Abstract
Exposure to fine ambient particulates (PM2.5) during gestation or neonatally has potent neurotoxic effects. While biological and behavioral data indicate a vulnerability to environmental pollutants across distinct neurodevelopmental windows, the behavioral consequences following exposure across the entire developmental period remain unknown. Moreover, several epidemiological studies support a link between developmental exposure to air pollution and an increased risk of later receiving a diagnosis of autism spectrum disorders (ASD), a neurodevelopmental disorder that persists throughout life. In the current study we sought to determine whether perinatal exposure to PM2.5 would reduce sociability and increase repetitive deficits in mice, two hallmark characteristics of ASD. Pregnant female B6C3 mice were exposed daily to concentrated ambient PM2.5 (CAPs) (135.8 μg/m3) or filtered air (3.1 μg/m3) throughout gestation followed by additional exposures to both dams and their litters from days 2-10 postpartum. Adult offspring were subsequently assessed for social and repetitive behaviors at 20 weeks of age. Daily perinatal exposure to CAPs significantly decreased sociability in male and female mice as measured by the social approach task; however, reductions in reciprocal social interaction and increased grooming behavior were only present in male offspring exposed to CAPs. These findings demonstrate that exposure to particulate air pollutants throughout early neurodevelopment induces long lasting behavioral deficits in a sex-dependent manner and may be an underlying cause of neurodevelopmental disorders such as ASD.
Keywords: Particulate Matter, Animal Model, Autism, ASD, Translational
1.0 Introduction
Autism spectrum disorders (ASD) are a class of neurodevelopmental disorders characterized by social-communication deficits and restricted/repetitive patterns of behavior (American Psychiatric Association, 2013) affecting an estimated 1 in 42 boys and 1 in 189 girls in the United States alone (Christensen et al. 2016). Although ASD is diagnosed during childhood, deficits persist throughout life and ASD-associated costs necessary for adult services are greater than those for children (Buescher et al., 2014), making it essential to understand ASD across all stages of the disorder. While the etiology of ASD remains unknown, epidemiological studies suggest that environmental factors like maternal exposure to air pollutants (Volk et al. 2011; Raz et al. 2015) contribute to ASD susceptibility.
Air pollution is composed of gases, metals, organic material, and ambient particulate matter (PM) (Costa et al. 2014). Fine ambient particulate matter (PM) is thought to be the most dangerous component of air pollution, causing neuroinflammation and oxidative damage (Block and Calderón-Garcidueñas 2009). Decreasing PM size corresponds with increasing severity of injury due to the ease with which smaller particles can enter tissues. PM smaller than 2.5μm in diameter (PM2.5), such as that found in diesel exhaust, accumulates near highways in major cities in the United States and other countries worldwide (Costa et al. 2014; 2017). In fact, epidemiological findings have linked residential proximity to a major highway during pregnancy with an increased childhood risk of an ASD diagnosis (Volk et al. 2011; Raz et al. 2015). More recently, a nation-wide case-control analysis across the continental United States revealed higher maternal exposure to PM2.5 throughout gestation was associated with greater odds of a child having ASD (Raz et al. 2015). Additionally, although the average PM2.5 exposure in the United States is below the World Health Organization's recommended level, many developing nations worldwide are well above this advised limit (WHO, 2016), potentially creating an at-risk environment for pregnant women. While these population-based studies are compelling, further non-human studies are necessary to demonstrate a direct causal relationship between air pollution PM and ASD.
Mouse models of in utero or neonatal pollutant exposure show altered neurotransmitter levels, neuroinflammation, impaired hippocampal-dependent memory, and depressive- or anxietylike behaviors in offspring. For example, gestational exposure to diesel exhaust (DE), a major source of PM2.5 pollution, affects neurotransmitter levels in the prefrontal cortex, striatum, hippocampus, hypothalamus, amygdala, cerebellum, and brainstem of offspring, and results in depressive- and anxiety-like behaviors in males specifically (Suzuki et al. 2010; Davis et al. 2013; Bolton et al. 2013; Thirtamara Rajamani et al. 2013; Yokota et al. 2013; 2016). Furthermore, gestational DE exposure combined with maternal stress reduce fear conditioned freezing behavior in adult offspring, indicating long-term impairment in hippocampal-dependent memory (Bolton et al. 2013). Neonatal exposure to fine and ultrafine concentrated ambient PM (≤100nm) differentially alters cytokine expression and neurotransmitter levels in female and male offspring and increases glial reactivity and hippocampal glutamate in males (Allen et al. 2014; 2017). While these findings highlight the neurotoxic effects of diesel exhaust on brain development, several questions remain regarding the behavioral consequences of PM2.5 exposure across gestational and postnatal periods. Specifically, most of the above studies fail to include female offspring in their analysis, and although the prevalence of ASD is greater in males, females are an important population that need equal consideration. Furthermore, no study to date has investigated the effects of developmental air pollution exposure on social and repetitive behaviors, core features of ASD and other neurodevelopmental disorders. Finally, experimental models of gestational or postnatal pollutant exposure focus on outcomes in juvenile and adolescent mice, but none have looked at the long term social behavioral consequences persisting into adulthood, which is essential when modeling a lifelong disorder. Therefore, a more complete understanding of the behavioral and developmental consequences resulting from pollutant exposure in utero will help elucidate potential mechanisms underlying the etiologies of neurodevelopmental disorders such as ASD that are characterized by similar behavioral deficits.
The current study assessed whether perinatal exposure to concentrated PM2.5 (CAPs, 135.8 μg/m3) alters well-validated measures of social and restricted/repetitive behaviors in adult male and female B6C3 hybrid mice (B6 × C3H). Specifically, social approach and reciprocal social interaction tasks allow for detection of broad and discrete social interactions, respectively, while increases in time spent self-grooming are often used to model the repetitive self-directed behaviors in individuals with ASD (Mason, 1991; Kelley, 2001; Crawley, 2007). Given that pollutant exposure affects neural circuitry involved in social cognition and repetitive behaviors (Adolphs, 2001; Kalueff et al., 2016), we hypothesized that early life CAPs exposure would decrease sociability and increase grooming behavior, and that this effect would be exacerbated in male compared to female offspring. To test this, pregnant dams were exposed to CAPs or filtered air (FA) throughout gestation and pups were exposed during lactation until postnatal day (P)10 to parallel the third trimester in human pregnancies (Dobbing and Sands 1979; Romijn et al. 1991). Adult offspring of both sexes were then assessed for ASD-like behavioral deficits using social approach, reciprocal social interaction, and grooming behavioral tasks.
2.0 Materials and Methods
2.1 Animals
Male and female B6C3F1 mice (Jackson Laboratory, Bar Harbor, Maine, USA) were purchased and initially housed and maintained for exposure at New York University, Department of Environmental Medicine (Tuxedo, NY) as previously described (Blum et al., In press). Each animal was housed in polycarbonate cages in temperature (20-23°C) and humidity (∼55% RH) controlled rooms on a 12 h light/dark cycle, and food and water were provided ad libitum. Females were housed in pairs and males individually for about 10 days. Upon the 2nd proestrus, one male and one female in proestrus were housed together in pairs. Seminal plugs were then checked 12-18 h after breeding pairs were co-housed (gestational day [G]-0.5), and upon presence of a plug, males were removed and females were separated into two exposure groups: Concentrated Ambient Particulate Matter ≤2.5μm (CAPs) or Filtered Air (FA). Food and water were restricted during exposure. A total of 25 litters were subjected to perinatal exposure (13 FA and 12 CAPs), and a subset of the offspring were raised until adulthood for behavioral analyses, FA n = 26 (13M/13F), CAPs n = 31 (15M/16F). Following perinatal CAPs exposure, parturition, and weaning, 1 – 3 male and female offspring from each litter were rehoused 2-4 mice per cage with sex- and treatment-matched cage mates (littermates when possible) and allowed to acclimate for 8 weeks. During this acclimation period, mice were observed daily for signs of aggression. Two male mice were removed from their cages and excluded from the study following evidence of aggression. Following acclimation, mice were transported in standard plastic cages in a temperature controlled van (World Courier, AmeriscourceBergen Corporation) to Mount Holyoke College, South Hadley, MA for behavioral assessment. Upon arrival at Mount Holyoke College, 16-week-old mice were group housed with previous cage mates in standard plastic cages and allowed 4 weeks to acclimate to the facilities prior to behavior testing. Mice were maintained at ambient room temperature on a 12 h light/dark cycle (lights on at 0800 hours). Food and water were provided ad libitum and all cages were given nestlets for enrichment. All behavioral procedures were performed during the first 4 h of the light cycle, and all procedures were approved by both New York University School of Medicine's and Mount Holyoke College's Institutional Animal Care and Use Committees.
2.2 Concentrated Fine-size Ambient Particulate System
After the presence of a seminal plug, female mice were separated and randomly sorted into one of two exposure groups, either CAPs or HEPA-filtered air (FA). Pregnant females were weighed each morning and exposed via whole body inhalation to either CAPs or FA using a modified Versatile Aerosol Concentration Enrichment System (VACES) developed by Sioutas et al. (1999) and modified by Maciejczyk et al. (2008). Ambient air was passed through an Aerotec 2 cyclone inlet to remove the majority of particles greater than 2.5μm in diameter then passed through silica gel and carbon filters to remove excess moisture and organic pollutants. PM aerosol was then chilled in a condenser tube and the remaining concentrated particles were passed over a warmed water bath to restore relative humidity. The concentrated aerosol was then divided into three streams for subsequent use: 27% of the particle flow was directed towards Teflon filters housed in Harvard impactors (Air Diagnostics and Engineering, Inc., Harrison, ME), 10% of the flow was directed towards a DataRam nephelometer to allow for continuous monitoring of CAPs mass concentration, and the remaining particles were streamed towards the animal exposure chamber. Mice in the control condition (FA) were exposed to house air passed through HEPA filters to remove approximately 98% of ambient particles prior to entering the VACES system.
2.3 Perinatal CAPs Exposure
Beginning on gestational day (G)0.5 female mice were exposed for 6 h/d, 7 d/wk until G17. Additionally, postnatal exposures were conducted starting 48 h after birth for 2 h/d, 7 d/wk, until P10. While gestational exposure was consistent with that previously used (Klocke et al., 2017), daily neonatal exposure time was reduced (compared to Allen et al., 2014) to minimize potential confounds of high CAPs exposure on neonatal lungs. To ensure no cross-contamination between treatment groups, mice were kept in cages equipped with HEPA filters. Daily samples of the exposure chamber were collected on Teflon filters (PALL Life Sciences Teflo, 37 mm, 2 μm pore, Ann Arbor, MI), gravimetrically weighed, and analyzed for exposure mass concentrations as previously described by (Blum et al. In Press). The average targeted CAPs concentration during exposure was approximately 11 times the ambient PM2.5 concentration in Sterling Forest (Tuxedo, NY).
2.4 X-Ray Fluorescence Spectroscopy
Mass concentrations of CAPs were determined daily using pre-weighed Teflon filters (37 mm, 0.2 μm pore size; Pall, Port Washington, New York). Particle-laden filters were equilibrated overnight in a temperature/humidity controlled room and weighed gravimetrically on a MT5 microbalance (Mettler Toledo, Hightstown, New Jersey). X-ray fluorescence spectroscopy (XRF) was used to determine the elemental composition of exposures by analyzing filters from every third exposure day on an ARL QUANT'X EDXRF Analyzer (ThermoFisher Scientific, Waltham, Massachusetts). Lot-matched, unexposed filters were used as blank controls.
2.5 Behavioral Assessments – Experimental Design
Experimental offspring were separated into four conditions: CAPs-female (n=16), CAPs-male (n=15), FA-female (n=13), FA-male (n=13). Twenty-week-old mice were evaluated for social and repetitive behaviors using the following tasks: three-chambered social approach, reciprocal social interaction, grooming, elevated plus maze, and open field. This age in mice is within the standard range of testing for social behavior and equivalent to adulthood in humans (Yang et al., 2011; Dutta and Sengupta, 2016). Behavioral analyses were conducted in a Latin Square design with at least 5 days between each task. All stimulus mice were sex- and weight-matched (±5 grams), between 15 – 21 weeks of age, and no stimulus mouse was used more than once for the same experimental animal in order to ensure novelty across social behavior tasks. All tasks were video recorded and analyzed using EthoVision XT 11.5 or manually scored (grooming only) by two independent raters blinded to treatment conditions.
2.5.1 Social Approach
Changes in social approach and social recognition behavior were measured between offspring of CAPs- and FA-exposed dams using a clean, polycarbonate, three-chambered social approach box (60cm × 40.5cm × 30cm). In a dimly lit room, experimental mice were first placed in the center chamber and allowed to habituate for 10 min followed by a habituation period in which mice were allowed to explore all three chambers for an additional 10 min. Mice were then returned to the center chamber and two identical wire cups were inverted and placed in the two side chambers with a novel object placed under one wire cup and a novel sex- and weight-matched (±5 grams) C57BL/6J mouse (Jackson Laboratory, Bar Harbor, ME) placed under the other wire cup. Experimental mice were video recorded and allowed to freely explore all chambers for 10 min and later scored using EthoVision XT 11.5 to evaluate social preference. Then, experimental mice were returned to the center chamber and the novel object was replaced with a second novel C57BL/6J mouse. Again, experimental mice were allowed to freely explore all chambers for 10 min and video recorded and scored using EthoVision XT 11.5 for evaluation of social recognition. For the social approach task, chamber entries, total time in each chamber, and total time sniffing the novel mouse and object were measured automatically. A sociability score was measured as: time spent in a social chamber – time spent in the novel object chamber. All testing chambers were thoroughly cleaned with 70% ethanol between each testing session.
2.5.2 Reciprocal Social Interaction and Locomotor Activity
Adult experimental offspring were evaluated for changes in interactive social behavior between offspring of CAPs and FA-exposed dams using the reciprocal social interaction (RSI) task. Mice were acclimated to a fully lit experimental room in their home cage for 30 min prior to the RSI task. Then, experimental mice were placed individually into clean plastic cages (40 × 50 × 20 cm) and allowed to habituate for 20 minutes. During this time mice were recorded for later analysis of locomotor behavior in an open field environment. Experimental mice were then quickly returned to the home cage and marked with blue (experimental mice) and pink (sex- and weight-matched (±5 grams) C57BL/6J-stimulus mice) hair chalk (OPAWZ, Ontario, CAN). Experimental and stimulus mice were placed in the RSI box at opposite ends, and allowed to interact for 20 min. Mice were recorded during this time and later scored for the amount of nose-to-nose, nose-to-body, anogenital sniffing, and total time in a social behavior using EthoVision XT 11.5 three-point body and social interaction modules.
2.5.3 Grooming
Experimental mice were tested for changes in repetitive behavior using a grooming task. Mice were placed individually in empty, clear plastic mouse cages and left to habituate for 10 min in a dimly lit room. Following the habituation period, mice were video recorded for an additional 10 min and later hand scored for self-grooming behavior by two individuals blinded to treatment condition. Grooming was defined as time spent licking paws, nose and/or face washing, or scratching fur with any foot. Inter-rater reliability was statistically confirmed using an interclass-correlation coefficient greater than 95%.
2.5.4 Elevated Plus Maze
To evaluate changes in general anxiety, mice were assessed using an elevated plus maze constructed of white Plexiglas in full light. The apparatus consisted of two open arms (30cm × 5cm × 0.5cm) and two perpendicular closed arms (30cm × 5cm × 15cm) extending from a central platform. The entire maze was elevated approximately 1 m from the floor. Mice were video recorded and placed in the central platform and allowed to freely explore the maze for 5 min. The videos were later scored using EthoVision XT 11.5 for the number of entries into each arm and the total time spent in each arm. Reductions in open arm exploration were interpreted as increased anxiety.
2.6 Statistical Analysis
All data were analyzed with linear mixed-effects models with maximum likelihood estimates and Type III sums of squares using R Statistics software version 3.3.3 (2016) and the “nlme” package. Model variations were assessed using the likelihood ratios test and the best model was selected based on the AIC/BIC criteria (Supplementary Materials). Based on the a priori hypothesis, treatment was selected as the most important factor. Maternal and pup weights were analyzed using autoregressive-1 (AR(1)) covariance structures with treatment as fixed effects. For repeated measures analysis of social approach and social recognition, treatment, sex, and chamber, as well as corresponding interactions, were set as fixed effects and litter as a random effect. Similarly, for reciprocal social interaction, grooming, elevated plus maze, and open field locomotor assays treatment, and sex, and treatment by sex interactions were set as fixed effects and litter as a random effect. For significant interactions, data were further analyzed using simple main-effects analysis and post hoc analyses were conducted when applicable using pairwise comparisons with Sidak corrections.
3.0 Results
3.1 Exposure Analysis
Mean CAPs concentrations were estimated by averaging particle weights from multiple Teflon filters across the treatment period. CAPs filters contained a 45-fold increase in the concentration of particulates (135.8 ± 13.17 μg/m3) compared to FA filters (3.1 ± 1.04 μg/m3) and eleven times the concentration found in the ambient air (11.9 ± 1.60 μg/m3). Filters from CAPs and FA chambers were further analyzed for particle deposition using X-ray fluorescence spectroscopy including sulfur, manganese, nickel, and lead. A total of 16 elements and metals were elevated in the CAPS air with the most abundant constituents being S, Na, Fe, Ca, Mg, and K (Table 1).
Table 1. X-Ray Flourecence Spectroscopy analysis of air constituents: Mean [±SD].
| Element | Ambient Air (ng/m3) | FA (ng/m3) | CAPs (ng/m3) | CAPs Fold Change from Ambient |
|---|---|---|---|---|
| Na | 130.67 [± 66.90] | 71.92 | 1019.59 [± 673.73] | 7.80 |
| Mg | 27.10 [± 12.18] | 20.376 | 241.36 [± 141.94] | 8.91 |
| S | 505.555 [± 313.99] | 8.37 | 5362.19 [± 4656.18] | 10.61 |
| K | 22.51 [± 11.24] | 1.53 | 200.35 [± 123.49] | 8.90 |
| Ca | 45.32 [± 35.49] | 9.07 | 300.68 [± 217.13] | 6.63 |
| Ti | 5.35 [± 5.61] | 2.08 | 22.34 [± 17.28] | 4.18 |
| Cr | 1.16 [± 1.00] | 0.54 | 10.25 [± 7.17] | 8.87 |
| Mn | 0.85 [± 0.65] | - | 12.20 [± 8.08] | 14.31 |
| Fe | 35.60 [± 24.09] | 3.93 | 343.18 [± 238.61] | 9.64 |
| Ni | 1.90 [± 1.77] | - | 7.10 [± 7.78] | 3.73 |
| Cu | 2.22 [± 1.23] | 5.37 | 39.21 [± 20.25] | 17.66 |
| Zn | 40.21 [± 48.28] | 3.12 | 66.84 [± 75.37] | 1.66 |
| Br | 10.58 [± 18.70] | - | 67.64 [± 53.59] | 6.39 |
| Sr | 1.49 [± 0.79] | 1.17 | 11.31 [± 7.25] | 7.60 |
| Lu | 5.16 [± 4.10] | 0.58 | 17.96 [± 28.30] | 3.48 |
| Pb | 1.26 [± 1.29] | 0.97 | 21.02 [± 25.02] | 16.73 |
Mn, Ni, Br were below the limit of detection in the FA condition
3.2 Maternal Weight, Pup Weight, and Litter Size
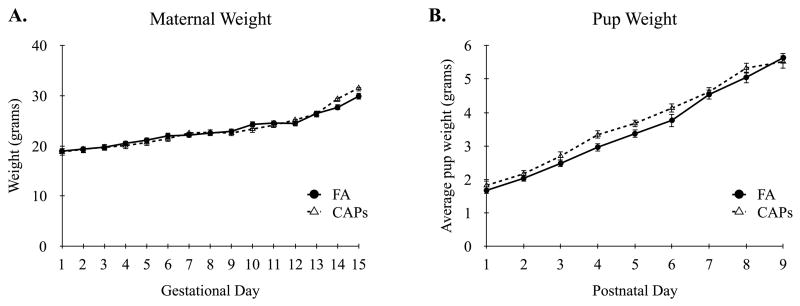
Analysis of dam weights throughout pregnancy showed typical growth curves over time, F(15, 240) = 67.12, p < 0.001, with no differences observed between treatment conditions, F(1, 23) = 0.028, p = 0.87 (Figure 1A). There were no differences in litter sizes between FA (M = 8.3, SD = 2.18) and CAPs dams (M = 9.0, SD = 2.27) at parturition, t(23) = 0.76, p = 0.45. The litters were weighed daily and growth curves estimated based on average pup weights. There was a significant main effect for time, F(8, 74) = 151.71, p < 0.001, with similar growth curves observed between treatment conditions as indicated by a non-significant time by treatment interaction, F(8, 74) = 0.84, p = 0.57 (Figure 1B).
Figure 1.
Maternal Weight and Pup Growth Curves. (A) Pregnant dams of both FA and CAPs groups showed similar weight gains throughout gestation. (B) There were no differences in average pup growth (i.e. weight) during the first nine days of postnatal development. Linear mixed-effects modeling with treatment and time as fixed effects. Data shown as mean ± SEM. Sample size: FA n = 26 pups (13M/13F; 13 dams), CAPs n = 31 pups (15M/16F; 12 dams).
3.3 Social Approach and Social Recognition
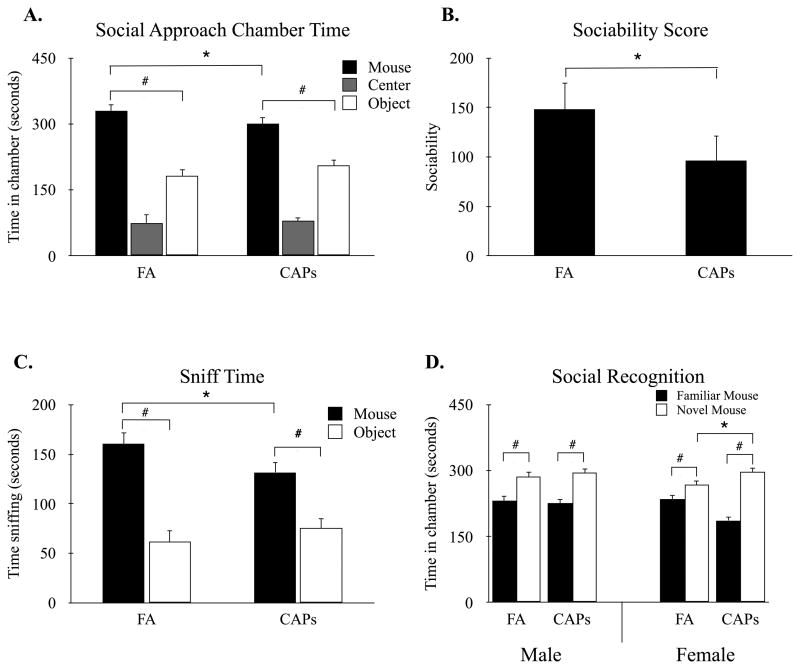
Social exploration in the social approach task was assessed using linear mixed-effects modeling. While there were no main effects for treatment or sex (Supplementary Materials), there was a significant main effect for chamber, F(1, 53) = 20.51, p < 0.001, with mice showing greater preference for the novel mouse chamber over novel object chamber (Figure 2A). Interestingly, there was a significant treatment by chamber interaction, F(1, 53) = 5.38, p < 0.05, suggesting differences in the magnitude of social exploration between the two treatment conditions. These treatment differences were independent of sex as indicated by a non-significant three-way interaction between chamber, treatment, and sex, F(1, 53) = 0.15, p = 0.70. Analysis of the interaction between treatment and chamber revealed that while both FA and CAPs mice showed preference for the novel mouse chamber over the novel object chamber, FA t(23) = 10.436, p < 0.001; CAPs F t(24) = 7.15, p < 0.001, there was a significant decrease in time spent in the mouse chamber in CAPs mice, t(23) = -2.20, p < 0.05 (Figure 2A). Similarly, there was a trend towards an increase in the time spent in the object chamber for CAPs mice compared to FA controls, but this increase did not reach statistical significance, t(23) = 1.91, p = 0.07.
Figure 2.
Offspring born to CAPs-exposed dams show reductions in social approach, but not in social recognition. (A) While both FA- and CAPs-exposed mice spent significantly more time in the novel mouse chamber compared to the novel object chamber, CAPs mice showed significantly reduced preference for the novel mouse compared to FA-treated controls. (B) Calculation of sociability score (time with mouse – time with object) further revealed CAPs mice had significantly decreased social motivation compared to sex- and age-matched FA controls. (C) FA- and CAPs exposed mice spent significantly more time sniffing the novel mouse compared to novel object, however CAPs mice displayed significantly decreased time sniffing the novel mouse compared to FA controls. (D) In the social recognition phase, mice in both exposure groups spent significantly more time in the chamber with the novel mouse compared to the chamber with the familiar mouse. Data were compared using a linear mixed-effects model with chamber, maternal treatment, and offspring sex as fixed effects and dam as the random effect (* p < 0.05 treatment; # p < 0.05 chamber). Data shown are means ± SEM. Sample size: FA n = 26 (13M/13F; 13 litters), CAPs n = 31 (15M/16F; 12 litters).
Assessment of sociability scores, as measured by the time in the novel mouse chamber minus the time in the novel object chamber, revealed a significant main effect for treatment, F(1, 23) = 4.71, p < 0.05, but no effect of sex on sociability scores (Supplementary Materials). Overall, CAPs-exposed male and female mice displayed reduced sociability scores compared to FA controls (Figure 2B). Importantly, there were no main effects for chamber entries, F(1, 52) = 0.55 p = 0.46, as well as a non-significant chamber by treatment interaction, F(1, 52) = 0.04 p = 0.84, indicating no differences in overall motor activity across conditions (data not shown).
In addition to chamber exploration, mice were assessed for the total time spent sniffing the novel object and novel mouse during the 10-minute social approach task. There was a significant treatment by chamber interaction, F(1, 23) = 10.39, p < 0.01, and this interaction was independent of sex as indicated by a non-significant chamber by treatment by sex interaction, F(1, 53) = 1.41, p = 0.24. Simple effects analysis of the treatment by chamber interaction indicated that male and female CAPs offspring spent significantly less time sniffing the novel mouse compared to FA control mice, t(23) = -2.68, p < 0.05 (Figure 2C). Conversely, there were no differences in time spent sniffing the novel object between FA and CAPs offspring, t(23) = 1.42, p = 0.17.
Finally, while CAPs exposure resulted in reductions in social approach, mice of both treatment conditions displayed species-typical preferences for a novel mouse in the social recognition task. Surprisingly, there was a three-way sex by chamber by treatment interaction that approached significance, F(1, 164) = 3.72, p = 0.055. Simple main effects analysis revealed a significant chamber by treatment interaction in female, F(1, 88) = 4.78, p = 0.03, but not male mice, F(1, 76) = 0.36, p = 0.55. Post hoc analysis of female mice further revealed a significant increase in novel mouse chamber exploration in CAPs mice compared to FA control females, t(23) = -2.60, p < 0.05 (Figure 2D).
3.4 Reciprocal Social Interaction
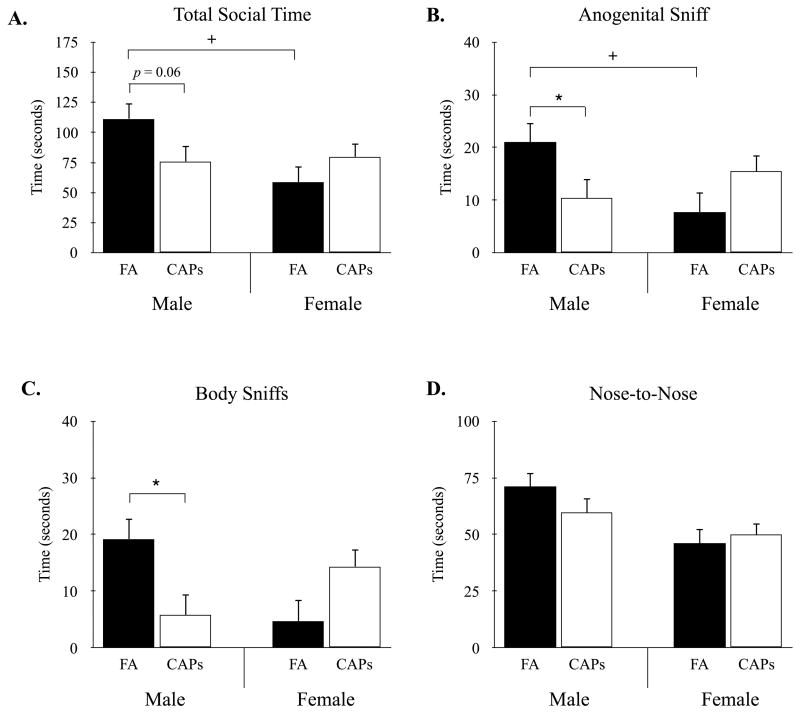
Mice were assessed for changes in species-specific social behaviors during a 20-min reciprocal social interaction task. For total social time, there was no main effect for treatment, F(1, 23) = 1.37, p = 0.25, or sex, F(1, 30) = 0.07, p = 0.79. However, there was a significant treatment by sex interaction for the total time spent engaging in a social interaction, F(1, 30) = 4.86, p < 0.05 (Figure 3A). Specifically, male CAPs-exposed mice showed a reduction in total social time compared to male FA-exposed offspring, t(23) = 1.92, p = 0.06 (Figure 3A), while no differences were observed between CAPs and FA female mice, t(23) = 1.7, p = 0.25. Moreover, male FA mice were observed to have a significantly higher total social time compared to female FA mice, t(30) = -2.80, p < 0.01. Differences in social interaction were most apparent in the total time mice spent engaging in anogenital sniffing and body sniffing (Figure 3B,C). Specifically, there was a significant treatment by sex interaction for anogenital sniffing, F(1, 28) = 5.38, p < 0.05 (Figure 3B), with CAPs exposure decreasing anogenital sniffing in male, t(23) = 2.03, p < 0.05, but not female mice, t(23) = 1.24, p = 0.23. In addition, male FA mice engaged in significantly more anogenital exploration compared to female FA mice, t(30) = -2.28, p < 0.05. For body sniffing, there was a significant treatment by sex interaction, F(1, 53) = 4.68, p < 0.05, with male CAPs showing decreased sniffing compared to FA controls (Figure 3C). However, differences between male and female FA mice did not reach statistical significance, t(30) = -1.88, p = 0.07 and there were no differences in body sniffing between female FA and CAPs mice, t(24) = 1.45, p = 0.16. Finally, male mice spent significantly more time engaging in nose-to-nose interactions compared to female mice, F(1, 23) = 8.53, p < 0.01. However, these differences were independent of treatment condition (Supplementary Materials), and there was a non-significant treatment by sex interaction (Figure 3D).
Figure 3.
CAPs exposure results in sex-dependent reductions in reciprocal social interactions. (A) Perinatal CAPs reduced social interaction time in male but not female offspring compared to FA controls. Female FA-exposed mice displayed reduced social interaction time compared to FA-treated males. (B) Male CAPs and female FA offspring displayed a significant reduction in anogenital sniffing behavior compared to FA male mice. (C) CAPs-exposure significantly decreased body sniffing in males compared to FA controls. (D) There were no differences in the total time spent in nose-to-nose sniffs between treatment conditions. Data were compared using a linear mixed-effects model with treatment and sex as fixed effects and dam as the random effect (* p < 0.05 treatment; + p < 0.05 sex). Data are means ± SEM. Sample size: FA n = 26 (13M/13F; 13 litters), CAPs n = 31 (15M/16F; 12 litters).
3.5 Grooming
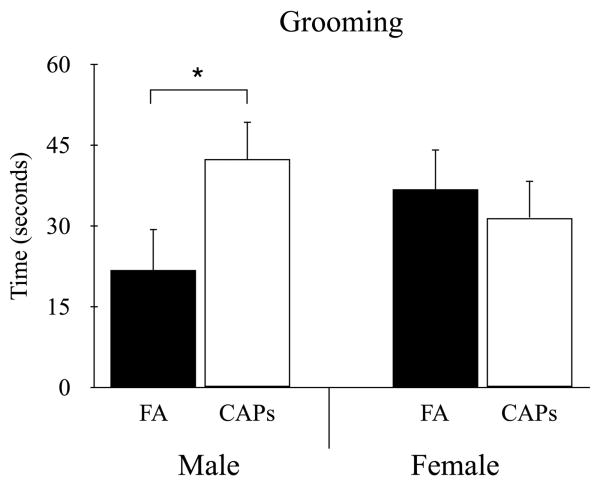
For total time grooming there was no main effect for treatment, F(1, 34) = 1.28, p = 0.27, or sex, F(1, 18) = 1.95, p = 0.18. However, there was a significant treatment by sex interaction, F(1, 28) = 5.80, p < 0.05, with male CAPs mice spending significantly more time grooming compared to male FA-exposed control mice t(18) = 2.26, p < 0.05 (Figure 4). This difference in repetitive grooming behavior between treatment groups was not observed in females, t(34) = 1.13, p = 0.27.
Figure 4.
Perinatal CAPs exposure increased grooming behavior in male offspring compared to FA-exposed controls. No differences in grooming were observed in females between treatment groups. Linear mixed-effects modeling with treatment and sex as fixed effects and litter as random effects (* p < 0.05). Data shown as mean ± SEM. Sample size: FA n = 26 (13M/13F; 13 litters), CAPs n = 31 (15M/16F; 12 litters).
3.6 Elevated Plus Maze
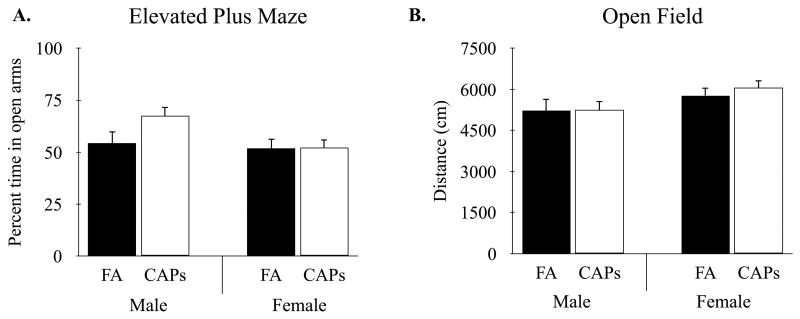
Mice were assessed for changes in anxiety-like behaviors in the elevated plus maze. There was a significant main effect for sex in the percent of time mice spent exploring the open arms of the arena, F(1, 30) = 5.59, p < 0.05 (Figure 5A). Specifically, male mice spent significantly more time in the open arms of the elevated plus maze compared to female mice, indicating a reduction in anxiety-like behavior among males. These sex-specific differences in open-arm exploration were independent of treatment. That is, there was no main effect for treatment and no treatment by sex interaction (Supplementary Materials), suggesting that perinatal CAPs exposure had no effect on anxiety-like behavior. Similarly, there were no differences between treatment groups in motor activity as measured by the number of entries into the open arms, F(1, 23) = 0.02, p = 0.87, as well as no significant interactions (data not shown).
Figure 5.
Mice exposed to CAPs showed no differences in non-ASD associated anxiety and locomotor behaviors. Perinatal CAPs exposure did not affect (A) open arm exploration in the elevated plus maze or (B) distance traveled in the open field task when compared to FA-exposed controls. Linear mixed-effects modeling with treatment and sex as fixed effects and litter as random effects. Data shown as mean ± SEM. Sample size: FA n = 26 (13M/13F; 13 litters), CAPs n = 31 (15M/16F; 12 litters).
3.7 Open Field
To determine whether early-life exposure to CAPs alters motor activity, mice were assessed for locomotor activity in the open field arena. There were no differences between treatment groups in total distance traveled, F(1, 36) = 0.83, p = 0.36 (Figure 5B), or average velocity F(1, 36) = 0.88, p = 0.35. However, there was a significant main effect for sex on distance traveled, F(1, 18) = 6.45, p < 0.05, and average velocity, F(1, 18) = 6.32, p < 0.05, with female mice exhibiting greater motor activity compared to male mice. Finally, there was a non-significant treatment by sex interaction for both distance traveled, F(1, 17) = 0.67, p = 0.42 and velocity, F(1, 53) = 0.68, p = 0.42.
Discussion
Daily perinatal exposure to CAPs produced ASD-like behavioral deficits in a sex-dependent manner, with male mice displaying more severe behavioral deficits compared to females. These sex-specific deficits in social interaction and increases in restricted/repetitive behaviors parallel the higher incidence of ASD reported in males compared to females (Christensen et al. 2016). This work extends previous findings utilizing gestational (Klocke et al. 2017) or early postnatal (Allen et al. 2014; 2017) CAPs exposure by combining both paradigms and exposing offspring throughout gestation and early neonatal life, a timeline that covers the equivalent of all three trimesters in human gestation (Dobbing and Sands 1979; Romijn et al. 1991). While the behavioral deficits found in the current study hold translational relevance for pregnant women exposed to elevated air pollution throughout gestation, it will be important for future studies to determine the significance of CAPs exposure during different developmental windows on offspring social behavior. Our findings using perinatal CAPs exposure at or below levels commonly seen in developing nations (World Health Organization 2016) support human epidemiological studies linking air pollution to ASD susceptibility (Windham et al. 2006; Volk et al. 2011; Raz et al. 2015), and suggest that this risk may be exacerbated in male offspring.
Interestingly, CAPs exposure decreased social approach behavior in both male and female mice without altering social recognition, while deficits in reciprocal social interaction were only observed in males. These findings reveal a potential sex-related specificity for CAPs treatment to impair social motivation over other aspects of social cognition, suggesting certain neural circuitry may be more susceptible to perinatal air pollutant exposure (Chevallier et al., 2012). These differences in sociability across behavioral assays likely reflect differences in the sensitivity of each assay in measuring social behavior. Specifically, the social approach task restricts species-typical interactions and is limited to measuring broad changes in approach behavior without the ability to detect discrete differences in the quality of more naturalistic interactions (Nadler et al. 2004). Conversely, the reciprocal social interaction task promotes more complex interactions between two freely behaving mice resulting in more sensitive detection of social behavior deficits. As a result, our automated reciprocal social interaction paradigm unbiasedly detected decreases in several social behavior postures in male CAPs mice only. Importantly, control male mice displayed higher levels of social interaction overall, with CAPs exposure reducing total social interaction time to a level similar to that observed in the control female mice. It is unclear whether these decreases in overall sociability in CAPs-exposed offspring are due to changes in pro- or anti-social encounters. Interestingly, manual review of the reciprocal social interaction videos revealed that five mice who exhibited aggression towards the stimulus mouse, all of which were FA-exposed males. This raises new questions as to whether perinatal CAPs exposure suppresses aggression specifically or other pro- or anti-social behaviors. Nonetheless, these sex-differences in baseline sociability are novel given that little is known about the social behavior profile of the B6C3 hybrid strain. Unlike more traditional mouse model strains, the B6C3F1 mice are the hybrid offspring of a C57BL/6 (female, B6) × C3H (male, C3) cross, and their offspring offer greater genetic variability and thus a more representative model of the genetic heterogeneity in the human population. While previous studies using C57BL/6 and C3H strains have reported high levels of sociability and sex-specific differences in social investigation (Moy et al. 2007; Holmes et al. 2011), our work is the first to demonstrate sex-specific differences in sociability within the B6C3 hybrid strain. Therefore, the genetic heterogeneity and more pronounced deficits observed in males support the translational potential of our findings as a valid model of PM-associated ASD neurodevelopment.
In addition to disrupting social behavior, perinatal exposure to CAPs increased stereotypical grooming in male offspring, a behavioral task associated with restricted/repetitive patterns of behavior in ASD (Crawley 2007). These increases in grooming behaviors occurred in the absence of changes in anxiety-like and locomotor behaviors. Our findings are closely in line with previous reports showing no changes in open arm exploration following prenatal exposure to diesel exhaust (6 × 50 μg) or urban air nanoparticles (350 μg/m3) (Davis et al. 2013; Bolton et al. 2013; 2014). Importantly, only when prenatal diesel exhaust exposure (6 × 50 μg) is combined with additional factors (i.e. maternal stress or metabolic challenges) are increases in anxiety-like behaviors observed (Bolton et al. 2013; 2014). Therefore, it is plausible that our model of perinatal CAPs exposure increases susceptibility to anxiety-like behavior, but these behavioral differences would only be observed when combined with an external/second stressor. Alternatively, the absence of any observed anxiety-like behaviors may be attributed to visual impairments associated with the C3H background strain. That is, a subset of mice carrying the retinal degeneration mutation from the C3H sire were likely blind by the age of weaning and, as a result, may have been less averse to the open arm of the elevated plus maze. Caution must therefore be taken when interpreting these results and future studies should assess anxiety-like behavior with tasks utilizing other sensory modalities. Our finding that CAPs exposure had no effect on locomotor behavior is in line with previous work showing no difference in spontaneous locomotor activity in males after gestational diesel exhaust exposure (90 μg/m3) at concentrations that are similar to ours (Yokota et al. 2016). This is in contrast to higher dose gestational diesel exhaust exposures (1.0 mg/m3) that are known to induce balance/coordination impairments (Yokota et al. 2013). These discrepancies underscore the importance of dosage when considering the behavioral impact of air pollution and suggest that lower doses may target complex social processes in the absence of global motor disturbances. Overall, our behavioral findings parallel those commonly found in ASD models and demonstrate the lasting consequences of perinatal CAPs exposure.
Elemental analysis of the CAPs air revealed significant elevations in several toxicants that are associated with ASD development (Grandjean and Landrigan 2006; Windham et al. 2006; Roberts et al. 2013; Schwartzer et al. 2013). These increases in heavy metal exposure are thought to contribute to maternal inflammation and decreased fetal micronutrient availability, both of which are hypothesized to contribute to the development of ASD (Nuttall 2017). Copper, for example, is a vital nutrient in the developing brain, but in excess has been associated with a later diagnosis of autism (Walker et al. 2011). Moreover, inhalation of copper nanoparticles (3.5 mg/m3) during gestation in mice results in maternal inflammation and long-term alterations in inflammatory cytokines in offspring (Adamcakova-Dodd et al. 2015), however this exposure level was much greater than copper levels in our CAPs treatment (39.21 ± 20.25 ng/m3). Similarly, maternal exposure to nickel, manganese or lead is associated with increased risk of autism development (Roberts et al. 2013), and the concentration of these constituents in our CAPs exposure fall within (nickel, 7.1 ± 7.79 ng/m3) or above (manganese, 12.2 ± 8.08 ng/m3; lead, 21.02 ± 25.02 ng/m3) the ranges reported. A previous study noted that children born in the San Francisco Bay Area and diagnosed with ASD on average were born in areas with slightly higher levels of nickel and lead compared to healthy controls (Windham et al. 2006). Furthermore, manganese exposure in mice induces an acute phase inflammatory response which in pregnant dams could be toxic to fetal neurodevelopment (Kobayashi et al. 2007). Importantly, maternal inflammation is linked to neurodevelopmental impairments in offspring (Estes and McAllister 2016) and mouse models have uncovered direct links between maternal inflammation and ASD-like behavioral deficits (Schwartzer et al. 2015; 2017). Taking together the links between individual heavy metal exposures, maternal inflammation, and increased risk of ASD-like behavioral deficits, the combination of elevated elements in our CAPs treatment may synergistically impact multiple mechanisms driving the behavioral deficits observed here. In fact, a recent report using a similar CAPs exposure paradigm exclusively during embryonic development revealed increases in neuroinflammation along with altered brain connectivity in offspring prenatally-exposed to CAPs (Klocke et al. 2017). These findings in combination with our behavioral outcomes shed light on how particular constituents of ambient urban air could produce neurological consequences on fetuses and neonates and highlights the importance of maternal environment on offspring neurodevelopment.
Overall, our findings support the link between air pollution and susceptibility to neurodevelopmental deficits. However, it is important to note that both experimental conditions (FA and CAPs) represent the extremes of typical air exposure conditions. Particulate matter from ambient air collected in Sterling Forest, Tuxedo, NY was concentrated to produce, on average, 11 times the levels typically found in ambient air within this location. Although care must be taken when interpreting the robust behavioral deficits described here, considering that the CAPS exposure level is above what would typically be experienced on a daily basis in the United States, PM2.5 exposure at or above our CAPs treatment used here (135.8 μg/m3) is commonly-experienced by individuals living in developing nations (World Health Organization 2016) and thus is likely to have an impact on gestational development worldwide. Additionally, our experimental design incorporated only 1 to 3 mice from each litter rather than measuring behavior from all littermates. While we used mixed-effects modeling to control for litter effects by nesting each animal within litter, the small number of offspring included within each litter introduces greater amounts of unexplained variability into the analysis and weakens the overall statistical power (Lazic and Essioux 2013). These limitations notwithstanding, our findings extend the list of behavioral deficits induced by perinatal pollutant exposure and demonstrate a novel sexual dimorphism in social and restricted/repetitive behaviors that closely model the sex-differences observed in the clinical population. The link between perinatal PM exposure and precipitation of ASD-like behavioral deficits supports ongoing epidemiological links between maternal exposure to particulate air pollution and increased risk of ASD (Windham et al. 2006; Volk et al. 2011; Raz et al. 2015). Future rodent studies should determine which gestational or neonatal windows confer the greatest susceptibility to developing social behavior deficits in an effort to uncover when during pregnancy fetal neurodevelopment may be most vulnerable to pollutant exposure. In addition, more attention needs to be directed at uncovering the biological mechanisms underlying these behavioral phenotypes to further our etiological understanding of neurodevelopmental disorders.
Supplementary Material
Highlights.
Mice were exposed to concentrated ambient particulate matter (CAPs) throughout gestation
Offspring displayed decreased social interactions in a sex-dependent manner
No changes were observed in social novelty recognition
CAPs increased repetitive grooming behaviors in male, but not female, mice
Acknowledgments
The authors would like to acknowledge Ms. Kathleen Byrne, Ms. Megan Johnson, Ms. Maddy Berkowitz-Cerasano, Ms. Katherine Suen, and Ms. Samantha Bilton for their technical expertise and Dr. Michael Lavine for his guidance on statistical analysis. The authors wish to further acknowledge the contributions of Ms. Carol Hoffman-Budde for assistance with the animal exposures, and Mr. Mianhua Zhong for assistance with the XRF analysis. Funding was provided by NIH (R15HD082638), the March of Dimes (21-F12-13), and NYU NIEHS Center (ES000260).
Abbreviations
- ASD
autism spectrum disorder
- CAPs
concentrated ambient particulates
- DE
diesel exhaust
- FA
filtered air
- PM
particulate matter
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamcakova-Dodd A, Monick MM, Powers LS, Gibson-Corley KN, Thorne PS. Effects of prenatal inhalation exposure to copper nanoparticles on murine dams and offspring. Part Fibre Toxicol. 2015 Oct 6;12(1):405. doi: 10.1186/s12989-015-0105-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Allen JL, Liu X, Pelkowski S, Palmer B, Conrad K, Oberdörster G, et al. Early Postnatal Exposure to Ultrafine Particulate Matter Air Pollution: Persistent Ventriculomegaly, Neurochemical Disruption, and Glial Activation Preferentially in Male Mice. Environ Health Perspect. 2014 Jun 5;122(9):939–945. doi: 10.1289/ehp.1307984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JL, Oberdorster G, Morris-Schaffer K, Wong C, Klocke C, Sobolewski M, et al. Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: Parallels with neuropathological and behavioral features of autism and other neurodevelopmental disorders. Neurotoxicology. 2017 Mar;59:140–154. doi: 10.1016/j.neuro.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. (5th ed.) 2013:31–33. [Google Scholar]
- Block ML, Calderón-Garcidueñas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends in Neurosciences. 2009 Aug 26;32(9):506–516. doi: 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum JL, Chen LC, Zelikoff JT. Exposure to ambient particulate matter during specific gestational periods produces adverse obstetric consequences in mice. Environ Health Perspect. 2017 Jul;125(7):077020-1–077020-13. doi: 10.1289/EHP1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Auten RL, Bilbo SD. Prenatal air pollution exposure induces sexually dimorphic fetal programming of metabolic and neuroinflammatory outcomes in adult offspring. Brain, Behavior, and Immunity. 2014 Mar;37:30–44. doi: 10.1016/j.bbi.2013.10.029. [DOI] [PubMed] [Google Scholar]
- Bolton JL, Huff NC, Smith SH, Mason SN, Auten RL, Bilbo SD. Maternal Stress and Effects of Prenatal Air Pollution on Offspring Mental Health Outcomes in Mice. Environ Health Perspect. 2013 Jul 3;121(9):1075–1082. doi: 10.1289/ehp.1306560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buescher AVS, Cidav Z, Knapp M, Mandell DS. Costs of Autism Spectrum Disorders in the United Kingdom and the United States. JAMA Pediatr. 2014;168(8):721–728. doi: 10.1001/jamapediatrics.2014.210. [DOI] [PubMed] [Google Scholar]
- Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The Social Motivation Theory of Autism. Trends Cogn Sci. 2012 Apr;16(4):231–239. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DL, Baio J, Braun KV, et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Surveill Summ. 2016;65(SS-3):1–23. doi: 10.15585/mmwr.ss6503a1. (No. SS-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roque P. Neurotoxicants Are in the Air: Convergence of Human, Animal, and In Vitro Studies on the Effects of Air Pollution on the Brain. Biomed Res Int. 2014;2014(8):1–8. doi: 10.1155/2014/736385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Cole TB, Coburn J, Chang YC, Dao K, Roqué PJ. Neurotoxicity of traffic-related air pollution. Neurotoxicology. 2017 Mar;59:133–139. doi: 10.1016/j.neuro.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. Mouse Behavioral Assays Relevant to the Symptoms of Autism. Brain Pathology. (2nd ed.) 2007 Oct;17(4):448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DA, Bortolato M, Godar SC, Sander TK, Iwata N, Pakbin P, et al. Prenatal Exposure to Urban Air Nanoparticles in Mice Causes Altered Neuronal Differentiation and Depression-Like Responses. PLoS ONE. 2013 May 29;8(5):e64128. doi: 10.1371/journal.pone.0064128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979 Mar 1;3(1):79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Dutta S, Sengupta P. Men and mice: Relating their ages. Life Sciences. 2016;152:244–248. doi: 10.1016/j.lfs.2015.10.025. [DOI] [PubMed] [Google Scholar]
- Estes ML, McAllister AK. Maternal immune activation: Implications for neuropsychiatric disorders. Science. 2016 Aug 20;353(6301):772–777. doi: 10.1126/science.aag3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. The Lancet. 2006 Dec;368(9553):2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- Holmes MM, Niel L, Anyan JJ, Griffith AT, Monks DA, Forger NG. Effects of Bax gene deletion on social behaviors and neural response to olfactory cues in mice. European Journal of Neuroscience. 2011 Oct 31;34(9):1492–1499. doi: 10.1111/j.1460-9568.2011.07881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff AV, Stewart AM, Song C, Berridge KC, Graybiel AM, Fentress JC. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat Rev Neurosci. 2016;17(1):45–59. doi: 10.1038/nrn.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE. Measurement of Rodent Stereotyped Behavior. Curr Protoc Neurosci. 2001;4:8.8.1–8.8.13. doi: 10.1002/0471142301.ns0808s04. 8.8. [DOI] [PubMed] [Google Scholar]
- Klocke C, Allen JL, Sobolewski M, Mayer-Pröschel M, Blum JL, Lauterstein D, et al. Neuropathological Consequences of Gestational Exposure to Concentrated Ambient Fine and Ultrafine Particles in the Mouse. Toxicological Sciences. 2017 Jan 13;156(2):492–508. doi: 10.1093/toxsci/kfx010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Kuroda J, Shibata N, Hasegawa T, Seko Y, Satoh M, et al. Induction of Metallothionein by Manganese Is Completely Dependent on Interleukin-6 Production. Journal of Pharmacology and Experimental Therapeutics. 2007;320(2):721–727. doi: 10.1124/jpet.106.112912. [DOI] [PubMed] [Google Scholar]
- Lazic SE, Essioux L. Improving basic and translational science by accounting for litter-to-litter variation in animal models. BMC Neuroscience. 2013 Mar 22;14(1):37. doi: 10.1186/1471-2202-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason GJ. Stereotypies: a critical review. Anim Behav. 1991;41:1015–1037. [Google Scholar]
- Maciejczyk P, Zhong M, Li Q, Xiong J, Nadziejko C, Chen LC. Effects of Subchronic Exposures to Concentrated Ambient Particles (CAPs) in Mice: II. The Design of a CAPs Exposure System for Biometric Telemetry Monitoring. Inhal Toxicol. 2008 Oct 6;17(4-5):189–197. doi: 10.1080/08958370590912743. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176(1):4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004 Sep 4;3(5):303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Nuttall JR. The plausibility of maternal toxicant exposure and nutritional status as contributing factors to the risk of autism spectrum disorders. Nutr Neurosci. 2017;20(4):209–218. doi: 10.1080/1028415X.2015.1103437. [DOI] [PubMed] [Google Scholar]
- Raz R, Roberts AL, Lyall K, Hart JE, Just AC, Laden F, et al. Autism Spectrum Disorder and Particulate Matter Air Pollution before, during, and after Pregnancy: A Nested Case–Control Analysis within the Nurses' Health Study II Cohort. Environ Health Perspect. 2015;123(3):264–270. doi: 10.1289/ehp.1408133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AL, Lyall K, Hart JE, Laden F, Just AC, Bobb JF, et al. Perinatal Air Pollutant Exposures and Autism Spectrum Disorder in the Children of Nurses' Health Study II Participants. Environ Health Perspect. 2013 Jun 18;121(8):978–984. doi: 10.1289/ehp.1206187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romijn HJ, Hofman MA, Gramsbergen A. At what age is the developing cerebral cortex of the rat comparable to that of the full-term newborn human baby? Early Hum Dev. 1991 Jul 1;26(1):61–67. doi: 10.1016/0378-3782(91)90044-4. [DOI] [PubMed] [Google Scholar]
- Schwartzer JJ, Careaga M, Chang C, Onore CE, Ashwood P. Allergic fetal priming leads to developmental, behavioral and neurobiological changes in mice. Translational Psychiatry. 2015 Apr 7;5(4):e543. doi: 10.1038/tp.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzer JJ, Careaga M, Coburn MA, Rose DR, Hughes HK, Ashwood P. Behavioral impact of maternal allergic-asthma in two genetically distinct mouse strains. Brain, Behavior, and Immunity. 2017 Jul;63:99–107. doi: 10.1016/j.bbi.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzer JJ, Koenig CM, Berman RF. Using mouse models of autism spectrum disorders to study the neurotoxicology of gene–environment interactions. Neurotoxicol Teratol. 2013 Mar;36:17–35. doi: 10.1016/j.ntt.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sioutas C, Kim S, Chang M. Development and evaluation of a prototype ultrafine particle concentrator. Journal of Aerosol Science. 1999 Sep;30(8):1001–1017. [Google Scholar]
- Suzuki T, Oshio S, Iwata M, Saburi H, Odagiri T, Udagawa T, et al. In utero exposure to a low concentration of diesel exhaust affects spontaneous locomotor activity and monoaminergic system in male mice. Part Fibre Toxicol. 2010;7(1):7. doi: 10.1186/1743-8977-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thirtamara Rajamani K, Doherty-Lyons S, Bolden C, Willis D, Hoffman C, Zelikoff J, et al. Prenatal and Early-Life Exposure to High-Level Diesel Exhaust Particles Leads to Increased Locomotor Activity and Repetitive Behaviors in Mice. Autism Res. 2013 Mar 11;6(4):248–257. doi: 10.1002/aur.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk HE, Hertz-Picciotto I, Delwiche L, Lurmann F, McConnell R. Residential Proximity to Freeways and Autism in the CHARGE Study. Environ Health Perspect. 2011;119(6):873–877. doi: 10.1289/ehp.1002835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LR, Rattigan M, Canterino J. A Case of Isolated Elevated Copper Levels during Pregnancy. J Pregnancy. 2011;2011(6):1–3. doi: 10.1155/2011/385767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windham GC, Zhang L, Gunier R, Croen LA, Grether JK. Autism Spectrum Disorders in Relation to Distribution of Hazardous Air Pollutants in the San Francisco Bay Area. Environ Health Perspect. 2006 Jun 21;114(9):1438–1444. doi: 10.1289/ehp.9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Ambient air pollution: A global assessment of exposure and burden of disease. World Health Organization; 2016. pp. 1–131. [Google Scholar]
- Yang M, Silverman JL, Crawley JN. Automated Three-Chambered Social Approach Task for Mice. Curr Protoc Neurosci. 2011 doi: 10.1002/0471142301.ns0826s56. Ch 8, Unit 8.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S, Moriya N, Iwata M, Umezawa M, Oshio S, Takeda K. Exposure to diesel exhaust during fetal period affects behavior and neurotransmitters in male offspring mice. J Toxicol Sci. 2013 Jan 30;38(1):13–23. doi: 10.2131/jts.38.13. [DOI] [PubMed] [Google Scholar]
- Yokota S, Oshio S, Moriya N, Takeda K. Social Isolation-Induced Territorial Aggression in Male Offspring Is Enhanced by Exposure to Diesel Exhaust during Pregnancy. PLoS ONE. 2016 Feb 26;11(2):e0149737. doi: 10.1371/journal.pone.0149737. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.