Abstract
Tsukamurella is an aerobic, Gram-positive and nonmotile bacterium. It was first isolated in 1941 from the mycetoma and ovaries of the bedbug. The primary strains were named Corynebacterium paurometabolum and Gordona aurantiaca and are different from the Collins et al., 1988 classification of the new Tsukamurella genus. Human infections with Tsukamurella species are rare because the species is a kind of saprophyte bacterium; however, most information regarding this species comes from case reports. Molecular markers for the identification Tsukamurella include sequencing of 16S rRNA, groEL, rpoB, secA1 and ssrA genes. Given the lack of information on the treatment of Tsukamurella infections, a combination of various antibiotic agents is recommended.
Keywords: Isolation, molecular identification, taxonomy, Tsukamurella
Introduction
Actinomycetes that have mycolic acid (chemotype IV) have been classified under genera such as Corynebacterium, Gordonia, Mycobacterium, Nocardia, Rhodococcus, Tsukamurella, Skermania and Williamsia [1]. As determined by 16S rRNA gene sequencing, these taxa have numerous features; Corynebacterineae comprise all these taxa, along with Turicella [2]. The genus Tsukamurella is an aerobic actinomycetes with a series of very long chains and unsaturated mycolic acid. It belongs to Actinobacteria, Actinomycetales, Corynebacterineae and Tsukamurellaceae. Tsukamurella was introduced in 1988 by Collins et al. according to the analysis of Japanese scientist Michico Tsukamura and of the studies of Steinhaus [3], [4], [5], [6]. Tsukamurella paurometabola was the first member of this genus, and it has a complex history. It was transferred from the genus Gordona to Rhodococcus and eventually classified as the genus Tsukamurella. The name Tsukamurella was selected to honor Michio Tsukamura, and paurometabolum (pauros, ‘small,’ and metabolos, ‘changeable’) was chosen because it had been described previously by Steinhaus [5] and refers to the fact that this bacterium is inactive in most phenotypic tests. On the basis of the rules of nomenclature, the name was then changed to paurometabola [4], [7]. The genus Tsukamurella consists of 11 species with available published names (http://www.bacterio.net/tsukamurella.html) [9]. Tsukamurella spp. are environmental saprophytes which are isolated from soil, arthropods, water, sludge foam and sponges [9]. They are opportunistic pathogens and can be spread through clinical instruments (e.g. catheters). They therefore can cause various infections in humans, such as pulmonary and cutaneous infections and meningitis; colonization also occurs in immunosuppressed individuals [1]. To date, nine species of the genus Tsukamurella have been isolated from human infections: inchonensis, paurometabola, strandjordii, tyrosinosolvens, pulmonis, hongkongensis and sinensis [10], [11]. Although Tsukamurella serpentis was recently isolated from the oral cavity of two venomous snakes (Naja atra) in China, there is no report of human infection by this species after being bitten [12].
History of Tsukamurella
One member of the aerobic actinomycetes with a series of very long chains and unsaturated mycolic acids that was created in 1988 is Tsukamurella. Tsukamurella is a Gram-positive, rod-shaped bacterium that is typically misidentified as Corynebacterium, Rhodococcus, Nocardia and some nontuberculous mycobacteria species, so molecular methods are necessary for their accurate identification. Members of the taxa that comprise mycolic acids with wall chemotype IV differ in some features; Tsukamurella consists of 64 to 78 carbon atoms, Nocardia 44 to 60, Mycobacterium 60 to 90 and Rhodococcus 34 to 64. The G+C content of Nocardia is 64 to 72 mol%, Mycobacterium 62 to 70 mol% and Rhodococcus 63 to 73 mol%, but Tsukamurella is 67 to 68 mol%, although some species are exceptions to these ranges. Tuberculostearic acid is another attribute that may be used to differentiate among genera. Tsukamurella, Nocardia, Mycobacterium and Rhodococcus include tuberculostearic acid, but Corynebacterium does not—again, with the exception of some species [4]. Steinhaus [5] in 1941 isolated an organism from the mycetoma and ovaries of bedbugs (Cimex lectularius) and named it Corynebacterium paurometabolum. The presence of meso-diaminopimelic acid and an arabinogalactan polymer also causes misidentification because strains that contain these substances resemble Corynebacterium. Unsaturated mycolic acid (68 to 76 carbon atoms and two to six double bonds) can be used to distinguish Tsukamurella species from Corynebacterium [4]. Tsukamura and Mizuno [6] in 1971 identified a similar species with long mycolic acid as Gordona aurantiaca. Rhodococciis aurantiacus was initially classified under the genus Rhodococcus, but because it did not have features that can be distinctly associated with the genus Rhodococcus, the species was omitted from this classification. The findings of Goodfellow et al. showed that Tsukamurella is similar to Mycobacterium and Nocardia, but because of very long series and unsaturated mycolic acid, it can differ from other mycolic acid–containing actinomycetes [4].
In accordance with the results of 16S rRNA gene analyses, Collins et al. [4] in 1988 reclassified C. paurometabolum and R. aurantiacus under a new genus, Tsukamurella. Apart from being a Gram-positive bacterium, Tsukamurella is a partially acid fast, obligatory aerobic bacterium that does not have a capsule or aerial hyphae. It is chemoorganotrophic, catalase positive and pyrazinamidase positive, and some species can produce acid of some sugars. Moreover, it is a non–spore forming, lysozyme-resistant bacterium with nonmotile, rod-shaped cells that can be straight or curved and can be seen in pairs or groups of coccobacillary forms by Gram stain. Some Tsukamurella species produce pigmented colonies; isolation by Löwenstein-Jensen and brain–heart infusion media indicated that Tsukamurella colonies are different. The species are long rods at the first stage of growth but later separate and form new rods. Colonies are visible at 24 to 72 hours' growth [13]. The optimal growth temperature of Tsukamurella is 25 to 35°C, although there are some exceptions (Table 1) [3]. The cell envelope of the bacterium consists of peptidoglycan, sugars (arabinose and galactose, as shown in a sugar analysis), phospholipids, fatty acids, mycolic acid and unsaturated menaquinones. Cell wall analysis has shown meso-diaminopimelic acid type A1 in the structure of peptidoglycan. The species also contains phosphatidyl ethanolamine and 10-methyloctadecanoid fatty acids, and it has a G+C content of 67 to 68 mol% [14]. Peptidoglycan consists of d-alanine, l-alanine, N-acetylglucosamine, d-glutamic acid and muramic acid. Phospholipids contain phosphatidylinositol, phosphatidylethanolamine and diphosphatidylglycerol. Fatty acids contain tuberculostearic acids [15].
Table 1.
Some phenotypic characteristics of Tsukamurella spp
| Name | OT (°C) | NaCl concentration (%) | Enzyme |
Carbon utilization |
Hydrolysis |
Study | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxi | Cata | Nit | Lip | Arab | Fru | Man | Sorb | Xly | Gala | Cello | Arabi | Dul | meso-Ery | Sali | Xanthine | Adenine | Hypoxanthine | Casein | Aesculin | Urea | Tyrosine | Tween 80 | ||||
| T. hongkongensis | ND | ND | − | + | ND | ND | − | ND | + | + | − | + | ND | + | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | [60] |
| T. inchonensis | 24–45 | 5% | ND | ND | − | ND | + | − | + | + | + | − | − | − | − | − | + | − | ND | + | − | + | + | − | ND | [61] |
| T. paurometabola | 10–35 | ND | ND | ND | ND | ND | − | + | − | + | + | − | ND | − | − | − | + | − | − | ND | − | ND | + | − | + | [4] |
| T. pseudospumae | 25–30 | ND | + | ND | ND | ND | + | + | − | + | + | − | − | + | − | − | − | − | ND | − | ND | + | − | + | ND | [62] |
| T. pulmonis | 24–37 | ND | ND | + | − | ND | ND | ND | + | + | − | ND | + | + | + | + | + | − | ND | + | − | − | + | − | + | [52] |
| T. sinensis | ND | ND | − | + | ND | ND | − | ND | − | − | − | − | ND | − | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | [60] |
| T. soli | 30 | 3.0% | − | + | − | + | − | + | ND | + | ND | ND | − | + | − | − | + | − | ND | − | − | + | + | + | + | [63] |
| T. spumae | 25–37 | ND | + | ND | ND | ND | + | − | + | + | + | − | + | + | + | + | − | − | ND | + | ND | ND | + | + | ND | [64] |
| T. strandjordae | 28–35 | 5% | ND | + | ND | ND | − | + | + | + | ND | + | ND | + | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | [11] |
| T. tyrosinosolvens | 24–37 | 5% | ND | ND | ND | ND | − | ND | + | + | − | + | − | ND | ND | − | ND | + | − | + | − | + | + | + | + | [65] |
| T. serpentis | 10,37 | 7% | − | + | ND | ND | + | + | + | + | + | + | ND | + | + | +/− | ND | − | ND | ND | ND | + | ND | − | ND | [12] |
+/− indicates different utilization.
Arab, arabinose; Arabit, arabitol; Cata, catalase; Cello, cellobiose; Dul, dulcitol; Fru, fructose; Gala, galactose; Lip, lipase; Man, mannitol; meso-Ery, meso-erythritol; ND, not determined; Nit, nitrate reductase; OT, optimum temperature; Oxi, oxidase; Sali, salicin; Sorb, sorbitol; Xly, xylose.
Infections Caused by Tsukamurella Species
To date there has been no decisive report that shows any specific virulence factor for this genus. Most information regarding human infection by Tsukamurella has derived from case reports. Given that such infection is not routine, it can be inferred to be a kind of nosocomial and sporadic infection. Infection with Tsukamurella spp. is rare and mostly caused by contact with infected catheters [16]. Some reports regarding Tsukamurella infections have identified the sources of infections as other mycolic acid–containing actinomycetes. Lung disease in immunodeficient patients has long been attributed to Tsukamurella infection. The most commonly reported sources of Tsukamurella infection include bacteraemia [17], [18], [19], [20], [21], [22], [23], [24], meningitis [25], peritonitis [26], keratitis [27], [28], [29], cutaneous infection [30], conjunctivitis [23], [29], [31], brain abscess [32], respiratory tract infection [29], [33], [34], [35], [36], [37], [38], catheter-related bloodstream infection [16], [19], [23], [29], [39], [40], [41], [42], [43], [44] and acute otitis media [16]. In addition, Tsukamurella is a threat to people with immunodeficiency, including HIV infection [34], [45]. The risk factors for such infection are renal failure, foreign bodies (e.g. clinical equipment) and most importantly immunosuppressive diseases [46]. Tuberculosis-like syndromes and symptoms include acute bronchitis, bacteraemic pneumonia, productive cough, haemoptysis and weight loss [47]. The isolation history of Tsukamurella spp. is shown in Table 2.
Table 2.
Isolation history of Tsukamurella spp
| Species | Introduced in year | First isolation in: |
G + C content (mol%) | Study | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clinical specimens |
Environmental samples |
|||||||||||||
| Mycetoma | Conjunctival swab | Lung | Sputum | Blood | Catheter | Soil | Deep-water marine sponge | Sludge foam | Insect | Venomous snake | ||||
| T. tyrosinosolvensa | 1997 | + | 73.6+ 0.03% | Yassin, Rainey et al., 1997 | ||||||||||
| T. hongkongensis | 2016 | + | + | 71.2 ± 2.3% | Teng, Tang et al., 2016 | |||||||||
| T. inchonensis | 1995 | + | + | 72% | Yassin, Rainey et al., 1995 | |||||||||
| T. pseudospumaea | 2004 | + | ND | Nam, Kim et al., 2004 | ||||||||||
| T. paurometabola | 1988 | + | + | ND | Collins et al., 1988 | |||||||||
| T. pulmonisa | 1996 | + | %69.8 | Yassin, Rainey et al., 1996 | ||||||||||
| T. sinensis | 2016 | + | + | 70.9 ± 2.2% | Teng, Tang et al., 2016 | |||||||||
| T. soli | 2010 | + | 70% | Weon, Yoo et al., 2010 | ||||||||||
| T. spumae | 2003 | + | 72% | Nam, Chun et al., 2003 | ||||||||||
| T. strandjordae | 2002 | + | ND | Kattar, Cookson et al., 2001 | ||||||||||
| T. serpentis | 2016 | + | 69.2 ± 1.5 | Tang, Teng 2016 | ||||||||||
ND, not determined.
See current taxonomy of Tsukamurella species.
Identification Methods
Phenotypic methods
Members of aerobic actinomycetes are increasing as a result of the discovery of new species, and clinical microbiologists will face problems identifying them. Phenotypic tests are the first way to distinguish among aerobic actinomycetes, although the spectra of these tests do not have all the varieties in laboratories to permit identification among species. For instance, analyzing whole-cell sugar is rarely used for identification in clinical laboratories. Staining of these bacteria in a medical laboratory is by Gram stain, which can differentiate a partially acid-fast organism from an acid-fast one. Most conventional and standard media are suitable for growing this bacterium. Blood agar, chocolate agar, brain–heart infusion agar, Sabouraud dextrose agar, Columbia agar with 5% defibrinated sheep's blood agar [12] and Löwenstein-Jensen medium will all support the growth of most aerobic actinomycetes. Aerobic conditions are recommended to better grow aerobic actinomycetes. Tsukamurella treatment by partially acid-fast stain shows weakly positive colony morphology; bacteria on conventional media are small and dry, with convex elevation, and form white, cream-coloured to orange colonies [48]. According to Bergey's Manual of Systematic Bacteriology, colonies of T. paurometabola are smooth and creamy and have a fried-egg appearance; other species can show a variety of colours. Optimal growth temperature is 25 to 37°C. As Steinhaus [5] has noted, semisolid medium with gelatin, carbohydrates and rabbit serum is the basic medium for isolation of T. paurometabola [15]. The species show partially acid-fast results, but some researchers believe that strongly acid-fast results have been observed in some species [3]. Matrix-assisted desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) may also be performed to help with identification [49], [50].
Molecular methods
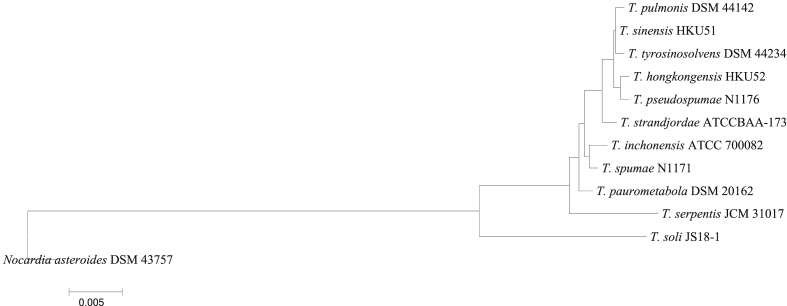
16S rRNA gene sequencing can be carried out to detect Tsukamurella spp. Park et al. [51] used primers 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1525R (5′-AAGGAGGTGWTCCARCC-3′) for the PCR amplification of the 16S rRNA gene. Through the PCR-mediated amplification of 16S rRNA gene and lipid analysis based on thin-layer chromatography, Yassin et al. [52] isolated T. pulmonis. Woo et al. [31] used the primers LPW57 (5′-AGTTTGATCCTGGCTCAG-3′) and LPW58 (5′-AGGCCCGGGAACGTATTCAC-3′). DNA-DNA hybridization is rarely used in clinical laboratories, although it is a standard molecular method for novel species identification. Via dot blot analysis, Kattar et al. [11] performed DNA-DNA hybridization and also carried out gas liquid chromatography, high-performance liquid chromatography and 16S rRNA gene sequencing with the primers 8FPL and DG74. Housekeeping genes such as hsp65 (heat shock protein 65), rpoB (RNA polymerase, β subunit), gyrB (DNA gyrase, subunit B), groEL (molecular chaperone GroEL) and the 16S–23S internal transcribed spacer are used to identify aerobic actinomycetes [3]. Teng et al. [53] used 16S rRNA, ssrA (small stable RNA), secA (secretory), rpoB and groEL for differentiation of Tsukamurella species; results indicated that only 16S rRNA and groEL were effective in indicating the exact species. Teng et al. [50] recommended the use of PCR–restriction fragment length polymorphism (RFLP) and 16S rRNA gene sequencing analysis or other advanced molecular methods to differentiate Tsukamurella from other similar genus. Pérez et al. [54], [55] also used 16S rRNA analysis, with PA (5′-AGAGTTTGATCCTGGCTCAG-3′) and PLO6 R (5′-GCGCTCGTTGCGGGACTTA ACC-3′) and Tsuka1(5′-CTACCTGCGCGACAACATG-3′), as well as Tsuka2 (5′-CGATCGTCTTCTTGCGGATG-3′) as primers for secA1 genes on microorganisms in blood from bloodstream infections, which indicated T. pulmonis. According to the evidence of Teng et al. [8], [53], the 16S rRNA gene was unsuccessful in differentiating T. sinensis from some strains such as T. pulmonis and T. tyrosinosolven; furthermore, secA1 failed to differentiate between Tsukamurella spumae and Tsukamurella pseudospumae. Two primers used for the hsp65 gene included TB11 (5′-ACCAACGATGGTGTGTCCAT-3′) and TB12 (5′-CTTGTCGAACCGCATACCCT-3′); the results indicated that T. spumae was the cause of an infection in that case report; for 16S rRNA gene sequencing, LPW27807 (5′-TGGCTCAGGACGAACGCT-3′) and LPW27808 (5′-GAGGTGATCCAGCCGCA-3′) were used [50]. PCR-RFLP was the first method adopted in Tsukamurella identification [50]. The 16S rRNA gene-based phylogenetic tree of Tsukamurella species was analysed by MEGA5 software [56] (Fig. 1).
Fig. 1.
Full gene sequencing (∼1500 bp fragment) of 16S rRNA gene-based phylogenetic tree of Tsukamurella species (standard isolates) computed by neighbour-joining analyses and Kimura two-parameter model. Support of each branch as determined from 1000 bootstrap samples. Bar 0.005 indicates one nucleotide substitution per 100 nucleotides.
Antibiotic Susceptibility Testing and Treatment
In the literature, there is little information about sensitivity to antibiotics in the genus Tsukamurella. The best antibiotic susceptibility testing is microbroth dilution, which was introduced by the Clinical and Laboratory Standards Institute [57]. Tsukamurella spp. are resistant to penicillin, oxacillin, piperacillin/tazobactam and cephalosporins, which are prescribed for treatment of nontuberculous mycobacteria infection, whereas Tsukamurella is susceptible to amikacin, ciprofloxacin, imipenem, doxycycline, linezolid and sulfamethoxazole [47], [58]. Because of the insufficiency of guidelines regarding the treatment of Tsukamurella infection, the combination of β-lactam and aminoglycoside antibiotic agents and the removal of catheters has been recommended for improved outcomes. Therefore, as a related taxon, Tsukamurella can be inactivated by the ribosylation of the 23-OH group of antibiotics [59]. Effective results resulted from a combination of β-lactam or macrolide with aminoglycoside antibiotic agents for a long treatment period [47]. Susceptibility testing is necessary for proper treatment of Tsukamurella infections [16].
Current Taxonomy of Tsukamurella Species
According to some new work by Teng et al. [50], some species such as T. tyrosinosolvens, T. pseudospumae and T. pulmonis have been misclassified. Too much similarity between some species of Tsukamurella in many molecular and phenotypic experiments was the reason that Teng et al. asked researchers to confirm the hypothesis that these three species in fact are the same type and misclassified. MALDI-TOF MS analysis, 16S rRNA gene sequencing, phylogenetic analysis, whole genome comparison, DNA-DNA hybridization and phenotypic characteristics are some of the studies Teng et al. performed. Research revealed that even though high similarity exists between T. tyrosinosolvens and T. carboxydivorans in the genomic analysis, some genomic islands and regions are present in the T. tyrosinosolvens genome. Further investigation and analysis indicated that these islands are mobile element proteins and other proteins related to phages. Although the T. tyrosinosolvens that they used their in research had been isolated from a patient with a cardiac pacemaker implant and the T. carboxydivorans had an environmental source (soil), more research is needed to confirm the pathogenic power of these genomic islands. After assessing similarity by G+C content, 16S rRNA gene sequencing, MALDI-TOF MS and phylogenomic analyses, reclassification was suggested for T. spongiae as T. pulmonis, T. carboxydivorans as T. tyrosinosolvens and finally T. sunchonensis as T. pseudospumae [50].
Conclusion
To our knowledge, this is the first review of the literature of Tsukamurella species. Despite the lack of information on this genus and its status as a kind of saprophyte, it has been confirmed as a cause of opportunistic infections. Members of this genus are increasingly being identified, thus highlighting the need to clarify its features for improved cooperation between doctors and microbiologists. The pathogenic mechanism and antibiotic resistance of Tsukamurella are unknown and therefore require more research that involves clinical samples and new detection methods. It is hoped that in the near future new molecular methods can reveal different aspects of Tsukamurella for the promotion of clinical perspectives and development of enhanced treatment options.
Conflict of Interest
None declared.
References
- 1.Dworkin M., Falkow S., Rosenberg E., Schleifer K.H., Stackebrandt E. vol. 3. Springer; New York: 2006. The prokaryotes. (Archaea Bacteria: firmicutes, actinomycetes). [Google Scholar]
- 2.Goodfellow M., Chun J., Stackebrandt E., Kroppenstedt R.M. Transfer of Tsukamurella wratislaviensis Goodfellow et al. 1995 to the genus Rhodococcus as Rhodococcus wratislaviensis comb. nov. Int J Syst Evol Microbiol. 2002;52:749–755. doi: 10.1099/00207713-52-3-749. [DOI] [PubMed] [Google Scholar]
- 3.Liu D. Taylor & Francis; New York: 2011. Molecular detection of human bacterial pathogens. [Google Scholar]
- 4.Collins M., Smida J., Dorsch M., Stackebrandt E. Tsukamurella gen. nov. harboring Corynebacterium paurometabolum and Rhodococcus aurantiacus. Int J Syst Evol Microbiol. 1988;38:385–391. [Google Scholar]
- 5.Steinhaus E.A. A study of the bacteria associated with thirty species of insects. J Bacteriol. 1941;42:757. doi: 10.1128/jb.42.6.757-790.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsukamura M., Mizuno S. A new species Gordona aurantiaca occurring in sputa of patients with pulmonary disease. Kekkaku (Tuberculosis) 1971;46:93–98. [PubMed] [Google Scholar]
- 7.Topley and Wilson's microbiology and microbial infections. vol. 8. New York: Wiley; 2006.
- 8.Teng J.L., Tang Y., Lau S.K., Woo P.C. Reply to Perez del Molino Bernal and Agüero Balbin, ‘seqA1 is a useful target for identification of Tsukamurella pulmonis.’. J Clin Microbiol. 2017;55:1592–1594. doi: 10.1128/JCM.00238-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization . IWA Publishing; London: 2004. Guidelines for drinking-water quality. [Google Scholar]
- 10.Cheung L.W. University of Hong Kong; 2014. Evaluation of gene targets for identification of Tsukamurella species. PhD diss. [Google Scholar]
- 11.Kattar M.M., Cookson B.T., Carlson L.C., Stiglich S.K., Schwartz M.A., Nguyen T.T. Tsukamurella strandjordae sp. nov., a proposed new species causing sepsis. J Clin Microbiol. 2001;39:1467–1476. doi: 10.1128/JCM.39.4.1467-1476.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang Y., Teng J.L., Cheung C.L., Ngan A.H., Huang Y., Wong S.S. Tsukamurella serpentis sp. nov., isolated from the oral cavity of Chinese cobras (Naja atra) Int J Syst Evol Microbiol. 2016;66:3329–3336. doi: 10.1099/ijsem.0.001187. [DOI] [PubMed] [Google Scholar]
- 13.Dworkin M., Falkow S., Rosenberg E., Schleifer K.H., Stackebrandt E. vol. 4. Springer; New York: 2006. The prokaryotes. (Bacteria: firmicutes, cyanobacteria). [Google Scholar]
- 14.Goodfellow M., Zakrzewska-Czerwinska J., Thomas E., Mordarski M., Ward A., James A. Polyphasic taxonomic study of the genera Gordona and Tsukamurella including the description of Tsukamurella wratislaviensis sp. nov. Zentralbl Bakteriol. 1991;275:162–178. doi: 10.1016/s0934-8840(11)80063-0. [DOI] [PubMed] [Google Scholar]
- 15.Parte A., Whitman W., Goodfellow M., Kämpfer P., Busse H.J., Trujillo M. vol. 5. Springer; New York: 2012. Bergey's manual of systematic bacteriology. (The Actinobacteria). [Google Scholar]
- 16.Liu C.Y., Lai C.C., Lee M.R., Lee Y.C., Huang Y.T., Liao C.H. Clinical characteristics of infections caused by Tsukamurella spp. and antimicrobial susceptibilities of the isolates. Int J Antimicrob Agents. 2011;38:534–537. doi: 10.1016/j.ijantimicag.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz M.A., Tabet S.R., Collier A.C., Wallis C.K., Carlson L.C., Nguyen T.T. Central venous catheter–related bacteremia due to Tsukamurella species in the immunocompromised host: a case series and review of the literature. Clin Infect Dis. 2002;35:e72–e77. doi: 10.1086/342561. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro C.L., Haft R.F., Gantz N.M., Doern G.V., Christenson J.C., O'Brien R. Tsukamurella paurometabolum: a novel pathogen causing catheter-related bacteremia in patients with cancer. Clin Infect Dis. 1992;14:200–203. doi: 10.1093/clinids/14.1.200. [DOI] [PubMed] [Google Scholar]
- 19.Chong Y., Lee K., Chon C.Y., Kim M.J., Kwon O.H., Lee H.J. Tsukamurella inchonensis bacteremia in a patient who ingested hydrochloric acid. Clin Infect Dis. 1997;24:1267–1268. doi: 10.1093/clinids/24.6.1267. [DOI] [PubMed] [Google Scholar]
- 20.Jones R.S., Fekete T., Truant A.L., Satishchandran V. Persistent bacteremia due to Tsukamurella paurometabolum in a patient undergoing hemodialysis: case report and review. Clin Infect Dis. 1994:830–832. doi: 10.1093/clinids/18.5.830. [DOI] [PubMed] [Google Scholar]
- 21.Shim H.E., Sung H., Baek S.M., Namgung S., Kim M.N., Kim Y.G. A case of catheter-related bacteremia of Tsukamurella pulmonis. Korean J Lab Med. 2009;29:41–47. doi: 10.3343/kjlm.2009.29.1.41. [DOI] [PubMed] [Google Scholar]
- 22.Clausen C., Wallis C.K. Bacteremia caused by Tsukamurella species. Clin Microbiol Newsl. 1994;16:6–8. [Google Scholar]
- 23.Esteban J., Calvo R., Molleja A., Soriano F. Isolation of Tsukamurella-like organisms from human samples: contamination, colonization, or infection? Clin Microbiol Newsl. 1998;20:6–8. [Google Scholar]
- 24.Elshibly S., Doherty J., Xu J., McClurg R., Rooney P., Millar B. Central line–related bacteraemia due to Tsukamurella tyrosinosolvens in a haematology patient. Ulster Med J. 2005;74:43. [PMC free article] [PubMed] [Google Scholar]
- 25.Prinz G., Ban E., Fekete S., Szabo Z. Meningitis caused by Gordona aurantiaca (Rhodococcus aurantiacus) J Clin Microbiol. 1985;22:472–474. doi: 10.1128/jcm.22.3.472-474.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaer A., Gadegbeku C. Tsukamurella peritonitis associated with continuous ambulatory peritoneal dialysis. Clin Nephrol. 2001;56:241–246. [PubMed] [Google Scholar]
- 27.Woo P.C., Fong A.H., Ngan A.H., Tam D.M., Teng J.L., Lau S.K. First report of Tsukamurella keratitis: association between T. tyrosinosolvens and T. pulmonis and ophthalmologic infections. J Clin Microbiol. 2009;47:1953–1956. doi: 10.1128/JCM.00424-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tam P.M., Young A.L., Cheng L., Congdon N., Lam P.T. Tsukamurella: an unrecognized mimic of atypical mycobacterial keratitis? The first case report. Cornea. 2010;29:362–364. doi: 10.1097/ICO.0b013e3181ae2594. [DOI] [PubMed] [Google Scholar]
- 29.Chen C.H., Lee C.T., Chang T.C. Tsukamurella tyrosinosolvens bacteremia with coinfection of Mycobacterium bovis pneumonia: case report and literature review. SpringerPlus. 2016;5:2033. doi: 10.1186/s40064-016-3707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Granel F., Lozniewski A., Barbaud A., Lion C., Dailloux M., Weber M. Cutaneous infection caused by Tsukamurella paurometabolum. Clin Infect Dis. 1996;23:839–840. doi: 10.1093/clinids/23.4.839. [DOI] [PubMed] [Google Scholar]
- 31.Woo P.C., Ngan A.H., Lau S.K., Yuen K.Y. Tsukamurella conjunctivitis: a novel clinical syndrome. J Clin Microbiol. 2003;41:3368–3371. doi: 10.1128/JCM.41.7.3368-3371.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheng W.H., Huang Y.T., Chang S.C., Hsueh P.R. Brain abscess caused by Tsukamurella tyrosinosolvens in an immunocompetent patient. J Clin Microbiol. 2009;47:1602–1604. doi: 10.1128/JCM.01932-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehta Y.B., Simonelli P., Goswami R., Bhanot N., Mehta Z. Tsukamurella infection: a rare cause of community-acquired pneumonia. Am J Med Sci. 2011;341:500–503. doi: 10.1097/MAJ.0b013e3182129d02. [DOI] [PubMed] [Google Scholar]
- 34.Alcaide M.L., Espinoza L., Abbo L. Cavitary pneumonia secondary to Tsukamurella in an AIDS patient. First case and a review of the literature. J Infect. 2004;49:17–19. doi: 10.1016/S0163-4453(03)00139-7. [DOI] [PubMed] [Google Scholar]
- 35.Ménard A., Degrange S., Peuchant O., Nguyen T.D.T., Dromer C., Maugein J. Tsukamurella tyrosinosolvens—an unusual case report of bacteremic pneumonia after lung transplantation. Ann Clin Microbiol Antimicrob. 2009;8:30. doi: 10.1186/1476-0711-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maalouf R., Mierau S.B., Moore T.A., Kaul A. First case report of community-acquired pneumonia due to Tsukamurella pulmonis. Ann Intern Med. 2009;150:147–148. doi: 10.7326/0003-4819-150-2-200901200-00022. [DOI] [PubMed] [Google Scholar]
- 37.de Jesus Perez V.A., Swigris J., Ruoss S.J. Coexistence of primary adenocarcinoma of the lung and Tsukamurella infection: a case report and review of the literature. J Med Case Rep. 2008;2:207. doi: 10.1186/1752-1947-2-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inchingolo R., Nardi I., Chiappini F., Macis G., Ardito F., Sali M. First case of Tsukamurella pulmonis infection in an immunocompetent patient. Respir Med CME. 2010;3:23–25. [Google Scholar]
- 39.Maertens J., Wattiau P., Verhaegen J., Boogaerts M., Verbist L., Wauters G. Catheter-related bacteremia due to Tsukamurella pulmonis. Clin Microbiol Infect. 1998;4:51–55. doi: 10.1111/j.1469-0691.1998.tb00336.x. [DOI] [PubMed] [Google Scholar]
- 40.del Molino Bernal I.C.P., Cano M.E., de la Fuente C.G., Martínez-Martínez L., López M., Fernández-Mazarrasa C. Tsukamurella pulmonis bloodstream infection identified by secA1 gene sequencing. J Clin Microbiol. 2015;53:743–745. doi: 10.1128/JCM.02545-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bouza E., Pérez-Parra A., Rosal M., Martín-Rabadán P., Rodríguez-Créixems M., Marín M. Tsukamurella: a cause of catheter-related bloodstream infections. Eur J Clin Microbiol Infect Dis. 2009;28:203–210. doi: 10.1007/s10096-008-0607-2. [DOI] [PubMed] [Google Scholar]
- 42.Lai K.K. A cancer patient with central venous catheter–related sepsis caused by Tsukamurella paurometabolum (Gordona aurantiaca) Clin Infect Dis. 1993:285–287. doi: 10.1093/clinids/17.2.285-a. [DOI] [PubMed] [Google Scholar]
- 43.Sheridan E.A., Warwick S., Chan A., Dall'Antonia M., Koliou M., Sefton A. Tsukamurella tyrosinosolvens intravascular catheter infection identified using 16S ribosomal DNA sequencing. Clin Infect Dis. 2003;36:e69–e70. doi: 10.1086/367654. [DOI] [PubMed] [Google Scholar]
- 44.Takebe I., Sawabe E., Ohkusu K., Tojo N., Tohda S. Catheter-related bloodstream infection by Tsukamurella inchonensis in an immunocompromised patient. J Clin Microbiol. 2014;52:2251–2253. doi: 10.1128/JCM.00421-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rey D., De Briel D., Heller R., Fraisse P., Partisani M., Leiva-Mena M. Tsukamurella and HIV infection. AIDS. 1995;9:1379. doi: 10.1097/00002030-199512000-00014. [DOI] [PubMed] [Google Scholar]
- 46.Almeida D.R., Miller D., Alfonso E.C. Tsukamurella: an emerging opportunistic ocular pathogen. Can J Ophthalmol. 2010;45:290–293. doi: 10.3129/i09-252. [DOI] [PubMed] [Google Scholar]
- 47.Savini V., Fazii P., Favaro M., Astolfi D., Polilli E., Pompilio A. Tuberculosis-like pneumonias by the aerobic actinomycetes Rhodococcus, Tsukamurella and Gordonia. Microbes Infect. 2012;14:401–410. doi: 10.1016/j.micinf.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 48.Conville P.S., Witebsky F.G. Nocardia, Rhodococcus, Gordonia, Actinomadura, Streptomyces, and other aerobic actinomycetes. In: Versalovic J., Carroll K.C., Funke G., Jorgensen J.H., Landry M.L., Warnock D.W., editors. Manual of clinical microbiology. 10th ed. American Society for Microbiology; Washington, DC: 2011. pp. 443–471. [Google Scholar]
- 49.Hsueh P.R., Lee T.F., Du S.H., Teng S.H., Liao C.H., Sheng W.H. Bruker Biotyper matrix-assisted laser desorption ionization–time of flight mass spectrometry system for identification of Nocardia, Rhodococcus, Kocuria, Gordonia, Tsukamurella, and Listeria species. J Clin Microbiol. 2014;52:2371–2379. doi: 10.1128/JCM.00456-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teng J.L., Tang Y., Huang Y., Guo F.B., Wei W., Chen J.H. Phylogenomic analyses and reclassification of species within the genus Tsukamurella: insights to species definition in the post-genomic era. Front Microbiol. 2016;7:1137. doi: 10.3389/fmicb.2016.01137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park S.W., Kim S.M., Park S.T., Kim Y.M. Tsukamurella carboxydivorans sp. nov., a carbon monoxide–oxidizing actinomycete. Int J Syst Evol Microbiol. 2009;59:1541–1544. doi: 10.1099/ijs.0.005959-0. [DOI] [PubMed] [Google Scholar]
- 52.Yassin A., Rainey F., Brzezinka H., Burghardt J., Rifai M., Seifert P. Tsukamurella pulmonis sp. nov. Int J Syst Evol Microbiol. 1996;46:429–436. doi: 10.1099/00207713-46-2-429. [DOI] [PubMed] [Google Scholar]
- 53.Teng J.L., Tang Y., Chiu T.H., Cheung C.L., Ngan A.H., Ngai C. The groEL gene is a promising target for species-level identification of Tsukamurella. J Clin Microbiol. 2017;55:649–653. doi: 10.1128/JCM.02260-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pérez del Molino Bernal I.C., Balbin J.A. seqA1 is a useful target for identification of Tsukamurella pulmonis. J Clin Microbiol. 2017;55:1591. doi: 10.1128/JCM.00198-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pérez del Molino Bernal I., Cano García M.E., García de la Fuente C., Martínez Martínez L., López M., Fernández Mazarrasa C. Tsukamurella pulmonis bloodstream infection identified by secA1 gene sequencing. J Clin Microbiol. 2015;53:743–745. doi: 10.1128/JCM.02545-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clinical and Laboratory Standards Institute . Clinical and Laboratory Standards Institute; Wayne, PA: 2011. Susceptibility testing of mycobacteria, Nocardia and other aerobic actinomycetes. Approved standard, second edition. CLSI document M24–A2. [PubMed] [Google Scholar]
- 58.Long S.S., Pickering L.K., Prober C.G. Elsevier/Saunders; Amsterdam: 2012. Principles and practice of pediatric infectious disease. [Google Scholar]
- 59.Georgiev V.S. Humana Press; New York: 2003. Opportunistic infections: treatment and prophylaxis. [Google Scholar]
- 60.Teng J.L., Tang Y., Wong S.S., Ngan A.H., Huang Y., Tsang C.C. Tsukamurella hongkongensis sp. nov. and Tsukamurella sinensis sp. nov., isolated from patients with keratitis, catheter-related bacteraemia and conjunctivitis. Int J Syst Evol Microbiol. 2016;66:391–397. doi: 10.1099/ijsem.0.000733. [DOI] [PubMed] [Google Scholar]
- 61.Yassin A., Rainey F., Brzezinka H., Burghardt J., Lee H., Schaal K. Tsukamurella inchonensis sp. nov. Int J Syst Evol Microbiol. 1995;45:522–527. doi: 10.1099/00207713-45-3-522. [DOI] [PubMed] [Google Scholar]
- 62.Nam S.W., Kim W., Chun J., Goodfellow M. Tsukamurella pseudospumae sp. nov., a novel actinomycete isolated from activated sludge foam. Int J Syst Evol Microbiol. 2004;54:1209–1212. doi: 10.1099/ijs.0.02939-0. [DOI] [PubMed] [Google Scholar]
- 63.Weon H.Y., Yoo S.H., Anandham R., Schumann P., Kroppenstedt R.M., Kwon S.W. Tsukamurella soli sp. nov., isolated from soil. Int J Syst Evol Microbiol. 2010;60:1667–1671. doi: 10.1099/ijs.0.014852-0. [DOI] [PubMed] [Google Scholar]
- 64.Nam S.W., Chun J., Kim S., Kim W., Zakrzewska-Czerwinska J., Goodfellow M. Tsukamurella spumae sp. nov., a novel actinomycete associated with foaming in activated sludge plants. Syst Appl Microbiol. 2003;26:367–375. doi: 10.1078/072320203322497392. [DOI] [PubMed] [Google Scholar]
- 65.Yassin A., Rainey F., Burghardt J., Brzezinka H., Schmitt S., Seifert P. Tsukamurella tyrosinosolvens sp. nov. Int J Syst Evol Microbiol. 1997;47:607–614. doi: 10.1099/00207713-47-3-607. [DOI] [PubMed] [Google Scholar]