Abstract
Background
Development of novel agents targeting the androgen axis has led to improved overall survival in castration-resistant prostate cancer (CRPC). This study aimed to investigate the optimal timing of treatment with one such agent, abiraterone acetate (AA), in Japanese patients.
Materials and methods
Between July 2014 and February 2016, 106 CRPC patients were administered AA in Nagoya City University Hospital, Nagoya, Japan and in four affiliated hospitals following failure of primary combined androgen blockade (CAB). Of these, records of 69 patients treated before chemotherapy were retrospectively analyzed. Patients were divided into two AA treatment groups: (1) first- or second-line after diagnosis of CRPC, designated the Early Group, and (2) third-line onwards, designated the Deferred Group. Prostate-specific antigen (PSA) response rate, ≥ 50% PSA decline rate with treatment, progression-free survival (PFS), and overall survival (OS) were compared between the two groups. National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0 was used to classify adverse events.
Results
In 24 patients in the Early Group and 45 patients in the Deferred Group, no significant differences in baseline parameters were observed between groups. PSA response rate, ≥ 50% PSA decline rate and PFS (but not OS) were significantly better in the Early Group than in the Deferred Group. Serum aspartate aminotransferase/alanine aminotransferase elevations were the most common Grade 3 treatment-related toxicities, and were clinically manageable. In subgroup analyses of the Early Group, comparison of first-line AA with second-line AA after flutamide treatment showed no changes in PSA response rate, PFS, or OS.
Conclusion
This study suggests improved favorable outcomes of first- or second-line AA treatment in Japanese chemotherapy-naïve CRPC patients after failed CAB; statistical confirmation of such improvement was evident for PFS, but not OS. In addition, early AA treatment exhibited an acceptable safety profile.
Keywords: abiraterone acetate, castration-resistant prostate cancer, combined androgen blockade
1. Introduction
Changes in lifestyle such as adoption of Western diets are possible contributing factors to the gradually observed increase in prostate cancer incidence and mortality in the Japanese population.1, 2, 3 Androgen deprivation therapy (ADT) remains the main first-line treatment for metastatic prostate cancer patients. However, the benefits of such treatment are short-lived, persisting only for a few years, by which time the disease undergoes transformation into metastatic castration-resistant prostate cancer (CRPC). Nonetheless, the development of several new drugs targeting the androgen axis has led to improvement in overall survival.4, 5, 6
Abiraterone acetate (AA) is one such new agent that selectively inhibits androgen synthesis in testes, adrenal glands, and tumor tissues by inhibition of cytochrome P450. AA has been approved in > 70 countries including Japan (in 2014) for treatment of chemotherapy-naïve metastatic CRPC patients and clinical studies have demonstrated its efficacy and safety.7, 8, 9
In accordance with Western guidelines such as those of the National Comprehensive Cancer Network (NCCN)10 and European Association of Urology (EAU),11 AA has been recommended as first-line treatment in metastatic CRPC patients after primary ADT. However, there are differences in approach between Western and Asian countries with regard to primary ADT. The NCCN and EAU guidelines do not generally recommend primary ADT for nonmetastatic prostate cancer patients, except in very high-risk disease, whereas Japanese guidelines include primary ADT as an option for all males except cases at very low risk. Furthermore, in Japan, combined androgen blockade (CAB) using antiandrogens such as bicalutamide and luteinizing hormone releasing hormone analogs has prevailed widely on the basis of several large multicenter randomized studies.12, 13, 14, 15, 16 In addition, vintage hormonal manipulation switching from bicalutamide to flutamide has frequently prevailed. Therefore, the present study sought to establish the optimal timing of treatment with AA in Japanese CRPC patients following failure of CAB. This was achieved by retrospective analysis of the efficacy and safety of AA use before chemotherapy in a multi-institutional context.
2. Materials and methods
2.1. Patients and treatment evaluation
A review was undertaken of 106 CRPC patients treated with oral AA (1,000 mg once daily) + prednisolone (5 mg twice daily) in five centers, including Nagoya City University Hospital, Nagoya, Japan, between July 2014 and February 2016. With Institutional Review Board approval in Nagoya City University Hospital, approved number was 60160035, the medical records of these patients were retrospectively analyzed. Sixty-nine of this cohort treated before chemotherapy were enrolled in the study. All patients had a histological diagnosis of prostate adenocarcinoma, which had progressed despite achieving castration-level values for testosterone by primary CAB treatment using a combination of oral bicalutamide 80 mg once daily and luteinizing hormone-releasing hormone agonist.
Clinical, biochemical, or radiographic progressive disease was defined according to Prostate Cancer Clinical Trials Working Group criteria.17 Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. The following variables were recorded following consultation of electronic patient medical records: patient age, initial prostate-specific antigen (PSA) levels, number of metastatic sites, prostate biopsy Gleason score, PSA response which was defined as the improvement of PSA levels at 12 weeks after AA treatment, absence/presence of PSA flare, nadir PSA level, and free survival (PFS) was measured from the start of the AA treatment until the time of radiographic or PSA progression. Patients were divided into two AA treatment groups: (1) first- or second-line following flutamide switching after diagnosis of free survival (PFS) was measured from the start of the AA treatment until the time of radiographic or PSA progression. Patients were divided into two AA treatment groups: (1) first- or second-line following flutamide switching after diagnosis of CRPC, designated the Early Group, and (2) third-line onwards, designated the Deferred Group. Statistical comparisons were made between these two groups. Clinical characteristics of all patients are listed in Table 1.
Table 1.
Comparison of patients' characteristics and response rate in early and deferred abiraterone groups
| Characteristics | Early Group (n = 24) | Deferred Group (n = 45) | P | |
|---|---|---|---|---|
| Median age, yr (range) | 71 (60–89) | 72 (45–89) | n.s. | |
| Initial serum PSA levels, ng/mL (range) | 194 (5.74–6,286) | 141 (2.70–6,969) | ||
| cN | 0 | 15 | 29 | n.s. |
| 1 | 9 | 16 | ||
| cM | 0 | 8 | 17 | n.s. |
| 1 | 16 | 28 | ||
| Position of AA treatment after failure of primary CAB | 1st | 9 | 0 | – |
| 2nd | 15 | 0 | ||
| 3rd | 0 | 10 | ||
| 4th | 0 | 11 | ||
| 5th onward | 0 | 24 | ||
| Pretreatment therapy of AA after failure of primary CAB | None | 9 | 0 | – |
| Flutamide | 15 | 45 | ||
| EMP | 0 | 45 | ||
| DEX | 0 | 35 | ||
| Glucocorticoid | 0 | 18 | ||
| Enz | 0 | 6 | ||
| Median duration of pretreatment before AA, mo (range) | 2.7 (0.5–21.9) | 27.5 (2.3–160.6) | – | |
| PSA response, n (%) | 19 (79.2) | 17 (37.8) | < 0.001*** | |
| 50% decline in PSA, n (%) | 11 (45.8) | 7 (15.6) | < 0.01** | |
**P < 0.01, ***P < 0.001 statistically significant.
AA, abiraterone acetate; CAB, combined androgen blockade; cM, clinical visceral metastasis; cN, clinical lymph node metastasis; DEX, dexamethasone; EMP, estramustine phosphate; Enz: enzalutamide; n.s., not significant; PSA, prostate-specific antigen.
2.2. Statistical analysis
Differences in categorical parameters were assessed using the Student t test. Cumulative rates were estimated using the Kaplan–Meier method, and the significance of differences between curves was tested by the log-rank test. Univariate and multivariate analyses employed the Cox proportional hazard regression model. A value of P < 0.05 was considered statistically significant. All data were analyzed using EZR software (Saitama Medical Center, Jichi Medical University, Yakushiji, Japan).
3. Results
3.1. Baseline population profile
Patient numbers in the Early and Deferred Groups, median ages of patients, and median initial PSA levels are shown in Table 1. Gleason score obtained by prostate needle biopsy was > 8 in all patients; most of whom also exhibited bone metastasis (approximate incidence almost 1 per site per group). There were no significant differences in clinical profile between the groups for initial diagnosis of CRPC. Median follow-up period from the diagnosis of CRPC was 15.3 (4.8–31.5) months in the Early Group and 40.8 (9.5–177.8) months in the Deferred Group, respectively.
3.2. Clinical response and outcomes of AA treatment
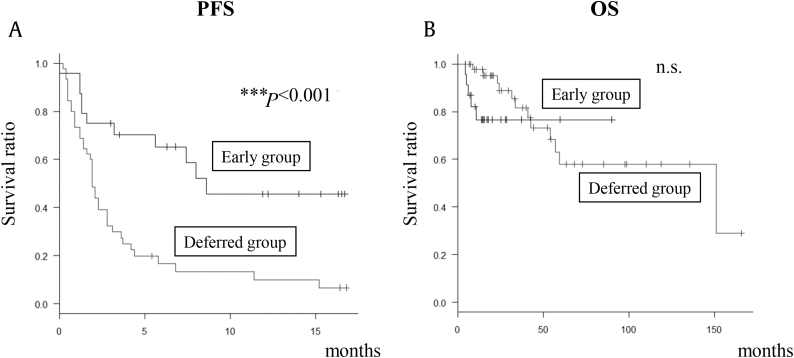
OS did not differ between the groups (Figs. 1A, 1B). Waterfall plot at the maximum PSA changes is shown in Fig. 2. As can be seen, the majority of patients had a decline in PSA level during this treatment period in the Early Group. Univariate and multivariate analyses of baseline parameters revealed that early use of AA was the only prognostic factor for PFS (Table 2).
Fig. 1.
PFS and OS in patients with CRPC treated with AA. (A) PFS in Early Group and Deferred Group. (B) OS in Early Group and Deferred Group. Early Group: first- or second-line AA after diagnosis of CRPC; Deferred Group: third-line AA onwards. ***P < 0.001 was statistically significant. AA, abiraterone acetate; CRPC, castration-resistant prostate cancer; n.s., not significant, OS, overall survival; PFS, progression-free survival.
Fig. 2.
Waterfall plot at maximum PSA changes from baseline after AA treatment. Early Group: first- or second-line AA after diagnosis of castration-resistant prostate cancer; Deferred Group: third-line AA onwards. AA, abiraterone acetate; PSA, prostate-specific antigen.
Table 2.
Univariate and multivariate analyses of baseline parameters, and progression-free survival in 69 patients treated with AA for castration-resistant prostate cancer
| Parameter | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Timing of AA treatment, early vs. deferred | 3.29 | 1.66–6.51 | <0.001*** | 2.99 | 1.35–6.64 | <0.01** |
| Age at initial diagnosis (yr), < 72 vs. ≥ 72 | 1.51 | 0.85–2.68 | 0.16 | 1.27 | 0.67–2.40 | 0.47 |
| Serum PSA levels at initial diagnosis (ng/mL), < 135 vs. ≥ 135 | 1.14 | 0.65–1.99 | 0.65 | 1.05 | 0.59–1.87 | 0.87 |
| Distant metastasis at initial diagnosis, yes vs. no | 1.11 | 0.61–2.01 | 0.73 | 0.97 | 0.52–1.82 | 0.92 |
| PSA flare after AA treatment, yes vs. no | 0.58 | 0.26–1.29 | 0.18 | 0.86 | 0.31–2.43 | 0.78 |
| Period of primary ADT to CRPC (mo), < 12 vs. ≥ 12 | 0.57 | 0.32–1.02 | 0.06 | 0.62 | 0.30–1.30 | 0.21 |
**P < 0.01, ***P < 0.001 indicates significant difference.
AA, abiraterone acetate; ADT, androgen deprivation therapy; CI, confidence interval; CRPC, castration-resistant prostate cancer; HR, hazard ratio; PSA, prostate-specific antigen.
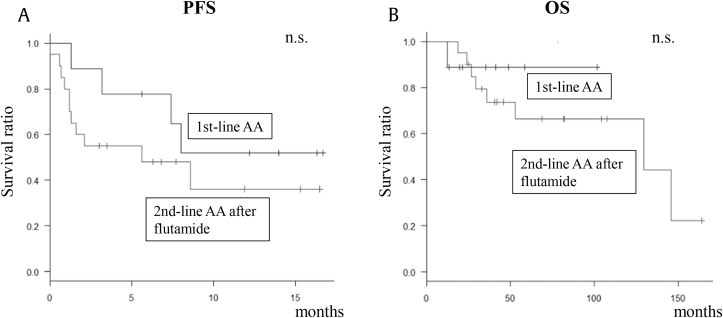
In subgroup analyses in the Early Group, all patients receiving second-line AA treatment after diagnosis of CRPC had previously been treated with flutamide as first-line therapy; therefore, the first-line group (n = 9) and those receiving second-line AA treatment after flutamide (n = 15) were analyzed. As shown in Table 3, baseline profiles did not differ significantly. In addition, there were no significant differences in PSA response or ≥ 50% PSA decline rates. Furthermore, PFS and OS did not differ significantly between the two groups (Figs. 3A, 3B).
Table 3.
Patient characteristics and response rate to first-line AA and second-line AA after flutamide
| Characteristics | 1st-line AA (n = 9) | 2nd-line AA after flutamide (n = 15) | P | |
|---|---|---|---|---|
| Median age, yr (range) | 71 (63–82) | 73 (60–89) | n.s. | |
| Initial serum PSA levels, ng/mL (range) | 222 (15.85–6,286) | 166 (5.73–3,980) | n.s. | |
| cN | 0 | 6 | 9 | n.s. |
| 1 | 3 | 6 | ||
| cM | 0 | 2 | 6 | n.s. |
| 1 | 7 | 9 | ||
| PSA response, n (%) | 8 (88.9) | 11 (73.3) | n.s. | |
| 50% decline in PSA, n (%) | 5 (55.6) | 6 (40.0) | n.s. | |
AA, abiraterone acetate; cM; cN; n.s., not significant; PSA, prostate-specific antigen.
Fig. 3.
PFS and OS in patients with castration-resistant prostate cancer treated with first- or second-line AA. (A) PFS in patients treated with first-line AA or second-line AA after flutamide. (B) OS in patients treated with first-line AA or second-line AA after flutamide. AA, abiraterone acetate; n.s., not significant; OS, overall survival; PFS, progression-free survival.
3.3. Adverse events
Table 4 shows the treatment-related extent of toxicity in all 69 patients treated with AA. In this cohort of chemotherapy-naïve patients, hepatic dysfunction, hypokalemia, and thrombocytopenia were Grade 3 adverse events. These analyses did not reveal any incidence of Grade 4 toxicity or treatment-related death.
Table 4.
Adverse events in 69 patients treated with abiraterone acetate for castration-resistant prostate cancer
| Toxicity | Grade (all cycles), No. of patients (%) |
|||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Hematological | ||||
| Hypokalemia | – | – | 1 (1.45) | – |
| Thrombocytopenia | – | – | 1 (1.45) | – |
| Nonhematological | ||||
| Increase in AST/ALT | 6 (8.70) | – | 3 (4.35) | – |
| Acneiform rash | 1 (1.45) | – | – | – |
| Hot flashes | 1 (1.45) | – | – | – |
| Hypertension | 1 (1.45) | – | – | – |
| Nausea/vomiting | – | 2 (2.90) | – | – |
| Glucose intolerance | – | 2 (2.90) | – | – |
| Localized edema | 2 (2.90) | – | – | – |
| Palpitations | 1 (1.45) | – | – | – |
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
4. Discussion
CAB with agents such as bicalutamide and luteinizing hormone-releasing hormone (LHRH) analogs has proved ineffective in CRPC patients. The present retrospective multi-institutional analysis of AA efficacy has demonstrated the benefit of first- or second-line AA treatment, as indicated by reductions in the PSA response and increases in PFS; AA also exhibited an acceptable safety profile. In the chemotherapy-naïve setting, total PSA response rate was 52.2% while ≥ 50% PSA decline rate was 26.1%. These results were marginally poorer than those obtained from a large study in non-Japanese chemotherapy-naïve CRPC patients (COU-AA-302)7 and in a similar Japanese Phase 2 study (JPN-201).8
Possible explanations for this difference include: (1) the fact that patients in the present study exhibited higher initial serum PSA levels; (2) a greater number of patients had higher Gleason scores; and (3) patients had received extensive ADT using steroids and the alkylating antineoplastic prodrug estramustine; thus, AA might not have been expected to have a significant effect in such very high risk populations. However, in spite of the high-risk nature of the patient population, first- or second-line AA treatment after the failure of primary CAB was more effective than deferred treatment and exhibited an acceptable safety profile.
The reason for the low incidence of adverse events in the present study compared with that of other studies was unclear; however, even if used in elderly patients, early timing of AA treatment should be recommended in terms of tumor response. The present results showed that OS did not differ significantly between the Early and Deferred Groups; this may have been in part because of the relatively short follow-up period in this study. Therefore, long-term analyses would be beneficial in strengthening the conclusions reached in this study.
Several clinical parameters have been measured in conjunction with PFS in CRPC, such as the period of primary ADT and of the PSA flare. The latter phenomenon is followed by a decrease in PSA levels shortly after initial treatment with agents such as LHRH analogs, AA, and taxane. Previous reports indicated that this phenomenon was observed in 5–30% of patients receiving an LHRH analog and in 8–20% of patients receiving taxane chemotherapy.18, 19, 20, 21, 22 However, its predictive value of prognosis or occurrence of adverse events is unclear. A number of reports of the PSA flare during AA treatment in CRPC patients have been published23, 24, 25; however, occurrence of such a phenomenon before docetaxel treatment, for example, was not associated with clinical outcomes. Also in the present study in the early AA setting, the PSA flare was not a prognostic factor for PFS. Novel biomarkers such as androgen receptor splice variant 7 in circulating tumor cells have recently been reported26, 27; this should facilitate accumulation of evidence regarding AA efficacy in Japanese patients in the future.
The present subgroup analyses used small sample sizes when comparing first-line AA treatment after primary CAB and second-line treatment after flutamide; however, PSA outcome and PFS were not significantly different. Although vintage hormonal manipulations such as use of flutamide are not recommended in recent guidelines, use of this agent has prevailed in Japan due to its low cost and low incidence of adverse events. Vintage hormonal manipulation switching from bicalutamide to flutamide has prevailed especially in Asian countries, therefore, based on the present data, a prospective study of sequential therapy with AA and flutamide has been initiated in CRPC patients after failure of primary CAB. It is hoped to report the results of this trial in due course.
There were several limitations to the present study. These included the typical shortcomings associated with a retrospective analysis, such as incompleteness of data collection, selection bias, and small sample size. However, it is believed that such limitations did not adversely affect the ability to capture the reported survival outcome with AA treatment. First- or second-line AA treatment in Japanese patients was beneficial even after the failure of primary CAB.
In conclusion, the outcomes of this study strongly suggest improved favorable outcomes on first- or second-line AA treatment in Japanese chemotherapy-naïve CRPC patients. This conclusion is supported by statistical analysis in terms of PFS. Furthermore, these analyses also suggest that second-line AA treatment after flutamide may be a feasible option in such CRPC patients.
Conflicts of interest
The authors wish to declare that they have no conflicts of interest.
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Center M.M., Jemal A., Lortet-Tieulent J., Ward E., Ferlay J., Brawley O. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–1092. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 4.Graham L., Schweizer M.T. Targeting persistent androgen receptor signaling in castration-resistant prostate cancer. Med Oncol. 2016;33:44. doi: 10.1007/s12032-016-0759-3. [DOI] [PubMed] [Google Scholar]
- 5.Chism D.D., De Silva D., Whang Y.E. Mechanisms of acquired resistance to androgen receptor targeting drugs in castration-resistant prostate cancer. Expert Rev Anticancer Ther. 2014;14:1369–1378. doi: 10.1586/14737140.2014.928594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crona D.J., Milowsky M.I., Whang Y.E. Androgen receptor targeting drugs in castration-resistant prostate cancer and mechanisms of resistance. Clin Pharmacol Ther. 2015;98:582–589. doi: 10.1002/cpt.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan C.J., Smith M.R., Fizazi K., Saad F., Mulders P.F., Sternberg C.N. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–160. doi: 10.1016/S1470-2045(14)71205-7. [DOI] [PubMed] [Google Scholar]
- 8.Matsubara N., Uemura H., Satoh T., Suzuki H., Nishiyama T., Uemura H. A phase 2 trial of abiraterone acetate in Japanese men with metastatic castration-resistant prostate cancer and without prior chemotherapy (JPN-201 study) Jpn J Clin Oncol. 2014;44:1216–1226. doi: 10.1093/jjco/hyu149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryan C.J., Shah S., Efstathiou E., Smith M.R., Taplin M.E., Bubley G.J. Phase II study of abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic response. Clin Cancer Res. 2011;17:4854–4861. doi: 10.1158/1078-0432.CCR-11-0815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohler J.L., Armstrong A.J., Bahnson R.R., D'Amico A.V., Davis B.J., Eastham J.A. Prostate cancer, version 1.2016. J Natl Compr Canc Netw. 2016;14:19–30. doi: 10.6004/jnccn.2016.0004. [DOI] [PubMed] [Google Scholar]
- 11.Cornford P., Bellmunt J., Bolla M., Briers E., De Santis M., Gross T. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol. 2017;71:630–642. doi: 10.1016/j.eururo.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Akaza H., Yamaguchi A., Matsuda T., Igawa M., Kumon H., Soeda A. Superior anti-tumor efficacy of bicalutamide 80 mg in combination with a luteinizing hormone-releasing hormone (LHRH) agonist versus LHRH agonist monotherapy as first-line treatment for advanced prostate cancer: interim results of a randomized study in Japanese patients. Jpn J Clin Oncol. 2004;34:20–28. doi: 10.1093/jjco/hyh001. [DOI] [PubMed] [Google Scholar]
- 13.Akaza H., Hinotsu S., Usami M., Arai Y., Kanetake H., Naito S. Combined androgen blockade with bicalutamide for advanced prostate cancer: long-term follow-up of a phase 3, double-blind, randomized study for survival. Cancer. 2009;115:3437–3445. doi: 10.1002/cncr.24395. [DOI] [PubMed] [Google Scholar]
- 14.Arai Y., Akaza H., Deguchi T., Fujisawa M., Hayashi M., Hirao Y. Evaluation of quality of life in patients with previously untreated advanced prostate cancer receiving maximum androgen blockade therapy or LHRHa monotherapy: a multicenter, randomized, double-blind, comparative study. J Cancer Res Clin Oncol. 2008;134:1385–1396. doi: 10.1007/s00432-008-0409-z. [DOI] [PubMed] [Google Scholar]
- 15.Klotz L., Schellhammer P., Carroll K. A re-assessment of the role of combined androgen blockade for advanced prostate cancer. BJU Int. 2004;93:1177–1182. doi: 10.1111/j.1464-410x.2004.04803.x. [DOI] [PubMed] [Google Scholar]
- 16.Labrie F., Dupont A., Belanger A., Cusan L., Lacourciere Y., Monfette G. New hormonal therapy in prostatic carcinoma: combined treatment with an LHRH agonist and an antiandrogen. Clin Invest Med. 1982;5:267–275. [PubMed] [Google Scholar]
- 17.Scher H.I., Halabi S., Tannock I., Morris M., Sternberg C.N., Carducci M.A. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahler C. Is disease flare a problem? Cancer. 1993;72:3799–3802. doi: 10.1002/1097-0142(19931215)72:12+<3799::aid-cncr2820721707>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Noguchi K., Uemura H., Harada M., Miura T., Moriyama M., Fukuoka H. Inhibition of PSA flare in prostate cancer patients by administration of flutamide for 2 weeks before initiation of treatment with slow-releasing LH-RH agonist. Int J Clin Oncol. 2001;6:29–33. doi: 10.1007/pl00012076. [DOI] [PubMed] [Google Scholar]
- 20.Nelius T., Filleur S. PSA surge/flare-up in patients with castration-refractory prostate cancer during the initial phase of chemotherapy. Prostate. 2009;69:1802–1807. doi: 10.1002/pros.21024. [DOI] [PubMed] [Google Scholar]
- 21.Angelergues A., Maillet D., Flechon A., Ozgüroglu M., Mercier F., Guillot A. Prostate-specific antigen flare induced by cabazitaxel-based chemotherapy in patients with metastatic castration-resistant prostate cancer. Eur J Cancer. 2014;50:1602–1609. doi: 10.1016/j.ejca.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Sugiono M., Winkler M.H., Okeke A.A., Benney M., Gillatt D.A. Bicalutamide vs cyproterone acetate in preventing flare with LHRH analogue therapy for prostate cancer – a pilot study. Prostate Cancer Prostatic Dis. 2005;8:91–94. doi: 10.1038/sj.pcan.4500784. [DOI] [PubMed] [Google Scholar]
- 23.Burgio S.L., Conteduca V., Rudnas B., Carrozza F., Campadelli E., Bianchi E. PSA flare with abiraterone in patients with metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2015;13:39–43. doi: 10.1016/j.clgc.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 24.Ueda Y., Matsubara N., Tabata K.I., Satoh T., Kamiya N., Suzuki H. Prostate-specific antigen flare phenomenon induced by abiraterone acetate in chemotherapy-naive patients with metastatic castration-resistant prostate cancer. Clin Genitourin Cancer. 2017;15:320–325. doi: 10.1016/j.clgc.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 25.Narmala S.K., Boulmay B.C. PSA flare after initiation of abiraterone acetate. J Community Support Oncol. 2014;12:191–192. doi: 10.12788/jcso.0045. [DOI] [PubMed] [Google Scholar]
- 26.Thadani-Mulero M., Portella L., Sun S., Sung M., Matov A., Vessella R.L. Androgen receptor splice variants determine taxane sensitivity in prostate cancer. Cancer Res. 2014;74:2270–2282. doi: 10.1158/0008-5472.CAN-13-2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shan X., Danet-Desnoyers G., Fung J.J., Kosaka A.H., Tan F., Perfito N. Registered report: androgen receptor splice variants determine taxane sensitivity in prostate cancer. PeerJ. 2015;3:e1232. doi: 10.7717/peerj.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]