Abstract
This expert working group report proposes an updated approach to subtype definition of vascular parkinsonism (VaP) based on a review of the existing literature. The persistent lack of consensus on clear terminology and inconsistent conceptual definition of VaP formed the impetus for the current expert recommendation report. The updated diagnostic approach intends to provide a comprehensive tool for clinical practice. The preamble for this initiative is that VaP can be diagnosed in individual patients with possible prognostic and therapeutic consequences and therefore should be recognized as a clinical entity. The diagnosis of VaP is based on the presence of clinical parkinsonism, with variable motor and non-motor signs that are corroborated by clinical, anatomic or imaging findings of cerebrovascular disease. Three VaP subtypes are presented: (1) The acute or subacute post-stroke VaP subtype presents with acute or subacute onset of parkinsonism, which is typically asymmetric and responds to dopaminergic drugs; (2) The more frequent insidious onset VaP subtype presents with progressive parkinsonism with prominent postural instability, gait impairment, corticospinal, cerebellar, pseudobulbar, cognitive and urinary symptoms and poor responsiveness to dopaminergic drugs. A higher-level gait disorder occurs frequently as a dominant manifestation in the clinical spectrum of insidious onset VaP, and (3) With the emergence of molecular imaging biomarkers in clinical practice, our diagnostic approach also allows for the recognition of mixed or overlapping syndromes of idiopathic Parkinson’s disease or other neurodegenerative parkinsonisms and comorbid CVD. Directions for future research are also discussed.
Keywords: Binswanger’s disease, cerebrovascular disease, gait, vascular parkinsonism, imaging
Preamble
Vascular parkinsonism (VaP) is another parkinsonian syndrome in need of more consistent definition. Although previous criteria for VaP have been formulated [1], the persistent lack of consensus on clear terminology and inconsistent conceptual definition of VaP in the existing literature formed the impetus for the current expert working group recommendation report. Despite the progress, in particular in the field of imaging methods, even very recent and well-elaborated reviews differed in the way to understand this term [2, 3]. Vizcarra et al. even called the entire concept into question [2], mainly because the white matter lesions seen on magnetic resonance imaging (MRI) are not always necessarily of vascular origin. Nevertheless, the term “pseudovascular” is inappropriate since it assumes that a different mechanism necessarily exists, which is not evident in most cases. It is clear that the diagnosis of VaP stems from the clinical observation that some parkinsonian patients cannot be classified in a category of neurodegenerative parkinsonism (or other reasonable alternative diagnosis) and where the presence of cerebrovascular impairment is the most parsimonious option.
The often contradictory views arise from the fact that we lack a clear understanding of pathological background of VaP. Clinico-pathological studies would solve many diagnostic doubts, however no sufficiently powered pathological study regarding VaP exist or can be anticipated in the foreseeable future. On the other hand, the emergence of molecular imaging techniques, such as dopamine transporter imaging, shows evidence of overlapping presence of vascular lesions and neurodegeneration in selected patients [4].
The expert working group met first time during the 9th International Congress on Vascular Dementia, in Ljubljana, Slovenia, in October 2015. As an international working group of experts in the field of movement disorders, vascular neurologists and brain MRI and nuclear medicine imaging specialists, we assumed the task to formulate an updated diagnostic approach for VaP subtypes. The preamble for this initiative is that VaP is a heterogeneous parkinsonian syndrome where clinical signs are variable, but their constellation can be diagnostic. More specifically, the working group recognizes that the diagnosis of VaP is based on convergence of clinical parkinsonism (and other clinical signs) with clinical and imaging findings consistent with cerebrovascular disease (CVD). Consequently, we propose more consistent terminology that will facilitate a diagnosis of discrete VaP subtypes. The recent emergence of molecular imaging in clinical practice will also allow for the first time the in vivo classification of a subtype of mixed neurodegenerative parkinsonisms and comorbic CVD. The updated diagnostic approach for VaP is intended to provide a comprehensive tool for clinical practice. It could also facilitate and harmonize further research to better define this entity, distinguish it from primary neurodegenerative parkinsonism and identify overlapping syndromes with mixed pathologies. We acknowledge the need to validate the proposed scheme in prospective studies and ultimate comparison with neuropathological findings.
Terms and definitions
The diagnosis of VaP is based on convergence of clinical parkinsonism with variable pyramidal and ataxic motor and non-motor signs, such as cognitive changes or bladder incontinence, that are corroborated by anatomic or imaging findings of CVD.
There are different subtypes of VaP:
(1) The acute/subacute post-stroke VaP subtype presents with (sub)acute onset of parkinsonism, which is typically asymmetric.
(2) The more frequent insidious onset VaP subtype presents with progressive parkinsonism with lack/insufficiency of levodopa responsiveness, with more prominent postural instability and gait difficulties, including a mixed shuffling-ataxic gait pattern, and upper motor neurons signs or incontinence.
(3) Mixed idiopathic Parkinson’s disease (PD) or other neurodegenerative parkinsonisms and CVD where comorbid vascular lesions may incrementally contribute to the parkinsonian impairments, especially postural instability and gait difficulties.
Methods
This expert working group reviewed articles published from 2006 to 2015 in English, cited in PUBMED database and in Googlescholar.com. Medical Subject Headings (MeSH) search terms included: vascular parkinsonism; cerebrovascular disease; lower body parkinsonism. The inclusion criteria for reviewing an article were: vascular parkinsonism and its definition, diagnostic criteria, epidemiological data, clinical features, symptoms and signs, brain imaging, scales, pathogenesis, risk factors, treatment, or differential diagnosis. The members of the team provided additional references that were not identified by the research strategy detailed above. Consequently, a total of 56 relevant articles served as basis of this expert working group report. Original literature that formed the basis for the concept of vascular parkinsonism, as cited in review articles [2, 3, 5], was used for a conceptual introduction to the topic or historical perspective.
History and epidemiology of vascular parkinsonism
L’hermitte and Cornil (1921) described parkinsonian syndromes associated with pseudobulbar, sphincter and cognitive symptoms as “lacunar” (discussed in [6]). Critchley (1929) coined the term “arteriosclerotic parkinsonism”, which was characterized by classical signs of rigidity, hypomimia, small stepped gait, absence of resting tremor, and often bilateral parkinsonian signs, associated with pseudobulbar palsy, dementia, bladder incontinence, pyramidal and cerebellar signs [7]. Subsequent authors introduced the terms “vascular parkinsonian syndrome” or “vascular parkinsonism” [8–10]. It has been suggested to replace the term “lower body parkinsonism” with “cerebrovascular gait disorder” characterized by a wide-based mixed ataxic-parkinsonian gait pattern [5]. More recently, it has been proposed to refine the term “pure vascular parkinsonism” as due to ischemic or hemorrhagic lesions in the substantia nigra, globus pallidus pars externa, thalamic ventrolateral nuclei, or nigrostriatal pathway versus pseudovascular parkinsonism [2] as a neurodegenerative parkinsonism with nonspecific neuroimaging signal abnormalities. In this respect, vascular pseudoparkinsonism would result from bilateral frontal strokes or apathetic depression from bilateral striatal lacunar strokes while pseudovascular pseudoparkinsonism would include higher-level gait disorders, including normal pressure hydrocephalus (NPH) [2]. Although VaP is defined as parkinsonism resulting from CVD, including in particular white matter lesions (white matter hyperintensities on MRI and lacunes of presumed vascular origin) [5, 8–10], it has been noted that the extent of CVD seen on imaging studies may not always correlate with the clinical phenotype and there is also poor correlation between microangiopathic brain disease and VaP from available clinicopathologic data [2]. Risk factors for cerebral small vessel disease were suggested to include high blood pressure, atherosclerosis, coronary artery disease, smoking, hyperhomocysteinemia, diabetes mellitus, sleep apnea and dyslipidemia [3] (Table 1). Cerebral small vessel disease can be asymptomatic or manifest as lower body parkinsonism, Binswanger’s disease, depression or urinary incontinence [3]. In the absence of clear etiological information, various terms have been used to describe progressive gait impairment in the elderly population with evidence of CVD as gait apraxia, senile gait, high-level gait disorder, psychogenic gait, fear of falling, or frontal lobe gait [3]. The epidemiology of VaP has been difficult to estimate because of discrepancies in diagnostic criteria, ascertainment methods, definition of study populations and because most studies consisted of cross-sectional samples in specialized centers. For example, VaP has been found to be present in about 3–5% in a post-mortem study of patients with parkinsonism [11]. From a series of 220 consecutive brain autopsies of patients with cerebral infarcts, only 5 (2–3%) had a clinical history of parkinsonian symptoms but there was an unclear association between these lesions and the clinical symptoms [12]. There were no correlations between both severity and lateralization of vascular lesions and lateralization and severity of the clinical symptoms [12]. More recently, a prospective cohort study reported the incident development of VaP over a mean period of 5.2 years in 15 patients (3%) out of 503 patients with cerebral small vessel disease who had no parkinsonism at baseline [13]. These data indicate that pure forms of VaP are relatively rare. On the other hand, VaP is often misdiagnosed. A study from the Queen Square Brain Bank reported 28 pathologically confirmed VaP cases of whom only six were diagnosed as VaP during life and the remainder as either PD or atypical parkinsonism [14]. Acute or subacute onset was seen in about one third of patients whereas the remainder had insidious onset.
Table 1.
Risk factors for cerebrovascular disease in VaPark 52
|
Pathophysiology of VaP
Proposed mechanisms of VaP include disruption of cortex-basal ganglia connections and impairment of long loop reflexes leading to impaired sensorimotor integration [3, 15]. The more rare and typically acute or subacute form of VaP can manifest itself when an ischemic or hemorrhagic stroke occurs in the substantia nigra or nigrostriatal pathway [2].
The more common and insidiously developing form of VaP may occur when typically widespread extra-nigrostriatal white or gray matter vascular lesions associate with parkinsonism. Although a distinct focal nigrostriatal deficit does not occur in this insidious type of VaP, it is assumed that parkinsonism occurs when the lesions disrupt connectivity in widespread neural systems underlying bipedal stance and gait that presumably include cortico-striatal-thalamo-cortical loops, interhemispheric fibers, striatothalamic-brainstem systems, and proper processing of multi-modal sensory information [2]. Several systemic medical disorders and lifestyle factors are known to increase the risk of CVD and are often present as comorbid conditions in VaP (Table 1).
Clinical diagnosis of VaP
VaP is a heterogeneous parkinsonian syndrome where clinical signs are variable but their constellation can be diagnostic. However, the individual clinical signs or symptoms in isolation are neither specific nor uniformly present [1, 5, 8, 10, 16].
1. Motor clinical signs of VaP
The typical presentation of the acute or subacute post-stroke subtype of VaP is due to ischemic or hemorrhagic lesions in the substantia nigra or nigrostriatal pathways leading to clinical hemi-parkinsonian signs related to the location of the acute or subacute lesion. Bilateral involvement is also possible. The appearance of post-stroke VaP is a rare non-progressive clinical condition timely related to the occurrence of the stroke. For example, there are several reports about cases of unilateral parkinsonism following infarcts in the territories of lenticulostriate artery [17, 18], and cerebral anterior artery in 6 patients [19]. On the other hand, in the Queen Square Brain Bank study from 28 cases of pathologically confirmed VaP one third had acute or subacute onset of parkinsonism [14].
The typical clinical picture the insidious onset of VaP includes a progressive disorder with a variable combination of symptoms including predominance of lower body symptoms with gait disorder, corticospinal findings, cerebellar signs, rigidity, cognitive impairment and urinary incontinence. Associated symptoms include early postural instability, falls, freezing of gait, rigidity in particular of lower limbs (with so-called ‘lead pipe’ rigidity more appreciable than ‘cogwheeling’ on examination), presence of upper motor neuron signs (less prominent symptoms in the upper limbs), pseudobulbar signs, dysphagia, dysarthria or involuntary laughter or crying, urinary difficulties, and cognitive decline [3]. Atypical or non-supportive features for VaP of the insidious type include responsiveness to levodopa, the presence of classic 4–5 Hz pill rolling resting tremor or visual hallucinations. The occurrence of individual symptoms was reported in several studies with convergent constellation of clinical symptoms [19, 20]. In a pathologically confirmed cohort of VaP bradykinesia was present in all cases, rigidity in 96%, falls in 76%, pyramidal signs in 54%, urinary incontinence in 50% and dementia in 39% but no visual hallucinations [14].
The shuffling-ataxic gait is characterized by a variable base, start and turn hesitation, short steps, shuffling, flexion of hips and knees and postural imbalance (for details see also below). It occurs frequently as an isolated or a dominant clinical sign in patients with vascular risk factors and imaging consistent with CVD. In this respect, it can be the early manifestation of the insidious onset VaP subtype before other symptoms are manifested and illustrate the spectrum nature of insidious onset VaP. The gait impairment would commonly progress to bilateral, fairly symmetric symptoms and mobility disturbances leading eventually to wheelchair bound impairment.
Mixed VaP/neurodegenerative parkinsonism and cerebrovascular disease (mixed PD/CVD subtype)
Patients meeting clinical diagnostic criteria for probable neurodegenerative parkinsonism - PD, diffuse Lewy body disease (DLB), progressive supranuclear palsy (PSP), multiple system atrophy (MSA), corticobasal syndrome (CBS), or non-tau frontotemporal dementia with parkinsonism and have imaging evidence consistent with CVD. The presence of abnormal striatal dopamine transporter imaging findings (not explained by focal nigrostriatal vascular lesions) and/or abnormal cardiac sympathetic nerve terminal imaging findings (not explained by diabetes mellitus or myocardial infarctions) will provide supportive evidence of this diagnostic subgroup. Findings of normal striatal dopamine transporter imaging or excellent response to dopaminergic medication would not support this mixed diagnostic entity. Figure 1 shows illustrative brain MRI and dopamine nerve terminal imaging examples of the proposed different VaP subtypes (Figure 1).
Figure 1.
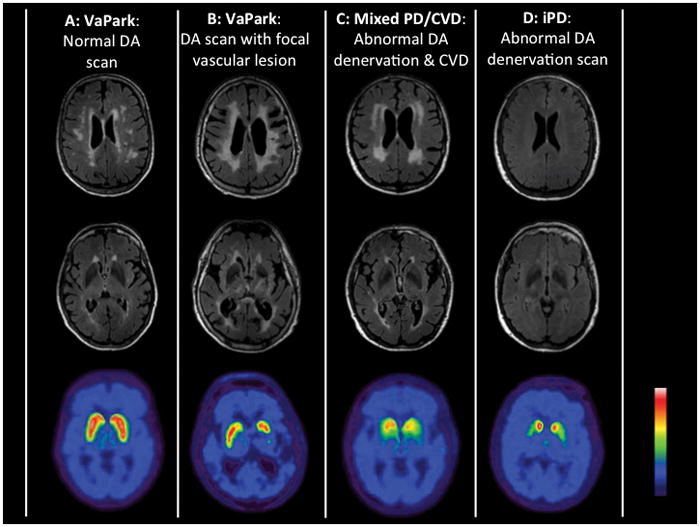
The composite figure illustrates MRI and dopamine nerve terminal imaging examples of proposed diagnostic classifications of pure VaP with either normal dopamine nerve terminal scan (A) or with spatial congruent vascular striatal lesion (B); mixed PD/CVD (C) and pure idiopathic PD (D).
A previous dopamine transporter PET and brain MRI study in patients with idiopathic PD and co-morbid vascular disease demonstrated that associated white matter disease is a greater determinant of axial motor impairments than nigrostriatal dopaminergic denervation [16]. Therefore, a clinical presentation characterized by postural instability and gait difficulties that appear out of proportion for the expected natural history of PD or for the degree of non-axial motor impairments would support a symptomatic manifestation of CVD in this sub-group. The presence of a wide-based gait and/or upper motor neuron signs would provide additional clinical support for this VaP subtype. Interestingly, a recent study in non-PD elderly showed that the clinical manifestations of (axial) parkinsonian in the setting of white matter lesions is dependent on the presence of a low synaptic dopamine polymorphism of the COMT gene (i.e., COMT val carriers), where as COMT met/met carriers did not manifest these symptoms despite the presence of vascular disease [21]. Implications of these observations suggest that PD subjects -who by their very nature of a hypo-dopaminergic state- may have a lower threshold for clinical manifestation of vascular disease.
2. Gait pattern in VaP - Cerebrovascular gait disorder
Abnormal gait would be the defining symptom for the majority of patients with the insidious onset subtype of VaP, where clinical features are progressive and sub-cortical ischemic changes are prominent [1, 10]. Gait disturbances have been reported to be the initial symptom in 90% of VaP patients [22]. The gait pattern in VaP has been variably described as cerebrovascular gait disorder, lower body parkinsonism, lower half parkinsonism, magnetic gait, frontal gait, “marche á petit pas”, slipping clutch syndrome, gait apraxia, vascular pseudoparkinsonism and mixed shuffling/ataxic gait [3, 5, 15, 20, 23–26]. The gait pattern of VaP has been described on the basis of clinical observations and various kinematic and gait analysis techniques [27, 28]. A key distinguishing feature from PD, is the wide based gait in VaP. The posture of patients with VaP may remain upright and the degree of flexion at knees and hips is less compared to PD [28].
Start hesitation and freezing are common features in VaP [10, 29]. Freezing may respond to external cues as in PD. Festination (tendency to move forward with increasingly rapid but smaller steps) is not common in VaP [7, 10]. Stride length in VaP gait is usually short, velocity slow and the patients have a tendency to shuffle [10, 30]. Disequilibrium and poor postural responses are common [3, 5, 10, 27]. Arm swings may be slightly diminished but often remain normal or may even become exaggerated [10]. In severe cases, apraxia of gait is seen where patients are unable to walk despite efforts coming from the upper body, and their feet appearing to be glued to the floor.
Patients with VaP can also show increased variability of stride length and times. The stride length is disproportionately decreased with slower gait and disproportionately increases with faster walking speed [27]. This phenomenon is similar to that seen in ataxia where an altered spatiotemporal gait strategy is needed to compensate for instability. Consequently, there is considerable overlap of parkinsonism and ataxic gait features in patients with VaP with some authors describing the gait pattern as “parkinsonian-ataxic” [15, 24].
3. Cognitive impairment and dementia
There is overlap between VaP and vascular dementia with common occurrence of cognitive impairment in VaP, in particular in the insidious onset subtype. Frequent occurrence of brain atrophy, white matter hyperintensities and lacunes of presumed vascular origin are common in both conditions that likely represent a spectrum disorder. It is possible that VaP and subcortical vascular dementia represent different aspects of a continuum of subcortical vascular encephalopathy (Binswanger’s disease) [3].
4. Urinary symptoms
Urinary symptoms in VaP are characterized by frequency, and later by incontinence [31]. The major underlying pathophysiology is detrusor overactivity likely due to lack of inhibitory frontal cortical control [31].
Neuroimaging in VaP
The diagnosis of CVD, which corroborates the clinical manifestations of VaP, is based on neuroimaging findings. Clinical symptoms of prior CVD, including a history of stroke, rapid or stepwise progression and presence of vascular risk factors provide supportive evidence. Acute strokes are typically seen in the acute/subacute post-stroke subtype of VaP whereas extensive more confluent subcortical small vessel disease is very common in the insidious onset subtype of VaP. Acute or subacute post-stroke VaP typically presents as unilateral clinical motor features of parkinsonism, after ischemic or hemorrhagic stroke affecting the contralateral substantia nigra and/or nigrostriatal pathway. Lesions in these areas generally occur due to occlusion of paramedian branches of mesencephalic arteries. The original criteria proposed by Zijlmans et al1. require a temporal association between the onset of clinical symptoms and CVD which only apply to the acute or subacute post-stroke VaP subtype [32].
The neuroimaging spectrum of CVD is rapidly increasing and includes the traditional white matter signal hyperintensities in MRI and lacunar infarcts but also microbleeds, enlarged Virchow Robin spaces and cortical and subcortical atrophy [33]. The imaging protocols and the description of cerebral small vessel lesions have been highly variable in the literature, which makes it difficult to compare results from different studies and may have led to spurious conclusions. In order to prevent diagnostic misclassification, centers of excellence have proposed standards of reporting vascular changes on neuroimaging, known as the STRIVE criteria [33]. These criteria also include the proposed operationalization of small vessel disease markers. The minimum MRI protocol requires T1, T2 and T2* weighted sequence (2D) and FLAIR, DWI and ADC acquisitions. A few cross-sectional studies showed a relation between small vessel disease and the presence of VaP [34]. With small vessel disease defined on the basis of the STRIVE criteria, a recent longitudinal study showed for the first time a significant relationship between the baseline presence of small vessel disease and the subsequent incidence of parkinsonism [13]. Meanwhile, new MRI acquisition protocols further deepen the spectrum of small vessel disease by investigating its manifestations beyond those seen with conventional MRI, including structural and functional connectivity, microinfarcts or microbleeds [35].
Computed tomography (CT) plays only a limited role in neuroimaging of VaP. Although leukoaraiosis and ventricular enlargement may be suggestive features, small lacunes or non-confluent white matter lesions may not be visible on CT scans and MRI seems to be the gold standard in the neuroimaging diagnosis of VaP. It is clear that the definitive role of brain vascular imaging changes remains to be further investigated as many elderly subjects have imaging changes of leukoencephalopathy yet are completely asymptomatic and also because of discrepancies between clinical symptoms and post-mortem vascular lesions remain [12, 17, 34]. It should be kept in mind, however, that pathology studies are by definition cross-sectional and usually include selected patient populations in advanced stages of their disease, preventing determination of causality.
Dopamine transporter imaging, such as FP-CIT SPECT (DaT SCAN™) is typically abnormal in cases with the acute or subacute post-stroke VaP subtype, where presynaptic striatal dopamine transporter deficiency is congruent with the focal stroke location. Striatal dopamine transporters are generally preserved in insidious onset VaP with the exception of spatial limited and rather symmetrical reduction congruent with striatal vascular disease [36]. Striatal dopamine transporter will be abnormal in patients with mixed neurodegenerative parkinsonism and comorbid CVD. Striatal dopamine transporter imaging assessment may help identifying those patients in whom dopaminergic therapy benefit is less likely: VaP with normal dopamine transporter SPECT, which is very unlikely to respond to dopaminergic drugs [4].
Differential diagnosis and mixed pathologies in VaP
Differential diagnoses of VaP include primary neurodegenerative parkinsonism due to α-synucleinopathy (PD, DLB, MSA), tau or other proteinopathies (such as PSP or CBS), NPH (idiopathic or secondary), myelopathies of non-vascular origin or frontal lobe lesions (tumors, demyelination) [2, 37]. The occurrence of typical symptoms of PD and simultaneous findings of CVD may indicate the coincidence of both diseases (Table 2) [5, 38, 39].
Table 2.
Clinical and imaging features in post-stroke and insidious VaPark subtypes and mixed VaPark/neurodegenerative parkinsonism.
| Diagnosis | Onset | Clinical presentation | Associated features | MRI findings |
|---|---|---|---|---|
| Acute/subacute Post-stroke VaPark | Acute/Subacute | 4–6 Hz resting/postural tremor (+/−) Rigidity with cogwheeling (−/+) Lower body predominance (−) Levodopa response (+) Dementia (−) Upper motor neuron signs (−/+) |
Asymmetry of neurological involvement, robust dopaminergic responsiveness | Contralateral stroke in substantia nigra or nigrostriatal pathway region |
| Insidious VaPark | Insidious | Tremor (−) Rigidity with cogwheeling (−) Predominance on lower body (+) Levodopa response (−) Dementia (+/−) Upper motor neuron signs (+) |
Predominantly symmetrical neurological involvement, cognitive or urinary symptoms | Deep white matter hyperintensities |
| Mixed neurodegenerative parkinsonism and CVD (mixed PD/CVD) | Insidious | 4–6 Hz resting/postural tremor (+/−) Rigidity with cogwheeling (+) Predominance on lower body (−/+) Levodopa response (+) Dementia (−/+) Upper motor neuron signs (−) |
Symmetrical or asymmetrical neurological involvement, cognitive or urinary symptoms | Deep white matter hyperintensities and/or strokes, lacunar infarcts |
Legend: (+) sign present; (−) sign absent; (−/+) sign can be present or absent, more likely absent; (+/−) sign can be present or absent, more likely present.
The clinical entities of NPH and frontal lobe lesions can usually be readily diagnosed by anatomic imaging supplemented by disease-specific ancillary laboratory tests. Specific clinical diagnostic criteria have been developed for primary neurodegenerative parkinsonisms, such as PD, DLB, PSP, MSA or CBS [9, 40–43]. Parkinsonism can also be present in non-tau frontotemporal dementia patients. Behavioral changes, language impairment, and motor neuron disease manifestations in these patients may be present [44, 45]. If clinical uncertainty about the differential diagnosis between VaP and these neurodegenerative parkinsonisms remains then supplemental testing can include presynaptic dopamine or cardiac sympathetic nerve terminal planar or SPECT cardiac imaging using MIBG [46, 47].
Another disorder characterized by gait disorder and incontinence is adult polyglucosan body disease [48] although the existence of peripheral neuropathy should alert to this possibility.
The co-occurrence of vascular disease and clinically manifest primary neurodegenerative parkinsonism is common resulting in mixed pathologies. For example, 19–50% of patients with autopsy confirmed PD were found to have cerebral vascular lesions at autopsy [11, 49, 50]. The comorbid presence of vascular lesions in PD has been selectively associated with abnormal gait and balance disturbances [16]. Similar mixed coincidence of vascular disease and neurodegenerative parkinsonisms can occur with other parkinsonian disorders, such as PSP, MSA or CBD.
Lewy bodies can be seen in 10 – 20% and β-amyloid plaques in up to 44% of cognitively and neurologically normal elderly, which may indicate prodromal proteinopathies [51, 52]. Therefore, the co-occurrence of vascular disease and (prodromal) neurodegenerative proteinopathies is expected to be common in the insidious onset sub-type of VaP as these generally occur in older individuals. Furthermore, cerebral amyloid angiopathy is positively correlated with Lewy body pathology in Lewy body disorders [53], illustrating additional overlap between vascular and neurodegenerative processes.
Older age is a significant risk factor for VaP [13] and normal aging is associated with substantial nigrostriatal dopaminergic degeneration, which can be as high as 7–8% per decade of adult life [54, 55]. Therefore, even in the absence of a primary neurodegenerative disorder, vascular lesions in the elderly are likely to become more symptomatic with greater nigrostriatal dopaminergic cell loss compared to younger age. Age-related amyloidopathy may also exacerbate axial motor burden, at least in PD [56]. In this respect, the expression of parkinsonism and associated non-motor symptoms in elderly subjects with VaP may be confounded by cumulative or interactive effects between vascular lesions, aging and common prodromal proteinopathies.
Conclusions, updated diagnostic approach and future directions
VaP can be diagnosed in individual patients with possible prognostic and therapeutic consequences and therefore should be recognized as a clinical entity. This expert working group report provides a summary of the history, clinical semiology, imaging, autopsy findings, and differential diagnosis. The lack of consensus on clear terminology and inconsistent conceptual definition of VaP in the existing literature formed the impetus for the current expert working group report. Based on a review of the recent literature, the expert panel has formulated an updated diagnostic approach for VaP (Table 3) by proposing more consistent nomenclature for the VaP subtypes. Acute or subacute onset of often asymmetric parkinsonism in the setting of an ischemic or hemorrhagic lesion in the brain stem or nigrostriatal pathways is the hallmark of the (sub)acute post-stroke VaP subtype. Insidious onset VaP subtype presents with developing parkinsonism with additional but variable pyramidal, ataxic and non-motor signs that are corroborated by imaging findings of CVD, most often cerebral small vessel disease, and not meeting diagnostic criteria for neurodegenerative parkinsonism; cerebrovascular gait disorder may be a prodromal or predominant manifestation of insidious VaP. Molecular imaging techniques not only allow the identification of more ‘pure’ sub-types of VaP but also recognize mixed or overlapping syndromes of neurodegenerative parkinsonism and comorbid CVD. Patients meeting clinical diagnostic criteria for probable neurodegenerative parkinsonism and also have imaging evidence of CVD in location(s) that is/are spatially congruent with presenting parkinsonism, in particular postural instability and gait difficulties, likely have the mixed PD/CVD subtype.
Table 3.
Updated diagnostic approach VaPark subtypes
| Acute/subacute Post-stroke VaPark | Insidious VaPark | Mixed neurodegenerative parkinsonism and CVD (mixed PD/CVD) | |
|---|---|---|---|
| Obligatory |
|
|
|
| Supportive |
|
|
|
| Non-supportive |
|
|
|
Legend: CBS, corticobasal syndrome; CVD, cerebrovascular disease; DLB, dementia with Lewy bodies; FTD, frontotemporal dementia; MSA, multiple system atrophy; PD, Parkinson disease; PSP, progressive supranuclear palsy.
The updated diagnostic approach to subtype definition of VaP is intended to facilitate and harmonize further research to better define this entity, distinguish it from primary neurodegenerative parkinsonism and identify overlapping syndromes with mixed pathologies. There is a clear need to validate the diagnostic approach in prospective longitudinal studies and ultimate comparison with neuropathological findings. Validation of these clinical criteria is needed to allow randomized therapeutic studies in VaP. There is a need for multimodal imaging or other biomarker studies to investigate cumulative or interactive effects between pure vascular lesions, aging and age-related prodromal neurodegenerative proteinopathies in the etiopathogenesis of VaP. There is also a critical unmet need to improve treatment of VaP. Therapy that minimizes the vascular risk factors is expected to slow down the rate of decline, but no studies have yet tested this hypothesis. Further research is also needed to identify the precise substrate for parkinsonism in insidious onset VaP, which may involve the investigation of possible hypoperfusion, inflammatory or excitatory toxic effects of primary vascular disease on neural circuitry relevant for mobility functions. Finally, increasing availability of vascular, neurotransmitter and proteinopathy biomarkers will facilitate the investigation of interactive effects between vascular disease, neurodegeneration and aging to better understand, diagnose and treat this special parkinsonian clinical entity.
In this manuscript we present our views on the clinical manifestations of VaP, but have not dealt with therapy of this disorder. Unfortunately, there are no good studies, which discuss these issues. In particular, we do not yet know whether aggressive treatment of the vascular risk factors can slow the progressive nature of the disease.
The assumption that the insidious form of VaP is indeed vascular in origin is based on the common existence of vascular risk factors in these elderly patients, and the interpretation of the white matter lesions seen on MRI as being of vascular origin. The limited data available supports this view but additional pathological reports are certainly needed.
Acknowledgments
The authors would like to thank Jacob Haugen, B.Sc., for editorial assistance.
Abbreviations
- CVD
Cerebrovascular disease
- CBS
corticobasal syndrome
- DLB
dementia with Lewy bodies
- MSA
multiple system atrophy
- PD
Parkinson’s disease
- NPH
normal pressure hydrocephalus
- PSP
progressive supranuclear palsy
- VaP
vascular parkinsonism
Footnotes
- Research Project: A. Conception, B. Organization, C. Execution
- Statistical Analysis: A. Design, B. Execution, C. Review and Critique
- Manuscript: A. Writing of the First Draft, B. Review and Critique
Ivan Rektor:1B,1C,3A,3B
Nicolaas I. Bohnen:1C,3A,3B
Amos D. Korczyn:1A,1B,1C,3A,3B
ViktoriaGryb: 3A,3B
Hrishikesh Kumar:3A,3B
Milica G. Kramberger:3A,3B
Frank-Erik de Leeuw:3A,3B
Zvezdan Pirtošek: 1C,3A,3B
Irena Rektorová:3A,3B
Ilana Schlesinger:3A,3B
Jaroslaw Slawek:3A,3B
Peter Valkovič: 3A,3B
Branislav Vesely: 3A,3B
Disclosures
Dr. Rektor receives supported by the Ministry of Education, Youth and Sports of the Czech Republic under the project CEITEC 2020 (LQ1601). Dr. de Leeuw received a VIDI innovational grant from the Netherlands Organization for Scientific Research (grant number 016.126.351). Dr. Bohnen receives research support from the National Institutes of Health [grant numbers P01 NS015655, R01 NS070856 and P50 NS091856], Michael J. Fox Foundation and the Department of Veterans Affairs [grant numbers I01 RX000317, I01 RX001631, I21 RX001587].
References
- 1.Zijlmans JC, Daniel SE, Hughes AJ, Revesz T, Lees AJ. Clinicopathological investigation of vascular parkinsonism, including clinical criteria for diagnosis. Mov Disord. 2004;19:630–40. doi: 10.1002/mds.20083. [DOI] [PubMed] [Google Scholar]
- 2.Vizcarra JA, Lang AE, Sethi KD, Espay AJ. Vascular Parkinsonism: deconstructing a syndrome. Mov Disord. 2015;30:886–94. doi: 10.1002/mds.26263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korczyn AD. Vascular parkinsonism--characteristics, pathogenesis and treatment. Nat Rev Neurol. 2015;11:319–26. doi: 10.1038/nrneurol.2015.61. [DOI] [PubMed] [Google Scholar]
- 4.Antonini A, Vitale C, Barone P, Cilia R, Righini A, Bonuccelli U, Abbruzzese G, Ramat S, Petrone A, Quatrale R, Marconi R, Ceravolo R, Stefani A, Lopiano L, Zappia M, Capus L, Morgante L, Tamma F, Tinazzi M, Colosimo C, Guerra UP. The relationship between cerebral vascular disease and parkinsonism: The VADO study. Parkinsonism Relat Disord. 2012;18:775–80. doi: 10.1016/j.parkreldis.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Rektor I, Rektorova I, Kubova D. Vascular parkinsonism--an update. J Neurol Sci. 2006;248:185–91. doi: 10.1016/j.jns.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 6.Nutt JG, Marsden CD, Thompson PD. Human walking and higher-level gait disorders, particularly in the elderly. Neurology. 1993;43:268–79. doi: 10.1212/wnl.43.2.268. [DOI] [PubMed] [Google Scholar]
- 7.Critchley M. Arteriosclerotic parkinsonism. Brain. 1929;52:23–83. [Google Scholar]
- 8.Fenelon G, Houeto JL. Vascular Parkinson syndromes: a controversial concept. Rev Neurol (Paris) 1998;154:291–302. [PubMed] [Google Scholar]
- 9.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winikates J, Jankovic J. Clinical correlates of vascular parkinsonism. Arch Neurol. 1999;56:98–102. doi: 10.1001/archneur.56.1.98. [DOI] [PubMed] [Google Scholar]
- 11.Jellinger KA. Prevalence of cerebrovascular lesions in Parkinson’s disease. A postmortem study. Acta Neuropathol. 2003;105:415–9. doi: 10.1007/s00401-002-0634-5. [DOI] [PubMed] [Google Scholar]
- 12.de Reuck J, Sieben G, de Coster W, vander Ecken H. Parkinsonism in patients with cerebral infarcts. Clin Neurol Neurosurg. 1980;82:177–85. doi: 10.1016/0303-8467(80)90035-9. [DOI] [PubMed] [Google Scholar]
- 13.van der Holst HM, van Uden IW, Tuladhar AM, de Laat KF, van Norden AG, Norris DG, van Dijk EJ, Esselink RA, Platel B, de Leeuw FE. Cerebral small vessel disease and incident parkinsonism: The RUN DMC study. Neurology. 2015;85:1569–77. doi: 10.1212/WNL.0000000000002082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glass PG, Lees AJ, Bacellar A, Zijlmans J, Katzenschlager R, Silveira-Moriyama L. The clinical features of pathologically confirmed vascular parkinsonism. J Neurol Neurosurg Psychiatry. 2012;83:1027–9. doi: 10.1136/jnnp-2012-302828. [DOI] [PubMed] [Google Scholar]
- 15.Thompson PD, Marsden CD. Gait disorder of subcortical arteriosclerotic encephalopathy: Binswanger’s disease. Mov Disord. 1987;2:1–8. doi: 10.1002/mds.870020101. [DOI] [PubMed] [Google Scholar]
- 16.Bohnen NI, Muller ML, Zarzhevsky N, Koeppe RA, Bogan CW, Kilbourn MR, Frey KA, Albin RL. Leucoaraiosis, nigrostriatal denervation and motor symptoms in Parkinson’s disease. Brain. 2011;134:2358–65. doi: 10.1093/brain/awr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fenelon G, Houeto JL. Unilateral parkinsonism following a large infarct in the territory of the lenticulostriate arteries. Mov Disord. 1997;12:1086–90. doi: 10.1002/mds.870120642. [DOI] [PubMed] [Google Scholar]
- 18.Murrow RW, Schweiger GD, Kepes JJ, Koller WC. Parkinsonism due to a basal ganglia lacunar state: clinicopathologic correlation. Neurology. 1990;40:897–900. doi: 10.1212/wnl.40.6.897. [DOI] [PubMed] [Google Scholar]
- 19.Kim JS. Involuntary movements after anterior cerebral artery territory infarction. Stroke. 2001;32:258–61. doi: 10.1161/01.str.32.1.258. [DOI] [PubMed] [Google Scholar]
- 20.Sibon I, Fenelon G, Quinn NP, Tison F. Vascular parkinsonism. J Neurol. 2004;251:513–24. doi: 10.1007/s00415-004-0421-4. [DOI] [PubMed] [Google Scholar]
- 21.Rosso AL, Bohnen NI, Launer LJ, Aizenstein HJ, Yaffe K, Rosano C. Vascular and dopaminergic contributions to mild parkinsonians signs in older adults. Neurology. 2018 doi: 10.1212/WNL.0000000000004842. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.FitzGerald PM, Jankovic J. Lower body parkinsonism: evidence for vascular etiology. Mov Disord. 1989;4:249–60. doi: 10.1002/mds.870040306. [DOI] [PubMed] [Google Scholar]
- 23.Gupta D, Kuruvilla A. Vascular parkinsonism: what makes it different? Postgrad Med J. 2011;87:829–36. doi: 10.1136/postgradmedj-2011-130051. [DOI] [PubMed] [Google Scholar]
- 24.Thompson PD, Nutt JG. Gait Disorders. In: Robert B, Gerald M, Joseph J, John C, editors. Bradley’s neurology in clinical practice. 6. Vol. 2012. Philadelphia, PA: Elsevier/Saunders; c2012. pp. 260–72. [Google Scholar]
- 25.Nutt JG. Classification of Gait and Balance Disorders. In: Ružika E, Hallett M, Jankovic J, editors. Advances in Neurology. Philadelphia, PA: Lippincott Williams and Wilkins; 2001. pp. 135–41. [PubMed] [Google Scholar]
- 26.Nutt JG. Higher-level gait disorders: an open frontier. Mov Disord. 2013;28:1560–5. doi: 10.1002/mds.25673. [DOI] [PubMed] [Google Scholar]
- 27.Ebersbach G, Sojer M, Valldeoriola F, Wissel J, Muller J, Tolosa E, Poewe W. Comparative analysis of gait in Parkinson’s disease, cerebellar ataxia and subcortical arteriosclerotic encephalopathy. Brain. 1999;122( Pt 7):1349–55. doi: 10.1093/brain/122.7.1349. [DOI] [PubMed] [Google Scholar]
- 28.Zijlmans JC, Poels PJ, Duysens J, van der Straaten J, Thien T, van’t Hof MA, Thijssen HO, Horstink MW. Quantitative gait analysis in patients with vascular parkinsonism. Mov Disord. 1996;11:501–8. doi: 10.1002/mds.870110505. [DOI] [PubMed] [Google Scholar]
- 29.Factor SA. The clinical spectrum of freezing of gait in atypical parkinsonism. Mov Disord. 2008;23(Suppl 2):S431–8. doi: 10.1002/mds.21849. [DOI] [PubMed] [Google Scholar]
- 30.Bazner H, Oster M, Daffertshofer M, Hennerici M. Assessment of gait in subcortical vascular encephalopathy by computerized analysis: a cross-sectional and longitudinal study. J Neurol. 2000;247:841–9. doi: 10.1007/s004150070070. [DOI] [PubMed] [Google Scholar]
- 31.Sakakibara R, Panicker J, Fowler CJ, Tateno F, Kishi M, Tsuyuzaki Y, Ogawa E, Uchiyama T, Yamamoto T. Vascular incontinence: incontinence in the elderly due to ischemic white matter changes. Neurol Int. 2012;4:e13. doi: 10.4081/ni.2012.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zijlmans JC. The role of imaging in the diagnosis of vascular parkinsonism. Neuroimaging Clin N Am. 2010;20:69–76. doi: 10.1016/j.nic.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw FE, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, Oostenbrugge R, Pantoni L, Speck O, Stephan BC, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M nEuroimaging STfRVco. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–38. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamanouchi H, Nagura H. Neurological signs and frontal white matter lesions in vascular parkinsonism. A clinicopathologic study. Stroke. 1997;28:965–9. doi: 10.1161/01.str.28.5.965. [DOI] [PubMed] [Google Scholar]
- 35.van Veluw SJ, Zwanenburg JJ, Rozemuller AJ, Luijten PR, Spliet WG, Biessels GJ. The spectrum of MR detectable cortical microinfarcts: a classification study with 7-tesla postmortem MRI and histopathology. J Cereb Blood Flow Metab. 2015;35:676–83. doi: 10.1038/jcbfm.2014.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zijlmans J, Evans A, Fontes F, Katzenschlager R, Gacinovic S, Lees AJ, Costa D. [123I] FP-CIT spect study in vascular parkinsonism and Parkinson’s disease. Mov Disord. 2007;22:1278–85. doi: 10.1002/mds.21479. [DOI] [PubMed] [Google Scholar]
- 37.Espay AJ, Narayan RK, Duker AP, Barrett ET, Jr, de Courten-Myers G. Lower-body parkinsonism: reconsidering the threshold for external lumbar drainage. Nat Clin Pract Neurol. 2008;4:50–5. doi: 10.1038/ncpneuro0688. [DOI] [PubMed] [Google Scholar]
- 38.Rektor I, Goldemund D, Sheardova K, Rektorova I, Michalkova Z, Dufek M. Vascular pathology in patients with idiopathic Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:24–9. doi: 10.1016/j.parkreldis.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 39.Rektor I, Goldemund D, Bednarik P, Sheardova K, Michalkova Z, Telecka S, Dufek M, Rektorova I. Impairment of brain vessels may contribute to mortality in patients with Parkinson’s disease. Mov Disord. 2012;27:1169–72. doi: 10.1002/mds.25066. [DOI] [PubMed] [Google Scholar]
- 40.McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 41.Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Durr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–6. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Litvan I, Agid Y, Jankovic J, Goetz C, Brandel JP, Lai EC, Wenning G, D’Olhaberriague L, Verny M, Chaudhuri KR, McKee A, Jellinger K, Bartko JJ, Mangone CA, Pearce RK. Accuracy of clinical criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome) Neurology. 1996;46:922–30. doi: 10.1212/wnl.46.4.922. [DOI] [PubMed] [Google Scholar]
- 43.Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, Boxer AL, Dickson DW, Grossman M, Hallett M, Josephs KA, Kertesz A, Lee SE, Miller BL, Reich SG, Riley DE, Tolosa E, Troster AI, Vidailhet M, Weiner WJ. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80:496–503. doi: 10.1212/WNL.0b013e31827f0fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leverenz JB, Yu CE, Montine TJ, Steinbart E, Bekris LM, Zabetian C, Kwong LK, Lee VM, Schellenberg GD, Bird TD. A novel progranulin mutation associated with variable clinical presentation and tau, TDP43 and alpha-synuclein pathology. Brain. 2007;130:1360–74. doi: 10.1093/brain/awm069. [DOI] [PubMed] [Google Scholar]
- 45.Mosca L, Lunetta C, Tarlarini C, Avemaria F, Maestri E, Melazzini M, Corbo M, Penco S. Wide phenotypic spectrum of the TARDBP gene: homozygosity of A382T mutation in a patient presenting with amyotrophic lateral sclerosis, Parkinson’s disease, and frontotemporal lobar degeneration, and in neurologically healthy subject. Neurobiol Aging. 2012;33:1846, e1–4. doi: 10.1016/j.neurobiolaging.2012.01.108. [DOI] [PubMed] [Google Scholar]
- 46.Navarro-Otano J, Gaig C, Muxi A, Lomena F, Compta Y, Buongiorno MT, Marti MJ, Tolosa E, Valldeoriola F. 123I-MIBG cardiac uptake, smell identification and 123I-FP-CIT SPECT in the differential diagnosis between vascular parkinsonism and Parkinson’s disease. Parkinsonism Relat Disord. 2014;20:192–7. doi: 10.1016/j.parkreldis.2013.10.025. [DOI] [PubMed] [Google Scholar]
- 47.Brigo F, Matinella A, Erro R, Tinazzi M. [(1)(2)(3)I]FP-CIT SPECT (DaTSCAN) may be a useful tool to differentiate between Parkinson’s disease and vascular or drug-induced parkinsonisms: a meta-analysis. Eur J Neurol. 2014;21:1369–e90. doi: 10.1111/ene.12444. [DOI] [PubMed] [Google Scholar]
- 48.Mochel F, Schiffmann R, Steenweg ME, Akman HO, Wallace M, Sedel F, Laforet P, Levy R, Powers JM, Demeret S, Maisonobe T, Froissart R, Da Nobrega BB, Fogel BL, Natowicz MR, Lubetzki C, Durr A, Brice A, Rosenmann H, Barash V, Kakhlon O, Gomori JM, van der Knaap MS, Lossos A. Adult polyglucosan body disease: Natural History and Key Magnetic Resonance Imaging Findings. Ann Neurol. 2012;72:433–41. doi: 10.1002/ana.23598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jellinger KA. The pathology of Parkinson’s disease. Adv Neurol. 2001;86:55–72. [PubMed] [Google Scholar]
- 50.Choi SA, Evidente VG, Caviness JN, Shill HA, Sabbagh MN, Connor DJ, Hentz JG, Adler CH, Beach TG. Are there differences in cerebral white matter lesion burdens between Parkinson’s disease patients with or without dementia? Acta Neuropathol. 2010;119:147–9. doi: 10.1007/s00401-009-0620-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FR, Visser PJ, Aalten P, Aarsland D, Alcolea D, Alexander M, Almdahl IS, Arnold SE, Baldeiras I, Barthel H, van Berckel BN, Bibeau K, Blennow K, Brooks DJ, van Buchem MA, Camus V, Cavedo E, Chen K, Chetelat G, Cohen AD, Drzezga A, Engelborghs S, Fagan AM, Fladby T, Fleisher AS, van der Flier WM, Ford L, Forster S, Fortea J, Foskett N, Frederiksen KS, Freund-Levi Y, Frisoni GB, Froelich L, Gabryelewicz T, Gill KD, Gkatzima O, Gomez-Tortosa E, Gordon MF, Grimmer T, Hampel H, Hausner L, Hellwig S, Herukka SK, Hildebrandt H, Ishihara L, Ivanoiu A, Jagust WJ, Johannsen P, Kandimalla R, Kapaki E, Klimkowicz-Mrowiec A, Klunk WE, Kohler S, Koglin N, Kornhuber J, Kramberger MG, Van Laere K, Landau SM, Lee DY, de Leon M, Lisetti V, Lleo A, Madsen K, Maier W, Marcusson J, Mattsson N, de Mendonca A, Meulenbroek O, Meyer PT, Mintun MA, Mok V, Molinuevo JL, Mollergard HM, Morris JC, Mroczko B, Van der Mussele S, Na DL, Newberg A, Nordberg A, Nordlund A, Novak GP, Paraskevas GP, Parnetti L, Perera G, Peters O, Popp J, Prabhakar S, Rabinovici GD, Ramakers IH, Rami L, Resende de Oliveira C, Rinne JO, Rodrigue KM, Rodriguez-Rodriguez E, Roe CM, Rot U, Rowe CC, Ruther E, Sabri O, Sanchez-Juan P, Santana I, Sarazin M, Schroder J, Schutte C, Seo SW, Soetewey F, Soininen H, Spiru L, Struyfs H, Teunissen CE, Tsolaki M, Vandenberghe R, Verbeek MM, Villemagne VL, Vos SJ, van Waalwijk van Doorn LJ, Waldemar G, Wallin A, Wallin AK, Wiltfang J, Wolk DA, Zboch M, Zetterberg H Amyloid Biomarker Study G. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313:1924–38. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–44. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 53.Ghebremedhin E, Rosenberger A, Rub U, Vuksic M, Berhe T, Bickeboller H, de Vos RA, Thal DR, Deller T. Inverse relationship between cerebrovascular lesions and severity of lewy body pathology in patients with lewy body diseases. J Neuropathol Exp Neurol. 2010;69:442–8. doi: 10.1097/NEN.0b013e3181d88e63. [DOI] [PubMed] [Google Scholar]
- 54.Frey KA, Koeppe RA, Kilbourn MR, Vander Borght TM, Albin RL, Gilman S, Kuhl DE. Presynaptic monoaminergic vesicles in Parkinson’s disease and normal aging. Ann Neurol. 1996;40:873–84. doi: 10.1002/ana.410400609. [DOI] [PubMed] [Google Scholar]
- 55.Bohnen NI, Muller ML, Kuwabara H, Cham R, Constantine GM, Studenski SA. Age-associated striatal dopaminergic denervation and falls in community-dwelling subjects. J Rehabil Res Dev. 2009;46:1045–52. doi: 10.1682/jrrd.2009.03.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muller ML, Frey KA, Petrou M, Kotagal V, Koeppe RA, Albin RL, Bohnen NI. beta-Amyloid and postural instability and gait difficulty in Parkinson’s disease at risk for dementia. Mov Disord. 2013;28:296–301. doi: 10.1002/mds.25213. [DOI] [PMC free article] [PubMed] [Google Scholar]


