Abstract
Background
Despite appropriate therapeutic interventions, progressive optic pathway glioma (OPG) in children may result in loss of vision and other neurologic morbidities. Molecularly targeted therapy against the MAP kinase pathway holds promise in improving outcomes while resulting in lower treatment-related toxicities. We report 2 children with refractory OPG who had a substantial and early reversal of their neurologic deficits and an impressive imaging response of their tumor to BRAFV600E inhibition therapy.
Methods
Two children with OPG (BRAFV600E mutated pilocytic astrocytoma) who did not respond to at least 1 frontline therapy were treated with the oral BRAFV600E inhibitor vemurafenib.
Results
Both children had substantial visual compromise before start of therapy, with one child additionally having motor deficits. Both had an early improvement in their vision, and the second child showed a demonstrable improvement in motor weakness. This was accompanied by a decrease in tumor size, which was sustained at 6 months from therapy. Neither child had significant toxicities except for mild skin sensitivity to vemurafenib.
Conclusions
BRAFV600E inhibitor therapy can potentially reverse visual and neurologic decline associated with progressive OPG. The clinico-radiologic response appears to be prompt and marked. Ongoing clinical trials using BRAFV600E inhibitors can help confirm these early promising findings.
Keywords: MAPK pathway, neurologic deficit, vision recovery, vemurafenib
Introduction
Most children with sporadic or non–neurofibromatosis-1 (NF-1) progressive optic pathway glioma (OPG) experience long-term visual deficits [1]. Although conventional chemotherapy may stabilize the disease and visual impairment, it rarely reverses existing visual or other neurologic deficits [2–4]. Furthermore, children with OPG experience considerable acute and chronic morbidities caused either by the disease or its therapy [5,6]. Less toxic approaches that prevent or reverse neurologic decline are urgently needed to improve the quality of life of these children.
Molecular studies have demonstrated the role of the MAPK pathway in pediatric gliomagenesis [7]. Targeted inhibition of BRAFV600E-mutant protein, an activator of the MAPK pathway, is beneficial in treating central nervous system (CNS) tumors that carry this mutation [8–11]. However, determining whether the mutation is present in an OPG requires biopsy. Traditionally, biopsies of OPG have been avoided due to the high risk of procedure-related complications such as deterioration of vision or vascular insults [12,13]. Surgical intervention is most often used to debulk the tumor when standard treatments have failed or for histologic confirmation when the tumor behaves atypically [13]. Herein, we report 2 patients with refractory OPG with severe visual compromise in which biopsy results guided radiation-sparing treatment decisions. Both patients had somatic targetable BRAFV600E mutations. Both experienced rapid and substantial reversal of neurologic deficits, including visual compromise, with the BRAFV600E inhibitor vemurafenib. Neither child had any stigmata or family history of NF-1. This report demonstrates the prompt and excellent clinical and imaging response to BRAFV600E inhibition in progressive OPG and makes a case for timely biopsy of these lesions in affected children who have not responded to frontline therapy. Written informed consent was obtained from families of both children for describing their clinical course and imaging findings.
Case 1
A 2.5-year-old boy presented with an unsteady gait and gradual loss of vision over a few months. He often ran into objects in his path. Neurologic examination revealed bilateral nystagmus and right cranial nerve VI palsy. Ophthalmologic evaluation demonstrated bilateral optic nerve atrophy. A formal visual acuity test could not be performed due to the patient’s young age, but he was able to fix on an object and follow it with both eyes open. Magnetic resonance imaging (MRI) of the brain and orbits demonstrated a large, expansive non-metastatic mass centered in the optic chiasm and hypothalamus with heterogeneous postcontrast enhancement (Fig. 1A). Given his severe visual compromise and young age, radiation therapy (RT) was not initiated. A stereotactic transtemporal tumor biopsy was done to potentially administer molecularly targeted medications in case conventional chemotherapy was not successful. There were no postoperative complications. Histologically, the lesion was identified as a pilocytic astrocytoma (PA), World Health Organization (WHO) grade I. Duplication or rearrangement of the BRAF gene (7q34) was not detected by fluorescence in situ hybridization, but the presence of a BRAFV600E mutation was confirmed by immunohistochemistry and targeted sequencing. A vincristine/carboplatin and oral temozolomide regimen was initiated [14].
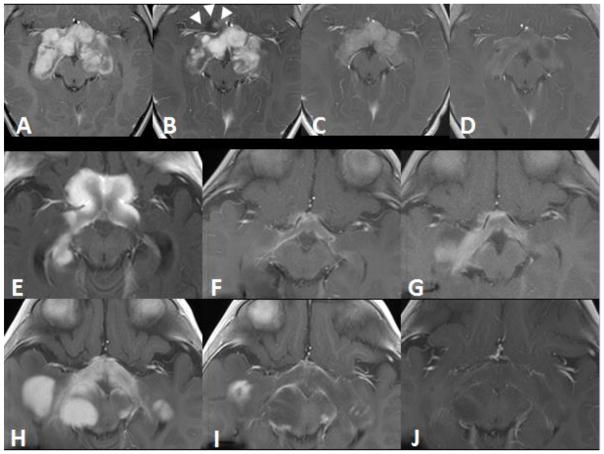
Fig. 1.
Axial contrast-enhanced T1-weighted MRIs in patients 1 (A–D) and 2 (E–J). Patient 1: (A) At baseline, a heterogeneously enhancing mass measuring 7.6×6.4×4.2 cm3 involved the optic chiasm, hypothalamus, and postchiasmatic optic pathways. (B) Six months after chemotherapy was initiated, overall enhancement in mass was slightly decreased and the tumor measured 7.3×6.2×4 cm3; however, a new anterior cystic/solid extension was apparent (arrowheads). (C) Eight weeks after initiating vemurafenib therapy, the lesion had decreased in size and enhancement. (D) At the 5-month follow-up, the tumor further decreased in size and enhancement and measured 5.9×4.4×1.8 cm3. Patient 2: (E) The baseline image shows enhancement and enlargement of the optic chiasm and hypothalamus, with tumor dimensions of 5.6×4.3×2.7 cm3. Also, the right optic pathway was larger than the left optic pathway. (F) After 13 months of chemotherapy, these features slowly improved. (G) At 15 months after initiating chemotherapy, the tumor had increased in size and enhancement. (H) Further progression was obvious after 1 year of weekly vinblastine therapy, and the tumor measured 5.1×9.4×4.5 cm3. (I) After 2 months of vemurafenib, there was dramatic improvement in the lesion. (J) At the 6-month follow-up, the tumor showed continued improvement and measured 4.1×9.2×4 cm3.
Six months from the start of therapy and after an initial period of stable symptoms, the patient’s vision worsened, and progression of the primary lesion was evident on imaging (Fig. 1B). The patient was then able to recognize objects at a distance of only 2 to 3 inches and did not recognize 1-inch pictures on visual testing. Due to his young age, concerns about the long-term toxicity associated with RT, and the positive BRAFV600E finding, we started oral vemurafenib (550 mg/m2, twice daily). Eight weeks after beginning therapy, the patient’s parents noticed that his vision had improved and he no longer ran into objects. Clinical examination revealed that the patient could once again fix on an object and follow it with each eye. He was able to identify 1-inch images at a 2- to 3-inch distance with both eyes open. MRI of the orbits demonstrated more than 50% reduction in tumor volume (Fig. 1C). This early impressive response continued, and tumor size reduced further after 5 months of therapy (Fig. 1D). The patient’s vision improved; he was able to recognize faces and colors and had minimal nystagmus. An ophthalmologic exam demonstrated persistent bilateral optic atrophy. Other than mild cutaneous photosensitivity, the patient experienced no substantial adverse effects.
Case 2
A 6-month-old male infant had an optic pathway and hypothalamic lesion consistent with a low-grade glioma (LGG) in a brain MRI obtained during an evaluation of bilateral nystagmus (Fig. 1E). The patient perceived light but did not track it. He had bilateral optic disc pallor and optic atrophy. MRIs showed evidence of leptomeningeal spread in the brain and cervical spine. A vincristine/carboplatin and oral temozolamide regimen, similar to that administered to the first patient, was initiated. The primary lesion progressively improved over 13 months with stable leptomeningeal disease (Fig. 1F). After approximately 15 months of therapy, the patient’s course was complicated by febrile neutropenia and Mycobacterium chelonae endocarditis, which was treated successfully with appropriate antibiotics. Soon thereafter, the primary lesion progressed (Fig. 1G). The patient was then treated with a weekly vinblastine regimen [15], which transiently stabilized the tumor.
One year into the weekly vinblastine therapy, the patient experienced left-sided weakness. He refused to walk and lost light perception. Imaging showed evidence of progression of the primary tumor (Fig. 1H). A regimen of thioguanine, procarbazine, CCNU, and vincristine [16] was administered for 3 months, but the disease continued to progress. Surgical debulking was considered a poor treatment choice because of the risk of substantial neurologic deficits and, given the patient’s metastatic disease, would at best provide only temporary symptom control. RT was also considered as a treatment option; however, in the setting of metastatic disease, the risk of craniospinal irradiation causing adverse long-term sequelae was high, given his young age and the extent of disease. Therefore, a stereotactic transtemporal tumor biopsy, similar to the first patient, was performed to determine whether the tumor expressed any molecular features that could be targeted. The post-operative period was uneventful. Histopathologic analysis confirmed a PA, WHO grade I. A BRAFV600E mutation was detected by immunohistochemistry and validated by targeted gene sequencing. Vemurafenib therapy was initiated, as done in the first patient. As he was unable to swallow pills, tablets were crushed and administered orally with food.
Two months after initiating vemurafenib, the patient regained left-sided movements and light perception in both eyes, with imaging evidence of improvement in the primary lesion and stable leptomeningeal disease (Fig. 1I). The patient’s mother communicated weekly improvements in his light perception. After 6 months of therapy, the patient had an almost normal range of spontaneous movements of his left extremity, albeit with mild weakness, and he could walk on his own but continued to demonstrate optic atrophy. MRIs showed steady decrease in the primary tumor size (Fig. 1J). As seen for the first patient, the only notable reported toxicity was cutaneous photosensitivity. The second patient experienced an episode of severe sunburn. He now avoids intense sun exposure and has not had any other adverse effects.
For safety monitoring, both patients continue to undergo periodic electrocardiograms and dermatologic evaluations due to the potential risk for QTc prolongation and squamous cell carcinoma, respectively, with vemurafenib therapy. Neither toxicity has been seen in both patients.
Discussion
Visual dysfunction contributes to the substantial morbidity experienced by children with OPG; for most patients, vision loss is not reversed by chemotherapy or RT [17,18,5]. RT produces more durable responses than does chemotherapy in children with LGG [16,19]. However, due to long-term toxicities associated with RT, especially in the very young [6], novel therapy is essential to treat these patients. Targeted therapy against the MAPK pathway holds promise for reversing neurologic dysfunction and sparing RT-associated toxicity [8,9,20,21].
In the presence of typical imaging features of an OPG in patients who do not have NF-1, biopsy confirmation is not routinely done before initiating tumor-directed therapy [22,16]. More recently, molecular studies of LGG have shown that activation of the MAPK pathway is a key event and driving force for most LGGs [7,23]. Tandem duplication of the BRAF oncogene at 7q34, with subsequent fusion to the KIAA1549 gene, is the most common aberration in PA (found in ~75% of cerebellar PAs) [24–26]. However, a smaller but substantial percentage of patients (10%) harbor the point mutation BRAFV600E [23]. Both aberrations result in formation of a mutant protein that constitutively activates the MAPK pathway through the RAS/RAF complex. Thus, both aberrations contribute to gliomagenesis, which makes them attractive targets for inhibitor therapy [24,23]. Although large clinical trials of BRAFV600E inhibitors for treatment of refractory pediatric LGG are ongoing (clinicaltrials.gov), extensive data prove the drug’s utility in adult melanoma. BRAF mutations occur in up to 60% of adult patients with melanomas [27], and BRAFV600E inhibitors improve the outcome and survival in those patients [28]. Furthermore, recent studies report profound responses to BRAF-inhibitor therapy in pediatric patients with brain tumors that express BRAFV600 mutations [20,21,8,11,10,9].
Our case series demonstrates the rapid and dramatic effect of BRAFV600E inhibitor vemurafenib in children with OPG. In patient 1, the tumor size reduced within 3 months of initiating vemurafenib therapy, and in patient 2, symptomatic recovery occurred even sooner. The response was even more pronounced at 6 months for both patients, thus confirming an ongoing effect of BRAFV600E inhibition on the tumors. Both patients had continued improvement in visual and neurologic deficits. Also, both tolerated the medication well, except for mild cutaneous toxicity, which is being symptomatically managed. Importantly, for treating young children who have difficulty swallowing pills, our experience with patient 2 demonstrates that vemurafenib can be powdered and still be effective. Thus, our case series and other reports indicate that vemurafenib has antitumor activity in primary CNS neoplasms [21,11,10,9].
Most clinical trials on OPG have used imaging response as a measure of treatment efficacy. The use of functional improvement, however, is a more useful measure of a clinical trial’s success. Despite this, an accurate and objective assessment of neurologic dysfunction, especially of vision changes, in young children is very challenging, as evidenced by the difficulty in measuring visual acuity in our first patient. The Response Evaluation in Neurofibromatosis and Schwannomatosis visual outcomes committee recommends the use of visual acuity assessment as the main functional end point for OPG clinical trials [29]. The committee also concludes that although it is vital to assess the optic disc for pallor and optic atrophy in children with OPG, the finding of optic atrophy in itself may not always correlate with visual acuity loss. Visual loss in OPG may be due to tumor infiltration or increased tumor vascularity, resulting in vasogenic edema [30]. Moreover, a chiastmatic/hypothalamic location, similar to that seen in our patients, is associated with a worse visual outcome in some studies [18,1]. Tumor shrinkage, with resultant decrease in pressure over the nerves, may have contributed to the reversal of neurologic decline in our patients.
This raises an important question regarding the timing of biopsy of suspected OPG in children who do not have NF-1. Most of these tumors are indolent and cause very gradual loss of vision in some patients. Hence, a careful follow-up with periodic eye examinations, without active intervention, is recommended for these children. However, in patients with progressive vision loss whose tumors did not respond to frontline chemotherapy, who may experience predictably greater toxicity with RT because of young age or require a large RT field because of tumor size, similar to our patients, biopsy needs to be judiciously considered to identify druggable targets. We propose that timely biopsy be performed and not deferred until after the failure of multiple chemotherapy regimens or RT. This approach will not only prevent the development of additional neurologic deficits but also avoid toxicities associated with those modalities. However, we recommend that biopsy be performed in institutions with appropriate pediatric neurosurgery expertise to reduce the risk of morbidity associated with the procedure.
Key questions about the optimal duration of BRAF inhibitor therapy and drug resistance in LGG remain unanswered. This radiologic response and clinical improvement need to be confirmed in large clinical trials that incorporate timely biopsy, when a child with OPG experiences neurologic worsening, and eligible children are treated with BRAFV600E inhibitors without deferring a biopsy until after failure of multiple modalities of therapy.
Acknowledgments
Funding: This work was supported by grant CA21765 from the National Institutes of Health, an unrestricted grant from Research to Prevent Blindness (MEH), and ALSAC.
The authors gratefully acknowledge Dr. Angela McArthur and Dr. Vani Shankar for scientific editing of the manuscript.
Footnotes
Compliance with ethical standards
Ethics approval: The study was approved by the institutional review board of St. Jude Children’s Research Hospital and the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate: Written informed consent was obtained from the patient families for review and publication of this report and any accompanying images.
Conflict of Interests: The authors declare no conflict of interest.
References
- 1.Wan MJ, Ullrich NJ, Manley PE, Kieran MW, Goumnerova LC, Heidary G. Long-term visual outcomes of optic pathway gliomas in pediatric patients without neurofibromatosis type 1. J Neurooncol. 2016 doi: 10.1007/s11060-016-2163-4. [DOI] [PubMed] [Google Scholar]
- 2.Kelly JP, Leary S, Khanna P, Weiss AH. Longitudinal measures of visual function, tumor volume, and prediction of visual outcomes after treatment of optic pathway gliomas. Ophthalmology. 2012;119:1231–1237. doi: 10.1016/j.ophtha.2011.12.035. [DOI] [PubMed] [Google Scholar]
- 3.Moreno L, Bautista F, Ashley S, Duncan C, Zacharoulis S. Does chemotherapy affect the visual outcome in children with optic pathway glioma? A systematic review of the evidence. Eur J Cancer. 2010;46:2253–2259. doi: 10.1016/j.ejca.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 4.Tow SL, Chandela S, Miller NR, Avellino AM. Long-term outcome in children with gliomas of the anterior visual pathway. Pediatr Neurol. 2003;28:262–270. doi: 10.1016/s0887-8994(02)00628-8. [DOI] [PubMed] [Google Scholar]
- 5.Terashima K, Chow K, Jones J, Ahern C, Jo E, Ellezam B, Paulino AC, Okcu MF, Su J, Adesina A, Mahajan A, Dauser R, Whitehead W, Lau C, Chintagumpala M. Long-term outcome of centrally located low-grade glioma in children. Cancer. 2013;119:2630–2638. doi: 10.1002/cncr.28110. [DOI] [PubMed] [Google Scholar]
- 6.Merchant TE, Conklin HM, Wu S, Lustig RH, Xiong X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27:3691–3697. doi: 10.1200/JCO.2008.21.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Wu G, Miller CP, Tatevossian RG, Dalton JD, Tang B, Orisme W, Punchihewa C, Parker M, Qaddoumi I, Boop FA, Lu C, Kandoth C, Ding L, Lee R, Huether R, Chen X, Hedlund E, Nagahawatte P, Rusch M, Boggs K, Cheng J, Becksfort J, Ma J, Song G, Li Y, Wei L, Wang J, Shurtleff S, Easton J, Zhao D, Fulton RS, Fulton LL, Dooling DJ, Vadodaria B, Mulder HL, Tang C, Ochoa K, Mullighan CG, Gajjar A, Kriwacki R, Sheer D, Gilbertson RJ, Mardis ER, Wilson RK, Downing JR, Baker SJ, Ellison DW St. Jude Children’s Research Hospital-Washington University Pediatric Cancer Genome P. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013;45:602–612. doi: 10.1038/ng.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lassaletta A, Guerreiro Stucklin A, Ramaswamy V, Zapotocky M, McKeown T, Hawkins C, Bouffet E, Tabori U. Profound clinical and radiological response to BRAF inhibition in a 2-month-old diencephalic child with hypothalamic/chiasmatic glioma. Pediatr Blood Cancer. 2016;63(11):2038–41. doi: 10.1002/pbc.26086. [DOI] [PubMed] [Google Scholar]
- 9.Rush S, Foreman N, Liu A. Brainstem ganglioglioma successfully treated with vemurafenib. J Clin Oncol. 2013;31:e159–160. doi: 10.1200/JCO.2012.44.1568. [DOI] [PubMed] [Google Scholar]
- 10.Robinson GW, Orr BA, Gajjar A. Complete clinical regression of a BRAF V600E-mutant pediatric glioblastoma multiforme after BRAF inhibitor therapy. BMC Cancer. 2014;14:258. doi: 10.1186/1471-2407-14-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee EQ, Ruland S, LeBoeuf NR, Wen PY, Santagata S. Successful Treatment of a Progressive BRAF V600E-Mutated Anaplastic Pleomorphic Xanthoastrocytoma With Vemurafenib Monotherapy. J Clin Oncol. 2016;34:e87–89. doi: 10.1200/JCO.2013.51.1766. [DOI] [PubMed] [Google Scholar]
- 12.Revere KE, Katowitz WR, Katowitz JA, Rorke-Adams L, Fisher MJ, Liu GT. Childhood Optic Nerve Glioma: Vision Loss Due to Biopsy. Ophthalmic plastic and reconstructive surgery. 2016;33(3S Suppl 1):S107–S109. doi: 10.1097/iop.0000000000000687. [DOI] [PubMed] [Google Scholar]
- 13.Sawamura Y, Kamada K, Kamoshima Y, Yamaguchi S, Tajima T, Tsubaki J, Fujimaki T. Role of surgery for optic pathway/hypothalamic astrocytomas in children. Neuro Oncol. 2008;10:725–733. doi: 10.1215/15228517-2008-033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chintagumpala M, Eckel SP, Krailo M, Morris M, Adesina A, Packer R, Lau C, Gajjar A. A pilot study using carboplatin, vincristine, and temozolomide in children with progressive/symptomatic low-grade glioma: a Children’s Oncology Group studydagger. Neuro Oncol. 2015;17:1132–1138. doi: 10.1093/neuonc/nov057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouffet E, Jakacki R, Goldman S, Hargrave D, Hawkins C, Shroff M, Hukin J, Bartels U, Foreman N, Kellie S, Hilden J, Etzl M, Wilson B, Stephens D, Tabori U, Baruchel S. Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J Clin Oncol. 2012;30:1358–1363. doi: 10.1200/JCO.2011.34.5843. [DOI] [PubMed] [Google Scholar]
- 16.Ater JL, Zhou T, Holmes E, Mazewski CM, Booth TN, Freyer DR, Lazarus KH, Packer RJ, Prados M, Sposto R, Vezina G, Wisoff JH, Pollack IF. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30:2641–2647. doi: 10.1200/JCO.2011.36.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Awdeh RM, Kiehna EN, Drewry RD, Kerr NC, Haik BG, Wu S, Xiong X, Merchant TE. Visual outcomes in pediatric optic pathway glioma after conformal radiation therapy. International journal of radiation oncology, biology, physics. 2012;84:46–51. doi: 10.1016/j.ijrobp.2011.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dodgshun AJ, Elder JE, Hansford JR, Sullivan MJ. Long-term visual outcome after chemotherapy for optic pathway glioma in children: Site and age are strongly predictive. Cancer. 2015;121:4190–4196. doi: 10.1002/cncr.29649. [DOI] [PubMed] [Google Scholar]
- 19.Merchant TE, Kun LE, Wu S, Xiong X, Sanford RA, Boop FA. Phase II trial of conformal radiation therapy for pediatric low-grade glioma. J Clin Oncol. 2009;27:3598–3604. doi: 10.1200/jco.2008.20.9494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aguilera D, Janss A, Mazewski C, Castellino RC, Schniederjan M, Hayes L, Brahma B, Fogelgren L, MacDonald TJ. Successful Retreatment of a Child with a Refractory Brainstem Ganglioglioma with Vemurafenib. Pediatr Blood Cancer. 2016;63:541–543. doi: 10.1002/pbc.25787. [DOI] [PubMed] [Google Scholar]
- 21.del Bufalo F, Carai A, Figa-Talamanca L, Pettorini B, Mallucci C, Giangaspero F, Antonelli M, Badiali M, Moi L, Bianco G, Cacchione A, Locatelli F, Ferretti E, Mastronuzzi A. Response of recurrent BRAFV600E mutated ganglioglioma to Vemurafenib as single agent. J Transl Med. 2014;12:356. doi: 10.1186/s12967-014-0356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker DA, Liu J, Kieran M, Jabado N, Picton S, Packer R, St Rose C. A multi-disciplinary consensus statement concerning surgical approaches to low-grade, high-grade astrocytomas and diffuse intrinsic pontine gliomas in childhood (CPN Paris 2011) using the Delphi method. Neuro Oncol. 2013;15:462–468. doi: 10.1093/neuonc/nos330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schindler G, Capper D, Meyer J, Janzarik W, Omran H, Herold-Mende C, Schmieder K, Wesseling P, Mawrin C, Hasselblatt M, Louis DN, Korshunov A, Pfister S, Hartmann C, Paulus W, Reifenberger G, von Deimling A. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121:397–405. doi: 10.1007/s00401-011-0802-6. [DOI] [PubMed] [Google Scholar]
- 24.Jones DT, Gronych J, Lichter P, Witt O, Pfister SM. MAPK pathway activation in pilocytic astrocytoma. Cell Mol Life Sci. 2012;69:1799–1811. doi: 10.1007/s00018-011-0898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacob K, Albrecht S, Sollier C, Faury D, Sader E, Montpetit A, Serre D, Hauser P, Garami M, Bognar L, Hanzely Z, Montes JL, Atkinson J, Farmer JP, Bouffet E, Hawkins C, Tabori U, Jabado N. Duplication of 7q34 is specific to juvenile pilocytic astrocytomas and a hallmark of cerebellar and optic pathway tumours. Br J Cancer. 2009;101:722–733. doi: 10.1038/sj.bjc.6605179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones DT, Kocialkowski S, Liu L, Pearson DM, Backlund LM, Ichimura K, Collins VP. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68:8673–8677. doi: 10.1158/0008-5472.CAN-08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 28.Hauschild A, Grob JJ, Demidov LV, Jouary T, Gutzmer R, Millward M, Rutkowski P, Blank CU, Miller WH, Jr, Kaempgen E, Martin-Algarra S, Karaszewska B, Mauch C, Chiarion-Sileni V, Martin AM, Swann S, Haney P, Mirakhur B, Guckert ME, Goodman V, Chapman PB. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 29.Fisher MJ, Avery RA, Allen JC, Ardern-Holmes SL, Bilaniuk LT, Ferner RE, Gutmann DH, Listernick R, Martin S, Ullrich NJ, Liu GT. Functional outcome measures for NF1-associated optic pathway glioma clinical trials. Neurology. 2013;81:S15–24. doi: 10.1212/01.wnl.0000435745.95155.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avery RA, Hwang EI, Jakacki RI, Packer RJ. Marked recovery of vision in children with optic pathway gliomas treated with bevacizumab. JAMA Ophthalmol. 2014;132:111–114. doi: 10.1001/jamaophthalmol.2013.5819. [DOI] [PubMed] [Google Scholar]