Abstract
In adult hematopoiesis, the hematopoietic stem cell (HSC) sits at the top of a hierarchy of hematopoietic progenitors responsible for generating the diverse repertoire of blood and immune cells. During embryonic development, however, the initial waves of hematopoiesis provide the first functioning blood cells of the developing embryo, such as primitive erythrocytes arising in the yolk sac, independently of HSC. In the field of developmental immunology, it has been recognized that some components of the immune system, such as B-1a lymphocytes, are uniquely produced during the embryonic and neonatal period, suggesting a “layered” development of immunity. Several recent studies have shed new light on the developmental origin of the layered immune system, suggesting complex and sometimes multiple contributions to unique populations of innate-like immune cells from both fetal HSC and earlier HSC-independent progenitors. In this review, we will attempt to synthesize these studies to provide an integrated model of developmental hematopoiesis and layered immunity that may offer new insight into the origin of HSC.
Keywords: Embryonic hematopoiesis, B-1a cell, innate immunity, hematopoietic stem cell (HSC), pre-HSC, yolk sac, aorta-gonad-mesonephros region (AGM), para-aortic splanchnopleura (P-Sp)
INTRODUCTION
Functionally defined, hematopoietic stem cells (HSC) have the unique ability to reconstitute the entire hematopoietic program of a conditioned adult recipient, which implies the capacity for homing and engraftment in the bone marrow niche, for life-long self-renewal, and for multilineage hematopoietic differentiation. During embryonic development, HSC arise from specialized hemogenic endothelium residing in arterial blood vessels in a process referred to as the endothelial to hematopoietic transition (EHT) (reviewed in (1)), before they undergo further maturation and expansion in the liver during the fetal period and finally home to the bone marrow. Complicating studies of the developmental ontogeny of HSC, embryonic hematopoiesis is characterized by unique waves of EHT giving rise to a series of transient progenitors prior to the generation of functional HSC, suggesting an HSC-independent origin in part serving to meet the immediate needs of the developing embryo. The initial waves exclusively emerge from extraembryonic yolk sac and are lacking in lymphoid potential. The earliest, primitive wave of hematopoiesis initiates by murine embryonic day E7.25 in the yolk sac blood islands, generating progenitors restricted to the production of primitive erythrocytes, megakaryocytes, and macrophages (2–5). Slightly thereafter by E8, erythromyeloid progenitors (EMPs) can be detected emerging within the blood vessels of the developing yolk sac, and give rise to definitive (adult-like) erythrocytes, megakaryocytes, and myeloid progeny that includes granulocytes in addition to macrophages (3, 6, 7).
The final wave of embryonic hematopoiesis, which is multilineage in that it encompasses lymphoid in addition to erythromyeloid potential, is thought to be responsible for generation of HSC. In contrast to the yolk sac-restricted primitive and EMP waves, this multilineage wave is detected from both extraembryonic and embryonic tissues. Freshly isolated cells that meet the functional definition of HSC by long-term, multilineage reconstitution following transplantation into immune-competent adult mice, are reliably detected only after about E11, in the aorta of the region known as the aorta-gonad-mesonephros (AGM) as well as in extraembryonic vessels including the vitelline and umbilical arteries (8–13). Counterintuitive to the concept that all multilineage progenitors are derived from HSC, studies over the last decades in search of the developmental origin of HSC have revealed the emergence of a complex array of progenitors with T and B lymphoid, lymphomyeloid, and multilineage potential that arise prior to detectable HSC (14–20). Furthermore, recent studies suggest that these early progenitors contribute to unique types of lymphocytes, such as B-1a B cells and certain subsets of γδ Τ-cells, which are primarily generated during the embryonic to neonatal period (15, 21–23). This has raised important questions about the developmental relationships of these early progenitors to later HSC. Do some of these early progenitors represent waves of hematopoiesis that, like primitive progenitors and EMP in the yolk sac, arise from distinct populations of hemogenic endothelium independently of HSC? Alternatively, do they arise from a common pool of HSC precursors, which are not yet competent to provide long-term multilineage engraftment in transplantation assays but are able to produce multilineage progeny including lymphoid cells in the early embryo? Finally, how do these early progenitors and subsequent fetal HSC differentially contribute to the unique lineages of fetal lymphocytes that constitute the layered immune model initially proposed by Herzenberg and Herzenberg (24–26)? Recent studies employing novel genetic models, lineage tracing, and clonal analysis, have provided new evidence to suggest increasing complexity in the emergence of HSC-independent progenitors and subsequent fetal HSC, and their relative contribution to “layered” immunity during development. Furthermore, these studies have challenged the dogma that HSC are the sole source of all hematopoietic lineages in the adult. The objective of this review is to summarize these recent advances and to offer a perspective on their implications for our current understanding of the origin of HSC.
Not all blood cells are the product of HSC: insights from development and evolution
Nishikawa et al. (27) proposed the concept of acaulescent (HSC-independent) hematopoiesis, where hematopoietic progenitor cells prior to the emergence of transplantable HSC are produced without stem cells. From a developmental perspective, the ability of the early embryo to generate functional blood cells by direct differentiation from mesodermal precursors, bypassing an intermediate HSC stage, is necessary to meet the immediate needs of the rapidly developing embryo. From an evolutionary perspective, the earliest waves of hematopoiesis recapitulate the formation of hematopoietic-like cells, such as amoebocytes, observed in some invertebrate organisms that lack an HSC equivalent. In this light, the existence of sequential “layers” of HSC-independent blood cell development in the early vertebrate embryo prior to HSC genesis is not surprising; however, our recognition of the complexity of layered hematopoiesis preceding HSC development in this regard has continued to evolve, and the contribution of these HSC-independent waves to some self-maintaining lineages of innate immune-like cells in the adult has gained recent attention.
HSC-independent primitive macrophages and EMP: Origin of tissue-resident macrophages
The first detectable hematopoietic progenitors during embryonic development arise in the yolk sac by mid primitive streak stage at E7.25 (2, 3). Referred to as primitive hematopoiesis, this initial wave produces primitive erythrocytes, megakaryocytes, and macrophages (3–5). The primitive erythrocytes, a transient population of initially nucleated erythrocytes expressing embryonic forms of globin, rapidly differentiate to form the first circulating blood cells of the developing embryo upon the initiation of circulation that connects the yolk sac vasculature to the embryo proper at around E8.25, providing for oxygen transport in the developing embryo. Primitive megakaryocytes, which share a common progenitor with primitive erythrocytes based on clonal colony-forming assays, provide the first circulating platelets for the embryo (5). Primitive macrophage progenitors, though detected concomitantly with primitive erythroid and megakaryocytes in the murine yolk sac, do not appear to share a common progenitor with the other primitive lineages based on clonal colony-forming assays. During embryonic development, macrophages play a central role in tissue remodeling, supporting erythrocyte maturation in the fetal liver, and instructing neuronal and vascular development (28–32).
A second wave of hematopoiesis arises in the yolk sac by E8, overlapping with the primitive wave but giving rise to distinct progenitors considered “definitive” based on their ability to generate adult-like erythrocytes with distinct globin expression patterns (6). This erythromyeloid progenitor (EMP) wave gives rise to clonal progenitors with erythroid, megakaryocyte and myeloid potential but not significant lymphoid potential, and can produce adult-like erythrocytes transiently upon transplantation (7). In contrast to the primitive wave, the EMP wave also has a broader myeloid potential that includes granulocyte in addition to macrophage production. Studies in Ncx1-deficient embryos, which lack a heartbeat and thus functional circulation, confirm that, like primitive hematopoiesis, the origin of EMP is restricted to the yolk sac, arising from hemogenic endothelium in both venous and arterial vessels of the developing yolk sac vasculature (33, 34). Lack of dependence on Notch signaling and blood flow (both of which are required for HSC production), as well as distinct temporal and spatial requirements for CBFβ, support the concept that EMPs arise from a separate population of hemogenic endothelial cells than those later giving rise to HSC in arterial vessels (33, 35–38).
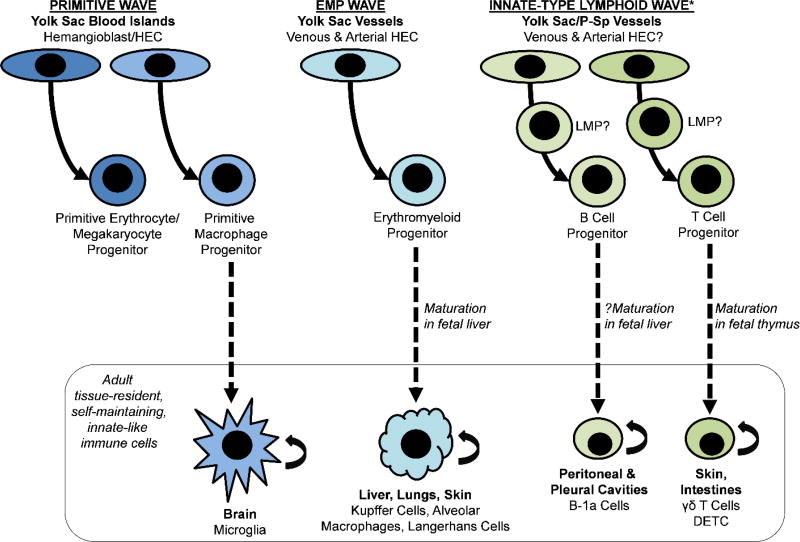
The primitive and EMP waves of yolk sac hematopoiesis give rise primarily to transient hematopoietic progeny that have unique and essential roles restricted to the developing embryo. However, recent lineage tracing studies have demonstrated the persistence of populations of tissue-resident macrophages derived from these waves of embryonic hematopoiesis in some adult tissues, including contribution of primitive macrophages to the microglia of the adult brain and EMP-derived macrophages to the Kupffer cells of the liver, alveolar macrophages of the lungs, and Langerhans cells of the epidermis. The distinct properties of these embryonic-derived tissue-resident macrophages, and the extent to which they can be replenished by HSC-derived monocytes in the setting of tissue injury, is the subject of much recent research given the essential functions of these unique populations in tissue homeostasis, innate immunity, and disease (39–42) (for current review, refer to (43)). Importantly, these studies have challenged the central dogma of HSC as the source of all hematopoietic lineages in the adult, providing evidence for maintenance and self-renewal of hematopoietic lineages from embryonic “layers” of developmental hematopoiesis independent of HSC (44) (Figure 1).
Figure 1. HSC-independent waves of embryonic hematopoiesis contribute to self-maintaining, tissue-resident populations of innate-type immune cells in adult tissues.
This includes microglia in the adult brain derived from primitive macrophage progenitors, tissue-resident macrophages (Kupffer cells, alveolar macrophages, and Langerhans cells) of the liver, lungs, and skin derived from erythromyeloid progenitors (EMP), some B-1a cells residing in the peritoneal and pleural cavities derived from embryonic B cell progenitors and/or lymphomyeloid progenitors (LMP), and some γδ T cells, including the subset of dendritic epidermal T cells (DETC), in skin and intestines derived from embryonic T cell progenitors and/or LMP. *It remains uncertain as to whether the earliest innate-type lymphoid wave is derived from the same lineage of hemogenic endothelium that later gives rise to HSC and/or from a distinct lineage of hemogenic endothelium (see Figure 2).
HSC-independent T lymphocytes: Origin of the first thymic pioneers and tissue-resident T cells
During embryogenesis, the thymic anlage develops from bilateral organ primordia at around E11 (45) at which time it is seeded with the earliest lymphoid progenitors (46–48). Consistent with the layered immune hypothesis, different waves of T cells carrying distinct T cell antigen receptors originate in the fetal thymus during development (49), some of which are uniquely produced from stem/progenitor cells in the embryonic and fetal periods, but not from adult HSC. These include certain subsets of γδ T cells, which ultimately reside largely in peripheral tissues such as gut and skin and bear restricted T cell receptors thought to function in first line defense to foreign antigens (for review see (50)). One γδ T cell subset, TCR-Vγ3 T cells (also known as dendritic epidermal T cells), functions uniquely in immune responses in the epidermis and can be produced only by stem/progenitor cells during embryonic and fetal development (23, 51). The distinct T lymphopoietic potentials of fetal and adult HSC/progenitors appear to be in part genetically controlled. For example, Lin28b, a negative regulator of the let-7 family of microRNA, is expressed in fetal HSC/progenitors but not in adult HSC. When overexpressed in adult bone marrow stem/progenitor cells, Lin28b can restore aspects of fetal lymphopoiesis, including efficient production of B-1a and γδ T cells (though not Vγ3 T cells) following transplantation (52).
Although there is disagreement as to whether the first progenitors colonizing the thymic anlage at E11 are lymphomyeloid progenitors or T cell-restricted progenitors (46–48), there is common speculation that these initial progenitors must be derived independently of HSC, since there are only 2–3 HSCs in the whole embryo at this stage (8). Consistent with an HSC-independent origin, progenitors with T cell potential have been detected prior to HSC emergence by numerous groups (18–20, 53, 54). Analysis in mice lacking functional circulation (Ncx1-deficient mice) confirmed T cell potential from both the yolk sac and embryo proper, including αβ T cells, ƴδ T cells, and Vγ3 T cells (23). Furthermore, this study detected T cell potential from E9.5 yolk sac progenitors as assayed following direct transplantation into immune-deficient mice, as well as T cell potential from both yolk sac and P-Sp following in vitro co-culture from cells phenotypically isolated as hemogenic endothelium (VE-Cadherin+CD41−), suggesting that autonomous generation of T cell progenitors by EHT may initially occur independently of HSC to provide the first progenitors colonizing the thymus (46, 47). Whether these early T cell progenitors share a common lineage of hemogenic endothelium with later HSC, or arise from distinct populations of hemogenic endothelium similar to the primitive and EMP waves, remains to be determined.
HSC-independent B lymphocytes: Origin of tissue-resident B1-a cells
The observation that fetal and adult progenitors give rise to B cells with distinct properties led to the initial proposition of the “layered” immune system hypothesis several decades ago (24, 25). Progenitors from the fetal liver are biased toward the production of a unique population of CD5+ B-cells, now referred to as B-1a cells, that reside mainly in peritoneal and pleural cavities and secrete natural IgM antibodies in a T cell-independent manner, contributing to innate-like immunity (26, 55). In contrast, stem/progenitor cells from the adult bone marrow primarily contribute to conventional B cells (B-2 lymphocytes) that constitute part of the adaptive immune system generating antigen-specific antibodies in a T cell-dependent manner. Until the seminal discovery of distinct B-1 specific progenitor cells in the fetal liver and fetal bone marrow (56, 57), it had long been debated whether B-1 and B-2 cells are derived from a common lymphocyte progenitor that differentiates depending on specific antigen exposures or they arise from separate progenitors. It is now generally recognized that B-1a cells are developmentally distinct from B-2 cells, but identifying the precise origin for B-1a progenitor cells during embryonic development has been challenging. Previous studies demonstrated the existence of unique progenitors with B-1, but not B-2, lymphocyte potential derived from yolk sac and P-Sp as early as E8.5, prior to HSC emergence (22). Furthermore, detection of these B-1 specific progenitors in HSC-deficient embryos, and their serial engraftment properties demonstrated by peritoneal transplantation in vivo, suggested an HSC-independent pathway of B-1a cell development (21). Parallel evidence from clonal transplantation studies of highly purified adult bone marrow HSC, and more recently from a study using inducible genetic labelling of HSC in situ, confirmed that adult HSC lack efficient contribution to the peritoneal B-1a compartment following transplantation or under physiologic conditions (58, 59), suggesting that B-1a cells may originate entirely independent of HSC.
Several studies examining B-1a cell production by fetal liver HSCs using different approaches, however, hint at an additional layer of complexity in the origin of B-1a cells, demonstrating that they can also be derived from at least a subset of fetal HSC (60, 61). Beaudin et al. (60) used a lineage reporter mouse, in which cells activating Flk2 expression are irreversibly labeled with GFP whereas those not having expressed Flk2 remain Tomato positive (Tom+), to define two distinct populations of fetal HSC based on history of Flk2 expression. The authors identified a “developmentally-restricted” Flk2+(GFP+) HSC that can provide long-term, multilineage and secondary engraftment in transplantation assays, thus meeting the functional definition of HSC. However, these Flk2+(GFP+) HSC displayed unique properties including lymphoid-biased engraftment and relative skewed contribution toward B-1a verses B-2 B cells in the peritoneum, and strikingly, they failed to significantly contribute to the long-term multilineage HSC that populate the bone marrow when traced into adult development in situ. In contrast, Flk2−(Tom+) HSC contributed to long-term HSC in the adult marrow and had lower B-1a contribution in transplantation assays. A separate group recently reported the use of barcoding technology to label fetal liver stage stem/progenitor cells (61). In this study, HSC were defined functionally by their clonal contribution to multilineage hematopoiesis (including short lived granulocytes) following transplantation into congenic strain mice. Detection of B-1a cell, B-2 cell, and granulocyte contribution in the majority of long-term engrafting clones sharing a common barcode, including those shown to provide robust secondary engraftment, confirmed a common origin for B-1a and B-2 cells in functionally defined fetal liver stage HSC. Loss of efficient B-1a cell engraftment following serial engraftment demonstrated that, over time, fetal liver HSC come to resemble adult HSC in this regard. In this study, direct transplantation of sorted, phenotypically defined E14.5 fetal liver HSC (LSK CD150+CD48−FLT3−) also confirmed a common clonal origin for peritoneal B-1a and B-2 cells at this stage, albeit only 2 of 40 mice transplanted were engrafted in this analysis. This is consistent with a prior study by Barber et al. in which neonatal (but not adult) HSC (sorted as LSK CD150+) could contribute to peritoneal B-1a engraftment, although a common clonal origin for B-1a and B-2 cells could not be confirmed as this study was based on transplantation of sorted populations of cells (62). In contrast, a recent study which incorporated transplantation of highly purified E15 fetal liver HSC (LSK CD150+CD48−CD45+CD41−) failed to demonstrate significant B-1a cell contribution (63). While further investigations will be needed to reconcile these studies, differences in the stages analyzed or gating strategies for isolating HSC could potentially account for differences in the functional properties of HSC in these studies (for further discussion on this topic, also refer to (64)).
Combined, these studies suggest heterogeneity in the fetal liver HSC pool, with biased contribution to B-1a cells from “developmentally-restricted” HSC relative to fetal HSC, which initially have limited B-1a potential that is lost over time as this pool expands to supply the HSC in the adult bone marrow. However, these studies do not address the lineage relationships of the various fetal liver stem/progenitor cells that differentially contribute to the B-1a cell compartment; specifically, do these cells have distinct origins or do they share a common precursor in early embryonic development? To address this question, we tested the B-1a cell potential of HSC precursors (pre-HSC) at the single cell level (65). We isolated and co-cultured single E9.5 to E11.5 pre-HSC from the P-Sp/AGM region with Akt-expressing AGM-derived endothelial cell (AGM-EC), which can promote their maturation to functional, engrafting HSC (66), and transplanted the clonal progeny into lethally irradiated congenic mice. Interestingly, all engrafted recipient mice displayed both B-1a and B-2 cell engraftment, indicating the presence of common precursors for B-1a and B-2 cells in the E9.5 to E11.5 P-Sp/AGM. This included clones that generated multilineage engraftment in primary and secondary transplants, suggesting a common precursor for B-1a and B-2 cells at the level of the pre-HSC. Interestingly, we noted a relative bias of clones derived from earlier time points (prior to E11) toward B-1a verses B-2 cell contribution in the peritoneum compared to later clones. This suggests that heterogeneity in the fetal liver HSC pool may originate as early as the pre-HSC stage, as most pre-HSC from the P-Sp/AGM are believed to seed the fetal liver before undergoing further maturation to HSC (67, 68). Based on our previous observation that YS/P-Sp-derived progenitors isolated prior to HSC emergence can also produce B-1a, but not B-2, cells following OP9 co-culture, it is also possible that this separate population of hemogenic precursors, independent of those that give rise to HSC, also contributes to B-1a progenitors in the fetal liver, consistent with their detection in the fetal liver of HSC-deficient Cbfβ−/−:Tek-GFP/Cbfβ embryos (21, 22). Regardless, these studies altogether support the existence of multiple origins, including HSC-independent and HSC-derived lineages, for B-1a cells during embryonic and fetal development. Determining the relative contributions of the HSC-independent YS/P-Sp B cell progenitors and pre-HSC/fetal HSC to tissue-resident B-1a cell compartments throughout development and in the adult will require lineage tracing studies that can uniquely mark HSC-independent B cell progenitors separate from HSC.
LMP, MPP, and pre-HSC: tracing the origin of multilineage hematopoiesis prior to HSC emergence
The above studies demonstrate the emergence of hematopoietic populations with T and B lymphoid potential from the yolk sac and P-Sp prior to and independent of HSC. However, distinguishing whether lineage-restricted B and T cells can emerge directly from hemogenic endothelium or rather transit through progenitors with multilineage or oligolineage potential requires clonal assays and lineage tracing studies. A recent report combining these approaches utilized a Rag1-GFP reporter mouse to identify a clonal Rag1-GFP+IL7Ra+ progenitor with restricted lymphoid and myeloid potential in the embryonic yolk sac and fetal liver (15). Lineage tracing confirmed the in vivo contribution of this lymphomyeloid progenitor (LMP) to fetal lymphocytes and myeloid cells, but not erythrocytes or megakaryocytes. Transcriptional analysis also demonstrated expression of lymphoid and myeloid-associated genes with concomitant downregulation of erythroid and megakaryocytes programs. Altogether, this study convincingly demonstrates the existence of an LMP-restricted progenitor in embryonic development arising prior to HSC, suggesting that at least in part B and T lymphocyte progenitors detected in the early yolk sac/P-Sp may transit through an LMP intermediate. However, although Rag1-GFP+IL7Ra+ progenitors with transcriptional profiles suggestive of LMP were detected as early as E9.5 in the yolk sac, the clonal in vitro lymphoid and myeloid potential of these progenitors at E9.5 was not assessed. Moreover, E9.5 yolk sac Rag1-GFP+IL7Ra+ progenitors were found to already express CD45 at this stage, in contrast to B and T lymphoid precursors previously described in the E9.5 yolk sac (21–23), thus the relationship of these various early yolk sac-derived progenitor populations requires further investigation.
Another recent study also identified clonal progenitors with true multilineage potential at a similar stage in development (14). Inlay and colleagues developed an in vitro assay for multipotency using an OP9 stroma with inducible expression of Notch ligand Delta1, which could simultaneously support the differentiation of erythroid, myeloid, megakaryocyte, B lymphocyte, and T lymphocyte fates from single cells. By this approach, the authors identified a phenotypically-defined population of clonal multipotent progenitors (MPP) that are detected simultaneously in the yolk sac and P-Sp/AGM as early as E9.5, although in far greater numbers from the yolk sac between E9.5 and E11.5. While cells isolated at E11.5 based on the identified MPP phenotype could contribute to multilineage hematopoiesis upon transplantation to adult immune-deficient recipients in this study, the relationship of the first emerging MPP at E9.5 and later HSC that can engraft immune-competent adult mice after E11, was not determined.
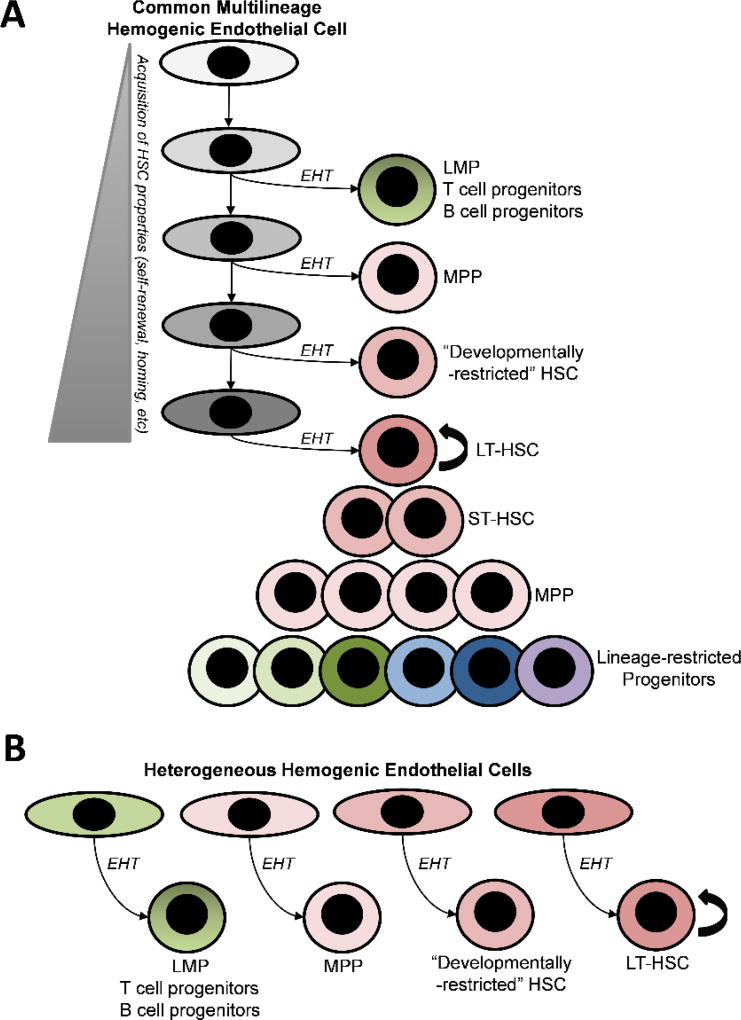
Although these studies together provide clear evidence for the emergence of progenitors with T and B lymphoid, LMP, and MPP activity prior to the emergence of HSC, the lineage relationship of these early multilineage progenitors to the precursors that eventually give rise to HSC remains obscure. Do they originate from a common pool of hemogenic endothelium or, like primitive progenitors and EMP, do they represent separate waves of hematopoiesis arising from lineages of hemogenic endothelial precursors distinct from those that give rise to HSC? Addressing this question highlights a critical challenge in the understanding of HSC ontogeny, which is that detection of HSC is based on a functional definition of long-term (or more stringently, serial) multilineage engraftability in adult immune-competent mice. Thus, in search of the developmental origin of HSC, a variety of surrogate assays have been utilized to identify HSC potential prior to detection of HSC that meet this functional definition. These include ex vivo maturation of HSC precursors (pre-HSC) to engrafting HSC by whole organ, re-aggregation, or stromal co-culture or in vivo transplantation assays using newborn or immune-deficient recipient mice that enable multilineage engraftment from hematopoietic precursor cells at earlier stages of development (54, 66, 68–74). Generally, these studies suggest that detection of pre-HSC activity coincides temporally with lymphoid/multilineage progenitor activity (17, 54, 66, 68–70, 75), which is also consistent with lineage tracing studies that genetically mark HSC precursors during this same window of development prior to the detection of transplantable HSC (76). However, those studies which have examined quantitative pre-HSC activity by clonal analysis or limiting dilution assays have found that initial pre-HSC activity prior to E10 is exceedingly rare (65, 67, 71), at most one per embryo equivalent, thus insufficient to account for T/B progenitor, LMP, and MPP activity during this same stage of development, although it cannot be ruled out that inefficiencies in the assays for pre-HSC may account for this discrepancy. Moreover, whereas the majority of LMP and MPP activity is quantitatively detected initially in the yolk sac, the pre-HSC activity which quantitatively accounts for the first wave of HSC in the fetal liver is detected predominantly in the AGM (67). Together, these studies thus suggest that initial lymphoid/LMP/MPP activity is not necessarily derived entirely from the same lineage of hemogenic endothelium that gives rise to HSC (Figure 2A), but may also be derived by direct differentiation from a heterogeneous population of hemogenic endothelium that in some cases may have more restricted hematopoietic potential (Figure 2B). Consistent with this, a recent clonal study of hemogenic endothelial precursors from the E9.5 to 11.5 P-Sp/AGM revealed significant heterogeneity in hematopoietic potential assessed by stromal co-culture (77). This heterogeneity may result from cell autonomous properties dependent on developmental ontogeny as well as from non-autonomous signals dependent on the niche microenvironment (egs, yolk sac verses P-Sp/AGM, dorso-ventral positioning in the aorta). Notably, this model, which provides for separate hemogenic endothelium with lymphoid/multilineage progenitor potential from hemogenic endothelium with pre-HSC/HSC potential, would also account for the ability to generate multiple hematopoietic lineages, including B and T cells, from embryonic stem cells using current protocols that have not generated detectable pre-HSC/HSC activity. Finally, this model could also account for the origin of HSC heterogeneity recently described in the fetal liver (60, 61), suggesting that unique populations of HSC with different self-renewal potentials and lineage biases (such as the “developmentally-restricted” fetal HSC described by Beaudin et al. (60)) could arise from distinct populations or waves of hemogenic endothelium. Supporting this concept, our clonal studies uncovered heterogeneity in functional aspects of pre-HSC, including their lineage potential and self-renewal capacity, for example revealing rare clones that can give rise to as many as several hundred long-term engrafting HSC following maturation and expansion in the AGM EC niche (65). A recent study using a novel technology that enabled tamoxifen-inducible DNA barcoding in E9.5 Tie2+ hemogenic precursors similarly revealed heterogeneous clonal contribution to adult hematopoiesis from barcoded precursors, some of which gave rise to several hundred HSC in the adult marrow pool (78). Thus, heterogeneity in hematopoietic potential may be inherent to hemogenic endothelium/pre-HSC at their inception, suggesting distinct waves of embryonic hematopoiesis may also contribute to a “layered” development of multilineage hematopoiesis and HSC genesis.
Figure 2. Models for the origin of multilineage progenitors that precede HSC during embryonic development.
Acaulescent (HSC-independent) progenitors, as originally proposed by Nishikawa et al. (27), can either originate from a common lineage of hemogenic endothelial cells that have the capacity to give rise to HSC (A) or from distinct lineages of hemogenic endothelial cells that are heterogeneous in their hematopoietic potential (B). In the first model, hemogenic endothelial cells with multilineage potential undergo developmental maturation to acquire additional properties necessary to generate long-term HSC (LT-HSC). Prior to full maturation, a hemogenic endothelial cell that undergoes endothelial to hematopoietic transition (EHT) would give rise to HSC-independent hematopoietic progeny. In the second model, lineages of hemogenic endothelial cells with distinct hematopoietic potentials originate from mesoderm in separate temporal or spatial waves, similar to those giving rise to primitive progenitors and EMP, thus producing different populations of HSC-independent progenitors or HSC. LMP (lymphomyeloid progenitor), MPP (multipotent progenitor), ST-HSC (short-term HSC).
Future directions
Building on foundational studies over the past decades which have explored the unique aspects of embryonic hematopoiesis, numerous recent studies discussed herein (and several others not included due to space constraints), employing a variety of novel strategies including fate mapping and clonal analysis, have revealed increasing complexity in the many layers of developmental hematopoiesis. These studies have provided new insights into HSC heterogeneity in the fetal liver, revealed both HSC-dependent and independent origins for some innate-like immune cells, such as B-1a lymphocytes, and identified persistence of some embryonic-derived, HSC-independent hematopoietic lineages in adult tissues. Rapidly advancing technologies in lineage tracing, such as use of genome editing to introduce barcodes over successive rounds of cell divisions (79), combined with high-throughput single cell transcriptional and epigenetic analysis (80, 81) should permit the comprehensive mapping of the lineage ancestry and transcriptional machinery regulating successive layers of embryonic hematopoiesis and culminating in the adult HSC. This advance would be transformative, for example revealing the precise contributions of HSC-independent waves to critical populations of immune cells in the adult, and identifying current barriers to efficient generation of long-term repopulating HSC from pluripotent stem cells. Ultimately, such knowledge could lead to novel therapeutic applications in disease modeling, gene editing, and cellular therapies for advancing treatments of hematologic and immunologic disorders.
HIGHLIGHTS.
During embryonic development, initial hematopoietic progenitors are generated from hemogenic endothelium in multiple waves independently of HSC.
Recent studies highlight the contribution of embryonic waves of HSC-independent hematopoiesis to unique innate-like immune cells that can persist as self-maintaining hematopoietic populations in adult tissues.
Recent studies suggest that distinct waves of hemogenic endothelium/HSC precursors may contribute to the production of heterogeneous populations of HSC in the fetal liver with distinct self-renewal properties and B cell lineage potentials.
Acknowledgments
We would like to acknowledge support from Alex’s Lemonade Stand Foundation and Hyundai Hope on Wheels Foundation (Brandon Hadland) and NIAID R01 AI121197 (Momoko Yoshimoto). This work was also supported by the National Institutes of Health NHLBI UO1 Grant #HL100395 and Ancillary Collaborative Grant #HL099997.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST DISCLOSURE
The authors declare no conflicts of interest pertaining to this manuscript.
References
- 1.Klaus A, Robin C. Embryonic hematopoiesis under microscopic observation. Dev Biol. 2017;428(2):318–27. doi: 10.1016/j.ydbio.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Moore MA, Metcalf D. Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony forming cells in the developing mouse embryo. Br J Haematol. 1970;18(3):279–96. doi: 10.1111/j.1365-2141.1970.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 3.Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126(22):5073–84. doi: 10.1242/dev.126.22.5073. Epub 1999/10/26. [DOI] [PubMed] [Google Scholar]
- 4.Xu MJ, Matsuoka S, Yang FC, Ebihara Y, Manabe A, Tanaka R, Eguchi M, Asano S, Nakahata T, Tsuji K. Evidence for the presence of murine primitive megakaryocytopoiesis in the early yolk sac. Blood. 2001;97(7):2016–22. doi: 10.1182/blood.v97.7.2016. [DOI] [PubMed] [Google Scholar]
- 5.Tober J, Koniski A, McGrath KE, Vemishetti R, Emerson R, de Mesy-Bentley KK, Waugh R, Palis J. The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis. Blood. 2007;109(4):1433–41. doi: 10.1182/blood-2006-06-031898. Epub 2006/10/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGrath KE, Frame JM, Fromm GJ, Koniski AD, Kingsley PD, Little J, Bulger M, Palis J. A transient definitive erythroid lineage with unique regulation of the β-globin locus in the mammalian embryo. Blood. 2011;117(17):4600–8. doi: 10.1182/blood-2010-12-325357. Epub 2011/03/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGrath KE, Frame JM, Fegan KH, Bowen JR, Conway SJ, Catherman SC, Kingsley PD, Koniski AD, Palis J. Distinct Sources of Hematopoietic Progenitors Emerge before HSCs and Provide Functional Blood Cells in the Mammalian Embryo. Cell Rep. 2015;11(12):1892–904. doi: 10.1016/j.celrep.2015.05.036. Epub 2015/06/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumaravelu P, Hook L, Morrison AM, Ure J, Zhao S, Zuyev S, Ansell J, Medvinsky A. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development. 2002;129(21):4891–9. doi: 10.1242/dev.129.21.4891. Epub 2002/10/25. [DOI] [PubMed] [Google Scholar]
- 9.Muller AM, Medvinsky A, Strouboulis J, Grosveld F, Dzierzak E. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1(4):291–301. doi: 10.1016/1074-7613(94)90081-7. doi: 1074-7613(94)90081-7 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Taoudi S, Morrison AM, Inoue H, Gribi R, Ure J, Medvinsky A. Progressive divergence of definitive haematopoietic stem cells from the endothelial compartment does not depend on contact with the foetal liver. Development. 2005;132(18):4179–91. doi: 10.1242/dev.01974. Epub 2005/08/17. [DOI] [PubMed] [Google Scholar]
- 11.Taylor E, Taoudi S, Medvinsky A. Hematopoietic stem cell activity in the aorta-gonad-mesonephros region enhances after mid-day 11 of mouse development. Int J Dev Biol. 2010;54(6–7):1055–60. doi: 10.1387/ijdb.103152et. [DOI] [PubMed] [Google Scholar]
- 12.Gordon-Keylock S, Sobiesiak M, Rybtsov S, Moore K, Medvinsky A. Mouse extraembryonic arterial vessels harbor precursors capable of maturing into definitive HSCs. Blood. 2013;122(14):2338–45. doi: 10.1182/blood-2012-12-470971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Bruijn MF, Speck NA, Peeters MC, Dzierzak E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J. 2000;19(11):2465–74. doi: 10.1093/emboj/19.11.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inlay MA, Serwold T, Mosley A, Fathman JW, Dimov IK, Seita J, Weissman IL. Identification of Multipotent Progenitors that Emerge Prior to Hematopoietic Stem Cells in Embryonic Development. Stem Cell Reports. 2014;2(4):457–72. doi: 10.1016/j.stemcr.2014.02.001. Epub 2014/04/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boiers C, Carrelha J, Lutteropp M, Luc S, Green JC, Azzoni E, Woll PS, Mead AJ, Hultquist A, Swiers G, Perdiguero EG, Macaulay IC, Melchiori L, Luis TC, Kharazi S, Bouriez-Jones T, Deng Q, Ponten A, Atkinson D, Jensen CT, Sitnicka E, Geissmann F, Godin I, Sandberg R, de Bruijn MF, Jacobsen SE. Lymphomyeloid contribution of an immune-restricted progenitor emerging prior to definitive hematopoietic stem cells. Cell Stem Cell. 2013;13(5):535–48. doi: 10.1016/j.stem.2013.08.012. Epub 2013/09/24. [DOI] [PubMed] [Google Scholar]
- 16.Godin I, Dieterlen-Lièvre F, Cumano A. B-lymphoid potential in pre-liver mouse embryo. Semin Immunol. 1995;7(3):131–41. doi: 10.1016/1044-5323(95)90041-1. [DOI] [PubMed] [Google Scholar]
- 17.Cumano A, Dieterlen-Lievre F, Godin I. Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura. Cell. 1996;86(6):907–16. doi: 10.1016/s0092-8674(00)80166-x. Epub 1996/09/20. doi: S0092-8674(00)80166-X [pii] [DOI] [PubMed] [Google Scholar]
- 18.Godin I, Dieterlen-Lievre F, Cumano A. Emergence of multipotent hemopoietic cells in the yolk sac and paraaortic splanchnopleura in mouse embryos, beginning at 8.5 days postcoitus. Proc Natl Acad Sci U S A. 1995;92(3):773–7. doi: 10.1073/pnas.92.3.773. Epub 1995/01/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu CP, Auerbach R. In vitro development of murine T cells from prethymic and preliver embryonic yolk sac hematopoietic stem cells. Development. 1991;113(4):1315–23. doi: 10.1242/dev.113.4.1315. [DOI] [PubMed] [Google Scholar]
- 20.Ohmura K, Kawamoto H, Fujimoto S, Ozaki S, Nakao K, Katsura Y. Emergence of T, B, and myeloid lineage-committed as well as multipotent hemopoietic progenitors in the aorta-gonad-mesonephros region of day 10 fetuses of the mouse. J Immunol. 1999;163(9):4788–95. [PubMed] [Google Scholar]
- 21.Kobayashi M, Shelley WC, Seo W, Vemula S, Lin Y, Liu Y, Kapur R, Taniuchi I, Yoshimoto M. Functional B-1 progenitor cells are present in the hematopoietic stem cell-deficient embryo and depend on Cbfβ for their development. Proc Natl Acad Sci U S A. 2014;111(33):12151–6. doi: 10.1073/pnas.1407370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshimoto M, Montecino-Rodriguez E, Ferkowicz MJ, Porayette P, Shelley WC, Conway SJ, Dorshkind K, Yoder MC. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc Natl Acad Sci U S A. 2011;108(4):1468–73. doi: 10.1073/pnas.1015841108. Epub 2011/01/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshimoto M, Porayette P, Glosson NL, Conway SJ, Carlesso N, Cardoso AA, Kaplan MH, Yoder MC. Autonomous murine T-cell progenitor production in the extra-embryonic yolk sac before HSC emergence. Blood. 2012;119(24):5706–14. doi: 10.1182/blood-2011-12-397489. Epub 2012/03/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herzenberg LA. Toward a layered immune system. Cell. 1989;59(6):953–4. doi: 10.1016/0092-8674(89)90748-4. Epub 1989/12/22. [DOI] [PubMed] [Google Scholar]
- 25.Herzenberg LA, Kantor AB. Layered evolution in the immune system. A model for the ontogeny and development of multiple lymphocyte lineages. Ann N Y Acad Sci. 1992;651:1–9. doi: 10.1111/j.1749-6632.1992.tb24588.x. [DOI] [PubMed] [Google Scholar]
- 26.Herzenberg LA. Layered evolution in the immune system: a view from history. Ann N Y Acad Sci. 2015;1362:1–5. doi: 10.1111/nyas.12795. Epub 2015/06/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishikawa SI, Fraser S, Fujimoto T, Endoh M, Nishikawa S, Ogawa M. All B cells are progeny of endothelial cells: a new perspective. Immunol Rev. 2000;175:112–9. Epub 2000/08/10. [PubMed] [Google Scholar]
- 28.Hopkinson-Woolley J, Hughes D, Gordon S, Martin P. Macrophage recruitment during limb development and wound healing in the embryonic and foetal mouse. J Cell Sci. 1994;107(Pt 5):1159–67. doi: 10.1242/jcs.107.5.1159. [DOI] [PubMed] [Google Scholar]
- 29.DeFalco T, Bhattacharya I, Williams AV, Sams DM, Capel B. Yolk-sac-derived macrophages regulate fetal testis vascularization and morphogenesis. Proc Natl Acad Sci U S A. 2014;111(23):E2384–93. doi: 10.1073/pnas.1400057111. Epub 2014/05/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Squarzoni P, Oller G, Hoeffel G, Pont-Lezica L, Rostaing P, Low D, Bessis A, Ginhoux F, Garel S. Microglia modulate wiring of the embryonic forebrain. Cell Rep. 2014;8(5):1271–9. doi: 10.1016/j.celrep.2014.07.042. Epub 2014/08/21. [DOI] [PubMed] [Google Scholar]
- 31.Tong CK, Vidyadaran S. Role of microglia in embryonic neurogenesis. Exp Biol Med (Maywood) 2016;241(15):1669–75. doi: 10.1177/1535370216664430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palis J. Interaction of the Macrophage and Primitive Erythroid Lineages in the Mammalian Embryo. Front Immunol. 2016;7:669. doi: 10.3389/fimmu.2016.00669. Epub 2017/01/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frame JM, Fegan KH, Conway SJ, McGrath KE, Palis J. Definitive Hematopoiesis in the Yolk Sac Emerges from Wnt-Responsive Hemogenic Endothelium Independently of Circulation and Arterial Identity. Stem Cells. 2016;34(2):431–44. doi: 10.1002/stem.2213. Epub 2015/10/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lux CT, Yoshimoto M, McGrath K, Conway SJ, Palis J, Yoder MC. All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac. Blood. 2008;111(7):3435–8. doi: 10.1182/blood-2007-08-107086. Epub 2007/10/13. doi: blood-2007-08-107086 [pii] 10.1182/blood-2007-08-107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hadland BK, Huppert SS, Kanungo J, Xue Y, Jiang R, Gridley T, Conlon RA, Cheng AM, Kopan R, Longmore GD. A requirement for Notch1 distinguishes 2 phases of definitive hematopoiesis during development. Blood. 2004;104(10):3097–105. doi: 10.1182/blood-2004-03-1224. Epub 2004/07/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumano K, Chiba S, Kunisato A, Sata M, Saito T, Nakagami-Yamaguchi E, Yamaguchi T, Masuda S, Shimizu K, Takahashi T, Ogawa S, Hamada Y, Hirai H. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity. 2003;18(5):699–711. doi: 10.1016/s1074-7613(03)00117-1. Epub 2003/05/20. [DOI] [PubMed] [Google Scholar]
- 37.Bertrand JY, Cisson JL, Stachura DL, Traver D. Notch signaling distinguishes 2 waves of definitive hematopoiesis in the zebrafish embryo. Blood. 2010;115(14):2777–83. doi: 10.1182/blood-2009-09-244590. Epub 2010/01/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen MJ, Li Y, De Obaldia ME, Yang Q, Yzaguirre AD, Yamada-Inagawa T, Vink CS, Bhandoola A, Dzierzak E, Speck NA. Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell stem cell. 2011;9(6):541–52. doi: 10.1016/j.stem.2011.10.003. Epub 2011/12/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–5. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, Leboeuf M, Low D, Oller G, Almeida F, Choy SH, Grisotto M, Renia L, Conway SJ, Stanley ER, Chan JK, Ng LG, Samokhvalov IM, Merad M, Ginhoux F. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J Exp Med. 2012;209(6):1167–81. doi: 10.1084/jem.20120340. Epub 2012/05/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu Y, Herndon JM, Sojka DK, Kim KW, Knolhoff BL, Zuo C, Cullinan DR, Luo J, Bearden AR, Lavine KJ, Yokoyama WM, Hawkins WG, Fields RC, Randolph GJ, DeNardo DG. Tissue-Resident Macrophages in Pancreatic Ductal Adenocarcinoma Originate from Embryonic Hematopoiesis and Promote Tumor Progression. Immunity. 2017;47(2):323–38.e6. doi: 10.1016/j.immuni.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, Frampton J, Liu KJ, Geissmann F. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336(6077):86–90. doi: 10.1126/science.1219179. Epub 2012/03/24. [DOI] [PubMed] [Google Scholar]
- 43.Ginhoux F, Guilliams M. Tissue-Resident Macrophage Ontogeny and Homeostasis. Immunity. 2016;44(3):439–49. doi: 10.1016/j.immuni.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 44.Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, García-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blackburn CC, Manley NR. Developing a new paradigm for thymus organogenesis. Nat Rev Immunol. 2004;4(4):278–89. doi: 10.1038/nri1331. [DOI] [PubMed] [Google Scholar]
- 46.Masuda K, Itoi M, Amagai T, Minato N, Katsura Y, Kawamoto H. Thymic anlage is colonized by progenitors restricted to T, NK, and dendritic cell lineages. J Immunol. 2005;174(5):2525–32. doi: 10.4049/jimmunol.174.5.2525. [DOI] [PubMed] [Google Scholar]
- 47.Ramond C, Berthault C, Burlen-Defranoux O, de Sousa AP, Guy-Grand D, Vieira P, Pereira P, Cumano A. Two waves of distinct hematopoietic progenitor cells colonize the fetal thymus. Nat Immunol. 2014;15(1):27–35. doi: 10.1038/ni.2782. Epub 2013/12/08. [DOI] [PubMed] [Google Scholar]
- 48.Luis TC, Luc S, Mizukami T, Boukarabila H, Thongjuea S, Woll PS, Azzoni E, Giustacchini A, Lutteropp M, Bouriez-Jones T, Vaidya H, Mead AJ, Atkinson D, Böiers C, Carrelha J, Macaulay IC, Patient R, Geissmann F, Nerlov C, Sandberg R, de Bruijn MF, Blackburn CC, Godin I, Jacobsen SE. Initial seeding of the embryonic thymus by immune-restricted lympho-myeloid progenitors. Nat Immunol. 2016;17(12):1424–35. doi: 10.1038/ni.3576. Epub 2016/10/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Havran WL, Allison JP. Developmentally ordered appearance of thymocytes expressing different T-cell antigen receptors. Nature. 1988;335(6189):443–5. doi: 10.1038/335443a0. [DOI] [PubMed] [Google Scholar]
- 50.Nielsen MM, Witherden DA, Havran WL. γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat Rev Immunol. 2017 doi: 10.1038/nri.2017.101. Epub 2017/09/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ikuta K, Kina T, MacNeil I, Uchida N, Peault B, Chien YH, Weissman IL. A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell. 1990;62(5):863–74. doi: 10.1016/0092-8674(90)90262-d. Epub 1990/09/07. doi: 0092-8674(90)90262-D [pii] [DOI] [PubMed] [Google Scholar]
- 52.Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo SA. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science. 2012;335(6073):1195–200. doi: 10.1126/science.1216557. Epub 2012/02/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishikawa SI, Nishikawa S, Kawamoto H, Yoshida H, Kizumoto M, Kataoka H, Katsura Y. In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity. 1998;8(6):761–9. doi: 10.1016/s1074-7613(00)80581-6. Epub 1998/07/09. doi: S1074-7613(00)80581-6 [pii] [DOI] [PubMed] [Google Scholar]
- 54.Cumano A, Ferraz JC, Klaine M, Di Santo JP, Godin I. Intraembryonic, but not yolk sac hematopoietic precursors, isolated before circulation, provide long-term multilineage reconstitution. Immunity. 2001;15(3):477–85. doi: 10.1016/s1074-7613(01)00190-x. [DOI] [PubMed] [Google Scholar]
- 55.Montecino-Rodriguez E, Dorshkind K. B-1 B cell development in the fetus and adult. Immunity. 2012;36(1):13–21. doi: 10.1016/j.immuni.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7(3):293–301. doi: 10.1038/ni1301. doi: ni1301 [pii] 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 57.Montecino-Rodriguez E, Dorshkind K. New perspectives in B-1 B cell development and function. Trends Immunol. 2006;27(9):428–33. doi: 10.1016/j.it.2006.07.005. doi: S1471-4906(06)00215-8 [pii] 10.1016/j.it.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 58.Ghosn EE, Yamamoto R, Hamanaka S, Yang Y, Herzenberg LA, Nakauchi H. Distinct B-cell lineage commitment distinguishes adult bone marrow hematopoietic stem cells. Proc Natl Acad Sci U S A. 2012;109(14):5394–8. doi: 10.1073/pnas.1121632109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sawai CM, Babovic S, Upadhaya S, Knapp DJ, Lavin Y, Lau CM, Goloborodko A, Feng J, Fujisaki J, Ding L, Mirny LA, Merad M, Eaves CJ, Reizis B. Hematopoietic Stem Cells Are the Major Source of Multilineage Hematopoiesis in Adult Animals. Immunity. 2016;45(3):597–609. doi: 10.1016/j.immuni.2016.08.007. Epub 2016/08/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Beaudin AE, Boyer SW, Perez-Cunningham J, Hernandez GE, Derderian SC, Jujjavarapu C, Aaserude E, MacKenzie T, Forsberg EC. A Transient Developmental Hematopoietic Stem Cell Gives Rise to Innate-like B and T Cells. Cell Stem Cell. 2016;19(6):768–83. doi: 10.1016/j.stem.2016.08.013. Epub 2016/09/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kristiansen TA, Jaensson Gyllenback E, Zriwil A, Bjorklund T, Daniel JA, Sitnicka E, Soneji S, Bryder D, Yuan J. Cellular Barcoding Links B-1a B Cell Potential to a Fetal Hematopoietic Stem Cell State at the Single-Cell Level. Immunity. 2016;45(2):346–57. doi: 10.1016/j.immuni.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 62.Barber CL, Montecino-Rodriguez E, Dorshkind K. Reduced production of B-1-specified common lymphoid progenitors results in diminished potential of adult marrow to generate B-1 cells. Proc Natl Acad Sci U S A. 2011;108(33):13700–4. doi: 10.1073/pnas.1107172108. Epub 2011/08/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ghosn EE, Waters J, Phillips M, Yamamoto R, Long BR, Yang Y, Gerstein R, Stoddart CA, Nakauchi H, Herzenberg LA. Fetal Hematopoietic Stem Cell Transplantation Fails to Fully Regenerate the B-Lymphocyte Compartment. Stem Cell Reports. 2016;6(1):137–49. doi: 10.1016/j.stemcr.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Beaudin AE, Forsberg EC. To B1a or not to B1a: do hematopoietic stem cells contribute to tissue-resident immune cells? Blood. 2016;128(24):2765–9. doi: 10.1182/blood-2016-10-697813. Epub 2016/10/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hadland BK, Varnum-Finney B, Mandal PK, Rossi DJ, Poulos MG, Butler JM, Rafii S, Yoder MC, Yoshimoto M, Bernstein ID. A Common Origin for B-1a and B-2 Lymphocytes in Clonal Pre-Hematopoietic Stem Cells. Stem Cell Reports. 2017;8(6):1563–72. doi: 10.1016/j.stemcr.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hadland BK, Varnum-Finney B, Poulos MG, Moon RT, Butler JM, Rafii S, Bernstein ID. Endothelium and NOTCH specify and amplify aorta-gonad-mesonephros-derived hematopoietic stem cells. J Clin Invest. 2015;125(5):2032–45. doi: 10.1172/JCI80137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rybtsov S, Ivanovs A, Zhao S, Medvinsky A. Concealed expansion of immature precursors underpins acute burst of adult HSC activity in foetal liver. Development. 2016;143(8):1284–9. doi: 10.1242/dev.131193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kieusseian A, Brunet de la Grange P, Burlen-Defranoux O, Godin I, Cumano A. Immature hematopoietic stem cells undergo maturation in the fetal liver. Development. 2012;139(19):3521–30. doi: 10.1242/dev.079210. Epub 2012/08/16. [DOI] [PubMed] [Google Scholar]
- 69.Yoder MC, Hiatt K, Mukherjee P. In vivo repopulating hematopoietic stem cells are present in the murine yolk sac at day 9.0 postcoitus. Proc Natl Acad Sci U S A. 1997;94(13):6776–80. doi: 10.1073/pnas.94.13.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoder MC, Hiatt K, Dutt P, Mukherjee P, Bodine DM, Orlic D. Characterization of definitive lymphohematopoietic stem cells in the day 9 murine yolk sac. Immunity. 1997;7(3):335–44. doi: 10.1016/s1074-7613(00)80355-6. Epub 1997/11/05. doi: S1074-7613(00)80355-6 [pii] [DOI] [PubMed] [Google Scholar]
- 71.Arora N, Wenzel PL, McKinney-Freeman SL, Ross SJ, Kim PG, Chou SS, Yoshimoto M, Yoder MC, Daley GQ. Effect of developmental stage of HSC and recipient on transplant outcomes. Dev Cell. 2014;29(5):621–8. doi: 10.1016/j.devcel.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoshimoto M, Porayette P, Yoder MC. Overcoming obstacles in the search for the site of hematopoietic stem cell emergence. Cell Stem Cell. 2008;3(6):583–6. doi: 10.1016/j.stem.2008.11.002. Epub 2008/12/02. doi: S1934-5909(08)00572-9 [pii] 10.1016/j.stem.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Medvinsky A, Dzierzak E. Definitive hematopoiesis is autonomously initiated by the AGM region. Cell. 1996;86(6):897–906. doi: 10.1016/s0092-8674(00)80165-8. [DOI] [PubMed] [Google Scholar]
- 74.Taoudi S, Gonneau C, Moore K, Sheridan JM, Blackburn CC, Taylor E, Medvinsky A. Extensive hematopoietic stem cell generation in the AGM region via maturation of VE-cadherin+CD45+ pre-definitive HSCs. Cell Stem Cell. 2008;3(1):99–108. doi: 10.1016/j.stem.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 75.Rybtsov S, Batsivari A, Bilotkach K, Paruzina D, Senserrich J, Nerushev O, Medvinsky A. Tracing the origin of the HSC hierarchy reveals an SCF-dependent, IL-3-independent CD43(−) embryonic precursor. Stem Cell Reports. 2014;3(3):489–501. doi: 10.1016/j.stemcr.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Samokhvalov IM, Samokhvalova NI, Nishikawa S. Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature. 2007;446(7139):1056–61. doi: 10.1038/nature05725. Epub 2007/03/21. [DOI] [PubMed] [Google Scholar]
- 77.Ganuza M, Hadland B, Chabot A, Li C, Kang G, Bernstein I, McKinney-Freeman S. Murine hemogenic endothelial precursors display heterogeneous hematopoietic potential ex vivo. Exp Hematol. 2017;51:25–35.e6. doi: 10.1016/j.exphem.2017.04.006. Epub 2017/04/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pei W, Feyerabend TB, Rössler J, Wang X, Postrach D, Busch K, Rode I, Klapproth K, Dietlein N, Quedenau C, Chen W, Sauer S, Wolf S, Höfer T, Rodewald HR. Polylox barcoding reveals haematopoietic stem cell fates realized in vivo. Nature. 2017;548(7668):456–60. doi: 10.1038/nature23653. Epub 2017/08/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McKenna A, Findlay GM, Gagnon JA, Horwitz MS, Schier AF, Shendure J. Whole-organism lineage tracing by combinatorial and cumulative genome editing. Science. 2016;353(6298):aaf7907. doi: 10.1126/science.aaf7907. Epub 2016/05/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cao J, Packer JS, Ramani V, Cusanovich DA, Huynh C, Daza R, Qiu X, Lee C, Furlan SN, Steemers FJ, Adey A, Waterston RH, Trapnell C, Shendure J. Comprehensive single-cell transcriptional profiling of a multicellular organism. Science. 2017;357(6352):661–7. doi: 10.1126/science.aam8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cusanovich DA, Daza R, Adey A, Pliner HA, Christiansen L, Gunderson KL, Steemers FJ, Trapnell C, Shendure J. Multiplex single cell profiling of chromatin accessibility by combinatorial cellular indexing. Science. 2015;348(6237):910–4. doi: 10.1126/science.aab1601. Epub 2015/05/07. [DOI] [PMC free article] [PubMed] [Google Scholar]