Abstract
Reporting of adverse events following immunization (AEFI) is a key component for functional vaccine safety monitoring system. The aim of our study is to document trends in the AEFI reporting ratio globally and across the six World Health Organization (WHO) regions. We describe the number of AEFI reports communicated each year through the World Health Organization/United Nations Children's Fund Joint Reporting Form on Immunization from 2000 to 2015. The AEFI reporting ratios (annual AEFI reports per 100,000 surviving infants) were calculated to identify WHO countries (n = 191 in 2000 and n = 194 by 2015) that met a minimal reporting ratio of 10, a target set by the Global Vaccine Action Plan for vaccine safety monitoring as a proxy measure for a functional AEFI reporting system. The number of countries reporting any AEFI fluctuated over time but with progress from 32 (17%) in 2000 to 124 (64%) in 2015. In 2015, the global average AEFI reporting ratio was 549 AEFI reports per 100,000 surviving infants. The number of countries with AEFI reporting ratios greater than 10 increased from 8 (4%) in 2000 to 81 (42%) in 2015. In 2015, 60% of countries in the WHO Region of the Americas reported at least 10 AEFI per 100,000 surviving infants, followed by 55% in European Region, 43% in Eastern Mediterranean Region, 33% in Western Pacific Region, 27% in South-East Asia Region and 21% in African Region. Overall, AEFI reporting has increased over the past sixteen years worldwide, but requires strengthening in a majority of low- and middle- income countries. The AEFI reporting ratio is useful for benchmarking and following trends over time; but does not provide information on the quality of the reporting system and does not guarantee capacity to detect and manage a vaccine safety problem at a national level. Additional efforts are required to ensure and improve data quality, AEFI reporting and surveillance of immunization safety in every country.
Keywords: Global vaccine safety blueprint, Global vaccine action plan, Adverse events following immunization, AEFI reporting, Vaccine safety surveillance
1. Background
The benefits of vaccination in saving lives and promoting health through reducing preventable diseases has been recognized worldwide, and vaccines currently prevent more than 2.5 million child deaths each year [1]. Even though vaccines are designed to be both safe and effective, adverse events following immunization (AEFI) do occur and need to be reported in order to identify problems and take appropriate corrective action [2]; examples include a withdraw of the RotaShield vaccine in 1999 after identifying its association with intussusception, and the recall of certain lots of meningococcus type C vaccine in 2009 due to contamination with Staphylococcus aureus [3], [4]. With progress in improving vaccination coverage and the addition of vaccines to national immunization schedules in recent years, it is estimated that almost twice the total number of doses of vaccine are administered in low- and middle-income countries (LMIC) compared to developed countries [5], [6]. As the number of doses administered increases, we can also expect to see an increased number of adverse events following immunizations (both true reactions and temporally coincidental events). Addressing these events requires functional vaccine safety monitoring systems [7], [8]. The Global Vaccine Action Plan (GVAP) identifies the establishment and strengthening of AEFI reporting systems as a priority activity for strengthening immunization programs, and defines the AEFI reporting ratio (number of AEFI reports per 100,000 surviving infants) as a performance indicator for monitoring progress [9]. Based on an empirical analysis of Joint Reporting Form on Immunization (JRF) data showing that countries with a sustainable passive vaccine safety surveillance system record at least 10 reports per 100,000 surviving infants per year, the target is currently set at that level [10].
Functional vaccine safety monitoring systems help public health authorities to address vaccine safety concerns in a timely manner, promoting confidence in immunization programs. Previous reports have documented the high variability of AEFI reporting across regions and countries, with a considerable number of countries without any AEFI reporting [11]. An analysis regarding indicators for the post-marketing surveillance of AEFI demonstrated that the global performance of AEFI reporting has shown positive but slow progress from 1997 to 2009 [6]. According to published data, 48% of all people in the world live in countries without functional safety monitoring systems for vaccines [6].
In 2011, the Global Vaccine Safety Blueprint (the Blueprint) was developed by the World Health Organization (WHO) and a group of partners as a strategy to further improve capacity of vaccine safety monitoring in LMIC [5], [6]. The mission of the Blueprint is “to optimize the safety of vaccines through effective use of pharmacovigilance principles and methods”. One of the Blueprint’s goals is “to assist LMIC to have at least minimal capacity for vaccine safety activities” [5]. The World Health Organization/ United Nations Children's Fund (WHO/UNICEF) JRF provides a mechanism for national authorities to report AEFI to the global level and also provides an opportunity to evaluate country capacity to meet minimal standards for vaccine safety monitoring.
Recently, the Global Advisory Committee on Vaccine Safety (GACVS) proposed that the sensitivity of AEFI surveillance should be assessed through a basic indicator [11]. The GVAP proposed an indicator as the ratio of annual AEFI reports to the number of surviving infants [11]. Our study provides a descriptive analysis of AEFI reporting using data from the WHO/UNICEF JRF [12] over the past 16 years among all WHO countries. The aim of this study is to document trends in the AEFI reporting ratio globally and across WHO regions.
2. Methods
2.1. Data source
We used data from the WHO/UNICEF JRF, a standard reporting format used to collect information from all Member States in the WHO Regions. The JRF has evolved from a limited set of information collected to capture a wide range of domains of standard performance, planning, financing to quality indicators such an AEFI indicator using aggregate data at global level. For purposes of this analysis, a Member State will be referred to as ‘country’ in this report. The JRF was developed through a consensus process among UNICEF, WHO, and selected ministries of health (MOH) to monitor immunization systems performance [12]. Information in the JRF is consider as the official report provided by the countries; information about number of AEFI cases in each country reported through the JRF has been available since 2000.
2.2. Analysis
Descriptive analysis was used to summarize the number of countries with complete data on AEFI reporting on the JRF from 2000 to 2015 by regions. We tabulated the total number of AEFI reported by all countries in each WHO region annually, from 2000 to 2015. Using JRF data from 2000 to 2015, we also tabulated global and regional ratios of AEFI reporting according to the number of surviving infants. The number of surviving infants was obtained from the United Nations Development Programme (UNDP) statistics on surviving infants [13]. AEFI reporting ratios were further examined by using 2015 data from each region and classifying AEFI reporting ratios into three categories: greater than or equal to 10 per 100,000 surviving infants, less than 10 per 100,000 surviving infants, and no information reported (i.e., country did not report any AEFI information through JRF).
3. Results
The number of countries reporting AEFI data through the JRF increased steadily from 32 (17%) of 191 countries in 2000–139 (72%) of 192 countries in 2004. From 2006 onwards, this number fluctuated between 122 (63%) and 134 (69%) (Table 1). By 2015, 124 (64%) of 194 countries reported AEFI data through the JRF. The WHO South-East Asia Region had a higher percentage of countries reporting AEFI data compared to other regions, with at least 10 (91%) out of 11 countries reporting during 2011–2014. The drop in number of Western Pacific countries reporting AEFI during 2005 illustrates how sensitive the JRF system is to completeness of data in a single year.
Table 1.
Number of countries reporting AEFI cases through the WHO/UNICEF Joint Reporting Form on Immunization, by WHO region, 2000–2015.
| Year | Country (N) | Countries reporting AEFI through JRF |
||||||
|---|---|---|---|---|---|---|---|---|
| Total N (%) |
African Regiona N (%) |
Region of the Americasb N (%) |
Eastern Mediterranean Regionc N (%) |
European Regiond N (%) |
South-East Asia Region e N (%) |
Western Pacific Regionf N (%) |
||
| 2015 | 194 | 124 (64) | 24 (51) | 29 (83) | 12 (57) | 34 (64) | 8 (73) | 17 (63) |
| 2014 | 194 | 130 (67) | 29 (62) | 27 (77) | 11 (52) | 37 (70) | 10 (91) | 16 (59) |
| 2013 | 194 | 133 (69) | 31 (66) | 28 (80) | 11 (52) | 37 (70) | 10 (91) | 16 (59) |
| 2012 | 194 | 129 (66) | 28 (60) | 25 (71) | 10 (48) | 37 (70) | 11 (100) | 18 (67) |
| 2011 | 194 | 129 (66) | 26 (55) | 27 (77) | 12 (57) | 37 (70) | 10 (91) | 17 (63) |
| 2010 | 193 | 125 (65) | 29 (63) | 26 (74) | 10 (48) | 38 (72) | 7 (64) | 15 (56) |
| 2009 | 193 | 122 (63) | 22 (48) | 28 (80) | 12 (57) | 35 (66) | 8 (73) | 17 (63) |
| 2008 | 193 | 126 (65) | 25 (54) | 28 (80) | 11 (52) | 38 (72) | 7 (64) | 17 (63) |
| 2007 | 193 | 134 (69) | 26 (57) | 30 (86) | 14 (67) | 36 (68) | 10 (91) | 18 (67) |
| 2006 | 193 | 128 (66) | 27 (59) | 21 (60) | 15 (71) | 39 (74) | 9 (82) | 17 (63) |
| 2005 | 192 | 106 (55) | 27 (59) | 20 (57) | 11 (52) | 37 (71) | 8 (73) | 3 (11) |
| 2004 | 192 | 139 (72) | 29 (63) | 30 (86) | 14 (67) | 42 (81) | 7 (64) | 17 (63) |
| 2003 | 192 | 120 (63) | 21 (46) | 25 (71) | 12 (57) | 35 (67) | 9 (82) | 18 (67) |
| 2002 | 192 | 98 (51) | 15 (33) | 19 (54) | 10 (48) | 34 (65) | 5 (45) | 15 (56) |
| 2001 | 191 | 72 (38) | 15 (33) | 15 (43) | 0 (0) | 28 (54) | 2 (20) | 12 (44) |
| 2000 | 191 | 32 (17) | 3 (7) | 12 (34) | 0 (0) | 11 (21) | 3 (30) | 3 (11) |
Abbreviations: AEFI, adverse events following immunization; WHO, World Health Organization; UNICEF, United Nations International Children's Emergency Fund.
Number of countries over year: 46 (2000–2010) and 47 (2011–2015).
Number of countries over year: 35 (2000–2015).
Number of countries over year: 21 (2000–2015).
Number of countries over year: 52 (2000–2005) and 53 (2006–2015).
Number of countries over year: 10 (2000–2001) and 11 (2002–2015).
Number of countries over year: 27 (2000–2015).
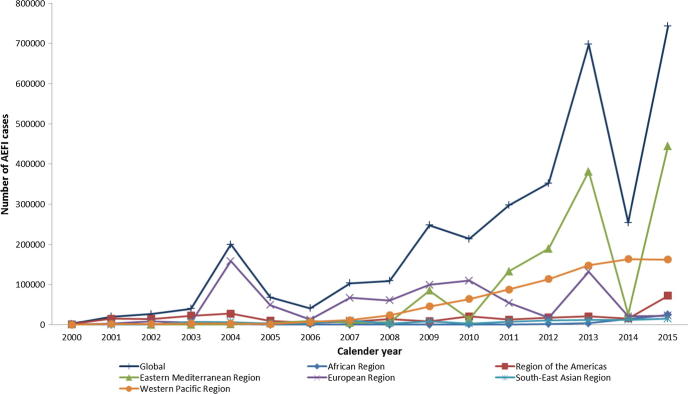
The global number of AEFI cases reported through the JRF increased during the period studied. This increase, however, was not regular, with four important peaks of AEFI reports apparent in 2004, 2009, 2013 and 2015 (Fig. 1). These peaks corresponded to high numbers of AEFI cases reported by single countries in the European Region during 2004 (Ukraine) and the Eastern Mediterranean Region during 2009 (Lebanon), 2013 (Egypt) and 2015 (Egypt). Individual annual peaks can reflect time-limited increases in AEFI reports because of mass vaccination campaigns or media attention. In the African Region, Region of the Americas, and South-East Asia Region the number of AEFI cases reported between 2000 and 2015 were consistently low with a very small increase in later years. Reported AEFI cases steadily increased in the Western Pacific Region from 2008 to 2015. AEFI cases reported in the European Region varied over time with low numbers of AEFI cases reported before 2004, but large number of AEFI cases reported in 2004 (Ukraine reported 119957 AEFI cases) and in 2013 (Uzbekistan reported 110951 AEFI cases), followed by a decrease in 2014 and 2015. Continuously low numbers of AEFI were reported in the Eastern Mediterranean Region before 2009. The first peak of AEFI cases in the Eastern Mediterranean Region occurred in 2009 (Lebanon reported 14628 AEFI cases), followed by a decrease in 2010, and another peak again in 2013 (Egypt reported 354043 AEFI cases), followed by a lower number in 2014 (Egypt reported 57 AEFI cases) and a new peak in 2015 (Egypt reported 414243 AEFI cases).
Fig. 1.
Trends in number of AEFI cases reported through the WHO/UNICEF Joint Reporting Form on Immunization, by WHO region, 2000–2015. (Abbreviations: AEFI, adverse events following immunization; WHO, World Health Organization; UNICEF, United Nations International Children's Emergency Fund.)
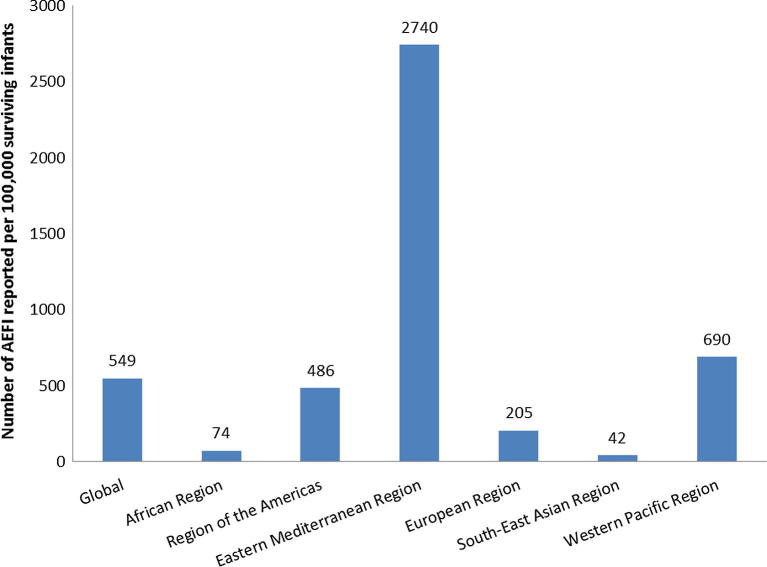
In 2015, the Eastern Mediterranean Region had the highest AEFI reporting ratio (2740 per 100,000 surviving infants) of all the regions, followed by the Western Pacific Region (690 per 100,000 surviving infants) (Fig. 2). Globally, the average AEFI reporting ratio in 2015 was 549 AEFI reports per 100,000 surviving infants. For all other regions, the aggregate ratio was lower than this average: European Region (205 AEFI reports per 100,000 surviving infants), Region of the Americas (486), African Region (74), and South-East Asia Region (42). In 2015, the Eastern Mediterranean Region accounted for the majority (60%) of all AEFI reports, followed by Western Pacific Region (22%), Region of the Americas (10%), African and European Regions (each 3%), and South-East Asia Region (2%). The high proportion of AEFI reports from the Western Pacific Region is driven by the figures from China, the most populous country in the Region. During the period studied, the number of AEFI reports from China increased from 1313 in 2005 to 152,066 in 2015 (no data available before 2004 and zero AEFI reports in 2004). China’s AEFI reporting ratio for 2015 was 926 per 100,000 surviving infants.
Fig. 2.
Number of AEFI cases reported per 100,000 surviving infants, by WHO region in 2015. (Abbreviations: AEFI, adverse events following immunization.)
In Table 2, we show the number of countries by region that had reported at least 10 AEFI per 100,000 surviving infants during 2015. During that year, a total of 81 (42%) countries exceeded the minimum threshold while 43 (22%) countries were below that threshold. The remaining 70 (36%) of all 194 WHO countries did not report any AEFI data. The percentage of countries with an AEFI reporting ratio of at least 10 per 100,000 surviving infants ranged from 21% (African Region) to 60% (Region of the Americas) in the six regions. Those with AEFI reporting ratios lower than 10 per 100, 000 surviving infants ranged from 9% (European Region) to 46% (South-East Asia Region). AEFI data were not available for 49% of countries in the African Region and 17% of countries in the Region of the Americas in 2015.
Table 2.
Number of countries with an AEFI reporting ratio above or below the target of 10 AEFI reports per 100, 000 surviving infants, by WHO region in 2015.
| WHO region | Total (N) | AEFI reporting ratio ≥10/100,000 surviving infants N (%) |
AEFI reporting ratio <10/100,000 surviving infants N (%) |
No AEFI data reported N (%) |
|---|---|---|---|---|
| African Region | 47 | 10 (21) | 14 (30) | 23 (49) |
| Region of the Americas | 35 | 21 (60) | 8 (23) | 6 (17) |
| Eastern Mediterranean Region | 21 | 9 (43) | 3 (14) | 9 (43) |
| European Region | 53 | 29 (55) | 5 (9) | 19 (36) |
| South-East Asia Region | 11 | 3 (27) | 5 (46) | 3 (27) |
| Western Pacific Region | 27 | 9 (33) | 8 (30) | 10 (37) |
| Global | 194 | 81 (42) | 43 (22) | 70 (36) |
Abbreviations: AEFI, adverse events following immunization.
AEFI reporting from the African Region since 2000 was low compared to all other WHO regions. AEFI reporting ratios calculated for the 2015 data from the African Region (total 47 countries), shows considerable variation by country. Burkina Faso (2407 cases per 100,000 surviving infants) had the highest AEFI reporting ratio in 2015, followed by Cameroon (229 cases per 100,000 surviving infants), while 35 countries reported AEFI reporting ratios lower than 10 per 100,000 surviving infants, including 23 countries with no AEFI data reported at all. In Burkina Faso, AEFI cases were reported during only 6 of the 16 years studied: and in the years 2014 and 2015, the number of AEFI reports was in significant excess (9529 and 16,192 cases, respectively) compared to any other year (1796, 16, 2 and 2216 cases in year 2004, 2005, 2006 and 2013).
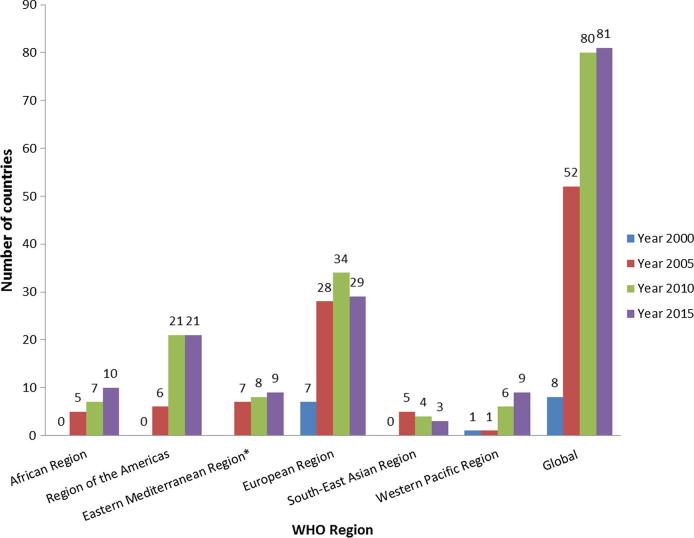
Trends in the number of countries in each region with AEFI reporting of at least 10 per 100,000 surviving infants in 2000, 2005, 2010 and 2015 is presented in Fig. 3. In 2015, the number of countries that reported at least 10 AEFI per 100,000 surviving infants was 10 times higher than in 2000 (8 countries vs 81 countries, respectively). During 2015, 85% (29/34) of European Region countries with available AEFI data reported at least 10 AEFI per 100,000 surviving infants, followed by the Eastern Mediterranean Region (9/12, 75%), the Region of the Americas (21/29, 72%), the Western Pacific Region (9/17, 53%), the African Region (10/24, 42%) and the South-East Asia Region (3/8, 38%).
Fig. 3.
Number of countries that reported at least 10 AEFI cases per 100,000 surviving infants, by WHO region in 2000, 2005, 2010 and 2015. (*Eastern Mediterranean Region has no AEFI data available in the year 2000. Abbreviations: AEFI, adverse events following immunization.)
4. Discussion
This analysis is the first to show trends of national AEFI data officially reported from countries through the JRF, and suggests that global vaccine safety monitoring system has generally improved over the past 16 years. AEFI reporting, however, remains non-existent or irregular in a majority of countries and large differences exist among regions. This report also illustrates the use of an AEFI reporting ratio and provides important information about the potential significance and limitations of such an indicator. As the first evaluation of the new GVAP target for AEFI monitoring (i.e., 10 AEFI reports per 100,000 surviving infants) [10], we found that in 2015, only 42% of countries achieved this minimal reporting ratio. This indicator will provide a useful initial milestone and if sustained over time, can reflect if a country has a basic system in place for reporting of safety concerns.
This analysis also documents important regional variations in AEFI reporting. The South-East Asia, Americas and European Regions have the largest proportion of countries that are reporting AEFI data to the JRF. Yet, large differences can still be observed in the numbers and ratios of AEFI reports across regions. For example, only three of eight countries from the South-East Asia Region provided AEFI information to the JRF meet the GVAP AEFI reporting ratio target. As a result, in countries with low or absent AEFI reporting, even increases in the number of vaccine doses administered will have no or limited effect on the reporting ratio. Variations in AEFI reporting requirements might in part explain this difference, for example, in some countries minor and serious AEFI cases are collected, although others only report serious AEFI cases. Regardless of the differences in reporting requirements among countries, spontaneous AEFI surveillance is always characterized by a large degree of under-reporting [14]. In addition, in many places, the culture of safety monitoring as a quality process for the programme is not in place. Instead, a “culture of fear” may be present in some countries, in which health staffs are afraid of being blamed or punished if they report AEFI [14]. This has been documented especially in LMIC, illustrating a lack of political commitment and insufficient training of health care workers [14].
In many countries, AEFI reporting has been observed to be irregular over time. One explanation for occasional peaks in reporting is supplemental immunization activities conducted in countries following AEFI emphasis and training sessions for a limited period of time. In other instances, new vaccine introductions have led to increased attention to safety monitoring and, on occasion, increased reporting might be due to a sense of insecurity towards a new vaccine. As occasional annual peaks in AEFI reports from individual countries can affect the global and regional totals, progress with vaccine safety capacity needs to be evaluated based on consistency in AEFI reports over time. The most consistent increase in AEFI reporting has been observed in China, reflecting a concerted effort by national authorities to improve their vaccine safety monitoring system. China’s current AEFI surveillance system was initiated in 2005, and from 2008 an online system has been used to collect nationwide data regarding vaccine safety. A national AEFI surveillance guideline was issued in 2010 [8], [15], at least partly because the Ministry of Health acknowledged the key role AEFI surveillance played during the large influenza vaccination campaign in the 2009 A (H1N1) influenza pandemic [16]. Since then, Chinese authorities have continuously improved their processes for investigating serious AEFI, monitoring safety signals and analysing the safety profile of their manufactured vaccines, including monitoring products used outside of the country such as the live attenuated SA 14-14-2 vaccine against Japanese encephalitis [17].
In our analysis, although not all countries that reported AEFI data reached the proposed minimum reporting ratio, it appears that in the two regions with established AEFI reporting (Region of the Americas and European Region), the proportion of countries that met this indicator was also the highest.
There are some limitations to our study. The AEFI reporting ratio indicator in itself is not a direct measure to assess if there is a functioning vaccine safety monitoring system in place in a country. Rather, this indicator indicates the capacity of AEFI reporting, not necessarily the quality of the system. It also does not allow for direct comparisons between countries, mainly because of varying country reporting requirements and definitions for AEFI. Variations in national immunization schedules and vaccine coverage make it difficult to select one denominator that would be applicable to all countries to allow comparisons. Moreover, we used the surviving infants obtained from UNDP as the denominator in calculating the AEFI reporting ratio, which implies that the focus of AEFI reporting is on vaccines administered during the first year of life. In many countries, however, vaccines are now used beyond that age group and AEFI reports may come from additional age groups. Although JRF data rely on information collected directly from countries, the consistency of reporting might vary. Vaccine safety data are collected by various units (e.g. immunization programs, regulatory authority, pharmacovigilance centres) within a country, yet not all of these units report AEFI cases through the JRF. As the JRF represents aggregate data, it is also impossible to verify the quality and completeness of the data.
AEFI reporting, examination of reports, investigation of serious cases and adequate response to AEFI cases are essential to ensure the quality and safety of immunization services [18]. Distinguishing serious AEFI cases from other AEFI cases would help to better understand the type of events for which safety monitoring is conducted. According to WHO, serious AEFI or clusters of AEFIs also require prompt investigation and, therefore, could lend themselves to further evaluation, in particular for completeness and timeliness.
Passive AEFI surveillance is one of the most basic components of a vaccine safety monitoring system. The ratio of number of AEFI reports to surviving infants provides an indication of reporting magnitude. Several more advanced indicators are required for monitoring specific AEFI reports, the quality of those reports and the quality of the response to serious AEFIs [6], [11]. For specific known vaccine reactions, the sensitivity and specificity of safety surveillance systems could also be monitored [19]. In considering enhancements to current JRF variables, inclusion of the source of safety surveillance data could provide valuable information on the level of information exchange between immunization programs and National Regulatory Authorities. With rapid advancement and progress in health information technologies, new information technology solutions that help to collate, transmit, analyse and process vaccine safety information will lead to more robust, consistent and timely vaccine safety monitoring systems in improving AEFI detection and reporting [20], [21].
5. Conclusions
AEFI reporting has increased over the past sixteen years in all parts of the world but remains non-existent or irregular in a majority of LMIC. The AEFI reporting ratio is useful for benchmarking and following trends over time; however, this ratio does not provide information on the quality of the reporting system and does not guarantee capacity to detect and manage a vaccine safety problem at a national level. Additional efforts are required to ensure and improve data quality, AEFI reporting and surveillance of immunization safety in every country.
Acknowledgments
Acknowledgement
We would like to thank Laura Conklin, Abigail Shefer, Kimberley Fox and Tom Shimabukuro for their constructive review of this manuscript.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
No conflict of interest.
Footnotes
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official positions of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry and of the World Health Organization.
References
- 1.WHO, UNICEF, World Bank. State of the world’s vaccines and immunization, 3rd ed. Geneva, World Health Organization, 2009: World Health Organization; 2009. Available from: <http://apps.who.int/iris/bitstream/10665/44169/1/9789241563864_eng.pdf>.
- 2.World Health Organization. Adverse events following immunization (AEFI); 2016. Available from: <http://www.who.int/vaccine_safety/initiative/detection/AEFI/en/>.
- 3.Murphy T.V., Gargiullo P.M., Massoudi M.S., Nelson D.B., Jumaan A.O., Okoro C.A. Intussusception among infants given an oral rotavirus vaccine. New England J Med. 2001;344(8):564–572. doi: 10.1056/NEJM200102223440804. [DOI] [PubMed] [Google Scholar]
- 4.CDC. Withdrawal of rotavirus vaccine recommendation; 1999. Available from: <https://www.cdc.gov/mmwr/preview/mmwrhtml/mm4843a5.htm>. [PubMed]
- 5.World Health Organization. Global vaccine safety blueprint; 2012. Available from: <http://extranet.who.int/iris/restricted/bitstream/10665/70919/1/WHO_IVB_12.07_eng.pdf?ua=1>.
- 6.World Health Organization. Global vaccine safety blueprint. The landscape analysis; 2012. Available from: <http://apps.who.int/iris/bitstream/10665/70854/1/WHO_IVB_12.04_eng.pdf>.
- 7.Amarasinghe A., Black S., Bonhoeffer J., Carvalho S.M., Dodoo A., Eskola J. Effective vaccine safety systems in all countries: a challenge for more equitable access to immunization. Vaccine. 2013;31(Suppl. 2):B108–B114. doi: 10.1016/j.vaccine.2012.10.119. [DOI] [PubMed] [Google Scholar]
- 8.Chen R.T., Shimabukuro T.T., Martin D.B., Zuber P.L., Weibel D.M., Sturkenboom M. Enhancing vaccine safety capacity globally: a lifecycle perspective. Vaccine. 2015;33(Suppl. 4):D46–D54. doi: 10.1016/j.vaccine.2015.06.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO. Performance indicators for vaccine safety monitoring systems; 2015. Available from: <http://www.who.int/vaccine_safety/committee/topics/global_AEFI_monitoring/Dec_2014/en/>.
- 10.World Health Organization. Global vaccine action plan. Secretariat annual report 2016; 2016. Available from: <http://www.who.int/immunization/global_vaccine_action_plan/gvap_secretariat_report_2016.pdf?ua=1>.
- 11.World Health Organization. Global advisory committee on vaccine safety, 3–4 December 2014; 2015. Available from: <http://www.who.int/vaccine_safety/committee/reports/wer9004.pdf?ua=1>.
- 12.World Health Organization. WHO/UNICEF joint reporting process; 2016. Available from: <http://www.who.int/immunization/monitoring_surveillance/routine/reporting/en/>.
- 13.United Nations Population Division. The 2015 revision of world population prospects; 2015. Available from: <https://esa.un.org/unpd/wpp/Download/Standard/Population/>.
- 14.Graham J.E., Borda-Rodriguez A., Huzair F., Zinck E. Capacity for a global vaccine safety system: the perspective of national regulatory authorities. Vaccine. 2012;30(33):4953–4959. doi: 10.1016/j.vaccine.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 15.Liu D., Wu W., Li K., Xu D., Ye J., Li L. Surveillance of adverse events following immunization in China: past, present, and future. Vaccine. 2015;33(32):4041–4046. doi: 10.1016/j.vaccine.2015.04.060. [DOI] [PubMed] [Google Scholar]
- 16.Liang X.F., Li L., Liu D.W., Li K.L., Wu W.D., Zhu B.P. Safety of influenza A (H1N1) vaccine in postmarketing surveillance in China. New England J Med. 2011;364(7):638–647. doi: 10.1056/NEJMoa1008553. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y., Dong D., Cheng G., Zuo S., Liu D., Du X. Post-marketing surveillance of live-attenuated Japanese encephalitis vaccine safety in China. Vaccine. 2014;32(44):5875–5879. doi: 10.1016/j.vaccine.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Global manual on surveillance of adverse events following immunization; 2014. Available from: <http://www.who.int/entity/vaccine_safety/publications/aefi_surveillance/en/>.
- 19.Kohl K.S., Magnus M., Ball R., Halsey N., Shadomy S., Farley T.A. Applicability, reliability, sensitivity, and specificity of six Brighton Collaboration standardized case definitions for adverse events following immunization. Vaccine. 2008;26(50):6349–6360. doi: 10.1016/j.vaccine.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Ateudjieu J., Stoll B., Nguefack-Tsague G., Tchangou C., Genton B. Vaccines safety; effect of supervision or SMS on reporting rates of adverse events following immunization (AEFI) with meningitis vaccine (MenAfriVac): a randomized controlled trial. Vaccine. 2014;32(43):5662–5668. doi: 10.1016/j.vaccine.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Tsafack M., Ateudjieu J. Improving community based AEFI (Adverse Events Following Immunization) reporting rate through telephone “beep” in a Cameroon health district: a randomized field trial. Pan Afr Med J. 2015;22:351. doi: 10.11604/pamj.2015.22.351.8368. [DOI] [PMC free article] [PubMed] [Google Scholar]