Abstract
Background
The aim of the paper is to investigate the outcomes of patients younger than 55 years in Victoria, Australia undergoing radical prostatectomy (RP) for prostate cancer.
Materials and methods
Data on all men undergoing RP in Victoria between January 1, 2004 and December 31, 2014 were obtained from the Victorian Cancer Registry. Tumor characteristics including Gleason grade, stage of disease (based on final pathology specimen), and cause of death were also obtained. Statistical analysis was performed using Chi-square test, Cox proportional hazards method, and Kaplan-Meier analysis.
Results
A total of 14,686 men underwent RP during the defined period. Of these men 109 were aged 35–44 years and 1,998 were aged 45–54 years. Men aged 35–44 years and 45–54 years were compared against men aged 55–74 years. The majority of men between the ages of 35 years and 44 years, and 45 years and 54 years had higher rates of Gleason ≤ 7 disease compared with men aged between 55 years and 74 years (92.7% vs. 86.8% vs. 79.3%; P < 0.01) and ≤ T2 disease (82.6% vs. 75.6% vs. 49.9%; P < 0.01) but similar median prostate-specific antigen values. On a multivariate analysis adjusting for Gleason score, T stage, and prostate-specific antigen, men aged 45–54 years and 55–64 years had 67% and 46% increase in overall survival, respectively, compared to men aged 65–74 years; but these differences were not seen in the 35–44 year age group. There were no differences in prostate cancer specific deaths between the groups. The 5- and 10-year overall survival outcomes were both higher for men aged 45–54 years compared to mean aged 55–74 years (97.9% vs. 95.9% and 94.9% vs. 85.3).
Conclusion
Men aged 45–54 years undergoing RP had better overall survival compared to men aged 55–74 years, but these effects were not seen in men aged 35–44 years. There were no differences in prostate cancer specific survival in these groups.
Keywords: Radical Prostatectomy, Survival Outcome, Young Men
1. Introduction
Prostate cancer remains the most common non skin malignancy diagnosed in Australian men, accounting for 25.2% of all new cancers diagnosed in men in 2016 and is the third leading cause of fatality from cancer.1 On a global scale, Australia ranks fifth of 15 countries in prostate cancer incidence, and the incidence of prostate cancer has been increasing.2, 3 The highest age-specific incidence occurs in men aged 65–69 years, with the incidence of prostate cancer increasing exponentially from 35 years of age onwards.1
Guidelines generally advocate screening be performed in men with at least a 10-year life expectancy.4, 5 In this regard, prostate cancer screening in younger men has potential to prevent prostate cancer deaths. Further, younger men are more likely to be amenable to aggressive treatment with a curative intent than those in an older age group owing to fewer comorbidities. However, most screening guidelines exist for men 50 years of age and older and prostate cancer screening is generally not routinely considered from 35 years of age, where incidence of the cancer first becomes notable.1
It has been shown that men older than 75 years have higher grades of disease and that age is an independent risk factor for poorer prostate cancer specific survival.6, 7, 8 At the same time, it has been reported in the United States that trends in prostate cancer are changing to affect a younger demographic.9 However, few studies have selectively focused on prostate cancer characteristics and outcomes in younger men, much less in the Australian context. Therefore, we aimed to investigate the outcomes of men younger than 55 years undergoing radical prostatectomy (RP) for the treatment of prostate cancer.
2. Materials and methods
2.1. Population characteristics
Victoria is the second largest state in Australia, with one fourth of the country's males at about 2,484,490 men. Data was obtained from the Victorian Cancer Registry, which receives mandatory cancer diagnosis notifications from 240 hospitals and pathology laboratories. The data includes patient demographics and detailed histopathological information.
Data on all men undergoing RP in Victoria between January 1, 2004 and December 31, 2014 were obtained from the Victorian Cancer Registry. Tumor characteristics including Gleason grade, stage of disease (based on histopathological examination and spread of tumor), and cause of death were acquired.
2.2. Statistical analyses
Chi-squared tests were used to compare patient and tumor characteristics. Univariate and multivariate survival analysis was performed using Kaplan-Meier survival curves and Cox-proportional hazard methods. Version 17.0 of the SPSS statistical software (SPSS Inc., Chicago, IL, USA) was used.
2.3. Ethics approval
No ethics approval was required for the data used in this study as the data included no identifying information. Data were released according to Victorian Cancer Registry Data Access Guidelines under a memorandum of understanding including conditions about data security, use, and destruction.
3. Results
A total of 14,686 men underwent RP between January 1, 2004 and December 31, 2014. Of these men, 109 were aged 35–44 years and 1,998 were aged 45–54 years. Younger men in the 35–44 years and 45–54 years age groups were compared against older men aged between 55 years and 74 years. The majority of men between the ages of 35 years and 44 years, and 45 years and 54 years had higher rates of Gleason ≤ 7 disease compared with men aged between 55 years and 74 years (92.7% vs. 86.8% vs. 79.3%; P < 0.01) and ≤ T2 disease (82.6% vs. 75.6% vs. 49.9%; P < 0.01) but similar median prostate-specific antigen (PSA) values (Table 1). A higher proportion of RP was performed in the metropolitan setting compared to rural, for all age categories.
Table 1.
Patient characteristics grouped by age.
| 35–44 yr | 45–54 yr | 55–64 yr | 65–74 yr | P | |
|---|---|---|---|---|---|
| Gleason score | 0.00 | ||||
| 6 | 51 (46.8) | 708 (35.4) | 1,919 (27.4) | 968 (18.5) | |
| 7 | 50 (45.9) | 1,027 (51.4) | 3,704 (53) | 2,949 (56.4) | |
| 8–10 | 2 (1.8) | 130 (6.5) | 679 (9.7) | 819 (15.7) | |
| Unknown | 6 (5.5) | 133 (6.7) | 689 (9.9) | 490 (9.4) | |
| Stage | 0.00 | ||||
| T1/T2 | 90 (82.6) | 1,510 (75.6) | 4,790 (68.5) | 3,209 (61.4) | |
| T3/4 | 19 (17.4) | 467 (23.4) | 2,131 (30.5) | 1,960 (37.5) | |
| Median PSA | 6.2 (2.2–14) | 6.3 (0.3–75) | 6.6 (0.2–95) | 6.4 (0.4–96) | NS |
| Location | |||||
| Metropolitan | 86 (78.9) | 1,504 (75.4) | 5,145 (73.7) | 3,892 (74.5) | NS |
| Rural | 23 (21.1) | 490 (24.6) | 1,836 (26.3) | 1,329 (25.5) | |
| Overall deaths | 2 | 47 | 262 | 329 | – |
| PC deaths | 1 | 16 | 58 | 58 | – |
NS, not significant, PC, prostate cancer; PSA, prostate-specific antigen.
3.1. Outcomes post RP
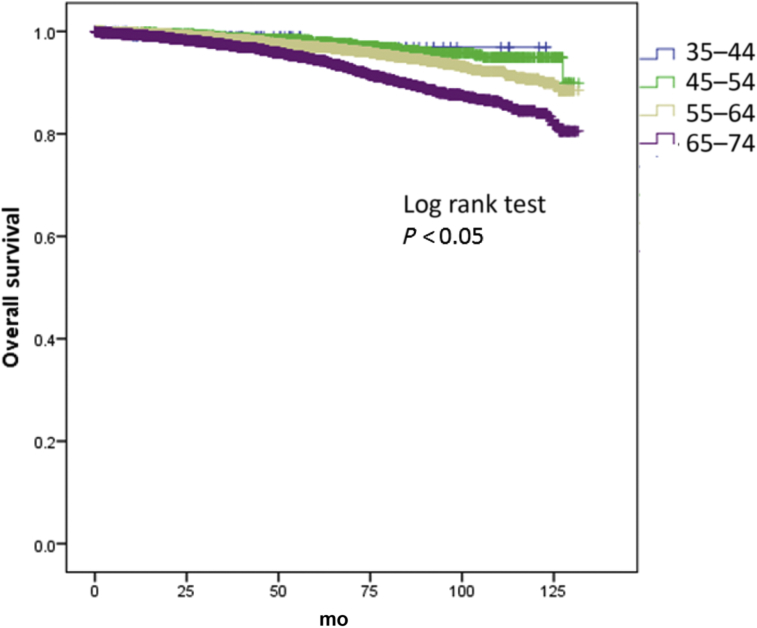
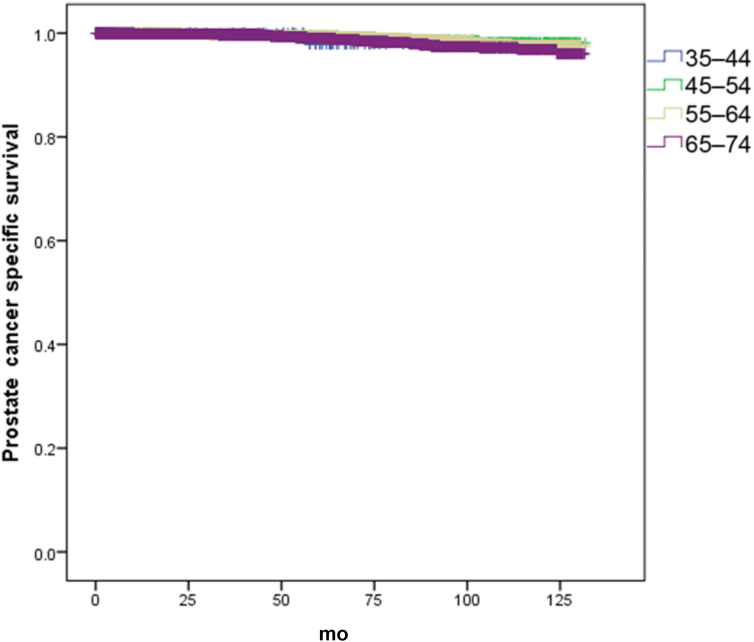
On a multivariate analysis adjusting for Gleason score, T stage, and PSA, men aged between 45–54 years and 55–64 years had 67% and 46% increase in overall survival (Fig. 1), respectively, compared to men aged 65–74 years (hazard ratio 0.33; 95% confidence interval 0.20–0.56; P < 0.05) and hazard ratio 0.54 (95% confidence interval 0.41–0.72). However, these differences were not seen in the 35–44 age group (Table 2). There were no differences in prostate cancer specific deaths between the groups (Table 3) (Fig. 2). The 5- and 10-year overall survival and prostate cancer specific rates are seen in Table 4.
Fig. 1.
Kaplan–Meier curve of overall survival of men undergoing radical prostatectomy (RP) by age groups.
Table 2.
Univariate and multivariate analysis of overall survival.
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| HR (95 CI) | P | HR (95 CI) | P | |
| Age (yr) | ||||
| 65–74 | 1.0 | – | 1.0 | – |
| 35–44 | 0.35 (0.09–1.40) | 0.13 | 0.99 (0.24–4.04) | 0.99 |
| 45–54 | 0.34 (0.25–0.47) | <0.05 | 0.33 (0.20–0.56) | <0.05 |
| 55–64 | 0.52 (0.45–0.62) | <0.05 | 0.54 (0.41–0.72) | <0.05 |
| Gleason score | ||||
| 6 | 1.0 | – | 1.0 | – |
| 7 | 0.92 (0.76–1.11) | 0.37 | 0.72 (0.51–1.00) | 0.05 |
| 8–10 | 2.14 (1.68–2.72) | <0.05 | 1.68 (1.10–2.56) | 0.02 |
| Unknown | 0.87 (0.67–1.12) | 0.28 | 0.59 (0.38–0.93) | 0.02 |
| Stage | ||||
| T1/T2 | 1.0 | – | 1.0 | – |
| T3/T4 | 1.30 (1.20–1.40) | <0.05 | 1.48 (1.11–1.96) | <0.05 |
| PSA | 0.98 (0.95–1.0) | 0.16 | 0.98 (0.96–1.0) | 0.17 |
| Location | ||||
| Metropolitan | – | – | 1.0 | – |
| Rural | – | – | 1.4 (1.09–1.89) | <0.05 |
CI, confidence interval; HR, hazard ratio; PSA, prostate-specific antigen.
Table 3.
Univariate and multivariate analysis of prostate cancer specific survival.
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| HR (95 CI) | P | HR (95 CI) | P | |
| Age (yr) | ||||
| 65–74 | 1.0 | – | 1.0 | – |
| 35–44 | 1.02 (0.14–7.40) | 0.98 | 3.39 (0.44–25.78) | 0.24 |
| 45–54 | 0.66 (0.38–1.15) | 0.14 | 0.58 (0.20–1.68) | 0.31 |
| 55–64 | 0.62 (0.45–0.94) | <0.05 | 1.04 (0.60–1.78) | 0.90 |
| Gleason score | ||||
| 6 | 1.0 | – | 1.0 | – |
| 7 | 1.72 (0.88–3.34) | 0.11 | 0.99 (0.40–2.46) | 0.99 |
| 8–10 | 20.56 (11.09–38.11) | <0.05 | 7.76 (3.19–18.90) | <0.05 |
| Unknown | 2.93 (1.44–5.94) | <0.05 | 1.36 (0.49–3.79) | 0.55 |
| Stage | ||||
| T1/T2 | 1.0 | – | 1.0 | – |
| T3/T4 | 10.39 (6.76–15.96) | <0.05 | 5.45 (2.94–10.10) | <0.05 |
| PSA | 0.99 (0.9–1.04) | 0.73 | 0.99 (0.94–1.05) | 0.82 |
| Location | ||||
| Metropolitan | 1.0 | – | 1.0 | – |
| Rural | 0.94 (0.64–1.39) | – | 0.74 | 0.37 |
CI, confidence interval; HR, hazard ratio; PSA, prostate-specific antigen.
Fig. 2.
Kaplan–Meier curve of prostate cancer specific survival of men undergoing radical prostatectomy (RP) by age groups.
Table 4.
Five- and 10-year survival rates of men undergoing radical prostatectomies.
| Overall survival | 5-year | 10-year |
|---|---|---|
| 35–44 | 96.9 | 96.9 |
| 45–54 | 97.9 | 94.9 |
| 55–64 | 96.9 | 90.7 |
| 65–74 | 94.5 | 84.0 |
| PC survival | ||
| 35–44 | 97.9 | 97.9 |
| 45–54 | 99.3 | 98.1 |
| 55–64 | 99.4 | 97.6 |
| 65–74 | 99.0 | 96.9 |
PC, prostate cancer.
4. Discussion
The differences in pathological and survival data we observed in males younger than 55 years as compared to those older than 55 years present unique opportunities and challenges. Prostate cancer is generally considered to be a disease of older men. In the era of prostate cancer screening, the incidence of prostate cancer increased nearly sixfold from 5.6 cases per 100,000 person-years to 32 cases per 100,000 person-years (1986–2008 data),10 and currently over 10% of new diagnoses that occur are in younger men aged 55 years and below.10, 11 Salinas et al12 observed that the greater proportion of cases diagnosed in those younger than 55 years are clinically localized, however males of a younger demographic are more likely to succumb to their cancer where it is of higher grade and stage.12 Therefore, this reflects a demographic of males with early onset prostate cancer that is distinct from the prostate cancer identified in older males that is beginning to be further explored.
In our analysis, we found that men aged 45–54 years with prostate cancer had lower grade disease and greater overall survival than their 55–74-year-old counterparts. This is consistent with the results of several other studies.13, 14, 15 Table 5 summarizes the results of several recent studies.13, 15, 16, 17, 18 In particular, a database study by Kinnear et al15 examined the outcomes of 7,018 men with prostate cancer in the state of South Australia, and found younger men had a greater proportion of T stage < 2 disease, lower median PSA, and higher rates of Gleason score < 7. This provides a good comparison of data from an interstate cohort.
Table 5.
Other recent studies of outcomes of younger men undergoing radical prostatectomies.
| Study | Year | No. of patients | Age (yr) | Overall survival | Prostate cancer survival | PSA free survival | Biochemical recurrence free survival |
|---|---|---|---|---|---|---|---|
| Freedland et al.13 | 2004 | 88 | <50 | – | – | 73% (5-yr) | – |
| Loeb et al.16 | 2008 | 55 | <40 | – | – | – | 94.8% |
| Lin et al.17 | 2009 | 30,338 | <55 | 85% (10-yr) | 87% (10-yr) | – | – |
| Becker et al.18 | 2014 | 175 | <50 | – | – | – | 63% (10-yr) |
| Kinnear et al.15 | 2016 | 182 | <50 | – | 98% | – | – |
PSA, prostate-specific antigen.
Interestingly, the survival difference between the age groups was not observed in the 35–44 age group. A population-based cohort study by Lin et al17 had similar findings. They studied the outcomes of 318,774 men aged 35–74 years with prostate cancer, and found that among all age groups with high grade and stage, the youngest men (aged 35–44 years) were at the highest risk of all-cause and cancer-specific death.17 Similarly, Thorstenson et al19 found a strong association between younger age and poor prognosis in men in whom metastatic disease was diagnosed before they were aged 50–55 years. This adds to the growing body of evidence of worse prognosis in the youngest group of men with prostate cancer.12 This prompts the theory that early onset prostate cancer may potentially be biologically different from cancer seen in older men.19
There are three well-recognized risk factors for prostate cancer: family history, age, and race. Family history is a risk factor of particular relevance to the discussion. Even though family history is responsible for a mere 9% of cases, early onset prostate cancer is likely to represent a larger proportion of hereditary disease than onset later than 55 years20 and has been shown to have a statistically significant greater number of alleles associated with increased risk of prostate cancer.21 Prostate cancer incidence increases with age more than any other form of cancer,22 but as shown in our results, the age at which the cancer is diagnosed can confer prognostic significance. In a population-based study of 1,476 Australian families with prostate cancer, Cui et al23 conducted segregation analyses and found that pattern best fit early onset prostate cancer was of autosomal dominantly inherited risk. Ongoing cancer genetics research has identified single nucleotide polymorphism in a transcription factor involved in early onset prostate cancer as well as the allele that is more significantly enhanced in these patients.12 Clearly, genetic inheritance has an important role in prostate cancer in the younger population. Further work could provide an opportunity to identify further genetic loci associated with early-onset disease, as well as explore the possibility of genetic testing to identify individuals at risk who may benefit from increased surveillance.
Younger men with biologically significant disease are more likely to undergo radical treatment, as they tend to have more favorable disease staging amenable to cure and fewer comorbidities.17 Longer life expectancy in these younger patients also means longer time to deal with possible side effects of treatment. Urinary incontinence and erectile dysfunction often have a greater impact on quality of life in the younger cohort. Becker et al18 reported continence rates of 97.4% in younger patients, and that age was an independent predictor of continence recovery at 1 year after RP. In addition, younger men retained more erectile function postoperatively, as well as favorable recovery of erectile function than older counterparts after surgery.18 Bilateral intraoperative nerve-sparing was found to be the most important predictor of postoperative erectile function recovery.18 These findings were corroborated by Labanaris et al.24 In their cohort, 92.6% of patients < 50 years underwent bilateral nerve-sparing RP, and reported 12-month continent and potency rates at 95.5% and 97.3%, respectively.24
There are some limitations to our study. Firstly, this dataset is representative of only one Australian state, so the applicability of the results to the wider population is uncertain but likely to be consistent given the nationalized health systems. Secondly, data on comorbidities, postoperative complications, and long-term oncological and functional outcomes were not available. This information would be particularly pertinent in a young cohort with longer life expectancy. Finally, like all database studies, our data is hostage to a few confounders and bias related to the observational nature of the study.
Overall, prostate cancer in younger males is an important clinical entity. We have shown in the Australian context that there are significant age-related differences in important parameters and outcomes, and these have been corroborated by numerous other analyses. Further research is required to ascertain the true health burden and economic costs of such findings, and for recommendations to be made particularly with regards to prostate cancer screening and management guidelines for prostate cancer detection, diagnosis, and management in the younger male population.
Conflicts of interest
None to be declared.
Acknowledgement
We would like to extend our gratitude to Vicky Thursfield, Cancer Control Information Manager Victorian Cancer Registry, for data provision.
References
- 1.AIHW . Australian Institute of Health and Welfare; Canberra: 2016. Australian Cancer Incidence and Mortality (ACIM) books: prostate cancer. [Google Scholar]
- 2.Hsing A.W., Tsao L., Devesa S.S. International trends and patterns of prostate cancer incidence and mortality. Int J Cancer. 2000;85:60–67. doi: 10.1002/(sici)1097-0215(20000101)85:1<60::aid-ijc11>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 3.Quinn M., Babb P. Patterns and trends in prostate cancer incidence, survival, prevalence and mortality. Part II individual countries. BJU Int. 2002;90:174–184. doi: 10.1046/j.1464-410x.2002.02823.x. [DOI] [PubMed] [Google Scholar]
- 4.RACGP . 8th ed. Royal Australian College of General Practitioners; East Melbourne: 2012. Guidelines for preventive activities in general practice. [Google Scholar]
- 5.PCFA-CCA . Prostate Cancer Foundation of Australia and Cancer Council Australia; Sydney: 2016. Draft clinical practice guidelines for PSA testing and early management of test-detected prostate cancer. [Google Scholar]
- 6.Shimizu H., Ross R.K., Bernstein L., Yatani R., Henderson B.E., Mack T.M. Cancers of the prostate and breast among Japanese and white immigrants in Los Angeles County. Br J Cancer. 1991;63:963–966. doi: 10.1038/bjc.1991.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsugane S., Gotlieb S.L.D., Laurenti R., Souza JMPd, Watanabe S. Cancer mortality among Japanese residents of the city of Säo Paulo, Brazil. Int J Cancer. 1990;45:436–439. doi: 10.1002/ijc.2910450310. [DOI] [PubMed] [Google Scholar]
- 8.Heidenreich A., Bellmunt J., Bolla M., Joniau S., Mason M., Matveev V. EAU guidelines on prostate cancer. Part I: screening, diagnosis, and treatment of clinically localised disease. Actas Urol Esp. 2011;35:501–514. doi: 10.1016/j.acuro.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Moul J.W. The evolving definition of advanced prostate cancer. Rev Urol. 2004;6(suppl 8):S10–S17. [PMC free article] [PubMed] [Google Scholar]
- 10.Howlader N.N.A., Krapcho M., Neyman N., Aminou R., Waldron W., Altekruse S.F. National Cancer Institute; Bethesda, MD: April, 2012. SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations) [Google Scholar]
- 11.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 12.Salinas C.A., Tsodikov A., Ishak-Howard M., Cooney K.A. Prostate cancer in young men: an important clinical entity. Nat Rev Urol. 2014;11:317–323. doi: 10.1038/nrurol.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freedland S.J., Presti J.C., Jr., Kane C.J., Aronson W.J., Terris M.K., Dorey F. Do younger men have better biochemical outcomes after radical prostatectomy? Urology. 2004;63:518–522. doi: 10.1016/j.urology.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 14.Samadi D.B., Sebrow D., Hobbs A.R., Bernstein A.N., Brajtbord J., Lavery H.J. Clinicopathological, functional, and immediate oncologic outcome assessment in men aged ≤50 years with prostate cancer after robotic prostatectomy. Urol Oncol. 2017;35 doi: 10.1016/j.urolonc.2016.07.016. 30.e17–30.e24. [DOI] [PubMed] [Google Scholar]
- 15.Kinnear N.J., Kichenadasse G., Plagakis S., O'Callaghan M.E., Kopsaftis T., Walsh S. Prostate cancer in men aged less than 50 years at diagnosis. World J Urol. 2016;34:1533–1539. doi: 10.1007/s00345-016-1824-4. [DOI] [PubMed] [Google Scholar]
- 16.Loeb S., Hernandez D.J., Mangold L.A., Humphreys E.B., Agro M., Walsh P.C. Progression after radical prostatectomy for men in their thirties compared to older men. BJU Int. 2008;101:1503–1506. doi: 10.1111/j.1464-410X.2008.07500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin D.W., Porter M., Montgomery B. Treatment and survival outcomes in young men diagnosed with prostate cancer: a population-based cohort study. Cancer. 2009;115:2863–2871. doi: 10.1002/cncr.24324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker A., Tennstedt P., Hansen J., Trinh Q.D., Kluth L., Atassi N. Functional and oncological outcomes of patients aged <50 years treated with radical prostatectomy for localised prostate cancer in a European population. BJU Int. 2014;114:38–45. doi: 10.1111/bju.12407. [DOI] [PubMed] [Google Scholar]
- 19.Thorstenson A., Garmo H., Adolfsson J., Bratt O. Cancer specific mortality in men diagnosed with prostate cancer before age 50 years: a nationwide population based study. J Urol. 2017;197:61–66. doi: 10.1016/j.juro.2016.06.080. [DOI] [PubMed] [Google Scholar]
- 20.Carter B.S., Beaty T.H., Steinberg G.D., Childs B., Walsh P.C. Mendelian inheritance of familial prostate cancer. Proc Natl Acad Sci USA. 1992;89:3367–3371. doi: 10.1073/pnas.89.8.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lange E.M., Salinas C.A., Zuhlke K.A., Ray A.M., Wang Y., Lu Y. Early onset prostate cancer has a significant genetic component. Prostate. 2012;72:147–156. doi: 10.1002/pros.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haas G.P., Sakr W.A. Epidemiology of prostate cancer. CA Cancer J Clin. 1997;47:273–287. doi: 10.3322/canjclin.47.5.273. [DOI] [PubMed] [Google Scholar]
- 23.Cui J., Staples M.P., Hopper J.L., English D.R., McCredie M.R., Giles G.G. Segregation analyses of 1,476 population-based Australian families affected by prostate cancer. Am J Hum Genet. 2001;68:1207–1218. doi: 10.1086/320114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labanaris A.P., Zugor V., Witt J.H. Robotic-assisted radical prostatectomy in men ≤50 years of age: surgical, oncological and functional outcomes. Anticancer Res. 2012;32:2097–2101. [PubMed] [Google Scholar]