Abstract
Orchids are diverse, occur in a wide range of habitats and dominate threatened species lists, but which orchids are threatened, where and by what? Using the International Union for Conservation of Nature Red List, we assessed the range and diversity of threats to orchids globally including identifying four threat syndromes: (1) terrestrial orchids in forests that are endemic to a country and threatened by illegal collecting; (2) orchids threatened by climate change, pollution, transportation and disturbance/development for tourism, and recreation activities, often in East Asia; (3) epiphytic orchids in Sub-Saharan Africa including Madagascar with diverse threats; and (4) South and Southeast Asia orchids threatened by land clearing for shifting agriculture. Despite limitations in the Red List data, the results highlight how conservation efforts can focus on clusters of co-occurring threats in regions while remaining aware of the trifecta of broad threats from plant collecting, land clearing and climate change.
Electronic supplementary material
The online version of this article (doi:10.1007/s13280-017-0964-0) contains supplementary material, which is available to authorized users.
Keywords: Conservation, Global biodiversity, Human impacts, Illegal plant collection, Threatened species
Introduction
Orchids are the world’s most diverse group of flowering plants with an estimated 26 567 species within 850–100 genera that account for 10% of angiosperms worldwide (Cribb et al. 2003; Jones 2006). Although individual orchid species often have narrow distributions, the family itself is widespread with orchids found on every continent except Antarctica (Jones 2006). They occur in a range of habitats including forests, wetlands, shrublands and grasslands and include terrestrial, epiphytic and lithophytic growth forms (Jones 2006). The increasing popularity of orchids among collectors, along with factors such as land clearing and climate change, means they are not only among the most diverse plants, but also among the most often threatened (Roberts and Dixon 2008).
The specific biological association of many orchid species with other organisms means that individual orchid species often occur as small isolated populations, heightening their risk of extinction (Swarts and Dixon 2009a). For example, many orchids rely on specific associations with fungi for germination, growth and nutrients (Swarts and Dixon 2009a). In addition, some orchids have very precise pollination mechanisms with elaborate systems for attracting pollinators, restricting the orchid’s distribution to that of its specific pollinators (Nilsson 1992; Cozzolino and Widmer 2005). Others, including many epiphytic orchids, have tight associations with their host plants, again limiting the orchid’s distribution to that of their host (Santos 2000). These factors contribute to many orchids facing increasing risks of extinction from a range of threatening processes (Swarts and Dixon 2009a).
Threats to plants, including orchids, are numerous and diverse and include those from co-occurring anthropogenic activities acting as threat syndromes (Burgman et al. 2007; IUCN 2016). Globally, habitat loss is recognised as the major threat to biodiversity with extensive areas of vegetation cleared annually, including in areas of high orchid diversity (Brummitt et al. 2015). Land clearing and other activities, including the construction of roads, can further fragment remaining vegetation adding to the threats for plants with specialist habitat requirements (Laurance et al. 2015). Habitat loss and fragmentation has already contributed to significant population declines in orchids. For example, in the UK, the distribution of many orchids has contracted by 50%, while in Estonia distributions have contracted by 25% (Kull and Hutchings 2006).
Increasing access to remote locations can amplify the threats not only from land clearing and fragmentation, but also by providing more opportunities for people to collect plants from the wild. Harvesting plants from the wild is a $US 21 thousand million industry with wild plant products used in medicines, horticulture and food (Rosen and Smith 2010). Some rare and threatened orchid species are specifically targeted by collectors due to their charismatic appearance, diversity of floral forms and natural rarity. This has contributed to the extinction of many species from attractive orchid genera such as Paphiopedilum and Cypripedium (Ballantyne and Pickering 2012; Phelps and Webb 2015; Hinsley et al. 2016). Although the trade in all wild orchids is restricted between countries under the Convention on International Trade in Endangered Species (CITES), illegal collection and underground markets in wild orchids within, and between, countries still flourish (Roberts and Dixon 2008).
Climate change is rapidly becoming the greatest threat to plants, including orchids (Seaton et al. 2010). It is projected that climate change will drive 15–37% of all taxa to extinction by 2050, including 56 000 endemic plant species in biodiversity hotspots (Thomas et al. 2004; Malcolm et al. 2006). Orchids are particularly at risk from climate change because of factors such as their narrow ranges and specific symbiotic associations with fungi and pollinators. Climate change is likely to further limit the availability of suitable habitats for orchids, as well as enhance existing threats such as those from droughts, fires and the spread of weeds (Gradstein 2008; Seaton et al. 2010). There is increasing recognition of all these threatening processes, but which are the most common threats to orchids globally, where, and do they co-occur as threat syndromes?
The aim of this study was to determine the most common threats to orchids globally and outline potential strategies to combat them. To do this, we used data from the International Union for Conservation of Nature (IUCN) Red List as it is the most comprehensive global database of threatened species, and because it uses a standardised set of criteria for listing species and threats (Rodrigues et al. 2006; Brummitt et al. 2015; IUCN 2016; Kull et al. 2016). Specifically, we assessed (1) the most common broad threats to orchids globally, (2) whether threats co-occur as threat syndromes, (3) where threats were more likely to occur, e.g. which threats are associated with which land regions, (4) whether there are patterns between orchid habitats and common threats, (5) the relationship between the growth forms of orchids (e.g. terrestrial, epiphytic or lithophytic) and threats, (6) what is threatening the most commonly listed orchid genera, and (7) finally, at a fine scale of threat category, which threats are correlated with land region, growth form, habitat and endemism?
Materials and Methods
There were 519 Orchidaceae species on the IUCN Red List listed as Critically Endangered (CR), Endangered (EN) or Vulnerable (VU) in October 2016. To assess global patterns in threats to orchids, we collected data for the 442 threatened orchid species with threat data on the Red List including the species name, IUCN status, habitat and where it occurs (e.g. land region, native countries and endemism), along with data on the 12 broad categories of threats and detailed threats for each category (Appendix Table S1). Additional data about the growth form of the orchids (e.g. if it was terrestrial, epiphytic or lithophytic) was obtained from the World Checklist of Selected Plant Families (WCSP 2016).
Data analysis
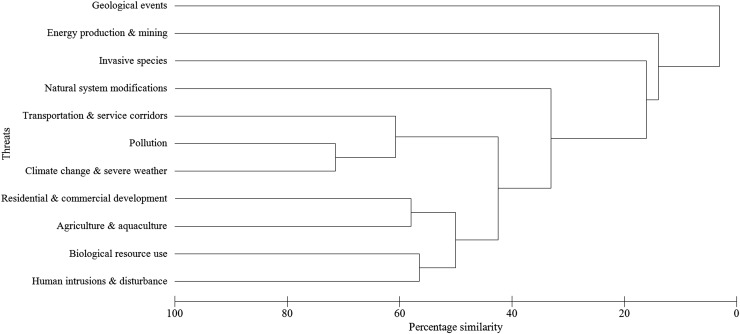
The total number and proportion of orchid species threatened globally were calculated for different regions, habitat, growth forms and genera. To assess if certain threats co-occurred among orchids, a hierarchical clustering of threats into groups was performed. The clustering used the results of a Bray–Curtis similarity matrix constructed in the ordination program Primer 6 with data on the broad threats for the 442 species of orchids (Clarke and Gorley 2006). The results were expressed visually as a dendrogram showing the percentage similarity among the threats based on the number of orchid species with the same threats.
To assess geographical patterns in threats, the distribution of orchids among regions was mapped for the four most common broad threats using ArcGIS (ESRI 2016). To determine whether there were significant differences in threats depending on whether the species occur within a protected area, and if they are endemic, a series of χ 2 analyses were performed using the statistical program RStudio (RStudio Team 2016). There were many threatened orchids from just two genera on the Red List: Paphiopedilum and Cypripedium. Therefore, patterns in threats for these two genera were analysed separately to see if they had distinct combinations of threats and locations compared to the rest of the genera. Finally, to explore potential threat syndromes and their relationship with these other factors in more detail, we repeated the dendrogram analysis using Red List data on more detailed levels of threats and combined this with information on land regions, growth forms and habitat types in the ordination program Primer 6 (Clarke and Gorley 2006).
Results
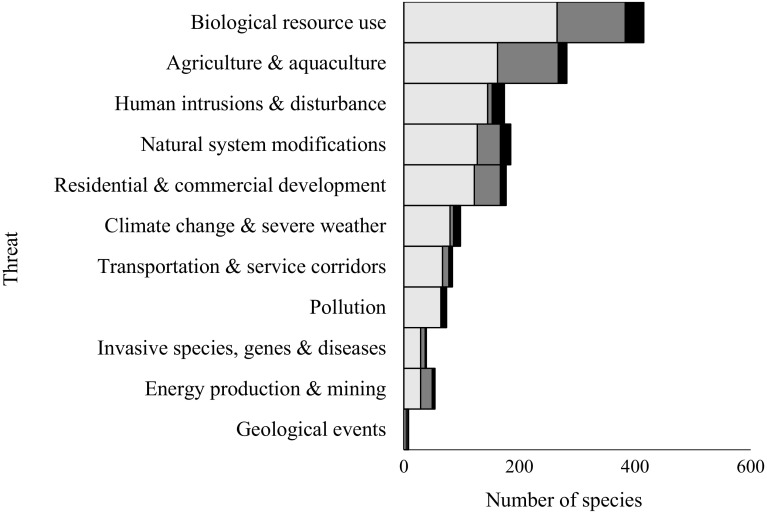
There is a wide diversity of threats to orchids globally, with orchids threatened by 11 of the 12 broad categories used in the IUCN Red List (Appendix Table S1). The most common threat was biological resource use (80% of the 442 species of orchids), followed by agriculture and aquaculture (53%) (Appendix Table S1). Human intrusion and disturbance affected 36% of the species, residential and commercial development threatened 35.5% of species, while modifications to natural systems affected 35% of the 442 orchids.
Most orchids were at risk from more than one type of threat, with an average of three threats per species and only 170 species listed with a single major threat. Some threats co-occurred as potential threat syndromes, indicating that many species are not only affected by one type of threat but commonly threatened by a pattern of co-occurring threatening processes (Fig. 1). Many orchids threatened by pollution, for example, were also threatened by climate change and from the impact of transport and service corridors (Fig. 1). Orchids threatened by residential and commercial development were often also threatened by agriculture and aquaculture while these two threats were both correlated with threats from biological use along with human intrusion and disturbance (Fig. 1). In contrast, the few species of orchids threatened by geological events (6 species), energy production and mining (45) or invasive species and diseases (36) were rarely also at risk from other threats.
Fig. 1.
Bray–Curtis similarity resemblance plot showing the levels of similarity among threats to the 442 orchid species on the IUCN Red List
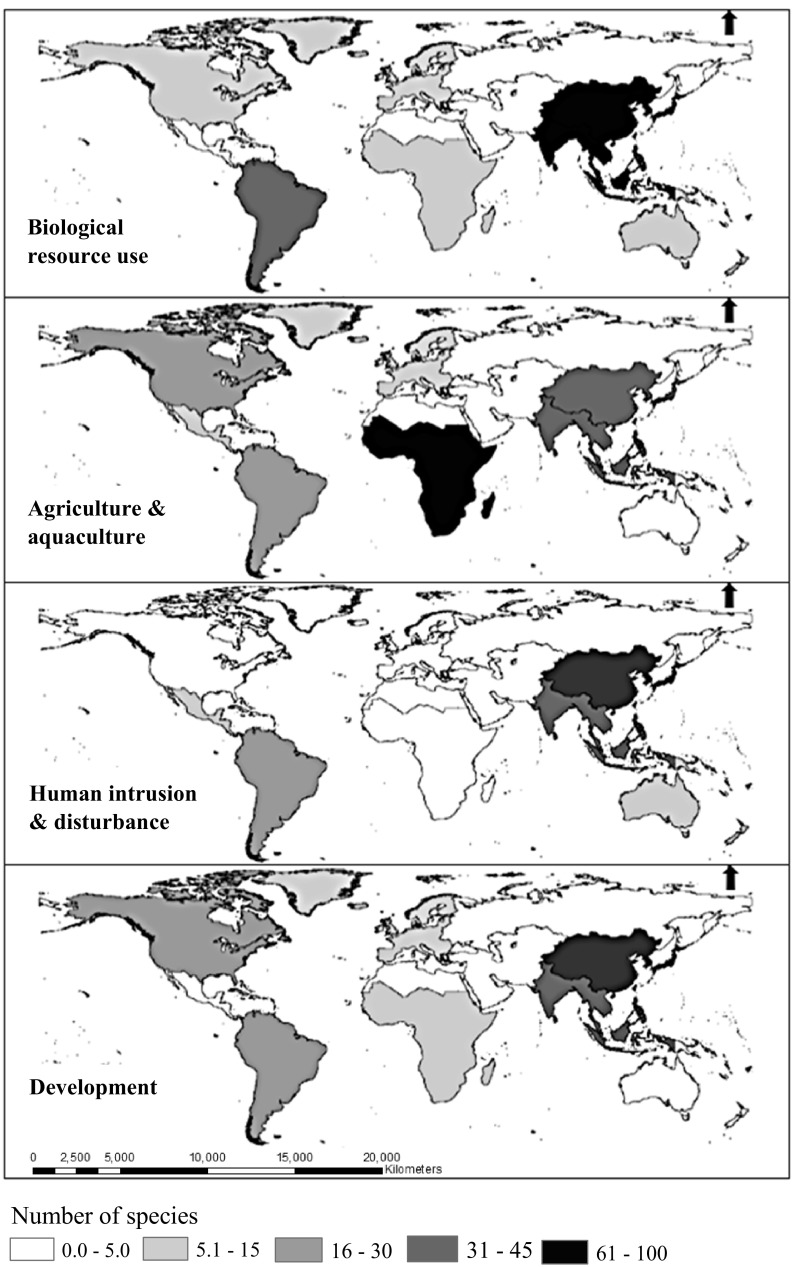
It was clear that threats to orchids varied among regions, both in terms of the types of threats, and how many species were affected (Fig. 2; Table 1). Sub-Saharan Africa had the highest number of threatened orchids (194 species) and the most common threats were agriculture and aquaculture (92 species), and modifications to natural systems (59 species). Over half of the Sub-Saharan Africa threatened orchids (98 species) were from Madagascar, with biological resource use threatening nearly all of them (91 species). Other regions with high numbers of threatened orchids included South and Southeast Asia (15% of orchids on the Red List), East Asia (14.5% of species) and South America (11% of species, Fig. 2). Orchids in these regions were most commonly affected by biological resource use, residential and commercial development, and human intrusion and disturbance (Fig. 2; Table 1).
Fig. 2.
The number of orchid species in different regions threatened by biological resource use, agriculture and aquaculture, human intrusion and disturbance or development, based on data for 442 species of orchids on the IUCN Red List
Table 1.
Threats to the 442 orchid species on the IUCN Red List per region, including the total number of species, the number endemic to a single country and the number of species in protected areas
| Sub-Saharan Africa | South and Southeast Asia | East Asia | South America | North America | Europe | Mesoamerica | Oceania | Caribbean Islands | North Africa | |
|---|---|---|---|---|---|---|---|---|---|---|
| Endemic | 104 | 47 | 30 | 32 | 1 | 15 | 1 | 10 | 5 | 2 |
| Protected area | 67 | 24 | 26 | 11 | 14 | 3 | 4 | 2 | 5 | 1 |
| Total listed with threats | 194 | 68 | 65 | 47 | 19 | 16 | 12 | 11 | 8 | 2 |
| Biological resource use | 130 | 68 | 65 | 43 | 12 | 12 | 10 | 7 | 5 | |
| Agriculture and aquaculture | 92 | 37 | 33 | 23 | 16 | 13 | 8 | 4 | 5 | 2 |
| Human intrusions and disturbance | 3 | 42 | 58 | 18 | 10 | 10 | 6 | 8 | 1 | 2 |
| Residential and commercial development | 13 | 34 | 46 | 18 | 18 | 13 | 5 | 2 | 8 | |
| Natural system modifications | 59 | 26 | 19 | 19 | 12 | 10 | 5 | 4 | 1 | |
| Climate change and severe weather | 4 | 19 | 47 | 2 | 7 | 1 | 3 | 2 | 1 | |
| Transportation and service corridors | 4 | 43 | 13 | 6 | 6 | 1 | 1 | 2 | 1 | |
| Pollution | 4 | 48 | 2 | 8 | 3 | 1 | 2 | |||
| Energy production and mining | 21 | 5 | 5 | 10 | 3 | 1 | ||||
| Invasive species, genes and diseases | 7 | 1 | 1 | 7 | 10 | 2 | 2 | 4 | 2 | |
| Geological events | 2 | 1 | 1 | 1 | 1 |
Over half of all threatened orchids had limited distributions, with 247 species naturally restricted to single countries (i.e. country endemics), but the proportion of country endemics varied significantly among regions (χ 2 tests, p < 0.001, Table 1). Just over half of the orchids from Sub-Saharan Africa were endemic to a country, including nearly all (94 species) of the orchids in Madagascar. Nearly all the European and Oceanian Red Listed orchids were also country endemics, while very few of those listed as threatened in North America and Mesoamerica were country endemics (Table 1). Country endemic orchids were more likely to be threatened by energy production and mining, biological resource use and natural systems modification (χ 2 tests, p < 0.001) than orchids that occur naturally in more than one country (Table 1).
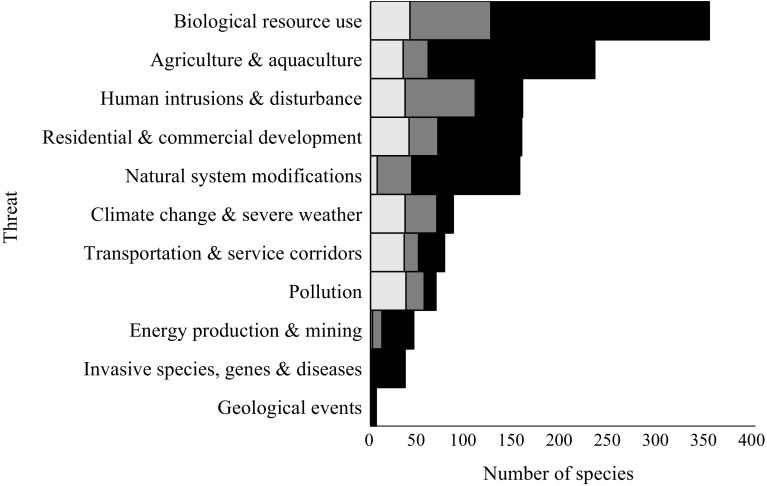
Growth forms of orchids, i.e. if they are terrestrial, epiphytic or lithophytic, differed in the types of threats they are exposed to (χ 2 tests, p < 0.001). Most of the Red List orchids are terrestrial and for these orchids biological resource use was the most common threat (82% of the 324 species), followed by agriculture and aquaculture (50% of species) and human intrusion and disturbance (45% of species, Fig. 3). Epiphytic orchids account for 162 of the Red Listed orchids. Proportionally fewer of these epiphytic orchids were threatened by biological resource use (73%) and development (28%) than terrestrial orchids, but they were more likely to be threatened by agriculture and aquaculture (65%) (Fig. 3). There are few lithophytic orchids on the Red List (37 species), of which 86% are threatened by biological resource use, 57% by human intrusion and disturbance and 48% by natural system modifications (Fig. 3).
Fig. 3.
The number of orchid species of each growth form affected by each of the major types of threats on the IUCN Red List, terrestrial species (light grey), epiphytic (mid grey) and lithophytic species (black)
Although there were 94 genera of orchids with at least one species on the Red List, two terrestrial and charismatic genera, Paphiopedilum and Cypripedium alone accounted for 125 of the Red List orchid species. Of the 101 recognised species of Paphiopedilum orchids (WCSP 2016), 84 are on the Red List. Like other orchids, they are mainly threatened by biological resource use (83% of species) and human intrusion and disturbance (72%). Fewer Paphiopedilum orchids were threatened by agriculture and aquaculture (31%) than expected based on the patterns for other orchid genera (Fig. 4).
Fig. 4.
The number of Cypripedium (light grey) and Paphiopedilum (mid grey) orchids affected by the 11 major types of threats on the IUCN Red List compared to all other genera (black)
There are 41 Red Listed Cypripedium species (out of the 64 species recognised internationally) (WCSP 2016). Individual Cypripedium species tended to be threatened by a wide diversity of factors, and were proportionally more likely to be threatened by pollution (90% of species), climate change and severe weather (88%) or transportation and service corridors (85%) than other orchids (Fig. 5). However, they were also still threatened by other common factors including biological resource use, human intrusion and disturbance and development (Fig. 4).
Fig. 5.
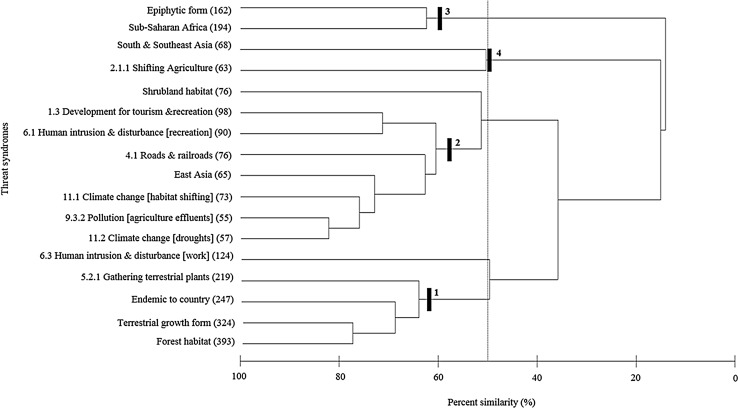
Bray–Curtis similarity resemblance plot showing the levels of similarity among threats, land regions, habitats, endemism and growth form to the 442 orchid species on the IUCN Red List and the four identified threat syndromes labelled 1–4 based on percent similarity, including (1) terrestrial species of orchids in forests that are endemic to at least one country and that are threatened by illegal plant collection; (2) orchids in East Asia affected by climate change, pollution, transportation including roads and railroads, and disturbance/development for tourism and recreation activities; (3) epiphytic species in Sub-Saharan Africa including in Madagascar, but with a range of threats; and (4) orchids in South and Southeast Asia that are affected by land clearing for non-timber agricultural crops
Finally, when we looked at threats at the finest scale on the Red List, including how they were correlated with land region, growth form and habitat, four threat syndromes were apparent (Fig. 5). They were (1) terrestrial species of orchids in forests that are endemic to at least one country and that are threatened by illegal plant collection; (2) orchids in East Asia affected by climate change, pollution, transportation including roads and railroads, and disturbance/development for tourism and recreation activities; (3) epiphytic species in Sub-Saharan Africa including in Madagascar, but with a range of threats; and (4) orchids in South and Southeast Asia that are affected by land clearing for non-timber agricultural crops.
Discussion
Common threats and threat syndromes
Many orchid species are threatened globally, particularly by biological resource use, agriculture and aquaculture, human intrusion and disturbance along with residential and commercial development. These threats were also found to be important for all plants in a recent review of threatening processes that also used IUCN Red List data (Brummitt et al. 2015). For orchids, however, human intrusion and disturbance was a more common threat than for some other types of plants.
Identifying which orchids are at risk of extinction and using data from well-regarded threatened species lists is important for conservation. These lists directly affect regulations and legal processes for the trade in orchids, affect approval for land clearing, shape management strategies, and inform the broader communities of the ongoing threats to these charismatic species (Burgman et al. 2007; Brummitt et al. 2015; Kull et al. 2016). Increasingly, researchers are interested in using such lists to identify threat syndromes which allow more targeted and effective management of threats than treating them as independent problems (Burgman et al. 2007). Here we found co-occurring threats to orchids which were associated with specific habitats, regions and growth forms allowing more targeted management of threats to orchids.
Threat syndrome one
Biological resource use was the most common threat for orchids, which is not surprising, as it includes the illegal collection of plants from the wild for medicine, food and trade (Subedi et al. 2013). Specifically, we found that illegal collecting of orchids was often a threat for terrestrial orchids in forests with limited distributions (e.g. country endemics) and, hence, form a threat syndrome. This combination of threat, growth form and habitat was important in several regions, with all listed orchids in South and Southeast Asia and East Asia threatened by biological resource use. This potentially reflects, at least in part, the extent to which orchids are used as medicinal plants within these regions. For example, a quarter of the native orchids in China and 347 species of orchids in Southeast Asia are collected for medicinal purposes, contributing to declines in orchid populations in the region (Liu et al. 2014; Phelps and Webb 2015).
The collecting of charismatic orchids for cultivation and propagation is also problematic, with the trade in orchids worth millions of dollars each year (NASS 2016). This is particularly important for orchids with charismatic flowers, such as many Paphiopedilum species, that have been the focus of collection by orchid enthusiasts for a long time (Phelps and Webb 2015). This has already driven some of these orchids, such as Paphiopedilum vietnamese, to extinction (Roberts and Dixon 2008) and collecting currently threatens nearly all extant Paphiopedilum species.
Strategies to reduce illegal plant collecting from the wild include (1) conservation reserves, (2) legal propagation of desirable orchids to help satisfy demand, (3) enforcement of restrictions on collecting and the trade in these orchids and (4) educating collectors and others to enhance support for orchid conservation. In addition to general protected areas, some countries have established specific orchid reserves to help reduce the demand for harvesting orchids from the wild. Establishing and supporting sustainable and legal orchid breeding/propagation programs can also satisfy the demand for some species of orchids including horticultural, medicinal and edible orchids (Subedi et al. 2013). For example, programs have been established in some areas in China to support the legal cultivation of attractive epiphytic, perennial orchids including Dendrobium species (Liu et al. 2014). In some countries, botanic gardens are involved in propagating rare orchids for use in their collections, for replanting in the wild, but also in some cases, for sale to the public to reduce the demand for wild orchids (Swarts and Dixon 2009b). Despite these types of approaches, enforcement of laws and regulations restricting the collection and trade in orchids remains important. With ongoing demand in new types and forms of orchids, it is critical that collectors and horticulturists adhere to regulations restricting the trade in some orchids including CITES (Hinsley et al. 2016). Finally, education is important and includes the work of orchid societies which raise awareness of their members about these issues, including what is threatening orchids, where, and how they can contribute to their conservation (Cuoco and Cronan 2009).
Threat syndrome two
Both climate change and pollution threaten orchids, including many of the same species of orchids. Climate change is increasingly threatening plants globally and orchids are no exception. Our study showed climate change, an important emerging threat, was strongly associated with threatened orchids in East Asia. For example, with climate change, suitable conditions for some orchids in the tropics of south-western China will only occur much higher up mountains (Liu et al. 2015). More broadly, climate change is known to be a major threat to specialist habitats such as cloud forests, threatening species restricted to such habitats such as epiphytic orchids (Foster 2001; Adhikari et al. 2012). With land clearing, some orchids may not be able to successfully disperse to suitable habitats, or there may no longer be forests at the higher altitude sites (Liu et al. 2015).
With climate change already affecting plant distributions in many regions and even greater changes predicted in the future, research modelling the effects of climate change on the distribution of orchids and their symbionts is important (Brundrett 2007). Dealing with the threat of climate change on the ground is likely to require integrated conservation strategies to maintain some existing habitat, but also possible translocation efforts to new habitats. Unfortunately, due to the specificity of orchids and their habitats, these approaches are not simple and may not always be feasible (Cuoco and Cronan 2009; Swarts and Dixon 2009a; Liu et al. 2015).
Many of the orchids threatened by climate change and pollution were also threatened by land clearing, trampling and fragmentation associated with disturbance and development for roads and railroads, tourism and recreation infrastructure and recreation activities among others. As the human population is expanding, increased pressure is being placed on natural areas, including areas with numerous threatened orchid species and high tourist visitation (Ballantyne and Pickering 2012). Consequently, there is an increase in road and infrastructure development, which have well-recognised impacts on many species of plants. These impacts include fragmenting otherwise intact natural habitats and increased competition from weeds (Parra-Tabla et al. 2011). Tourism and recreation, both from infrastructure and activities, is also increasingly recognised as a threat to many plants including orchids (Wraith and Pickering 2017). Orchids are often the focus of specialised tourism, known as orchid tourism, with many groups targeting threatened species to photograph or collect. This orchid tourism causes trampling damage to the orchids and surrounding vegetation from hiking and other activities such as four wheel driving (Light and MacConaill 2007).
As the threats within this syndrome are large-scale issues, solutions also need to be large scale. These solutions include identifying and protecting natural areas of high conservation value with joint management by governments, non-government agencies, landholders and the general public (Laurance et al. 2015). Appropriate management within vulnerable areas will help conserve threatened species, including orchids, which are suffering the impacts of this threat syndrome.
Threat syndromes three and four
The final two threat syndromes were more specific and geographically based than those above. They included epiphytic orchids in Sub-Saharan Africa, including Madagascar, that were threatened by a range of factors, and orchids in South and Southeast Asia threatened by shifting agriculture, a form of land clearing.
That there are many threatened orchids in Madagascar is not surprising as the country is renowned for floral diversity, much of which is both endemic to the country and threatened (Cribb and Hermans 2007; Brummitt et al. 2015). What is interesting is that it is often the epiphytic species that are at risk. One of the more common threatening processes for epiphytic orchids in Sub-Saharan Africa was land clearing from agriculture and deforestation (Harper et al. 2007). Epiphytic orchids often have specific associations with host trees, and hence the recovery of their populations post disturbance is correlated with the regeneration of host trees, which is slow if at all (Adhikari et al. 2012). Therefore, conserving primary forest habitat is important (Gradstein 2008) as it will help conserve orchids as well as other charismatic species.
Terrestrial orchids occurring in South and Southeast Asia were commonly affected by land clearing for shifting agriculture which is not unexpected as this practice is a known contributor to deforestation in the region (Rasul and Thapa 2003). Although shifting agriculture is still common in the region, agriculture practices are increasingly transitioning to more secure and permanent methods, but these still have impacts on biodiversity from clearing of natural habitat. Financial and social support for more sustainable agriculture practices is important in these regions and more generally to protect threatened species (Tilman et al. 2002).
More broadly than just these examples, land clearing is a key threatening process for orchids globally. In the IUCN Red List, it appeared in different broad threat categories, such as logging in biological resource use, clearing for agriculture in agriculture and aquaculture, and clearing and fragmentation associated with urbanisation in the broader residential and commercial development category. Overall, land clearing threatens many forest orchids in South and Southeast Asia and tropical areas of Sub-Saharan Africa, including some parts of Madagascar. The high specificity, localised populations and intrinsic rarity of orchids make land clearing a common and severe threat to orchid populations (Swarts and Dixon 2009a; Swarts et al. 2010; Ballantyne and Pickering 2012).
Limiting deforestation and other types of land clearing and securing more protected areas will help protect forest-dependent terrestrial and epiphytic orchids. For example, increasing conservation efforts in Sub-Saharan Africa is important, including the expansion of protected areas to include populations of threatened orchids is important as only a third of the threatened orchids in the region are currently in protected areas, including those in Madagascar (Mittermeier et al. 2005).
Limitations
Using global databases, such as the IUCN Red List, for research and conservation has some limitations, and as such, this current assessment of threats to orchids should be treated as indicative rather than a definitive assessment of global patterns. Although the IUCN Red List is regarded as a useful tool for the management of threatened species (Brummitt et al. 2015) and is often used in global reviews of threats (Ripple et al. 2017), it should be used with some caution (Possingham et al. 2002; Rodrigues et al. 2006). A key issue with relying upon the database is an underrepresentation of threatened species for some regions and groups of organisms. Lags in adding species to the Red List occur for some countries and regions. These delays are often the result of the interplay between a lack of funding, different levels of government cooperation in listing, different priorities for government conservation authorities, and/or a lack of research identifying species at threat and by what (Schatz 2009). Focused efforts on research and listing from key biodiversity hotspots will help address some of these gaps.
Even for some countries with a good track record in research and with governments that recognise the importance of threatened species, there can still be an underrepresentation of data in the IUCN Red List data. Australia is a clear example of this, with only five species of orchids from Australia on the Red List, even though there are 200 species of orchids on the Australian Government’s own list of threatened plants (Rankin et al. 2015; Australian Government 2016).
Limitations regarding the amount of threat data available for some species of orchids were also evident in the Red List, with 77 orchid species on the list lacking threat data. When threat data were available, they were not always comprehensive, as data on the overall severity and impact of some threats were missing. Information on the severity of a threat is important for interpreting the relative importance of threats and determining which need immediate mitigation. These limitations concomitantly limited our capacity to assess the full range of orchid species and their threats. Adding more species and more comprehensive, updated data will enhancing the accuracy and usefulness of the IUCN Red list as a resource for assessing global patterns in biodiversity (Rodrigues et al. 2006).
Conclusions
Orchids are threatened globally, most often terrestrial orchids in forests. Major threats to orchids on the IUCN Red List include illegal collecting (biological resource use), habitat loss and fragmentation (agriculture and aquaculture, human intrusion and disturbances and development), climate change and natural system modifications. Many of these threats co-occurred and we identified four threat syndromes which require integrated management and conservation efforts. Highlighting threat syndromes on a global scale can create more consistent conservation planning and help focus efforts on the specific threats in a given region.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Biographies
Jenna Wraith
is a Doctoral Candidate in the Environmental Futures Research Institute and in the School of Environment at Griffith University. Her research interests include conservation ecology, global biodiversity and climate change.
Catherine Pickering
is a Professor in the Environmental Futures Research Institute and in the School of Environment at Griffith University, Australia. Her research interest includes botany, ecology, park management, climate change and research methodology including systematic quantitative literature reviews.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s13280-017-0964-0) contains supplementary material, which is available to authorized users.
Contributor Information
Jenna Wraith, Email: jenna.wraith@griffithuni.edu.au.
Catherine Pickering, Email: c.pickering@griffith.edu.au.
References
- Adhikari YP, Fischer HS, Fischer A. Host tree utilization by epiphytic orchids in different land-use intensities in Kathmandu Valley, Nepal. Plant Ecology. 2012;213:1393–1412. doi: 10.1007/s11258-012-0099-0. [DOI] [Google Scholar]
- Australian Government . Species listed under the Environment Protection and Biodiversity Conservation Act 1999 (EPBC Act) Canberra: Australian Government; 2016. [Google Scholar]
- Ballantyne M, Pickering C. Ecotourism as a threatening process for wild orchids. Journal of Ecotourism. 2012;11:34–47. doi: 10.1080/14724049.2011.628398. [DOI] [Google Scholar]
- Brummitt NA, Bachman SP, Griffiths-Lee J, Lutz M, Moat JF, Farjon A, Donaldson JS, Hilton-Taylor C, et al. Green plants in the red: A baseline global assessment for the IUCN sampled Red List index for plants. PLoS ONE. 2015;10:e0135152. doi: 10.1371/journal.pone.0135152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundrett MC. Scientific approaches to Australian temperate terrestrial orchid conservation. Australian Journal of Botany. 2007;55:293–307. doi: 10.1071/BT06131. [DOI] [Google Scholar]
- Burgman MA, Keith D, Hopper SD, Widyatmoko D, Drill C. Threat syndromes and conservation of the Australian flora. Biological Conservation. 2007;134:73–82. doi: 10.1016/j.biocon.2006.08.005. [DOI] [Google Scholar]
- Clarke KR, Gorley RN. PRIMER-E version 6. Plymouth: PRIMER-E; 2006. [Google Scholar]
- Cozzolino S, Widmer A. Orchid diversity: An evolutionary consequence of deception? Trends in Ecology and Evolution. 2005;20:487–494. doi: 10.1016/j.tree.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Cribb PJ, Hermans J. The conservation of Madagascar’s orchids. A model for an integrated conservation project. Lankesteriana. 2007;7:1–2. [Google Scholar]
- Cribb PJ, Kell SP, Dixon KW, Barrett RL. Orchid conservation: A global perspective. In: Dixon KW, Kell SP, Barrett RL, Cribb PJ, editors. Orchid conservation. Kota Kinabalu: Natural History Publications; 2003. pp. 1–24. [Google Scholar]
- Cuoco LB, Cronan JB. Orchidaceae: Using a globalized commodity to promote conservation and sustainable economic development in southern Ecuador. Journal of Sustainable Forestry. 2009;28:799–824. doi: 10.1080/10549810902936623. [DOI] [Google Scholar]
- ESRI . ArcGIS release 10.4.1. Redlands: Environmental Systems Research Institute; 2016. [Google Scholar]
- Foster P. The potential negative impacts of global climate change on tropical montane cloud forests. Earth-Science Reviews. 2001;55:73–106. doi: 10.1016/S0012-8252(01)00056-3. [DOI] [Google Scholar]
- Gradstein SR. Epiphytes of tropical montane forests—Impact of deforestation and climate change. Göttingen Centre for Biodiversity and Ecology. Biodiversity and Ecology Series. 2008;2:51–65. [Google Scholar]
- Harper GJ, Steininger MK, Tucker CJ, Juhn D, Hawkins F. Fifty years of deforestation and forest fragmentation in Madagascar. Environmental Conservation. 2007;34:325–333. doi: 10.1017/S0376892907004262. [DOI] [Google Scholar]
- Hinsley, A., A. Nuno, M. Ridout, F.A.V. St John, and D.L. Roberts. 2016. Estimating the extent of cites noncompliance among traders and end-consumers; lessons from the global orchid trade. Conservation Letters. doi:10.1111/conl.12316. Accessed 1 Sep 2017.
- IUCN. 2016. The IUCN Red List of Threatened Species. http://www.iucnredlist.org. Accessed 25 Sep 2016.
- Jones DL. In: A complete guide to native orchids of Australia, including the island territories. Savage A, editor. Sydney: Reed New Holland; 2006. pp. 12–20. [Google Scholar]
- Kull T, Hutchings MJ. A comparative analysis of decline in the distribution ranges of orchid species in Estonia and the United Kingdom. Biological Conservation. 2006;129:31–39. doi: 10.1016/j.biocon.2005.09.046. [DOI] [Google Scholar]
- Kull T, Selgis U, Peciña MV, Metsare M, Ilves A, Tali K, Sepp K, Kull K, Shefferson RP. Factors influencing IUCN threat levels to orchids across Europe on the basis of national red lists. Ecology and Evolution. 2016;6:6245–6265. doi: 10.1002/ece3.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurance WF, Peletier-Jellema A, Geenen B, Koster H, Verweij P, Van Dijck P, Lovejoy TE, Schleicher J, Van Kuijk M. Reducing the global environmental impacts of rapid infrastructure expansion. Current Biology. 2015;25:R259–R262. doi: 10.1016/j.cub.2015.02.050. [DOI] [PubMed] [Google Scholar]
- Light M, MacConaill M. Effects of trampling on a terrestrial orchid environment. Lankesteriana. 2007;7:1–2. [Google Scholar]
- Liu H, Luo Y-B, Heinen J, Bhat M, Liu Z-J. Eat your orchid and have it too: A potentially new conservation formula for Chinese epiphytic medicinal orchids. Biodiversity and Conservation. 2014;23:1215–1228. doi: 10.1007/s10531-014-0661-2. [DOI] [Google Scholar]
- Liu Q, Chen J, Corlett RT, Fan X, Yu D, Yang H, Gao JY. Orchid conservation in the biodiversity hotspot of southwestern China. Conservation Biology. 2015;29:1563–1572. doi: 10.1111/cobi.12584. [DOI] [PubMed] [Google Scholar]
- Malcolm JR, Liu C, Neilson RP, Hansen L, Hannah LEE. Global warming and extinctions of endemic species from biodiversity hotspots. Conservation Biology. 2006;20:538–548. doi: 10.1111/j.1523-1739.2006.00364.x. [DOI] [PubMed] [Google Scholar]
- Mittermeier RA, Hawkins F, Rajaobelina S, Langrand O. Wilderness conservation in a biodiversity hotspot. International Journal of Wilderness. 2005;11:42–46. [Google Scholar]
- National Agricultural Statistics Service, NASS. 2016. Floriculture crops. Washington, DC: United States Department of Agriculture 41, 51.
- Nilsson LA. Orchid pollination biology. Trends in Ecology and Evolution. 1992;7:255–259. doi: 10.1016/0169-5347(92)90170-G. [DOI] [PubMed] [Google Scholar]
- Parra-Tabla V, Vargas CF, Naval C, Calvo LM, Ollerton J. Population status and reproductive success of an endangered epiphytic orchid in a fragmented landscape. Biotropica. 2011;43:640–647. doi: 10.1111/j.1744-7429.2011.00752.x. [DOI] [Google Scholar]
- Phelps J, Webb EL. “Invisible” wildlife trades: Southeast Asia’s undocumented illegal trade in wild ornamental plants. Biological Conservation. 2015;186:296–305. doi: 10.1016/j.biocon.2015.03.030. [DOI] [Google Scholar]
- Possingham HP, Andelman SJ, Burgman MA, Medellín RA, Master LL, Keith DA. Limits to the use of threatened species lists. Trends in Ecology and Evolution. 2002;17:503–507. doi: 10.1016/S0169-5347(02)02614-9. [DOI] [Google Scholar]
- Rankin BL, Ballantyne M, Pickering CM. Tourism and recreation listed as a threat for a wide diversity of vascular plants: A continental scale review. Journal of Environmental Management. 2015;154:293–298. doi: 10.1016/j.jenvman.2014.10.035. [DOI] [PubMed] [Google Scholar]
- Rasul G, Thapa GB. Shifting cultivation in the mountains of South and Southeast Asia: Regional patterns and factors influencing the change. Land Degradation and Development. 2003;14:495–508. doi: 10.1002/ldr.570. [DOI] [Google Scholar]
- Ripple WJ, Wolf C, Newsome TM, Hoffmann M, Wirsing AJ, McCauley DJ. Extinction risk is most acute for the world’s largest and smallest vertebrates. Proceedings of the National Academy of Sciences of USA. 2017 doi: 10.1073/pnas.1702078114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DL, Dixon KW. Orchids. Current Biology. 2008;18:R325–R329. doi: 10.1016/j.cub.2008.02.026. [DOI] [PubMed] [Google Scholar]
- Rodrigues AS, Pilgrim JD, Lamoreux JF, Hoffmann M, Brooks TM. The value of the IUCN Red List for conservation. Trends in Ecology and Evolution. 2006;21:71–76. doi: 10.1016/j.tree.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Rosen GE, Smith KF. Summarizing the evidence on the international trade in illegal wildlife. EcoHealth. 2010;7:24–32. doi: 10.1007/s10393-010-0317-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RStudio Team . RStudio: Integrated development for R. Boston: RStudio, Inc.; 2016. [Google Scholar]
- Santos FD. Orchid preference for host tree genera in a Nicaraguan tropical rain forest. Selbyana. 2000;21:25–29. [Google Scholar]
- Schatz GE. Plants on the IUCN Red List: Setting priorities to inform conservation. Trends in Plant Science. 2009;14:638–642. doi: 10.1016/j.tplants.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Seaton PT, Hu H, Perner H, Pritchard HW. Ex situ conservation of orchids in a warming world. The Botanical Review. 2010;76:193–203. doi: 10.1007/s12229-010-9048-6. [DOI] [Google Scholar]
- Subedi A, Kunwar B, Choi Y, Dai Y, van Andel T, Chaudhary RP, de Boer HJ, Gravendeel B. Collection and trade of wild-harvested orchids in Nepal. Journal of Ethnobiology and Ethnomedicine. 2013;9:64. doi: 10.1186/1746-4269-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarts ND, Dixon KW. Terrestrial orchid conservation in the age of extinction. Annals of Botany. 2009;104:543–556. doi: 10.1093/aob/mcp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarts ND, Dixon KW. Perspectives on orchid conservation in botanic gardens. Trends in Plant Science. 2009;14:590–598. doi: 10.1016/j.tplants.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Swarts ND, Sinclair EA, Francis A, Dixon KW. Ecological specialization in mycorrhizal symbiosis leads to rarity in an endangered orchid. Molecular Ecology. 2010;19:3226–3242. doi: 10.1111/j.1365-294X.2010.04736.x. [DOI] [PubMed] [Google Scholar]
- Thomas CD, Cameron A, Green RE, Bakkenes M, Beaumont LJ, Collingham YC, Erasmus BFN, De Siqueira MF, et al. Extinction risk from climate change. Nature. 2004;427:145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S. Agricultural sustainability and intensive production practices. Nature. 2002;418:671. doi: 10.1038/nature01014. [DOI] [PubMed] [Google Scholar]
- WCSP. 2016. World Checklist of Selected Plant Families. Facilitated by the Royal Botanic Gardens, Kew. http://apps.kew.org/wcsp/. Accessed 30 Sep 2016.
- Wraith J, Pickering C. Tourism and recreation a global threat to orchids. Biodiversity and Conservation. 2017;10531:1–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.