Abstract
Background
FOXP1 is a pleiotropic protein that plays important roles in immune responses (B-cell development regulation and differentiation of monocyte), organ development (cardiac valves, lung, and esophagus), and neuronal development. Besides being the primary regulator of normal human tissue development, FOXP1 also plays a role in tumorigenesis. However, the potential value of FOXP1 expression in tumor prognosis remains controversial. FOXP1 expression was assessed in tumor cells (TCs) and stromal cells (SCs) of cutaneous melanomas with the aim of analyzing the associations between FOXP1 expression and clinicopathological characteristics. We believe this article to be the first report analyzing the correlations between FOXP1 expression and clinicopathological, as well as histological, characteristics in melanoma.
Materials and methods
In total, 96 formalin-fixed, paraffin-embedded primary cutaneous melanoma tissue specimens were subjected to immunohistochemical analysis for FOXP1, and the results were correlated with classical clinicopathological features and patient survival.
Results
FOXP1 overexpression in TCs was strongly associated with the presence of metastases in sentinel lymph nodes (p=0.0003, OR=11.66) and positive status of regional lymph nodes (p=0.0006, OR=22.15). In 96% (52 of 54) of patients presenting with low FOXP1 expression, no clinical or histopathological features of lymphatic dissemination were observed. However, thinner and nonulcerated tumors were reported to have increased numbers of FOXP1-positive SCs. In addition, a strong association was observed between FOXP1 upregulation in SCs and the absence of regional lymph node metastases. There was a significant correlation between FOXP1 upregulation in TCs and shorter cancer-specific overall survival (log-rank test, p=0.0040) and disease-free survival (log-rank test, p=0.0021). FOXP1 expression was confirmed in multivariate analysis as a factor that significantly unfavorably impacts prognosis in melanoma patients (HR=3.14, p=0.0299, adjusted for age, Breslow thickness, and sex).
Conclusion
The findings from this study indicate that FOXP1 has a major role in melanoma progression, which makes it a candidate for molecular target-based cancer therapy.
Keywords: dermatopathology, melanocytic lesion, microenvironment, tumor biology, melanomagenesis
Introduction
Despite the fact that cutaneous melanoma makes up for as few as 4% of all diagnosed skin cancers, it is the cause of 75% of skin cancer deaths.1 Despite being curable when diagnosed in the early stage, it still poses a challenge in the more advanced cases.2 The incidence of melanoma has been significantly increasing over the recent years.1–3 Therefore, no effort is spared to clarify the mechanisms of melanomagenesis as well as to determine the risk and prognostic factors and thus to improve the effectiveness of melanoma treatment, which, despite the discovery of novel agents, still poses difficulties.2–4
FOX proteins constitute a large family of transcription factors playing a pivotal role during ontogenesis as well as in the adult life.5 FOX proteins bind to DNA through a forkhead domain (FHD), also known as winged helix domain.5,6 FHD consists of three N-terminal α-helix regions, three β-strands, and two loops (called “wings” or “wing domains”) toward its C-terminal region.5–7 In order to simplify the nomenclature, classification, and identification of the FOX proteins, a unified nomenclature was established in 2000. FOX transcription factors were divided into subfamilies designated by a letter.8 Members of the same subfamily were given an additional Arabic numeral.8 Studies conducted to date identified 19 FOX subfamilies (A–S).5
The FOXP group (one of the subfamilies) differs structurally and shows a broader spectrum of functions in comparison with other FOX subfamilies.7 The FHD is C-terminally located and contains deletions in its wing regions.6,7 Apart from that, FOXP transcription factors share zinc finger and leucine zipper motif, which gives them the ability to form heterodimers.6,7 At the molecular level, FOXP proteins act mainly as transcriptional repressors.7 FOX protein P1 (FOXP1) has an important function in neuronal development.9 Mutations of its gene, FOXP1, located on chromosome 3p14.1,7 can result in the development of autism spectrum disorder, intellectual disability, speech and language deficits as well as motor development delay.9 FOXP1 is also engaged in lung and esophagus morphogenesis, as well as in B-cell development.7,10 The widely researched role of FOXP1 in carcinogenesis is of great importance, although still unclear to some extent.
On the one hand, FOXP1 acts as an oncogene.7 Its overexpression in diffuse large B-cell lymphomas (DLBCLs) and marginal zone lymphomas is associated with poor patient prognosis.7,11 Moreover, the overexpression of FOXP1 in mucosa-associated lymphoid tissue lymphomas is related to a high risk of transformation into DLBCLs.7,12 It is suggested that downregulated expression of FOXP1 might also be prognostically valuable in the case of all lymphomas originating from lymphocytes.11 FOXP1 influences many aspects of lymphomagenesis. First, it promotes cell survival by repressing S1PR2 signaling. Apoptotic processes are also suppressed thanks to cooperation between FOXP1 and nuclear factor-κB, which is the case in activated B-cell like subtype DLBCL (ABC-DLBCL) cells. FOXP1 overexpression also disrupts terminal B-cell differentiation in ABC-DLBCL cells by inhibiting major histocompatibility complex class II expression.13 Furthermore, overexpressed FOXP1 activates Wnt/β-catenin signaling in germinal center B-cell like subtype DLBCL (GC-DLBCL) cells by forming a complex with TCF7L2 and CBP at Wnt target gene promoters and by enhancing β-catenin acetylation by CBP. Therefore, FOXP1 is a key factor in DLBCL pathogenesis, which occurs in both subtypes of this neoplasm.13 However, it has been shown that ABC-DLBCL cells are more dependent on FOXP1 compared with GC-DLBCL ones. In one study, two ABC-DLBCL cell lines (TMD8 and HBL-1) underwent FOXP1 knockdown resulting in their apoptosis, while the FOXP1 knockdown in GC-DLBCL cell lines did not induce cell death.14
Interestingly, FOXP1 may also be a tumor suppressor.7 Decreased FOXP1 protein expression is an unfavorable factor for solid tumors (breast cancer, prostate cancer, endometrial cancer, non-small-cell lung carcinoma, colorectal cancer, epithelial ovarian cancer, and neuroblastoma).11 The mechanisms of suppressive influence of FOXP1 have not been fully understood yet, but recent studies have shed light on some oncogenetic aspects.
This study intended to examine FOXP1 expression and prognostic significance in primary skin melanomas. We analyzed correlations between FOXP1 immunoreactivity in tumoral and stromal compartments and detailed clinicopathological parameters. We believe that to date no other report has been published on the correlations between FOXP1 expression in melanoma and clinicopathological as well as histological characteristics of this tumor.
Materials and methods
Patients
Our study group was composed of 96 cutaneous melanoma patients treated at the Lower Silesian Oncology Center in Wroclaw, Poland, diagnosed in 2005–2010. Patients were enrolled in this study based on the availability of their medical documentation and tissue material, which included paraffin blocks and histopathology slides. Comprehensive clinical data were retrieved from the archival medical records, and data concerning the diagnostic and therapeutic procedures used were sourced from the cancer outpatient clinic at the Lower Silesian Oncology Center and Lower Silesian Cancer Registry, as well as Civil Register Office.
This study was reviewed and approved by the ethics committee of the Wroclaw Medical University, Wroclaw, Poland (No 478/2017). The study was retrospectively performed, and additional informed consent was not required by the ethics committee (the patients did not personally participate in the study, and the results of the planned study did not have any influence on the original treatment of these patients because it had already been finished). This study was performed in accordance with the Declaration of Helsinki.
Records were reviewed for clinical and pathological data (age and gender), primary tumor (pT) location, tumor stratification according to pT, the presence or absence of nodal and distant metastases, information on disease recurrence, and sentinel lymph node biopsy (SLNB) procedures (Table 1).
Table 1.
Correlations between clinicopathological parameters and FOXP1 expression in neoplastic and stromal compartments of primary tumor
| Clinical parameters | FOXP1 immunoreactivity
|
|||||
|---|---|---|---|---|---|---|
| Tumor cells
|
Stromal cells
|
|||||
| Low (n=54) | High (n=42) | p-value | Absent (n=18) | Present (n=78) | p-value | |
| Age (21–79 years); mean=56.58±15.45; median=58.00 | 56.52±16.43; 59.50 | 56.88±14.29; 59.50 | 0.9205a | 55.22±15.88; 58.00 | 57.12±15.44; 58.00 | 0.6898a |
| Gender | ||||||
| Female | 20 (37.04) | 19 (45.24) | 0.4173b | 5 (27.78) | 34 (43.59) | 0.2902c |
| Male | 34 (62.96) | 23 (54.76) | 13 (72.22) | 44 (56.41) | ||
| pT location | ||||||
| Head/neck | 2 (3.70) | 1 (2.38) | 0.4443d | 1 (5.56) | 2 (2.56) | 0.3401d |
| Extremities | 26 (48.15) | 15 (35.71) | 9 (50.00) | 32 (41.03) | ||
| Hand/foot | 6 (11.11) | 8 (19.05) | 4 (22.22) | 10 (12.82) | ||
| Trunk | 20 (37.04) | 18 (42.86) | 4 (22.22) | 34 (43.59) | ||
| pT | ||||||
| pT1 | 25 (46.30) | 9 (21.43) | 0.0440e | 1 (5.56) | 33 (42.31) | 0.0015e |
| pT2 | 10 (18.52) | 8 (19.05) | 4 (22.22) | 14 (17.95) | ||
| pT3 | 7 (12.96) | 16 (38.10) | 5 (27.78) | 18 (23.08) | ||
| pT4 | 12 (22.22) | 9 (21.43) | 8 (44.44) | 13 (16.67) | ||
| Regional lymph nodes status (pN) | ||||||
| No metastases (pN−) | 52 (96.30) | 29 (69.05) | 0.0003c | 12 (66.67) | 69 (88.46) | 0.0326b |
| Metastases present (pN+) | 2 (3.70) | 13 (30.95) | 6 (33.33) | 9 (11.54) | ||
| Distant metastases (pM) | ||||||
| No metastases (pM−) | 53 (98.15) | 38 (90.48) | 0.1645c | 15 (83.33) | 76 (97.44) | 0.1546c |
| Metastases present (pM+) | 1 (1.85) | 4 (9.52) | 3 (16.67) | 2 (2.56) | ||
| SNLB status (55 patients) | ||||||
| No metastases (SNLB−) | 32 (96.97) | 13 (59.09) | 0.0006c | 5 (62.50) | 40 (85.11) | 0.1492c |
| Metastases present (SNLB+) | 1 (3.03) | 9 (40.91) | 3 (37.50) | 7 (14.89) | ||
Notes: Data presented as mean ± SD; median or n (%).
p-value of the Mann–Whitney U-test;
p-value of the Pearson’s χ2 test;
p-value of the Fisher’s exact test;
p-value of the Freeman–Halton extension of the Fisher’s exact probability test;
p-value of the Cochran–Armitage test for trend; statistically significant results (p<0.05) are given in bold.
Abbreviations: pT, primary tumor; SLNB, sentinel lymph node biopsy.
Archival formalin-fixed, paraffin-embedded tumor specimens were analyzed. Specifically, all H&E-stained sections of the pT were examined independently by two pathologists who reported data such as Breslow thickness, Clark level, histological type, mitotic rate (the number of mitotic figures per 1 mm2), the presence of ulceration, lymphangioinvasion, microsatellitosis, the intensity of tumor-infiltrating lymphocytes (TILs), and microscopic evidence of regression (Table 2).
Table 2.
Correlations between histological characteristics of melanoma primary tumors and FOXP1 expression in neoplastic and stromal compartments
| Histopathological parameters | FOXP1 immunoreactivity
|
|||||
|---|---|---|---|---|---|---|
| Tumor cells
|
Stromal cells
|
|||||
| Low (n=54) | High (n=42) | p-value | Absent (n=18) | Present (n=78) | p-value | |
| Breslow thickness (mm) | 1.20 (0.70–4.00) | 2.50 (1.20–4.00) | 0.0649a | 3.85 (2.00–6.00) | 1.80 (0.75–4.00) | 0.0021a |
| Clark level | ||||||
| I | 0 (0.00) | 0 (0.00) | 0.0529b | 0 (0.00) | 0 (0.00) | 0.0112b |
| II | 14 (25.93) | 3 (7.14) | 2 (11.11) | 15 (19.23) | ||
| III | 24 (44.44) | 22 (52.38) | 5 (27.78) | 41 (52.56) | ||
| IV | 12 (22.22) | 12 (28.57) | 7 (38.89) | 17 (21.79) | ||
| V | 4 (7.41) | 5 (11.90) | 4 (22.22) | 5 (6.41) | ||
| Histological type | ||||||
| Superficial spreading melanoma | 38 (70.37) | 26 (61.90) | 0.5673c | 9 (50.00) | 55 (70.51) | 0.2601c |
| Nodular malignant melanoma | 14 (25.93) | 15 (35.71) | 8 (44.44) | 21 (26.92) | ||
| Acral lentiginous melanoma | 2 (3.70) | 1 (2.38) | 1 (5.56) | 2 (2.56) | ||
| Mitotic rate | ||||||
| 0 | 28 (51.85) | 16 (38.10) | 0.1796d | 5 (27.78) | 39 (50.00) | 0.1171e |
| ≥1 | 26 (48.15) | 26 (61.90) | 13 (72.22) | 39 (50.00) | ||
| Ulceration | ||||||
| No | 34 (62.96) | 18 (42.86) | 0.0640e | 5 (27.78) | 47 (60.26) | 0.0177e |
| Yes | 20 (37.04) | 24 (57.14) | 13 (72.22) | 31 (39.74) | ||
| TILs | ||||||
| No | 8 (14.81) | 9 (21.43) | 0.2533c | 4 (22.22) | 13 (16.67) | 0.5749c |
| Nonbrisk | 15 (27.78) | 16 (38.10) | 7 (38.89) | 24 (30.77) | ||
| Brisk | 31 (57.41) | 17 (40.48) | 7 (38.89) | 41 (52.56) | ||
| Microsatellitosis | ||||||
| No | 50 (92.59) | 41 (97.62) | 0.3818e | 16 (88.89) | 75 (96.15) | 0.2346e |
| Yes | 4 (7.41) | 1 (2.38) | 2 (11.11) | 3 (3.85) | ||
| Tumor regression | ||||||
| No | 52 (96.30) | 37 (88.10) | 0.2340e | 15 (83.33) | 74 (94.87) | 0.1195e |
| Yes | 2 (3.70) | 5 (11.90) | 3 (16.67) | 4 (5.13) | ||
Notes: Data presented as median (1: quartile–3: quartile) or n (%).
p-value of the Mann–Whitney U-test;
p-value of the Cochran–Armitage test for trend;
p-value of the Freeman–Halton extension of the Fisher’s exact probability test;
p-value of the Pearson’s χ2 test;
p-value of the Fisher’s exact test; statistically significant results (p<0.05) are given in bold.
Abbreviation: TILs, tumor-infiltrating lymphocytes.
Immunohistochemistry (IHC)
Immunohistochemical evaluation of FOXP1 expression (mouse monoclonal antibody, clone 3F12, LS-C114491; dilution 1:150; LifeSpan Biosciences, Seattle, WA, USA) was performed on 4-µm-thick paraffin sections mounted on silanized slides (code number S 3003; Dako Denmark A/S, Glostrup, Denmark). The sections were then deparaffinized, rehydrated, and subjected to heat-induced epitope unmasking. pT Link was the module applied for this purpose (EnVision™ Target Retrieval Solution was used for 20- to 40-minute incubation at 97°C). Immunohistochemical test was performed by using Autostainer Link and EnVision™ FLEX/HRP (SM802; Dako Denmark A/S).
Evaluation of IHC
FOXP1 was expressed in two cellular compartments, namely TCs and SCs, both as a cytoplasmic distribution. A different IHC reaction assessment system was applied for every cell type.
FOXP1 expression in TCs was evaluated by using a semi-quantitative method with the two immunohistochemical reaction parameters: the percentage of cells with a positive cytoplasmic reaction (the percentage of reactive tissue) and reaction intensity. The final immunohistochemical reaction results were calculated by using the semi-quantitative immunoreactive score (IRS) scale devised by Remmele and Stenger15 in which the percentage of stained cells (0–4 points) and the intensity of reaction (0–3 points) were taken into account. The IRS that has a value of 0–12 points is the result of the multiplication of the scores for these two parameters.
Five hot spots with the highest intensity of a corresponding IHC reaction at a magnification of 400× (high-power field [HPF]) were evaluated to determine FOXP1 immunoreactivity in SCs. Final interpretation of IHC reaction with anti-FOXP1 antibody was based on nonmalignant morphology of cells, namely small, normochromatic nuclei; no nuclear shape aberrations; no nucleoli; and normal nuclear/cytoplasmic ratio. The mean number of FOXP1-positive cells was calculated in each case, and subsequently, the tumors were grouped into three categories: no FOXP1 expression in SCs (the mean number of positive SCs <1/HPF), low expression in SCs (the mean number of positive SCs ≥1/HPF and <20/HPF), and high expression in SCs (the mean number of positive SCs ≥20/HPF).
Statistical analysis
For the purpose of statistical analysis, we introduced two divisions of the whole group. First division was based on the IRS of TCs. We used the median score of IRS=4 as a cutoff point. Patients whose specimens scored higher (IRS >4) were classified as a group with high FOXP1 expression, and those who scored ≤4 were a group with low FOXP1 expression. With regard to FOXP1 expression in stromal compartment, we compared cases with no FOXP1 expression in SCs to cases with present FOXP1 expression in SCs (tumors with low and high expressions, as described above, were grouped together). Nominal variables were compared by using χ2 tests or Fisher’s exact tests depending on the number of cases; pT location was compared by using the Freeman–Halton extension of the Fisher’s exact probability test. We implemented Cochran–Armitage statistics to test pT and Clark level variables. Comparisons of continuous variables between groups were made by using Mann–Whitney U-tests. Spearman’s rank correlation was used for correlation testing. Survival analysis was performed with log-rank tests (univariate tests) and Cox proportional hazard models for multivariate analysis. Multivariate models were constructed to evaluate the impact of FOXP1 expression adjusted for age, gender, pT, Breslow thickness, and Clark level. The threshold of statistical significance was established at p=0.05.
Results
FOXP1 expression in melanoma cells and stromal compartment
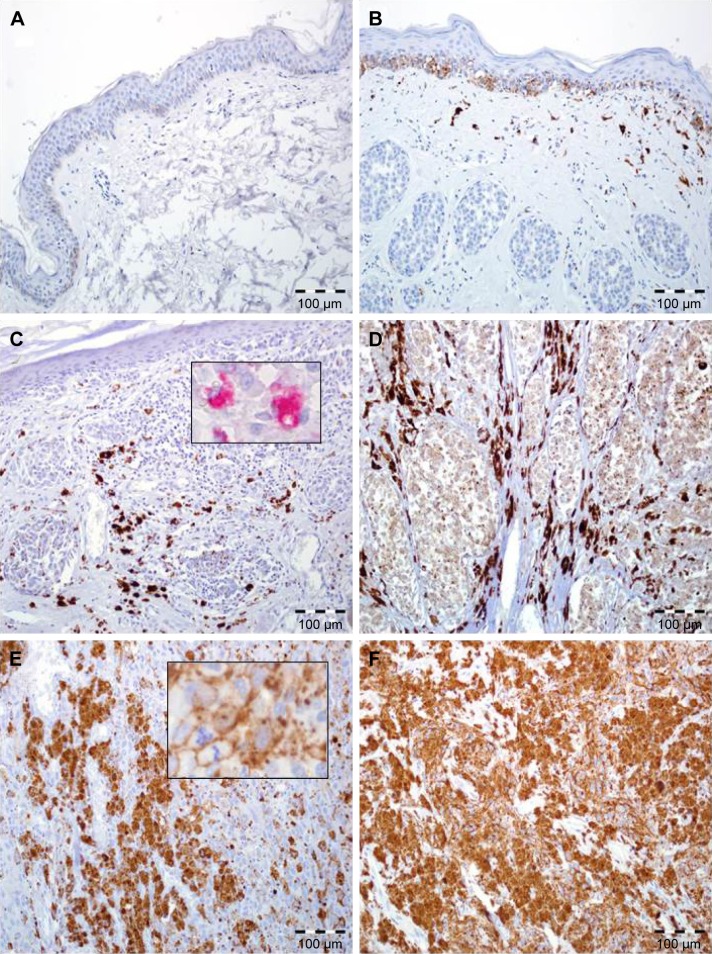
Elevated expression of FOXP1 in TCs, defined as IRS >4, was observed in 44% (42 of 96) of cases, while the remaining 56% (54 of 96) of cases demonstrated no/low expression. Mean IRS for FOXP1 expression in TCs was 4.67±3.7 (median=4; Figure 1).
Figure 1.
FOXP1 expression in skin melanoma patients.
Notes: (A) Normal skin without FOXP1 immunoreactivity (200×, hematoxylin). (B) Intermediate expression of FOXP1 in the basal epidermal cells and normal melanocytes with lack of immunoreactivity in nests of benign melanocytes in intradermal skin nevus. Note the moderate reaction in macrophages and fibroblasts (200×, hematoxylin). (C) Diffuse, strong FOXP1 reactivity in stromal cells (predominantly in tumor-associated macrophages) with lack of expression in malignant melanocytes; inset: immunohistochemical reaction (red chromogen) with anti-CD68 antibody (200×, 600×, hematoxylin). (D) Enhanced FOXP1 expression in stromal cells with low immunoreactivity in melanoma cells (200×, hematoxylin). (E) High FOXP1 expression in neoplastic melanocytes, higher magnification: FOXP1-positive melanoma cells with atypical mitotic figure (200×, 600×, hematoxylin). (F) Diffuse, strong FOXP1 reactivity in melanoma cells with lack of expression in stromal compartment (200×, hematoxylin).
FOXP1 expression in stromal compartment was observed in 81.25% of pTs (78 of 96); 18.75% (18 of 96) of cases had no FOXP1-expressing SCs (Figure 1).
Analysis of correlations between FOXP1 expression in melanoma cells and clinicopathological parameters
Increased FOXP1 expression was correlated with a more advanced pT (p=0.0440; Table 1). Furthermore, there was a significant association between elevated FOXP1 expression and the presence of regional nodal metastases (p=0.0003, OR=11.66, 95% CI=2.46–55.27; Table 1). Among 55 subjects who underwent SLNB procedure, high IRS was more frequently observed in patients presenting with sentinel lymph node metastases (p=0.0006, OR=22.15, 95% CI=2.54–192.92; Table 1). No associations were observed between FOXP1 expression in TCs and other basic clinical parameters (ie, age, gender, pT location, and the presence of distant metastases; Table 1).
We observed no statistically significant associations between FOXP1 IRS and commonly assessed histopathological characteristics, ie, Breslow thickness, Clark level, histological type, mitotic rate, the presence of ulceration, TIL grade, the presence of microsatellitosis, and tumor regression (Table 2).
Analysis of correlations between FOXP1 expression in the stromal compartment and clinicopathological parameters
Stromal expression of FOXP1 was observed predominantly in thin melanomas (p=0.0015; Table 1). Moreover, in tumors with high FOXP1 expression in SCs (88.46%, 69 of 78; p=0.0326; Table 1), no metastases in regional lymph nodes were identified. An association between high FOXP1 expression in SCs and low Breslow thickness (p=0.0021; Table 2) and less advanced Clark level (p=0.0112; Table 2) was observed. A correlation between FOXP1 immunoreactivity in SCs and lack of ulceration of the pT (p=0.0177; Table 2) was also observed. However, no associations between immuno expression of FOXP1 in SCs and other histopathological parameters (histological type, mitotic rate, TIL grade, the presence of microsatellitosis, and tumor regression) were found (Table 2). We did not observe any correlation between FOXP1 expression in SCs and age, gender, or the location of primary lesion (Table 1).
Impact of FOXP1 immunoreactivity in melanoma cells and stromal compartment on 5-year survival
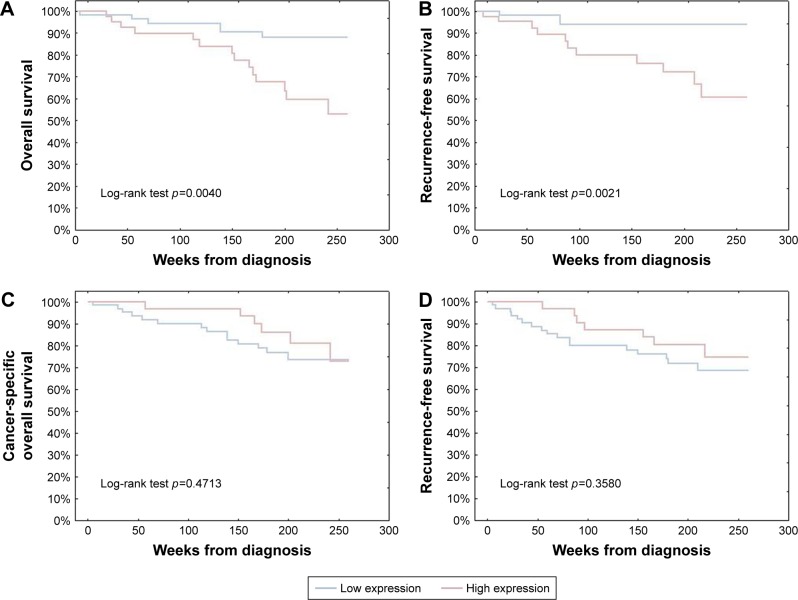
FOXP1 overexpression in melanoma cells was strongly correlated with shorter cancer-specific overall survival (CSOS) and disease-free survival (DFS; HR=3.80, p=0.0063; and HR=3.28, p=0.0062, respectively, in univariate Cox analysis). No significant influence of FOXP1 expression in SCs on melanoma patients’ long-term survival (Figure 2) was observed in Kaplan–Meier analysis.
Figure 2.
Kaplan–Meier analysis of the prognostic impact of FOXP1 expression in cutaneous melanoma patients.
Notes: Upregulation of FOXP1 in melanoma cells was significantly associated with shorter CSOS and DFS (A and B, respectively). Elevated numbers of FOXP1-positive stromal cells had no significant impact on CSOS and DFS (C and D, respectively).
Abbreviations: CSOS, cancer-specific overall survival; DFS, disease-free survival.
Clinicopathological parameters and survival of melanoma patients – multivariable Cox analysis
It was demonstrated that the independent unfavorable prognostic factors that have a statistically significant effect on cutaneous melanoma patients’ survival in the context of CSOS are Breslow thickness (p=0.01, HR=1.16, 95% CI=1.04–1.31) and high expression of FOXP1 in melanoma cells (p=0.03, HR=0.32, 95% CI=0.11–0.89; Table 3). Similar results were observed in the context of DFS, but interestingly age also had a significant impact on shorter DFS (p=0.04, HR=0.97, 95% CI=0.94–0.99; Table 3).
Table 3.
Multivariable Cox regression analysis
| Cancer-specific overall survival
|
Disease-free survival
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio | Lower 95% CI | Upper 95% CI | p-value | Hazard ratio | Lower 95% CI | Upper 95% CI | p-value | |
| Age (years) | 0.9963 | 0.9664 | 1.0272 | 0.8134 | 0.9692 | 0.9411 | 0.9982 | 0.0373 |
| Breslow (mm) | 1.1628 | 1.0364 | 1.3046 | 0.0102 | 1.2699 | 1.1170 | 1.4437 | 0.0003 |
| Gender | 0.6060 | 0.2361 | 1.5552 | 0.2975 | 0.4576 | 0.1874 | 1.1178 | 0.0863 |
| FOXP1 expression in SCs | 1.2602 | 0.4641 | 3.4217 | 0.6500 | 1.5159 | 0.5905 | 3.8916 | 0.3872 |
| FOXP1 expression in TCs | 0.3181 | 0.1131 | 0.8946 | 0.0299 | 0.2828 | 0.1106 | 0.7229 | 0.0084 |
Note: Statistically significant results (p<0.05) are given in bold.
Abbreviations: SCs, stromal cells; TCs, tumor cells.
Discussion
FOXP1 expression in two compartments of melanoma tumors – TCs and SCs – was analyzed in this study. Statistical analysis explored the associations between the expression of FOXP1 and clinicopathological characteristics. High expression of FOXP1 in melanoma cells was demonstrated to be strongly correlated with thick primary tumor and the presence of metastases in regional lymph nodes. Moreover, enhanced FOXP1 immunoreactivity in TCs was significantly associated with shorter CSOS and DFS. On the other hand, the presence of FOXP1 in stromal compartment was connected with thin nonulcerated melanoma and no regional lymph node metastases.
The results seem to be contradictory to the belief that elevated level of FOXP1 protein is a favorable prognostic factor in solid tumors. However, results similar to ours were observed in hepatocellular carcinoma (HCC) study group, where FOXP1 overexpression was correlated with an aggressive cancer phenotype.16 Moreover, it was proven that overexpressed FOXP1 could be used as a biomarker of early HCC in liver cirrhosis associated with hepatitis B virus infection.17 This phenomenon was further investigated, and silencing of FOXP1 gene impaired the HCC cell growth ability in vitro, while knockdown of the gene decreased HCC cells’ tumorigenicity.18 Downregulation of FOXP1 resulted in G1/S cycle arrest with reduced expression of total Rb, phosphorylated Rb, and E2F1, while expression of cyclin-dependent kinase 4 (CDK4), cyclin-dependent kinase 6 (CDK6), and cyclin D1 did not change noticeably.18 These results suggest that FOXP1 overexpression inhibits G1/S cycle arrest by promoting Rb phosphorylation via CDK4- and CDK6-independent pathway.18 The same mechanism could be responsible for worse survival in patients with melanoma exhibiting increased expression of FOXP1 protein. This discrepancy should be clarified in more advanced clinicopathological studies.
In our study, we observed FOXP1 expression only in the cytoplasmic compartment with no nuclear immunoreactivity. In HCC, FOXP1 was also predominantly present in the cytoplasm (both nuclear staining and cytoplasmic staining were present only in a few samples).16 This finding was correlated with higher tumor diameter, higher serum α-fetoprotein levels, and more advanced stage in tumor, node, and metastasis classification, which is why FOXP1 is considered a novel independent negative prognostic factor in this neoplasm.16 Moreover, cytoplasmic expression of FOXP1 in endometrial cancer cells was correlated with increased HIF1-α expression and deeper myometrial invasion, while nuclear immunore-activity of FOXP1 was associated with the expression of estrogen receptor-α.19 The abovementioned results combined with our findings indicate that FOXP1 may function as an oncogene in solid neoplasms.
Studies on ovarian cancer have cast new light on the function of FOXP1 protein in the progression of tumor. In one study, higher expression of FOXP1 resulted in increased cell migration and the patients being unresponsive to paclitaxel or cisplatin treatment, while underexpression of FOXP1 exerted opposite effects.20 FOXP1 gene upregulation in chemoresistant ovarian tissues was also demonstrated by Kim et al.21 Another study showed downregulation of FOXP1 in the nuclear compartment and increased expression in the cytoplasmic compartment with an increasing degree of malignancy and tumor staging.22
It is worth noting that detailed analysis of the references revealed that the majority of studies concentrated on FOXP1 as a prognostic factor in human tumors, suggesting that decreased FOXP1 expression was correlated with poor clinical outcome.19,22–27 Most of these studies were discussed and summarized in a meta-analysis done by Xiao et al in 2016, where the FOXP1 protein was shown to act as a tumor suppressor in solid neoplasms.11
In our study, overexpression of FOXP1 protein in TCs resulted in more advanced tumor stage and poorer prognosis, while elevated expression of FOXP1 in SCs (predominantly in tumor-associated macrophages [TAMs]) was associated with lower tumor stage. Metabolic activity of TAMs in the local immune environment is thought to support melanomagenesis and immune escape.28–30 Macrophages, however, constitute a heterogeneous cell population with dynamically changing phenotype during carcinogenesis. M1 cells are antineoplastic agents, promoting inflammatory response via tumor necrosis factor-α, while macrophages with M2 phenotype help the tumor avoid immune surveillance.30 Initially, M1-type macrophages mediate response to tumor antigens, yet they often rapidly switch to M2 phenotype; this phenomenon is promoted by cytokines produced and secreted by TCs.31,32 FOXP1 is known to play a pivotal role in monocyte differentiation and macrophage functions. Overexpression of this protein inhibits these processes by repression of c-Fms/M-CSFR (receptor for macrophage colony-stimulating factor).33 Furthermore, Epstein–Barr virus–encoded miR-BART11 was observed to promote carcinogenesis of inflammation-induced nasopharyngeal carcinoma (NPC) and gastric cancer (GC), targeting FOXP1 molecule, resulting in its downregulation.34 Conversely, overexpression of FOXP1 inhibited differentiation of monocytes and thus NPC and GC cell growth.34 This finding would be concordant with our observations regarding the association between elevated FOXP1 expression in SCs and less advanced melanoma stage. However, since no in vivo or in vitro studies have been performed in the context of stromal FOXP1 immunoreactivity in cutaneous melanoma, further studies are necessary to identify molecular mechanisms involved in this process.
Conclusion
FOXP1’s function in solid neoplasms can depend on its cellular distribution – the nuclear one may inhibit tumorigenesis, while the cytoplasmic localization may result in oncogenesis. The oncogenic mechanism of FOXP1 can depend on Rb phosphorylation as it was shown in HCC.18 One cannot exclude other factors, eg, cooperation with other proteins or signaling cascades typical of particular tumors which could influence FOXP1’s role as either an oncogene or a tumor suppressor.7 It is also possible that there are several isoforms of FOXP1, indistinguishable by IHC, and that the tumorigenetic activity depends on the isoform type.7 Moreover, the role of FOXP1 in SCs is also of paramount importance, as the overexpressed protein contributes to hindering of monocyte differentiation and thus inhibiting tumor growth.33,34 Taken together, the data suggest that the nature of FOXP1 is far more complex than believed hitherto, and its role in tumorigenesis merits further investigation, particularly in terms of the development of new, effective antineoplastic drugs targeted on FOXP1.
Acknowledgments
The abstract of this paper was presented at the 29th European Congress of Pathology, September 2–6, 2017, Amsterdam, and was published in the Virchows Archiv. 2017;471(Suppl.1):s.S1 poz.OFP-01-003. This research was financed through a statutory subsidy by the Polish Minister of Science and Higher Education as a part of grants ST.B130.16.049 and ST.C280.17.010 (record numbers in the Simple system). Wojciech Fendler and Konrad Pagacz were supported by the First Team Project funded by Smart Growth Operational Programme and the Foundation for Polish Science.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Arrangoiz R, Dorantes J, Cordera F, Juarez MM, Paquentin EM, de León EL. Melanoma review: epidemiology, risk factors, diagnosis and staging. J Cancer Treat Res. 2016;4(1):1–15. [Google Scholar]
- 2.Niezgoda A, Niezgoda P, Czajkowski R. Novel approaches to treatment of advanced melanoma: a review on targeted therapy and immunotherapy. Biomed Res Int. 2015;2015:16. doi: 10.1155/2015/851387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garbe C, Eigentler TK, Keilholz U, Hauschild A, Kirkwood JM. Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist. 2011;16(1):5–24. doi: 10.1634/theoncologist.2010-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maverakis E, Cornelius LA, Bowen GM, et al. Metastatic melanoma – a review of current and future treatment options. Acta Derm Venereol. 2015;95(5):516–524. doi: 10.2340/00015555-2035. [DOI] [PubMed] [Google Scholar]
- 5.Benayoun BA, Caburet S, Veitia RA. Forkhead transcription factors: key players in health and disease. Trends Genet. 2011;27(6):224–232. doi: 10.1016/j.tig.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Stroud JC, Wu Y, Bates DL, et al. Structure of the forkhead domain of FOXP2 bound to DNA structure. Structure. 2006;14(1):159–166. doi: 10.1016/j.str.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Koon HB, Ippolito GC, Banham AH, Tucker PW. FOXP1: a potential therapeutic target in cancer. Expert Opin Ther Targets. 2007;11(7):955–965. doi: 10.1517/14728222.11.7.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaestner KH, Knöchel W, Martínez DE. Unified nomenclature for the winged helix/forkhead transcription factors. Genes Dev. 2000;14(2):142–146. [PubMed] [Google Scholar]
- 9.Bacon C, Schneider M, Le Magueresse C, et al. Brain-specific Foxp1 deletion impairs neuronal development and causes autistic-like behaviour. Mol Psychiatry. 2015;20(5):632–639. doi: 10.1038/mp.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shu W, Lu MM, Zhang Y, Tucker PW, Zhou D, Morrisey EE. Foxp2 and Foxp1 cooperatively regulate lung and esophagus development. Development. 2007;134(10):1991–2000. doi: 10.1242/dev.02846. [DOI] [PubMed] [Google Scholar]
- 11.Xiao J, He B, Zou Y, et al. Prognostic value of decreased FOXP1 protein expression in various tumors: a systematic review and meta-analysis. Sci Rep. 2016;6:30437. doi: 10.1038/srep30437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He M, Gao L, Zhang S, et al. Prognostic significance of miR-34a and its target proteins of FOXP1, p53, and BCL2 in gastric MALT lymphoma and DLBCL. Gastric Cancer. 2014;17(3):431–441. doi: 10.1007/s10120-013-0313-3. [DOI] [PubMed] [Google Scholar]
- 13.Gascoyne DM, Banham AH. The significance of FOXP1 in diffuse large B-cell lymphoma. Leuk Lymphoma. 2017;58(5):1037–1051. doi: 10.1080/10428194.2016.1228932. [DOI] [PubMed] [Google Scholar]
- 14.Dekker JD, Parka D, Shaffer AL, 3rd, et al. Subtype-specific addiction of the activated B-cell subset of diffuse large B-cell lymphoma to FOXP1. Proc Natl Acad Sci U S A. 2016;113(5):E577–E586. doi: 10.1073/pnas.1524677113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Remmele W, Stegner HE. Vorschlag zur einheitlichen Definition eines Immunreaktiven Scores (IRS) für den Östrogenrezeptornachweis (ER-ICA) im Mammakarcinomgewebe [Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue] Pathologe. 1987;8(3):138–140. German. [PubMed] [Google Scholar]
- 16.Zhang Y, Zhang S, Wang X, et al. Prognostic significance of FOXP1 as an oncogene in hepatocellular carcinoma. J Clin Pathol. 2012;65(6):528–533. doi: 10.1136/jclinpath-2011-200547. [DOI] [PubMed] [Google Scholar]
- 17.Li F, Liu T, Xiao CY, Yu JX, Lu LG, Xu MY. FOXP1 and SPINK1 reflect the risk of cirrhosis progression to HCC with HBV infection. Biomed Pharmacother. 2015;72:103–108. doi: 10.1016/j.biopha.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Sun J, Cui M, et al. Downregulation of FOXP1 inhibits cell proliferation in hepatocellular carcinoma by inducing G1/S phase cell cycle arrest. Int J Mol Sci. 2016;17(9) doi: 10.3390/ijms17091501. pii: E1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giatromanolaki A, Koukourakis MI, Sivridis E, Gatter KC, Harris AL, Banham AH. Loss of expression and nuclear/cytoplasmic localization of the FOXP1 forkhead transcription factor are common events in early endometrial cancer: relationship with estrogen receptors and HIF-1a expression. Modern Pathol. 2006;19(1):9–16. doi: 10.1038/modpathol.3800494. [DOI] [PubMed] [Google Scholar]
- 20.Choi EJ, Seo EJ, Kim DK, et al. FOXP1 functions as an oncogene in promoting cancer stem cell-like characteristics in ovarian cancer cells. Oncotarget. 2016;7(3):3506–3519. doi: 10.18632/oncotarget.6510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YS, Hwan JD, Bae S, Bae DH, Shick WA. Identification of differentially expressed genes using an annealing control primer system in stage III serous ovarian carcinoma. BMC Cancer. 2010;10:576. doi: 10.1186/1471-2407-10-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Z, Zhu L, Gao J, et al. Expression of FOXP1 in epithelial ovarian cancer (EOC) and its correlation with chemotherapy resistance and prognosis. Tumor Biol. 2015;36(9):7269–7275. doi: 10.1007/s13277-015-3383-5. [DOI] [PubMed] [Google Scholar]
- 23.Fox SB, Brown P, Han C, et al. Expression of the forkhead transcription factor FOXP1 is associated with estrogen receptor and improved survival in primary human breast carcinomas. Clin Cancer Res. 2004;10(10):3521–3527. doi: 10.1158/1078-0432.CCR-03-0461. [DOI] [PubMed] [Google Scholar]
- 24.Ijichi N, Shigekawa T, Ikeda K, et al. Association of double-positive FOXA1 and FOXP1 immunoreactivities with favorable prognosis of tamoxifen-treated breast cancer patients. Horm Cancer. 2012;3(4):147–159. doi: 10.1007/s12672-012-0111-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng J, Zhang X, Zhu H, Wang X, Ni S, Huang J. High expression of FoxP1 is associated with improved survival in patients with non–small cell lung cancer. Am J Clin Pathol. 2012;138(2):230–235. doi: 10.1309/AJCPDHQFNYJZ01YG. [DOI] [PubMed] [Google Scholar]
- 26.De Smedt L, Palmans S, Govaere O, et al. Expression of FOXP1 and colorectal cancer prognosis. Lab Med. 2015;46(4):299–311. doi: 10.1309/LM7IHV2NJI1PHMXC. [DOI] [PubMed] [Google Scholar]
- 27.Ackermann S, Kocak H, Hero B, et al. FOXP1 inhibits cell growth and attenuates tumorigenicity of neuroblastoma. BMC Cancer. 2014;14:840. doi: 10.1186/1471-2407-14-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hussein MR. Tumour-associated macrophages and melanoma tumourigenesis: integrating the complexity. Int J Exp Pathol. 2006;87(3):163–176. doi: 10.1111/j.1365-2613.2006.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22(2):231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Nazimek K, Bryniarski K. Aktywność biologiczna makrofagów w zdrowiu i chorobie [The biological activity of macrophages in health and disease] Postepy Hig Med Dosw (online) 2012;66:507–520. doi: 10.5604/17322693.1004080. Polish. [DOI] [PubMed] [Google Scholar]
- 31.Fairweather D, Cihakova D. Alternatively activated macrophages in infection and autoimmunity. J Autoimmun. 2009;33(3–4):222–230. doi: 10.1016/j.jaut.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zamarron BF, Chen W. Dual roles of immune cells and their factors in cancer development and progression. Int J Biol Sci. 2011;7(5):651–658. doi: 10.7150/ijbs.7.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi C, Sakuma M, Mooroka T, et al. Down-regulation of the forkhead transcription factor Foxp1 is required for monocyte differentiation and macrophage function. Blood. 2008;112(12):4699–4711. doi: 10.1182/blood-2008-01-137018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song Y, Li X, Zeng Z, et al. Epstein-Barr virus encoded miR-BART11 promotes inflammation-induced carcinogenesis by targeting FOXP1. Oncotarget. 2016;7(24):36783–36799. doi: 10.18632/oncotarget.9170. [DOI] [PMC free article] [PubMed] [Google Scholar]