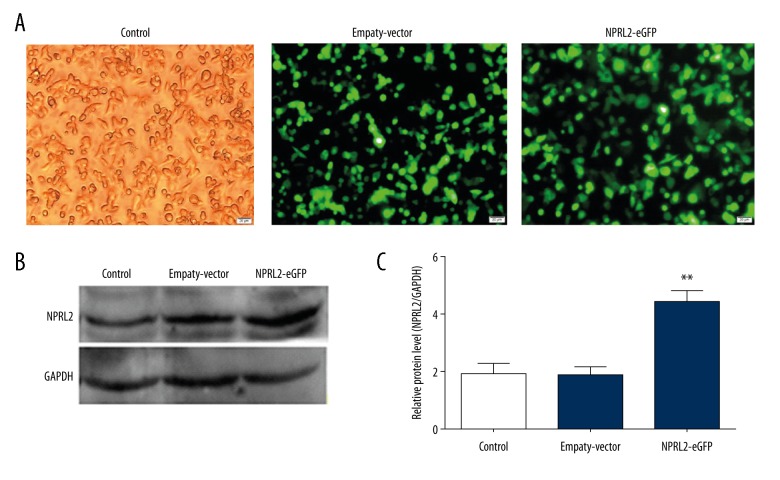
The HCT116 cells were divided into 3 groups: the negative control, empty vector-stably transfected, and NPRL2-stably transfected cell groups. Cells expressed enhanced green fluorescence protein (GFP) after transfection with the lentiviral vectors. After 72 h, the transfection effect was examined using fluorescence microscopy (Figure 1A). Protein expression was further confirmed by Western blotting (Figure 1B, 1C).
Figure 1.
Lentiviral transduction of NPRL2. Monitoring of the transduction efficiency of HCT116 cells by light and fluorescence microscopy (A). Western blot analysis was used to assess transfection efficiency (B, C). Results in bar graph are represented as mean ± SD from three different triplicate experiments. **P<0.01. (Control, cells without transfection; Empty vector (EV), cells transfected with empty vector; NPRL2-eGFP, cells transfected with NPRL2-overexpression vector).
NPRL2 overexpression enhances CPT-11 sensitivity of CRC cells
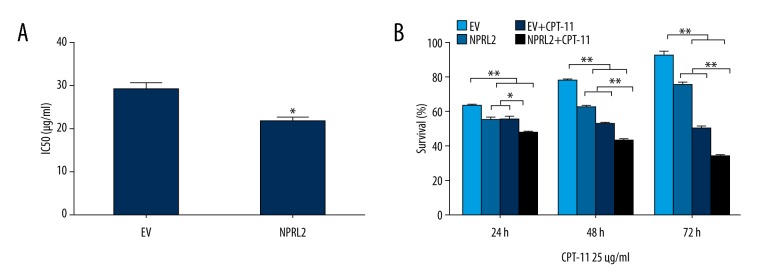
To further study the potential function of NPRL2 in CPT-11–induced cytotoxicity, a CCK8 assay was performed on the transfected cell lines. The IC50 of CPT-11 was reduced in cells transduced with NPRL2 compared to the cells transduced with the empty vector (P<0.05), which suggests that NPRL2 overexpression improves the sensitivity of CPT-11 in HCT116 cells (Figure 2A). Cell survival was examined after empty vector or NPRL2 transduction and following the treatment of 25 μg/ml of CPT-11 in HCT116 cells for 24 h, 48 h, and 72 h. Results revealed that the impacts of NPRL2 on CPT-11 sensitivity were time-dependent (Figure 2B).
Figure 2.
NPRL2 overexpression enhances CPT-11 sensitivity of CRC cells. The IC50 of CPT-11 was lower in cells transduced with NPRL2 than in cells transduced with empty vector (EV) (A). HCT116 cells treated with empty vector (EV) (with or without 25 μg/ml CPT-11) or with NPRL2 (with or without 25 μg/ml CPT-11) for an additional 24, 48 and 72 h, and cell viability was detected by a CCK8 assay (B). Results in bar graphs are represented as mean ±SD from three different triplicate experiments. * P<0.05; ** P<0.01.
NPRL2 overexpression enhances CPT-11 sensitivity by prompting apoptosis in CRC cells
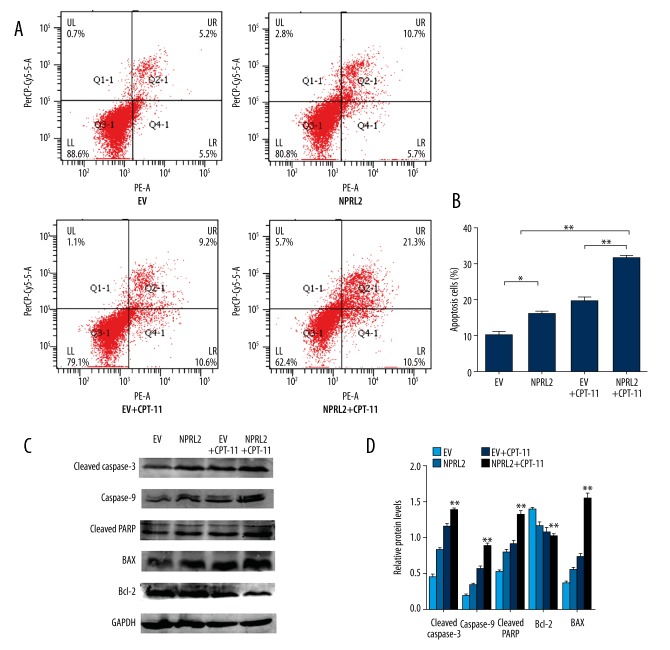
To establish if NPRL2 affected apoptosis, the HCT116 cells transduced with NPRL2 or the empty vector were treated with CPT-11 for 48 h. According to the flow cytometry results, the cells that had been treated with NPRL2 had a higher apoptosis rate than the cells treated with the empty vector. In addition, combined NPRL2 and CPT-11 treatment resulted in significantly greater apoptosis than with either treatment alone (Figure 3A, 3B). To establish the effect of NPRL2 on cell apoptosis, we used Western blotting to evaluate the stimulation of apoptotic proteins in HCT116 cells treated with the empty vector, NPRL2, the empty vector plus CPT-11, or NPRL2 plus CPT-11. The cells with NPRL2 and CPT-11 had higher levels of cleaved caspase-3, caspase-9, cleaved PARP, and BAX, and a decreased level of Bcl-2 compared to the cells with the empty vector, NPRL2, and the empty vector plus CPT-11 (Figure 3C, 3D).
Figure 3.
NPRL2 overexpression enhances CPT-11 sensitivity by prompting apoptosis in CRC cells. HCT116 cells were treated with empty vector (EV), NPRL2, combined empty vector with CPT-11 or combined NPRL2 with CPT-11 for 48 h, and cell apoptosis was determined by Annexin V-PE/7AAD (A). Apoptotic rate of HCT116 cells were calculated (B). The protein levels of cleaved caspase-3, caspase-9, cleaved PARP, BAX and Bcl-2 in HCT116 cells treated with empty vector (EV), NPRL2, empty vector plus CPT-11 or NPRL2 plus CPT-11 for 48 h were revealed by Western blotting. GADPH served as loading Control (C, D). Results in bar graphs are represented as mean ±SD from three different triplicate experiments. * P<0.05; ** P<0.01.
NPRL2 overexpression enhances sensitivity to CPT-11 by increasing γ-H2AX expression in CRC cells
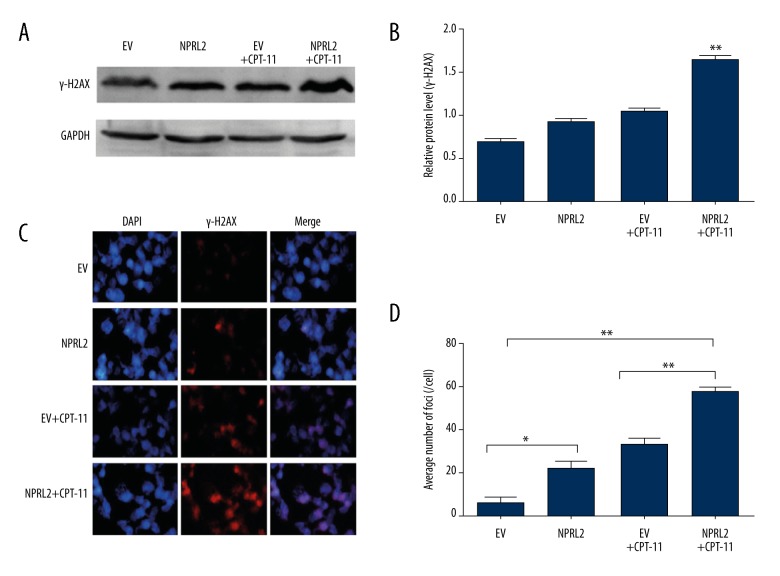
We initially utilized Western blotting to evaluate γ-H2AX expression (Figure 4A). In the HCT116 cells treated with the empty vector, NPRL2, the empty vector plus CPT-11, or NPRL2 plus CPT-11 for 24 h, γ-H2AX protein levels were most significantly elevated in the cells treated with NPRL2 plus CPT-11 (Figure 4B).
Figure 4.
Overexpression enhances sensitivity to CPT-11 by increasing γ-H2AX expression in CRC cells. The protein levels of γ-H2AX in HCT116 cells treated with empty vector (EV), NPRL2, empty vector (EV) plus CPT-11 or NPRL2 plus CPT-11 for 24 h were evaluated by Western blotting. GADPH served as loading Control (A, B). γ-H2AX expression were examined in cells treated with empty vector (EV), NPRL2, empty vector (EV) + CPT-11 or NPRL2 + CPT-11 for 24 h by immunofluorescence staining (C). The bar graph summarizing nuclear foci distribution in the above four groups (D). Results in bar graphs are represented as mean ±SD from three different triplicate experiments. * P<0.05; ** P<0.01.
Next, we analyzed γ-H2AX foci formation using immunofluorescence staining after treatment with the empty vector, NPRL2, the empty vector plus CPT-11, or NPRL2 plus CPT-11 for 24 h in HCT116 cells (Figure 4C). The cells exposed to NPRL2 plus CPT-11 showed more γ-H2AX foci than the cells treated with the empty vector, NPRL2, or the empty vector plus CPT-11 (Figure 4D).
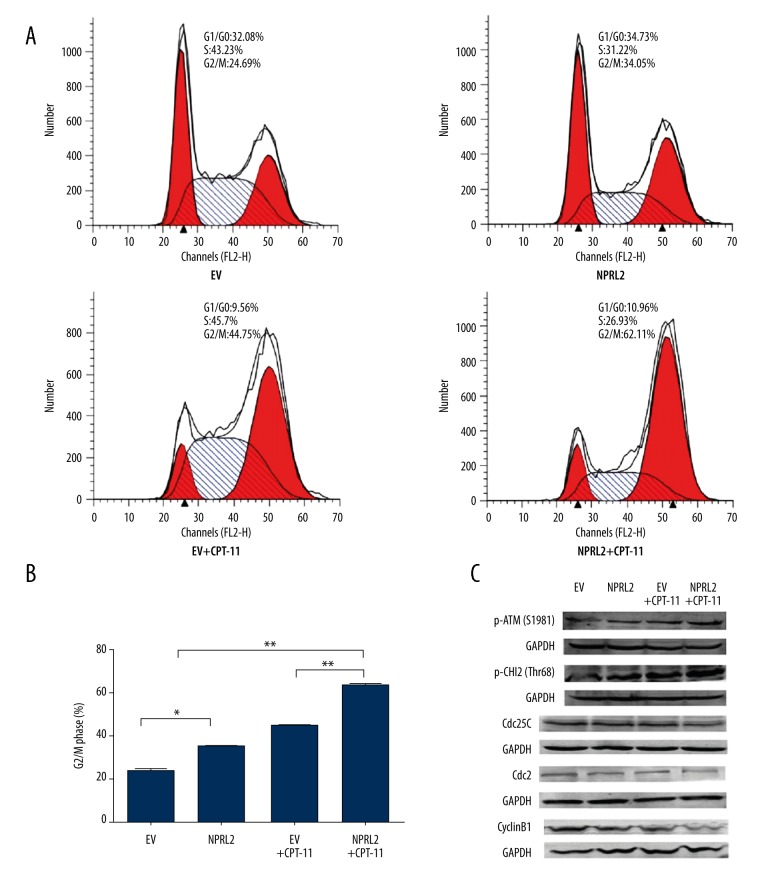
NPRL2 overexpression enhances sensitivity to CPT-11 by inducing G2/M cell cycle arrest in CRC cells
To study whether NPRL2 regulates cell cycle progression in CRC cells, HCT116 cells were treated with the empty vector, NPRL2, the empty vector plus CPT-11, or NPRL2 plus CPT-11 for 48 h and analyzed by flow cytometry (Figure 5A). The results suggested that NPRL2 plus CPT-11 led to a more pronounced accumulation of cells in G2/M and a greater shift toward G2/M arrest than in the cells treated with the empty vector, NPRL2, or the empty vector plus CPT-11 (Figure 5B).
Figure 5.
NPRL2 overexpression enhances sensitivity to CPT-11 by inducing G2/M cell cycle arrest and activating the DNA damage checkpoint pathway in CRC cells. Cell cycle distribution in HCT116 cells treated with empty vector (EV) (with or without CPT-11) or with NPRL2 (with or without CPT-11) for 48 h (A). Bar graphs of HCT116 cells cycle distribution (B). HCT116 cells treated with an empty vector (EV), NPRL2, empty vector (EV) plus CPT-11 or NPRL2 plus CPT-11 were harvested at 48 h and then the expression of p-ATM (S1981), p-Chk2 (Thr68), Cdc25C, Cyclin B1 and Cdc2 were analyzed by Western blotting. GADPH served as loading Control (C). Results in bar graph are represented as mean ±SD from three different triplicate experiments. * P<0.05; ** P<0.01.
NPRL2 overexpression increases sensitivity to CPT-11 by activating the DNA damage checkpoint pathway
We then examined the impact of NPRL2 on cell cycle regulatory proteins. The cells were exposed to the empty vector, NPRL2, the empty vector plus CPT-11, or NPRL2 plus CPT-11, and then the p-ATM (S1981), p-Chk2 (Thr68), Cdc25C, Cdc2, and Cyclin B1 levels were ascertained by Western blotting. The cells treated with NPRL2 plus CPT-11 had higher levels of p-ATM (S1981) and p-Chk2 (Thr68) than the cells treated with the empty vector, NPRL2, or the empty vector plus CPT-11. Conversely, the lower levels of Cdc25C, Cdc2, and Cyclin B1 in the cells treated with NPRL2 plus CTP-11 may explain the greater shift toward G2/M arrest (Figure 5C).
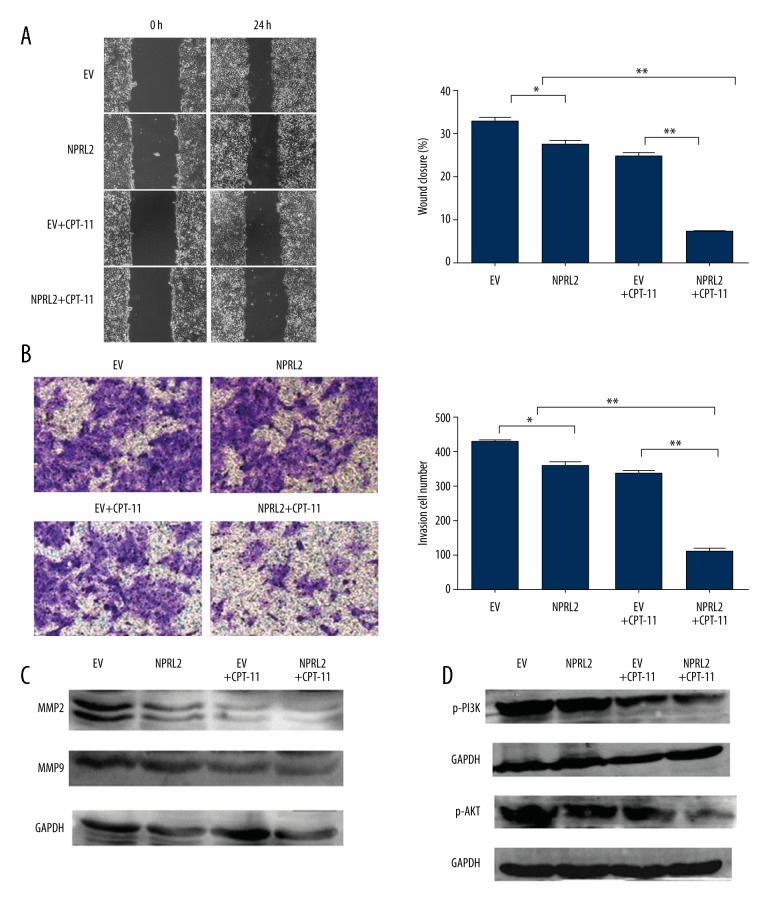
NPRL2 overexpression improves CPT-11–induced inhibition of invasion and migration through the down-regulation of PI3K/AKT pathway-dependent MMP reduction
To evaluate the role of NPRL2 in prevention of invasion and movement of HCT116 cells, the impacts of NPRL2 on the ability of HCT116 cells to move around were examined with a wound-healing assay. After 24 h, the wound-healing assay showed that the migration rate of the cells treated with the combination of CPT-11 and NPRL2 was more strongly inhibited than those with the empty vector, NPRL2, or the empty vector plus CPT-11 (Figure 6A). The impact of NPRL2 on HCT116 cell invasion through Matrigel was also assayed. After 24 h, we discovered that the CPT-11 and NPRL2 amalgam revealed much greater inhibition of the invasive ability of HCT116 colon cancer cells compared to the empty vector, NPRL2, or the empty vector plus CPT-11 (Figure 6B). Further, the expression levels of the MMP-2 and MMP-9 proteins, evaluated by Western blotting, were significantly down-regulated in the CPT-11 and NPRL2 amalgam in contrast to the empty vector, NPRL2, and the empty vector plus CPT-11 (Figure 6C). To additionally examine the latent mechanism of the CPT-11 and NPRL2 amalgam on cell movement and invasion, the PI3K/AKT signaling pathway in HCT116 cells was investigated. Western blot analysis showed that the cells treated with NPRL2 plus CPT-11 showed significantly greater down-regulation of p-PI3K and p-AKT than the cells treated with the empty vector, NPRL2, or the empty vector plus CPT-11 (Figure 6D).
Figure 6.
NPRL2 overexpression improves CPT-11–induced inhibition of invasion and migration through the down-regulation of PI3K/AKT pathway-dependent MMP reduction. Changes of migratory potential were detected by wound-healing assay. The cells were pre-treated empty vector (EV) (with or without CPT-11) or with NPRL2 (with or without CPT-11) for 24 h (A). Transwell assay were done in HCT116 cells treated with empty vector (EV), NPRL2, empty vector (EV) plus CPT-11 or NPRL2 plus CPT-11 for 24 h (B). The protein levels of MMP2, MMP9, p-PI3K and p-AKT in HCT116 cells treated empty vector (EV) (with or without CPT-11) or with NPRL2 (with or without CPT-11) for 24 h were quantified by Western blotting (C, D). Results in bar graphs are represented as mean ± SD from three different triplicate experiments. * P<0.05; ** P<0.01.
Discussion
The NPRL2 gene has strong antitumor activity in vitro and in vivo by limiting proliferation and prompting apoptosis [10]. Yogurtcu et al. reported that down-regulation of the NPRL2 gene may contribute to the progression of colon cancer [11]. NPRL2 expression level was negatively correlated with the survival rate of patients with osteosarcoma and hepatocellular carcinoma (HCC), indicating its value as an independent prognostic marker [12,13]. Furthermore, intratumoral injection of a recombinant adenoviral or lentiviral vector containing NPRL2 gene significantly suppressed tumor growth and tumor progression and metastasis in various human lung cancer and glioma mouse models [10,14]. A prior evaluation revealed that NPRL2, as a molecular therapeutic target for enhancing and resensitizing the reaction to cisplatin, has been documented in lung cancer [15,16]. In addition, NPRL2 expression increased cancer cell sensitivity to several anticancer drugs, including doxorubicin, Taxol, etoposide (VP-16), and 17-(allylamino)-17-demethoxygeldanamycin (17-AAG) [5,17]. However, whether NPRL2 enhances the sensitivity to CPT-11 in colon cancer cells remains unknown.
In the present study we examined the therapeutic impacts of NPRL2 plus CPT-11 in HCT116 cells and investigated the mechanism of action. The results of this study suggest that NPRL2 substantially improved CPT-11 activity in HCT116 cells by inducing cell apoptosis, G2/M arrest, and impeding cell proliferation and cell movement and invasion. An examination of the molecular mechanism of NPRL2 improvement of CPT-11 sensitivity demonstrated that γ-H2AX plays an essential role has a pivotal part because γ-H2AX protein levels were significantly elevated in the cells treated with NPRL2 plus CPT-11 compared to the cells treated with the empty vector, NPRL2, or the empty vector plus CPT-11. γ-H2AX is thought to be a sensitive biomarker for DNA double-strand breaks (DSBs) and is reported to be stimulated by ATM [18]. These discoveries indicate that the sensitizing impact of NPRL2 for CPT-11 might be connected to the induction of DNA DSBs. The majority of antitumor therapies function by causing DSBs. γ-H2AX formation is induced by apoptotic DNA fragmentation, which is crucial for apoptosis [19]. Our outcomes suggest that combination treatment therapy with NPRL2 and CPT-11 improves γ-H2AX expression and prompts potent apoptosis. Cells from mice that do not have the H2AX gene have a G2/M checkpoint flaw close to that in ATM-lacking cells exposed to diminished doses of ionizing radiation [20]. Tu et al. discovered that H2AX phosphorylation happens throughout cell cycle progression and that γ-H2AX levels are elevated upon the initiation of the G2/M phase in Hela cells [21]. Jayachandran et al. have documented that the amalgam of NPRL2 and cisplatin can elevate γ-H2AX expression and prompt cell arrest in the G2/M phase in lung cancer cells [22]. Our data revealed that the combination of NPRL2 plus CPT-11 significantly prompts G2/M phase cell cycle arrest in HCT116 cells, which is tightly connected to the expression of the γ-H2AX protein.
ATM, is a phospholipid acyl enzyme-3-kinase that functions in reaction to the DNA damage, especially DNA DSBs. ATM motivates the proteins pertinent to the cell cycle and DNA damage signaling. Motivated ATM then stimulates Chk1 and Chk2 activity by phosphorylation [21]. Cdc25C can be inactivated by phosphorylation at Ser216 by Chk1, and, following sequestration of Cdc25C in the cytoplasm by binding Chk2 with members of the 14-3-3 protein family, halts early mitosis [23]. Cdc25C phosphatase motivates cellular initiation into mitosis by stimulating the cyclin-dependent kinase Cdc2/cyclin B1 complex [24,25]. In our study, a combination treatment of NPRL2 and CPT-11 increased the activation of the phosphorylation ATM and Chk2 kinases and subsequently resulted in the down-regulation of Cdc25C, Cdc2, and Cyclin B1 expression.
MMPs, in particular MMP2 and MMP9, are critical to tumor cell movement and invasion [26]. Huang et al. documented that the down-regulation of NPRL2 stimulates PDK1-Akt1 and adds to the malignant development of glioma cells [14]. The PI3K/AKT pathway is a crucial signaling pathway that is responsible for cancer migration and invasion [27]. Inhibition of PI3K and AKT results in decreased MMP2 and MMP9 expression in cancer cells [28]. A prior evaluation by our team revealed that NPRL2 can prevent the invasion of CRC cells [29]. Nevertheless, the impact of NPRL2 in tandem with chemotherapeutic agents on invasion and movement has yet to be elucidated. Thus, in this study, we further revealed that combined treatment with NPRL2 and CPT-11 efficiently prevented the invasion and movement of the HCT116 cells in vitro. We also showed that NPRL2 plus CPT-11 significantly limited the activity of MMP2 and MMP9, and inhibited the PI3K/AKT signaling pathway, which plays a role in impeding movement and invasion of NPRL2 in HCT116 cells.
Conclusions
NPRL2 overexpression improves CPT-11 sensitivity by impeding the proliferation, movement, and invasion of cancer cells and inducing apoptosis and G2/M cell cycle arrest. We also revealed that NPRL2 overexpression in colon cancer cells improves the sensitivity to CPT-11 by stimulating essential elements of the DNA damage checkpoint pathway. These results are a substantial advance in comprehending the antitumor impacts of NPRL2 and also offering novel knowledge on the molecular mechanisms underlying the impacts of NPRL2. NPRL2 has potential use as a molecular therapeutic agent to treat individuals with CRC and to assist them in overcoming resistance to CPT-11.
Footnotes
Source of support: This work was supported by Harbin Medical University research funds (No. YJSCX2015-24HYD)
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Lerman MI, Minna JD. The 630-kb lung cancer homozygous deletion region on human chromosome 3p21.3: Identification and evaluation of the resident candidate tumor suppressor genes. The International Lung Cancer Chromosome 3p21.3 Tumor Suppressor Gene Consortium. Cancer Res. 2000;60:6116–33. [PubMed] [Google Scholar]
- 3.Li J, Wang F, Haraldson K, et al. Functional characterization of the candidate tumor suppressor gene NPRL2/G21 located in 3p21.3C. Cancer Res. 2004;64:6438–43. doi: 10.1158/0008-5472.CAN-03-3869. [DOI] [PubMed] [Google Scholar]
- 4.Liu AY, Liu DG, Du YJ, et al. Relationship between tumor and peripheral blood NPRL2 mRNA levels in patients with colorectal adenoma and colorectal cancer. Cancer Biol Ther. 2014;15(5):489–95. doi: 10.4161/cbt.28016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schenk PW, Brok M, Boersma AW, et al. Anticancer drug resistance induced by disruption of the Saccharomyces cerevisiae NPR2 gene: A novel component involved in cisplatin-and doxorubicin-provoked cell kill. Mol Pharmacol. 2003;64:259–68. doi: 10.1124/mol.64.2.259. [DOI] [PubMed] [Google Scholar]
- 6.Sabir SR, Sahota NK, Jones GDD, Fry AM. Loss of Nek11 prevents G2/M arrest and promotes cell death in HCT116 colorectal cancer cells exposed to therapeutic DNA damaging agents. PLoS One. 2015;10(10):e0140975. doi: 10.1371/journal.pone.0140975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomicic MT, Kaina B. Topoisomerase degradation, DSB repair, p53 and IAPs in cancer cell resistance to camptothecin-like topoisomerase I inhibitors. Biochim Biophys Acta. 2013;1835:11–27. doi: 10.1016/j.bbcan.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Liu MN, Liu AY, Du YJ, et al. Nitrogen permease regulator-like 2 enhances sensitivity to oxaliplatin in colon cancer cells. Mol Med Rep. 2015;12(1):1189–96. doi: 10.3892/mmr.2015.3495. [DOI] [PubMed] [Google Scholar]
- 9.Patel BB, Majumdar AP. Synergistic role of curcumin with current therapeutics in colorectal cancer: minireview. Nutr Cancer. 2009;61:842–46. doi: 10.1080/01635580903285106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji L, Nishizaki M, Gao B, et al. Expression of several genes in the human chromosome 3p21.3 homozygous deletion region by an adenovirus vector results in tumor suppressor activities in vitro and in vivo. Cancer Res. 2002;62:2715–20. [PMC free article] [PubMed] [Google Scholar]
- 11.Yogurtcu B, Hatemi I, Aydin I, Buyru N. NPRL2 gene expression in the progression of colon tumors. Genet Mol Res. 2012;11:4810–16. doi: 10.4238/2012.September.12.3. [DOI] [PubMed] [Google Scholar]
- 12.Gao Y, Wang J, Fan G. NPRL2 is an independent prognostic factor of osteosarcoma. Cancer Biomark. 12:2012–2013. 31–36. doi: 10.3233/CBM-120290. [DOI] [PubMed] [Google Scholar]
- 13.Otani S, Takeda S, Yamada S, et al. The tumor suppressor NPRL2 in hepatocellular carcinoma plays an important role in progression and can be served as an independent prognostic factor. J Surg Oncol. 2009;100:358–63. doi: 10.1002/jso.21241. [DOI] [PubMed] [Google Scholar]
- 14.Huang N, Cheng S, Mi X, et al. Downregulation of nitrogen permease regulator like-2 activates PDk1-AkT1 and contributes to the malignant growth of glioma cells. Mol Carcinog. 2016;55(11):1613–26. doi: 10.1002/mc.22413. [DOI] [PubMed] [Google Scholar]
- 15.Ueda K, Kawashima H, Ohtani S, et al. The 3p21.3 tumor suppressor NPRL2 plays an important role in cisplatin-induced resistance in human non-small-cell lung cancer cells. Cancer Res. 2006;66:9682–90. doi: 10.1158/0008-5472.CAN-06-1483. [DOI] [PubMed] [Google Scholar]
- 16.Niedner H, Christen R, Lin X, et al. Identification of genes that mediate sensitivity to cisplatin. Mol Pharmacol. 2001;60:1153–60. [PubMed] [Google Scholar]
- 17.Kurata A, Katayama R, Watanabe T, et al. TUSC4/NPRL2, a novel PDK1-interacting protein, inhibits PDK1 tyrosine phosphorylation and its downstream signaling. Cancer Sci. 2008;99(9):1827–34. doi: 10.1111/j.1349-7006.2008.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu C, Zhu F, Cho YY, et al. Cell apoptosis: requirement of H2AX in DNA ladder formation, but not for the activation of caspase-3. Mol Cell. 2006;23:121–32. doi: 10.1016/j.molcel.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogakou EP, Nieves-Neira W, Boon C, et al. Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. J Biol Chem. 2000;275(13):9390–95. doi: 10.1074/jbc.275.13.9390. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez-Capetillo O, Chen HT, Celeste A, et al. DNA damage-induced G2-M checkpoint activation by histone H2AX and 53BP1. Nat Cell Biol. 2002;4:993–97. doi: 10.1038/ncb884. [DOI] [PubMed] [Google Scholar]
- 21.Tu WZ, Li B, Huang B, et al. γ-H2AX foci formation in the absence of DNA damage: Mitotic H2AX phosphorylation is mediated by the DNA-PKcs/CHK2 pathway. FEBS Lett. 2013;587(21):3437–43. doi: 10.1016/j.febslet.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 22.Jayachandran G, Ueda K, Wang B, et al. NPRL2 sensitizes human non-small cell lung cancer (NSCLC) cells to cisplatin treatment by regulating key components in the DNA repair pathway. PLoS One. 2010;5(8):e11994. doi: 10.1371/journal.pone.0011994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng CY, Graves PR, Thoma RS, et al. Mitotic and G2 checkpoint control: Regulation of 14-3-3 protein binding by phosphorylation of Cdc25C on serine-216. Science. 1997;277:1501–5. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- 24.Tse AN, Carvajal R, Schwartz GK. Targeting checkpoint kinase 1 in cancer therapeutics. Clin Cancer Res. 2007;13:1955–60. doi: 10.1158/1078-0432.CCR-06-2793. [DOI] [PubMed] [Google Scholar]
- 25.Eymin B, Claverie P, Salon C, et al. p14ARF triggers G2 arrest through ERK mediated Cdc25C phosphorylation, ubiquitination and proteasomal degradation. Cell Cycle. 2006;5:759–65. doi: 10.4161/cc.5.7.2625. [DOI] [PubMed] [Google Scholar]
- 26.Guan H, Cai J, Zhang N, et al. Sp1 is upregulated in human glioma, promotes MMP-2-mediated cell invasion and predicts poor clinical outcome. Int J Cancer. 2012;130:593–601. doi: 10.1002/ijc.26049. [DOI] [PubMed] [Google Scholar]
- 27.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–49. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 28.Tseng CH, Tzeng CC, Chiu CC, et al. Discovery of 2-[2-(5-nitrofuran-2-yl) vinyl] quinoline derivatives as a novel type of antimetastatic agents. Bioorg Med Chem. 2015;23:141–48. doi: 10.1016/j.bmc.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 29.Liu AY, Liu MN, Pei FH, et al. Functional characterization of the nitrogen permease regulator-like-2 candidate tumor suppressor gene in colorectal cancer cell lines. Mol Med Rep. 2015;12(3):3487–93. doi: 10.3892/mmr.2015.3881. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]