Abstract
Quinoa cultivation has been expanded around the world in the last decade and is considered an exceptional crop with the potential of contributing to food security worldwide. The exceptional nutritional value of quinoa seeds relies on their high protein content, their amino acid profile that includes a good balance of essential amino acids, the mineral composition and the presence of antioxidants and other important nutrients such as fiber or vitamins. Although several studies have pointed to the influence of different environmental stresses in certain nutritional components little attention has been paid to the effect of the agroecological context on the nutritional properties of the seeds what may strongly impact on the consumer food’s quality. Thus, aiming to evaluate the effect of the agroecological conditions on the nutritional profile of quinoa seeds we analyzed three quinoa cultivars (Salcedo-INIA, Titicaca and Regalona) at different locations (Spain, Peru and Chile). The results revealed that several nutritional parameters such as the amino acid profile, the protein content, the mineral composition and the phytate amount in the seeds depend on the location and cultivar while other parameters such as saponin or fiber were more stable across locations. Our results support the notion that nutritional characteristics of seeds may be determined by seed’s origin and further analysis are needed to define the exact mechanisms that control the changes in the seeds nutritional properties.
Keywords: Quinoa, Seed, Agroecological conditions, Nutritional properties
Introduction
Chenopodium quinoa Willd., commonly known as quinoa, belongs to the family Amaranthaceae (Cusack, 1984). Quinoa is mainly growing in the arid and semi-arid areas of the Andean region of South America although its cultivation has been expanded worldwide (Choukr-Allah et al., 2016; Bazile et al., 2016). It is well adapted to extreme conditions including water deficits, low temperatures, salinity and poor soils and it can grow at sea level up to elevations of 4,000 m above the sea (Adolf, Jacobsen & Shabala, 2013; Jacobsen, Mujica & Jensen, 2003; Jacobsen et al., 2007; Ruiz et al., 2014). In the last years, quinoa has gained worldwide attention due to the remarkable nutritional properties of its seeds, that include high protein content and essential amino acids (including lysine), fats, flavonoids, vitamins and minerals and as a gluten-free product (Alvarez-Jubete, Arendt & Gallagher, 2010; Lutz, Martínez & Martínez, 2013; Paśko et al., 2008; Gómez-Caravaca et al., 2012). The seeds, leaves, tender stems and inflorescences can be consumed in the human diet and as animal feed. Also, quinoa leaves are rich in phenolic compounds (including ferulic, sinapinic, gallic acid, kaempferol, isorhamnetin, or rutin) that possess antioxidant and anticancer properties (Gawlik-Dziki et al., 2013). Due to the high nutritional value of quinoa, its genetic diversity and its great adaptability to stressful environments, it has been considered an exceptional crop with the potential of contributing to food security worldwide (Bazile et al., 2016).
The quality of the seeds and other plant organs is a complex trait that results from the interaction of genetic and environmental factors (Wimalasekera, 2015). Breeding programs in quinoa have mainly focused on the generation of better environmentally adapted plants with improved resistance to mildew aiming to develop high-yielding varieties allowing the worldwide crop expansion (Bazile, Jacobsen & Verniau, 2016; Zurita-Silva et al., 2014). Less attention has been paid to the seed nutritional properties when developing new quinoa varieties. However, the improvement of quinoa seed quality is challenging and key for food security and has been almost exclusively focused on generating hybrid varieties with lower saponin contents (sweet varieties) (Zurita-Silva et al., 2014).
The impact of agroecological conditions or agronomic practices on the quinoa nutritional quality has been little explored. Nonetheless, several studies point to the importance of environmental or agronomical factors affecting the nutritional properties (such as the amino acid profile or the protein content) of quinoa seeds including drought, salinity or the cultivation area (Prado et al., 2014a; Gonzalez et al., 2012; Bascuñan Godoy et al., 2015; Wu et al., 2016; Aloisi et al., 2016). Still, the information regarding the mechanisms that control seed nutritional properties is scarce.
Overall, knowing that the nutritional properties of quinoa seeds are determined by the concentration of nutrients (i.e., amino acid content), their balance and quality (i.e., protein quality or the amino acid profile) and their bioavailability (determined by components such as phytate content), our working hypothesis states that the environmental and climatic factors as well as the agronomical practices used can significantly affect the nutritional quality of quinoa seeds. In order to analyze the impact of these factors, three quinoa cultivars were selected (Salcedo-INIA, Titicaca and Regalona) and their agronomical performance and nutritional characteristics were evaluated at different locations (Spain, Peru and Chile). The results revealed that several nutritional parameters such as the protein content, the amino acid profile or the mineral composition of the seeds change with the location and cultivar while other parameters such as saponin, remained unchanged among the varieties and locations studied.
Material and Methods
Plant material, experimental design and locations
Quinoa (Chenopodium quinoa Willd.) seeds of cultivars Regalona (registered variety of BAER, Chile), Salcedo-INIA (experimental station of Illpa—Puno, Peru, Mujica, Izquierdo & Marathee, 2001) and Titicaca (generously provided by Dr. Jacobsen of Copenhagen University) were selected to evaluate their agronomic potential and seed nutritional traits at three locations with different agroecological conditions: El Pobo (Teruel, Spain), Arequipa (Peru) and Río Hurtado (Chile). The field experiment at El Pobo (40.50°N, 0.86°W, 1,399 m a.s.l.) (Spain) was carried out under rainfed conditions between May and October 2016 within a range of temperatures between 24.1 and 4.7 °C on average (registering a maximum of 32.3 °C and a minimum of −2.8 °C) and 194 mm total precipitation during the mentioned period. The field trial at Río Hurtado (30.3°S, 70.6°W, 1,462 m a.s.l.) (Chile), was carried out between November 2015 and April 2016 within a range of temperatures between 11 and 25 °C on average (registering a maximum of 34 °C and a minimum of 3.7 °C). The total precipitation during that period was 150 mm and the irrigation was applied using drip lines that released 4 L m−1 h−1 according to Martinez et al. (2009). In Arequipa (16°S, 71°W, 2,355 m a.s.l.) the experiment was carried out under irrigation between March and July 2016 with an average temperature of 14 °C (registering a maximum of 28.8 °C and a minimum of 4.2 °C) and 15.3 mm total precipitation.
The soil type in Spain (a clay-silty-loam soil) presented a pH of 7.9, 4.8% organic matter, 3 dS m−1 of electrical conductivity (EC) of the saturated paste and phosporous (P) and potassium (K) equivalent to 37 and 438 mg kg−1, respectively. The soil type in Chile (a loamy alluvial Entisol) presented a pH of 7.8, 7.7% of organic matter, 2.6 dS m−1of EC and content of P and exchangeable K equivalent to 49.96 and 237 mg kg−1, respectively. The soil type in Arequipa (Peru) (a loam soil) presented 4.89% of organic matter, 2.25 dS m−1 of EC, a pH of 6.95 and a content of 39.31 mg kg−1 of P and 624.96 mg kg−1 of K.
The experimental design consisted in randomized blocks (8 m2 per block, 4 m × 2 m, L × W) with 4 replications in each location using the three varieties (Regalona, Salcedo-INIA and Titicaca). Each block was composed of 4 rows of 4 m in length (row spacing = 50 cm). Seeds were directly germinated in the soil with a sowing density of 10 kg/ha between 1 and 2.5 cm depth.
Quantitative multi-elemental analysis
Quantitative multi-elemental analysis by inductively coupled plasma (ICP) spectrometry was used to determine total content of calcium (Ca), magnesium (Mg), iron (Fe), sodium (Na), zinc (Zn), P and K contents in C. quinoa seeds. Seed samples were firstly grinded to a fine powder. The samples were then submitted to the Interdepartmental Investigation Service Laboratory at the Universidad Autónoma de Madrid (SIdI-UAM, Madrid, Spain). Samples were digested in a microwave oven and subsequently analyzed using the equipment ICP-MS NexION 300XX (Perkin Elmer Inc., Hopkinton, MA, USA).
Total phytate content
Total phytate content was determined using Myo-Inositol Hexakisphosphate Determination method. This method involved acid extraction of myo-inositol phosphates from 0.5 g of flour (ground seeds) in 20 mL of HCl 0.66 M with vigorously stirring at room temperature overnight followed by treatment with a phytase and alkaline phosphatase (K-PHYT 07/11; Megazyme, Bray, Wicklow, Ireland). The total phosphate released was proportional to myo-inositol hexakisphosphate in non-processed seeds. It was measured using a colorimetric method with ammonium molybdate reactive to form 12-molybdophosphoric acid, which was subsequently reduced under acidic conditions to molybdenum blue. The amount of molybdenum blue formed in the reaction was proportional to the amount of free phosphate present in the sample and was measured at 655 nm using a spectrophotometer SPECTROstar nano (BMG LabTech GmbH, Ortenberg, Germany). Phosphorus was quantified interpolating from a calibration curve using standards of known phosphorus concentration. The samples were done by triplicate and the results were expressed in grams of phytic acid per 100 g of seeds in dry matter.
Protein content
The Kjeldahl method with a conversion factor of 6.25 by AOAC method no 960.52 (Association of Official Analytical Chemists International, 2016) was employed to quantify the total crude protein content of the quinoa seed samples. All determinations were done in triplicate.
Amino acid quantification
Liquid chromatography mass spectrometry (LC/MS) was used to determine free amino acid content of C. quinoa seeds. Seed samples were grinded to a fine powder. Free amino acid was extracted as described previously by Hacham, Avraham & Amir (2002). One hundred fifty mg of seed powder was homogenized in 400 µL water:chloroform:methanol (3:5:12 v/v) and centrifuged at 14,000 rpm for 2 min. This step was repeated twice and both supernatants were combined and mixed with 200 µL chloroform and 300 µL of water. The resulting mixture was centrifuged at 14,000 rpm for 2 min. The supernatant (corresponding to the water:methanol phase) was subjected to speed-vac to dry and resuspended in 100 µL miliQ H2O.
Free amino acid extracts were submitted to the Chromatography Laboratory at the SIdI-UAM (Spain) for analysis. Amino acid determination was carried out using HPLC-MS with an Agilent system detector composed by an 1,100 series HPLC coupled to a single 6,120 Quadrupole. For the chromatographic separation, 5 µL were injected in an ACE 5 AQ (250 × 4.6 mm, 5 µm) thermostated column at 30 °C with 0.4 mL/min flow rate and binary gradient elution. The elution was performed in H2O with 0.1% formic acid (v/v) as eluent “A” and acetonitrile (ACN) with 0.1% formic acid (v/v) as eluent “B”. The gradient program was as follows for eluent B: 0 min, 0%; 30 min, 100%; 35 min; 100%; 36 min, 0%; 55 min, 0%. The ionization parameters were as follows: positive atmospheric pressure chemical ionization (APcI+), fragmentor voltage 40 V, capillary voltage 2.0 kV, charging voltage 2.0 kV, nebulizer pressure 20 psig, drying gas flow 5 L/min at 350 °C, vaporizer temperature 250 °C, and corona current 4 µA. Data was recorded scanning from 50 to 250 Da.
Ferric reducing antioxidant power (FRAP) assay
The FRAP assay was used to determine the antioxidant capacity of seed samples. The procedure described by Benzie & Strain (1996) was used with some modifications. Briefly, 30 µL sample aliquots were mixed with 90 µL of distilled water and 900 µL of freshly prepared FRAP reagent at 37 °C (2.5 mL of a 10 mmol/L 2,4,6-tripyridyl-s-triazine (TPTZ) solution in 40 mmol/L HCl with 2.5 mL of 20 mmol/L FeCl3 and 25 mL of 0.3 mol/ L acetate buffer at a pH of 3.6). The absorbance of the reaction mixture was measured spectrophotometrically (atomic absorption spectroscopy (PinAAcle 900F FL HSN, WinLab32 software; Perkin Elmer Inc., Hopkinton, MA, USA) at 593 nm following incubation at 37 °C for 2 h. FRAP concentration was calculated from a calibration curve obtained by linear regression and the results are expressed in Trolox equivalents (mmol TE 100g−1). The reference used was the synthetic antioxidant Trolox at a concentration of 100 to 1500 mmol in 80% methanol solution, which was tested under the same conditions.
Fiber and saponin determination
For fiber determination samples were submitted to the SERBILAC laboratory (Universidad Nacional de San Agustín de Arequipa, Peru). Fiber content was determined following the protocol described in AOAC Methods 2016 (Association of Official Analytical Chemists International, 2016). Samples were submitted to the Laboratory of quality control (Universidad Nacional del Altiplano Puno, Peru). Total saponin content was determined spectrophotometrically at 528 nm using an SQ-2802 single beam scanning spectrophotometer (UNICO) (Lozano et al., 2012). The concentration of saponin was read off from a standard curve of different concentrations of saponin (Calbiochem, CAS 8047-15-2, Darmstadt, Germany) (from 0.5 to 7.5 mg/ L) dissolved in an aqueous solution.
Statistical analysis
Results are presented as Mean value ± Standard Deviation or Error. Pairwise comparisons were done by using Student t-test at a probability level of 5% (P < 0.05). Multiple comparisons were done by one-way analysis of variance (ANOVA) followed by Duncan or Tukey HSD post-hocs to analyze the quantitative data at a probability level of 5% (P < 0.05). The JMP® (ver.11.0) statistical package (SAS Institute) and the Free Software R were used for the statistical analyses.
Results
Variations in agronomical traits of Chenopodium quinoa cultivars under different agroecological conditions
Among the three quinoa varieties studied, differences were found in terms of seed yield when consider total yield and seed weight per plant (Table 1). Although a larger total yield (Kg/Ha) was achieved by Salcedo-INIA grown in Peru followed by Titicaca grown in Chile, this was due to a larger plant density in these two varieties and not because of a better variety performance as revealed by the seed weight per plant (that was the smallest in Salcedo-INIA from Peru). Also, the harvest index parameter values did not differ among the varieties tested. On the contrary, Titicaca in Spain yield less seeds than Regalona (t-Student, P < 0.05) and Regalona showed the largest seed weight per plant among cultivars and locations. Besides, Titicaca and Regalona in Peru were unable to yield seeds and Salcedo-INIA in Spain showed important seed losses due to seed dehiscence detected at harvesting related to defects in the timing of maturity, the uniformity of maturity and the lack drydown of plant at seed maturity.
Table 1. Agronomical traits of three Chenopodium quinoa cultivars growing under different agroecological conditions.
Different letters indicate statistical differences at a P < 0.05 (Duncan t-test).
| Variety | Location | Yield kg/ha | Seed weight per plant (g) | Harvest index | Plant height (m) | Stem diameter (cm) | Panicle length (cm) | Panicle diameter (cm) | Plant weight (g) | Days to flowering | Days to maturity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Regalona | Chile | 2,402.33 ± 465.28bc | 40.33 ± 13.80ab | 0.42 ± 0.22a | 1.30 ± 0.06ab | 15.33 ± 1.53abc | 17.00 ± 1.00d | 11.00 ± 1.00b | 103.00 ± 18.08ab | 70 | 165 |
| Salcedo | Chile | 2,743.33 ± 80.13b | 36.67 ± 7.02bc | 0.47 ± 0.09a | 0.84 ± 0.09e | 11.33 ± 1.53e | 31.66 ± 3.51b | 7.00 ± 0.00d | 78.00 ± 2.65cd | 100 | 180 |
| Titicaca | Chile | 4,300.33 ± 1841.58a | 42.67 ± 9.71ab | 0.50 ± 0.00a | 1.04 ± 0.09d | 12.00 ± 1.00de | 22.33 ± 2.08c | 7.00 ± 0.00d | 86.00 ± 19.31bc | 50 | 105 |
| Salcedo | Peru | 5,170.00 ± 142.82a | 26.40 ± 1.14c | 0.40 ± 0.00a | 1.36 ± 0.01a | 18.25 ± 0.50a | 35.50 ± 1.29a | 15.60 ± 0.71a | 66.00 ± 2.58d | 65 | 145 |
| Regalona | Spain | 2,606.00 ± 0.00bc | 50.60 ± 9.58a | 0.47 ± 0.04a | 1.25 ± 0.05bc | 14.80 ± 2.28bcd | 20.00 ± 2.55cd | 12.00 ± 1.58b | 107.80 ± 15.06a | 63 | 138 |
| Salcedo | Spain | a | a | a | 1.36 ± 0.06a | 16.80 ± 2.77ab | 36.00 ± 2.55a | 9.00 ± 1.00c | a | 92 | 187 |
| Titicaca | Spain | 1,526.00 ± 0.00c | 37.20 ± 4.66abc | 0.42 ± 0.03a | 1.15 ± 0.03c | 12.20 ± 1.30cde | 28.00 ± 2.35b | 7.00 ± 1.00d | 89.60 ± 7.67abc | 51 | 119 |
Notes.
Seed losses were detected due to defects related to the timing of maturity, the uniformity of maturity and the drydown of plant at seed maturity. Titicaca and Regalona were unable to grow in Peru at the time the experiment was performed.
Most of the morphological traits varied with the location and among cultivars (Table 1). Salcedo-INIA cultivar showed the smallest plants in Chile but the biggest plants in Spain. Salcedo-INIA presented the largest stem diameter and panicle length in Peru and Spain while the stem diameter of Regalona was the largest in Chile. Salcedo-INIA showed the biggest panicle length among varieties in the different locations. Plant weight did not differ among locations but varieties as shown in Table 1.
Regarding the analysis of phenological traits, days to flowering and days to maturity were evaluated. Titicaca showed the shortest time to flowering and to maturity at both locations (Chile and Spain), followed by Regalona. Salcedo-INIA presented the largest time to flowering and maturity (approximately 95 days and 184, respectively) in Spain and Chile while reduced the days to flowering and maturity in Peru.
Mineral composition and phytate content in C. quinoa seeds
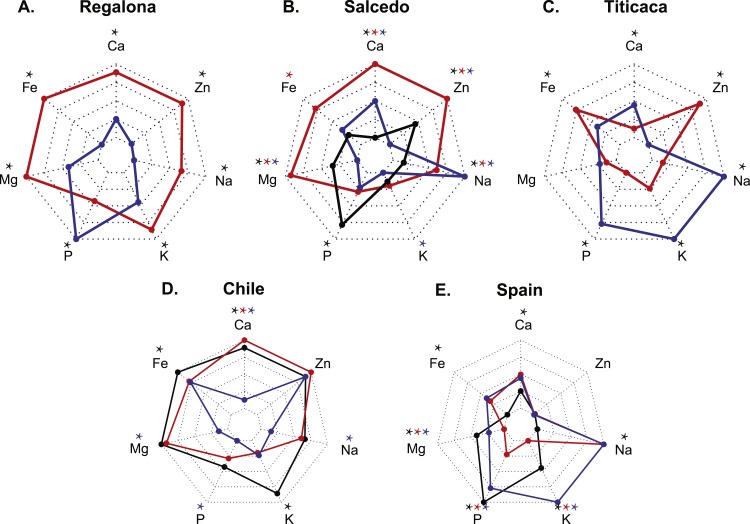
Quantitative multi-elemental analysis was performed aiming to assess differences in the seed mineral composition among cultivars and locations due to distinct agroecological conditions. When analyzing the effect of the location in each genotype (Figs. 1A–1C), it was observed that Regalona seeds stored larger amounts of mineral nutrients in Chile with exception of P. Salcedo-INIA showed larger quantities of Mg, Fe, Ca and Zn in Peru, while in Chile presented the largest amount of P and the lowest of Na. Titicaca in Spain had larger amounts of Ca, K, P and Na but less amount of Fe and Zn comparing the same variety grown in Chile.
Figure 1. Mineral composition of three cultivars of C. quinoa seeds growing at three different locations.
Quantitative multi-elemental analysis was performed using inductively coupled plasma (ICP) spectrometry and the total content of P, K, Ca, Mg, Fe, Na and Zn were determined. Three cultivars (Regalona, Salcedo-INIA and Titicaca) grown at three different locations (Peru, Chile and Spain) were used to evaluate changes in the mineral composition associated with different agroecological conditions. (A–C) Mineral composition of one variety comparing among countries (Red: Chile; Black: Peru; Blue: Spain): (A) Regalona, (B) Salcedo-INIA and (C) Titicaca. (D–E) Mineral composition of seeds from Spain or Chile comparing among varieties (Red: Regalona; Black: Salcedo-INIA; Blue: Titicaca): (D) Chile and (E) Spain. Values are presented as the Mean relative to the maximum and minimum values for each element (n = 4). Asterisks indicate statistical differences at a P < 0.05 (Tukey t-test or Student t-Test when comparing pairs). When more than two samples are compared, colored asterisks indicate the sample that is statistically significant.
Differences among varieties were also found in each location (Figs. 1C and 1D). In Chile, Regalona cultivar stored the highest amounts of Ca followed by Salcedo-INIA. Salcedo-INIA presented the largest amounts of Fe and K. Titicaca in Chile showed the lowest contents in Ca, P, Mg and Na compared to the other two cultivars. In Spain, Titicaca showed the highest level of K. Salcedo-INIA grown in Spain showed the highest amounts of P and Mg but smallest of Fe, Ca and Na. All the cultivars grown in Spain or Chile showed similar Zn contents.
Overall, a larger accumulation of Mg and Fe tend to appear in Chile. Zn contents changed with the location but remains unchanged within cultivars. Generally, the type of cultivar and location affects the content of certain minerals, indicating that both factors (variety and location) are determinants of the mineral composition of quinoa seeds.
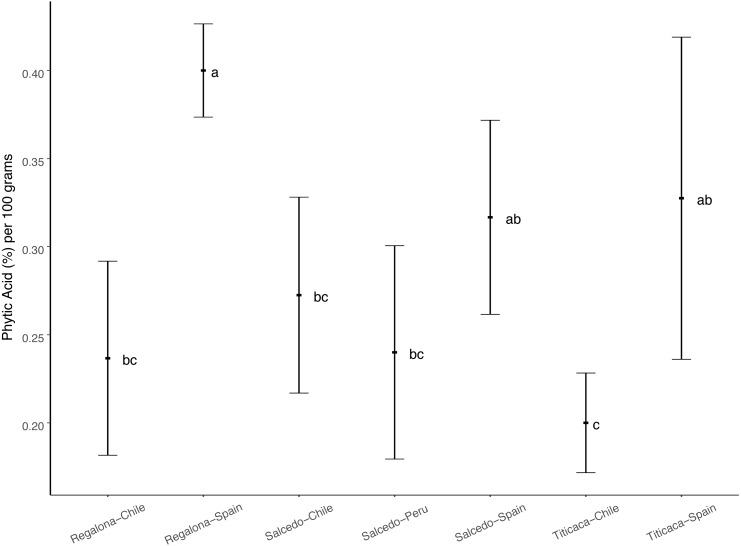
Phytic acid is considered an important seed component conditioning the nutritional properties of seeds (Lott et al., 2000). The analysis of phytate content in quinoa seeds (Fig. 2) revealed that Regalona seeds from Spain showed the largest phytate content, followed by the Salcedo-INIA and Titicaca seeds from this country. Titicaca seeds from Chile presented the smallest phytate content. The fact that no differences were found among cultivars in the same location but a given genotype vary among locations suggests that phytic acid content in quinoa seeds might be determined by environmental factors and not by the type of cultivar.
Figure 2. Phytic acid content among three varieties of C. quinoa seeds growing at different locations.
Phytic acid content of quinoa seeds was determined using the myo-inositol hexakisphosphate method. Phytic acid is presented as the % of phytate per 100 grams of seeds. Values are Mean ± Stnd. Dev. (n = 4). Different letters indicate statistical differences at a P < 0.05 (Duncan t-test).
Total Protein content and free amino acid profile of quinoa seeds obtained from Chile, Peru and Spain
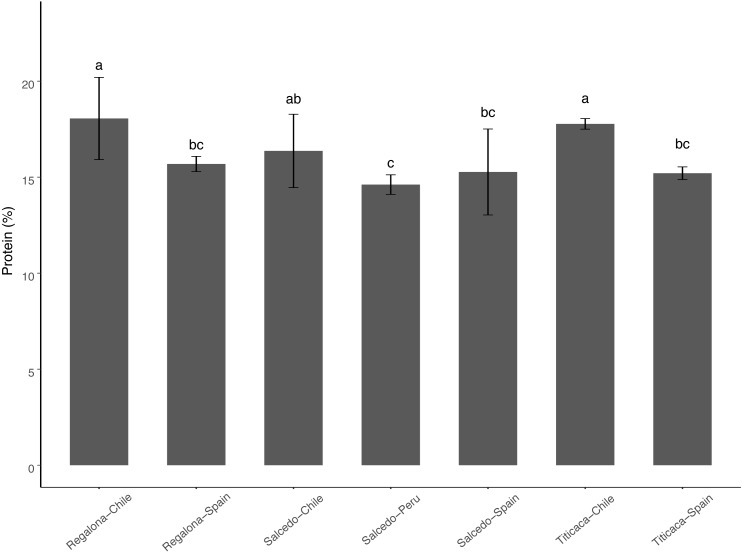
The range of total protein content was found between 14 and 17% among the cultivars and the locations analyzed (Fig. 3). No differences were found when comparing among varieties in a particular location. Consistently, seeds from Chile showed a higher total protein content comparing to Spain or Peru. These results might suggest that agroecological conditions can influence on the protein content of the seeds.
Figure 3. Total Protein content of C. quinoa seeds.
Total protein content was determined by using the Kjeldahl method in seeds of Regalona, Salcedo-INIA and Titicaca cultivars grown in Chile, Peru or Spain. Values are Mean ± Stnd. Dev. (n = 3). Different letters indicate statistical differences at a P < 0.05 (Duncan t-test).
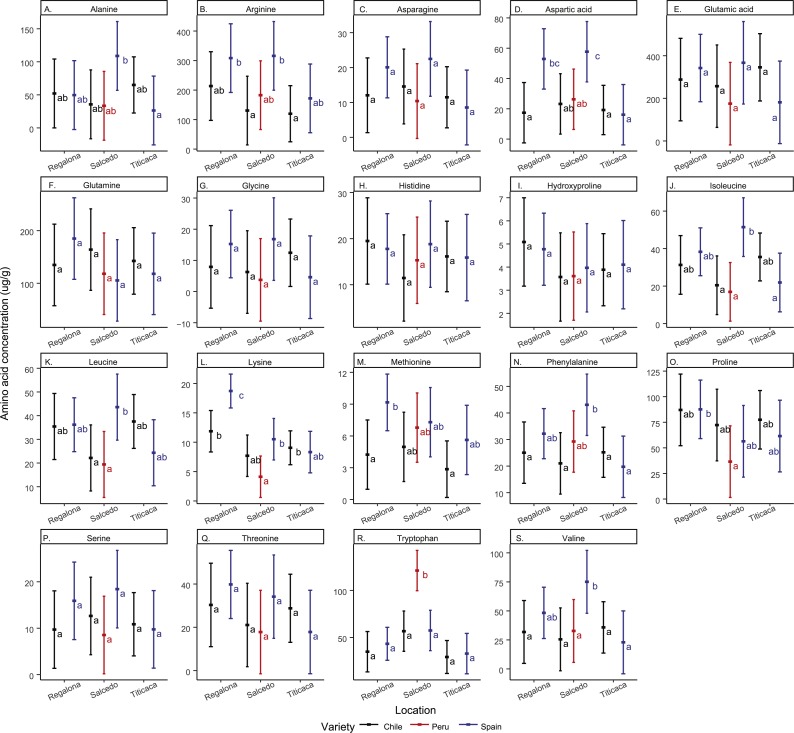
Regarding amino acid composition, the most abundant amino acids found in the quinoa seeds analyzed in the present study were arginine and glutamic acid (Fig. 4 and Table S2). Asparagine, glutamic acid, glutamine, histidine, glycine, hydroxyproline, serine and threonine and remained unchanged in all cultivars and locations. The rest of the amino acids quantified showed differences among cultivars and/or locations. For instance, Regalona seeds grown in Spain showed higher contents of arginine, aspartic acid, lysine and methionine compared to the Chilean Regalona seeds. Spanish Salcedo-INIA seeds showed larger contents of aspartic acid, isoleucine, leucine and valine compared to the Peruvian and Chilean Salcedo-INIA seeds. Arginine and phenylalanine showed higher contents in Salcedo-INIA seeds obtained from Spain when compared with the seeds from Chile but no differences were detected in the contents of these two amino acids between Spain and Peru.
Figure 4. Free amino acid composition of C. quinoa seeds from different cultivars and locations.
Amino acid contents of a pool of 10 g of C. quinoa seeds were determined by using LC/MS. Seeds of different cultivars including Regalona, Salcedo-INIA and Titicaca were obtained from different locations (Chile, Peru or Spain). (A) Alanine, (B) arginine, (C) asparagine, (D) aspartic acid, (E) glutamic acid, (F) glutamine, (G) glycine, (H) histidine, (I) hydroxyproline, (J) isoleucine, (K) leucine, (L) lysine, (M) methionine, (N) phenylalanine, (O) proline, (P) serine, (Q) threonine, (R) tryptophan and (S) valine. Values are Mean ± SE (n = 4). Different letters indicate statistical differences at a P < 0.05 (Tukey t-test).
Noteworthy, an elevated amount of tryptophan was found in Salcedo-INIA from Peru, amount that was superior to any of the cultivars analyzed. In Spain, the amount of lysine in Regalona and valine in Salcedo-INIA were higher when compared to other cultivars or locations. In contrast, the content of amino acids in Titicaca did not change significantly among cultivars nor locations.
When analyzing the amino acid content by location it was observed that the varieties grown in Chile did not present differences in their amino acid content. However, inter cultivar differences were observed in the amino acid contents in the Spanish quinoa seeds for alanine, asparagine, isoleucine, lysine and valine.
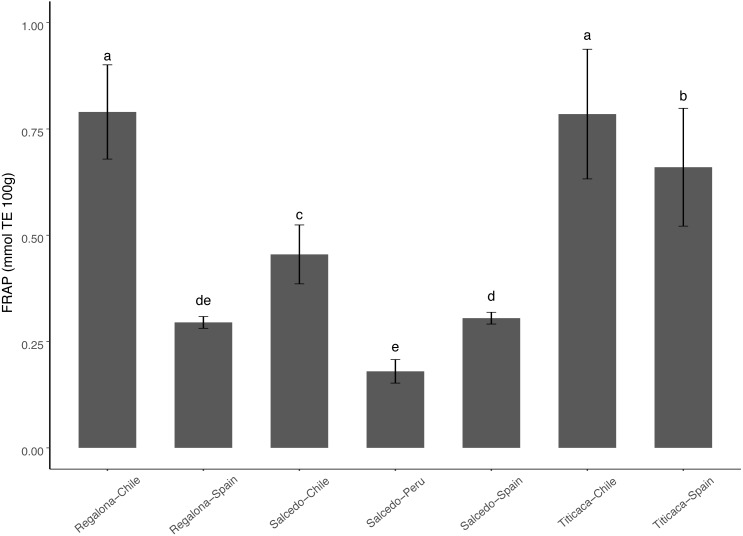
Antioxidant properties of quinoa seeds in different cultivars and locations
Antioxidant capacity varied among cultivars and locations ranging from 0.75 to 0.15 mmol TE 100 g−1 (Fig. 5). Titicaca and Regalona seeds from Chile presented the highest antioxidant activity measured as FRAP, while Salcedo-INIA from Peru presented the lowest values. The big differences observed between the antioxidant properties of Regalona at the two locations studied did not appear in Titicaca nor Salcedo-INIA (differences among locations in these two cultivars were smaller) what would indicate that changes among cultivars appear when evaluating the effect of the agroecological conditions on the antioxidant properties.
Figure 5. Variation of antioxidant properties of C. quinoa seeds of different cultivars and locations.
Antioxidant properties were determined by using FRAP in seeds of Regalona, Salcedo-INIA and Titicaca cultivars grown in Chile, Peru or Spain. Values are Mean ± Stnd. Dev. (n = 3). Different letters indicate statistical differences at a P < 0.05 (Duncan t-test).
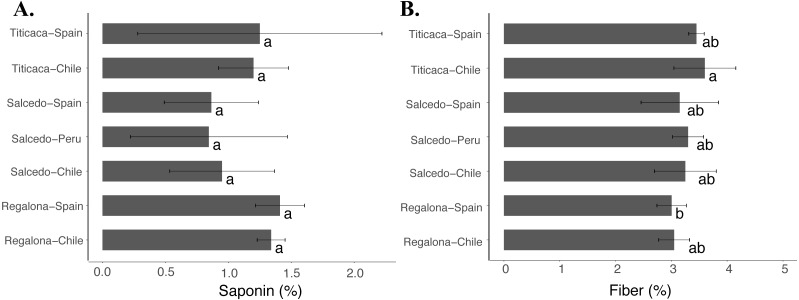
Fiber and saponin contents
Although no differences were found in the saponin content (Fig. 6A) among the cultivars or locations, Titicaca seeds from Chile showed larger fiber contents compared to Regalona seeds obtained from Spain (Fig. 6B). These results might indicate that fiber might be more susceptible to variations associated with changes in agroecological conditions despite no big differences were found among cultivars and locations.
Figure 6. Saponin and fiber contents of C. quinoa seeds from three cultivars grown at three different locations.
(A) Saponin and (B) fiber contents were determined in Regalona, Salcedo-INIA and Titicaca seeds obtained at three different locations (Chile, Peru or Spain). Values are Mean ± Stnd. Dev. (n = 3). Different letters indicate statistical differences at a P < 0.05 (Duncan test).
Discussion
Quinoa is able to grow under a wide variety of environmental conditions and to tolerate a broad range of stresses which, in addition to the excellent nutritional properties of its seeds, makes this crop an attractive and feasible option from an agronomic perspective (Jancurová, Minarovičová & Dandár, 2009; Filho et al., 2017). Numerous studies have been published in the last years describing the effect of different abiotic stresses on quinoa, however, the analysis of how quinoa responds to a certain environment altering the nutritional properties of the seeds has been little explored. Here, we have analyzed how different agroecological conditions can induce distinct agronomical and nutritional responses depending on the variety of quinoa considered. We observed that despite some traits were largely influenced by the genotype, such as the phenological characters (Bertero et al., 2004), others were more sensitive to the interaction between the genotype and the environment resulting in specific responses.
The analysis of the mineral composition is crucial when considering the nutritional quality of the seeds (Vaz Patto et al., 2015). The mineral concentration of the quinoa seeds evaluated in the present study was found within a range similar to what was previously reported in quinoa (Table S1) (Prado et al., 2014b; Miranda et al., 2013b; Miranda et al., 2013a). Interestingly, the accumulation of some minerals was heavily influenced by the location considered. For instance, the amount of Ca varied up to 2.6 times among locations in Salcedo-INIA cultivar. These variations could significantly impact on the consumer. For instance, the dietary reference intake (DRI) for Fe in women between 19 and 30 years old stablished by the US government (U.S. Department of Health and Human Services, U.S. Department of Agriculture, 2015) corresponds to 18 mg/day which could be covered with the uptake of 200 g of the Regalona seeds from Chile instead of the 400 g required when consuming Regalona seeds from Spain. The differences observed in the mineral composition may be caused by a strong effect of the environment, and we hypothesized that one of the main contributing factors could be the soil composition. Previous studies growing quinoa under different agroecological conditions are in agreement with our results and hypothesis (Prado et al., 2014b; Miranda et al., 2013a), and can be extended as well to the effects observed on the grains and seeds of other crop species such as wheat, pea and corn (Kotlarz et al., 2011; Gomez-Becerra et al., 2010; Gu et al., 2015).
One of the most attractive features of quinoa is the high protein content and the good balance of amino acids in the seeds (Filho et al., 2017). Both were shown to rely on nitrogen metabolism which is known to be regulated by agroecological factors such as abiotic stresses (i.e., water stress) or soil factors (i.e., nitrogen fertilization) (Bascuñan Godoy et al., 2015; Geren, 2015; Thanapornpoonpong et al., 2008; Varisi et al., 2008). Although the total protein content was found within a range already described for quinoa seeds (between 15 and 20%) (Jancurová, Minarovičová & Dandár, 2009; Miranda et al., 2013b), our results suggest that the environmental conditions could determine the protein content found in quinoa. Similarly, Gonzalez and coworkers found changes in the protein content of ten quinoa cultivars growing in two different agroecological regions (Gonzalez et al., 2012). Other studies, however, have shown no differences in the protein content when comparing quinoa seeds growing in two environments (Miranda et al., 2013a), suggesting that the interaction of environmental conditions and genotype plays an important role modulating the protein content in the seeds.
The analysis of the seed free amino acid profile revealed also variations associated with the cultivar and location. The presence of essential amino acids (EAA), including methionine, threonine or lysine, contributes to the high nutritional properties of the quinoa seeds and can vary when plants are subjected to abiotic stress (Bascuñan Godoy et al., 2015; Joshi et al., 2010). Among the EAA analyzed in this study, only threonine and histidine remained stable in all cases, and changes were observed in the rest of EAA. These results support previous findings that claimed that the EAA content can vary significantly depending on the genotype and seed’s origin (Gonzalez et al., 2012). Thanapornpoonpong and coworkers found that nitrogen availability determines not only the protein content but also the amino acid composition of quinoa seeds (Thanapornpoonpong et al., 2008). Taken all together, these findings suggest that a complex genotype ×environment interaction alters nitrogen metabolism resulting in seed nutritional differences. Nonetheless, the main and specific factors contributing to these changes remain to be elucidated.
Different bioactive components contribute to the antioxidant capacity of quinoa seeds including polyphenols, flavonoids and vitamins (A, B and E) (Filho et al., 2017), which may prevent cancer, cardiovascular and other, chronic diseases (Tang & Tsao, 2017). The amount of these phytochemicals in quinoa seems to be genotype-specific and could vary significantly under stressful conditions (Bascuñan Godoy et al., 2015; Aloisi et al., 2016). Our results showed differences among cultivars and locations in agreement with the results obtained by Miranda and co-workers (Miranda et al., 2013a), what highlights the importance of the environmental factors conditioning the accumulation of antioxidants in the seeds. In the present study, was especially noticeable the antioxidant capacity of the Chilean cultivars that tended to have the greatest values when comparing among locations.
The total fiber content in quinoa seeds was found within the characteristic range (Filho et al., 2017). Little variation was observed among cultivars and locations suggesting that this parameter might be less sensitive to agroecological variations. Nonetheless, Miranda and coworkers reported that the changes in fiber contents only occurred in the soluble dietary fraction and no alteration was detected in the total fiber, indicating that both fractions might be affected differently (Miranda et al., 2013a).
Saponin and phytic acid have been traditionally considered antinutrients that diminish the nutritional value of seeds due to their ability to alter the absorption of minerals (Jancurová, Minarovičová & Dandár, 2009; Ruales & Nair, 1993). However, several studies have pointed to the beneficial effects associated to these two compounds (Yao et al., 2014). In the case of saponin different breeding programs have aimed to develop quinoa varieties with a lower saponin content trying to increase the palatability of the seeds increasing consumers acceptance (Zurita-Silva et al., 2014; Nowak, Du & Charrondière, 2016). Besides being determined by the variety, recent evidences have suggested that external factors might impact on saponin contents of quinoa seeds. For instance, it was reported that the saponin content of Q52 variety diminished under water or salinity stress (Gómez-Caravaca et al., 2012). Also, it was shown that the saponin content was altered in Regalona seeds when growing at different locations (Miranda et al., 2013a). Nonetheless, our results did not find differences in the saponin content in any of the cultivars or locations analyzed supporting the hypothesis that claims that this trait is largely determined by the variety more than being environmentally regulated.
A different response was observed regarding phytic acid whose content varied with the location suggesting that this might modulate its accumulation. To our knowledge, no previous studies have been carried out evaluating the effect of environmental factors in quinoa seed phytate composition. In oat, barley and dry beans was described a strong effect of agroecological conditions in their phytate content (Miller, Youngs & Oplinger, 1980; Dai et al., 2007; Wang et al., 2017). However, the exact factors that cause these changes remain unclear. Considering that phytic acid contributes extensively to the nutritional profile of quinoa seeds it should be stressed that further studies are needed in the field to deeply analyze the contributing environmental factors involved in phytate seed accumulation.
Conclusions
Our results highlight that different agroecological conditions could significantly alter the agronomical and nutritional properties of quinoa what impacts on the seed quality. Although not all the nutritional traits evaluated varied to the same extent, one can affirm that both the variety and location determined the mineral composition, the amino acid profile, the protein content and the antioxidant capacity of the quinoa seeds. Therefore, when evaluating the nutritional quality of quinoa seeds and in order to provide precise nutritional information to the consumer we should consider the cultivar and the agroecological context. This work also provides valuable information that could be used in breeding programs to maximize the potential of this crop by defining stable varieties and/or environments from a nutritional point of view.
Supplemental Information
Values are Mean ± Stnd Dev (n = 4).
Different letters indicate statistical differences at a P < 0.05 (Duncan t-test). Values represent Mean ± SE.
FRAP and protein content were determined as described in the Methods section. Data is presented in Figs. 3 and 5.
Free amino acids were extracted from quinoa Seeds and analyze according to what is described in the Methods section. Data is presented in Fig. 4.
Agronomical parameters presented in Table 1 were determined in the three quinoa cultivars at the three locations.
Fiber and Saponin were determined in quinoa seeds as described in the Methods section. Data is presented in figures 6 and 7.
Mineral composition of seeds was determined as described in the Methods section and final data is presented in Fig. 1.
Phytate was determined in seeds according to the methodology described in Methods. Final data is presented in Fig. 2.
Acknowledgments
We thank Dr. Sven Jacobsen from the University of Copenhagen for providing us with the Titicaca Chenopodium quinoa seeds used in this study. We also thank Karla Miranda Ramos for the technical assistance with the phytic acid determination, Rosa Sedano Pérez for her technical assistance with the amino acid determination, Inmaculada Rivas Ramírez for her technical assistance with the minerals quantification and Susana Vilariño for the stimulating discussions that have helped improving this manuscript.
Funding Statement
This work was supported by the CEAL-AL/2015-27 Banco Santander-UAM grant (Spain), the PROMETEO/2017/189 grant from the Generalitat Valenciana (Spain) and the Juan de la Cierva Fellowship Program (JCI-2012-14172) (MINECO, Spain) (to María Reguera). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
María Reguera conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Carlos Manuel Conesa conceived and designed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Alejandro Gil-Gómez analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Claudia Mónika Haros and Vilbett Briones-Labarca performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Miguel Ángel Pérez-Casas, Rodrigo Álvarez and Katherine Pinto performed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Luis Bolaños and Luisa Bascuñán-Godoy conceived and designed the experiments, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Ildefonso Bonilla conceived and designed the experiments, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft, read critically the manuscript.
Ángel Mujica conceived and designed the experiments, performed the experiments, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data are provided in the Supplemental Files.
References
- Adolf, Jacobsen & Shabala (2013).Adolf VI, Jacobsen S-E, Shabala S. Salt tolerance mechanisms in quinoa (Chenopodium quinoa Willd. Environmental and Experimental Botany. 2013;92:43–54. doi: 10.1016/j.envexpbot.2012.07.004. [DOI] [Google Scholar]
- Aloisi et al. (2016).Aloisi I, Parrotta L, Ruiz KB, Landi C, Bini L, Cai G, Biondi S, Del Duca S. New insight into quinoa seed quality under salinity: changes in proteomic and amino acid profiles, phenolic content, and antioxidant activity of protein extracts. Frontiers in Plant Science. 2016;7 doi: 10.3389/fpls.2016.00656. Article 656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Jubete, Arendt & Gallagher (2010).Alvarez-Jubete L, Arendt EK, Gallagher E. Nutritive value of pseudocereals and their increasing use as functional gluten-free ingredients. Trends in Food Science and Technology. 2010;21:106–113. doi: 10.1016/j.tifs.2009.10.014. [DOI] [Google Scholar]
- Association of Official Analytical Chemists International (2016).Association of Official Analytical Chemists (AOAC) International . Official methods of analysis of AOAC International. AOAC International; Rockville: 2016. [Google Scholar]
- Bascuñan Godoy et al. (2015).Bascuñan Godoy L, Reguera M, Abdel-Tawab YM, Blumwald E. Water deficit stress-induced changes in carbon and nitrogen partitioning in Chenopodium quinoa willd. Planta. 2015;243:591–603. doi: 10.1007/s00425-015-2424-z. [DOI] [PubMed] [Google Scholar]
- Bazile, Jacobsen & Verniau (2016).Bazile D, Jacobsen S-E, Verniau A. The global expansion of quinoa: trends and limits. Frontiers in Plant Science. 2016;7 doi: 10.3389/fpls.2016.00622. Article 622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazile et al. (2016).Bazile D, Pulvento C, Verniau A, Al-Nusairi MS, Ba D, Breidy J, Hassan L, Mohammed MI, Mambetov O, Otambekova M, Sepahvand NA, Shams A, Souici D, Miri K, Padulosi S. Worldwide evaluations of quinoa: preliminary results from post international year of quinoa FAO projects in nine countries. Frontiers in Plant Science. 2016;7 doi: 10.3389/fpls.2016.00850. Article 850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzie & Strain (1996).Benzie J, Strain J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical Biochemistry. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Bertero et al. (2004).Bertero HD, De La Vega AJ, Correa G, Jacobsen SE, Mujica A. Genotype and genotype-by-environment interaction effects for grain yield and grain size of quinoa (Chenopodium quinoa Willd.) as revealed by pattern analysis of international multi-environment trials. Field Crops Research. 2004;89:299–318. doi: 10.1016/j.fcr.2004.02.006. [DOI] [Google Scholar]
- Choukr-Allah et al. (2016).Choukr-Allah R, Rao NK, Hirich A, Shahid M, Alshankiti A, Toderich K, Gill S, Butt KUR. Quinoa for marginal environments: toward future food and nutritional security in MENA and central asia regions. Plant Science. 2016;7 doi: 10.3389/fpls.2016.00346. Article 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack (1984).Cusack D. Quinua: grain of the Incas. Ecologist. 1984;14:21–31. [Google Scholar]
- Dai et al. (2007).Dai F, Wang J, Zhang S, Xu Z, Zhang G. Genotypic and environmental variation in phytic acid content and its relation to protein content and malt quality in barley. Food Chemistry. 2007;105:606–611. doi: 10.1016/j.foodchem.2007.04.019. [DOI] [Google Scholar]
- Filho et al. (2017).Filho AMM, Pirozi MR, Borges JTDS, Pinheiro Sant’Ana HM, Chaves JBP, Coimbra JSDR. Quinoa: nutritional, functional, and antinutritional aspects. Critical Reviews in Food Science and Nutrition. 2017;57:1618–1630. doi: 10.1080/10408398.2014.1001811. [DOI] [PubMed] [Google Scholar]
- Gawlik-Dziki et al. (2013).Gawlik-Dziki U, Świeca M, Sułkowski M, Dziki D, Baraniak B, Czyz J. Antioxidant and anticancer activities of Chenopodium quinoa leaves extracts—in vitro study. Food and Chemical Toxicology. 2013;57:154–160. doi: 10.1016/j.fct.2013.03.023. [DOI] [PubMed] [Google Scholar]
- Geren (2015).Geren H. Effects of different nitrogen levels on the grain yield and some yield components of quinoa (Chenopodium quinoa Willd.) under menditerranean climatic conditions. Turkish Journal of Field Crops. 2015;20:59–64. doi: 10.17557/.39586. [DOI] [Google Scholar]
- Gomez-Becerra et al. (2010).Gomez-Becerra HF, Yazici A, Ozturk L, Budak H, Peleg Z, Morgounov A, Fahima T, Saranga Y, Cakmak I. Genetic variation and environmental stability of grain mineral nutrient concentrations in Triticum dicoccoides under five environments. Euphytica. 2010;171:39–52. doi: 10.1007/s10681-009-9987-3. [DOI] [Google Scholar]
- Gómez-Caravaca et al. (2012).Gómez-Caravaca AM, Iafelice G, Lavini A, Pulvento C, Caboni MF, Marconi E. Phenolic compounds and saponins in quinoa samples (Chenopodium quinoa Willd.) grown under different saline and nonsaline irrigation regimens. Journal of Agricultural and Food Chemistry. 2012;60:4620–4627. doi: 10.1021/jf3002125. [DOI] [PubMed] [Google Scholar]
- Gonzalez et al. (2012).Gonzalez JA, Konishi Y, Bruno M, Valoy M, Prado FE. Interrelationships among seed yield. total protein and amino acid composition of ten quinoa (Chenopodium Quinoa) cultivars from two different agroecological regions. Journal of the Science of Food and Agriculture. 2012;92:1222–1229. doi: 10.1002/jsfa.4686. [DOI] [PubMed] [Google Scholar]
- Gu et al. (2015).Gu R, Chen F, Liu B, Wang X, Liu J, Li P, Pan Q, Pace J, Soomro AA, Lübberstedt T, Mi G, Yuan L. Comprehensive phenotypic analysis and quantitative trait locus identification for grain mineral concentration, content, and yield in maize (Zea mays L.) Theoretical and Applied Genetics. 2015;128:1777–1789. doi: 10.1007/s00122-015-2546-5. [DOI] [PubMed] [Google Scholar]
- Hacham, Avraham & Amir (2002).Hacham Y, Avraham T, Amir R. The N-Terminal Region of Arabidopsis cystathionine gamma-synthase plays an important regulatory role in methionine metabolism. Plant Physiology. 2002;128:454–462. doi: 10.1104/pp.010819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen et al. (2007).Jacobsen S-E, Monteros C, Corcuera LJ, Bravo LA, Christiansen JL, Mujica A. Frost resistance mechanisms in quinoa (Chenopodium quinoa Willd. 2007. [DOI]
- Jacobsen, Mujica & Jensen (2003).Jacobsen S-E, Mujica A, Jensen CR. The Resistance of quinoa (Chenopodium quinoa Willd.) to adverse abiotic factors. Food Reviews International. 2003;19:99–109. doi: 10.1081/FRI-120018872. [DOI] [Google Scholar]
- Jancurová, Minarovičová & Dandár (2009).Jancurová M, Minarovičová L, Dandár A. Quinoa—a review. Czech Journal of Food Sciences. 2009;27:71–79. doi: 10.4314/tjpr.v5i1.14634. [DOI] [Google Scholar]
- Joshi et al. (2010).Joshi V, Joung J-G, Fei Z, Jander G. Interdependence of threonine, methionine and isoleucine metabolism in plants: accumulation and transcriptional regulation under abiotic stress. Amino Acids. 2010;39:933–947. doi: 10.1007/s00726-010-0505-7. [DOI] [PubMed] [Google Scholar]
- Kotlarz et al. (2011).Kotlarz A, Sujak A, Strobel W, Grzesiak W. Chemical composition and nutritive value of protein of the pea seeds—effect of harvesting year and variety. Vegetable Crops Research Bulletin. 2011;75:57–69. doi: 10.2478/v10032-011-0018-2. [DOI] [Google Scholar]
- Lott et al. (2000).Lott J, Ockendena I, Raboya V, Battena GD. Phytic acid and phosphorus in crop seeds and fruits: a global estimate. Seed Science Research. 2000;10:11–33. doi: 10.1017/S0960258500000039. [DOI] [Google Scholar]
- Lozano et al. (2012).Lozano M, Ticona E, Carrasco C, Flores Y, Almanza G. Cuantificación de Saponinas en Residuos de Quinua Real Chenopodium Quinoa Willd. Revista Boliviana de Química. 2012;29(2):128–135. [Google Scholar]
- Lutz, Martínez & Martínez (2013).Lutz M, Martínez A, Martínez EA. Daidzein and Genistein Contents in seeds of quinoa (Chenopodium quinoa Willd.) from local ecotypes grown in arid Chile. Industrial Crops and Products. 2013;49:117–121. doi: 10.1016/j.indcrop.2013.04.023. [DOI] [Google Scholar]
- Martinez et al. (2009).Martinez EA, Veas E, Jorquera C, San Martin R, Jara P. Re-introduction of quinoa into arid Chile: cultivation of two lowland races under extremely low irrigation. Journal of Agronomy and Crop Science. 2009;195:1–10. doi: 10.1111/j.1439-037X.2008.00332.x. [DOI] [Google Scholar]
- Miller, Youngs & Oplinger (1980).Miller GA, Youngs VL, Oplinger ES. Environmental and cultivar effects on oat phytic acid concentration. Cereal Chemistry. 1980;57:189–191. [Google Scholar]
- Miranda et al. (2013a).Miranda M, Vega-Gálvez A, Martínez EA, López J, Marín R, Aranda M, Fuentes F. Influence of contrasting environments on seed composition of two quinoa genotypes: nutritional and functional properties. Chilean Journal of Agricultural Research. 2013a;73:06–07. doi: 10.4067/S0718-58392013000200004. [DOI] [Google Scholar]
- Miranda et al. (2013b).Miranda M, Vega-Gálvez A, Quispe-fuentes I, Rodríguez MJ. Nutritional aspects of six quinoa (Chenopodium quinoa WILLD.) ecotypes from three geographical areas of Chile. Chilean Journal of Agricultural Research. 2013b;72:175–181. doi: 10.4067/S0718-58392012000200002. [DOI] [Google Scholar]
- Mujica, Izquierdo & Marathee (2001).Mujica J, Izquierdo A, Marathee JP. Quinoa (Chenopodium quinoa will) ancestral cultivo andino, alimento del presente y futuro. Food and Agriculture Organization, Universidad Nacional del Altiplano de Puno; Santiago: 2001. Origen y descripción de la quinua; pp. 9–53. [Google Scholar]
- Nowak, Du & Charrondière (2016).Nowak V, Du J, Charrondière UR. Assessment of the nutritional composition of quinoa (Chenopodium quinoa Willd.) Food Chemistry. 2016;193:47–54. doi: 10.1016/j.foodchem.2015.02.111. [DOI] [PubMed] [Google Scholar]
- Paśko et al. (2008).Paśko P, Sajewicz M, Gorinstein S, Zachwieja Z. Analysis of selected phenolic acids and flavonoids in Amaranthus cruentus and Chenopodium quinoa seeds and sprouts by HPLC. Acta Chromatographica. 2008;20:661–672. doi: 10.1556/AChrom.20.2008.4.11. [DOI] [Google Scholar]
- Prado et al. (2014a).Prado FE, Fernández-Turiel JL, Tsarouchi M, Psaras GK, González JA. Variation of seed mineral concentrations in seven quinoa cultivars grown in two agroecological sites. Cereal Chemistry Journal. 2014a;91:453–459. doi: 10.1094/CCHEM-08-13-0157-R. [DOI] [Google Scholar]
- Prado et al. (2014b).Prado FE, Fernández-Turiel JL, Tsarouchi M, Psaras GK, González JA. Variation of seed mineral concentrations in seven quinoa cultivars grown in two agroecological sites. Cereal Chemistry. 2014b;91:453–459. doi: 10.1094/CCHEM-08-13-0157-R. [DOI] [Google Scholar]
- Ruales & Nair (1993).Ruales J, Nair BM. Saponins, phytic acid tannins and protease inhibitors in quinoa (Chenopodium quinoa, Willd) seeds. Food Chemistry. 48:137–143. doi: 10.1016/0308-8146(93)90048-K. [DOI] [Google Scholar]
- Ruiz et al. (2014).Ruiz KB, Biondi S, Oses R, Acuña Rodriguez IS, Antognoni F, Martinez-Mosqueira EA, Coulibaly A, Canahua-Murillo A, Pinto M, Zurita-Silva A, Bazile D, Jacobsen S-E, Molina-Montenegro MA. Quinoa biodiversity and sustainability for food security under climate change: a review. Agronomy for Sustainable Development. 2014;34(2):349–359. doi: 10.1007/s13593-013-0195-0. [DOI] [Google Scholar]
- Tang & Tsao (2017).Tang Y, Tsao R. Phytochemicals in quinoa and amaranth grains and their antioxidant, anti-inflammatory, and potential health beneficial effects: a review. Molecular Nutrition & Food Research. 2017;61:1600767. doi: 10.1002/mnfr.201600767. [DOI] [PubMed] [Google Scholar]
- Thanapornpoonpong et al. (2008).Thanapornpoonpong SN, Vearasilp S, Pawelzik E, Gorinstein S. Influence of various nitrogen applications on protein and amino acid profiles of amaranth and quinoa. Journal of Agricultural and Food Chemistry. 2008;56:11464–11470. doi: 10.1021/jf802673x. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, U.S. Department of Agriculture (2015).U.S. Department of Health and Human Services, U.S. Department of Agriculture 2015–2020 Dietary guidelines for Americans. Eighth Edition. 2015. http://health.gov/dietaryguidelines/2015/guidelines/ [2 January 2018]. http://health.gov/dietaryguidelines/2015/guidelines/
- Varisi et al. (2008).Varisi VA, Camargos LS, Aguiar LF, Christofoleti RM, Medici LO, Azevedo RA. Lysine biosynthesis and nitrogen metabolism in quinoa (Chenopodium quinoa): study of enzymes and nitrogen-containing compounds. Plant Physiology and Biochemistry. 2008;46:11–18. doi: 10.1016/j.plaphy.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Vaz Patto et al. (2015).Vaz Patto MC, Amarowicz R, Aryee ANA, Boye JI, Chung H-J, Martín-Cabrejas MA, Domoney C. Achievements and Challenges in Improving the Nutritional Quality of Food Legumes. Critical Reviews in Plant Science. 2015;34:105–143. doi: 10.1080/07352689.2014.897907. [DOI] [Google Scholar]
- Wang et al. (2017).Wang N, Hou A, Santos J, Maximiuk L. Effects of cultivar growing location and year on physicochemical and cooking characteristics of dry beans (Phaseolus vulgaris) Cereal Chemistry. 2017;94:128–134. doi: 10.1094/CCHEM-04-16-0124-FI. [DOI] [Google Scholar]
- Wimalasekera (2015).Wimalasekera R. Role of seed quality in improving crop yields. In: Hakeem KR, editor. Crop production and global environmental issues. Springer International Publishing; Cham: 2015. pp. 153–168. [DOI] [Google Scholar]
- Wu et al. (2016).Wu G, Peterson AJ, Morris CF, Murphy KM. Quinoa seed quality response to sodium chloride and sodium sulfate salinity. Frontiers in Plant Science. 2016;7 doi: 10.3389/fpls.2016.00790. Article 790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao et al. (2014).Yao Y, Yang X, Shi Z, Ren G. Anti-Inflammatory activity of saponins from quinoa (Chenopodium quinoa Willd.) seeds in lipopolysaccharide-stimulated raw 2647 macrophages cells. Journal of Food Science. 2014;79(5):H1018–H1023. doi: 10.1111/1750-3841.12425. [DOI] [PubMed] [Google Scholar]
- Zurita-Silva et al. (2014).Zurita-Silva A, Fuentes F, Zamora P, Jacobsen S-E, Schwember AR. Breeding quinoa (Chenopodium quinoa Willd.): potential and perspectives. Molecular Breeding. 2014;34:13–30. doi: 10.1007/s11032-014-0023-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Values are Mean ± Stnd Dev (n = 4).
Different letters indicate statistical differences at a P < 0.05 (Duncan t-test). Values represent Mean ± SE.
FRAP and protein content were determined as described in the Methods section. Data is presented in Figs. 3 and 5.
Free amino acids were extracted from quinoa Seeds and analyze according to what is described in the Methods section. Data is presented in Fig. 4.
Agronomical parameters presented in Table 1 were determined in the three quinoa cultivars at the three locations.
Fiber and Saponin were determined in quinoa seeds as described in the Methods section. Data is presented in figures 6 and 7.
Mineral composition of seeds was determined as described in the Methods section and final data is presented in Fig. 1.
Phytate was determined in seeds according to the methodology described in Methods. Final data is presented in Fig. 2.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are provided in the Supplemental Files.